Abstract
Background
Type 1 diabetes is a condition in which the pancreas produces little or no insulin. People with type 1 diabetes must manage their blood glucose levels by monitoring the amount of glucose in their blood and administering appropriate amounts of insulin via injection or an insulin pump. Continuous glucose monitoring may be beneficial compared to self-monitoring of blood glucose using a blood glucose meter. It provides insight into a person's blood glucose levels on a continuous basis, and can identify whether blood glucose levels are trending up or down.
Methods
We conducted a health technology assessment, which included an evaluation of clinical benefit, value for money, and patient preferences related to continuous glucose monitoring. We compared continuous glucose monitoring with self-monitoring of blood glucose using a finger-prick and a blood glucose meter. We performed a systematic literature search for studies published since January 1, 2010. We created a Markov model projecting the lifetime horizon of adults with type 1 diabetes, and performed a budget impact analysis from the perspective of the health care payer. We also conducted interviews and focus group discussions with people who self-manage their type 1 diabetes or support the management of a child with type 1 diabetes.
Results
Twenty studies were included in the clinical evidence review. Compared with self-monitoring of blood glucose, continuous glucose monitoring improved the percentage of time patients spent in the target glycemic range by 9.6% (95% confidence interval 8.0–11.2) to 10.0% (95% confidence interval 6.75–13.25) and decreased the number of severe hypoglycemic events.
Continuous glucose monitoring was associated with higher costs and small increases in health benefits (quality-adjusted life-years). Incremental cost-effectiveness ratios (ICERs) ranged from $592,206 to $1,108,812 per quality-adjusted life-year gained in analyses comparing four continuous glucose monitoring interventions to usual care. However, the uncertainty around the ICERs was large. The net budget impact of publicly funding continuous glucose monitoring assuming a 20% annual increase in adoption of continuous glucose monitoring would range from $8.5 million in year 1 to $16.2 million in year 5.
Patient engagement surrounding the topic of continuous glucose monitoring was robust. Patients perceived that these devices provided important social, emotional, and medical and safety benefits in managing type 1 diabetes, especially in children.
Conclusions
Continuous glucose monitoring was more effective than self-monitoring of blood glucose in managing type 1 diabetes for some outcomes, such as time spent in the target glucose range and time spent outside the target glucose range (moderate certainty in this evidence). We were less certain that continuous glucose monitoring would reduce the number of severe hypoglycemic events. Compared with self-monitoring of blood glucose, the costs of continuous glucose monitoring were higher, with only small increases in health benefits. Publicly funding continuous glucose monitoring for the type 1 diabetes population in Ontario would result in additional costs to the health system over the next 5 years. Adult patients and parents of children with type 1 diabetes reported very positive experiences with continuous glucose monitoring. The high ongoing cost of continuous glucose monitoring devices was seen as the greatest barrier to their widespread use.
OBJECTIVE
This health technology assessment evaluated the clinical benefit, cost-effectiveness, and patient experiences of continuous glucose monitoring compared with usual care (i.e., self-monitoring of blood glucose using a finger-prick and a blood glucose meter) for the management of type 1 diabetes.
BACKGROUND
Health Condition
In Canada, approximately 3.4 million people live with diabetes. It is uncertain how many of those have type 1 diabetes. Some estimates from manufacturers of continuous glucose monitors in Canada, report that approximately 180,000 Canadians have type 1 diabetes, of whom 70,000 live in Ontario. Other estimates suggest that more than 300,000 people in Canada have type 1 diabetes, 150,000 of whom are in Ontario.1,2
In type 1 diabetes, the beta cells (insulin-producing cells) in the pancreas are damaged.3 The role of insulin in the body is to promote entry of glucose into the tissue cells. Inside the cell, glucose is metabolized to release energy, crucial for cell functioning. In most cases, type 1 diabetes is caused by an autoimmune process (the immune system attacks its own cells), resulting in a loss of beta cells. This eventually leads to high levels of glucose in the blood, affecting protein synthesis (protein-building) and other metabolic disorders such as diabetic ketoacidosis (too much acid in the blood).3 Over the long term, people with diabetes can experience serious complications, including kidney disease, heart disease, stroke, nerve damage, and damage to the eyes, leading to blindness.1
Diabetes is considered one of the most burdensome diseases for health care systems because of the time and resource costs related to managing diabetes and its complications.4
Clinical Need and Target Population
Patients with type 1 diabetes manage their blood glucose levels by frequently monitoring the amount of glucose in their blood and administering appropriate amounts of insulin to keep their blood glucose levels in the target range. Hyperglycemia (high blood glucose) can result in the long-term diabetes complications listed above. Hypoglycemia (low blood glucose) may lead to loss of consciousness, seizure, or coma.1
Type 1 diabetes affects people of all ages and genders. It is the most common type of diabetes in children and teens, accounting for at least 85% of diabetes cases in patients aged less than 20 years.5
Current Treatment Options
Typically, people with type 1 diabetes self-monitor their blood glucose levels using a blood glucose meter. Blood glucose levels are usually expressed in millimoles per litre (mmol/L) or milligrams per decilitre (mg/dL). To measure blood glucose levels with a blood glucose meter, a person must prick their finger and squeeze a drop of blood onto a test strip inserted into the meter. The meter then provides a readout of the blood glucose level. People with type 1 diabetes who use a meter usually take readings at regular intervals, including before meals, after meals, before and after physical activity, before driving, and during the night.
A useful laboratory measure for assessing long-term blood glucose management is glycated hemoglobin (A1C), which estimates average blood glucose concentrations over a period of 3 months. This is commonly expressed in terms of National Glycohemoglobin Standardization Program units (%), International Federation of Clinical Chemistry units (mmol/mol), or estimated average glucose (mg/dL). Diabetes Canada (formerly the Canadian Diabetes Association) recommends an optimal A1C of ≤7% to prevent the long-term complications of diabetes.6
Health Technology Under Review
Continuous glucose monitoring provides an opportunity for patients to monitor their blood glucose levels more frequently. It is aimed at helping people with diabetes gain a better understanding of their blood glucose control in real time.
Continuous monitoring of blood glucose levels can be used with multiple daily injections of insulin or an insulin pump. Continuous glucose monitors can be separate from an insulin pump (called standalone continuous glucose monitors) or they can be part of a system that is integrated with an insulin pump (called a sensor-augmented insulin pump).7
Continuous glucose monitors consist of a sensor inserted underneath the skin, a transmitter, and a small monitor. Every few minutes, the sensor measures blood glucose levels in the interstitial fluid8 (fluid that surrounds tissue cells) and sends readings via the transmitter to the monitor, which displays the information.8 For some models, the information can also be transmitted to other devices using Bluetooth technology, so that family members or other caregivers can access blood glucose information.
Continuous glucose monitors that are currently licenced in Canada require regular finger-prick testing to calibrate, usually every 12 hours.7 Continuous glucose monitors that do not require calibration with a finger-prick are expected to reach the market in 2018. The sensors for continuous glucose monitors are intended to be used for no more than 7 days and must be replaced regularly.7 Sensors that last 4 months are in development.9
Regulatory Information
As of November 2016, Health Canada had granted licenses for continuous glucose monitors from two manufacturers. Medtronic (Brampton, Ontario) and Dexcom (San Diego, California) have licences for several generations of devices. For this assessment, we reviewed any Medtronic or Dexcom device that has been included in peer-reviewed publications since 2010. Table 1 summarizes the devices that have Health Canada licences and met the inclusion criteria for this assessment.
Table 1:
Summary of Included Devices
| Manufacturer | Device | Year | License Number |
|---|---|---|---|
| Dexcom | G4 | 2013, 2014 | 91189 |
| G5 | 2016 | 97937 | |
| Medtronic | Glucose sensor | 2000, 2009 | 20654 |
| REAL-TIME transmitter | 2007, 2009, 2013, 2016 | 73839 | |
| Enlite glucose sensor | 2013 | 90691 | |
| 630G | 2016 | 97802 |
Source: Health Canada.10
Medtronic offers a sensor-augmented insulin pump. The continuous glucose monitor is integrated with the pump and includes a “low glucose suspend” feature, which shuts off the administration of insulin for up to 2 hours when blood glucose levels are below a predetermined threshold and the patient is not responding to alerts. This feature may be beneficial for patients with nocturnal (nighttime) hypoglycemia or hypoglycemia unawareness.
The Dexcom continuous glucose monitor is a standalone device, but it can be integrated with the Animas Vibe insulin pump (Animas Corporation, West Chester, Pennsylvania).
Because the scope of this assessment was limited to continuous glucose monitoring devices by manufacturers with Health Canada licences at the time of writing, devices such as the Dexcom SEVEN and the Abbott FreeStyle Navigator were not included in this health technology assessment. Because this assessment was focused on devices used to support patients’ continuous monitoring of their blood glucose levels, devices such as the iPRO2 CGM system (license number 85706) and the Abbot Freestyle Libre Pro (license number 97934), which are used only by health care professionals, were also excluded from this assessment.
Ontario Context
Most patients in Ontario are not reimbursed for the cost of purchasing a continuous glucose monitor. Individuals must pay out of pocket or have private insurance that covers these devices.
In Ontario, the cost of a continuous glucose monitor is publicly funded for people who qualify for the Ontario Disability Support Program and the Mandatory Special Necessities benefit, Ministry of Community and Social Services.11 The Ontario Public Drugs Program offers reimbursement for 3,000 blood glucose test strips per year for certain populations who use insulin to manage their diabetes (i.e., people aged 65 years or older; people who qualify for the Ontario Disability Support Program; Ontario Works recipients; clients of the Trillium Drug Program; residents of long-term care homes or homes for special care; and individuals enrolled in home care).
Ontario's Assistive Devices Program provides funding assistance for insulin pumps for people with type 1 diabetes who are unable to achieve good blood glucose control with multiple daily injections alone.12 To be eligible, patients must have demonstrated good adherence to diabetes management prior to starting pump therapy. Adults must have been on multiple daily injections for 1 year prior to starting insulin pump therapy; pediatric patients are not required to be on multiple daily injections. Since the cost of an insulin pump is covered as an insured device in Ontario, patients who use an insulin pump with integrated continuous glucose monitoring capabilities need to pay for only the continuous glucose monitoring transmitters and sensors.
International Context
Continuous glucose monitoring is in widespread use around the world, and many insurance providers offer some funding. Table 2 summarizes Canadian and international funding options for continuous glucose monitoring.
Table 2:
International Funding of Continuous Glucose Monitoring
| Country | Reimbursement Plan | Details of Fundinga |
|---|---|---|
| Canada | Regional funding and private insurance programs | Limited funding regionally; some funding through private insurance companies |
| Ontario | Assistive Devices Program, Ministry of Health and Long-Term Care | Funding of pump costs for insulin pumps with integrated continuous glucose monitors |
| Some private insurance companies | Details vary by insurance company | |
| Czech Republic | Patient capitation model | Partial funding for continuous glucose monitoring devices |
| France | National insurance funding | Funding for continuous glucose monitoring devices |
| Germany | National insurance funding | Funding for continuous glucose monitoring devices |
| Netherlands | Regional insurance funding | Partial funding for continuous glucose monitoring devices |
| Norway | Tenders; regional | Funding for continuous glucose monitoring devices |
| Slovenia | National reimbursement funding | Funding for continuous glucose monitoring devices for the pediatric population only |
| Sweden | Regional insurance funding | Funding for continuous glucose monitoring devices |
| Switzerland | National reimbursement funding | Funding for continuous glucose monitoring devices |
| United Kingdom | National Institute for Health and Care Excellence diagnostic assessment | National Health Service funds under specific circumstances; the National Institute for Health and Care Excellence guideline strongly recommends the use of continuous glucose monitoring in young people with impaired hypoglycemic awareness or frequent severe hypoglycemic events and adults who meet certain criteria.13,14 |
| United States | Some private insurance companiesb | Details differ depending on insurance company |
| Medicare | Funding for therapeutic continuous glucose monitoring devices |
Information was gathered in part from Dexcom (San Diego, California).
Blue Cross/Blue Shield, Aetna, Cigna, Humana, United Healthcare, Kaiser Permanente, Wellpoint.15
CLINICAL EVIDENCE
Research Question
Compared with usual care (i.e., self-monitoring of blood glucose using a blood glucose meter), what is the effectiveness of continuous glucose monitoring (using standalone devices or integrated with insulin pumps) in the management of type 1 diabetes?
Methods
Research questions are developed by Health Quality Ontario in consultation with clinical experts, patients, health care providers, and other health system stakeholders.
Clinical Literature Search
We performed a literature search on January 24, 2017, to retrieve studies published from January 1, 2010, to the search date. We used the Ovid interface to search the following databases: MEDLINE, Embase, Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, Health Technology Assessment, National Health Service Economic Evaluation Database (NHSEED), and Database of Abstracts of Reviews of Effects (DARE); and we used the EBSCOhost interface to search the Cumulative Index to Nursing & Allied Health Literature (CINAHL). Medical librarians developed the search strategies using controlled vocabulary (i.e., Medical Subject Headings) and relevant keywords. The final search strategy was peer reviewed using the PRESS Checklist.16 We created database auto-alerts in MEDLINE, Embase, and CINAHL and monitored them for the duration of the health technology assessment review, until February 28, 2017.
We performed targeted grey literature searching of health technology assessment agency sites and clinical trial registries. See Appendix 1 for the literature search strategies, including all search terms.
Clinical experts and manufacturers suggested that since continuous glucose monitoring technology has evolved over time, the cut-off year for our literature search should be 2010.
Literature Screening
A single reviewer reviewed the abstracts and, for those studies meeting the eligibility criteria, we obtained full-text articles.
Types of Studies
We included randomized, controlled studies and observational studies that examined (1) the effectiveness of standalone continuous glucose monitors compared with standalone self-monitoring of blood glucose or (2) the effectiveness of continuous glucose monitors integrated with insulin pumps compared with insulin self-management strategies involving insulin pumps or multiple daily injections.
We did not include before-after studies, editorials, case series, or commentaries.
Types of Participants
We included studies of patients with type 1 diabetes. We also considered subgroup analyses by age category.
Types of Interventions
A continuous glucose monitor is any device that provides continuous monitoring of blood glucose, with the results available at any time for patient review. This device may be used alone in conjunction with an insulin pump or multiple daily injections, or it may be integrated into an insulin pump (sensor-augmented pump). Continuous glucose monitors may include additional features, such as high/low glucose alarms or a low glucose suspend option (for sensor-augmented pumps).
Types of Settings
We considered the outpatient setting, with devices used by patients to support management of their blood glucose levels.
Types of Outcome Measures
Time-related glucose variability: The time a patient spends inside (or outside) the target glucose range is usually preferred to A1C as a measurement of overall glucose management, because A1C can be misleading. Patients may spend their day swinging between high and low blood glucose levels; using A1C, which measures the 3-month blood glucose average, may mask this variability
Hypoglycemia: Hypoglycemia is categorized by severity. Hypoglycemia occurs when blood glucose levels fall below 4 mmol/L. Severe hypoglycemia is associated with adverse outcomes for patients. Severe outcomes require the assistance of another person and include seizure, loss of consciousness, and hospitalization
A1C levels: Despite the limitations of A1C (see above), it is commonly used by researchers to evaluate diabetes management. It can provide a good indication of long-term blood glucose levels, since blood cells survive in the body for 3 to 4 months. Diabetes Canada recommends that A1C levels not exceed 7.0%6
User satisfaction: We considered patient satisfaction, with a preference for validated measures of overall satisfaction and health-related quality of life. We also included parent or guardian satisfaction where available
Data Extraction
We extracted relevant data on study characteristics and risk-of-bias items. We used a data form to collect study information about:
Sources (i.e., citation information, contact details, study type)
Characteristics of participants, interventions, and comparators
Methods (i.e., study design, study duration in years, participant allocation, allocation sequence concealment, blinding, reporting of missing data, reporting of outcomes, and whether the study compared two or more groups)
Outcomes (i.e., outcomes measured, number of participants for each outcome, number of participants missing for each outcome, outcome definition and source of information, unit of measurement, upper and lower limits [for scales], and times at which outcomes were assessed)
We contacted study authors for clarification as needed.
Health Equity
During scoping, we did not identify any reported health inequities in relation to continuous glucose monitoring for patients with type 1 diabetes. Nonetheless, whenever available, we have reported distributional characteristics for people likely to be affected by equity, as outlined in PROGRESS-Plus.17
Statistical Analysis
Analysis was done using Review Manager.18 We did not conduct meta-analyses because of heterogeneity in the populations, interventions, and outcomes reported in the included studies. Instead, we have presented narrative syntheses. Where specific outcomes reported were consistent across included studies, we used forest plots for visual purposes, but did not pool estimates. Wherever possible, we reported effect sizes, along with 95% confidence intervals.
Quality of Evidence
We evaluated the quality level of the evidence for each outcome according to the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) guidelines.19,20. We then rated the studies based on the following considerations: risk of bias, inconsistency, indirectness, imprecision, publication bias, magnitude of effect, and dose-response gradient. We determined the overall quality to be high, moderate, low, or very low using a step-wise, structural methodology. The quality level reflects our certainty about the evidence.
We assessed the risk of bias for each study individually using the Cochrane Risk of Bias Tool to assess randomized controlled trials, and the Risk of Bias Assessment Tool for Non-randomized Studies (RoBANS) for observational studies (Appendix 2).21,22
Expert Consultation
Throughout this project, we sought expert consultation on the use of continuous glucose monitoring. Experts consulted included physicians who specialize in endocrinology and diabetes, in both adult and pediatric populations. We also consulted people from industry, specifically Medtronic and Dexcom representatives. The roles of the expert advisors were to inform us of the appropriate use of the technology, contextualize the evidence, and provide insight for our health technology assessment.
Results
Literature Search
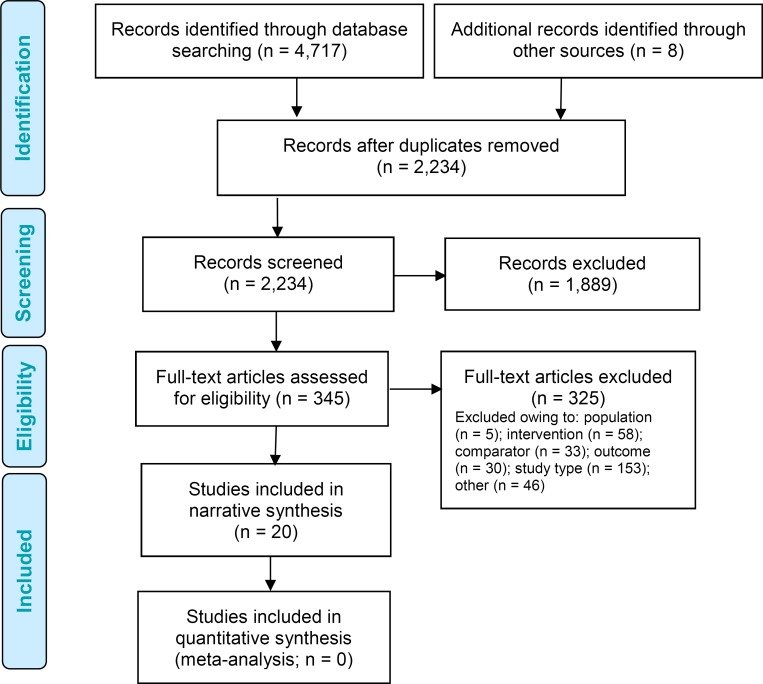
The literature search yielded 2,234 citations published between January 1, 2010, and January 24, 2017, after removing duplicates. We reviewed titles and abstracts to identify potentially relevant articles. We obtained the full texts of these articles for further assessment. We searched the reference lists of the included studies, along with health technology assessment websites and other sources, to identify additional relevant studies. Eight citations were added, and 20 full text studies were included in the narrative synthesis.
Figure 1 presents the flow diagram for the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA).
Figure 1: PRISMA Flow Diagram—Clinical Search Strategy.
Source: Adapted from Moher et al.23
Abbreviation: PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Details of the included studies are summarized in Table 3. The studies varied by continuous glucose monitoring device, inclusion criteria, patient age, and follow-up period. We identified 16 randomized controlled trials24–39 and four observational studies.40–43 Four studies exclusively focused on pediatric populations.26,29,34,37
Table 3:
Summary of Included Studies
| Author, Year Setting | Study Design (Trial Name)a CGM Device | Recruitment Period | Inclusion Criteria | Sample Size, I/C Intervention | Control | Study Period | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age, y | Diagnosis | Glucose Control | Insulin Therapy | Other | |||||||
| Beck et al, 201724 United States 24 sites |
RCT (DIAMOND) Dexcom G4 | October 2014–May 2016 | ≥ 25 | Type 1 diabetes > 1 year | A1C between 7.5% and 10.0% | MDI | Not pregnant | 105/53 | CGM | SMBG | 24 weeks |
| Bergenstal et al, 201025 United States and Canada 30 sites |
RCT (STAR 3) Medtronic MiniMed Paradigm REAL-Time | January 2007–December 2008 | 1–70 | Type 1 diabetes ≥ 3 months | A1C between 7.4% and 9.5% | MDI | NA | 244/241 | SAP | MDI with SMBG | 1 year |
| Bukara-Radujkovic et al, 201126 Bosnia and Herzegovina 1 site |
RCT Medtronic MiniMed | 2006–2007 | 5–18 | Type 1 diabetes ≥ 1 year | A1C ≥ 8% | MDI | NA | 40/40 | CGM | SMBG | 6 months |
| Hermanides et al, 201127 Europe 8 sites |
RCT Medtronic MiniMed Paradigm REAL-Time | April 2007–January 2009 | 18–65 | Type 1 diabetes ≥ 1 year | A1C ≥ 8.2% | MDI | NA | 43/35 | SAP | MDI with SMBG | 26 weeks |
| Hommel et al, 201428 Europe 8 sites |
RCT, crossover (SWITCH) Medtronic MiniMed Paradigm REAL-Time | January 2008–July 2010 | 6–70 | Type 1 diabetes ≥ 1 year | A1C between 7.5% and 9.5% | CSII > 6 months | CGM-naïve | 153 (total sample size) | Sensor on | Sensor off | 17 months |
| Kordonouri et al, 201229 Europe 5 sites |
RCT (ONSET) Medtronic MiniMed Paradigm REAL-Time | February 2007–October 2008 | 1–16 | Type 1 diabetes ≥ 1 year | NR | CSII | NA | 80/80 | SAP | CSII with SMBG | 1 year |
| Langeland et al, 201230 Norway 1 site |
RCT, crossover Medtronic MiniMed Guardian REAL-Time | January 2009–March 2009 | 18–50 | Type 1 diabetes > 3 years | A1C between 7% and 10% | MDI or CSII | > 1 serious hypoglycemic event in previous 6 months Untreated concomitant disease | 30 (total sample size) | CGM | SMBG | 20 weeks; 4 weeks of intervention, 8 weeks of washout before crossover |
| Lind et al, 201731 Sweden 15 sites |
RCT, crossover (GOLD) Dexcom G4 | February 2014–June 2016 | ≥ 18 | Type 1 diabetes > 1 year | A1C ≥ 7.5% | MDI | NA | 142 (total sample size) | CGM | Usual care | 26 weeks of intervention, 17 weeks of washout before crossover |
| Little et al, 201432 United Kingdom 5 sites |
RCT, 2 × 2 crossover (HypoCOMPaSS) Medtronic REAL-Time | NR | 18–74 | Type 1 diabetes, C-peptide negative | Impaired hypoglycemia awareness | NR | NA | 96 (total sample size) | CGM with MDI CGM with CSII | SMBG with MDI SMBG with CSII | 24 weeks |
| Ly et al, 201333 Australiab |
RCT Medtronic Paradigm Veo | December 2009–January 2012 | 4–50 | Type 1 diabetes | Hypoglycemia unawareness/ impaired awareness | CSII > 6 months | Not pregnant | 46/49 | SAP with low glucose suspend | CSII with SMBG | 6 months |
| McQueen et al, 201440 United States 1 site |
Retrospective cohort Medtronic MiniMed Paradigm REAL-Time or Dexcom device | 2006–2011 | ≥ 18 | Type 1 diabetes | NR | NR | Not pregnant | 66/67 | CGM with SMBG | SMBG | Up to 10 months |
| Olivier et al, 201434 Canada 2 sites |
Pilot RCT Medtronic MiniMed Paradigm REAL-Time | February 2009–January 2011 | 5–18 | Type1 diabetes ≥ 1 year | NR | Injection therapy | NA | 10/10 | CGM with CSII | CSII with delayed CGM | 4 months |
| Quiros et al, 201541 Europe 8 sites |
Retrospective observational study of RCT (SWITCH) Medtronic MiniMed Paradigm REAL-Time | January 2008–July 2010 | 6–70 | Type 1 diabetes ≥ 1 year | A1C between 7.5% and 9.5% | CSII > 6 months | NA | 20 (total sample size) | SAP | CSII | 3 years |
| Radermecker et al, 201042 Belgium 1 site |
Prospective observational controlled trial Medtronic Guardian REAL-Time | NR | Adults | Type 1 diabetes ≥ 1 year | ≥ 6 capillary glucose recordings of < 60 mg/dL in 14 days | CSII > 1 year | NA | 13 (total sample size) | CGM | SMBG | 12 weeks |
| Rosenlund et al, 201535 Denmark 2 sites |
RCT Medtronic MiniMed Paradigm Veo | February 2012–December 2014 | 18–75 | Type 1 diabetes | A1C ≥ 7.5% | MDI | GFR at least 45 mL/min/ 1.73 m2 No other concomitant disease; no pregnancy | 26/29 | SAP | MDI with SMBG | 1 year |
| Rubin and Peyrot, 201236 United States and Canada 30 sites |
RCT (STAR 3) Medtronic MiniMed Paradigm REAL-Time | January 2007–December 2008 | 7–70 | Type 1 diabetes ≥ 3 months | A1C between 7.4% and 9.5% | MDI | < 2 hypoglycemic events in previous year Not pregnant | 243/238 | SAP | MDI with SMBG | 1 year |
| Slover et al, 201237 United States and Canada 30 sites |
RCT (STAR 3) Medtronic MiniMed Paradigm REAL-Time | January 2007–December 2008 | 7–18 | Type 1 diabetes ≥ 3 months | A1C between 7.4% and 9.5% | MDI | < 2 hypoglycemic events in previous year | 78/78 | SAP | MDI with SMBG | 1 year |
| Soupal et al, 201643 Czech Republic 1 site |
Prospective controlled trial Medtronic MiniMed Paradigm Veo | NR | > 18 | Type 1 diabetes > 2 years | A1C between 7% and 10% | MDI or CSII | No concomitant disease; not pregnant or planning pregnancy | 27/38 | SAP CGM with MDI | SMBG with CSII SMBG with MDI | 52 weeks |
| Tumminia et al, 201538 Italy 1 site |
RCT, crossover Medtronic MiniMed Guardian REAL-Time | January–March 2012 | 18–60 | Type 1 diabetes | A1C > 8% | MDI or CSII | Middle-class socioeconomic status; no concomitant disease; not pregnant or planning pregnancy | 20 (total sample size) | CGM | SMBG | 14 months; 6 months of intervention, 2 months of washout before crossover |
| van Beers et al, 201639 Netherlands 2 sites |
RCT (IN CONTROL) Medtronic MiniMed Paradigm Veo | March 2013–February 2014 | 18–75 | Type 1 diabetes | Impaired hypoglycemia awareness | CSII or MDI | No concomitant disease; not pregnant | 26/26 | CGM | SMBG | 44 weeks; 16 weeks of intervention, 12 weeks of washout before crossover |
Abbreviations: A1C, glycated hemoglobin; CGM, continuous glucose monitoring; CSII, continuous subcutaneous insulin infusion (insulin pump); GFR, glomerular filtration rate; I/C, intervention/control; MDI, multiple daily injections; NA, not applicable; NR, not reported; RCT, randomized controlled trial; SAP, sensor-augmented pump; SMBG, self-management of blood glucose.
