Abstract
Introduction
Delirium predicts higher long-term cognitive morbidity. We previously identified a cohort of patients with spontaneous intracerebral hemorrhage and delirium, and found worse outcomes in Health Related Quality of Life (HRQoL) in the domain of Cognitive Function. We tested the hypothesis that agitation would have additional prognostic significance on later Cognitive Function HRQoL.
Materials and Method
Prospective identification of 174 patients with acute intracerebral hemorrhage, measuring stroke severity, agitation, and delirium with a standardized protocol and measures. HRQoL was assessed using the Neuro-QOL at 28 days, three months, and one year. Functional outcomes were measured with the modified Rankin Scale.
Results
Among the 81 patients with health related quality of life follow up data available, patients who had agitation and delirium had worse Cognitive Function HRQoL scores at 28 days (T-scores for delirium with agitation 20.9 ± 7.3, delirium without agitation 30.4 ± 16.5, agitation without delirium 36.6 ± 17.5, neither agitated nor delirious 40.3 ± 15.9, P=0.03), and at 1 year (P=0.006). The effect persisted in mixed models after correction for severity of neurologic injury, age, and time of assessment (P=0.0006), and was not associated with medication use, seizures or infection.
Conclusions
The presence of agitation with delirium in patients with intracerebral hemorrhage may predict higher risk of unfavorable cognitive outcomes up to one year later.
Keywords: Cognition Disorders, Delirium, Hyperkinesis, Psychomotor Agitation, Quality of Life, Stroke
Introduction
Hospitalized patients with delirium have high morbidity and mortality, and there has been increasing evidence of the particular impact on long-term cognitive function. (1–3) Hyperactive, hypoactive, and mixed subtypes of delirium have been described both historically and in DSM V, but there are limited data describing the prognostic implications of motor subtypes on cognition. (4–9) The etiology of delirium can be complex, and its varied pathophysiologic processes complicate study and can limit generalizability of outcomes. (10) While most reports describe delirium in patients with global conditions such as sepsis (11), focal brain injuries such as neurological disorders are common, and delirium in this context is an interesting model because there is relatively limited systemic inflammation, infection, or confounding medication administration. Delirium has been demonstrated to be common in acute ischemic stroke, estimated at 11–50%, and presages poorer functional outcomes. (12,13)
In the recent past, long term stroke outcomes research was based largely on motor symptoms, but the NIH recently developed the Neuro-QOL (Neuro-QOL) and the Patient Reported Outcomes Measurement Information System (PROMIS) to provide state-of-the-art outcomes that reflect the perspective of patients and caregivers, (14,15) and these have been validated against the current standard for outcomes assessment. Using these validated measures on a cohort of patients with intracerebral hemorrhage (ICH, spontaneous bleeding into brain tissue), we previously reported that patients with delirium had poorer functional outcomes at 28 days, and worse Cognitive Function Health Related Quality of Life (HRQoL) at 28 days, three months and one year than similar patients without delirium. (16) We then hypothesized that motor agitation with delirium (hyperactive delirium) would have additional prognostic significance.
Materials and Method
We prospectively identified 174 patients with acute ICH admitted from December 2009 through October 2014. All patients were diagnosed with ICH by a board-certified neurologist using computed tomography. Patients were excluded if their ICH was attributable to trauma, hemorrhagic conversion of ischemic stroke, or structural lesions (aneurysm, tumor, arteriovenous malformation or vessel dissection). Patients received nursing care with non-pharmacologic methods to prevent and address delirium, such as frequent reorientation and employing the participation of loved ones, as part of hospital protocol.
All patients with ICH were admitted to the Neuro/Spine ICU (NSICU) with a standardized order set. We prospectively recorded baseline demographic, past medical history, clinical data, and follow-up data. (16) Electroencephalographic (EEG) monitoring was routinely performed in unresponsive patients and interpreted by a board-certified epileptologist to evaluate for the presence of subclinical seizures. We assessed patients with the National Institutes of Health (NIH) Stroke Scale (NIHSS), a validated neurologic examination ranging from 0 (no deficit) to 42 (worst possible score), with a score of 8 or more indicating a moderately severe deficit. (17) We also assessed the ICH Score, a validated severity of injury scale from 0 (least severe) to 6 (most severe). (18)
Delirium and Agitation Assessment
The Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) was used to assess delirium, which has been validated for use in patients with acute stroke and neurological injury, including ICH. (19,12) As previously noted, a positive CAM-ICU indicated the presence of a change from the patient’s new baseline mental status (established on admission after ICH symptom onset) plus inattention and either an altered level of consciousness or disorganized thinking as previously described.
