Abstract
Vascular endothelial growth factor and its receptor (VEGF-VEGFR) system play a critical role in the regulation of angiogenesis and lymphangiogenesis in vertebrates. Each of the VEGF has specific receptors, which it activates by binding to the extracellular domain of the receptors, and, thus, regulates the angiogenic balance in the early embryonic and adult stages. However, de-regulation of the VEGF-VEGFR implicates directly in various diseases, particularly cancer. Moreover, tumor growth needs a dedicated blood supply to provide oxygen and other essential nutrients. Tumor metastasis requires blood vessels to carry tumors to distant sites, where they can implant and begin the growth of secondary tumors. Thus, investigation of signaling systems related to the human disease, such as VEGF-VEGFR, will facilitate the development of treatments for such illnesses.
Keywords: Angiogenesis, Drugs, Signal transduction, Tumor growth and metastasis, Vascular endothelial growth factor (VEGF)
INTRODUCTION
Angiogenesis, the physiological process through which new vessels form from pre-existing vessels, is responsible for most, if not all, blood vessel growth during development (1, 2). Various angiogenic proteins, including fibroblast growth factors (FGFs), vascular endothelial growth factors (VEGFs/VEGFRs), angiopoietin/Tie receptors, platelet-derived growth factors (PDGFs/PDGFRs), and EphrinB2/EphB4 (3–8) result in the stimulation of angiogenesis. This process is tightly regulated depending on the balance of pro- and anti-angiogenic factors (9). However, if the angiogenesis is not properly controlled, various diseases are induced. For example, excessive angiogenesis can lead to chronic disease states such as tumor growth and metastasis, and several disease, such as ulcers and ischemic heart disease, are the result of insufficient angiogenesis (10). Among the angiogenic proteins, VEGF-VEGFR is a crucial regulator of pathological angiogenesis such as in cancer as well as physiological vasculogenesis and angiogenesis in early embryonic and adult stages (11).
VEGFs bind to the VEGFRs on the cell surface, and stimulate cellular responses by causing the receptors to dimerize and become activated through transphosphorylation (12). When cells are deficient of oxygen, namely in hypoxia, the cell produces hypoxia-inducible factor (HIF), which can stimulate the release of VEGF. Thus, hypoxia may be an essential regulator of VEGF expression. Additionally, several diseases characterized by excess angiogenesis are associated with hypoxia-driven de-regulated VEGF expression (12, 13). Several antiangiogenic drugs target the VEGF-VEGFR system, including VEGF-neutralizing antibody (bevacizumab), small molecule kinase inhibitors (sunitinib, sorafenib, and apatinib), and humanized monoclonal antibody targeting the extracellular domain of the VEGFR (ramucirumab). However, the resistance mechanisms of cancer and the side effects of drugs limit the use of these drugs in chemotherapy (14). Consequently, a more detailed investigation focused on the pathological angiogenesis, as a therapeutic target, is required for the development of safe and continuously available drugs.