Some studies have been given a trial nickname; where that exists, it has been listed to help identify multiple publications on the same study.
Number of sites not provided.
Results for Time-Related Glucose Variability
We examined glucose variability as a measure of time spent in or out of the target glycemic (normoglycemic) range. Results for time spent in the target glycemic range are presented in Table 4. Results from two randomized controlled trials favoured continuous glucose monitoring over control.
Table 4:
Results for Time Spent in Target Glycemic Range
| Author, Year | Measure of Glucose Variability | Resultsa | Differencea | P-valuea | |
|---|---|---|---|---|---|
| Intervention | Control | ||||
| Adult Population, RCTs | |||||
| Beck et al, 201724 | Mean minutes per day within the target range of 70–180 mg/dL | 736 (SE 7.59) Change from baseline: | 650 (SE 7.61) Change from baseline: 0 min | Adjusted mean difference: 77 (99% CI 6–147) | .005 |
| van Beers et al, 201639 | Mean % time spent in normoglycemia (4.0–10.0 mmol/L) | +76 min 65.0 (95% CI 62.8–67.3) | 55.4 (95% CI 53.1–57.7) | 9.6 (95% CI 8.0–11.2) | < .01 |
| Adult Population, Observational Study | |||||
| Soupal et al, 201643 | Mean % time spent between 4.0 and 10.0 mmol/L | 69 (SE 2.12) | 59 (SE 2.46) | 10 (95% CI 6.75–13.25) | Study authors reported difference as not significantb |
Abbreviations: CI, confidence interval; RCT, randomized controlled trial; SE, standard error.
CIs, P-values, and SE were calculated by the authors of this health technology assessment.
Repeated calculations conducted by the authors of this health technology assessment yielded a significant P-value.
The quality of the evidence for time spent in the target glycemic range was moderate for the randomized controlled trials and very low for the observational study. Details of the GRADE assessment can be found in Appendix 2.
Table 5 summarizes the findings for studies that evaluated time spent outside the target glycemic range. Overall, results favoured continuous glucose monitoring over control.
Table 5:
Results for Time Spent Outside of Target Glycemic Range
| Author, Year | Measure of Glucose Variability | Results | Difference | P-value | |
|---|---|---|---|---|---|
| Intervention | Control | ||||
| Time Spent in Hypoglycemic Range, Adult Population, RCTs | |||||
| Beck et al, 201724a | Median minutes per day in hypoglycemia (< 60 mg/dL) | 20 (IQR 9–30) | 40 (IQR 16–68) | −20 | .002b |
| Hermanides et al, 201127 | Mean % of time in hypoglycemia (< 4.0 mmol/L) | 2.7 (SE 0.53) | 2.5 (SE 0.6) | LSM difference at baseline and end of study: 0.2 (95% CI −1.6 to 1.7) | .96 |
| Ly et al, 201333a | Median % of time in hypoglycemia (< 60 mg/dL) | Dayc: 1.5 (IQR 0.9–3.7) Nightc: 2.4 (IQR 0.4–5.3) | Dayc: 3.3 (IQR 1.6–5.9) Nightc: 6.2 (IQR 4.2–9.9) | Dayc: −1.8 Nightc: −3.8 | Dayc: .01b Nightc: < .001b |
| van Beers et al, 201639a | Mean hours per day in hypoglycemia (≤ 3.9 mmol/L) | 1.6 (95% CI 1.3–2.0) | 2.7 (95% CI 2.4–3.1) | Mean difference −1.1 (95% CI −1.4 to −0.8) | < .001 |
| Time Spent in Hyperglycemic Range, Adult Population, RCTs | |||||
| Beck et al, 201724a | Median minutes per day in hyperglycemia (> 250 mg/dL) | 223 (IQR 128–383) | 347 (IQR 241–429) | −124 | < .001b |
| Hermanides et al, 201127 | Mean % of time in hyperglycemia (> 11.1 mmol/L) | 21.6 (SE 1.91) | 38.2 (SE 3.58) | LSM difference between groups at baseline and end of study: −17.3 (95% CI −25.1 to −9.5) | < .001 |
Abbreviations: CI, confidence interval; IQR, interquartile range; LSM, least square mean; RCT, randomized controlled trial; SE, standard error.
Select results are presented; additional thresholds and permutations of similar results are available in the original study.
Authors reported the P-value for the mean difference; the comparison is for the median difference.
Day, 6 a.m. to 10 p.m.; night, 10 p.m. to 6 a.m.
The quality of the evidence for time spent outside the target glycemic range was moderate for the randomized controlled trials in adults. Details of the GRADE assessment can be found in Appendix 2.
Results for Hypoglycemia
Table 6 summarizes the results for hypoglycemia and severe hypoglycemia. Because of variations in how hypoglycemia was reported between studies, it was difficult to develop a summary conclusion. However, in general there did not seem to be a substantial difference in hypoglycemic outcomes between the continuous glucose monitoring groups and the control groups in both adult and pediatric populations.
Table 6:
Results for Hypoglycemia
| Author, Year | Measure of Hypoglycemia | Results | Difference | P-value | |
|---|---|---|---|---|---|
| Intervention | Control | ||||
| Adult Population, RCTs | |||||
| Bergenstal et al, 201025 | AUC of rate of patients having blood glucose < 50 mg/dL per day | 0.02 (SE 0.03)a | 0.03 (SE 0.07)a | −0.01 (SE 0.003)a | .16b |
| Hermanides et al, 201127 | Mean number of hypoglycemic episodes (< 4.0 mmol/L) per day | 0.7 (SE 0.11)a | 0.6 (SE 0.12)a | 0.1 (95% CI −0.2 to 0.5)a | .40 |
| Langeland et al, 201230 | Mean number of hypoglycemic episodes (≤ 3.1 mmol/L) per 4 weeks | 8.2 (SE 0.41)a | 7.3 (SE 0.36)a | 0.9 (95% CI 0.85–0.95)a | .67c |
| Tumminia et al, 201538 | AUC of rate of patients having blood glucose < 70 mg/dL per day | Owing to concerns with the statistical analyses, results are not reportedd | NS | ||
| Adult Population, Observational Studies | |||||
| Radermecker et al, 201042 | Mean decrease from baseline in number of hypoglycemic episodes (< 60 mg/dL) per 14 days | 6.2 (95% CI 2.2–10.2) | 0.67 (95% CI −4.7 to 6.0) | Mean difference 5.3 (95% CI −0.49 to 11.55)a | .85a |
| Soupal et al, 201643 | Mean reduction of % time spent in hypoglycemia | 6 (SE 0.87)a | 7 (SE 1.18)a,e | −1 (SE 2.39)d | .68 |
| Pediatric Population, RCTs | |||||
| Bergenstal et al, 201025 | AUC of rate of patients having blood glucose < 50 mg/dL | Owing to concerns with the statistical analyses, results are not reportedd | .64 | ||
| Bukara-Radujkovic et al, 201126 | Difference in average number of hypoglycemic episodes (< 3.5 mmol/L) per day | 0.223 | 0.175 | 0.048 | NR |
| Slover et al, 201137 | AUC of rate of patients having blood glucose < 60 mg/dL per day (change from baseline)e | Age 7–12: 0.05 (SD 0.08) Age 13–18: −0.05 (SD 0.08) | Age 7–12: 0.03 (SD 0.06) Age 13–18: −0.05 (SD 0.09) | Age 7–12: 0.02 (SD 0.16) Age 13–18: 0 (SD 0.15) | Age 7–12: .05 Age 13–18: .87 |
Abbreviations: AUC, area under the curve; CI, confidence interval; NR, not reported; NS, not significant; RCT, randomized controlled trial; SD, standard deviation; SE, standard error.
Calculations for SE and CI were conducted by the authors of this health technology assessment.
The reported P-value was adjusted for baseline differences, but the SE is for the unadjusted difference.
We could not replicate results for this P-value based on the methods and data reported by the authors.
Data were skewed, but the authors used statistical methods that are valid only under a symmetric assumption. Statistical results were questionable.
The study included both patients on insulin pumps and those on multiple daily injections, but these results were for only patients on multiple daily injections.
The quality of the evidence for hypoglycemia was low for the randomized controlled trials in adults, and very low for the observational studies in adults and randomized controlled trials in children. Details of the GRADE assessment can be found in Appendix 2.
Table 7 summarizes findings for severe hypoglycemic events. Results were generally in favour of continuous glucose monitoring.
Table 7:
Results for Severe Hypoglycemic Events
| Author, Year | Measure of Severe Hypoglycemic Events | Results | Difference | P-value | |
|---|---|---|---|---|---|
| Intervention | Control | ||||
| Adult Population, RCTs | |||||
| Little et al, 201432 | Severe hypoglycemia requiring the assistance of another person, annualized rate | 0.8 (SD 1.8) | 0.9 (SD 2.1) | −0.1 (SD 3.63) | .95 |
| Ly et al, 201333a | Severe hypoglycemia, including seizure or coma, 6-month rate per 100 patient-months (change from baseline) | −1.8 | 0.1 | −1.5 (95% CI −2.7 to −0.3)a | .02b |
| van Beers et al, 201639 | Number of severe hypoglycemic events | 14 | 34 | −20 | .033b |
Abbreviations: CI, confidence interval; RCT, randomized controlled trial; SD, standard deviation.
Results generated from a statistical model.
Computed using a nonparametric statistical test.
The quality of the evidence for severe hypoglycemic events was low for the randomized controlled trials in adults. Details of the GRADE assessment can be found in Appendix 2.
Results for A1C Levels
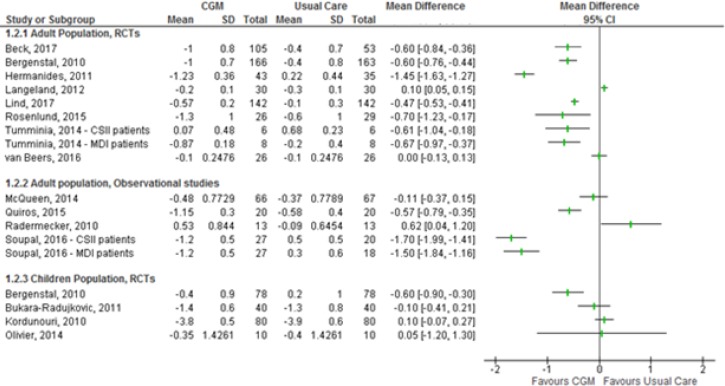
Studies comparing average A1C levels reported results in two ways: change in average A1C levels from baseline, and average A1C levels at the end of the study. The former approach accounts for baseline differences in A1C levels; as a result, our assessment focused only on results derived using this approach. Results for the difference in change in blood glucose levels from baseline to end of study are summarized in Figure 2.
Figure 2: Changes in A1C from Baseline to End of Study.
Sources: Data from Beck et al,24 Bergenstal et al,25 Bukara-Radujkovic et al,26 Hermanides et al,27 Kordonouri et al,29 Langeland et al,30 Lind et al,31 McQueen et al,40 Olivier et al,34 Quiros et al,41 Radermecker et al,42 Rosenlund et al,35 Soupal et al,43 Tumminia et al,38 and van Beers et al.39
Abbreviations: A1C, glycated hemoglobin; CGM, continuous glucose monitoring; CI, confidence interval; CSII, continuous subcutaneous insulin infusion (insulin pump); MDI, multiple daily injections; RCT, randomized controlled trial; SD, standard deviation.
Note: Olivier et al34 and Quiros et al41 reported results for a combined population of adults and children. Tumminia et al38 reported a crossover design, but their analysis was based on a before-after design.
Based on the overall results, continuous glucose monitoring led to a greater reduction in A1C levels than usual care. However, the average A1C values at the end of follow-up were higher than 7% for all studies—above the threshold set by the Diabetes Canada guidelines.6 As a result, we do not regard the reduction in A1C observed above as clinically important. However, Beck et al24 reported that 18% of people who used continuous glucose monitoring achieved an A1C ≤ 7.0%; only 2% of the usual care group reached this threshold.
The quality of the evidence for changes in A1C levels was moderate for randomized controlled trials in adults, low for randomized controlled trials in children, and very low for observational studies in adults. Details of the GRADE assessment can be found in Appendix 2.
Results for User Satisfaction
Of the studies that reported user satisfaction, most used well-known measures of quality of life, often measures specific to diabetes. Table 8 presents results reported in the individual studies.
Table 8:
Results for User Satisfaction
| Author, Year | Measure of User Satisfaction | Results | Difference | P-value | |
|---|---|---|---|---|---|
| Intervention | Control | ||||
| Results for Adult Population, RCTs | |||||
| Beck et al, 201724 | CGM satisfaction survey, mean score | 4.2 (SD 0.4) | NR | NR | NR |
| Hermanides et al, 201127 | Problem areas in diabetes scale | 21.0 (SD 19.3) | 23.7 (SD 19.4) | LSM change from baseline: −7.9 (95% CI −15.1 to −0.61) | .03 |
| Hypoglycemia fear survey | 24.1 (SD 20.2) | 20.3 (SD 16.9) | LSM change from baseline: −3.2 (95% CI −10.0 to 3.7) | .36 | |
| DTSQ | 32.4 (SD 3.5) | 23.8 (SD 6.2) | LSM change from baseline: 9.3 (95% CI 7.3–11.3) | < .001 | |
| Hommel et al, 201428 | DTSQ status version, overall treatment satisfaction | NR | NR | 1.16 | .010 |
| Langeland et al, 201230 | DTSQ change version, change in total score SF-36, change in total average | 3.93 (SD 8.00) −0.3 (SD 8.5) | 5.74 (SD 5.83) −0.3 (SD 9.8) | −1.81 (SD 16.14)a 0 (SD 16.14)a | .47 .35 |
| Lind et al, 201731 | DTSQ status version, scale total | 30.21 (95% CI 29.47–30.96) | 26.62 (95% CI 25.61–27.64) | 3.43 (95% CI 2.31–4.54) | < .001 |
| Little et al, 201432 | DTSQ total satisfaction | 30 (SD 5) | 30 (SD 5) | — | .79 |
| Rubin and Peyrot, 201236 | SF-36, change from baseline | MCS: 0.05 PCS: 1.22 | MCS: −1.26 PCS: 0.26 | MCS: −1.21a PCS: 0.96a | NR NR |
| Results for Adult Population, Observational Studies | |||||
| Radermecker et al, 201042 | DQOL total score, change from baseline | −2.3 (95% CI −6.4 to 1.7) | 0.7 (95% CI −2.5 to 3.8) | −3.0 (95% CI −7.67 to 1.68)a | .22a |
| Results for Pediatric Population, RCTs | |||||
| Hommel et al, 201428 | PedsQL overall health-related quality of life | NR | NR | Child self-rating: −0.31 (SD 0.84) Parent proxy rating: −3.92 (SD 1.18)b | Child self-rating: .84 Parent proxy rating: .002 |
| Kordonouri et al, 201229 | KIDSCREEN-27 psychological well-being | Child self-report: 50.4 (SD 9.2) Proxy/parent: 47.8 (SD 9.3) | Child self-report: 50.3 (SD 10.8) Proxy/parent: 48.6 (SD 10.3) | Child self-report: 0.1 (SD 18.56)a Proxy/parent: −0.8 (SD 18.74)a | Child self-report: .905 Proxy/parent: .826 |
| Olivier et al, 201434 | DTSQ change in total score | NR | NR | −9 (95% CI −16 to −1) | .02 |
| Rubin and Peyrot, 201236 | PedsQL overall score, change from baseline | Child self-report: 0.33 Caregiver: 40.19 | Child self-report: 1.19 Caregiver: 5.07 | Child self-report: 29.14 Caregiver: 35.12 | Child self-report: .001 Caregiver: < .001 |
Abbreviations: CGM, continuous glucose monitoring; CI, confidence interval; DQOL, Diabetes Quality of Life [questionnaire]; DTSQ, Diabetes Treatment Satisfaction Questionnaire; LSM, least square mean; MCS, mental composite score; NR, not reported; PCS, physical composite score; PedsQL, pediatric quality of life inventory; RCT, randomized controlled trials; SD, standard deviation; SF-36, 36-Item Short Form Health Survey.
Results calculated based on information published in original studies.
Publication noted that this was not a clinically meaningful difference.
Results were inconsistent across studies, probably reflecting differences in types of outcomes and survey tools. Therefore, we rated the quality of the evidence for user satisfaction as low for randomized controlled trials in adults and children, and very low in observational studies in adults. Details of the GRADE assessment can be found in Appendix 2.
Summary
We included 20 studies that reported on the use of continuous glucose monitors (as standalone devices or integrated with insulin pumps) compared with usual care. Usual care was typically defined as self-monitoring of blood glucose levels using a finger-prick blood glucose meter.
We did not perform meta-analyses because of the heterogeneity of populations and interventions. All results for outcomes of interest have been summarized narratively (Table 9).
Table 9:
Summary of Findings
| Outcome | Finding | GRADE |
|---|---|---|
| Time-related glucose variability | Continuous glucose monitoring was more effective than usual care in terms of increased time spent in the target glycemic range | Moderate to very low |
| Continuous glucose monitoring was more effective than usual care in terms of decreasing time spent outside the target glycemic range | Moderate | |
| Hypoglycemia | There was no substantial difference in hypoglycemic outcomes between patients in the continuous glucose monitoring group and those in the usual care group | Low to very low |
| Continuous glucose monitoring was more effective than usual care in reducing severe hypoglycemic events | Low | |
| A1C levels | Results favoured continuous glucose monitoring over usual care in the reduction of A1C levels from baseline | Moderate to very low |
| User satisfaction | Findings on end-of-study user satisfaction with continuous glucose monitoring compared with usual care were inconsistent | Low to very low |
| Findings on children, parent, and caregiver satisfaction with continuous glucose monitoring compared with usual care were inconsistent | Low |
Abbreviations: A1C, glycated hemoglobin; GRADE, Grading of Recommendations Assessment, Development, and Evaluation.
We applied the GRADE criteria to assess the quality of evidence (Appendix 2).19 We applied GRADE to randomized controlled trials in adult populations, observational studies in adult populations, and randomized controlled trials of child populations separately for each outcome, where reported. There were no observational studies in child populations.
Discussion
Main Findings and Clinical Relevance
Continuous glucose monitoring was more effective than self-monitoring of blood glucose for the management of type 1 diabetes, as demonstrated by outcomes such as time spent in target glucose range and severe hypoglycemic events. Interestingly, the majority of the reviewed studies were unable to demonstrate the same effect for hypoglycemia.
Studies evaluating the impact of continuous glucose monitoring on user satisfaction yielded mixed results. These findings may be partly explained by the fact that wearing a sensor or a pump may be perceived as an interruption to children's normal activities. Engaging with parents, caregivers, and children to understand the most practical way to monitor blood glucose is important for effective management of type 1 diabetes. In addition, to avoid diabetes complications, controlling diabetes at a younger age can reduce the risk of metabolic memory,44 a condition characterized by persistent diabetes complications despite tight glucose control. Details about parent and child preferences with regard to diabetes management in the Ontario context are provided in the Patient, Caregiver, and Public Engagement section of this health technology assessment.
We noted several limitations from the primary studies. First, studies that evaluated the effectiveness of continuous glucose monitoring in reducing hyperglycemia recruited patients with high A1C levels. A substantial decrease in A1C would have been required from the patients in these studies to meet the 7% threshold recommended by many experts and clinical practice guidelines. Although the majority of studies did demonstrate a reduction in A1C, the average decrease was not enough to meet the threshold. Second, some studies expressed concerns about missing data.28,36,37 To address the problem, these studies imputed outcomes by carrying forward the observation from the last visit, but treated the imputed values as if they were real during analysis. In doing so, these studies may have overestimated the precision of point estimates and introduced outcome classification errors. Third, the statistical methods used in some studies25,32,38 yielded estimates with ranges that covered implausible values. Specifically, the reported standard deviations were larger than the point estimates, suggesting that the area under the curve or the number of hypoglycemic episodes could be negative. As a result, we could not determine the true precision of the point estimates for these studies. Finally, the definition of usual care for some studies included the use of an insulin pump, a mode of insulin administration that is used less in Ontario (there are about 14,000 insulin pump users in Ontario). This means results from these studies may not accurately reflect the effectiveness of continuous glucose monitoring in Ontario, where usual care generally involves multiple daily injections. A comparison of different methods of insulin administration was beyond the scope of this health technology assessment.
Real-World Use of Continuous Glucose Monitoring
Some studies29,31,35,39 enrolled only patients who exceeded a certain threshold of adherence with glucose monitoring; as a result, the level of adherence in the controlled setting of these studies was likely to be higher than in the general population. Several survey studies have examined adherence and reasons for discontinuation of continuous glucose monitoring in the real world.45–49 They demonstrated that patients do not use continuous glucose monitoring 100% of the time, and that use tends to taper off over time. The main reasons reported for discontinued use were cost; discomfort with wearing the devices, including sensors falling off; and finding the alarms disruptive.
Ongoing Studies
During scoping, we identified 35 studies on clinicaltrials.gov related to continuous glucose monitoring, glycemic control, and type 1 diabetes. However, we determined that the current literature was sufficient to evaluate the clinical effectiveness of continuous glucose monitoring.
Conclusions
Based on moderate certainty in the evidence, we found that continuous glucose monitoring was more effective than self-monitoring of blood glucose in managing type 1 diabetes for some outcomes, such as time spent in target glucose range and time spent outside target glucose range. Similar findings were obtained for the outcome of severe hypoglycemic events, although there was low certainty in the evidence for this outcome.
ECONOMIC EVIDENCE
Research Question
What is the cost-effectiveness of continuous glucose monitoring compared with self-monitoring of blood glucose in patients with type 1 diabetes?
