Abstract
Sustained retention in HIV medical care is a key health behavior for the long-term health of people living with HIV (PLWH). Approximately 60% of PLWH in the U.S. are poorly retained in HIV care, yet to date, the few available evidence-based retention-promoting interventions are resource and time intensive to implement. The current study describes the feasibility and acceptability of a theory-based retention-promoting intervention designed to meet the needs of a busy clinical care setting. 60 Minutes for Health reflects a low-resource single-session intervention, implemented by a health educator, to PLWH who have had a recent gap in care (≥6-months) in the past 18-months. Intervention content was informed by a situated application of the Information Motivation Behavioral Skills Model and delivered using a Motivational Interviewing-based format. The intervention uses a workbook to guide a series of activities that: 1) Identify and reduce misinformation guiding HIV care attendance. 2) Enhance motivation to maintain care via personal health goals. 3) Build skills for coping with emotional distress related to living with HIV. 4) Increase self-efficacy for navigating the logistics of maintaining care amidst competing priorities. A small feasibility pilot of this intervention protocol was conducted to assess its potential to improve retention in care and to obtain estimates for a larger-scale efficacy trial. Participants were randomized to the 60-minute intervention session (n=8), or a theory-based time-and-attention control session focused on diet and nutrition (n=8). Medical records were abstracted to evaluate changes in participants’ retention in care status at 12- and 24-months post-intervention. Findings suggest the intervention is both feasible and acceptable to implement with poorly retained PLWH in a clinic setting. Post-intervention a larger proportion of intervention participants were retained in care (12-months: 87.5%, 24-months: 62.5%), compared control participants (12-months: 50.0%, 24-months: 25.0%). Future work should aim to evaluate a larger-scale efficacy trial.
Keywords: HIV, Retention in Care, Brief Intervention, Theory-based
INTRODUCTION
Retention in care for people living with HIV (PLWH) supports improved individual health, viral suppression, and reduced risk of subsequent HIV transmission (Giordano et al., 2007; Mayer, 2011). In the U.S., approximately 60% of PLWH are poorly retained in HIV care (Bradley et al., 2014), with 63% of all new HIV infections attributed to this group (Skarbinski et al., 2015). Efforts to improve retention in HIV care are critical to individual- and public health, reflecting a top priority of the National HIV/AIDS Strategy (Office of National AIDS Policy, 2015).
Increased retention-promotion efforts have identified few efficacious strategies over the past decade (Higa, Crepaz, & Mullins, 2016); most of which are relatively resource and time intensive to implement. Such strategies include modifying patient monitoring systems (Bove, Golden, Dhanireddy, Harrington, & Dombrowski, 2015; Robbins et al., 2012), or changing how clinics coordinate and deliver patient care (Davila et al., 2013; Enriquez et al., 2008; Lucas et al., 2010). Other strategies seek to reach patients through enhanced social marketing and support services (Hightow-Weidman, Smith, Valera, Matthews, & Lyons, 2011), or require large time commitments from clinic staff to maintain frequent contact with patients (Bove et al., 2015; Craw et al., 2008; Gardner et al., 2005; Gardner et al., 2014; Irvine et al., 2015). One exception was the Stay Connected intervention which promoted significant, though modest, improvements in retention outcomes through coordinated messages about the importance of retention in care via brochures, clinic posters, and brief provider-delivered messages (Gardner et al., 2012).
Additional retention strategies designed to work within the time and resource constraints of existing medical systems could help to increase the number of adequately retained PLWH in the U.S. To address this need, 60 Minutes for Health, a theory-based, low-resource, single-session intervention was developed to be implemented in a busy clinic setting by lay staff to patients with a gap in care (≥6-months) over the past 18-months. This study describes and evaluates a small acceptability and feasibility pilot of the 60 Minutes for Health intervention.
METHODS
Trial Design
The 60 Minutes for Health protocol was implemented using a rigorous randomized time-and-attention control trial. Participants could have one of three affiliations with the medical system in which this trial took place: 1) Accessing affiliated HIV care, 2) Accessing affiliated substance use treatment only, or 3) No longer accessing any affiliated services (non-affiliated participants). Randomization was blocked in groups of six by participants’ affiliation status. Within each block, randomization allocated participants (1:1) to one of two theory-based 60-minute sessions focused on retention in HIV care (intervention) or diet and nutrition (control).
Setting
The intervention was piloted in the Bronx, NY in affiliation with a large medical system that provides integrated HIV care and access to in-house ancillary services (e.g., mental health, adherence support) at seven community-based clinics and one substance use treatment clinic. These sites predominately serve a low-income ethnic/racial minority population. As a feasibility pilot, intervention sessions were held in exam rooms or office space at two different clinical settings.
Participants
Eligibilie participants were: 1) ≥18 years old, 2) HIV-positive and have initiated HIV care ≥24-months before recruitment, 3) comfortable communicating in English for ~3 hours, and 4) ‘poorly retained’ (i.e., having a gap in care of ≥6-months over the previous 18-months).