In this review, we describe the structural and functional information regarding the VEGF-VEGFR system to increase understanding of angiogenesis in physiological and pathological processes.
STRUCTURE AND BIOCHEMICAL PROPERTIES OF VEGFRs WITH ITS LIGANDS
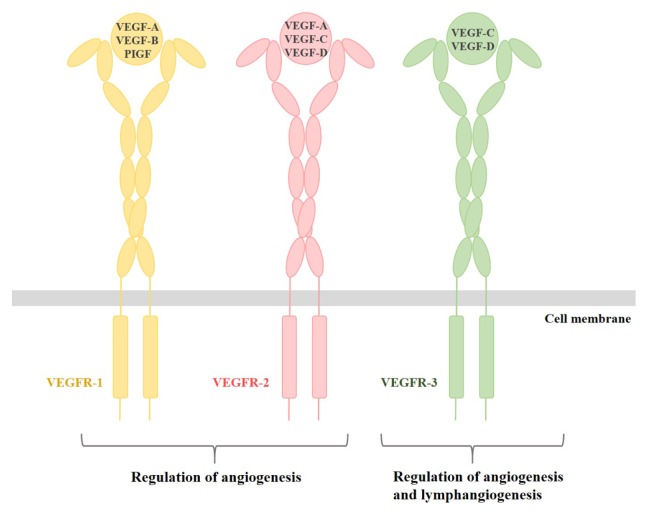
Genes encoding novel tyrosine kinase receptors were isolated in the early 1990s, and the tyrosine kinase receptors that positively and negatively regulate the formation of blood and lymph vessels were denoted VEGFRs (15, 16). Three genes are encoding three full-length receptors (VEGFR-1, -2, and -3) and one soluble molecule (sVEGFR-1), and most VEGFRs show similar overall structures that comprise of three primary domains. VEGFRs are typically composed of an extracellular ligand-binding domain (ECD) with a seven immunoglobulin (Ig)-like domain, a transmembrane domain and a tyrosine kinase domain split by a kinase insert and a carboxy terminus (Fig. 1A) (11, 17). The kinase domains of VEGFRs are the most conserved region, with high sequence identities (78–80%). The VEGF-VEGFR system plays a central role in the regulation of tumor angiogenesis and can be a potential target for anti-angiogenic therapy. There are five VEGF family members (VEGF-A, VEGF-B, VEGF-C, VEGF-D, and placental growth factor) encoded from the mammalian genome (3, 18). Moreover, alternative splicing of primary RNA transcripts from the VEGF gene family generates various isoforms. For example, the isoforms of human VEGF-A are labeled as VEGF-A121, VEGF-A145, VEGF-A165, VEGF-A189 and VEGF-A206, and homodimeric VEGF-B exists as two different transcripts, VEGF-B167 and VEGF-B186 (19). Among them, VEGF-A (known as VEGF) is one of the most critical factors for blood vessel formation during early embryogenesis (11). VEGF-A binds to Ig domains 2 and 3 localized in the ECD of VEGFR-1 and VEGFR-2 (20, 21). Interestingly, the affinity of VEGF-A to VEGFR-1 is about one order of magnitude higher than that to VEGFR-2, but the tyrosine kinase activity of VEGFR-2 in response to VEGF-A is much higher than that of VEGFR-1 (17, 22). VEGF-B and placenta growth factor (PIGF) bind to VEGFR-1, but their mechanisms that activate the receptor are different (23). Specifically, VEGF-B stimulates Tyr1213 phosphorylation of VEGFR-1, whereas PIGF stimulates Tyr1309 phosphorylation (24). VEGF-C and VEGF-D are specific ligands for VEGFR-3, which plays a critical role in angiogenesis and lymphangiogenesis in adults (Fig. 2) (25).
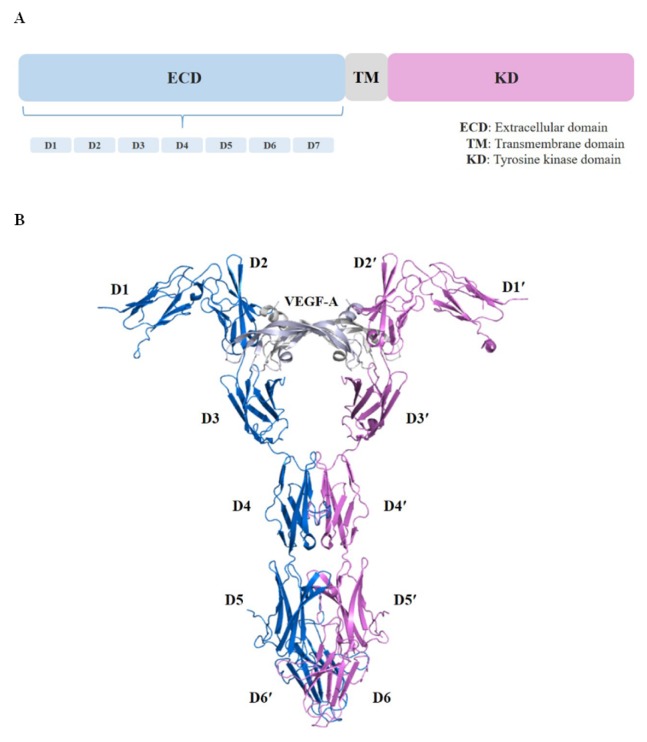
Fig. 1.
Structure of the VEGFR-1 extracellular domain in complex with VEGF-A. (A) Schematic representation of the domain organization of VEGFR is shown. (B) Complex crystal structure of VEGFR-1 extracellular domain with VEGF-A (PDB ID: 5T89) is shown. We have shown the structure in a ribbon representation with each chain depicted by a different color. The chains of the VEGF-A homodimer are shown in light blue and gray, and the VEGFR-1 D1–D6 chains in deep blue and magenta.
Fig. 2.
Schematic illustration of the VEGF-VEGFR system. The VEGF family including VEGF-A, VEGF-B, VEGF-C, VEGF-D and PIGF binds to its specific receptor. We have depicted its ligands in yellow, VEGFR-2 and its ligands in pink, and VEGFR-3 and its ligands in green.