Methods
Economic Literature Search
We performed an economic literature search on January 25, 2017, for studies published from January 1, 2010, to the search date. We applied methodological filters to the clinical search to limit retrieval to economic evaluations and studies on cost, quality of life, and health utilities.50
Database auto-alerts were created in MEDLINE, Embase, and CINAHL and monitored for the duration of the health technology assessment review. We performed targeted grey literature searching of health technology assessment agency sites, clinical trial registries, and Tufts Cost-Effectiveness Analysis Registry. See Clinical Evidence, Literature Search, above, for further details on methods used. See Appendix 1 for literature search strategies, including all search terms.
Finally, we reviewed reference lists of included economic literature for any additional relevant studies not identified through the systematic search.
Literature Screening
A single reviewer reviewed titles and abstracts, and, for those studies meeting the eligibility criteria, we obtained full-text articles. For studies containing several comparators, we extracted only the results for the comparison of interest.
Types of Studies
We included cost-effectiveness or cost-utility analyses that compared continuous glucose monitoring with self-monitoring of blood glucose in adults and children with type 1 diabetes. We examined economic studies that fulfilled the described entry criteria and that had a follow-up time or time horizon of 1 year or greater.
We did not include abstracts, letters, editorials, unpublished studies, or noncomparative studies reporting the costs of continuous glucose monitoring.
Types of Participants
The population of interest was patients with type 1 diabetes, including those with hypoglycemia unawareness.
Types of Interventions
Continuous glucose monitoring can be performed using different devices and technologies (see Background). We looked at studies that compared self-monitoring of blood glucose plus either multiple daily injections or an insulin pump with one or more continuous glucose monitoring interventions:
Continuous glucose monitoring plus multiple daily injections
Continuous glucose monitoring plus insulin pump
Sensor-augmented pump (continuous glucose monitoring integrated with an insulin pump)
Sensor-augmented pump with a low-glucose suspend feature
Types of Outcomes Measures
We examined the following outcomes: incremental costs, incremental quality-adjusted life-years (QALYs), incremental cost-effectiveness ratios (ICERs), and incremental net benefit.
Data Extraction
We extracted relevant data on the following:
Source (i.e., name, location, year)
Populations and comparators
Interventions
Outcomes (i.e., health outcomes, costs, and ICERs)
Study Applicability and Limitations
We determined the usefulness of each identified study for decision-making by applying a modified applicability checklist for economic evaluations that was originally developed by the National Institute for Health and Care Excellence (NICE) in the United Kingdom. The original checklist is used to inform development of the institute's clinical guidelines. We modified the wording of the questions to remove references to guidelines and to make them Ontario-specific.
We separated the checklist into two sections. In the first, we assessed the applicability of the study to our research question. A summary of the studies judged to be directly applicable, partially applicable, or not applicable to the research question are shown in Appendix 3. If the study was deemed directly or partially applicable to the research question, we assessed the limitations of the study (minor, potentially serious, or very serious) using the second section of the checklist.
Results
Literature Search
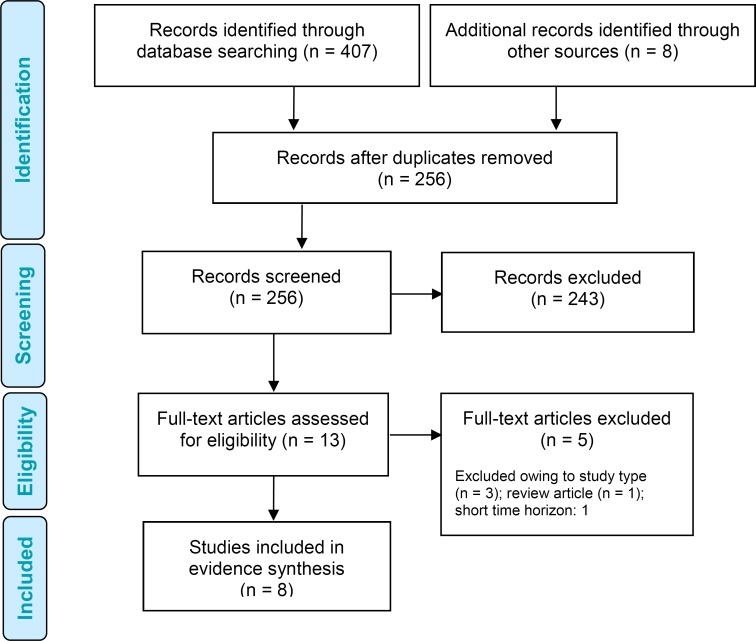
The literature search yielded 256 citations published between January 1, 2010, and January 25, 2017 (with duplicates removed). We excluded a total of 243 articles based on information in the title and abstract. We then obtained the full texts of 13 potentially relevant articles for further assessment. Figure 3 presents the flow diagram for the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA).
Figure 3: PRISMA Flow Diagram—Economic Search Strategy.
Source: Adapted from Moher et al.23
Eight studies (seven cost-utility analyses51–57 and one health technology assessment report by the National Institute for Health and Care Excellence58) met the inclusion criteria (Table 10). All studies were based on models. Three cost-utility analyses studies were from United States,51,52,57 two studies were from the United Kingdom,53,58 and one each was from Sweden,54 France,55 and Denmark.56 No studies were done in children with type 1 diabetes.
Table 10:
Results of the Economic Literature Review—Summary
| Name, Year, Location | Study Design and Perspective | Population | Intervention/ Comparator | Results | ||
|---|---|---|---|---|---|---|
| Health Outcomes | Costs | Cost-Effectiveness | ||||
| Huang et al, 2010,57 United States |
|
Cohort 1: adults with T1D aged ≥ 25 years and A1C ≥ 7.0% Cohort 2: all ages with A1C ≤ 7.0% | CGM SMBG | Cohort 1 Total QALYs: SMBG 13.75; CGM 14.35 QALYs gained: 0.60 Cohort 2 Total QALYs: SMBG 16.69; CGM 17.80 QALYs gained: 1.11 Annual discount rate: 3% | 2007 US dollars Cohort 1 Total costs: SMBG $601,070; CGM $659,837 Incremental cost for CGM: $58,767 vs. SMBG Cohort 2 Total costs: SMBG $2,111,539; CGM $2,198,925 Incremental cost for CGM: $87,386 vs. SMBG Annual discount rate: 3% | Cohort 1 ICER: $98,679 per QALY gained vs. SMBG Cohort 2 ICER: $87,386 per QALY gained vs. SMBG |
| Kamble et al, 2012,52 United States |
|
Adults with inadequately controlled T1D; mean age of 41.3 years; mean A1C 8.3% | SAP SMBG MDI | Total QALYs: SAP 10.794; SMBG MDI 10.418 QALYs gained: 0.376 Annual discount rate: 3% | 2010 US dollars 3-day sensors Total costs: SMBG MDI $167,170; SAP $253,493 Incremental cost for SAP: $86,324 vs. SMBG MDI 6-day sensors Total costs: SMBG MDI $167,170; SAP $230,352 Incremental cost for SAP: $63,182 vs. SMBG MDI Annual discount rate: 3% | 3-day sensors ICER: $229,675 per QALY gained vs. SMBG MDI 6-day sensors ICER: $168,104 per QALY gained vs. SMBG MDI |
| McQueen et al, 2011,51 United States |
|
20-year history of T1D, mean age of 40 years | CGM + SMBG with intensive insulin therapy SMBG with intensive insulin therapy | Total QALYs: SMBG 10.289; CGM + SMBG 10.812 QALYs gained: 0.52 Annual discount rate: 3% | 2007 US dollars Total costs: SMBG $470,583; CGM + SMBG $494,135 Incremental cost for CGM + SMBG: $23,552 vs. SMBG alone Annual discount rate: 3% | ICER: $45,033 per QALY gained vs. SMBG alone |
| Riemsma et al, 2016,58 United Kingdom |
|
27-year history of T1D; mean age of 42 years; 38% male | SAP SMBG MDI or SMBG CSII | SAP vs. SMBG MDI Total QALYs: SMBG MDI 11.4146; SAP 12.0604 QALYs gained: 0.6458 SAP vs. SMBG CSII Total QALYs: SMBG CSII 11.9756; SAP 12.0604 QALYs gained: 0.0849 Annual discount rate: 1.5% | 2014 British pounds SAP vs. SMBG MDI Total costs: SMBG MDI £61,070; SAP £147,150 Incremental cost for SAP: £86,100 vs. SMBG MDI SAP vs. SMBG CSII Total costs: SMBG CSII £90,436; SAP £147,150 Incremental cost for SAP: £56,713 vs. SMBG CSII Annual discount rate: 3.5% | SAP vs. SMBG MDI ICER: £133,323 per QALY vs. SMBG MDI SAP vs. SMBG CSII ICER: £668,789 per QALY vs. SMBG CSII |
| Roze et al, 2016,53 France |
|
Patients with T1D; mean age 27 years; mean duration of diabetes 13 years; mean A1C 10% | SAP LGS SMBG CSII | Total QALYs: SAP LGS 17.88; SMBG CSII 14.89 QALYs gained: 2.99 Annual discount rate: 1.5% | 2013 British pounds Total costs: SAP LGS £125,559; SMBG CSII £88,991 Incremental cost for SAP LGS: £36,568 vs. SMBG CSII Annual discount rate: 3.5% | ICER: £12,233 per QALY gained vs. SMBG CSII |
| Roze et al, 2015,54 France |
|
Patients with T1D; mean age 27 years; mean duration of diabetes 13 years; mean A1C 8.6% | SAP SMPG CSII | Total QALYs: SAP 13.05; SMPG CSII 12.29 QALYs gained: 0.76 Annual discount rate: 3% | 2013 Swedish kronor (SEK) Total costs: SAP SEK 868,897; SMPG CSII SEK 453,791 Incremental cost for SAP: SEK 415,106 vs. SMPG CSII Annual discount rate: 3% | ICER: SEK 60,332 per QALY gained vs. SMPG CSII |
| Roze et al, 2016,55 France |
|
Patients with T1D; mean age 36 years; mean duration of diabetes 17 years; mean A1C 9.0% | SAP LGS SMBG CSII | Uncontrolled A1C at baseline Total QALYs: SAP LGS 10.55; SMBG CSII 9.36 QALYs gained: 1.19 Elevated risk for hypoglycemic events Total QALYs: SAP LGS 18.46; SMBG CSII 18.30 QALYs gained: 2.99 Annual discount rate: 4% | 2014 euros Uncontrolled A1C at baseline Total costs: SAP LGS ζ84,972; SMBG CSII ζ49,171 Incremental cost for SAP LGS: ζ35,801 vs. SMBG CSII Elevated risk for hypoglycemic events Total costs: SAP LGS ζ88,680; SMBG CSII ζ57,097 Incremental cost for SAP LGS: ζ31,583 vs. SMBG CSII Annual discount rate: 4% | Uncontrolled A1C at baseline ICER: ζ30,163 per QALY gained vs. SMBG CSII Elevated risk for hypoglycemic events ICER: ζ22,005 per QALY gained vs. SMBG CSII |
| Roze et al, 2017,56 France |
|
Cohort 1: people with T1D and hyperglycemia (baseline A1C 8.1%) Cohort 2: people with T1D at increased risk for hypoglycemic events (owing to impaired awareness of hypoglycemia) | SAP LGS SMBG CSII | Cohort 1 Total QALYs: SAP LGS 12.44; SMBG CSII 10.99 QALYs gained: 1.45 Cohort 2 Total QALYs: SAP LGS 13.08; SMBG CSII 11.20 QALYs gained: 1.88 Annual discount rate: 3% | 2015 Danish kroner (DKK) Cohort 1 Total costs: SAP LGS DKK 2,027,316; SMBG CSII DKK 1,801,293 Incremental cost for SAP LGS: DKK 226,023 vs. SMBG CSII Cohort 2 Total costs: SAP LGS DKK 2,277,868; SMBG CSII DKK 2,109,186 Incremental cost for SAP LGS: DKK 168,682 vs. SMBG CSII Annual discount rate: 3% | Cohort 1 ICER: DKK 156,082 per QALY gained vs. SMBG CSII Cohort 2 ICER: DKK 89,868 per QALY gained vs. SMBG CSII |
Abbreviations: A1C, glycated hemoglobin; CGM, continuous glucose monitoring; CSII, continuous subcutaneous insulin infusion (insulin pump); ICER, incremental cost-effectiveness ratio; LGS, low-glucose suspend [feature]; MDI, multiple daily injections; NHS, National Health Service; QALY, quality-adjusted life-year; SAP, sensor-augmented pump; SMBG, self-monitoring of blood glucose; SMPG, self-monitoring of plasma glucose; T1D, type 1 diabetes.
We excluded five studies: one review of the economic literature,59 one study with a 6-month time horizon,60 and three costing studies.61–63 The costing studies were focused on the costs of self-monitoring of blood glucose only, the implications of averting severe hypoglycemic events, and patient time spent on diabetes-related care.
Review of Included Economic Studies
Applicability and Limitations of the Included Studies
We assessed the methodological quality of the included studies using an applicability checklist (Appendix 3).
All studies were deemed partially applicable to our research question, because they were partially similar to our base case population and comparators. However, we found no studies that evaluated continuous glucose monitoring from the perspective of Ontario's public health care payer, so the results could not be directly translated to the Ontario context.
All studies included important outcomes related to continuous glucose monitoring and insulin infusion. All studies except those by McQueen et al51 and Riemsma et al58 were sponsored by device manufacturers.
All eight studies had important limitations, including the estimation of transition probabilities and treatment effects from various study populations. Also, they did not fully capture hypoglycemic events and two did not specify the use of insulin infusion. The majority of the studies used the Center for Outcomes Research and Evaluation model, which was based on the Diabetes Control and Complications Trial64 and the United Kingdom Prospective Diabetes Study,65 and was developed to reflect the natural history of type 1 diabetes.
Discussion
Of the eight eligible studies:
Three compared continuous glucose monitoring plus a sensor-augmented pump with self-monitoring of blood glucose plus either multiple daily injections52,58 or insulin pump therapy54,58
Three compared continuous glucose monitoring plus a low-glucose suspend feature with self-monitoring of blood glucose plus insulin pump therapy53,55,56
McQueen et al51 evaluated the cost-effectiveness of continuous glucose monitoring plus self-monitoring of blood glucose versus self-monitoring of blood glucose alone. Both interventions were accompanied by intensive insulin therapy. Inputs to their economic model were obtained mainly from the Diabetes Control and Complications Trial,64 the UK Prospective Diabetes Study,65 and the Wisconsin Epidemiologic Study of Diabetic Retinopathy.66
Huang et al57 evaluated the cost-effectiveness of continuous glucose monitoring versus self-monitoring of blood glucose. The authors based their effectiveness data on the Juvenile Diabetes Research Foundation continuous glucose monitoring trials67–69 and the Diabetes Control and Complications Trial,64 and they took information on diabetes complications from a modelling study of type 2 diabetes.70 The authors concluded that continuous glucose monitoring was cost-effective for an adult population aged ≥ 25 years with A1C levels ≥ 7.0%, assuming a willingness-to-pay threshold of $100,000 USD/QALY gained. Continuous glucose monitoring was more cost-effective for all age groups with A1C levels ≤ 7.0%.57 They found that if the benefits of continuous glucose monitoring were not extended long-term, the ICER would exceed $700,000 USD/QALY gained and would not be cost-effective at commonly used thresholds.
Riemsma et al58 evaluated the cost-effectiveness of several technologies, including continuous glucose monitoring integrated with a sensor-augmented pump; self-monitoring of blood glucose plus multiple daily injections; and self-monitoring of blood glucose plus an insulin pump. They obtained short-term effectiveness data on continuous glucose monitoring from a meta-analysis of 19 clinical trials and long-term effectiveness data from the Diabetes Control and Complications Trial,64 the UK Prospective Diabetes Study,65 and other literature sources (this meta-analysis was not applicable to the clinical evidence review in this health technology assessment). The authors found that continuous glucose monitoring with a sensor-augmented pump was not cost-effective compared to self-monitoring of blood glucose plus either multiple daily injections or an insulin pump. The study assumed treatment effects to be the mean reduction in A1C from baseline to 12 months. The report concluded that self-monitoring of blood glucose plus multiple daily injections was the most cost-effective option, given the current United Kingdom threshold of £30,000 GBP/QALY gained.58
Kamble et al52 evaluated the cost-effectiveness of continuous glucose monitoring plus a sensor-augmented pump compared with self-monitoring of blood glucose plus multiple daily injections. The authors derived the efficacy of continuous glucose monitoring with a sensor-augmented pump from the STAR 3 adult cohort.25 They found that continuous glucose monitoring with a sensor-augmented pump did not represent good value for money in adults when considering (1) the significant and ongoing costs associated with continuous glucose monitoring; and (2) the costs of long-term complications in relation to the expected health benefits of 0.376 QALYs.
Roze et al evaluated the cost-effectiveness of continuous glucose monitoring compared with self-monitoring of plasma glucose plus insulin pump therapy. They performed four studies, from the perspectives of the United Kingdom,53 Sweden,54 France,55 and Denmark.56 All but one54 used continuous glucose monitoring with a low-glucose suspend feature. The authors used the Center for Outcomes Research and Evaluation diabetes model to determine the cost-effectiveness of continuous glucose monitoring over a lifetime horizon. They derived the clinical effectiveness of continuous glucose monitoring from a patient-level meta-analysis71 and a Swedish observational study on type 2 diabetes.72 Overall, the conclusion from all four economic evaluations was that continuous glucose monitoring was likely to be cost-effective compared with self-monitoring of blood glucose and insulin pump therapy.
Overall, the results from the economic evidence review were mixed. McQueen et al51 demonstrated the cost-effectiveness of continuous glucose monitoring versus self-monitoring of blood glucose (when both interventions were accompanied by intensive insulin therapy, type not specified) at an empirical threshold of $50,000 USD/QALY gained. However, the authors may have modelled a constant decreasing rate of complications from the start of continuous glucose monitoring to approximately 33 years, resulting in relatively favourable ICER values.
Huang et al57 also demonstrated the cost-effectiveness of continuous glucose monitoring versus self-monitoring of blood glucose, but for a much higher empirical threshold of $100,000 USD/QALY gained, which might not be applicable to Canadian settings. As well, the authors did not specify the method of insulin infusion and found considerable uncertainties around the ICER.
Economic evaluations by Roze et al also demonstrated the cost-effectiveness of continuous glucose monitoring with a low-glucose suspend feature versus self-monitoring of blood glucose and insulin pump from the perspectives of the United Kingdom,53 Sweden,54 France,55 and Denmark.56
In contrast, Riemsma et al58 showed that newer technologies—standalone continuous glucose monitoring and sensor-augmented pumps—were not cost-effective compared with the current standard of self-monitoring of blood glucose plus multiple daily injections.
Lastly, Kamble et al52 found unfavourable cost-effectiveness results for a sensor-augmented pump versus self-monitoring of blood glucose plus multiple daily injections.
We found no economic evaluations of continuous glucose monitoring in children with type 1 diabetes.
Conclusions
The economic evidence showed mixed results when comparing continuous glucose monitoring with self-monitoring of blood glucose. All studies indicated that continuous glucose monitoring was more effective but also more costly. No studies were conducted in children with type 1 diabetes. No study was conducted from the Ontario or Canadian health care perspective, and many had methodological limitations and uncertainties in the results.
PRIMARY ECONOMIC EVALUATION
The published economic evaluations identified in the economic evidence review addressed our interventions of interest, but none of them took a Canadian perspective. Owing to these limitations, we conducted a primary economic evaluation.
Research Question
What is the cost-effectiveness of continuous glucose monitoring compared with self-monitoring of blood glucose in adult patients with type 1 diabetes from the perspective of the Ontario Ministry of Health and Long-Term Care?
Methods
The information presented in this report follows the reporting standards set out by the Consolidated Health Economic Evaluation Reporting Standards statement.73
Type of Analysis
We performed cost-utility and cost-effectiveness analyses. Our cost-effectiveness analysis assessed the cost per life-year saved. Our cost-utility analysis assessed the cost per QALY gained.
Target Population
The target population was adult patients, mean age of 27 years, mean A1C of 8.8%, diagnosed with type 1 diabetes and treated on average for 6 years (range 1 to 15 years).64,74
Our target population was based on the Diabetes Control and Complications Trial and the follow-up Epidemiology of Diabetes Interventions and Complications study (n = 1,411), the only randomized controlled trial to follow patients with type 1 diabetes for more than 20 years and report diabetes-related complications.64,74 The mean age and mean A1C of our target population at baseline and for the disease duration were assumed from the control arm of the Diabetes Control and Complications Trial. The study population had an average baseline A1C that was higher than that reported in some studies of continuous glucose monitoring, but reflected that of the average diabetes population, which tends to keep blood glucose levels higher to avoid severe hypoglycemic events.
We were unable to develop an economic evaluation of continuous glucose monitoring in children, owing to a lack of data on utilities and probabilities for children with type 1 diabetes.
Perspective
We conducted this analysis from the perspective of the Ontario Ministry of Health and Long-Term Care.
Interventions
We conducted four economic evaluations of continuous glucose monitoring compared with self-monitoring of blood glucose. We took this approach (1) because the clinical review excluded studies that compared continuous glucose monitoring devices with each other and (2) so that we could consider continuous glucose monitoring devices as a class, without regard to manufacturer or type.
Our review of the economic literature assessed eight possible interventions used in clinical practice (see Economic Evidence Review, Types of Interventions). However, because of a lack of clinical evidence, our evaluation was limited to four interventions. Appendix 4 provides our reasons for including the four interventions and the associated references for the selected studies. Table 11 summarizes the interventions evaluated in the economic model.
Table 11:
Disease Interventions and Comparators Evaluated in the Primary Economic Model
| Intervention | Comparator |
|---|---|
| Standalone CGM device plus multiple daily injections | SMBG plus multiple daily injections |
| Sensor-augmented pump | SMBG plus multiple daily injections |
| Standalone CGM device plus insulin pump | SMBG plus insulin pump |
| Sensor-augmented pump | SMBG plus insulin pump |
Abbreviations: CGM, continuous glucose monitoring; SMBG, self-monitoring of blood glucose.
We conducted a pairwise comparison (i.e., two at a time) of continuous glucose monitoring and self-monitoring of blood glucose. We considered continuous glucose monitoring devices approved in Canada and produced in 2010 or later. We did not rank the different continuous glucose monitoring devices by cost-effectiveness. For more details about the technologies we assessed, see the Background and Clinical Evidence Review sections.
Discounting and Time Horizon
We applied an annual discount rate of 1.5% to both costs and QALYs.75 We used a lifelong time horizon for all analyses.
Model Structure
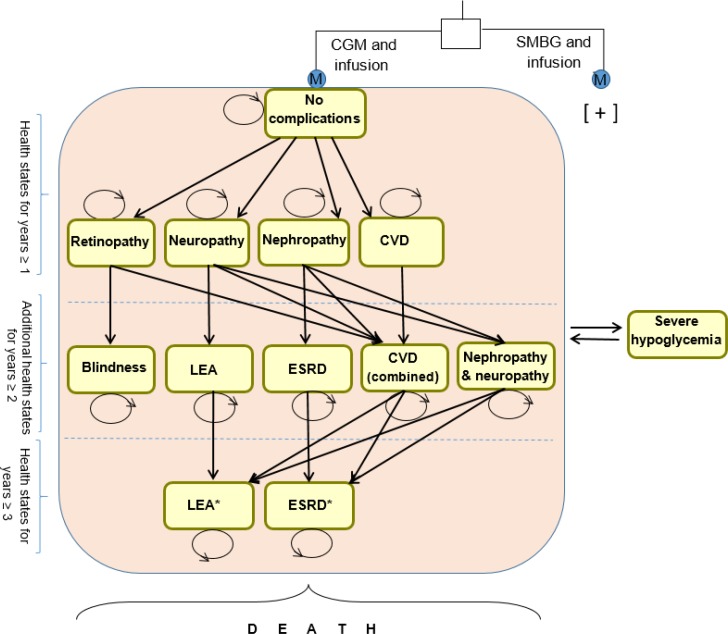
We adapted a transition-state model structure developed by McQueen et al51 for patients with type 1 diabetes, and we used a Markov cohort model with a 1-year cycle to explore long-term disease progression. Our model included more health states than the McQueen et al model.51 We also included long-term diabetes complications and short-term acute complications (such as severe hypoglycemia).
Our model consisted of 14 health states (Figure 4). All patients started in the “no complications” state. From the first year onward, they:
Figure 4: Continuous Glucose Monitoring Versus Self-Monitoring of Blood Glucose—Long-Term Markov Model of Complications in Patients With Type 1 Diabetesa.