Recruitment and Enrollment
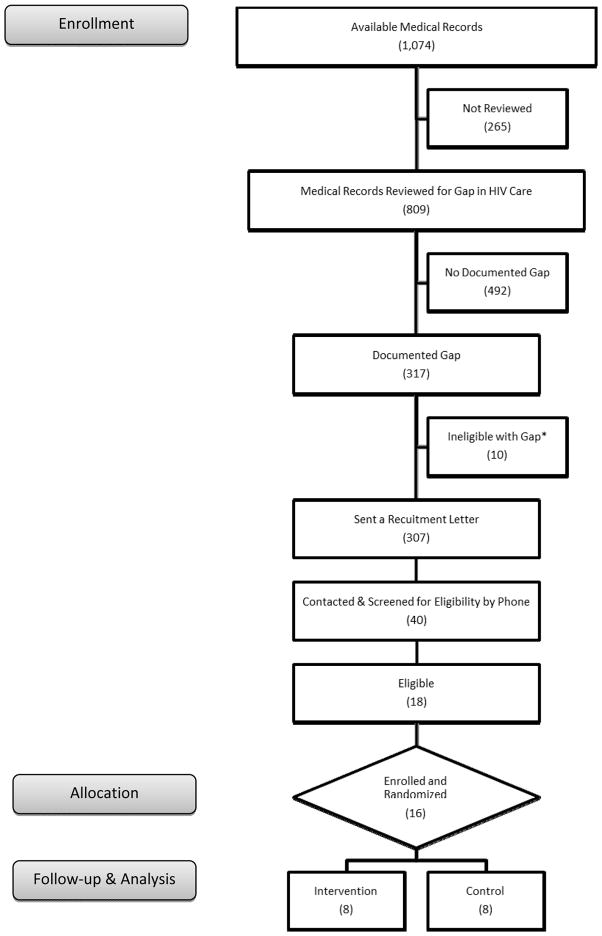
Recruitment occurred from 08-April-2013 to 24-May-2013 and from 08-July-2013 to 16-August-2013; 13-weeks total (Figure 1). In that time, medical chart reviews identified 307 poorly retained patients, and 40 were reached by phone to be screened for eligibility. Eighteen of those screened were eligible, and 16 were enrolled (1 declined participation, 1 lacked transportation to study site).
Figure 1. Recruitment and Enrollment of Poorly Retained Patients Living with HIV (N=16).
* Monolingual Spanish-speaking or recently diagnosed HIV-positive (≤ 24-months)
Procedures
All participant procedures were completed in a single visit totaling ≤ 3 hours. Eligible participants were invited to one of two participating clinical care settings where they were: 1) consented, 2) completed a 30-minute pre-test assessment via Audio-Computer Assisted Self-Interview (ACASI), 3) immediately randomized to participate in either the intervention or control condition for a 60-minute interactive session, 4) completed a 30-minute immediate post-test ACASI-delivered assessment, and 5) remunerated $45 for their time and travel. Study procedures were approved by the affiliated institutional review board.
Intervention
Theoretical Framework
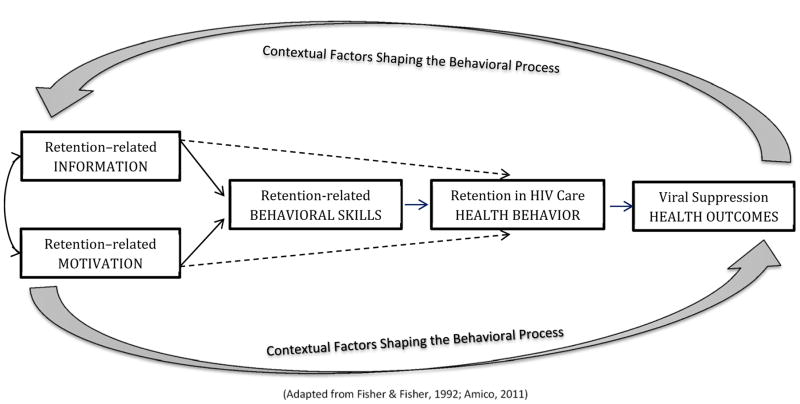
Intervention development was informed by a situated application of the Information, Motivation, Behavioral Skills (sIMB) model (Amico, 2011; J. D. Fisher, Fisher, Amico, & Harman, 2006; J. D. Fisher & Fisher, 1992). This model (Figure 2) proposes that enhancing HIV care-related information and bolstering personal and social motivation for engaging in care will support building the requisite level of behavioral skills needed to access routine HIV care over time and across diverse situations. In turn, overcoming these deficits will promote stronger retention resulting in improved health outcomes. These improvements are hypothesized to create a feedback loop reinforcing sustained retention in HIV care (Amico, 2011; W. A. Fisher, Fisher, & Harman, 2003). To situate this behavioral process, elicitation work examined how contexts known to impact retention might inform the types of information, motivation, and behavioral skills in need of targeted intervention (Smith, Fisher, Cunningham, & Amico, 2012). These contextual factors included substance use, depression, transportation, competing priorities, and how one feels about living with a life-long, often stigmatized HIV diagnosis.
Figure 2.
A situated application of the Information, Motivation, Behavioral Skills (sIMB) Model applied to Retention in HIV Care
The elicitation work (Smith et al., 2012) identified a need to target HIV care-related misinformation, especially ways it feeds into implicit rules (i.e., heuristics) guiding decisions to delay HIV care (e.g., I feel OK, so there is no reason to see my HIV doctor). This also included a need to address participants’ misperceptions that they ‘never miss appointments,’ or ‘don’t go that long without seeing their doctor’ despite documented gaps in their medical records. Attitudes and beliefs in need of targeted motivational support reflected being less concerned about HIV than other physical health conditions (e.g., diabetes, hypertension), feeling that depression or active substance use negated their own- or their provider’s ability to address their HIV, and feeling distressed by the physical and emotional changes experienced in relation to living with HIV. Likewise, low self-efficacy for coping with negative feelings, and for prioritizing HIV care appointments when faced with competing priorities or faulty heuristics, suggested that stronger behavioral skills are needed to overcome these information and motivational deficits.
Intervention Approach
The intervention was designed to minimize time and resource constraints of a busy clinic. A health educator guides participants through the semi-structured intervention activities during a 60-minute visit--the maximum billable time frame for a health education session. An illustrated workbook was developed to be accessible to a range of literacy levels, while its ‘portability’ minimizes disruptions to clinic flow and receipt of care. This flexibility allows the 60-minute intervention session to be implemented to patients presenting with a recent gap in HIV care in the clinic setting as soon as space and time are available.
Intervention Delivery
A Motivational Interviewing (MI) -based communication approach (Miller & Rose, 2009; Rollnick & Miller, 1995) is used to engage participants in the intervention activities through a non-judgmental, collaborative conversation that positions them as the ‘expert’ on the situations affecting their HIV care decisions. This enables the health educator to elicit sIMB deficits contributing to poor retention while allowing participants to define what meaningful steps are needed to address these deficits. This MI-based approach has a long-standing history of being successfully paired with interventions informed by the Information, Motivation, Behavioral Skills Model (J. D. Fisher et al., 2006; Konkle-Parker, Erlen, Dubbert, & May, 2012) and similar single-session interventions targeting HIV prevention and treatment behaviors (Outlaw et al., 2010; Safren et al., 2001; Simbayi et al., 2004; Wolfers, de Wit, Hospers, Richardus, & de Zwart, 2009).