To date, many structural studies of the VEGF/VEGFR complex based on single-particle electron microscopy, small-angle X-ray scattering, and X-ray crystallography show how the ligand binds to the membrane distal Ig domains. Moreover, studies of other Ig domains of the VEGFR suggest the possibility of receptor-receptor interactions (19, 26–29). The first complete and recently reported VEGF/VEGFR ECD complex structure provides insightful information regarding the ligand binding and ligand-induced homotypic interactions of VEGFR (30). The structure of full-length VEGFR-1 ECD in complex with VEGF-A exists as two sets of 1:1 complexes in the asymmetric unit and two receptors linked by the dimeric VEGF-A bound to the Ig domains (Fig. 1B) (30). Unlike previous VEGFR-1 complex structures that contained only Ig domain 2, the recently reported complex structures include the complete ECD of the receptor with VEGF-A that interacts with both Ig domains 2 and 3 of VEGFR-1 (19, 30–32). The results of these studies also suggest that the homotypic receptor-receptor contacts in Ig domains 4–7 increase the binding affinity of VEGFR-1 ECD for VEGF-A based on the finding that the binding affinity is 20 times higher in the presence of homotypic interactions (30). Moreover, researchers have conducted many studies targeting the structure-based design of VEGFR-2 inhibitors as therapeutic agents since the crystal structure of the catalytic kinase domain of VEGFR-2 was determined (33–36). The overall fold and catalytic residue positions of the VEGFR-2 kinase domain are similar to those observed in other tyrosine-kinase structures. There are two lobes (N-lobe and C-lobe), and the catalysis of phosphotransfer takes place in the cleft between the two lobes (34). Despite differences in the kinase activity of VEGFRs in response to its ligands, the available structural information regarding the kinase domains of VEGFR-1 and VEGFR-3 remains sparse. Thus, more detailed investigations based on the molecular structure of the remaining VEGFR kinase domains are required to improve understanding of their catalytic and signal transduction mechanisms.
BIOLOGICAL FUNCTION OF VEGF-VEGFR SYSTEM
VEGF-VEGFR system is crucial to vascular development and neovascularization in physiological and pathological processes of both embryos and adults, and many studies have investigated anti-VEGF-VEGFR molecules disturbing signal transduction by the VEGF-VEGFR system to improve anti-angiogenic therapy (12). VEGFR-1 is expressed in vascular endothelial cells and non-endothelial cells, including haematopoietic stem cells, macrophages, and monocytes. Fong et al. reported that VEGFR-1 knockout mice died at embryonic day 8.5–9.0 because of overgrowth of endothelial cells and disorganization of blood vessels in the embryo (37). Moreover, to identify how VEGFR-1 negatively regulates angiogenesis during early embryogenesis, mice expressing only the VEGFR-1 extracellular and transmembrane domains were generated. Interestingly, angiogenesis in mice was almost average, indicating that the ECD of VEGFR-1, not the kinase domain, plays a critical role as a suppressor of vascular formation by trapping VEGF-A and thereby preventing VEGFR-2 activation (38). Autophosphorylation on tyrosine residues of VEGFR-1 and coupling to intracellular signal transducers can trigger weak signals for growth and survival of endothelial cells and pericytes, as well as for cell migration of macrophages (17). Phospholipase C (PLCγ) involved in the mitogen-activated protein kinase (MAPK) pathway adheres to the phosphorylated Tyr1169 of VEGFR-1 for regulation of endothelial cell proliferation (39, 40). The p85 subunit of phosphoinositide 3-kinase (PI3K) has also been reported to bind to the activated and phosphorylated VEGFR-1 (41).