Level of arousal was assessed using the Richmond Agitation-Sedation Scale (RASS). (20) The RASS is scored from −5 (unresponsive) to +4 (combative, violent). For intubated patients, NSICU protocol treatment goal was 0 (alert and calm) to −2 (briefly awakens with eye contact to voice). A RASS of 2 (frequent non-purposeful movement or ventilator dys-synchrony) or greater was considered to indicate agitation.
The CAM-ICU and RASS were assessed twice per day by trained nursing staff during inpatient admission. CAM-ICU assessment and RASS assessment appear on the bedside ICU nurse’s task list every shift and require a response. These methods have been previously described. (16) We consider patients to have delirium with agitation if there was a positive CAM-ICU and RASS of 2 or more at any pointduring the hospital stay. Delirium without agitation indicates a positive CAM-ICU at any time with all RASS scores of less than 2 during the hospital stay. Agitation without delirium indicates a RASS of 2 or more, no positive CAM-ICU assessments, and at least one negative CAM-ICU assessment during the hospital stay, e.g., the patient could be assessed with the CAM-ICU at least once. If the RASS remained less than 2 and all CAM-ICU were negative during the entirety of the admission, the patient was considered neither agitated nor delirious. The CAM-ICU was marked as “unable to assess” if the patient could not be assessed with the CAM-ICU during the hospital stay due to unresponsiveness; if all CAM-ICU attempts were scored as “unable to assess” this indicated permanent coma and a dismal prognosis (see Results).
Follow Up Assessments
We assessed HRQoL with Neuro-QOL, a validated set of self-reported or proxy-reported measures that assess HRQoL for neurologic, oncologic and other health conditions. (14) Neuro-QoL was developed alongside of the NIH Patient Reported Outcomes Measurement Information System (PROMIS). (14,15) The Neuro-QoL domain of Cognitive Function assesses difficulty with tasks such as balancing a checkbook, remembering a list of errands, keeping track of important documents such as bills or insurance policies, and following instructions for medications. Results are expressed as T scores, where a score of 50 is centered on the mean of the general U.S. population (standard deviation=10); e.g., a score of 40 indicates HRQoL in the domain of Cognitive Function one standard deviation below the US population mean. Additional information regarding the development, validation and psychometrics are available at www.nihpromis.org and www.neuroqol.org.
Telephone interviews were available to patients without Internet access or by preference. Neuro-QOL was separately validated for proxy report as part of its development, and we have previously observed that correction for proxy report did not substantively change results in this clinical scenario. (21,22) We assessed HRQoL outcomes with Neuro-QoL at 28 days, three months, and one year after ICH symptom onset as previously described, either by the patient/caregiver on the web, or with study staff reading the questions and recording the responses by telephone. (16) Medications administered to the cohort were electronically retrieved.
The standard functional outcome score after stroke and ICH is the modified Rankin Scale (mRS), a validated measure of overall disability scored from 0 (no symptoms) to 6 (death) obtained by a structured interview. (23,24) A score of 2 or more indicates some motor disability, and a score of 4 or more indicates dependence on others for all self care. Patients or surrogates were queried at the time of admission about patient level of function immediately prior to ICH for baseline mRS. The mRS was subsequently assessed with the patient or a caregiver at discharge or at 14 days, and coincidentally with HRQoL assessment 28 days, three months and twelve months.
Standard Protocol Approvals, Registrations, and Patient Consents
The Institutional Review Board approved the study. Written informed consent was obtained from all individual participants included in the study, either from the patient or a legally authorized representative. The IRB issued an exemption from consent for patients who died while in the hospital, and non-communicative patients for whom a legally authorized representative could be not located.
Statistical Analysis
Differences in proportion were assessed with X2, using Fisher’s exact test when appropriate. Differences among group means were assessed for statistical significance using analysis of variance (ANOVA); multiple comparisons were corrected with the Least Significant Differences technique when the omnibus F-test was statistically significant. Assessments of continuous data normality were guided by histogram inspection and the Kolmogorov-Smirnov test, with significant deviations from normality triggering non-parametric statistics. When data were not sufficiently normally distributed, differences were assessed using the Kruskal-Wallis or Mann-Whitney U tests, as appropriate. When estimating the effect of agitation and delirium on T Scores of Cognitive Function HRQoL, we used mixed models to account for the dependency among T-scores induced by repeated assessments within individuals over time. Time of assessment was modeled as a fixed effect, and patients were considered random effects; the model also controlled for age and stroke severity measured with the NIH Stroke Scale as previously described. (16, 25) We have previously found that using the ICH Score (a composite score of ICH severity) rather than NIH Stroke Scale and age leads to unstable models. (26)
We carried out logistic regression on predictors of no disability (defined as mRS 0 or 1, as previously described). (16) We were unable to analyze the impact of delirium on functional impairment, measured with the mRS, with ordinal regression because the proportional odds assumption did not hold.