Abbreviations: CGM, continuous glucose monitoring; CVD, cardiovascular disease, ESRD, end-stage renal disease; LEA, lower-extremity amputation; SMBG, self-monitoring of blood glucose.
aStraight arrows represent progression to a severe health state; curved arrows represent remaining in the same health state; straight arrows to and from severe hypoglycemia represent the probability of having a hypoglycemic event in any health state. All health states except no complications, retinopathy, neuropathy, and blindness had excess mortality. Infusion includes multiple daily injections or insulin pump. The model structure was adopted from McQueen et al.51
*Combined health states.
Stayed in the “no complications” state
-
Transitioned into one of the initial four diabetes complication health states:
∘ Retinopathy
∘ Neuropathy
∘ Nephropathy
∘ Cardiovascular disease
Died because of diabetes complications or other causes (i.e., entered the absorbing death state)
Patients in any health state could have a severe hypoglycemic event. We counted a number of severe hypoglycemic events for each health state. To account for the episodic nature of hypoglycemia, we estimated the probability of multiple hypoglycemic events for a 1-year cycle, based on the published literature.76
From the second year onward, patients could:
-
Move to a more severe condition state:
∘ Blindness
∘ Lower-extremity amputation
∘ End-stage renal disease
-
Move to a combined complication health state:
∘ Nephropathy and cardiovascular disease
∘ Neuropathy and cardiovascular disease
∘ Retinopathy and cardiovascular disease
∘ Neuropathy and nephropathy
Enter the death state
From the third year onward, patients with nephropathy and cardiovascular disease, neuropathy and cardiovascular disease, or neuropathy and nephropathy could die or transition to the most severe health states:
Lower-extremity amputation
End-stage renal disease
After the third year, patients were in the no complications state or had transitioned to any of the complication health states. Figure 4 provides a simplified schematic of the Markov model.
The Markov health states were based on a description of clinical outcomes from the Diabetes Control and Complications Trial.76 The macrovascular (cardiovascular disease) and microvascular (retinopathy, nephropathy, and neuropathy) complications of type 1 diabetes occur in later stages of the disease. Therefore, concomitant health states have attributes of both cardiovascular disease and retinopathy, nephropathy, or neuropathy.
No complications: Patients in this state are free from long-term major adverse events but can have short-term severe hypoglycemic events. They may experience microvascular or macrovascular complications over time, or die from any cause76
Retinopathy: Patients in this state have a growth of easily torn new blood vessels in the retina, as well as macular edema (swelling of part of the retina), which can lead to severe vision loss or blindness. This state includes patients with proliferative diabetic retinopathy or worse, patients with clinically significant macular edema, and patients undergoing photocoagulation therapy76
Neuropathy: Patients in this state have abnormal and decreased sensation, usually starting in the feet and later in the fingers and hands. When combined with damaged blood vessels, neuropathy can lead to a diabetic foot ulcer, with a high probability of lower-extremity amputation in the later stages76
Nephropathy: Patients in this state have kidney damage. This can lead to chronic renal failure, eventually requiring dialysis. Nephropathy is defined as an albumin excretion rate of 300 mg/24 hours or higher, a serum creatinine level of 2 mg/dL or higher, or the need for dialysis or renal transplantation76
Cardiovascular disease: Patients in this state have conditions that involve narrowed or blocked blood vessels. They might experience any of the following: myocardial infarction (heart attack); stroke; or death secondary to cardiovascular disease, angina, or revascularization (e.g., vascular bypass or angioplasty)76
Severe hypoglycemic event: Severe hypoglycemia is an acute complication of diabetes. A severe hypoglycemic event can result in loss of consciousness or seizure, and risk is known to increase with intensive therapy76
Blindness: Diabetic retinopathy can eventually lead to blindness. Patients in the blindness state have, at most, one-tenth of normal vision in their better eye, even when wearing corrective lenses77
Lower-extremity amputation: Patients in this state undergo amputation of the leg—either above or below the knee—to remove tissue that is ischemic (does not have enough blood supply), infected, or necrotic (dead), or because of an untreatable ulcer. Amputation can be a life-saving procedure76
End-stage renal disease: Patients in this state are in the final stage of chronic kidney disease. Their kidneys no longer function well enough to meet the needs of daily life. Treatments are dialysis or kidney transplantation76
Death: At any point in the model timeline, a patient could die. Death could be the result of diabetes, but all health states are susceptible to death from other causes. This is the absorbing health state
Main Assumptions
The major assumptions for our model were as follows:
Reduction of A1C with continuous glucose monitoring was associated with a decrease in risk of diabetes-related complications present in the first 12 months, but then the benefit of continuous glucose monitoring slowly declined over a patient's lifetime
Patients who used continuous glucose monitoring would have better quality of life because they would have less or no worry about hypoglycemia. A hypoglycemic event could occur in any health state and occurred at a constant probability over time, conditional to the treatment strategy
Our target population was treated for an average of 6 years (range 1 to 15 years)64,74 before entering the model. To simplify, we assumed that a certain proportion of patients could enter more severe health states, including lower-extremity amputation and end-stage renal disease, as of the second model cycle. We tested this assumption in a scenario analysis by delaying complications for 10 years
Model Parameters
Natural History
We obtained transition probabilities for diabetes complications from the best available literature sources. The Diabetes Control and Complications Trial and the Epidemiology of Diabetes Interventions and Complications study provided more than 20 years of follow-up for a cohort of 1,411 patients with type 1 diabetes who received either intensive or conventional treatment.76 We derived risk functions for no complications to retinopathy, nephropathy, and cardiovascular disease for the first year and subsequent years using a Weibull function fitted to the data (Table 12).
Table 12:
Risk Functions for Type 1 Diabetes Used in the Markov Model
| Change of Health State | Study, Year | Function | λ (Scale ÷ Intercept) | γ (Shape ÷ Slope) | R2 (Goodness of Fit) |
|---|---|---|---|---|---|
| No complications to retinopathy | DCCT, 200976 | Weibull | 0.000025 | 2.98542 | 0.988251 |
| No complications to nephropathy | 0.000823 | 1.66710 | 0.955383 | ||
| No complications to CVD | 0.000086 | 2.07549 | 0.91064 |
Abbreviations: CVD, cardiovascular disease; DCCT, Diabetes Control and Complications Trial.
We also derived transition probabilities from no complications to neuropathy for the first year from the Diabetes Control and Complications Trial and the Epidemiology of Diabetes Interventions and Complications study74 (Table 13). We obtained probabilities for severe hypoglycemia from the same studies,76 which reported event rates over 18.5 years of follow-up.
Table 13:
Annual Transition Probabilities for the Type 1 Diabetes Markov Model
| Parameter | Mean Value | 95% CI | Source |
|---|---|---|---|
| First Year Onward | |||
| No complications to neuropathy | 0.0235 | 0.0218–0.0252 | DCCT, 201474 |
| Severe hypoglycemic event (acute event from any health state) | 0.0982 | 0.0909–0.1036 | DCCT, 200976 |
| Second Year Onward | |||
| Retinopathy to blindness | 0.0064 | 0.0062–0.0066 | Early Treatment Diabetic Retinopathy Study Research Group, 199178 |
| Neuropathy to lower-extremity amputation | 0.1200 | 0.1104–0.1296 | Jonasson et al, 200879 |
| Nephropathy to end-stage renal disease | 0.072 | 0.006–0.008 | McQueen et al, 201151 Eastman et al, 199770 |
| Neuropathy to CVDa | 0.0200 | 0.0188–0.0212 | Klein et al, 200466 |
| Neuropathy to nephropathy | 0.097 | 0.0943–0.0997 | Wu et al, 199880 |
| Retinopathy to CVDa | 0.0155 | 0.0146–0.0164 | Klein et al, 200466 |
| Nephropathy to CVDa | 0.0224 | 0.0210–0.0238 | Klein et al, 200466 |
Abbreviations: CI, confidence interval; CVD, cardiovascular disease; DCCT, Diabetes Control and Complications Trial.
Transition probability of having CVD concomitant with retinopathy, nephropathy, or neuropathy.
From the second year, patients could have multiple complications. We obtained transition probabilities for cardiovascular disease concomitant with retinopathy, nephropathy, or neuropathy from Klein et al,66 who provided 20 years of evidence from the Wisconsin Epidemiologic Study of Diabetic Retinopathy. We obtained the transition probability for neuropathy to nephropathy from Wu et al.80 We obtained the transition probability from nephropathy to end-stage renal disease from the nephropathy diabetes model,70 based on the Wisconsin Epidemiologic Study of Diabetic Retinopathy.51,81 We derived the probability of blindness for patients with proliferative diabetic retinopathy from the Early Treatment Diabetic Retinopathy Study Research Group.78 We estimated the probability of lower-extremity amputation from patients with peripheral neuropathy in a study by Jonasson et al.79
From the third year, patients could move from cardiovascular disease concomitant with retinopathy, nephropathy, or neuropathy to the most severe health states of lower-extremity amputation and end-stage renal disease. Transition probabilities for those states were the same as for the second year.
Intervention Effects
We examined the effectiveness of continuous glucose monitoring in lowering A1C levels from baseline and reducing the number of severe hypoglycemic events.
We estimated the intervention effect of continuous glucose monitoring as the percentage mean reduction from the baseline A1C value. We assumed a mean baseline value of 8.8% for our base case population, consistent with A1C values for the relevant population in clinical studies64,74 and in studies included in the clinical evidence section of this health technology assessment (Figures 1 and 2). Table 14 shows the mean change in A1C values from baseline to the end of each study for continuous glucose monitoring, calculated for all interventions.
Table 14:
Mean A1C Levels, Changes From Baseline With Continuous Glucose Monitoringa
| Study, Year | Intervention and Comparator | Mean A1C, Baseline (95% CI) | Mean A1C, End of Study (95% CI) | Change (95% CI) |
|---|---|---|---|---|
| Lind et al, 201731 | Standalone CGM plus multiple daily injections vs. SMBG plus multiple daily injections | 8.49 (8.41–8.57) | 7.92 (7.79–8.05) | −0.57 (−0.78 to −0.41) |
| Bergenstal et al, 201025 | Sensor-augmented pump vs. SMBG plus multiple daily injections | 8.30 (8.27–8.33) | 7.30 (7.25–7.35) | −1.00 (−1.08 to −0.92) |
| Quiros et al, 201541 | Sensor-augmented pump vs. SMBG plus insulin pump | 8.47 (8.33–8.61) | 7.38 (7.24–7.52) | −1.09 (−1.37 to −0.81) |
| Tumminia et al, 201538 | Standalone CGM plus insulin pump vs. SMBG plus insulin pump | 8.5 (8.38–8.62) | 7.82 (7.74–7.90) | −0.68 (−1.04 to −0.18) |
Abbreviations: CGM, continuous glucose monitoring; CI, confidence interval; SMBG, self-monitoring of blood glucose.
Note: Values used for deterministic analysis. Point estimates from Tumminia et al38 were not statistically or clinically meaningful.
We estimated the intervention effect for severe hypoglycemic events as the difference in mean event rates per patient-year between the treatment and control arms. The clinical evidence review revealed no statistically significant difference in rates of severe hypoglycemic events between patients using continuous glucose monitoring and patients receiving usual care. To apply the risk reduction for all continuous glucose monitoring interventions in the model, we selected data from a study by Bergenstal et al25 because of its higher methodological quality, and because its sample size was the largest. The rate of severe hypoglycemia was 15.31 episodes per 100 person-years in the continuous glucose monitoring group and 17.62 episodes per 100 person-years in the control group.76 Based on this ratio, we estimated the relative risk (RR) of severe hypoglycemia to be 0.869 (95% CI 0.476–1.586) for continuous glucose monitoring.
However, the average baseline A1C levels in the studies that reported severe hypoglycemia25,33,39 were well above the target A1C threshold for optimal blood glucose management (> 7%). Hence, the findings from these studies may not reflect the experience of patients at high risk of hypoglycemia. To make sure that patients at highest risk were not overlooked, we conducted sensitivity analyses to cover a wide range of baseline A1C levels, using estimates from two suggested studies (RR 0.17433 and RR 0.69539).
Risk Reduction
Similar to many published economic models51,65 and one health technology assessment,58 we represented the efficacy of continuous glucose monitoring using risk reductions for short-term acute events (severe hypoglycemia) and long-term micro- and macrovascular complications resulting from reduced mean A1C levels.
We estimated risk reductions for complication rates based on data from the Diabetes Control and Complications Trial64,76 which reported the effects of intensive treatment (administration of insulin three or more times per day via pump or injection, self-monitoring of blood glucose at least four times per day, dietary intake, and exercise) on A1C levels and the progression of long-term complications.
We obtained initial relative risks for long-term diabetes complications such as retinopathy, nephropathy, neuropathy, and cardiovascular disease from the Diabetes Control and Complications Trial64,76 and Martin et al.82 Assuming a log-linear relationship, we estimated the effect of continuous glucose monitoring on reducing diabetes complications through the change in relative risk for each 1% reduction in mean A1C.83 Appendix 5 (Table A9) provides detailed examples of these calculations using data from the DIAMOND24 and GOLD31 trials.
We obtained a percentage change in A1C for continuous glucose monitoring from studies suggested by the clinical evidence review (Figure 2).
The relative risks for long-term diabetes complications used in the economic model are shown in Table 15.
Table 15:
Change in A1C From Baseline and Risk Reduction for Diabetes Complications
| Study, Year | Intervention and Comparator | % Decrease in A1C From Baseline (95% CI)a | Diabetes Complications, RR (95% CI) | |||
|---|---|---|---|---|---|---|
| Retinopathy | Nephropathy | Neuropathy | CVD | |||
| Lind et al, 201731 | Standalone CGM plus multiple daily injections vs. SMBG plus multiple daily | 6.71 (6.02–7.42) | 0.794 (0.775–0.813) | 0.847 (0.832–0.862) | 0.769 (0.748–0.790) | 0.859 (0.845–0.872) |
| Bergenstal et al, 201025 | Sensor-augmented pump vs. SMBG plus multiple daily injections | 12.05 (11.77–12.33) | 0.661 (0.655–0.667) | 0.742 (0.737–0.747) | 0.624 (0.618–0.631) | 0.761 (0.756–0.766) |
| Quiros et al, 201541 | Sensor-augmented pump vs. SMBG plus insulin pump | 12.87 (12.66–13.09) | 0.643 (0.638–0.647) | 0.727 (0.723–0.731) | 0.605 (0.599–0.610) | 0.747 (0.743–0.750) |
| Tumminia et al, 201538 | Standalone CGM plus insulin pump vs. SMBG plus insulin pump | 8.00 (7.63–8.36) | 0.760 (0.750–0.769) | 0.820 (0.813–0.828) | 0.731 (0.721–0.742) | 0.834 (0.827–0.841) |
Mortality
We modelled mortality based on diabetes complications and death from other causes. We estimated mortality rates owing to acute complications (including severe hypoglycemia and coma) and long-term diabetes complications using data from the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study, following patients for 27 years.84 We used these estimates to model mortality owing to diabetes complications after the first year (Table 16).
Table 16:
Annual Mortality for Diabetes Complications States
| Parameter | Mean Mortality | 95% CI | Source |
|---|---|---|---|
| First Year Onward | |||
| Nephropathy | 0.0036 | 0.0033–0.0039 | DCCT, 201584 |
| Severe hypoglycemic event | 0.0063 | 0.0058–0.0068 | DCCT, 201584 |
| Death (all-cause mortality, no diabetes), age 27–54 years | 0.0020 | 0.0019–0.0021 | DCCT, 201584 |
| Second Year Onward | |||
| Lower-extremity amputation | 0.093 | 0.0845–0.1015 | Vamos et al, 201085 |
| End-stage renal disease | 0.1640 | 0.1613–0.1667 | Wolowacz et al, 201586 |
| Neuropathy and nephropathy | 0.0036 | 0.0033–0.0039 | DCCT, 201584 |
Abbreviations: CI, confidence interval; DCCT, Diabetes Control and Complications Trial.
For cardiovascular disease alone and cardiovascular disease combined with microvascular complications, we used time-dependent probabilities of death following the onset of congestive heart failure from the Center for Outcomes Research and Evaluation diabetes model65 (Appendix 6, Table A10).
We used the perioperative mortality of patients who underwent amputation to model excess mortality in the lower-extremity amputation state.85
We obtained an excess mortality rate for end-stage renal disease from Wolowacz et al,86 who examined death rates for diabetes in the United Kingdom Renal Registry.
We calculated age-specific mortality rates for the population without diabetes and estimated nonspecific mortality rates by subtracting diabetes-related deaths from total deaths from all causes.87
We used mortality rates from the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study to calibrate diabetes and nondiabetes mortality rates estimated by the model.84
Utilities
Table 17 presents all health state utility values used in the base case analysis.
Table 17:
Utilities for Complications Health States
| Health State | Mean Utility Value | 95% CI | Source |
|---|---|---|---|
| No complications | 0.814 | 0.710–0.918 | Clarke et al, 2002,88 Currie et al, 200689 |
| Severe hypoglycemic eventa | −0.021 | −0.122 to −0.010 | Currie et al, 200690 |
| Nephropathy | 0.575 | 0.566–0.584 | McQueen et al, 201151 |
| Neuropathy | 0.624 | 0.609–0.639 | Palmer et al, 200465 |
| Retinopathy | 0.612 | 0.603–0.621 | McQueen et al, 201151 |
| CVD | 0.685 | 0.628–0.742 | Palmer et al, 200465 |
| Blindness | 0.569 | 0.493–0.645 | Palmer et al, 200465 |
| Lower-extremity amputation | 0.534 | 0.525–0.543 | McQueen et al, 201151 |
| End-stage renal disease | 0.490 | 0.450–0.540 | Tengs and Wallace, 200091 |
| Combined Health States | |||
| Neuropathy and nephropathy | 0.557 | 0.528–0.577 | Sullivan and Ghushchyan, 2006,92 McQueen et al, 201151 |
| Neuropathy and CVD | 0.544 | 0.532–0.567 | McQueen et al, 201151 |
| Nephropathy and CVD | 0.516 | 0.488–0.531 | McQueen et al, 201151 |
| Retinopathy and CVD | 0.553 | 0.525–0.572 | McQueen et al, 201151 |
| Lower-extremity amputation and CVD | 0.511 | 0.487–0.534 | Assumption |
| End-stage renal disease and CVD | 0.447 | 0.424–0.471 | Assumption |
Abbreviations: CI, confidence interval; CVD, cardiovascular disease.
Duration of the event and disutility associated with it is explained in the text below.
We obtained the utility for diabetes with no complications from the United Kingdom Prospective Diabetes Study (n = 3,192), which used the EuroQoL EQ-5D instrument to estimate health-related quality of life in patients with diabetes.88,89 We obtained utilities for diabetes complications such as nephropathy, retinopathy, and lower-extremity amputation from diabetes patients in the US Medical Expenditure Panel Survey (n = 2,778), based on the EuroQoL EQ-5D92 and from a modelling study by McQueen et al.51 We obtained utility values for cardiovascular disease, blindness, and neuropathy from the United Kingdom Prospective Diabetes Study88 and the Center for Outcomes Research and Evaluation diabetes model.65 We used a utility value for myocardial infarction in the cardiovascular disease health state.88 We obtained the utility for end-stage renal disease (related to hemodialysis) from Tengs and Wallace.91 We assumed the highest utility decrement (reduction) for combined health states.
The occurrence and severity of hypoglycemic symptoms were associated with increased patient worry about hypoglycemia and lower health-related quality of life over 1 year of the model cycle. We used a disutility estimate of 0.047 from Currie et al,90 who examined the fear of hypoglycemia in a survey of 1,305 patients with confirmed type 1 diabetes. The same trial reported a disutility value of 0.122 for the most severe hypoglycemic events. We used both values for our base case analysis.
The evidence was unclear about the duration of severe hypoglycemia. It could last less than an hour, or it could last longer and require an emergency department visit or hospitalization. We assumed that if patients felt unwell owing to a severe hypoglycemic event, they would take time off work. According to a European survey, people who experienced a severe hypoglycemic event took 4 to 7 days off work.93 We used this period of time to account and adjust for the duration and episodic nature of severe hypoglycemic events over 1 year of the model cycle.
We made the following annual adjustments for severe hypoglycemic events. We assumed 5.5 days off work as the duration of a severe hypoglycemic event, and we calculated an annual disutility as follows:
Using data from another European online survey,94 we established the proportion of patients with diabetes (40%) who were very worried about hypoglycemia. In this way, we ensured that the total disutility value included the event itself and the fear of hypoglycemia (adjusted for the episodic nature of the event and productivity loss owing to the event):
These calculations allowed us to develop the overall model disutility for a severe hypoglycemic event (Table 17).
Cost Parameters
Table 18 presents estimates of the health care costs used in our base case analysis. We obtained health care costs for nephropathy, neuropathy, and retinopathy from O'Brien et al.95 We estimated costs for cardiovascular disease, blindness, lower-extremity amputation, and end-stage renal disease from the Ontario Diabetes Economic Model.96 This model reflected actual resource-use profiles for a large prospective cohort of people with diabetes (N = 734,113) over 10 years. We calculated costs for cardiovascular disease as an average cost of all costs for ischemic heart disease, myocardial infarction, heart failure, and stroke.96 We obtained costs for severe hypoglycemic events with an inpatient visit from the Ontario Case Costing Initiative (2014 dollars).97 When necessary, we inflated costs to 2017 dollars using the Consumer Price Index.14 There was no direct evidence that continuous glucose monitoring prevented hospitalizations owing to hypoglycemic events,25,33,39 so we did not consider this possibility in our model.
Table 18:
Average Annual Per-Patient Cost of Diabetes Complications and Interventions in Ontario
| Mean Cost ($)a | Source | ||
|---|---|---|---|
| First Year | Subsequent Years | ||
| Health State: Diabetes-Related Complications | |||
| Nephropathy | 80 | 13 | O'Brien et al 200395 |
| Neuropathy | 192 | 192 | O'Brien et al 200395 |
| Retinopathy | 492 | 52 | O'Brien et al 200395 |
| Severe hypoglycemic event (in-patient visit) | 3,775 | 3,775 | McQueen et al, 201597 |
| Cardiovascular disease | 18,682 | 4,072 | O'Reilly et al, 200796 |
| Blindness | 3,483 | 2,482 | O'Reilly et al, 200796 |
| Lower-extremity amputation | 43,984 | 6,024 | O'Reilly et al, 200796 |
| End-stage renal disease | 28,221 | 12,808 | O'Reilly et al, 200796 |
| Intervention (Trade Name) | |||
| Sensor-augmented pump (Dexcom G4 Platinum + Animas Vibe) | 11,811 | 11,811 | Dexcom and Animasb |
| Sensor-augmented pump (Dexcom G5 Mobile + Animas Vibe) | 11,534 | 11,534 | Dexcom and Animasb |
| Sensor-augmented pump with a low-glucose suspend feature (MiniMed Veo) | 9,211 | 9,211 | Medtronicb |
| Standalone CGM plus multiple daily injections (Dexcom G4 Platinum) | 10,097 | 10,097 | Dexcomb |
| Standalone CGM plus multiple daily injections (Dexcom G5 Mobile) | 8,587 | 8,587 | Dexcomb |
| SMBG plus insulin pump (Animas Vibe or MiniMed Veo) | 6,817 | 6,817 | Medtronic and Animasb |
| SMBG plus multiple daily injections | 3,677 | 3,677 | Estimatec |
Abbreviations: CGM, continuous glucose monitoring; SMBG, self-monitoring of blood glucose.
We used 25% of the mean cost for calculations of standard error in our probabilistic sensitivity analysis. All costs are presented in 2017 Canadian dollars.
Manufacturer information.
See Appendix 6, Table A13, for more details.
We assumed that the annual cost of diabetes treatment included the following:
Insulin
Diabetes treatment supplies
Continuous glucose monitoring device and supplies
Insulin pump system approved by Health Canada
We obtained the annual cost of insulin treatment with multiple daily injections from a systematic review and mixed-treatment comparison meta-analysis report by the Canadian Agency for Drugs and Technologies in Health98 (Appendix 6, Table A11). We used the cost of insulin lispro (Humalog) cartridges to calculate treatment with an insulin pump (Appendix 6, Table A12). We obtained the annual cost of diabetes treatment supplies, such as needles and syringes, from a Shoppers Drug Mart pharmacy. All details of treatment supplies calculations are presented in Appendix 6, Table A13.
Dexcom provided costs for a standalone continuous glucose monitoring device and supplies. Animas and Medtronic provided insulin pump costs. We used device warranty times to calculate the annual costs of continuous glucose monitoring devices and insulin pumps. Appendix 6, Table A14, details the costs of the diabetes technologies and supplies. Appendix 6, Table A15, details diabetes treatment costs for the seven interventions presented in Table 18.
Analyses
Base Case Analysis
In the base case analysis, we applied a deterministic approach and used actual values or mean values as the model inputs. We presented the results as incremental costs (difference in costs) and incremental QALYs (difference in quality-adjusted life-years) for each continuous glucose monitoring device compared with self-monitoring of blood glucose.