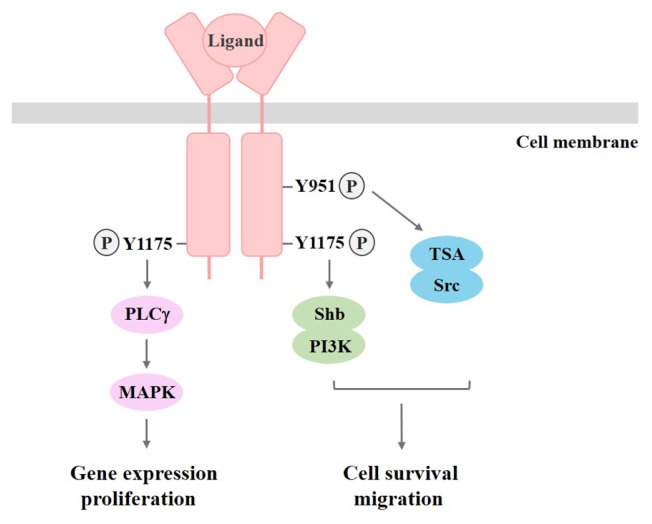
The VEGFR-2 expression is detected in not only vascular endothelial and lymphatic endothelial cells, but also megakaryocytes and haematopoietic stem cells (42). In VEGFR-2 knockout mice, there were defects in vasculogenesis and haematopoietic development, resulting in death at embryonic stage 8.5–9.0 (43). These results show that VEGFR-2 acts as a positive signal transducer in growth and differentiation of endothelial cells. Consequently, these findings indicate that VEGFR-1 and VEGFR-2 collaborate in the regulation of vascular formation as a negative and positive regulator, respectively (17). Among the autophosphorylated tyrosine residues in VEGFR-2, phosphorylated Tyr1175 leads to binding of PLCγ, which stimulates the MAPK pathway involved in the regulation of DNA synthesis, and binding of PI3K involved in cell survival (17, 44). It has also been reported that Tyr951, another phosphorylated residue in VEGFR-2, leads to adaptation of T cell-specific adapter (TSA), which regulates actin stress fiber organization and migratory responses of endothelial cells by associating with the cytoplasmic tyrosine kinase Src (Fig. 3) (45). VEGFR-3 is primarily expressed in lymphatic endothelial cells, and activation of VEGFR-3 by interaction with VEGF-C results in proliferation, migration, and survival of lymphatic endothelial cells. Additionally, VEGFR-3 plays an essential role in the development of the vascular network and the cardiovascular system during embryonic development (46, 47). There are five tyrosine phosphorylation sites in the VEGFR-3 kinase domain, and the receptor mainly mediates activation of the MAPK pathway (17, 48).
Fig. 3.
Signaling pathways activated by VEGFR2. The phosphorylation of tyrosine residues creates docking sites for the recruitment of downstream signaling effectors. Subsequently, signaling cascades activated by VEGFR2 can regulate gene expression, cell proliferation, survival, and migration.
ANGIOGENESIS AND ANTI-ANGIOGENIC THERAPY IN CANCER
Uncontrolled cell growth and proliferation cause cancer, one of the most common diseases in humans. There are several biological hallmarks of cancer, including self-sufficiency in growth signals, insensitivity to anti-growth signals, evading apoptosis, limitless replicative potential, sustained angiogenesis, tissue invasion and metastasis, abnormal metabolic pathways, evading the immune system, and genome instability (49). Blood vessel growth is essential for the growth and metastasis of solid tumors; thus, angiogenesis is considered one of the most critical targets for investigation of tumor therapy (50). The VEGF-VEGFR system is known as a primary regulator of tumor angiogenesis, and inactivation of the system has been reported in a variety of human diseases such as tumor angiogenesis, tumor-dependent ascites formation, metastasis, and inflammatory diseases including rheumatoid arthritis, rheumatoid psoriasis, hyperthyroidism and atherosclerosis (3, 18). VEGFR-1 may contribute to pathological angiogenesis by stimulating the activation of endothelial cells and the recruitment of bone marrow progenitor cells (51, 52). Additionally, sVEGFR-1 expressed in the trophoblast layer is a splice variant of VEGFR-1, and may play a critical role in the formation of a regulatory barrier against abnormal vascular permeability and abnormal angiogenesis (11). The finding that artificial overexpression of sVEGFR-1 in a pregnant rat model induces hypertension and proteinuria strongly suggests that increased sVEGFR-1 is a crucial causative factor of the preeclampsia symptoms (hypertension and proteinuria) (53). VEGFR-2 has also been directly linked to tumor angiogenesis and blood vessel-dependent metastasis. Specifically, VEGFR-2 is upregulated under the hypoxic stress that occurs during the rapid growth of tumor cells (11). Either dysfunction or increased activation of VEGFR-3 can be involved in human pathological conditions. Inactivation of VEGFR-3 can aggravate congenital lymphedema that results from decreased transport capacity of the lymphatic vessels and features chronic and disabling swelling of tissues (54, 55). Another lymphedema caused by filariasis, trauma or infection may be treated with VEGF-C, alleviating the increased activation of VEGFR-3 (17).