With 15 patients in each of the four groups, we anticipated 80% power to detect a 5 point difference in T Scores using mixed effects models (PASS v.12, NCSS Inc, Kaysville, UT). The analysis was carried out using standard statistical software (SPSS v. 21; IBM, Armonk, NY; NCSS v. 9, NCSS Inc., Kaysville, UT). A statistician from Neuro-QoL and the PROMIS Statistical Center who was not involved in the acquisition of data (J.L.B.) directed and reviewed the statistical analysis, with additional review for accuracy by author (RA).
Results
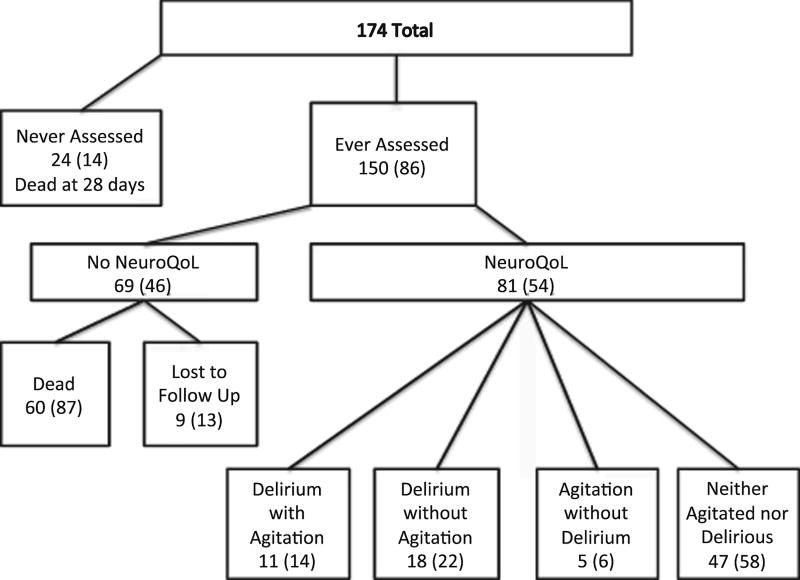
Demographic data are shown in Table 1. Of 174 enrolled patients, 150 were assessable using the CAM-ICU during the hospital stay and were considered “ever-assessed.” The other 24 patients were never assessable due to neurologic injury from ICH, and all were dead at 28 days. NIHSS and ICH in patients with follow up data had less severe ICH and NIHSS scores. Of the 150 ever-assessed patients, 53 (30%) patients had delirium (e.g., at least one CAM-ICU assessment was positive, “ever delirious”) and 97 (56%) were never delirious (e.g., all assessable CAM-ICU assessments were negative). Of the total cohort, 146 (84%) had a mRS of 0 (no symptoms) at baseline prior to ICH.
Table 1.
Demographics
| Variable | All Patients | NeuroQoL Patients | P value |
|---|---|---|---|
| N | 174 | 81 | |
| Age (mean) | 63.5 | 61 | 0.1 |
| Women | 82 (47) | 33 (41) | 0.2 |
| Race/Ethnicity | |||
| African-American | 75 (43) | 28 (35) | 0.1 |
| Caucasian | 81 (47) | 42 (52) | |
| Other | 18 (10) | 11 (14) | |
| NIHSS on admit | 11 | 7 | 0.02 |
| GCS on admit | 13.5 [8–15] | 14 [13–15] | 0.01 |
| Comatose on admit (GCS<=8) | 49 (28) | 8 (10) | <0.00001 |
| Comorbidity | |||
| CAD | 19 (10) | 6 (7) | |
| Atrial fibrillation | 13 (7) | 3 (3) | |
| Hypertension | 127 (73) | 61 (75) | |
| Diabetes mellitus | 30 (20) | 12 (15) | |
| Delirium/Agitation | 0.0002 | ||
| CAM ICU−/RASS− Neither agitated nor delirious | 83 (48) | 47 (58) | |
| CAM ICU+/RASS− Delirium without agitation | 36 (21) | 18 (22) | |
| CAM ICU−/RASS+ Agitation without delirium | 14 (8) | 5 (6) | |
| CAM ICU+/RASS+ Delirium with Agitation | 17 (10) | 11 (14) | |
| Never Assessable | 24 (14) | 0 |
Data are N (%) or median [Q1 – Q3] as appropriate. Patients who were never assessable were all dead by 28 days, and thus had no HRQoL scores.
Of the 150 patients that were ever-assessed, 81 had HRQoL follow up data. As in Table 1 and Figure 1, agitation and delirium measures for the 81 patients with follow up data for one year were as follows: Neither agitated nor delirious (CAM ICU-/RASS-) = 47 (58%); Delirium without agitation (CAM ICU+/RASS-) = 18 (22%); Agitation without delirium (CAM ICU-/RASS+) = 5 (6%); Delirium with Agitation (CAM ICU+/RASS+) = 11 (14%).