Sensitivity Analyses
A deterministic method may provide the most reliable estimate of cost-effectiveness based on the best available data, but it does not consider the uncertainty of inputs to the model or the possibility of other clinical scenarios. As a result, we performed sensitivity analyses to address the uncertainty of model inputs and clinical scenarios.
We assessed variability and uncertainty in two ways. We conducted one-way sensitivity analyses using plausible ranges of high and low values for the model variables (as suggested by the literature). We also conducted probabilistic sensitivity analyses by assigning distributions to model parameters. In the probabilistic sensitivity analyses, we used gamma distribution to represent the uncertainty of the cost parameters, because cost data are skewed and cannot be negative.99 We used beta distributions for probabilities and utilities, because those estimates are confined to a range of 0 to 1.100 We used uniform distribution where a mean estimate of utility value was associated with a high standard error. In Monte Carlo probabilistic simulations, all parameters were randomly sampled from their assigned distributions for a cohort of 1,000 patients. We also estimated the likelihood of each treatment strategy being optimal across a range of willingness-to-pay thresholds.
The estimates used in our one-way sensitivity analyses are presented in Table 19.
Table 19:
Sensitivity Analyses for Continuous Glucose Monitoring Model
| Parameters | Mean Value | Plausible Range | Source | |
|---|---|---|---|---|
| Minimum | Maximum | |||
| Discount rates, % | 1.5 | 0 | 5.0 | Paulden et al75 |
| Annual Transition Probabilities | ||||
| First year onward | ||||
| No complications to retinopathy (exponential) | 0.0764 | 0.0461 | 0.0829 | DCCT, 2009,76 DCCT, 201474 |
| No complications to nephropathy (exponential) | 0.0094 | 0.0008 | 0.0526 | DCCT, 200976 |
| No complications to neuropathy | 0.0235 | 0.0218 | 0.0252 | DCCT, 200976 |
| No complications to CVD (exponential) | 0.0045 | 0.0310 | 0.0084 | Hoerger et al, 2004,81 DCCT, 2005101 |
| No complications to severe hypoglycemic event | 0.0982 | 0.0526 | 0.1513 | DCCT, 200976 |
| Second year onward | ||||
| Retinopathy to blindness | 0.0064 | 0.0010 | 0.1010 | Hoerger et al, 2004,81 McQueen et al, 201151 |
| Neuropathy to lower-extremity amputation | 0.1200 | 0.0620 | 0.1690 | Hoerger et al, 2004,81 McQueen et al, 201151 |
| Nephropathy to end-stage renal disease | 0.072 | 0.0041 | 0.096 | Hoerger et al, 2004,81 McQueen et al, 201151 |
| Neuropathy to CVD | 0.0200 | 0.0160 | 0.0440 | Hoerger et al, 2004,81 McQueen et al, 201151 |
| Neuropathy to nephropathy | 0.0970 | 0.0550 | 0.1490 | Wu et al, 199880 |
| Retinopathy to CVD | 0.0155 | 0.0100 | 0.0430 | Klein et al, 2004,66 McQueen et al, 201151 |
| Nephropathy to CVD | 0.0224 | 0.0130 | 0.0340 | Klein et al, 2004,66 McQueen et al, 201151 |
| Utilities | ||||
| No complications | 0.814 | 0.710 | 0.918 | Clarke et al, 2002,88 Currie et al, 200690 |
| Nephropathy | 0.575 | 0.545 | 0.606 | Sullivan et al, 200692 |
| Neuropathy | 0.624 | 0.573 | 0.632 | McQueen et al, 2011,51 Palmer et al, 200465 |
| Retinopathy | 0.612 | 0.581 | 0.643 | McQueen et al, 2011,51 Sullivan et al, 200692 |
| CVD | 0.685 | 0.513 | 0.742 | Clarke et al, 2002,88 and Palmer et al, 200465 |
| Severe hypoglycemia | 0.66 | 0.544 | 0.764 | Vexiau et al, 2008,102 Marrett et al, 2009,103 Currie et al, 200690 |
| Blindness | 0.569 | 0.540 | 0.734 | Clarke et al, 2002,88 and Palmer et al, 200465 |
| Lower-extremity amputation | 0.534 | 0.425 | 0.644 | Clarke et al, 2002,88 Sullivan et al, 200692 |
| End-stage renal disease | 0.49 | 0.45 | 0.53 | Tengs and Wallace, 200091 |
| Cost, First Year, 2017 Canadian Dollars | ||||
| No complications | 2,262 | 1,667 | 2,262 | McQueen et al, 2015,97 O'Reilly et al, 200796 |
| Nephropathy | 80 | 70 | 90 | McQueen et al, 2015,97 O'Brien et al 2003,95 assumption |
| Neuropathy | 192 | 150 | 213 | O'Brien et al, 2003,95 assumption |
| Retinopathy | 492 | 400 | 642 | McQueen et al, 2015,97 O'Brien et al 2003,95 assumption |
| CVD | 18,682 | 7,471 | 24,170 | OCCI, O'Brien et al, 200395 |
| Severe hypoglycemia | 3,775 | 1,500 | 4,000 | Assumption, OCCI |
| Blindness | 3,483 | 2,738 | 5,000 | McQueen et al, 2015,97 O'Brien et al, 2003,95 assumption |
| Lower-extremity amputation | 43,984 | 31,884 | 50,000 | McQueen et al, 2015,97 O'Brien et al 2003,95 assumption |
| End-stage renal disease | 28,221 | 25,841 | 81,769 | McQueen et al, 2015,97 O'Brien et al 200395 |
| Relative Risk From Lowering A1C by 1% | ||||
| Nephropathy | 0.038 | 0.032 | 0.043 | DCCT, 2009,76 DCCT, 201474 |
| Neuropathy | 0.025 | 0.020 | 0.030 | DCCT, 2009,76 DCCT, 201474 |
| Retinopathy | 0.029 | 0.023 | 0.034 | DCCT, 2009,76 DCCT, 201474 |
| CVD | 0.040 | 0.035 | 0.045 | DCCT, 2009,76 DCCT, 201474 |
| Severe hypoglycemia | 0.061 | 0.055 | 0.066 | DCCT, 2009,76 DCCT, 201474 |
Abbreviations: A1C, glycated hemoglobin; CVD, cardiovascular disease; DCCT, Diabetes Control and Complications Trial; OCCI, Ontario Case Costing Initiative.
Scenario Analyses
In addition to the sensitivity analyses, we conducted several scenario analyses to explore the effects of the most sensitive parameters to the cost-effectiveness results. These scenarios examined the robustness of our results in the face of changes to the relative risk of severe hypoglycemic events and the costs of continuous glucose monitoring devices.
Scenario 1: Variations of Relative Risks for Severe Hypoglycemia Events
In the base case analysis, we used the relative risk of severe hypoglycemia associated with continuous glucose monitoring (RR 0.869) as estimated by Bergenstal et al.25 Because this estimate was based on a single study, we examined how changes in the probability of a severe hypoglycemic event would influence our base case results. We assumed that the estimated risk reduction would be 20% to 80% of the relative risk:
RR 0.695, a 20% reduction of the effect
RR 0.521, a 40% reduction of the effect
RR 0.348, a 60% reduction of the effect
RR 0.174, an 80% reduction of the effect
We estimated a range of relative risks for patients with hypoglycemia unawareness.
Scenario 2: Reductions in Costs of Continuous Glucose Monitoring Devices
In scenario 2, we examined the cost of continuous glucose monitoring devices, assuming reductions of 30%, 20%, and 10%.
Scenario 3: Government Funding
In scenario 3, we examined the effect of cost reductions specific to Ontario. In Ontario, provincial funding of insulin, supplies, and blood glucose testing strips varies depending on the age and eligibility of the patient. We considered several scenarios from a patient perspective with various levels of government funding (Table 20):
Table 20:
Cost to Patient of Technologies With Various Levels of Government Funding
| Intervention (Trade Name) | Cost to the Patient, $ | ||||
|---|---|---|---|---|---|
| Intervention | Insulin Not Covered, Pump Covered | Insulin and Pump Covered | Insulin, Pump, and 75% of Sensors Covered | Pump and 75% of Sensors Covered | |
| Sensor-augmented pump (Dexcom G4 Platinum + Animas Vibe) | 11,811 | 7,520 | 6,786 | 5,681 | 6,415 |
| Sensor-augmented pump (Dexcom G5 Mobile + Animas Vibe) | 11,534 | 7,243 | 6,509 | 5,404 | 6,138 |
| Sensor-augmented pump with a low-glucose suspend feature (MiniMed Veo) | 9,211 | 4,920 | 4,186 | 3,406 | 4,140 |
| Standalone CGM plus multiple daily injections (Dexcom G4 Platinum) | 10,097 | 10,097 | 8,749 | 7,644 | 8,992 |
| Standalone CGM plus multiple daily injections (Dexcom G5 Mobile) | 8,587 | 8,587 | 7,239 | 6,134 | 7,482 |
| SMBG plus insulin pump (Animas Vibe or MiniMed Veo) | 6,817 | 2,333 | 1,599 | 1,599 | 2,333 |
| SMBG plus multiple daily injections | 3,677 | 3,677 | 2,329 | 2,329 | 3,677 |
Abbreviations: CGM, continuous glucose monitoring; SMBG, self-monitoring of blood glucose.
Insulin not funded but insulin pump funded
Funding for insulin and insulin pump
Funding for insulin, pump, and 75% of the sensor costs
Funding for insulin pump and 75% of the sensor costs
Scenario 4: Structural Assumption of Treatment Effect to Be Constant for the Rest of a Patient's Life
In our base case scenario, we assumed that a reduction in the risk of diabetes-related complications would occur within the first 12 months. This was similar to the approach taken in the health technology assessment by the National Institute for Health and Care Excellence.58 In scenario 4, we examined the treatment effect of a reduction in A1C owing to continuous glucose monitoring if it were constant (and maximized) for the rest of a patient's life.
Scenario 5: Delay of Severe Diabetes Complications for 10 Years
According to our model structure, from year 2 onward, a certain proportion of patients could enter more severe diabetes complication health states, including lower-extremity amputation and end-stage renal disease. Although our target population was treated for about 6 years (range 1 to 15 years), in scenario 5, we tested the effect of delaying the development of severe complications for another 10 years.
Generalizability
The findings of this economic analysis are generalizable to adults with type 1 diabetes, but not to children or pregnant women with type 1 diabetes. They may be used to guide decision-making about the specific patient populations addressed in the trials we investigated.
Expert Consultation
Throughout the development of this model, we consulted clinicians who specialize in treating type 1 diabetes and have experience with continuous glucose monitoring. The role of these expert advisors was to review the structure and inputs of the economic model and confirm that the information reasonably reflected the clinical setting. The statements, conclusions, and views expressed in this report do not necessary represent the views of the consulted experts.
Results
Base Case Analysis
The results of the base case analysis are presented in Table 21. Costs, life-years, and QALYs were higher with continuous glucose monitoring than with self-monitoring of blood glucose (usual care). All strategies were associated with ICERs that are generally considered to be very high, suggesting that, compared with self-monitoring of blood glucose, continuous glucose monitoring is not cost-effective at commonly used willingness-to-pay thresholds.
Table 21:
Base Case Analysis
| Intervention | Average Total Costs, $ | Average Total Effects, QALYs | Average Total Effects, LYs | Incremental Cost,a $ | Incremental Effect,b QALYs | Incremental Effect, LYs | ICER $/QALY | ICER $/LY |
|---|---|---|---|---|---|---|---|---|
| Standalone CGM Plus Multiple Daily Injections vs. SMBG Plus Multiple Daily Injections | ||||||||
| SMBG + MDI | 125,586 | 18.812 | 26.411 | |||||
| CGM + MDI | 229,413 | 18.906 | 26.520 | 103,827 | 0.094 | 0.109 | 1,108,812 | 951,152 |
| Sensor-Augmented Pump vs. SMBG Plus Multiple Daily Injectionsc | ||||||||
| SMBG + MDI | 125,586 | 18.812 | 26.411 | |||||
| SAP | 258,306 | 18.944 | 26.564 | 132,720 | 0.132 | 0.153 | 1,007,909 | 868,881 |
| Standalone CGM Plus Insulin Pump vs. SMBG Plus Insulin Pump | ||||||||
| SMBG + insulin pump | 177,320 | 18.812 | 26.411 | |||||
| CGM + insulin pump | 257,947 | 18.916 | 26.531 | 80,627 | 0.104 | 0.121 | 778,687 | 669,059 |
| Sensor-Augmented Pump vs. SMBG Plus Insulin Pump | ||||||||
| SMBG + insulin pump | 177,320 | 18.812 | 26.411 | |||||
| SAP | 258,373 | 18.949 | 26.570 | 81,052 | 0.137 | 0.159 | 592,206 | 510,755 |
Abbreviations: CGM, continuous glucose monitoring; ICER, incremental cost effectiveness ratio; LY, life-year; MDI, multiple daily injections; QALY, quality-adjusted life year; SAP, sensor-augmented pump; SMBG, self-monitoring of blood glucose.
Incremental cost = average cost (strategy B) - average cost (strategy A); Costs include all components of direct medical costs without accounting for government funding of insulin treatment, insulin pump, and blood glucose test strips
Incremental effect = average effect (strategy B) - average effect (strategy A).
Difference in outcomes between sensor-augmented pump and self-monitoring of blood glucose plus multiple daily injections was owing to a difference in the relative risk of diabetes complications.
Sensitivity Analyses
One-Way Sensitivity Analysis
We conducted deterministic one-way sensitivity analyses for each parameter using their plausible ranges. Figure 5 shows the results for standalone continuous glucose monitoring with multiple daily injections versus self-monitoring of blood glucose with multiple daily injections. Our model was most sensitive to the relative risk of neuropathy, the relative risk of severe hypoglycemic events, treatment costs, discount rates, and the probability of severe hypoglycemia. Our model was less sensitive to mortality, costs and utilities of complications, and costs of severe hypoglycemic events. One-way sensitivity analyses showed consistent results among all continuous glucose monitoring interventions considered (Appendix 7).
Figure 5: One-Way Sensitivity Analysis: Continuous Glucose Monitoring With Multiple Daily Injections Versus Self-Monitoring of Blood Glucose With Multiple Daily Injections.
Abbreviations: CGM, continuous glucose monitoring; CVD, cardiovascular disease; ICER, incremental cost-effectiveness ratio; LEA, lower-extremity amputation; MDI, multiple daily injections; QALY, quality-adjusted life-year; RR, relative risk; SMBG, self-monitoring of blood glucose.
Note: Bars indicate ICER value and directions obtained using the ranges presented in parentheses. Bars indicate ICER values and directions obtained by ranging the model parameters value presented in parentheses. Blue bars indicate ICER values at the upper end of the range, and orange bars indicate ICERs at the lower end. For some parameters, the upper value led to a reduction in ICERs and the lower value led to an increases ICERs.
Probabilistic Sensitivity Analysis
Compared with self-monitoring of blood glucose (usual care), the probability of continuous glucose monitoring being cost-effective was low (Appendix 7, Figure A4). At a willingness-to-pay threshold of $50,000/QALY, the four continuous glucose monitoring interventions had a very small chance of being cost-effective, from 0.1% to 6.6%. There was also large uncertainty around the ICERs associated with these interventions.
Scenario Analyses
Scenario 1: Variations in Relative Risk of a Severe Hypoglycemic Event
The ICERs for the included interventions were higher than $50,000/QALY gained, even considering a favourable relative risk of 0.174 (Table 22). The lowest ICERs were for continuous glucose monitoring with a sensor-augmented pump versus self-monitoring of blood glucose plus an insulin pump. For people with hypoglycemia unawareness, who have a four- to five-fold increased risk of severe hypoglycemia104,105 (RR 0.521 or RR 0.348), ICERs ranged from $262,255/QALY gained to $571,199/QALY gained.
Table 22:
Scenario 1 Resultsa
| Intervention | Average Total Costs, $ | Average Total Effects, QALYs | Incremental Cost, $ | Incremental Effect, QALYs | ICER, $/QALY |
|---|---|---|---|---|---|
| RR of Severe Hypoglycemic Event 0.695 (Coefficient 0.8) | |||||
| Standalone CGM plus multiple daily injections vs. SMBG plus multiple daily injections | |||||
| SMBG + MDI | 125,586 | 18.812 | |||
| CGM + MDI | 225,987 | 18.950 | 100,401 | 0.138 | 728,356 |
| Sensor-augmented pump vs. SMBG plus multiple daily injections | |||||
| SMBG + MDI | 125,586 | 18.812 | |||
| SAP | 254,923 | 18.988 | 129,336 | 0.176 | 734,908 |
| Standalone CGM plus insulin pump vs. SMBG plus insulin pump | |||||
| SMBG + insulin pump | 177,320 | 18.812 | |||
| CGM + insulin pump | 254,558 | 18.960 | 77,237 | 0.148 | 522,665 |
| Sensor-augmented pump vs. SMBG plus insulin pump | |||||
| SMBG + insulin pump | 177,320 | 18.812 | |||
| SAP | 254,992 | 18.993 | 77,672 | 0.181 | 428,747 |
| RR of Severe Hypoglycemic Event 0.521 (Coefficient 0.6) | |||||
| Standalone CGM plus multiple daily injections vs. SMBG plus multiple daily injections | |||||
| SMBG + MDI | 125,586 | 18.812 | |||
| CGM + MDI | 222,540 | 18.994 | 96,954 | 0.182 | 532,066 |
| Sensor-augmented pump vs. SMBG plus multiple daily injections | |||||
| SMBG + MDI | 125,586 | 18.812 | |||
| SAP | 251,519 | 19.033 | 125,932 | 0.220 | 571,199 |
| Standalone CGM plus insulin pump vs. SMBG plus insulin pump | |||||
| SMBG + insulin pump | 177,320 | 18.812 | |||
| CGM + insulin pump | 251,149 | 19.004 | 73,828 | 0.192 | 384,162 |
| Sensor-augmented pump vs. SMBG plus insulin pump | |||||
| SMBG + insulin pump | 177,320 | 18.812 | |||
| SAP | 251,594 | 19.038 | 74,273 | 0.226 | 329,228 |
| RR of Severe Hypoglycemic Event 0.348 (Coefficient 0.4) | |||||
| Standalone CGM plus multiple daily injections vs. SMBG plus multiple daily injections | |||||
| SMBG + MDI | 125,586 | 18.812 | |||
| CGM + MDI | 219,096 | 19.039 | 93,509 | 0.226 | 412,870 |
| Sensor-augmented pump vs. SMBG plus multiple daily injections | |||||
| SMBG + MDI | 125,586 | 18.812 | |||
| SAP | 248,116 | 19.077 | 122,530 | 0.265 | 462,659 |
| Standalone CGM plus insulin pump vs. SMBG plus insulin pump | |||||
| SMBG + insulin pump | 177,320 | 18.812 | |||
| CGM + insulin pump | 247,741 | 19.049 | 70,420 | 0.236 | 297,797 |
| Sensor-augmented pump vs. SMBG plus insulin pump | |||||
| SMBG + insulin pump | 177,320 | 18.812 | |||
| SAP | 248,177 | 19.082 | 70,856 | 0.270 | 262,255 |
| RR of Severe Hypoglycemic Event 0.174 (Coefficient 0.2) | |||||
| Standalone CGM plus multiple daily injections vs. SMBG plus multiple daily injections | |||||
| SMBG + MDI | 125,586 | 18.812 | |||
| CGM + MDI | 215,612 | 19.083 | 90,026 | 0.271 | 332,015 |
| Sensor-augmented pump vs. SMBG plus multiple daily injections | |||||
| SMBG + MDI | 125,586 | 18.812 | |||
| SAP | 244,675 | 19.122 | 119,089 | 0.310 | 384,647 |
| Standalone CGM plus insulin pump vs. SMBG plus insulin pump | |||||
| SMBG + insulin pump | 177,320 | 18.812 | |||
| CGM + insulin pump | 244,295 | 19.093 | 66,974 | 0.281 | 238,205 |
| Sensor-augmented pump vs. SMBG plus insulin pump | |||||
| SMBG + insulin pump | 177,320 | 18.812 | |||
| SAP | 244,741 | 19.127 | 67,421 | 0.315 | 214,098 |
Abbreviations: CGM, continuous glucose monitoring; ICER, incremental cost-effectiveness ratio; MDI, multiple daily injections; QALY, quality-adjusted life-year; RR, relative risk; SAP, sensor-augmented pump, SMBG, self-monitoring of blood glucose.
We used a range of coefficients to estimate the sensitivity of the model results to the parameter “RR of severe hypoglycemic event.” We applied different coefficients to the base case RR (0.869) to include all potential scenarios. Relatively low ICERs are shown in bold.
Scenario 2: Reductions in Costs of Continuous Glucose Monitoring Devices
Scenario 2 analyzed the costs of continuous glucose monitoring devices, assuming reductions of 30%, 20%, or 10% (Table 23). Despite significant cost reductions, none of the pairwise comparisons of continuous glucose monitoring versus self-monitoring of blood glucose were cost-effective at common willingness-to-pay thresholds (i.e., $50,000/QALY or $100,000/QALY). At a 30% device cost reduction, ICERs ranged from $383,667/QALY gained to $791,249/QALY gained, suggesting that continuous glucose monitoring was not cost-effective.
Table 23:
Scenario 2 Results
| Intervention | Average Total Costs, $ | Average Total Effects, QALYs | Incremental Cost, $ | Incremental Effect, QALYs | ICER $/QALY |
|---|---|---|---|---|---|
| 30% CGM Device Cost Reduction | |||||
| Standalone CGM plus multiple daily injections vs. SMBG plus multiple daily injections | |||||
| SMBG + MDI | 125,586 | 18.812 | |||
| CGM + MDI | 197,492 | 18.906 | 71,906 | 0.094 | 767,913 |
| Sensor-augmented pump vs. SMBG plus multiple daily injections | |||||
| SMBG + MDI | 125,586 | 18.812 | |||
| SAP | 229,777 | 18.944 | 104,191 | 0.132 | 791,249 |
| Standalone CGM plus insulin pump vs. SMBG plus insulin pump | |||||
| SMBG + insulin pump | 177,320 | 18.812 | |||
| CGM + insulin pump | 229,483 | 18.916 | 52,163 | 0.104 | 503,786 |
| Sensor-augmented pump vs. SMBG plus insulin pump | |||||
| SMBG + insulin pump | 177,320 | 18.812 | |||
| SAP | 229,831 | 18.949 | 52,511 | 0.137 | 383,667 |
| 20% CGM Device Cost Reduction | |||||
| Standalone CGM plus multiple daily injections vs. SMBG plus multiple daily injections | |||||
| SMBG + MDI | 125,586 | 18.812 | |||
| CGM + MDI | 208,132 | 18.906 | 82,546 | 0.094 | 881,546 |
| Sensor-augmented pump vs. SMBG plus multiple daily injections | |||||
| SMBG + MDI | 125,586 | 18.812 | |||
| SAP | 239,287 | 18.944 | 113,700 | 0.132 | 863,469 |
| Standalone CGM plus insulin pump vs. SMBG plus insulin pump | |||||
| SMBG + insulin pump | 177,320 | 18.812 | |||
| CGM + insulin pump | 238,971 | 18.916 | 61,651 | 0.104 | 595,419 |
| Sensor-augmented pump vs. SMBG plus insulin pump | |||||
| SMBG + insulin pump | 177,320 | 18.812 | |||
| SAP | 239,345 | 18.949 | 62,024 | 0.137 | 453,180 |
| 10% CGM Device Cost Reduction | |||||
| Standalone CGM plus multiple daily injections vs. SMBG plus multiple daily injections | |||||
| SMBG + MDI | 125,586 | 18.812 | |||
| CGM + MDI | 218,773 | 18.906 | 93,187 | 0.094 | 995,179 |
| Sensor-augmented pump vs. SMBG plus multiple daily injections | |||||
| SMBG + MDI | 125,586 | 18.812 | |||
| SAP | 248,796 | 18.944 | 123,210 | 0.132 | 935,689 |
| Standalone CGM plus insulin pump vs. SMBG plus insulin pump | |||||
| SMBG + insulin pump | 177,320 | 18.812 | |||
| CGM + insulin pump | 248,459 | 18.916 | 71,139 | 0.104 | 687,053 |
| Sensor-augmented pump vs. SMBG plus insulin pump | |||||
| SMBG + insulin pump | 177,320 | 18.812 | |||
| SAP | 248,859 | 18.949 | 71,538 | 0.137 | 522,693 |
Abbreviations: CGM, continuous glucose monitoring; MDI, multiple daily injections; QALY, quality-adjusted life-years; SAP, sensor-augmented pump; SMBG, self-monitoring of blood glucose.