The VEGF-VEGFR system has been confirmed to be useful as a target of new drugs to suppress a range of diseases, particularly malignancies. There are several anti-angiogenic compounds including VEGF-neutralizing antibody (bevacizumab) and tyrosine kinase inhibitor (sunitinib and sorafenib), which inhibit growth and metastasis of tumors. When tumors show drug-resistance to standard cytotoxic therapy, anti-angiogenic compounds may be the ideal drugs for treating cancer patients (11). Bevacizumab is a humanized monoclonal antibody targeting VEGF-A that can selectively neutralize VEGF-A, but not other VEGF family members. The FDA approved Bevacizumab in 2004 for the treatment of cancer. However, it was withdrawn in late 2011 because it has no clear efficacy data on overall survival in large-scale phase III clinical researches such as E2100, AVADO and RIBBON-1 clinical trials (11, 56). Bevacizumab has some adverse effects that can be life-threatening, including hypertension, proteinuria, rhinorrhagia, thrombosis and bleeding (57). Additionally, certain cancers are resistance to bevacizumab through several mechanisms, such as the enhancement of alternative pro-angiogenic signaling pathways, recruitment of bone marrow-derived pro-angiogenic cells to the tumor, and increasing of pericyte in tumor (58). Sunitinib malate and sorafenib tosylate can selectively target some protein receptors, including VEGFRs, and inhibit their kinase activity. Moreover, they can be widely applied because they cause few adverse reactions (59). In addition, the development of other anti-VEGF-VEGFR drugs such as VEGF-Trap and humanized anti-VEGFR antibodies is consistently ongoing to overcome adverse drug effects. Recent studies suggest that VEGF pathway appears to be useful for prognosis of several cancers patients including breast cancer and is also conducted as the most critical pathway regulating liver and lymph node metastasis of breast cancer (60–62). Therefore, we can use VEGF-VEGFR system as a potential target of new drugs, and more detailed structure-based insightful information regarding the VEGF-VEGFR system is essential to improve the anti-angiogenic therapy for the improved quality of life of cancer patients.
ACKNOWLEDGEMENTS
This research was financially supported by the 2017 Post-Doc. Development Program of Pusan National University and Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2017R1A6A3A11028281).
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicting interests.
REFERENCES
- 1.Birbrair A, Zhang T, Wang ZM, Messi ML, Mintz A, Delbono O. Pericytes at the intersection between tissue regeneration and pathology. Clin Sci (Lond) 2015;128:81–93. doi: 10.1042/CS20140278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Birbrair A, Zhang T, Wang ZM, et al. Type-2 pericytes participate in normal and tumoral angiogenesis. Am J Physiol Cell Physiol. 2014;307:C25–38. doi: 10.1152/ajpcell.00084.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alitalo K, Carmeliet P. Molecular mechanisms of lymphangiogenesis in health and disease. Cancer Cell. 2002;1:219–227. doi: 10.1016/S1535-6108(02)00051-X. [DOI] [PubMed] [Google Scholar]
- 4.Davis S, Aldrich TH, Jones PF, et al. Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell. 1996;87:1161–1169. doi: 10.1016/S0092-8674(00)81812-7. [DOI] [PubMed] [Google Scholar]
- 5.Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997;18:4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- 6.Heldin CH, Westermark B. Mechanism of action and in vivo role of platelet-derived growth factor. Physiol Rev. 1999;79:1283–1316. doi: 10.1152/physrev.1999.79.4.1283. [DOI] [PubMed] [Google Scholar]
- 7.Shibuya M, Ito N, Claesson-Welsh L. Structure and function of vascular endothelial growth factor receptor-1 and -2. Curr Top Microbiol Immunol. 1999;237:59–83. doi: 10.1007/978-3-642-59953-8_4. [DOI] [PubMed] [Google Scholar]
- 8.Wang HU, Chen ZF, Anderson DJ. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell. 1998;93:741–753. doi: 10.1016/S0092-8674(00)81436-1. [DOI] [PubMed] [Google Scholar]