Figure 1.
As agitation is a component of the CAM-ICU and is generally considered indicative of delirium, we tested the hypothesis that agitation and delirium were associated. As expected, there was an association between agitation (RASS ≥ 2) and delirium (positive CAM-ICU, Likelihood ratio 6.4, p=0.01). Thus, it would be inappropriate to consider these variables as independent in subsequent analyses.
T scores for Cognitive Function HRQoL were significantly different at 28 days and one year (Table 2) between categories of delirium and agitation, with the worse scores in patients who had delirium with agitation.
Table 2.
Means of T Scores for Cognitive Function HRQoL, stratified by time of assessment and category of agitation and/or delirium. T Scores at 28 days and 12 months were different between groups. Differences between groups over time were significant in multivariate models (see text for details).
| Delirium with Agitation |
Delirium without Agitation |
Agitation without Delirium |
Neither Agitated nor Delirious |
N | P-value | |
|---|---|---|---|---|---|---|
| 28 days | 20.9 ± 7.3 | 30.4 ±16.5 | 36.6 ±17.5 | 40.4 ± 15.9 | 56 | 0.03 |
| 3 months | 24.8 ± 10.7 | 40.4 ± 19.0 | 49.2* | 37.9 ± 14 | 35 | 0.3 |
| 12 months | 20.5 ± 11.1 | 34.2 ± 12.9 | 44.0 ± 12.2 | 36.7 ± 14.6 | 31 | 0.002 |
No SD was calculated for this cell due to insufficient N.
In mixed models, the summary variable for delirium with agitation (delirium with agitation, agitation without delirium, delirium without agitation, neither agitated nor delirious) was associated with T Scores for Cognitive Function HRQoL (p=0.0006) after controlling for age (p=0.2 per year, 95%CI 0.02 - 0.4, p=0.02), NIHSS (1.3 per point, 95%CI 0.9 - 1.7, P<0.0001) and time of assessment. After correction for multiple comparisons, patients who had delirium with agitation had significantly worse T Scores in Cognitive Function HRQoL than the three other categories (p=0.006). We repeated the analysis classifying delirium and agitation as independent variables with an interaction term for delirium * agitation, and found similar results (i.e., the interaction term was significant, p=0.003, indicating that the combination provided additional predictive accuracy in the model).
As might be expected, there was a bias in that patients who did not have HRQoL measurements at follow-up were more likely to be dead than those with follow up data (p<0.001). Of the 69 patients without HRQoL follow-up data available, 60 died. Most mortality was early: 35 were dead by 14 days, an additional 11 were dead at 28 days, an additional eight were dead at three months, and the remainder were dead at one year. Of 81 patients with HRQoL data available, 34 were completed by telephone with study staff via interview, 24 by the patient on the Internet, and the remainder by a family member/caregiver on the internet, i.e., most HRQoL data was entered (47 of 81, 58%) by the patient or a family member/caregiver without assistance from study staff.
Patients who had delirium with or without agitation had a higher (worse) functional impairment, measured with the mRS, at 28 days (134 patients assessed at 28 days) compared to those who were not delirious, 4 [4 – 5] vs. 3 [2 – 4] (p=0.03). All patients who experienced delirium with agitation (i.e., positive CAM-ICU and RASS ≥ 2) had a mRS score of 2 or more at 28 days compared to other categories of CAM-ICU and RASS assessments (p=0.01).
To exclude the possibility that severity of neurologic injury would confound the classification of delirium with agitation, we compared the ICH Score between categories of agitation and delirium. The ICH Score was not different between the categories of agitation and delirium (p=0.3 by Kruskal-Wallis test), nor was the initial NIH Stroke Scale (a standardized neurologic examination, p=0.2 by Kruskal-Wallis test), nor age (p=0.8 by ANOVA).
Because time on the ventilator is potentially associated with more sedation and a higher risk of delirium, we compared the number of ventilator-free days (days the patient was alive and breathing without mechanical assistance) between categories of delirium with agitation. There was no significant difference in ventilator-free days between groups (p = 0.2). Among assessable patients, the median number of ventilator free days was 14, i.e., assessable patients typically did not require mechanical ventilatory support from the first 14 days after ICH onset.
Further, we compared the use of medications that might be associated with delirium with agitation. As noted in Table 3, midazolam, fentanyl, diazepam, phenytoin and levetiracetam administration was not different between groups. Benzodiazepines were sparingly used, if at all. As would be expected, more haloperidol was administered to patients that were classified as having delirium with agitation, although doses were modest, cumulatively under 2 mg. Of the six patients who received haloperidol, four had delirium with agitation, one had agitation without delirium, and one was neither agitated nor delirious. There was no association between haloperidol administration and T Scores for Cognitive Function HRQoL (p=0.6).