Retention-promotion Intervention
The theory-based intervention activities (Table 1) guide participants through four distinct sections developed to identify and address critical sIMB deficits (Amico, 2011; Smith et al., 2012). Section 1. Focusing on my physical health aims to normalize retention in care as a challenging long-term health behavior, identify and correct retention-related misinformation and faulty heuristics participants use to decide whether to attend or delay routine HIV care visits. This section further elicits participants’ physical health priorities that might be leveraged to improve retention in care. Section 2. Focusing on my emotional health seeks to address previous findings that how one feels about living with HIV can present as a major motivational and behavioral skills barrier to sustained retention in care (Smith et al., 2012). Specifically, participants explore emotions they frequently feel about living with HIV and how those feelings might facilitate or impede routine HIV care visits. They then identify and practice behavioral skills for coping with these emotions. To strengthen these skills, participants are provided with materials to practice brief affect-management exercises at home. Section 3. Building on my HIV care history helps participants to identify when they experienced gaps in care over the previous 18-months and explores motivations, behavioral skills, and contexts (e.g., competing priorities, substance use) affecting their recent retention history. This discussion is used to strategize how best to navigate similar challenges and leverage personal strengths to promote better retention in the following 12-months. Section 4. Achieving my personal health goals works to integrate the previous discussions to support participants in identifying a personal health goal, and in developing a targeted action plan for building participants’ information, motivation, behavioral skills, and resources needed to attain this goal.
Table 1. 60-Minutes for Health.
Intervention Workbook Sections, Time Allocations, Goals and Activities
| Section 1 Focusing on my physical health |
Section2 Focusing on my emotional health |
Section3 Building on my HIV care history |
Section 4 Achieving my personal health goals |
|---|---|---|---|
|
| |||
| Time: 10 minutes | Time: 20 minutes | Time: 15 minutes | Time: 15 minutes |
|
| |||
|
Goals and
Activities: Normalize and acknowledge retention in care challenges
|
Goals and
Activities: Identify the types of emotions, both positive and negative, participants frequently feel about living with HIV
|
Goals and
Activities: Elicit contexts aiding or impeding the participants’ retention in HIV care history over the previous 18-months
|
Goals and
Activities: Build on the ways participants see their physical and mental health as relating to their retention in care to identify a personal health goal
|
To strengthen these behavioral skills, participants are provided a printed instructions and an audio-CD containing brief affect management exercises to implement at home.
Time-and-Attention Control Condition
This session was adapted from Project Eban’s health promotion arm (El-Bassel et al., 2011; Jemmott, 2008) because it is theory-based, informed by Bandura’s Social Cognitive Theory (Bandura, 1998), developed for a similar target population, and successfully improved participants’ diet and nutrition behaviors. Adaptations were made to enhance the visual presentation of the diet and nutrition content vis-à-vis the development of a workbook, and to allow the intervention to be delivered through the same MI-based approach in an equally engaging and interactive one-on-one 60-minute session. To complement home-based skills building activities, participants were given a set of measuring cups and a booklet of culturally-aligned healthy recipes. These adaptations facilitated a meaningful and rigorous time-and-attention comparison condition.
Measures
Study data were collected from ACASI-delivered immediate pre-post assessments and participants’ medical records. Descriptive pre-test data included: sociodemographics, HIV treatment history, mental health items developed for this study, and barriers to HIV care (Kalichman, Catz, & Ramachandran, 1999). Participants self-reported any current mental health diagnosis and whether they were accessing treatment (medications, therapy) for that condition (1=Yes, 0=No). The Addiction Severity Index (McLellan et al., 1992) was used to assess substance use in the past 30-days (1=Yes, 0=No: drinking to the point of intoxication, use of cannabis or illicit drugs [cocaine, heroin, other opiates]). Physical health measures included a validated 1-item measure of perceived health (poor, fair, good, excellent) (Bowling, 2005), and the total number of self-reported comorbid health diagnoses commonly affecting PLWH (Range: 0–7) (Chu et al., 2011; Crum et al., 2006).
Post-test ACASI data collected our primary outcomes, acceptability and feasibility, on a range of metrics adapted from previous studies (Calvin, 2010; Zauszniewski, 2012) to reflect the structure of the current intervention. Participants responded to 21-items based on their respective participation in the intervention or control session (see Table 4). Acceptability of the program’s content and delivery measured: 1) the overall program (1-item), 2) the program’s topic (Retention or Diet and Nutrition; 3-items), 3) the facilitator (3-items), and 4) the workbook activities (3-items). Acceptability of program participation measured: 5) the program’s appeal (3-items), 6) perceived costs and benefits of participation (4-items), and any experiences of 7) physical (1-item) or 8) emotional distress (1-item) related to participation. Program feasibility measures reflected: 1) participants’ perceived ability to implement what they learned in the next 6-months (1-item), and 2) participants’ ability to finish the entire 60-minture program in a single-session (1-item; 1=Yes, 0=No). With one exception, responses were given on a 5-point Likert-type scale and recoded so that more favorable assessments are reflected in higher ratings (1=Least favorable, 5=Most favorable). A mean composite score was created for sub-scales with ≥3-items. Mean scores for the eight acceptability metrics and the two feasibility metrics were computed for the total sample and each study arm.
Table 4.