- 9.Smith GA, Fearnley GW, Harrison MA, Tomlinson DC, Wheatcroft SB, Ponnambalam S. Vascular endothelial growth factors: multitasking functionality in metabolism, health and disease. J Inherit Metab Dis. 2015;38:753–763. doi: 10.1007/s10545-015-9838-4. [DOI] [PubMed] [Google Scholar]
- 10.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shibuya M. Vascular endothelial growth factor and its receptor system: physiological functions in angiogenesis and pathological roles in various diseases. J Biochem. 2013;153:13–19. doi: 10.1093/jb/mvs136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cross MJ, Dixelius J, Matsumoto T, Claesson-Welsh L. VEGF-receptor signal transduction. Trends Biochem Sci. 2003;28:488–494. doi: 10.1016/S0968-0004(03)00193-2. [DOI] [PubMed] [Google Scholar]
- 13.Harris AL. Hypoxia--a key regulatory factor in tumour growth. Nat Rev Cancer. 2002;2:38–47. doi: 10.1038/nrc704. [DOI] [PubMed] [Google Scholar]
- 14.Fontanella C, Ongaro E, Bolzonello S, Guardascione M, Fasola G, Aprile G. Clinical advances in the development of novel VEGFR2 inhibitors. Ann Transl Med. 2014;2:123. doi: 10.3978/j.issn.2305-5839.2014.08.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Vries C, Escobedo JA, Ueno H, Houck K, Ferrara N, Williams LT. The fms-like tyrosine kinase, a receptor for vascular endothelial growth factor. Science. 1992;255:989–991. doi: 10.1126/science.1312256. [DOI] [PubMed] [Google Scholar]
- 16.Terman BI, Carrion ME, Kovacs E, Rasmussen BA, Eddy RL, Shows TB. Identification of a new endothelial cell growth factor receptor tyrosine kinase. Oncogene. 1991;6:1677–1683. [PubMed] [Google Scholar]
- 17.Shibuya M, Claesson-Welsh L. Signal transduction by VEGF receptors in regulation of angiogenesis and lymphangiogenesis. Exp Cell Res. 2006;312:549–560. doi: 10.1016/j.yexcr.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 18.Shibuya M. Involvement of Flt-1 (VEGF receptor-1) in cancer and preeclampsia. Proc Jpn Acad Ser B Phys Biol Sci. 2011;87:167–178. doi: 10.2183/pjab.87.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iyer S, Darley PI, Acharya KR. Structural insights into the binding of vascular endothelial growth factor-B by VEGFR-1(D2): recognition and specificity. J Biol Chem. 2010;285:23779–23789. doi: 10.1074/jbc.M110.130658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davis-Smyth T, Chen H, Park J, Presta LG, Ferrara N. The second immunoglobulin-like domain of the VEGF tyrosine kinase receptor Flt-1 determines ligand binding and may initiate a signal transduction cascade. EMBO J. 1996;15:4919–4927. [PMC free article] [PubMed] [Google Scholar]
- 21.Tanaka K, Yamaguchi S, Sawano A, Shibuya M. Characterization of the extracellular domain in vascular endothelial growth factor receptor-1 (Flt-1 tyrosine kinase) Jpn J Cancer Res. 1997;88:867–876. doi: 10.1111/j.1349-7006.1997.tb00463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Waltenberger J, Claesson-Welsh L, Siegbahn A, Shibuya M, Heldin CH. Different signal transduction properties of KDR and Flt1, two receptors for vascular endothelial growth factor. J Biol Chem. 1994;269:26988–26995. [PubMed] [Google Scholar]
- 23.Roskoski R., Jr VEGF receptor protein-tyrosine kinases: structure and regulation. Biochem Biophys Res Commun. 2008;375:287–291. doi: 10.1016/j.bbrc.2008.07.121. [DOI] [PubMed] [Google Scholar]
- 24.Autiero M, Waltenberger J, Communi D, et al. Role of PlGF in the intra- and intermolecular cross talk between the VEGF receptors Flt1 and Flk1. Nat Med. 2003;9:936–943. doi: 10.1038/nm884. [DOI] [PubMed] [Google Scholar]
- 25.Roskoski R., Jr Vascular endothelial growth factor (VEGF) signaling in tumor progression. Crit Rev Oncol Hematol. 2007;62:179–213. doi: 10.1016/j.critrevonc.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 26.Brozzo MS, Bjelic S, Kisko K, et al. Thermodynamic and structural description of allosterically regulated VEGFR-2 dimerization. Blood. 2012;119:1781–1788. doi: 10.1182/blood-2011-11-390922. [DOI] [PubMed] [Google Scholar]