Scenario 3: Government Funding
Scenario 3 considered funding 75% of the cost of sensors, the usual practice by the Ministry of Health and Long-Term Care (Table 24). Continuous glucose monitoring was not cost-effective, even in the most favourable scenario from the purchaser perspective, with ICERs ranging from $402,619/QALY gained to $911,819/QALY gained.
Table 24:
Scenario 3 Results
| Intervention | Average Total Costs, $ | Average Total Effects, QALYs | Incremental Cost, $ | Incremental Effect, QALYs | ICER $/QALY |
|---|---|---|---|---|---|
| Insulin and Insulin Pump Covered | |||||
| Standalone CGM plus multiple daily injections vs. SMBG plus multiple daily injections | |||||
| SMBG + MDI | 125,586 | 18.812 | |||
| CGM + MDI | 229,413 | 18.906 | 103,827 | 0.094 | 1,108,812 |
| Sensor-augmented pump vs. SMBG plus multiple daily injections | |||||
| SMBG + MDI | 125,586 | 18.812 | |||
| SAP | 186,966 | 18.944 | 61,380 | 0.132 | 466,133 |
| Standalone CGM plus insulin pump vs. SMBG plus insulin pump | |||||
| SMBG + insulin pump | 103,443 | 18.812 | |||
| CGM + insulin pump | 186,771 | 18.916 | 83,328 | 0.104 | 804,781 |
| Sensor-augmented pump vs. SMBG plus insulin pump | |||||
| SMBG + insulin pump | 103,443 | 18.812 | |||
| SAP | 187,002 | 18.949 | 83,559 | 0.137 | 610,523 |
| Insulin Not Covered, Insulin Pump Covered | |||||
| Standalone CGM plus multiple daily injections vs. SMBG plus multiple daily injections | |||||
| SMBG + MDI | 103,372 | 18.812 | |||
| CGM + MDI | 207,067 | 18.906 | 103,695 | 0.094 | 1,107,402 |
| Sensor-augmented pump vs. SMBG plus multiple daily injections | |||||
| SMBG + MDI | 103,372 | 18.812 | |||
| SAP | 174,759 | 18.944 | 71,388 | 0.132 | 542,135 |
| Standalone CGM plus insulin pump vs. SMBG plus insulin pump | |||||
| SMBG + insulin pump | 91,344 | 18.812 | |||
| CGM + insulin pump | 174,593 | 18.916 | 83,248 | 0.104 | 804,007 |
| Sensor-augmented pump vs. SMBG plus insulin pump | |||||
| SMBG + insulin pump | 91,344 | 18.812 | |||
| SAP | 174,790 | 18.949 | 83,446 | 0.137 | 609,694 |
| Insulin Pump and 75% of Sensors Covered | |||||
| Standalone CGM plus multiple daily injections vs. SMBG plus multiple daily injections | |||||
| SMBG + MDI | 125,586 | 18.812 | |||
| CGM + MDI | 211,099 | 18.906 | 85,513 | 0.094 | 913,229 |
| Sensor-augmented pump vs. SMBG plus multiple daily injections | |||||
| SMBG + MDI | 125,586 | 18.812 | |||
| SAP | 168,595 | 18.944 | 43,008 | 0.132 | 326,617 |
| Standalone CGM plus insulin pump vs. SMBG plus insulin pump | |||||
| SMBG + insulin pump | 103,443 | 18.812 | |||
| CGM + insulin pump | 168,442 | 18.916 | 65,000 | 0.104 | 627,762 |
| Sensor-augmented pump vs. SMBG plus insulin pump | |||||
| SMBG + insulin pump | 103,443 | 18.812 | |||
| SAP | 168,623 | 18.949 | 65,180 | 0.137 | 476,237 |
| Insulin, Insulin Pump, and 75% of Sensors Covered | |||||
| Standalone CGM plus multiple daily injections vs. SMBG plus multiple daily injections | |||||
| SMBG + MDI | 103,372 | 18.812 | |||
| CGM + MDI | 188,753 | 18.906 | 85,381 | 0.094 | 911,819 |
| Sensor-augmented pump vs. SMBG plus multiple daily injections | |||||
| SMBG + MDI | 103,372 | 18.812 | |||
| SAP | 156,388 | 18.944 | 53,016 | 0.132 | 402,619 |
| Standalone CGM plus insulin pump vs. SMBG plus insulin pump | |||||
| SMBG + insulin pump | 91,344 | 18.812 | |||
| CGM + insulin pump | 156,264 | 18.916 | 64,919 | 0.104 | 626,988 |
| Sensor-augmented pump vs. SMBG plus insulin pump | |||||
| SMBG + insulin pump | 91,344 | 18.812 | |||
| SAP | 156,411 | 18.949 | 65,067 | 0.137 | 475,407 |
Abbreviations: CGM, continuous glucose monitoring; MDI, multiple daily injections; QALY, quality-adjusted life year; SAP, sensor augmented pump; SMBG, self-monitoring of blood glucose.
Scenario 4: Structural Treatment Effect Constant for Rest of a Patient's Life
Scenario 4 analyzed the structural assumption of a treatment effect if it were constant for the rest of a patient's life (Table 25). In this scenario, ICERs decreased by 10 to 12 times compared with the base case, ranging from $58,379/QALY gained to $112,979/QALY gained.
Table 25:
Scenario 4 Results
| Intervention | Average Total Costs, $ | Average Total Effects, QALYs | Incremental Cost, $ | Incremental Effect, QALYs | ICER $/QALY |
|---|---|---|---|---|---|
| Standalone CGM Plus Multiple Daily Injections vs. SMBG Plus Multiple Daily Injections | |||||
| SMBG + MDI | 125,586 | 18.812 | |||
| CGM + MDI | 241,703 | 19.840 | 116,117 | 1.0278 | 112,979 |
| Sensor-Augmented Pump vs. SMBG Plus Multiple Daily Injections | |||||
| SMBG + MDI | 125,586 | 18.812 | |||
| SAP | 285,827 | 20.593 | 160,240 | 1.7805 | 89,995 |
| Standalone CGM Plus Insulin Pump vs. SMBG Plus Insulin Pump | |||||
| SMBG + insulin pump | 177,320 | 18.812 | |||
| CGM + insulin pump | 275,981 | 20.025 | 98,661 | 1.2125 | 81,369 |
| Sensor-Augmented Pump vs. SMBG Plus Insulin Pump | |||||
| SMBG + insulin pump | 177,320 | 18.812 | |||
| SAP | 287,841 | 20.705 | 110,520 | 1.8931 | 58,379 |
Abbreviations: CGM, continuous glucose monitoring; MDI, multiple daily injections; QALY, quality-adjusted life-year; SAP, sensor-augmented pump; SMBG, self-monitoring of blood glucose.
Scenario 5: Delay of Severe Diabetes Complications for 10 Years
In scenario 5, severe diabetes-related complications occurred only after the 10th cycle (16 years after diagnosis) to reflect current clinical realities (Table 26). Compared with the base case, a delay in entering a severe diabetes health state until after the 10th cycle increased ICER values by 10% to 30%, depending on the continuous glucose monitoring intervention. This finding was owing to a decrease in incremental effect.
Table 26:
Scenario 5 Results
| Intervention | Average Total Costs, $ | Average Total Effects, QALYs | Incremental Cost, $ | Incremental Effect, QALYs | ICER $/QALY |
|---|---|---|---|---|---|
| Standalone CGM Plus Multiple Daily Injections vs. SMBG Plus Multiple Daily Injections | |||||
| SMBG + MDI | 130,228 | 19.279 | |||
| CGM + MDI | 235,081 | 19.354 | 104,853 | 0.075 | 1,397,670 |
| Sensor-Augmented Pump vs. SMBG Plus Multiple Daily Injections | |||||
| SMBG + MDI | 130,228 | 19.279 | |||
| SAP | 264,115 | 19.380 | 133,887 | 0.101 | 1,328,239 |
| Standalone CGM Plus Insulin Pump vs. SMBG Plus Insulin Pump | |||||
| SMBG + insulin pump | 182,585 | 19.279 | |||
| CGM + insulin pump | 263,892 | 19.361 | 81,307 | 0.082 | 994,805 |
| Sensor-Augmented Pump vs. SMBG Plus Insulin Pump | |||||
| SMBG + insulin pump | 182,585 | 19.279 | |||
| SAP | 264,156 | 19.384 | 81,571 | 0.104 | 781,967 |
Abbreviations: CGM, continuous glucose monitoring; MDI, multiple daily injections; QALY, quality-adjusted life-year; SAP, sensor-augmented pump; SMBG, self-monitoring of blood glucose.
Discussion
Our cost-effectiveness analyses showed that various continuous glucose monitoring interventions were not cost-effective compared with self-monitoring of blood glucose (usual care) at common willingness-to-pay thresholds. All continuous glucose monitoring interventions were more effective but more costly than the corresponding self-monitoring of blood glucose strategies. They were associated with ICERs ranging from $592,206/QALY gained to $1,108,812/QALY gained, much higher than the empirical threshold of $50,000/QALY gained.
The cost-effectiveness results were most sensitive to the relative risk of neuropathy, the risk reduction of severe hypoglycemic events, and the cost of the continuous glucose monitoring device. The results were less sensitive to excess mortality, the costs or utilities of diabetes complications, and the costs of severe hypoglycemic events. Our findings remained robust in all scenario analyses except one, which assumed lifelong effectiveness of continuous glucose monitoring and continuing risk reduction for all complications over a patient's lifetime. Some experts may argue that this is the most realistic scenario, and some economic evaluations have used this approach.
Our results were in agreement with the findings of several cost-effectiveness analyses. A recent health technology assessment by NICE concluded that self-monitoring of blood glucose combined with multiple daily injections was cost-effective compared to continuous glucose monitoring and different insulin infusion options.31,58 The results for the NICE base case model ranged from £133,323/QALY gained to £730,501/QALY gained ($221,316 CAD/QALY gained to $1,210,495 CAD/QALY gained using an exchange rate of £1 = $1.66 CAD, assessed July 14, 2017). In general, these findings were in line with our results. Differences were related to variations in modelling approach, including the model structure (we used a more simplified approach, while NICE used the CORE global model, including 17 diabetes complication sub-models) and model parameters (we used pairwise comparison for clinical effectiveness, and NICE used indirect comparison). As well, the costs used in the model were different: we used the Ontario health care perspective, and NICE used a United Kingdom perspective.
In our model, the benefits of continuous glucose monitoring were limited to reductions in A1C and fewer severe hypoglycemic events. Lower A1C and better management of A1C lowers the probability of diabetes complications in the long term. Scenario analyses conducted by Huang et al57 limited to glucose-lowering, with a subsequent reduction in diabetes risk complications, resulted in ICERs from $701,397 USD/QALY gained to $1,185,384 USD/QALY gained. The authors concluded that the benefits from improved glycemic control were relatively small, because complications occurred later in a patient's life, so the benefits of complication reduction were heavily discounted.57
Other studies by Roze et al53–56 and McQueen et al51 demonstrated the cost-effectiveness of continuous glucose monitoring compared with self-monitoring of blood glucose. These authors may have modelled a continually decreasing rate of complications in the long term with continuous glucose monitoring.
Limitations
Although we used a comprehensive modelling approach, our study had several limitations.
We simplified the clinical pathway of the disease, and although we modelled the most important stages of the disease, we did not model all possible stages.
Evidence for the clinical effectiveness of standalone continuous glucose monitoring in reducing severe hypoglycemia was unclear. A systematic review and meta-analysis of randomized trials indicated that data for the incidence of severe or nocturnal hypoglycemia were sparse and imprecise.106 The clinical evidence review in this health technology assessment found that there was low certainty about the effectiveness of continuous glucose monitoring to reduce the number of severe hypoglycemic events compared with self-monitoring of blood glucose.
We obtained treatment effects owing to reductions in A1C and severe hypoglycemic events from the clinical evidence review, but we obtained consequent risk reductions for diabetes complications from the Diabetes Control and Complications Trial.64
The treatment pattern used in the Diabetes Control and Complications Trial might not match the current standard. The Diabetes Control and Complications Trial (reported in 1993) was a long-term, multicentre randomized clinical trial with a long-term follow-up study (Epidemiology of Diabetes Interventions and Complications study).64,74 These studies were the only ones to follow patients with type 1 diabetes for more than 25 years. The long-term outcomes from these studies provide a reliable sense of the clinical course expected with modern therapy at the time of the trial, but not with more recent treatment regimens.76
The level of treatment adherence achieved during the Diabetes Control and Complications Trial64 might not be achieved by the general population of patients with type 1 diabetes, so the long-term benefits from the reduction of diabetes complications might be smaller.
We were unable to rank the continuous glucose monitoring devices, because studies that examined and compared devices were excluded from the clinical evidence review.
Owing to the scarcity of utility data and probabilities for children with type 1 diabetes, we did not develop a model for this population. Numerous assumptions would have been required to generate such a model, leading to vast uncertainties.
Finally, our model accounted for the potential impact of severe hypoglycemic events and improved glycemic control in people with type 1 diabetes. We modelled impacts from the reduction of severe hypoglycemic events and from decreased patient worry through improvements in health-related quality of life. The clinical evidence showed a significant decrease in severe hypoglycemic events, but there was a high level of uncertainty with these results (based on the GRADE analysis).
Our results were in agreement with results from other published studies. Future research should evaluate the rates of severe hypoglycemic events more precisely. Severe hypoglycemia should be reported uniformly, with standard cut-offs and definitions.106 Preferably, results should be reported per patient-year instead of per event.106
Conclusion
Compared with self-monitoring of blood glucose (usual care), our base case estimate was that continuous glucose monitoring provides modest incremental benefit at substantial incremental cost. However, there was considerable uncertainty about value for money, given the nature of the available evidence.
BUDGET IMPACT ANALYSIS
We conducted a budget impact analysis from the perspective of the Ontario Ministry of Health and Long-Term Care to estimate the cost burden of publicly funding continuous glucose monitoring devices over the next 5 years. All costs are reported in 2017 Canadian dollars. We forecasted the 5-year budget impact starting from 2018 to address current changes in policy regulation related to government funding of prescription medications for children and youth up to age 24 years.107 Although we did not conduct a primary economic evaluation in children owing to a lack of data, we did consider a subgroup of the population younger than 24 years in the budget impact analysis. Our reporting and analysis are in accordance with the 2012 International Society for Pharmacoeconomics and Outcomes Research (ISPOR) good practice guidelines for budget impact analyses.108
Research Question
What is the 5-year budget impact of publicly funding continuous glucose monitoring devices in patients with type 1 diabetes in the context of the Ontario Ministry of Health and Long-Term Care?
Methods
Target Population
We forecasted the prevalence of diabetes for Ontario's population based on data from the Canadian Diabetes Cost Model, developed by the Canadian Diabetes Association.109 We applied an annual prevalence rate increase of 0.31% for 2018 to 2022. We obtained a projection of the Ontario population from Statistics Canada.110 According to the Canadian Diabetes Association, the prevalence of type 1 diabetes ranges from 5% to 10%.
Data from a budget impact analysis by a continuous glucose monitoring manufacturer indicated that about 113,000 people in Ontario had type 1 diabetes in 2017.111 The manufacturer used the median prevalence (i.e., 7.5%) from the range provided by the Canadian Diabetes Association, above. However, our experts suggested that the prevalence of type 1 diabetes among adults in Ontario in 2017 is 6%; we used that figure to calculate the target population for our model (Table 27). According to Statistics Canada, the number of patients with type 1 diabetes is expected to increase over the next 5 years, from 90,288 in 2017 to 105,932 in 2022.110
Table 27:
Ontario Population and Estimated Prevalence of Type 1 Diabetes
| Population/Prevalence | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 |
|---|---|---|---|---|---|---|
| Ontario population, n | 13,920,500 | 14,004,100 | 14,081,900 | 14,154,600 | 14,222,100 | 14,284,300 |
| Projected prevalence of diabetes in Ontario, % | 10.81 | 11.12 | 11.43 | 11.74 | 12.05 | 12.36 |
| Ontario population with diabetes (type 1 and type 2), n | 1,504,806 | 1,557,256 | 1,609,561 | 1,661,750 | 1,713,763 | 1,765,539 |
| Prevalence of type 1 diabetes, % (range 5% to 10%) | 6 | 6 | 6 | 6 | 6 | 6 |
| Ontario population with type 1 diabetes (estimate),a n | 90,288 | 93,435 | 96,574 | 99,705 | 102,826 | 105,932 |
| Ontario population with hypoglycemia unawareness,a n (25% of people with type 1 diabetes) | 22,572 | 23,359 | 24,143 | 24,926 | 25,706 | 26,483 |
Values used in the budget impact analysis.
Based on consultations with experts (endocrinologists, diabetes educators, and manufacturers of continuous glucose monitoring devices), we identified a subgroup of people with hypoglycemia unawareness who would benefit most from continuous glucose monitoring. The prevalence of hypoglycemia unawareness in type 1 diabetes ranges from 20% to 29%.105,112,113 We used a median prevalence of 25% in our model (Table 27).
In 2018, the Ontario government will start a new pharmacare program that will cover drug costs (including blood glucose test strips and insulin) for children and youth up to 24 years of age.107 We applied this change to our costing of insulin and test strips for people in this age group. To account for the change in drug funding, we grouped our target populations into three categories (0–24 years, 25–64 years, and 65+ years) and estimated the number of people with type 1 diabetes for each age subgroup (Table 28). See Appendix 8 (Tables A16 and A17) for estimates of government funding by age group.
Table 28:
Ontario Populations with Diabetes, Type 1 Diabetes, and Hypoglycemia Unawareness, by Age Group
| Age, y | 2018 | 2019 | 2020 | 2021 | 2022 |
|---|---|---|---|---|---|
| Ontario Population With Diabetes | |||||
| 0–24 | 438,362 | 448,102 | 457,426 | 466,974 | 477,133 |
| 25–64 | 848,956 | 874,784 | 899,789 | 923,958 | 946,319 |
| 65+ | 269,927 | 286,676 | 304,512 | 322,856 | 342,112 |
| Totala | 1,557,245 | 1,609,561 | 1,661,727 | 1,713,787 | 1,765,564 |
| Ontario Population With Type 1 Diabetes (Prevalence 6%) | |||||
| 0–24 | 26,302 | 26,886 | 27,446 | 28,018 | 28,628 |
| 25–64 | 50,937 | 52,487 | 53,987 | 55,437 | 56,779 |
| 65+ | 16,196 | 17,201 | 18,271 | 19,371 | 20,527 |
| Totala | 93,435 | 96,574 | 99,704 | 102,827 | 105,934 |
| Ontario Population With Hypoglycemia Unawareness (Prevalence 25% of People with Type 1 Diabetes) | |||||
| 0–24 | 6,575 | 6,722 | 6,861 | 7,005 | 7,157 |
| 25–64 | 12,734 | 13,122 | 13,497 | 13,859 | 14,195 |
| 65+ | 4,049 | 4,300 | 4,568 | 4,843 | 5,132 |
| Totala | 23,359 | 24,143 | 24,926 | 25,707 | 26,483 |
Total estimates might not match with those in Table 28 due to rounding.
Resource
We received information from two main manufacturers of continuous glucose monitoring devices: Dexcom (standalone continuous glucose monitoring devices) and Medtronic (sensor-augmented pump with or without a low-glucose suspend feature). Based on this information, we estimated the annual use of continuous glucose monitoring interventions in two scenarios: manufacturer-suggested projections and conservative projections with 20% annual increase in uptake based on the number of current users (Figure 8 and Appendix 8, Table A18).
Figure 6: Continuous Glucose Monitoring—Current Use and Projection Scenarios in Ontario, 2018–2022.
Source: Information for current use and manufacturer projections provided by Dexcom and Medtronic in January 2017.
Abbreviations: CGM, continuous glucose monitoring; CSII, continuous subcutaneous insulin infusion (insulin pump); MDI, multiple daily injections; SAP, sensor-augmented pump.
Based on Dexcom's projections, the number of patients in Ontario to use standalone continuous glucose monitoring will be about 3,800 to 4,500 in 5 years. This projection was based on a 5% adoption rate at the introduction of continuous glucose monitoring in the United States. Currently, about 800 people use standalone continuous glucose monitoring devices in Ontario. We estimated that a 40% annual increase in uptake would be needed to reach the projected goal.
Based on data from Medtronic, about 1,300 people use continuous glucose monitoring devices integrated with a sensor-augmented pump. Of these, 1,170 have a sensor-augmented pump with a low-glucose suspend feature. Those using a sensor-augmented pump and continuous glucose monitoring are the most motivated, because they fear hypoglycemia (Appendix 8, Table A19). These patients pay out of pocket or through private insurance for continuous glucose monitoring sensors. Based on the experiences of other countries that fund continuous glucose monitoring sensors, sensor use tends to reach a plateau after 5 years, at approximately 30% of sensor-augmented pump users. Medtronic projected a 25% annual increase in the use of sensor-augmented pumps with continuous glucose monitoring.
Table 29 presents the number of patients projected to use standalone continuing glucose monitoring or sensor-augmented pumps with continuous glucose monitoring based on manufacturer information.
Table 29:
Estimated Annual Number of Patients Using Continuous Glucose Monitoring in Ontario, Manufacturer Projections
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | |
|---|---|---|---|---|---|
| Standalone CGM + multiple daily injections | 610 | 855 | 1,196 | 1,675 | 2,345 |
| Standalone CGM + insulin pump | 497 | 696 | 974 | 1,364 | 1,909 |
| Total standalone CGM devices | 1,107 | 1,550 | 2,171 | 3,039 | 4,254 |
| Sensor-augmented pumps | 1,768 | 2,163 | 2,700 | 3,276 | 3,650 |
| Total CGM users | 2,875 | 3,714 | 4,870 | 6,314 | 7,904 |
Abbreviation: CGM, continuous glucose monitoring.
Totals may appear inexact due to rounding.
However, we used a conservative 20% annual increase of adoption of continuous glucose monitoring based on the number of current users (Table 30) for the following reasons:
Table 30:
Estimated Annual Number of Patients Using Continuous Glucose Monitoring in Ontario, Conservative Projections
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | |
|---|---|---|---|---|---|
| Standalone CGM + multiple daily injections | 523 | 628 | 753 | 904 | 1,085 |
| Standalone CGM + insulin pump | 426 | 511 | 613 | 736 | 883 |
| Total standalone CGM devices | 949 | 1,139 | 1,367 | 1,640 | 1,968 |
| Sensor-augmented pumps | 1,560 | 1,872 | 2,246 | 2,696 | 3,235 |
| Total CGM users | 2,509 | 3,011 | 3,613 | 4,336 | 5,203 |
Abbreviation: CGM, continuous glucose monitoring.
Dexcom obtained its adoption rate for standalone continuous glucose monitoring devices from progressive hospitals with higher-than-average adoption rates
The infrastructure for continuous glucose monitoring in Canada is still under development; more educators, manuals, and technical support are required for wide implementation
Canadian Costs
Reference Case
We assessed the budget impact of funding continuous glucose monitoring for three patient populations:
The total number of people projected to use continuous glucose monitoring (Table 30)
The Ontario population with type 1 diabetes and hypoglycemia unawareness (Table 28)
The entire Ontario type 1 diabetes population (Table 28)
The Ontario government provides funding to people with diabetes, covering some of the medical costs associated with diabetes management. Support varies by age group, type of diabetes, and type of insulin used (Appendix 8, Table A20).
Beginning in January 1, 2018, the provincial government will fund prescription medications for children and youth 24 years of age or younger.107 This government also funds most types of insulin and blood glucose testing strips for those 65 years or older. For people over age 65, the government also provides an annual grant of $170, paid once per year, for the purchase of needles and syringes to inject insulin.
According to the Ministry of Health and Long-Term Care, approximately 17% of people in Ontario do not have drug funding through private plans or the Ontario Drug Benefit program. Patients who are not eligible for the Ontario Drug Benefit program can receive a 75% reimbursement for test strips and lancets, up to a maximum of $920 per year, through the Ontario Monitoring for Health Program.114
The Assistive Devices Program covers the cost of insulin pumps and related supplies for people of all ages with type 1 diabetes who meet the program's medical eligibility criteria.
Estimated annual per-patient costs for continuous glucose monitoring with various types of government funding for type 1 diabetes presented in Table 31.