Post-test 60 Minutes for Health Intervention Acceptability and Feasibility
| Total Sample (N=16) | Retention Arm (n=8) | Diet/Nutrition Arm (n=8) | Difference Test | |
|---|---|---|---|---|
| M (SD) | M (SD) | M (SD) | p-value* | |
| Acceptability: Program Content and Delivery | ||||
| Overall 60-minute Program | 4.75 (0.45) | 4.75 (0.46) | 4.75 (0.46) | p= .999 |
| 1. Overall, how would you rate this program? | ||||
| Program Topic Score | 4.35 (0.49) | 4.29 (0.45) | 4.42 (0.56) | p= .630 |
| 2. The program has been helpful to me | ||||
| 3. I was satisfied with the information the program provided | ||||
| 4. I think the program was personally relevant | ||||
| Program Facilitator Score | 4.77 (0.40) | 4.83 (0.36) | 4.71 (0.45) | p= .549 |
| 5. I think the program facilitator was understanding | ||||
| 6. I think the program facilitator communicated clearly | ||||
| 7. I think the program facilitator was friendly | ||||
| Workbook Activities Score | 4.56 (0.45) | 4.63 (0.45) | 4.50 (0.47) | p= .597 |
| 8. The workbook exercises helped me learn about myself | ||||
| 9. I think the workbook exercises were easy to complete | ||||
| 10. I think the workbook exercises were interesting | ||||
| Acceptability: Program Participation | ||||
| Appeal of the 60-minute program | 4.46 (0.47) | 4.29 (0.45) | 4.63 (0.45) | p=.162 |
| 11. I would recommend this program to a friend | ||||
| 12. I would be interested in continuing a program like this | ||||
| 13. I am satisfied that I took part in this program | ||||
| Costs and Benefits of participation | 4.19 (0.48) | 4.16 (0.27) | 4.22 (0.65) | p=.806 |
| 14. I think there were too many exercises (R) | ||||
| 15. I think the program time requirements were reasonable | ||||
| 16. I think the exercises were worthwhile | ||||
| 17. I think this program is something I needed | ||||
| Physical distress from participation | 4.81 (0.54) | 4.88 (0.35) | 4.75 (0.71) | p= .662 |
| 18. Did your participation cause you any physical distress? | ||||
| Emotional distress from participation | 4.75 (0.58) | 4.88 (0.35) | 4.63 (0.74) | p= .405 |
| 19. Did your participation cause you any emotional distress? | ||||
| Program Feasibility | ||||
| 20. It will be hard to implement what I learned in the next 6-month(R) | 3.44 (1.31) | 3.63 (1.19) | 3.25 (1.49) | p= .586 |
| 21. I was able to finish the entire 60-minute program today | Yes (n = 16) | Yes (n = 8) | Yes (n = 8) | a |
Response Scale: 1=least favorable - 5=most favorable.
2-tail significance test (p< .05).
Difference test could not be computed.
Reverse coded.
Medical records data were abstracted by study staff with clinical experience at baseline and 24-months post-baseline. These data were used to document our secondary outcome, retention in care across three 12-month intervals: 1) 12-months pre-intervention, 2) 12-moths post-intervention, and 3) 24-months post-intervention (i.e., months 13–24). HIV care visits were defined as documented visits with an antiretroviral-monitoring provider. For each 12-month interval, retention in care was evaluated by first computing the number of quarters (3-month intervals) with a documented HIV care visit, and then by documenting (1=Yes, 0=No) whether the participant met the HRSA definition of retention in HIV care (≥2 HIV care visits separated by at least 90-days in a 12-month interval) (HRSA, Updated January 2015). Since patients intermittently attended HIV care, HIV viral load and CD4 values were not reliably available, yielding missing data during gaps in care. Prior research has established a consistent association between retention in care and clinical outcomes (Giordano et al., 2007; Mugavero et al., 2009; Mugavero et al., 2012). As a feasibility pilot, our focus was specifically on retention in care following the intervention, and HIV lab data were not abstracted.
Statistical Analysis
Descriptive statistics were used to characterize the study sample, our primary outcomes (mean intervention acceptability and feasibility scores), and retention in care (secondary outcome). Group differences by study arm on these metrics were further explored using bivariate statistics. While we did not anticipate comparisons to reach conventional levels of statistical significance in this small pilot, we applied these tests to explore the adequacy of randomization on all pre-intervention metrics (p< .05, 2-tailed). They were also used to explore potential differences in participants’ retention in care post-intervention, hypothesizing that we would observe trends towards better retention in the intervention arm compared to the control (p< .05, 1-tail).
RESULTS
Participant Characteristics
In general, participants in both study arms were similar (Table 2). The majority were middle-aged (M=48.75, SD=10.76), female (62.5%), and identified as either Hispanic/Latino (37.5%) or non-Hispanic Black (62.5%). Most had less than a high school education (62.5%) and were unemployed (43.8%) or on disability (31.3%), while >80% earned <$20,000 annually but stably housed. Randomization allocated all Lesbian/Gay/Bisexual (LGB) participants (n=3) to the intervention (p=.055).
Table 2.