- 27.Leppanen VM, Tvorogov D, Kisko K, et al. Structural and mechanistic insights into VEGF receptor 3 ligand binding and activation. Proc Natl Acad Sci U S A. 2013;110:12960–12965. doi: 10.1073/pnas.1301415110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ruch C, Skiniotis G, Steinmetz MO, Walz T, Ballmer-Hofer K. Structure of a VEGF-VEGF receptor complex determined by electron microscopy. Nat Struct Mol Biol. 2007;14:249–250. doi: 10.1038/nsmb1202. [DOI] [PubMed] [Google Scholar]
- 29.Yang Y, Xie P, Opatowsky Y, Schlessinger J. Direct contacts between extracellular membrane-proximal domains are required for VEGF receptor activation and cell signaling. Proc Natl Acad Sci U S A. 2010;107:1906–1911. doi: 10.1073/pnas.0914052107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Markovic-Mueller S, Stuttfeld E, Asthana M, et al. Structure of the Full-length VEGFR-1 Extracellular Domain in Complex with VEGF-A. Structure. 2017;25:341–352. doi: 10.1016/j.str.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 31.Christinger HW, Fuh G, de Vos AM, Wiesmann C. The crystal structure of placental growth factor in complex with domain 2 of vascular endothelial growth factor receptor-1. J Biol Chem. 2004;279:10382–10388. doi: 10.1074/jbc.M313237200. [DOI] [PubMed] [Google Scholar]
- 32.Wiesmann C, Fuh G, Christinger HW, Eigenbrot C, Wells JA, de Vos AM. Crystal structure at 1.7 A resolution of VEGF in complex with domain 2 of the Flt-1 receptor. Cell. 1997;91:695–704. doi: 10.1016/S0092-8674(00)80456-0. [DOI] [PubMed] [Google Scholar]
- 33.McTigue M, Murray BW, Chen JH, Deng YL, Solowiej J, Kania RS. Molecular conformations, interactions, and properties associated with drug efficiency and clinical performance among VEGFR TK inhibitors. Proc Natl Acad Sci U S A. 2012;109:18281–18289. doi: 10.1073/pnas.1207759109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McTigue MA, Wickersham JA, Pinko C, et al. Crystal structure of the kinase domain of human vascular endothelial growth factor receptor 2: a key enzyme in angiogenesis. Structure. 1999;7:319–330. doi: 10.1016/S0969-2126(99)80042-2. [DOI] [PubMed] [Google Scholar]
- 35.Okamoto K, Ikemori-Kawada M, Jestel A, et al. Distinct binding mode of multikinase inhibitor lenvatinib revealed by biochemical characterization. ACS Med Chem Lett. 2015;6:89–94. doi: 10.1021/ml500394m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oguro Y, Miyamoto N, Okada K, et al. Design, synthesis, and evaluation of 5-methyl-4-phenoxy-5H-pyrrolo[3,2-d]pyrimidine derivatives: novel VEGFR2 kinase inhibitors binding to inactive kinase conformation. Bioorg Med Chem. 2010;18:7260–7273. doi: 10.1016/j.bmc.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 37.Fong GH, Rossant J, Gertsenstein M, Breitman ML. Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature. 1995;376:66–70. doi: 10.1038/376066a0. [DOI] [PubMed] [Google Scholar]
- 38.Hiratsuka S, Minowa O, Kuno J, Noda T, Shibuya M. Flt-1 lacking the tyrosine kinase domain is sufficient for normal development and angiogenesis in mice. Proc Natl Acad Sci U S A. 1998;95:9349–9354. doi: 10.1073/pnas.95.16.9349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Landgren E, Schiller P, Cao Y, Claesson-Welsh L. Placenta growth factor stimulates MAP kinase and mitogenicity but not phospholipase C-gamma and migration of endothelial cells expressing Flt 1. Oncogene. 1998;16:359–367. doi: 10.1038/sj.onc.1201545. [DOI] [PubMed] [Google Scholar]
- 40.Sawano A, Takahashi T, Yamaguchi S, Shibuya M. The phosphorylated 1169-tyrosine containing region of flt-1 kinase (VEGFR-1) is a major binding site for PLCgamma. Biochem Biophys Res Commun. 1997;238:487–491. doi: 10.1006/bbrc.1997.7327. [DOI] [PubMed] [Google Scholar]
- 41.Cunningham SA, Waxham MN, Arrate PM, Brock TA. Interaction of the Flt-1 tyrosine kinase receptor with the p85 subunit of phosphatidylinositol 3-kinase. Mapping of a novel site involved in binding. J Biol Chem. 1995;270:20254–20257. doi: 10.1074/jbc.270.35.20254. [DOI] [PubMed] [Google Scholar]
- 42.Katoh O, Tauchi H, Kawaishi K, Kimura A, Satow Y. Expression of the vascular endothelial growth factor (VEGF) receptor gene, KDR, in hematopoietic cells and inhibitory effect of VEGF on apoptotic cell death caused by ionizing radiation. Cancer Res. 1995;55:5687–5692. [PubMed] [Google Scholar]