Table 3.
Cumulative medication administration with delirium and agitation status represented as median interquartile ranges. Only the cumulative administration of haloperidol was different between groups. When the cumulative administration was corrected for ICU length of stay, there were no differences between groups for any of the medications.
| Variables | Delirium with Agitation |
Agitation without Delirium |
Delirium without Agitation |
Neither Agitated nor Delirious |
P-value |
|---|---|---|---|---|---|
| Diazepam, mg | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0.9 |
| Fentanyl, mcg | 0 (0–250) | 106 (0–4132) | 0 (0–156) | 0 (0–100) | 0.1 |
| Haloperidol, mg | 0 (0–1.375) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0.000025 |
| Levetiracetam, mg | 0 (0–6500) | 12750 (0–20625) | 0 (0–1750) | 0-(0–7750) | 0.15 |
| Lorazepam, mg | 0 (0–1.25) | 0 (0–1.125) | 0 (0–0.875) | 0 (0–1) | 0.8 |
| Midazolam, mg | 0.5 (0–3.5) | 0 (0–2.5) | 0 (0–1) | 0 (0–1.375) | 0.8 |
| Phenytoin, mg | 0 (0–0) | 0 (0–2150) | 0- (0–0) | 0 (0–0) | 0.4 |
Conclusion
We found that survivors of ICH who had delirium with agitation had worse T Scores in Cognitive Function HRQoL up to a year after ICH than patients who had delirium without agitation, or patients without delirium. These data demonstrate that agitation could have additional prognostic implications for long term Cognitive Function HRQoL. (16)
While most patients with agitation were also classified as having delirium symptoms, the small number of patients who had agitation without delirium requires further study and clarification. The presence of agitation is also assessed within the CAM-ICU, and is thus a component of the diagnosis of delirium, although not an essential one in the scoring algorithm. (19, 27) In this study, “agitation without delirium” signifies an elevated RASS without corresponding positive CAM-ICU assessment, likely indicating the patient was able to complete an attention task despite agitation. Underscoring the general acceptance that agitation and delirium are related to each other, and acknowledging that agitation and delirium symptoms were correlated in our data, we analyzed agitation and delirium as one variable (delirium with agitation, delirium without agitation, etc.) rather than as two separate variables. (Accounting for agitation and delirium separately revealed a significant interaction between them.) Further validated ICU measurements for subtypes of delirium might be helpful, although other evidence based delirium assessments such as the ICDSC usually agree with the CAM-ICU. (28)
In a large study of four intensive care units, patients with hypoactive delirium had better cognitive HRQoL than those with other delirium subtypes, similar to the results described here. (29) A positive CAM-ICU prompted treatment with haloperidol per their protocol. The effect of haloperidol on Cognitive Function HRQoL in our patient population is not clear, as we had too few patients treated with haloperidol for a meaningful analysis. Our study also found different frequency of motor subtypes than their population of surgical and trauma critical care patients. (7)
Acute confusion due to seizure activity could be misdiagnosed for delirium, however, we found few patients with seizures despite routine EEG monitoring, making this unlikely. Additionally, seizure medications were not associated with agitation or delirium in this cohort. Seizures are common after ICH, and additional EEG patterns and the incidence of delirium would be of interest and are a topic for future research. (30)
There are several important limitations to these data. Patients all had ICH, which may limit generalizability to other clinical scenarios. The CAM-ICU and the RASS, however, have been previously shown to be assessable in neurologically injured patients as well as medical/surgical ICU populations, (13, 20) and our results broadly agree with previous work covering multiple ICUs. While Neuro-QoL measures could not be administered to a substantial number of patients, this was generally due to death, an all-too-common consequence of ICH. This may bias our results toward patients with less severe ICH. We do not have data specifically measuring baseline neurocognitive performance in our population, although the large majority of patients were asymptomatic prior to ICH (i.e., baseline mRS = 0 prior to ICH), so widespread cognitive decline prior to ICH is highly unlikely in our cohort. Caregivers sometimes reported Neuro-QoL outcomes on behalf of a patient unable to report for him/herself, however, Neuro-QOL measures were validated for proxy report as part of their development, and this did not meaningfully impact our results. (17, 23)
It is possible that we missed some delirium, which may be underdiagnosed, especially for patients without agitation; our study utilized trained nursing staff and obtained rates of delirium consistent with other reports. (30,31) More patients might have been classified as delirious if the CAM-ICU were assessed more frequently, but it is also possible that delirium lasting less than twelve hours may be less likely to effect outcomes. Delirium in patients with ICH is typically short lived, usually a single day, as opposed to several days of delirium in many medical and surgical ICU patients. (1,32)
The location of a brain lesion might make the development of delirium more or less likely. Right-hemisphere subcortical white matter, superior longitudinal fasciculus and parahippocampal gyrus, indicated increased odds of delirium in patients with ICH (OR=13, 95 % CI 3.9–43.3, P < 0.001). (33)
Neuro-QoL assesses patient- and caregiver-reported HRQoL for Cognitive Function and other specific domains of HRQoL. These outcomes are complementary to traditionally obtained functional outcomes such as the mRS and highlight the patient’s ability to complete cognitive tasks of daily independent life, such as balancing a checkbook, running errands and planning for events out of the routine. While we found that all patients who had delirium with agitation had some level ofmotor disability, HRQoL provides additional insights to outcomes that might be missed by a summary scale. (34)
Conclusions
Delirium with agitation, as determined by standard rating scales, was independently predictive of worse Cognitive Function HRQoL up to a year later in survivors of ICH. The presence of agitation in addition to delirium may have important diagnostic and prognostic implications, and measures of agitation should be considered in future research on delirium.