Baseline Participant Characteristics and Contextual Experiences
| Demographic Items | Total Sample (N=16) | Retention Arm (n=8) | Diet/Nutrition Arm (n=8) | Difference Test | |||
|---|---|---|---|---|---|---|---|
| N | (%) | N | (%) | N | (%) | p-value* | |
| Age (years) | M=48.75, SD=10.76 | M=47.25, SD=12.22 | M=50.25, SD=9.66 | p= .595 | |||
| Female | 10 | (62.5%) | 5 | (62.5%) | 5 | (62.5%) | p= .999 |
| Race/Ethnicity | |||||||
| Non-Hispanic Black | 10 | (62.5%) | 6 | (75.0%) | 4 | (50.0%) | p= .608 |
| Hispanic Ethnicity | 6 | (37.5%) | 2 | (25.0%) | 4 | (50.0%) | p= .608 |
| Sexual Orientation | p= .055 | ||||||
| Heterosexual | 13 | (81.2%) | 5 | (62.5%) | 8 | (100.0%) | |
| LGB | 3 | (18.8%) | 3 | (25.0%) | 0 | (0.0%) | |
| Highest Level of Education | p= .328 | ||||||
| < Diploma/GED | 10 | (62.5%) | 4 | (50.0%) | 6 | (75.0%) | |
| Employment Status | p= .505 | ||||||
| Disabled/Unemployed | 12 | (75.0%) | 5 | (62.5%) | 7 | (87.5%) | |
| Estimated Annual Income | p=. 522 | ||||||
| ≤ $20,000 | 13 | (81.2%) | 6 | (75.0%) | 7 | (87.5%) | |
| Current Housing Situation | p=. 643 | ||||||
| Rent/Own | 13 | (81.3%) | 6 | (75.0%) | 7 | (87.5%) | |
| Mental Health Status | |||||||
| Current Diagnosis | 8 | (50.0%) | 4 | (50.0%) | 4 | (50.0%) | p=. 999 |
| Taking medications | 4 | (25.0%) | 2 | (25.0%) | 2 | (25.0%) | p=. 999 |
| In Therapy | 5 | (31.3%) | 2 | (25.0%) | 3 | (37.5%) | p=. 999 |
| Substance Use Status | |||||||
| Alcohol Intoxication† | 2 | (12.5%) | 2 | (25.0%) | 0 | (0.0%) | p= .467 |
| Cannabis† | 5 | (31.3%) | 2 | (25.0%) | 3 | (37.5%) | p= .999 |
| Illicit drugs† | 4 | (25.0%) | 1 | (12.5%) | 3 | (37.5%) | p= .569 |
| Subjective Health | p= .143 | ||||||
| Poor | 0 | (0.0%) | |||||
| Fair | 7 | (43.8%) | 2 | (25.0%) | 5 | (62.5%) | |
| Good | 9 | (56.3%) | 6 | (75.0%) | 3 | (37.5%) | |
| Excellent | 0 | (0.0%) | |||||
| No. Health Conditions | M= 2.19, SD= 1.60 | M= 2.00, SD= 1.07 | M= 2.38, SD= 2.07 | p= .655 | |||
2-tail significance test (p< .05).
LGB= Lesbian, Gay, Bisexual; SRO= Single-room Occupancy;
Past 30-days.
Regarding access to HIV medical care (Table 3), all but one participant had health insurance coverage for all 12-months before baseline. Participants randomized to the control arm had been living with HIV an average of 5 years longer than the intervention arm (p=.047), and reported slightly more transportation-related barriers in the past 6-months (p≥.119). No other factors were found to be statistically significant or clinically remarkable.
Table 3.
Baseline HIV and Treatment Access Histories
| Total Sample (N=16) | Retention Arm (n=8) | Nutrition/Diet Arm (n=8) | Difference Test | ||||
|---|---|---|---|---|---|---|---|
| N | (%) | N | (%) | N | (%) | p-value* | |
| Years Living with HIV | M= 16.31, SD= 5.30 | M= 13.88, SD= 5.14 | M= 18.75, SD= 3.69 | p= .047 | |||
| Years in HIV Care | M= 13.63, SD= 5.03 | M= 12.38, SD= 5.61 | M= 14.88, SD= 4.39 | p= .337 | |||
| Usual Source of HIV Care | p= .999 | ||||||
| Affiliated Care Site | 11 | (68.8%) | 6 | (75.0%) | 5 | (62.5%) | |
| Non-Affiliated Care Site | 5 | (31.3%) | 2 | (25.0%) | 3 | (37.5%) | |
| Self-Reported HAART Status | |||||||
| On HAART | 16 | (100.0%) | 8 | (100.0%) | 8 | (100.0%) | a |
| Non-adherent past 4 weeks | 10 | (62.5%) | 5 | (62.5%) | 5 | (62.5%) | p= .999 |
| Insurance Status Past 12-Months | p= .279 | ||||||
| Without insurance part-time | 1 | (6.3%) | 0 | (0.0%) | 1 | (12.5%) | |
| Public insurance full-time | 13 | (81.3%) | 6 | (75.0%) | 7 | (87.5%) | |
| Public & private full-time | 2 | (12.5%) | 2 | (25.0%) | 0 | (0.0%) | |
| HIV Care Access Barriers Past 6-Months† | |||||||
| Unable to pay for HIV Treatment | 0 | (0.0%) | a | ||||
| Didn’t have transportation to HIV care | 3 | (18.8%) | 0 | (0.0%) | 3 | (37.5%) | p= .200 |
| Unable to pay for travel to HIV care | 6 | (37.5%) | 1 | (12.5%) | 5 | (62.5%) | p= .119 |
| Inconvenient HIV clinic hours | 3 | (18.8%) | 1 | (12.5%) | 2 | (25.0%) | p= .999 |
| No longer cared enough about myself | 1 | (6.3%) | 0 | (0.0%) | 1 | (12.5%) | p= .999 |
| Didn’t have child care to get HIV care | 1 | (6.3%) | 0 | (0.0%) | 1 | (12.5%) | p= .999 |
Responses given on a 5-point Likert-type scale (1= Not at all difficult, 5= Very difficult}.
2-tail significance test (p< .05).
Difference test could not be computed.
Intervention Acceptability and Feasibility
Post-intervention (Table 4), participants in both arms provided equally favorable (p≥.549) acceptability ratings (1=Lease favorable to 5=Most favorable) regarding the overall program (M=4.75, SD=0.45), the program’s respective topic (Retention or Diet and Nutrition; M=4.35, SD=0.49), the program facilitator (M=4.77, SD=0.40), and workbook activities (M=4.56, SD=0.45). Similarly, participants in both arms equally (p≥.405) perceived the costs and benefits of participating in the program were reasonable (M=4.19, SD=0.48), and reported little-to-no physical (M=4.81, SD=0.54) or mental distress (M=4.75, SD=0.58) resulting from their participation. However, control participants (M=4.63, SD=0.45) rated their 60-minute program as slightly more appealing than intervention participants (M=4.29, SD=0.45; t= −1.48, p=.162). Feasibility and fidelity ratings indicated all session activities were completed in the allotted time, though participants in both conditions thought it would be somewhat difficult to implement what they had learned in the next 6-months (M=3.44, SD=1.31; t=0.56, p=.586). No other ratings were found to be statistically significant or potentially reflective of differential experiences by study arm.