Table 31:
Estimated Annual Per-Patient Costs of Continuous Glucose Monitoring When Insulin Pump, Insulin, and Supplies Are Publicly Funded
| Intervention | Technology | Year 1, $ | Year 2, $ | Year 3, $ | Year 4, $ | Year 5, $ |
|---|---|---|---|---|---|---|
| Comparators | ||||||
| SMBG + multiple daily injections | — | 2,329 | 2,692 | 2,952 | 3,134 | 3,251 |
| SMBG + insulin pump | Animas Vibe | 1,599 | 1,963 | 2,234 | 2,432 | 2,566 |
| Interventions | ||||||
| CGM + multiple daily injections | Dexcom G4/G5 | 7,994a | 8,340a | 8,479a | 8,529a | 8,506a |
| CGM + insulin pump | Integrated | 6,647b | 6,974b | 7,150b | 7,232b | 7,241b |
| SAP with LGS | MiniMed Veo | 4,186 | 4,493 | 4,731 | 4,873 | 4,940 |
| SAP or SAP with LGS | 10% SAP; 90% SAP with LGS | 4,432 | 4,741 | 4,973 | 5,109 | 5,170 |
| Average CGM device | 40% CGM + multiple daily injections; 60% SAP | 5,857 | 6,181 | 6,376 | 6,477 | 6,505 |
Abbreviations: CGM, continuous glucose monitoring; LGS, low-glucose suspend [feature]; SAP, sensor-augmented pump; SMBG, self-monitoring of blood glucose.
Average cost of Dexcom G4 Platinum and Dexcom G5 Mobile.
Average cost of Dexcom G4 Platinum + Animas Vibe and Dexcom G5 Mobile + Animas Vibe.
We calculated the amount of existing government support for diabetes supplies, blood glucose test strips, and insulin for the different age groups and patient populations (Appendix 8, Tables A16 and A17).
Scenarios
We estimated the annual costs per patient if continuous glucose monitoring were publicly funded by the Ministry of Health and Long-Term Care. We calculated annual per-patient costs for the following scenarios:
Scenario 1: All direct medical costs (Table 32). We calculated average annual costs per patient from years 1 to 5 based on our model estimates (from deterministic non-discounted cost-utility analyses; see Primary Economic Evaluation).
Scenario 2: Funding for insulin, pump, and 75% of sensors (Table 33)
Table 32:
Estimated Annual Per-Patient Costs (All Direct Medical Costs) of Continuous Glucose Monitoring in Ontario
| Intervention | Technology | Year 1, $ | Year 2, $ | Year 3, $ | Year 4, $ | Year 5, $ |
|---|---|---|---|---|---|---|
| Comparators | ||||||
| SMBG + multiple daily injections | — | 3,677 | 4,039 | 4,277 | 4,431 | 4,515 |
| SMBG + insulin pump | Animas Vibe | 6,817 | 7,175 | 7,365 | 7,450 | 7,458 |
| Interventions | ||||||
| CGM + multiple daily injections | Dexcom G4/G5 | 9,342a | 9,693a | 9,810a | 9,830a | 9,775a |
| CGM + insulin pump | Integrated | 11,673b | 12,075b | 12,126b | 12,090b | 11,980b |
| SAP with LGS | MiniMed Veo | 9,211 | 9,531 | 9,673 | 9,701 | 9,649 |
| SAP or SAP with LGS | 10% SAP; 90% SAP with LGS | 9,457 | 9,785 | 9,918 | 9,940 | 9,882 |
| Average for CGM devices | 40% CGM + multiple daily injections; 60% CGM + SAP | 9,411 | 9,748 | 9,875 | 9,896 | 9,839 |
Abbreviations: CGM, continuous glucose monitoring; LGS, low-glucose suspend [feature]; SAP, sensor-augmented pump; SMBG, self-monitoring of blood glucose.
Average cost of Dexcom G4 Platinum and Dexcom G5 Mobile.
Average cost of Dexcom G4 Platinum + Animas Vibe and Dexcom G5 Mobile + Animas Vibe.
Table 33:
Estimated Annual Per-Patient Costs of Continuous Glucose Monitoring when Insulin Pump, Insulin, and 75% of Sensor Costs Are Publicly Funded
| Intervention | Technology | Year 1, $ | Year 2, $ | Year 3, $ | Year 4, $ | Year 5, $ |
|---|---|---|---|---|---|---|
| Comparators | ||||||
| SMBG + multiple daily injections | — | 2,329 | 2,692 | 2,952 | 3,134 | 3,251 |
| SMBG + insulin pump | Animas Vibe | 1,599 | 1,963 | 2,234 | 2,432 | 2,566 |
| Interventions | ||||||
| CGM + multiple daily injections | Dexcom G4/G5 | 6,889a | 7,240a | 7,389a | 7,461a | 7,465a |
| CGM + insulin pump | Integrated | 5,542b | 5,893b | 6,058b | 6,157b | 6,194b |
| SAP with LGS | MiniMed Veo | 3,406 | 3,725 | 3,959 | 4,112 | 4,199 |
| SAP or SAP with LGS | 10% SAP; 90% SAP with LGS | 3,619 | 3,942 | 4,169 | 4,317 | 4,399 |
| Average CGM device | 40% CGM + multiple daily injections; 60% CGM + SAP | 4,927 | 5,261 | 5,457 | 5,574 | 5,625 |
Abbreviations: CGM, continuous glucose monitoring; LGS, low-glucose suspend [feature]; SAP, sensor-augmented pump; SMBG, self-monitoring of blood glucose.
Average cost of Dexcom G4 Platinum and Dexcom G5 Mobile.
Average cost of Dexcom G4 Platinum + Animas Vibe and Dexcom G5 Mobile + Animas Vibe.
For these scenarios, we calculated the funding for blood glucose test strips by age group, because funding varies by patient age.
We also considered a third scenario—a hypothetical situation in which device costs underwent price negotiations, and calculated the budget impact corresponding to device cost reductions of 10%, 20% or 30% (Table 34).
Table 34:
Estimated Annual Costs of Continuous Glucose Monitoring Devices With Cost-Reduction Scenarios
| Strategies | Device Cost, $ | |||
|---|---|---|---|---|
| Base Case | 10% Reduction | 20% Reduction | 30% Reduction | |
| CGM + multiple daily injections (average) | 9,342 | 8,408 | 7,474 | 6,539 |
| SAP (average) | 11,673 | 10,505 | 9,338 | 8,171 |
| SAP (± LGS) | 9,211 | 8,290 | 7,369 | 6,448 |
| Average cost of CGM | 9,411 | 8,470 | 7,529 | 6,588 |
Abbreviations: CGM, continuous glucose monitoring; LGS, low glucose suspend [feature]; SAP, sensor-augmented pump; SMBG, self-monitoring of blood glucose.
Analysis
To address all possible scenarios in Ontario, we conducted the following budget impact analyses:
-
Three reference case analyses to estimate the net budget impact of continuous glucose monitoring compared with self-monitoring of blood glucose after accounting for government funding of insulin treatment, insulin pump, and blood glucose test strips:
∘ The total number of people projected to use continuous glucose monitoring, based on a 20% annual increase of adoption in Ontario
∘ The Ontario population with hypoglycemia unawareness
∘ The entire Ontario type 1 diabetes population
Two sensitivity analyses to estimate the net budget impact of considering all direct medical costs (scenario 1) and different levels of government funding (insulin, insulin pump, and blood glucose test strips, plus 75% of sensor costs; scenario 2) by age group
Various price negotiation scenarios for the continuous glucose monitoring device (30%, 20% and 10% price reductions; scenario 3)
Four sensitivity analyses to estimate the net budget impact of price reductions for continuous glucose monitoring devices and 75% of sensor costs (scenario 4)
Sensitivity analyses of the base case and all above-mentioned scenarios, based on Dexcom's projected 40% annual increase of adoption in Ontario (scenario 5)
We calculated the net budget impact cumulatively: annual costs included the costs of new continuous glucose monitoring devices and expenses for devices introduced in previous years.
Results
Reference Case
Table 35 shows the net budget impact for the reference case, using data on continuous glucose monitoring uptake, the percentage of patients with hypoglycemia unawareness (Tables 28 and 30), the entire type 1 diabetes population, and annual per-patient costs of technologies (Table 31). Appendix 8, Tables A21 to 23, provide the net budget impact by age group.
Table 35:
Net Budget Impact of Funding Continuous Glucose Monitoring in Ontario
| Intervention | Year 1, $ | Year 2, $ | Year 3, $ | Year 4, $ | Year 5, $ |
|---|---|---|---|---|---|
| Funding CGM Based on a Conservative Projection of a 20% Annual Increase | |||||
| CGM | 14,192,322 | 17,835,103 | 22,441,206 | 27,412,678 | 31,857,150 |
| SMBG | 5,701,743 | 7,953,809 | 10,498,720 | 13,226,191 | 15,621,231 |
| Net budget impact | 8,490,579 | 9,881,295 | 11,942,486 | 14,186,487 | 16,235,919 |
| Funding CGM for Entire Population With Hypoglycemia Unawareness | |||||
| CGM | 120,487,478 | 131,981,755 | 140,758,669 | 147,524,997 | 152,658,439 |
| SMBG | 41,693,554 | 51,518,633 | 59,307,520 | 65,546,416 | 70,408,816 |
| Net budget impact | 78,793,925 | 80,463,123 | 81,451,149 | 81,978,581 | 82,249,622 |
| Funding CGM for the Entire Type 1 Diabetes Population | |||||
| CGM | 481,949,914 | 527,927,021 | 563,034,675 | 590,099,986 | 610,633,755 |
| SMBG | 166,774,215 | 206,074,531 | 237,230,078 | 262,185,663 | 281,753,568 |
| Net budget impact | 315,175,698 | 321,852,490 | 325,804,597 | 327,914,323 | 328,880,187 |
Abbreviations: CGM, continuous glucose monitoring; SMBG, self-monitoring of blood glucose.
Scenarios
Scenario 1: All Direct Medical Costs
We assessed the budget impact of funding continuous glucose monitoring when considering all direct medical costs (excludes government funding for insulin, insulin pump, and blood glucose test strips) by age group (Table 36).
Table 36:
All Direct Medical Costs
| Age,a y | Year 1, $ | Year 2, $ | Year 3, $ | Year 4, $ | Year 5, $ |
|---|---|---|---|---|---|
| Funding CGM Based on a Conservative Projection of a 20% Annual Increase | |||||
| All ages | 13,663,640 | 16,350,489 | 19,342,798 | 22,893,882 | 27,137,269 |
| 0–24 | 3,846,289 | 4,602,632 | 5,444,962 | 6,444,585 | 7,639,091 |
| 25–65 | 7,448,947 | 8,913,725 | 10,545,030 | 12,480,958 | 14,794,307 |
| 65+ | 2,368,403 | 2,834,131 | 3,352,807 | 3,968,338 | 4,703,871 |
| Funding CGM for Entire Population With Hypoglycemia Unawareness | |||||
| All ages | 121,695,684 | 125,149,848 | 126,682,327 | 127,706,376 | 128,424,590 |
| 0–24 | 34,257,109 | 35,229,450 | 35,660,840 | 35,949,108 | 36,151,284 |
| 25–65 | 66,344,309 | 68,227,400 | 69,062,855 | 69,621,132 | 70,012,677 |
| 65+ | 21,094,266 | 21,692,998 | 21,958,632 | 22,136,137 | 22,260,629 |
| Funding CGM for Entire Type 1 Diabetes Population | |||||
| All ages | 486,782,736 | 500,599,393 | 506,729,308 | 510,825,506 | 513,308,356 |
| 0–24 | 137,028,436 | 140,917,800 | 142,643,359 | 143,796,431 | 144,495,349 |
| 25–65 | 265,377,235 | 272,909,602 | 276,251,421 | 278,484,528 | 279,838,092 |
| 65+ | 84,377,065 | 86,771,991 | 87,834,527 | 88,544,547 | 88,974,915 |
Abbreviations: CGM, continuous glucose monitoring; NBI, net budget impact.
Ages 0–24 years, full funding of blood glucose test strips (annual cost $1,243); 25–65 years, strips funded to a maximum of $920; 65+ years, full funding of strips and $170 grant for syringes and needles.
Scenario 2: Government Funding for the Insulin, Pump, Strips, and 75% of Sensor Costs, by Age Group
Because government funding is for patients with type 1 diabetes and would involve only the addition of 75% sensor costs, the net budget impact of this scenario (Table 37) was much lower than that of the reference case and scenario 1. Appendix 8, Tables A24 to A26, provide the calculation details. Appendix 8, Table A27, provides details on adopting continuous glucose monitoring for the population with hypoglycemia awareness using a conservative projection.
Table 37:
Net Budget Impact of Funding Continuous Glucose Monitoring in Ontario by Age Group—Funding for Insulin, Pump, Strips, and 75% of Sensor Costs
| Age,a y | Year 1, $ | Year 2, $ | Year 3, $ | Year 4, $ | Year 5, $ |
|---|---|---|---|---|---|
| Funding CGM Based on a Conservative Projection of a 20% Annual Increase | |||||
| All ages | 6,258,114 | 7,212,880 | 8,616,558 | 10,212,713 | 11,746,912 |
| 0–24 | 1,738,689 | 2,003,952 | 2,393,935 | 2,837,394 | 3,263,640 |
| 25–65 | 3,397,447 | 3,915,777 | 4,677,815 | 5,544,347 | 6,377,243 |
| 65+ | 1,121,977 | 1,293,151 | 1,544,808 | 1,830,972 | 2,106,029 |
| Funding CGM for Entire Population With Hypoglycemia Unawareness | |||||
| All ages | 62,186,324 | 63,630,543 | 64,296,591 | 64,842,725 | 65,295,437 |
| 0–24 | 17,499,740 | 17,721,986 | 17,688,562 | 17,646,273 | 17,613,612 |
| 25–65 | 33,898,320 | 34,600,771 | 34,804,315 | 34,934,408 | 34,960,287 |
| 65+ | 10,788,265 | 11,307,785 | 11,803,714 | 12,262,045 | 12,721,538 |
| Funding CGM for Entire Type 1 Diabetes Population | |||||
| All ages | 248,745,298 | 254,522,171 | 257,186,365 | 259,370,901 | 261,063,447 |
| 0–24 | 69,998,960 | 70,887,946 | 70,754,249 | 70,585,090 | 70,421,580 |
| 25–65 | 135,593,278 | 138,403,086 | 139,217,260 | 139,737,631 | 139,776,924 |
| 65+ | 43,153,060 | 45,231,139 | 47,214,856 | 49,048,180 | 50,864,943 |
Abbreviations: CGM, continuous glucose monitoring; NBI, net budget impact.
Ages 0–24 years, full funding of blood glucose test strips (annual cost $1,243); 25–65 years, strips funded to a maximum $920; 65+ years, full funding of strips and $170 grant for syringes and needles.
Scenario 3: Cost Reductions for Continuous Glucose Monitoring Devices
Table 38 presents the budget impact of including only the costs associated with the continuous glucose monitoring device (i.e., transmitter, sensors, and batteries). Public funding of continuous glucose monitoring would motivate more people to use it, which would stimulate production growth and potentially decrease the costs of production, especially the costs of sensors. Appendix 8, Table A28 provides details of estimated reductions in the cost of continuous glucose monitoring devices.
Table 38:
Net Budget Impact of Cost Reductions for Continuous Glucose Monitoring Devices
| Intervention | Year 1, $ | Year 2, $ | Year 3, $ | Year 4, $ | Year 5, $ |
|---|---|---|---|---|---|
| Funding CGM Based on a Conservative Projection of a 20% Annual Increase | |||||
| Device cost | 13,663,640 | 16,396,368 | 19,675,642 | 23,610,770 | 28,332,924 |
| Device cost + 75% sensor cost | 11,396,928 | 13,676,314 | 16,411,577 | 19,693,892 | 23,632,670 |
| Device cost reduced by 30% | 6,394,826 | 7,673,791 | 9,208,550 | 11,050,260 | 13,260,312 |
| Funding CGM for Entire Population With Hypoglycemia Unawareness | |||||
| Device cost | 121,695,684 | 125,704,689 | 129,684,091 | 133,627,989 | 137,530,346 |
| Device cost + 75% sensor cost | 99,975,005 | 103,254,454 | 106,505,923 | 109,724,341 | 112,904,518 |
| Device cost reduced by 30% | 55,746,044 | 57,539,926 | 59,309,134 | 61,050,287 | 62,759,917 |
| Funding CGM for Entire Type 1 Diabetes Population | |||||
| Device cost | 486,782,736 | 502,818,757 | 518,736,363 | 534,511,956 | 549,760,390 |
| Device cost + 75% sensor cost | 399,900,019 | 413,017,815 | 426,023,690 | 438,897,364 | 451,257,077 |
| Device cost reduced by 30% | 222,984,175 | 230,159,706 | 237,236,538 | 244,201,146 | 250,678,673 |
Abbreviations: CGM, continuous glucose monitoring.
We also conducted scenarios involving device cost reductions of 10%, 20%, and 30%. The budget impact of device cost reductions are presented in Appendix 8, Tables A29 to A31. The cost reduction by 30% showed a significant reduction in net budget impact.
Scenario 4: Cost Reductions for Continuous Glucose Monitoring Devices and Government Funding
Appendix 8, Table A32, presents the results of scenario 4, which estimated the budget impact of device cost reductions plus government funding for insulin, insulin pump, blood glucose test strips, and 75% of sensors.
The additional investment to implement this scenario in Ontario, assuming a conservative annual adoption rate of 20%, would be as follows:
If device costs were reduced as suggested above, the net budget impact would range from $1.9 million to $8.7 million
If continuous glucose monitoring were funded for those with hypoglycemia unawareness, the net budget impact would range from $23.1 million to $54.3 million
If continuous glucose monitoring were funded for the entire type 1 diabetes population across all cost reduction scenarios, the net budget impact would range from $92.6 million to $217.1 million
Scenario 5: 40% Annual Increase in Adoption of Continuous Glucose Monitoring in Ontario
The net budget impact would vary from $9.3 million to $27.5 million over the next 5 years (Appendix 8, Table A33). This amount significantly declines when considering various scenarios of government support or reductions in device and sensor costs. Appendix 8, Table A34, also presents the net budget impact of funding continuous glucose monitoring for the entire type 1 diabetes population and for those with hypoglycemia unawareness.
Discussion
The budget impact of funding continuous glucose monitoring would be large compared to the budget impact of many other novel technologies, because of the large number of people with type 1 diabetes and the high cost of supplies (sensors) for continuous glucose monitoring. Based on a projected 20% annual increase in uptake (starting from 2,091 current users), funding continuous glucose monitoring over the next 5 years would cost the province $8.5 million in year 1 to $16.2 million in year 5. Funding continuous glucose monitoring devices for the entire population with hypoglycemia unawareness (26,483 users) could result in extra spending of $78.8 million in year 1 to $82.2 million in year 5.
Of the 5,203 new users of continuous glucose monitoring projected in the next 5 years, an estimated 62% would use integrated monitoring (continuous glucose monitoring with a sensor-augmented pump, with or without a low-glucose suspend feature), and 38% would use standalone continuous glucose monitoring. Budget spending would be expected to increase over time with higher uptake.
A budget impact analysis of introducing Dexcom continuous glucose monitoring in patients with type 1 diabetes with hypoglycemia unawareness in Ontario111,115 showed a cost savings of $140 million over 5 years. These results were not in line with our findings for several possible reasons.
First, in the Dexcom analysis, the clinical benefits of continuous glucose monitoring were associated with a reduction in emergency department visits and hospital admissions related to severe hypoglycemia patients with in type 1 diabetes. As well, these data were obtained from a European online survey.93 The survey evaluated the burden of hypoglycemia in insulin-treated patients with diabetes but did not specify methods of blood glucose monitoring. Linking those reductions in emergency department visits and hospital admissions with the use of continuous glucose monitoring may not have been clinically valid.
Second, the potential for continuous glucose monitoring to reduce the number of severe hypoglycemic events and lower A1C versus self-monitoring of blood glucose was unclear. Data on the incidence of severe hypoglycemic events were obtained from short-term (less than 1 year) studies24,31,39 that showed mixed results. In addition, the rates of severe hypoglycemic events, typically identified as events requiring the assistance of another person (e.g., a seizure, loss of consciousness, or hospitalization), were not properly reported. Longitudinal data from the Diabetes Control and Complications Trial demonstrated that tight control of A1C is associated with a reduction in long-term complications but also with an increased risk of severe hypoglycemic events.76 The clinical evidence review in this health technology assessment was in agreement with other studies, showing no statistically significant difference in rates of severe hypoglycemic events among patients who used continuous glucose monitoring versus those who used self-monitoring of blood glucose.106
Third, we considered patients with hypoglycemia unawareness as a subgroup in our budget impact analysis but did not assess this group in the primary economic evaluation, owing to limited clinical evidence for this population. However, we do agree that patients with hypoglycemia unawareness could benefit most from using continuous glucose monitoring. More research is required to assess the clinical effectiveness of continuous glucose monitoring in patients with hypoglycemia unawareness.
Finally, our primary economic evaluation showed that the cost of continuous glucose monitoring devices was one of the main drivers of the model results. Costs for continuous glucose monitoring are three to four times higher than those for self-monitoring of blood glucose.
Our model-based budget impact analysis provided robust costing parameters and was based on a rigorous systematic review of the best quality evidence to minimize possible biases. Using real-world data from randomized controlled studies was a more robust approach than calculating budget impact based on hypothetical scenarios, as in the Dexcom analysis.
Conclusions
If continuous glucose monitoring were publicly funded in Ontario as an alternative to self-monitoring of blood glucose in patients with type 1 diabetes, the net budget impact for the province would be $8.5 million to $16.2 million over the next 5 years, assuming a 20% increase in adoption each year. Funding continuous glucose monitoring for the entire population with hypoglycemia unawareness would lead to a net budget impact of $78.8 million to $82.2 million over the next 5 years.
PATIENT, CAREGIVER, AND PUBLIC ENGAGEMENT
Objective
The objective of this analysis was to explore the underlying values, needs, impacts, and preferences of those who have lived experience with type 1 diabetes. The treatment focus was continuous glucose monitoring versus usual care (self-monitoring of blood glucose using a finger-stick and a blood glucose meter).
Background
Patient, caregiver, and public engagement provides a unique source of information about people's experiences of a health condition and the health technologies or interventions used to manage or treat that health condition. It includes the impact of the condition and its treatment on the patient, the patient's family and other caregivers, and the patient's personal environment. It also provides insights into how a health condition is managed by the province's health system.
Information shared from lived experience can also identify gaps or limitations in published research (e.g., sometimes typical outcome measures do not reflect what is important to those with lived experience).116–118 Additionally, lived experience can provide information and perspectives on the ethical and social values implications of health technologies or interventions.
Because the needs, priorities, preferences, and values of those with lived experience in Ontario are not often adequately explored in published literature, we contact and speak directly with people who live with a given health condition, including those who may have experience with the intervention we are exploring.
Type 1 diabetes has a significant impact on people with diabetes and their families, and it substantially affects their quality of life. It is estimated that more than 300,000 people in Canada live with type 1 diabetes, 150,000 of whom are in Ontario.1,2 This disease strikes both young and old, requires daily management, and lasts for a lifetime.
For this project, we spoke with people who have lived experience: patients with type 1 diabetes and their families. For children with type 1 diabetes, we spoke to their parents about the impact of the disease.
A large number of those we spoke to had experience managing their diabetes using continuous glucose monitoring. Gaining an understanding of the day-to-day experience of managing diabetes, including people's experience with continuous glucose monitoring, helps us assess the potential value of this technology from the perspective of patients and caregivers.
Methods
Engagement Plan
The engagement plan for this health technology assessment focused on consultation to examine the experiences of patients with type 1 diabetes and those of their families and other caregivers, including their experience with continuous glucose monitoring. We engaged people face-to-face, via phone interviews, through written interview responses, and in focus groups.
Primarily, we used qualitative interviews, because this method of engagement allows us to explore the meaning of central themes in the experiences of patients with type 1 diabetes, as well as those of their families and caregivers. Our main task in interviewing is to understand what people tell us and gain an understanding of the story behind their experiences.119 The sensitive nature of exploring people's experiences of a health condition and their quality of life are other factors that support our primary choice of an interview methodology.
We also held two focus groups, which allowed for more thematic discussion of issues relating to type 1 diabetes in a supportive group environment. Focus groups were split into two main patient populations: adults with type 1 diabetes and parents of children with type 1 diabetes. In comparison to qualitative interviews, focus group discussions focused on commonalities and differences, and on broader experiences of diabetes management and continuous glucose monitoring, rather than on individual stories.
Participant Recruitment
We used an approach called purposive sampling,120–123 which involves actively reaching out to patients, families, and caregivers with direct experience of the health condition and health technology or intervention being reviewed. We approached a variety of partner organizations, health clinics, diabetes support associations, and foundations to spread the word about this engagement activity and to make contact with patients, families, and caregivers, including those with experience of type 1 diabetes and continuous glucose monitoring.
Inclusion Criteria
We sought to speak with patients with type 1 diabetes and their families who actively manage their diabetes. Patients were not required to have direct experience with continuous glucose monitoring.
We sought broad geographic, cultural, and socioeconomic representations to elicit possible equity issues in accessing and using continuous glucose monitoring devices.
Exclusion Criteria
We did not set specific exclusion criteria.