Table 4.
Agitation and ICH Contingency Table for patients with follow up Neuro QoL data
| RASS (−) | RASS (+) | |
|---|---|---|
| CAM (−) | Neither agitated nor delirious | Agitation without delirium |
| CAM (+) | Delirium without agitation | Delirium with agitation |
Acknowledgments
Dr. Naidech has consulted for the FDA (Center for Drug Evaluation and Research). Dr. Cella, and Ms Beaumont have received prior funding from NINDS for Neuro-QoL, contract HHSN271201200036C.
This project was supported by grant number K18HS023437 from the Agency for Healthcare Research and Quality to Dr. Naidech. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality.
The institution received funding from Northwestern University’s Clinical and Translational Sciences (UL1RR025741) and from the Agency for Healthcare Research and Quality. The infrastructure for automated retrieval was funded in part by National Institutes of Health through a grant to Northwestern University’s Clinical and Translational Sciences (UL1RR025741).
Dr. Liotta has received compensation from Edwards Life Sciences, Direct Flow Medical, and SAGE therapeutics for work performed as a clinical trials collaborator unrelated to this manuscript.
Dr. Maas received funding from the National Institute of Neurologic Disorders and Stroke (NINDS) grant K23NS092975.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
The remaining authors have disclosed that they do not have any potential conflicts of interest. Disclosure of off-label product use is limited to the use of haloperidol for agitation in the intensive care unit, and is mentioned only to demonstrate that it did not affect study results.
References
- 1.Pandharipande PP, Girard TD, Jackson JC, Morandi A, Thompson JL, Pun BT, Brummel NE, Hughes CG, Vasilevskis EE, Shintani AK, Moons KG, Geevarghese SK, Canonico A, Hopkins RO, Bernard GR, Dittus RS, Ely EW, BRAIN-ICU Study Investigators Long-term cognitive impairment after critical illness. N Engl J Med. 2013 Oct 3;369(14):1306–16. doi: 10.1056/NEJMoa1301372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brummel NE, Jackson JC, Pandharipande PP, Thompson JL, Shintani AK, Dittus RS, Gill TM, Bernard GR, Ely EW, Girard TD. Delirium in the ICU and subsequent long-term disability among survivors of mechanical ventilation. Crit Care Med. 2014 Feb;42(2):369–77. doi: 10.1097/CCM.0b013e3182a645bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saczynski JS, Marcantonio ER, Quach L, Fong TG, Gross A, Inouye SK, Jones RN. Cognitive trajectories after postoperative delirium. N Engl J Med. 2012 Jul 5;367(1):30–9. doi: 10.1056/NEJMoa1112923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boettger Soenke, William Breitbart. "Phenomenology of the subtypes of delirium: Phenomenological differences between hyperactive and hypoactive delirium". Palliative and Supportive Care. 2011 Jun;9(2):129–35. doi: 10.1017/S1478951510000672. [DOI] [PubMed] [Google Scholar]
- 5.American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5™ (5th ed.) Arlington, VA: American Psychiatric Publishing, Inc.; 2013. [Google Scholar]
- 6.Yang FM, Marcantonio ER, Inouye SK, Kiely DK, Rudolph JL, Fearing MA, Jones RN. Phenomenological subtypes of delirium in older persons: patterns, prevalence, and prognosis. Psychosomatics. 2009 May-Jun;50(3):248–54. doi: 10.1176/appi.psy.50.3.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pandharipande P, Cotton BA, Shintani A, Thompson J, Costabile S, Pun BT, Dittus R, Ely EW. Motoric subtypes of delirium in mechanically ventilated surgical and trauma intensive care unit patients. Intensive Care Medicine. 2007 Oct;33(10):1726–31. doi: 10.1007/s00134-007-0687-y. [DOI] [PubMed] [Google Scholar]
- 8.Marcantonio E, Duthie E, Resnick NM. Delirium severity and psychomotor types: their relationship with outcomes after hip fracture repair. J Am Geriatr Soc. 2002 May;50(5):850–7. doi: 10.1046/j.1532-5415.2002.50210.x. [DOI] [PubMed] [Google Scholar]