Retention Outcomes
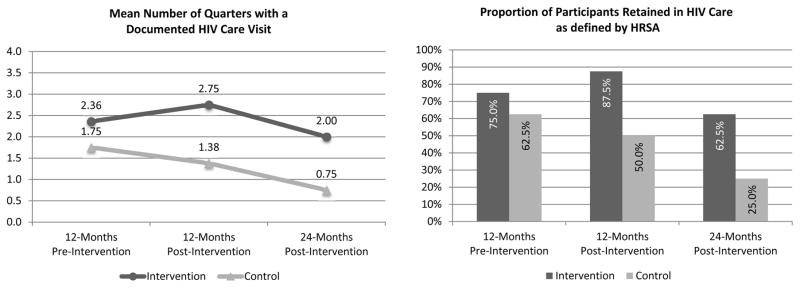
Compared to the control (M=1.75, SD=1.58), participants randomized to the intervention (M=2.36, SD=1.19) had slightly better retention in the 12-months prior to baseline (Figure 3), though there was no trend toward significant group diferences on either retention metric (Number of quarters with a documented visit: t(14)=0.094, p(2-tail)=.386; Proportion meeting the HRSA definition: p(2-tail)=.999). Over the first 12-month period post-intervention, participants randomized to the intervention demonstrated substantially better retention in care compared to the control, where retention declined. Specifically, compared to control participants (M=1.38, SD=1.60), intervention participants (M=2.75, SD=1.28) had significantly more quarters with a documented HIV care visit (t(14)=1.898, p(1-tail)=.039), and a larger proportion met the HRSA definition of retention (Intervention: 87.5%, Control: 50.0%; p(1-tail)=.141). Over the second 12-month post-intervention follow-up period, retention declined in both groups, but remained higher in the intervention arm. Specifically, compared to the control (M=0.75, SD=1.16), intervention participants (M=2.00, SD=1.20) continued to have significantly more quarters with a documented HIV care visit (t(14)=2.118, p(1-tail)=.027), and a larger proportion met the HRSA definition of retention (Intervention: 62.5%, Control: 25.0%; p(1-tail)= .157).
Figure 3.
Pre-Post Evaluation of Participants Retention in HIV Care Status
DISCUSSION
Findings suggest the 60 Minutes for Health intervention is both feasible and acceptable to implement with poorly retained HIV-positive patients. This pilot reflects a practical, theory-based behavioral intervention, rigorously designed to promote retention in HIV care in a busy clinical setting. Although caution is needed when interpreting results with this small sample, the 60 Minutes for Health intervention may facilitate more sustained retention in HIV care over time for patients, who in the absence of intervention, are likely to fall out of HIV care, as occurred among participants randomized to the control arm.
Overall, poorly retained participants enrolled in the 60-Minutes for Health intervention favorably evaluated their experiences. Findings suggest participants are willing to engage in targeted intervention activities with a lay staff member that where feasibly implemented in a busy clinical setting within 60-minutes. Participants perceived some difficulty implementing what they had learned in the intervention, likely reflecting a more comprehensive recognition of the challenges that have affected their retention in care to-date, as we observed better retention in care, relative to baseline, among intervention participants. Given how practical this intervention is to implement, if found to be efficacious in a larger-scale trial, it could be easily scaled up for use in clinical settings.
As a feasibility pilot, we are unable to assess the efficacy of the 60 Minutes for Health intervention in improving retention in care. The rigorous randomized time-and-attention control design lends strength to the positive retention in care trends observed at 12- and 24-months post-intervention. Outdated contact information may have limited our ability to recuit patients lost-to-follow-up due to more substantial transportation, substance use, or mental health barriers. Additional strategies may be needed to address such barriers, as the current intervention was designed to prevent subsequent lost-to-follow-up among poorly retained patients cycling through clinical care. This pilot occurred within an integrated HIV care setting, which may limit generalizability to sites with fewer ancillary services. As this study did not collect laboratory data independent of participants’ regular HIV care visits, we are limited in our ability to speak to the intervention’s potential indirect effect on participants’ viral load status.
Despite these limitations, the current study provides promising data. In line with the most recent National HIV/AIDS Strategy’s top priorities (Office of National AIDS Policy, 2015), our findings suggest a practical behavioral intervention that might support sustained retention in care behaviors among patients sub-optimally retained in HIV care. While larger structural solutions are still needed, 60 Minutes for Health may afford critical real-time support that can be leveraged for patients at-risk-of dropping out of care. The potential efficacy of the 60 Minutes for Health intervention should be tested in a larger-scale efficacy trial.
Acknowledgments
The authors wish to thank the participants, clinic staff, and providers who shared their knowledge, time, and energy with this project in hopes of supporting better outcomes for their peers and their patients. The authors have no financial interests or benefits to disclose arising from the direct applications of this research.
Funding Source: This work was supported by NIH under NIMH [F31 MH093264 ], NIDA [K24 DA036955 and K01 DA039767], and NIMHD [L60 MD009353], as well as seed grants from the University of Connecticut’s Institute for Collaboration on Health, Intervention, and Policy, the Society of Behavioral Medicine, and the American Psychological Association’s Division 38 (Health Psychology).
Footnotes
Disclosure statement: The authors have no financial disclosures to make as a result of this research.
Contributor Information
Laramie R. Smith, Division of Global Public Health, Department of Medicine; University of California, San Diego School of Medicine; 9500 Gilman Drive #0507, La Jolla, CA 92093-0507; United States
K. Rivet Amico, Department of Health Behavior and Health Education, School of Public Health, University of Michigan; 1415 Washington Heights, Ann Harbor, Michigan, 48109-2029; United States.