- 43.Shalaby F, Rossant J, Yamaguchi TP, et al. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature. 1995;376:62–66. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- 44.Holmqvist K, Cross MJ, Rolny C, et al. The adaptor protein shb binds to tyrosine 1175 in vascular endothelial growth factor (VEGF) receptor-2 and regulates VEGF-dependent cellular migration. J Biol Chem. 2004;279:22267–22275. doi: 10.1074/jbc.M312729200. [DOI] [PubMed] [Google Scholar]
- 45.Matsumoto T, Bohman S, Dixelius J, et al. VEGF receptor-2 Y951 signaling and a role for the adapter molecule TSAd in tumor angiogenesis. EMBO J. 2005;24:2342–2353. doi: 10.1038/sj.emboj.7600709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Karkkainen MJ, Haiko P, Sainio K, et al. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat Immunol. 2004;5:74–80. doi: 10.1038/ni1013. [DOI] [PubMed] [Google Scholar]
- 47.Makinen T, Veikkola T, Mustjoki S, et al. Isolated lymphatic endothelial cells transduce growth, survival and migratory signals via the VEGF-C/D receptor VEGFR-3. EMBO J. 2001;20:4762–4773. doi: 10.1093/emboj/20.17.4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dixelius J, Makinen T, Wirzenius M, et al. Ligand-induced vascular endothelial growth factor receptor-3 (VEGFR-3) heterodimerization with VEGFR-2 in primary lymphatic endothelial cells regulates tyrosine phosphorylation sites. J Biol Chem. 2003;278:40973–40979. doi: 10.1074/jbc.M304499200. [DOI] [PubMed] [Google Scholar]
- 49.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 50.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 51.Carmeliet P, Moons L, Luttun A, et al. Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat Med. 2001;7:575–583. doi: 10.1038/87904. [DOI] [PubMed] [Google Scholar]
- 52.Hattori K, Heissig B, Wu Y, et al. Placental growth factor reconstitutes hematopoiesis by recruiting VEGFR1(+) stem cells from bone-marrow microenvironment. Nat Med. 2002;8:841–849. doi: 10.1038/nm740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maynard SE, Min JY, Merchan J, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Irrthum A, Karkkainen MJ, Devriendt K, Alitalo K, Vikkula M. Congenital hereditary lymphedema caused by a mutation that inactivates VEGFR3 tyrosine kinase. Am J Hum Genet. 2000;67:295–301. doi: 10.1086/303019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rockson SG. Lymphedema. Am J Med. 2001;110:288–295. doi: 10.1016/S0002-9343(00)00727-0. [DOI] [PubMed] [Google Scholar]
- 56.Gerber HP, Ferrara N. Pharmacology and pharmacodynamics of bevacizumab as monotherapy or in combination with cytotoxic therapy in preclinical studies. Cancer Res. 2005;65:671–680. [PubMed] [Google Scholar]
- 57.Quesada AR, Munoz-Chapuli R, Medina MA. Anti-angiogenic drugs: from bench to clinical trials. Med Res Rev. 2006;26:483–530. doi: 10.1002/med.20059. [DOI] [PubMed] [Google Scholar]
- 58.Piao Y, Henry V, Tiao N, et al. Targeting intercellular adhesion molecule-1 prolongs survival in mice bearing bevacizumab-resistant glioblastoma. Oncotarget. 2017;8:96970–96983. doi: 10.18632/oncotarget.18859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Motzer RJ, Michaelson MD, Redman BG, et al. Activity of SU11248, a multitargeted inhibitor of vascular endothelial growth factor receptor and platelet-derived growth factor receptor, in patients with metastatic renal cell carcinoma. J Clin Oncol. 2006;24:16–24. doi: 10.1200/JCO.2005.02.2574. [DOI] [PubMed] [Google Scholar]
- 60.Chen X, Zheng Z, Chen L, Zheng H. MAPK, NFkappaB, and VEGF signaling pathways regulate breast cancer liver metastasis. Oncotarget. 2017;8:101452–101460. doi: 10.18632/oncotarget.20843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen Y, Liu Y, Wang Y, et al. Quantification of STAT3 and VEGF expression for molecular diagnosis of lymph node metastasis in breast cancer. Medicine (Baltimore) 2017;96:e8488. doi: 10.1097/MD.0000000000008488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schirosi L, De Summa S, Tommasi S, et al. VEGF and TWIST1 in a 16-biomarker immunoprofile useful for prognosis of breast cancer patients. Int J Cancer. 2017;141:1901–1911. doi: 10.1002/ijc.30868. [DOI] [PubMed] [Google Scholar]