- 9.Meagher DJ, Leonard M, Donnelly S, Conroy M, Adamis D, Trzepacz PT. A longitudinal study of motor subtypes in delirium: relationship with other phenomenology, etiology, medication exposure and prognosis. Journal of Psychosomatic Research. 2011;71(6):395–403. doi: 10.1016/j.jpsychores.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 10.Maldonado JR. Neuropathogenesis of delirium: review of current etiologic theories and common pathways. Am J Geriatr Psychiatry. 2013 Dec;21(12):1190–222. doi: 10.1016/j.jagp.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 11.Pun BT, Ely EW. The importance of diagnosing and managing ICU delirium. Chest. 2007 Aug;132(2):624–36. doi: 10.1378/chest.06-1795. [DOI] [PubMed] [Google Scholar]
- 12.Mitasova A, Kostalova M, Bednarik J, Michalcakova R, Kasparek T, Balabanova P, Dusek L, Vohanka S, Ely EW. Poststroke delirium incidence and outcomes: validation of the confusion assessment method for the intensive care unit (CAM-ICU) Crit Care Med. 2012;40:484–490. doi: 10.1097/CCM.0b013e318232da12. [DOI] [PubMed] [Google Scholar]
- 13.Oldenbeuving AW, de Kort PLM, Jansen BPW, Algra A, Kappelle LJ, Roks G. Delirium in the acute phase after stroke: incidence, risk factors, and outcome. Neurology. 2011;76:993–999. doi: 10.1212/WNL.0b013e318210411f. [DOI] [PubMed] [Google Scholar]
- 14.Cella D, Lai JS, Nowinski CJ, Victorson D, Peterman A, Miller D, Bethoux F, Heinemann A, Rubin S, Cavazos JE, Reder AT, Sufit R, Simuni T, Holmes GL, Siderowf A, Wojna V, Bode R, McKinney N, Podrabsky T, Wortman K, Choi S, Gershon R, Rothrock N, Moy C. Neuro-QOL: brief measures of health-related quality of life for clinical research in neurology. Neurology. 2012 Jun 5;78(23):1860–7. doi: 10.1212/WNL.0b013e318258f744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cella D, Yount S, Rothrock N, Gershon R, Cook K, Reeve B, Ader D, Fries JF, Bruce B, Rose M, PROMIS Cooperative Group The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. Journal of clinical epidemiology. 2010 Nov;63(11):1179–94. doi: 10.1016/j.jclinepi.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naidech AM, Beaumont JL, Rosenberg NF, Maas MB, Kosteva AR, Ault ML, Cella D, Ely EW. Intracerebral Hemorrhage and Delirium Symptoms Length of Stay, Function, and Quality of Life in a 114-Patient Cohort. American Journal of Respiratory Critical Care Medicine. 2013 Dec 1;188(11):1331–7. doi: 10.1164/rccm.201307-1256OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brott T, Adams HP, Jr, Olinger CP, Marler JR, Barsan WG, Biller J, Spilker J, Holleran R, Eberle R, Hertzberg V, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. 1989;20:864–870. doi: 10.1161/01.str.20.7.864. [DOI] [PubMed] [Google Scholar]
- 18.Hemphill JC, Bonovich DC, Besmertis L, Manley GT, Johnston SC. The ICH score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke. 2001 Apr;32(4):891–7. doi: 10.1161/01.str.32.4.891. [DOI] [PubMed] [Google Scholar]
- 19.Ely EW, Margolin R, Francis J, May L, Truman B, Dittus R, Speroff T, Gautam S, Bernard GR, Inouye SK. Evaluation of delirium in critically ill patients: validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) Crit Care Med. 2001 Jul;29(7):1370–9. doi: 10.1097/00003246-200107000-00012. [DOI] [PubMed] [Google Scholar]
- 20.Ely EW, Truman B, Shintani A, Thomason JW, et al. Monitoring sedation status over time in ICU patients: reliability and validity of the Richmond Agitation-Sedation Scale (RASS) JAMA. 2003 Jun 11;289(22):2983–91. doi: 10.1001/jama.289.22.2983. [DOI] [PubMed] [Google Scholar]
- 21.Liu H, Cella D, Gershon R, Shen J, Morales LS, Riley W, Hays RD. Representativeness of the PROMIS Internet panel. J Clin Epidemiol. 2010 Nov;63(11):1169–78. doi: 10.1016/j.jclinepi.2009.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Naidech AM, Beaumont JL, Berman M, Liotta E, Maas MB, Prabhakaran S, Kording K, Holl J, Cella D. Web-Based Assessment of Outcomes After Subarachnoid and Intracerebral Hemorrhage: A New Patient Centered Option for Outcomes Assessment. Neurocrit Care. 2015 Aug;23(1):7. doi: 10.1007/s12028-014-0098-1. [DOI] [PubMed] [Google Scholar]