Jeffrey D. Fisher, Institute for Collaboration on Health, Intervention, and Policy, University of Connecticut, 2006 Hillside Rd. U-1248, Storrs, CT, 06269-1248; United States
Chinazo O. Cunningham, Albert Einstein College of Medicine, 111 E. 210th Street Bronx, NY, 10467; United States
References
- Amico KR. A situated-information motivation behavioral skills model of care initiation and maintenance (sIMB-CIM): An IMB model based approach to understanding and intervening in engagement in care for chronic medical conditions. Journal of Health Psychology. 2011;16(7):1071–1081. doi: 10.1177/1359105311398727. [DOI] [PubMed] [Google Scholar]
- Bandura A. Health promotion from the perspective of social cognitive theory. Psychology and Health. 1998;13(4):623–649. [Google Scholar]
- Bove JM, Golden MR, Dhanireddy S, Harrington RD, Dombrowski JC. Outcomes of a clinic-based surveillance-informed intervention to relink patients to HIV care. Journal of Acquired Immune Deficiency Syndromes (1999) 2015;70(3):262–268. doi: 10.1097/QAI.0000000000000707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowling A. Just one question: If one question works, why ask several? Journal of Epidemiology and Community Health. 2005;59(5):342–345. doi: 10.1136/jech.2004.021204. 59/5/342 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley H, Hall HI, Wolitski RJ, Van Handel MM, Stone AE, LaFlam M, … Frazier EL. Vital signs: HIV diagnosis, care, and treatment among persons living with HIV—United states, 2011. MMWR Morb Mortal Wkly Rep. 2014;63(47):1113–1117. [PMC free article] [PubMed] [Google Scholar]
- Calvin CL. Unpublished PhD. Boston College, William F. School of Nursing; 2010. Feasibility, acceptability, and preliminary efficacy of a cognitive training intervention for postoperateive cardiac surgical patients. [Google Scholar]
- Chu C, Umanski G, Blank A, Meissner P, Grossberg R, Selwyn PA. Comorbidity-related treatment outcomes among HIV-infected adults in the bronx, NY. Journal of Urban Health. 2011;88(3):507–516. doi: 10.1007/s11524-010-9540-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craw JA, Gardner LI, Marks G, Rapp RC, Bosshart J, Duffus WA, … Safford LA. Brief strengths-based case management promotes entry into HIV medical care: Results of the antiretroviral treatment access study-II. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2008;47(5):597. doi: 10.1097/QAI.0b013e3181684c51. [DOI] [PubMed] [Google Scholar]
- Crum NF, Riffenburgh RH, Wegner S, Agan BK, Tasker SA, Spooner KM, … Wallace MR. Comparisons of causes of death and mortality rates among HIV-infected persons: Analysis of the pre-, early, and late HAART (highly active antiretroviral therapy) eras. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2006;41(2):194. doi: 10.1097/01.qai.0000179459.31562.16. [DOI] [PubMed] [Google Scholar]
- Davila JA, Miertschin N, Sansgiry S, Schwarzwald H, Henley C, Giordano TP. Centralization of HIV services in HIV-positive african-american and hispanic youth improves retention in care. AIDS Care. 2013;25(2):202–206. doi: 10.1080/09540121.2012.689811. [DOI] [PubMed] [Google Scholar]
- El-Bassel N, Jemmott JB, III, Landis JR, Pequegnat W, Wingood GM, Wyatt GE, Bellamy SL. Intervention to influence behaviors linked to risk of chronic diseases: A multisite randomized controlled trial with african-american HIV-serodiscordant heterosexual couples. Archives of Internal Medicine. 2011;171(8):728–736. doi: 10.1001/archinternmed.2011.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enriquez M, Farnan R, Cheng A, Almeida A, Valle DD, Pulido-Parra M, Flores G. Impact of a bilingual/bicultural care team on HIV-related health outcomes. Journal of the Association of Nurses in AIDS Care. 2008;19(4):295–301. doi: 10.1016/j.jana.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Fisher JD, Fisher WA, Amico KR, Harman JJ. An information-motivation-behavioral skills model of adherence to antiretroviral therapy. Health Psychology. 2006;25(4):462–473. doi: 10.1037/0278-6133.25.4.462. [DOI] [PubMed] [Google Scholar]
- Fisher WA, Fisher JD, Harman J. Social psychological foundations of health and illness. Malden, MA: Blackwell Publishing; 2003. The information-motivation-behavioral skills model: A general social psychological approach to understanding and promoting health behavior; pp. 82–106. [Google Scholar]
- Fisher JD, Fisher WA. Changing AIDS-risk behavior. Psychological Bulletin. 1992;111(3):455–474. doi: 10.1037/0033-2909.111.3.455. [DOI] [PubMed] [Google Scholar]
- Gardner LI, Marks G, Craw JA, Wilson TE, Drainoni M, Moore RD, … Holman S. A low-effort, clinic-wide intervention improves attendance for HIV primary care. Clinical Infectious Diseases. 2012;55(8):1124–1134. doi: 10.1093/cid/cis623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner LI, Metsch LR, Anderson-Mahoney P, Loughlin AM, Del Rio C, Strathdee S, … Holmberg SD. Efficacy of a brief case management intervention to link recently diagnosed HIV-infected persons to care. Aids. 2005;19(4):423–431. doi: 10.1097/01.aids.0000161772.51900.eb. [DOI] [PubMed] [Google Scholar]
- Gardner LI, Giordano TP, Marks G, Wilson TE, Craw JA, Drainoni ML … Retention in Care Study Group. Enhanced personal contact with HIV patients improves retention in primary care: A randomized trial in 6 US HIV clinics. Clinical Infectious Diseases : An Official Publication of the Infectious Diseases Society of America. 2014;59(5):725–734. doi: 10.1093/cid/ciu357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano T, Gifford A, White J, Clinton A, Suarez-Almazor M, Rabeneck L, Hartman C, … Morgan R. Retention in care: A challenge to survival with HIV infection. Clinical Infectious Diseases. 2007;44(11):1493–1499. doi: 10.1086/516778. [DOI] [PubMed] [Google Scholar]