- 23.Banks JL, Marotta CA. Outcomes Validity and Reliability of the modified Rankin Scale: implications for stroke clinical trials, a literature review and synthesis. Stroke. 2007 Mar;38(3):1091–6. doi: 10.1161/01.STR.0000258355.23810.c6. [DOI] [PubMed] [Google Scholar]
- 24.Saver JL, Filip B, Hamilton S, Yanes A, Craig S, Cho M, Conwit R, Starkman S, FAST-MAG Investigators and Coordinators Improving the reliability of stroke disability grading in clinical trials and clinical practice: the Rankin Focused Assessment (RFA) Stroke. 2010 May;41(5):992–5. doi: 10.1161/STROKEAHA.109.571364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shintani AK, Girard TD, Eden SK, Arbogast PG, Moons KG, Ely EW. Immortal time bias in critical care research: application of time- varying Cox regression for observational cohort studies. Crit Care Med. 2009;37:2939–2945. doi: 10.1097/CCM.0b013e3181b7fbbb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Naidech AM, Garg RK, Liebling S, Levasseur K, Macken MP, Schuele SU, Batjer HH. Anticonvulsant use and outcomes after intracerebral hemorrhage. Stroke. 2009;40:3810–3815. doi: 10.1161/STROKEAHA.109.559948. [DOI] [PubMed] [Google Scholar]
- 27.Peterson JF, Pun BT, Dittus RS, Thomason JW, Jackson JC, Shintani AK, Ely E. Delirium and its motoric subtypes: a study of 614 critically ill patients. Journal of the American Geriatrics Society. 2006;54(3):479–484. doi: 10.1111/j.1532-5415.2005.00621.x. [DOI] [PubMed] [Google Scholar]
- 28.Plaschke K, von Haken R, Scholz M, Engelhardt R, Brobeil A, Martin E, Weigand MA. Comparison of the confusion assessment method for the intensive care unit (CAM-ICU) with the Intensive Care Delirium Screening Checklist (ICDSC) for delirium in critical care patients gives high agreement rate(s) Intensive Care Med. 2008 Mar;34(3):431–6. doi: 10.1007/s00134-007-0920-8. [DOI] [PubMed] [Google Scholar]
- 29.van den Boogaard M, Schoonhoven L, Evers AW, van der Hoeven JG, van Achterberg T, Pickkers P. Delirium in critically ill patients: Impact on long-term health-related quality of life and cognitive functioning. Critical care med. 2012;40(1):112–118. doi: 10.1097/CCM.0b013e31822e9fc9. [DOI] [PubMed] [Google Scholar]
- 30.Vespa PM, O’Phelan K, Shah M, Mirabelli J, Starkman S, Kidwell C, Saver J, Nuwer MR, Frazee JG, McArthur DA, et al. Acute seizures after intracerebral hemorrhage: a factor in progressive midline shift and outcome. Neurology. 2003;60:1441–1446. doi: 10.1212/01.wnl.0000063316.47591.b4. [DOI] [PubMed] [Google Scholar]
- 31.Shehabi Y, Riker RR, Bokesch PM, Wisemandle W, Shintani A, Ely EW, SEDCOM (Safety and Efficacy of Dexmedetomidine Compared With Midazolam) Study Group Delirium duration and mortality in lightly sedated, mechanically ventilated intensive care patients. Crit Care Med. 2010 Dec;38(12):2311–8. doi: 10.1097/CCM.0b013e3181f85759. [DOI] [PubMed] [Google Scholar]
- 32.Meagher DJ, Leonard M, Donnelly S, Conroy M, Adamis D, Trzepacz PT. A longitudinal study of motor subtypes in delirium: frequency and stability during episodes. Journal of Psychosomatic Research. 2012;72(3):236–241. doi: 10.1016/j.jpsychores.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 33.Naidech AM, Polnaszek KL, Berman MD, Voss JL. Hematoma Locations Predicting Delirium Symptoms After Intracerebral Hemorrhage. Neurocrit Care. 2016 Jun;24(3):397–403. doi: 10.1007/s12028-015-0210-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Naidech AM, Beaumont JL, Berman M, Francis B, Liotta E, Maas MB, Prabhakaran S, Holl J, Cella D. Dichotomous "Good Outcome" Indicates Mobility More Than Cognitive or Social Quality of Life. Crit Care Med. 2015 Aug;43(8):1654–1659. doi: 10.1097/CCM.0000000000001082. [DOI] [PMC free article] [PubMed] [Google Scholar]