- Higa DH, Crepaz N, Mullins MM. Identifying best practices for increasing linkage to, retention, and re-engagement in HIV medical care: Findings from a systematic review, 1996–2014. AIDS and Behavior. 2016;20(5):951–966. doi: 10.1007/s10461-015-1204-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hightow-Weidman LB, Smith JC, Valera E, Matthews DD, Lyons P. Keeping them in “STYLE”: Finding, linking, and retaining young HIV-positive black and latino men who have sex with men in care. AIDS Patient Care and STDs. 2011;25(1):37–45. doi: 10.1089/apc.2010.0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine MK, Chamberlin SA, Robbins RS, Myers JE, Braunstein SL, Mitts BJ, … Nash D. Improvements in HIV care engagement and viral load suppression following enrollment in a comprehensive HIV care coordination program. Clinical Infectious Diseases : An Official Publication of the Infectious Diseases Society of America. 2015;60(2):298–310. doi: 10.1093/cid/ciu783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemmott JB. Eban health promotion intervention: Conceptual basis and procedures: NIMH multisite HIV/STD prevention trial for african american couples group. Journal of Acquired Immune Deficiency Syndromes (1999) 2008;49(Suppl 1):S28–34. doi: 10.1097/QAI.0b013e3181842548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalichman SC, Catz S, Ramachandran B. Barriers to HIV/AIDS treatment and treatment adherence among african-american adults with disadvantaged education. Journal of the National Medical Association. 1999;91(8):439–446. [PMC free article] [PubMed] [Google Scholar]
- Konkle-Parker DJ, Erlen JA, Dubbert PM, May W. Pilot testing of an HIV medication adherence intervention in a public clinic in the deep south. Journal of the American Academy of Nurse Practitioners. 2012;24(8):488–498. doi: 10.1111/j.1745-7599.2012.00712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas GM, Chaudhry A, Hsu J, Woodson T, Lau B, Olsen Y, … Barditch-Crovo P. Clinic-based treatment of opioid-dependent HIV-infected patients versus referral to an opioid treatment program: A randomized trial. Annals of Internal Medicine. 2010;152(11):704–711. doi: 10.1059/0003-4819-152-11-201006010-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer KH. Introduction: Linkage, engagement, and retention in HIV care: Essential for optimal individual-and community-level outcomes in the era of highly active antiretroviral therapy. Clinical Infectious Diseases. 2011;52(suppl 2):S205–S207. doi: 10.1093/cid/ciq043. [DOI] [PubMed] [Google Scholar]
- McLellan A, Kushner H, Metzger D, Peters R, Smith I, Grissom G, … Argeriou M. The fifth edition of the addiction severity index. J Subst Abuse Treat. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- Miller WR, Rose GS. Toward a theory of motivational interviewing. The American Psychologist. 2009;64(6):527–537. doi: 10.1037/a0016830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugavero MJ, Lin HY, Allison JJ, Giordano TP, Willig JH, Raper JL, … Davies S. Racial disparities in HIV virologic failure: Do missed visits matter? JAIDS Journal of Acquired Immune Deficiency Syndromes. 2009;50(1):100–108. doi: 10.1097/QAI.0b013e31818d5c37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugavero MJ, Amico KR, Westfall AO, Crane HM, Zinski A, Willig JH, … Saag MS. Early retention in HIV care and viral load suppression: Implications for a test and treat approach to HIV prevention. Journal of Acquired Immune Deficiency Syndromes (1999) 2012;59(1):86–93. doi: 10.1097/QAI.0b013e318236f7d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Office of National AIDS Policy. National HIV/AIDS strategy for the united states:Updated through 2020. Washington, DC: Office of National AIDS Policy; 2015. [Google Scholar]
- Outlaw AY, Naar-King S, Parsons JT, Green-Jones M, Janisse H, Secord E. Using motivational interviewing in HIV field outreach with young african american men who have sex with men: A randomized clinical trial. American Journal of Public Health. 2010;100(Suppl 1):S146–51. doi: 10.2105/AJPH.2009.166991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins GK, Lester W, Johnson KL, Chang Y, Estey G, Surrao D, … Meigs JB. Efficacy of a clinical decision-support system in an HIV practice: A randomized trial. Annals of Internal Medicine. 2012;157(11):757–766. doi: 10.7326/0003-4819-157-11-201212040-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollnick S, Miller WR. What is motivational interviewing? Behavioural and Cognitive Psychotherapy. 1995;23:325–334. doi: 10.1017/S1352465809005128. [DOI] [PubMed] [Google Scholar]
- Safren SA, Otto MW, Worth JL, Salomon E, Johnson W, Mayer K, Boswell S. Two strategies to increase adherence to HIV antiretroviral medication: Life-steps and medication monitoring. Behaviour Research and Therapy. 2001;39(10):1151–1162. doi: 10.1016/s0005-7967(00)00091-7. [DOI] [PubMed] [Google Scholar]
- Simbayi LC, Kalichman SC, Skinner D, Jooste S, Cain D, Cherry C, … Bok W. Theory-based HIV risk reduction counseling for sexually transmitted infection clinic patients in cape town, south africa. Sexually Transmitted Diseases. 2004;31(12):727–733. doi: 10.1097/01.olq.0000145849.35655.f1. [DOI] [PubMed] [Google Scholar]
- Skarbinski J, Rosenberg E, Paz-Bailey G, Hall HI, Rose CE, Viall AH, … Mermin JH. Human immunodeficiency virus transmission at each step of the care continuum in the united states. JAMA Internal Medicine. 2015;175(4):588–596. doi: 10.1001/jamainternmed.2014.8180. [DOI] [PubMed] [Google Scholar]
- Smith LR, Fisher JD, Cunningham CO, Amico KR. Understanding the behavioral determinants of retention in HIV care: A qualitative evaluation of a situated information, motivation, behavioral skills model of care initiation and maintenance. AIDS Patient Care and STDs. 2012;26(6):344–355. doi: 10.1089/apc.2011.0388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services, Health Research and Services Administration (HRSA) HIV/AIDS bureau performance measures: HIV medical visit frequency. (Updated January 2015) (No. National Quality Forum #: 2079) [Google Scholar]
- Wolfers ME, de Wit JB, Hospers HJ, Richardus JH, de Zwart O. Effects of a short individually tailored counselling session for HIV prevention in gay and bisexual men receiving hepatitis B vaccination. BMC Public Health. 2009;9:255. doi: 10.1186/1471-2458-9-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zauszniewski JA. Intervention development: Assessing critical paramfeters from the intervention recipient’s perspective. Applied Nursing Research. 2012;25(1):31–39. doi: 10.1016/j.apnr.2010.06.002. [DOI] [PubMed] [Google Scholar]