Participants
We conducted interviews and focus groups with 59 individuals. We interviewed 45 patients and families one on one, either in person or over the phone. We conducted two interviews via written correspondence, and we held two six-person focus groups.
Those interviewed included adults with type 1 diabetes and parents of children with type 1 diabetes. The children ranged in age from less than 2 years old to 16 years old. We recruited participants from across Ontario.
The majority of participants had direct experience with continuous glucose monitoring. Because no participants received continuous glucose monitoring devices immediately upon diagnosis of type 1 diabetes, they were able to compare their experiences of diabetes management with and without these devices.
Approach
At the beginning of the interview and focus groups, we explained the role of Health Quality Ontario, the purpose of the health technology assessment, the risks of participation, and how personal health information would be protected. We gave this information to participants both verbally and in a printed letter of information (Appendix 9). We then obtained participants’ verbal consent before starting the interview and focus groups. With participants’ consent, we audio-recorded interviews and then had the recordings transcribed.
Interviews lasted 20 to 90 minutes. They were loosely structured and consisted of a series of open-ended questions. Questions were based on a list developed by the Health Technology Assessment International Interest Group on Patient and Citizen Involvement in Health Technology Assessment.124 Questions focused on the impact of type 1 diabetes on patients’ and families’ quality of life, their experiences with treatment options, and their perceptions of the benefits or limitations of using continuous glucose monitoring to manage diabetes. See Appendix 9 for our interview guide.
The focus groups lasted approximately 90 minutes each. They were loosely structured and guided by a series of open-ended questions, based on the interview guide. Questions focused on commonalities and differences in diabetes management and continuous glucose monitoring, allowing members to explore themes surrounding the topic.
Data Extraction and Analysis
We used a modified version of a grounded-theory methodology to analyze interview transcripts, focus group transcripts, and survey results. The grounded-theory approach allowed us to organize and compare information across participants. This method consisted of a repetitive process of obtaining, documenting, and analyzing responses while simultaneously collecting, analyzing, and comparing information.125,126 We used the qualitative data analysis software program NVivo (QSR International, Doncaster, Victoria, Australia) to identify and interpret patterns in interview, focus group, and survey data. The patterns we identified then allowed us to highlight the impact of health conditions and treatments on the patients, family members, and caregivers we interviewed.
Results
Lived Experience of Type 1 Diabetes
During the interviews and focus groups, patients with type 1 diabetes and their family members repeatedly emphasized the daily burden and stress of managing this disease. People with type 1 diabetes have to regularly monitor their blood glucose levels and make adjustments by administering insulin. Therefore, multiple times per day, every day, year after year, patients and families must make calculations and decisions about the amount of insulin to take. Participants emphasized that while these calculations and injections may eventually become routine, they are of vital medical importance, and errors can have grave health consequences in both the short and long term.
Diagnosis
Adult patients with type 1 diabetes and parents of children with type 1 diabetes described the overwhelming and emotional experience of diagnosis. Often, they had no previous experience or knowledge of type 1 diabetes. A short hospital stay, which included rapid education about diabetes and its management, was a common experience. They often encountered a steep learning curve in attempting to understand the disease and its day-to-day medical management:
Yeah, it's insane. Absolutely. My husband and I, we were discharged after about an hour of training. After spending a weekend in the hospital, we had the clinic people come in and train us, and they sent us home. And even though I'm a nurse and we're both fairly intelligent people, we sat in bed that night and we're like, “What the heck are we doing?”
I had no idea. I knew there was a difference between type 1 and type 2, and I knew the basics about it, but I had no idea the daily management that is involved in type 1 diabetes.
Adult patients and parents of newly diagnosed children reported quickly learning that daily management of diabetes extends beyond merely injecting insulin. Adjustments to food choices and activity levels were new concerns they needed to learn about:
You have to learn how to count carbs and everything, and estimate … For me, I like everything to be an exact science, but I had to learn that nothing is an exact science with this disease. It's kind of trial and error, and it's so unpredictable.
The [blood glucose] was so unpredictable with his activity level. Being so little, we just didn't know what to expect or how different foods were going to react.
You have to try and eliminate reasons why this is happening and adjust basal insulin rates and bolus insulin rates, and it was just a different language to me. You know, you just start researching, and basically I've spent the last 2 years online reading and reading.
While newly diagnosed adults tended to focus on the informational burden of the diagnosis, parents of children often spoke of its emotional impact. These parents repeatedly reported fearing and doubting their ability to keep their children alive and healthy. Managing this challenging disease in young children, with little background knowledge, was daunting:
So, it was really hard at first with the multiple finger pokes and the daily injections. There were months where we would have to—my husband would have to hold her down and basically restrain her while I gave her injections.
But the emotions were overwhelming, and as a parent, you're trying to hold it together, because all the education was done in front of my child. I would have preferred a moment where they took her away and just let me digest it—you know, say everything I was feeling, because you can't say, “I'm scared.”
…Because you're trying to stay brave for your child whose whole world was turned upside down.
We were diagnosed over a weekend. Friday was our education, and then we had to return Monday and Tuesday for more education … That was the scariest weekend of my life, because I did not feel I was equipped to take care of my child, for the first time ever. And I have three kids, and it was the first time ever in my life as a mother I felt very ill-equipped.
While patients and families expressed appreciation for the support the medical system provided at the time of diagnosis, they often still felt the support was inadequate, owing to the overwhelming nature of the medical management that diabetes requires. Patients and families often reported feelings of abandonment, of being left to self-manage the disease with a perceived lack of proper training or preparation:
So, it was very traumatizing and very upsetting, but even then we didn't really understand what this meant. They said, “They have diabetes,” and we thought, “Okay, she'll have to have a shot every day.” Like, that's kind of all we thought that this disease was. We had no idea just how complex it is and how it can affect every aspect of what she does in her day-to-day life.
The clinic was good in terms of giving the basic information that we needed, and they gave us pretty much all we could chew at that time; because it's overwhelming. There's a lot of content that's coming at you, and you basically are getting trained to be a nurse in a week.
Day-to-Day Impact of Type 1 Diabetes
While events surrounding the diagnosis of type 1 diabetes were unique to each person, those interviewed consistently reported the overwhelming nature of the diagnosis. Similarly, almost all of those interviewed referred to significant and profound changes in their daily life, including their quality of life. The descriptions of this impact generally fell into three categories: social, emotional, and other.
Social Impact
The social impact of type 1 diabetes was more commonly reported among parents of children with type 1 diabetes than among adults with the disease. People who were diagnosed as adults spoke of adjustments they had to make in their work life or in social settings, but overall expressed less social impact:
But I do notice that I do a lot of exercise, a lot of walking. I belong to quite a few groups that go out and walk around and do things like that, and I have to make sure that I stop everybody. “Sorry, guys, you're going to have to wait. I've got to test.” And I have to do it about every 20 minutes when I'm out.
On the other hand, children who were newly diagnosed faced increased social challenges, at a time when social interactions are less established and comfortable. Several parents spoke of wanting to minimize the changes brought on by type 1 diabetes. As much as possible, they did not want the disease to change their child's life:
For her, it was important to be back with her friends, and because we didn't disrupt her life so much, she just kind of kept going.
She has a couple of other kids in the school … in high school specifically, who refused to talk about their diabetes or refused to tell anybody that they had it, because they felt that they were an outcast.
Parents of children with type 1 diabetes reported that a particularly challenging time for their children was during school. Parents would often be required to educate classmates, teachers, and administrators about type 1 diabetes and how their children would manage it. For younger children, this required that more responsibility be placed on teachers. For older children, it could mean a disruption in class while the child managed their blood glucose:
So I become quite a momma bear and threatened—not threatened—but explained to them what could happen … I did [it] quite a few times. I insisted on always training the teachers and training the staff.
And [my daughter with type 1 diabetes] was actually saying to me yesterday how it really bothered her that no matter how many times she would say something to a teacher and how many times they were great and they understood about the diabetes, they would still turn to her and say, “Why are you eating in class?”
When the boys were younger, they had to go to the office to check their blood sugar or inject—when they were first diagnosed, before they were on a pump—because they didn't want to scare any of the other kids.
Additionally, children were involved in sports and physical activities at school. This required extra-careful management of sugar and insulin to avoid catastrophic low blood glucose levels:
Well, it just affects every aspect of our lives … especially for her. She plays volleyball, a higher-level volleyball, and she has to plan hours in advance for the physical activities. And there's even gym class or, you know, a school trip where they walk somewhere, she has to—an hour in advance—make sure her blood sugar is at a safe level to sustain that physical activity so she doesn't go low in the middle of it. So she has to plan pretty far in advance.
Emotional Impact
Parents consistently reported the emotional impact of type 1 diabetes. They often spoke of the near-constant fear and anxiety they experienced in caring for their child. The health of their child became something never to be taken for granted, and parents often reported wrestling with the daily struggle of keeping their child healthy and the fear of failure. This emotional burden had a large impact on the parents, their children, and their extended families:
And, yeah, just a feeling of, “I can't control this. I can't control this disease for [my son].” And, of course, then there's the [thought], “Is my kid going to die from a low blood sugar that I slept through, that I didn't catch?”
And it's incredibly … it's hard to describe, but incredibly frustrating, and there's some shame involved in … not being able to do this properly for your child. Even though you know in your rational mind, it's not really possible. But you're always striving to do a better job for your child, for sure.
So there was all that, and as a result, I wasn't sleeping within the first month, I don't think—and that was contributing to my anxiety and my inability to go to work, and it was seriously impacting our quality of life as a family.
And, you know, that level of fear and anxiety takes a toll. Takes a toll on [my son], takes a toll on us, takes a toll on your relationship. You know, it can become your focus. Because it's your child.
Additionally, parents spoke of their contrasting desires to keep their children safe by observing them and testing their blood glucose, but also wanting to allow them independence and to be as “normal” as possible. Parents also reflected on the challenge of ensuring their children were responsible and diligent in their diabetes management. In young children, parents took on this responsibility. However, as their child grew older and gained more independence, parents attempted to cultivate a sense of responsibility in their child to manage their own diabetes, without eliciting resentment or rebellion. Parents reported the emotional burden that this caused and the challenges in allowing their child to manage their condition independently, knowing the potential long-term consequences of poor diabetes management:
The hardest thing for a parent to do is giving their child their independence with a type 1.
I think she might have been maybe 10 or 11, and she went out to a movie with her friends, and she was having so many problems with her blood sugar. I dropped her off, and then I cried because I knew she would have no idea what the movie was about. Her friends were all excited that all the parents let them go to a movie for the first time by themselves, and [my daughter] was going to be there testing her blood sugar and not being able to eat the popcorn…
It scares me because … my boys are just, like, “Yeah, whatever, it's diabetes.” In my head, all I can picture is my friend Kevin; he's got multiple amputations because he didn't care enough. And that's what I don't want for my boys. And, of course, I panic and think about it, like, “Hey, by the way guys, got to check your sugar more.”
To help alleviate these emotional burdens, parents spoke of linking with other parents, often through social media. They often received comfort from other parents. Parents found a huge benefit in being able to reach out and speak about their concerns, their fears, and the challenges they faced in managing their child's diabetes:
For me, a big piece was the emotional healing that I was going through … I needed to know that we weren't alone, because it does feel very isolating if you don't know anybody who's doing this, who's dealing with this. And I wanted to be able to do it better. And so by connecting with other families—there's just a lot of learning to be had.
Other Impacts
Changes to sleeping patterns was the most common impact mentioned by parents of children with type 1 diabetes. Informed of the risk of hypoglycemia overnight (through their health providers or their own research), parents reported the need to wake up multiple times to test their child's blood glucose and make any necessary corrections. This had huge impact on both the parents and the child. A large number of parents reported being so worried about their child's blood glucose that they intentionally kept levels high, reasoning that the long-term effects of high blood glucose were not as dire as the immediate effects of a hypoglycemic event:
Absolutely, and the worst-case scenario is a low in the middle of the night. So, some people who don't have continuous glucose monitoring go with the 12, three, and six program, which means for the duration of the time their child lives in their house, they're going to wake up at midnight, three, and six and check him.
And we weren't getting sleep because we never got to the point [of comfort]. Like, we'd check her at midnight, we'd check her at three, and we kept doing this and doing this, and we were never comfortable not checking her.
It was just not as good management, because we ran him higher. That was the way to be safe. He sat at 14 to 16, 14 to 18, overnight because that was the way to keep him safe, because it's so dangerous for him to go low.
Information About Continuous Glucose Monitoring
Rarely did adult patients or parents of children with type 1 diabetes report that they first heard of continuous glucose monitoring at the time of diagnosis. In fact, patients and families often reported that the information they received about continuous glucose monitoring did not initially come from their health care provider. Often, it was first conveyed through social media support groups for patients and families with type 1 diabetes. Sometimes, continuous glucose monitoring devices were introduced when patients sought out an insulin pump.
While the initial information may not have come from health care providers, both parents and adult patients reported seeking the opinion and approval of their health care team before choosing to invest in continuous glucose monitoring technology:
I didn't even know it existed. I basically sat there and thought, “There's got to be something, some kind of technology out there, that will make this easier,” and I was really surprised the clinic, like I said, didn't suggest it. It's so expensive.
And then obviously we learned about continuous glucose monitoring when we were learning about the pump, because they go hand in hand very often.
Well, we spoke fairly extensively to [a doctor] at Sick Kids, and he spoke of the benefits and any kinds of disadvantages, so it was good advice from him. But I think it was more just our own understanding [that made us choose continuous glucose monitoring].
Barriers to Using Continuous Glucose Monitoring
Financial Barriers
Adult patients and parents of children with type 1 diabetes were asked about their perceptions of the existing barriers to more widespread use of continuous glucose monitoring, and what barriers they had encountered. They consistently reported that the greatest barrier was cost. Even patients who reported that they used their devices in a limited, targeted way to simply learn about their blood glucose patterns often said that they would use the devices more if they were cheaper:
Like I said, we learned about continuous glucose monitoring when she went on the pump, but it wasn't ever something we'd considered, because, well, we're not paying for that, right? And we don't have any outside medical insurance either. We're self-employed farmers, so, you know, it's completely out of pocket.
Patients and parents spoke often of the compromises they made to afford continuous glucose monitoring, such as extending the sensors beyond their recommended usage:
The sensors are only supposed to be used for a week, and if you want to you can go online and find out how to extend that sensor to maybe 2 weeks or 3 weeks, depending on how old the kid is and how active they are. That's what people do.
That money could be groceries or set aside for their education, and that we won't be able to afford, because we're paying for diabetes supplies, so it's a catch-22 for a lot of families, I think. We want it! What are we going to do? Like, in the future, the kids don't go to [college] because we were trying to save their life now with continuous glucose monitoring? Yeah.
Many of those interviewed expressed their gratitude that they had private insurance or could purchase a continuous glucose monitoring device, but acknowledged that there were many families and individuals for whom the cost is simply prohibitive. Often, owing to the social and online connectivity around dealing with type 1 diabetes management, those interviewed knew of other families who could not afford continuous glucose monitoring devices. When interviewed, those who could not afford the devices often expressed their emotional pain and frustration at not being able to provide the care they felt their child required:
You know, we're fortunate in the sense that we can afford it, right? Thankfully. And I say that all the time, because there are families that just can't.
I couldn't possibly afford to pay for that, although if I didn't have the coverage, knowing what I know now and having used it for the last 3½ years, we can't live without it. This is a tool that we absolutely have to have to take care of my child.
She's been wanting it for 2 years, and she just can't afford [a continuous glucose monitoring device]. She actually texted me a month or two ago because overnight her son had had a severe hypoglycemic episode with a seizure, and they had to call 911. He was OK; it doesn't seem to be any permanent damage or anything like that, but it was shattering for her. I mean, she's doing nights all alone, she's doing all of it alone. Those are the stories for me where I think, “There's got to be a better way,” and that a continuous glucose monitoring device shouldn't be a luxury item. It should be accessible for everybody, in my mind.
And you want, you really want, to do your best, the best for your child. You want to offer your child the best that's available, right? And sometimes you can't, and that's horrible, right? You should be able to give them the best kind of therapy.
On occasion, when speaking of the prohibitive cost of continuous glucose monitoring, patients lamented that their insulin pump was funded by insurance or through government funding, but not the continuous glucose monitoring device. A large number of patients spoke about choosing a continuous glucose monitoring device over a pump, if given the choice:
And we love the benefit of the pump. Like, it's very easy to just roll with whatever she wants to eat, and it's very convenient. But we have always said if we ever have to give up a device, it would be the pump. We would never give up the continuous glucose monitoring device, because that information that we have access to is invaluable to us.
I would give up my pump before my continuous glucose monitoring device if I had to. Yeah. And pumps are great. They offer us a ton of flexibility. But from a safety and peace-of-mind standpoint, the continuous glucose monitoring device is my comfort.
Other Barriers
While cost was the barrier most often mentioned by adult patients and parents of children with type 1 diabetes, it was not the only barrier. A number of those interviewed spoke about the lack of information about continuous glucose monitoring. People also felt that perhaps adult patients who had managed their diabetes one way for many years were reluctant to use a newer technology:
I have a mom with type 1 and a son with type 1, so two generations apart. My mom uses multiple daily injections. She doesn't use a pump. While she is really, really glad that [my son] has a continuous glucose monitoring device, she doesn't have one. Well, for her, cost is a huge barrier for the device. And she's been type 1 since she was 51; she's now 75. So there is a feeling of, “I've done this.”
And I think that there would be the unknown, maybe just not enough information or not having tried it yet to see what the benefits were.
I mean not everybody can afford [it], and the knowledge is not there. I mean, obviously, the older generation probably doesn't even know that this is out there, that it's available, that it's easy. People might be intimidated by technology.
Use of Continuous Glucose Monitoring
Those who were able to afford and use continuous glucose monitoring reported that the benefits were numerous and important. However, occasionally the value of continuous glucose monitoring was different for adult patients and parents. Often, this difference could be attributed to the level of comfort in managing diabetes. Adults may have been managing their diabetes for many years, and continuous glucose monitoring was only the latest in a long line of tools used to assist in their management. For parents of newly diagnosed children, however, continuous glucose monitoring was more than a tool. It served as way to keep their child safe and healthy.
Many of those interviewed—both adults and parents of children with type 1 diabetes—spoke of an unauthorized feature of a particular continuous glucose monitoring device, known as Nightscout. With this feature, it was possible to program the device to wirelessly transmit blood glucose readings to other electronic devices, such as smart phones or smart watches. This feature was originally developed by parents and has since spread widely via the Internet and social media.
Nightscout was not an original feature of this particular continuous glucose monitoring device, but a large number of people with diabetes and parents reported its benefits. The next generation of continuous glucose monitoring devices is expected to include the ability to transmit readings wirelessly, much as Nightscout has done for the older device.
Overall, the benefits of continuous glucose monitoring, including the Nightscout feature, fell into three general categories: social, emotional, and medical and safety benefits.
Social Benefits
Adult patients who used a continuous glucose monitoring device spoke less often about its social benefits than parents of children with diabetes. Being able to check blood glucose levels discreetly instead of using finger pricks and a blood glucose meter was mentioned as a nice option, but adult patients placed less emphasis on it. They seemed less concerned with the social impact of their diabetes management, although they appreciated the ability to manage it discreetly. In addition, they reported that continuous glucose monitoring could have a positive impact on their employment, especially if work required long periods in a car or travelling:
I'm 50 years old. I don't really care about people around me, so if they don't like me testing in front of them, that's too bad, whether I'm in a restaurant or in a board meeting. I mean, it's a little bit uncomfortable, and you have to interrupt people in the middle of their lectures or whatever, [and] everybody stops and looks at you. At this point, I don't really worry about it.
What I see with continuous glucose monitoring is the freedom of being able to know what your glucose is at all times, for exercising … sometimes it hinders me in doing things because I have to drag along my test kit. I have to stop in the middle of my walks or my exercise, in the middle of a class, where I have to interrupt everybody.
In contrast, parents of children with type 1 diabetes often spoke of the social freedom that continuous glucose monitoring provided for their children. They perceived it as being very beneficial for their child's quality of life. Parents often expressed the desire to minimize the impact that diabetes would have on their child's life—to allow their child to be as “normal” as possible. Many parents felt that continuous glucose monitoring allowed their child to get closer to this ideal; they could manage their diabetes in a way that was as socially unobtrusive as possible.
Parents also felt more comfortable allowing their child a larger degree of freedom. The Nightscout feature mentioned above enhanced this by allowing remote monitoring of blood glucose levels:
It gives her independence … She didn't have her mother constantly over her shoulder. And what happens when mom's constantly over your shoulder after your diagnosis? You start to resent type 1. You know what I mean?
But now … I can say yes a lot more, she can have freedom to be out. And there's also a—you know, as a 13-year-old girl, with anything, you want to be the same as everybody else. You don't want to stand out at all.
Diabetes interrupts and disrupts; continuous glucose monitoring took away a lot of that disruption and interruption.
This social benefit extended to others in the circle of the child's care. Parents reported that teachers, sport coaches, friends, and other family members were more comfortable being responsible for a child with type 1 diabetes when that child had a continuous glucose monitoring device. Continuous glucose monitoring allowed for easier management of diabetes, providing greater information about blood glucose levels and reporting trends, thereby reducing the potential need for drastic intervention by carers:
[With Nightscout,] we're alerted when she's high or low, so we can help her deal with it from anywhere. So that comforts her coaches and her teachers and everybody as well, knowing that she's going to be alerted and she's not just going to drop and hit the floor.
And I have a friend who I'm eternally grateful to, because continuous glucose monitoring made her comfortable enough to say, “I want to have [your son] for a sleepover,” and we'd never had that before, ever. So it meant that he could go and do things that kids do all the time, and it meant that we could back off a little.
Emotional Benefits
Many parents reported the fear, anxiety, and sense of failure they felt in trying to care for their child with type 1 diabetes. Constantly trying to manage the fluctuations of blood glucose was described as exhausting and frustrating. Parents who were able to provide continuous glucose monitoring for their child reported a noticeable reduction in these emotions. Continuous glucose monitoring allowed a sense of safety and security that had been lacking.
Parents also reported their increased comfort in allowing their child to grow and manage their diabetes more independently because of continuous glucose monitoring:
Then, after my maternity leave ended, I thought, “You know, I can't put her in someone else's care. I can't relinquish this and trust that she will still be okay.” But with [continuous glucose monitoring] and getting comfortable with that, we started to be able to relinquish some of that need to monitor her ourselves. She actually went to preschool, which we didn't think she would do.
So as much as it gave to me as a parent, in that safety net and not worrying, it gave to my child that sense … the normalizing of what it's like to be a normal 10-year-old kid …
We've kind of said it, but just to reiterate, it has changed our lives, especially for [our son] for nighttime, especially for school. It's just that peace of mind. Just to have other people look after him, it gives us peace of mind, too.
My job is to raise my children to be good people and to be the best they can be. But with my daughter, it felt good to teach her how to manage her chronic lifelong illness. And having the continuous glucose monitor there to explain to an 8-, 9-, 10-year-old what happens when you eat, it's concrete. That was a turning point as well for us, because she started to connect what she eats with her blood sugar.
While the emotional benefits of continuous glucose monitoring were reported most often by parents of children with diabetes, a number of adult patients also remarked on the comfort it provided:
I also suffer from stress and anxiety, which is caused a lot by my health issues, and knowing what your blood sugar is all the time really helps, because, you know, I would test 10 to 20 times a day to keep my blood sugar in very good control. And so, being able to look at my [continuous glucose monitoring device] all the time, and being able to know what my blood sugar is, has definitely improved my life, [and] the way I live my life.
If either [my son with type 1 diabetes] or I don't have [continuous glucose monitoring] for even a couple of hours, we feel really weird, because we're so used to having information. And it's almost like, well, I can't survive without it, but, yeah, I find it really beneficial as well.
Medical and Safety Benefits
Beyond the social and emotional benefits of continuous glucose monitoring, both adults and parents of children with type 1 diabetes were far more likely to report the perceived medical and safety benefits of the device. Adult patients who had been managing their diabetes for many years often described continuous glucose monitoring as a useful educational tool to learn about their own body and how different factors affected their blood glucose levels:
This is just another tool that helps keep me away from hospitals.
Given my background, I know these continuous glucose monitoring devices are just a tool. They're never to be trusted 100%; so we still test his blood sugar when we don't get the reading. It's more helpful, I find, to get the trends, to find out when he goes up and when he goes down.
This ability to see trends of blood glucose levels rather than the isolated data points provided by finger-prick monitoring was a common feature perceived to have enormous medical benefit. This perception on the part of patients and parents was consistent with clinical findings on the benefits of continuous glucose monitoring. People reported that knowing trends allowed for more aggressive insulin dosing; parents and adult patients could use the trend data to adjust dosing immediately, rather than waiting for the next finger prick. This was felt to be especially helpful in children—parents felt that changes in hormones, activities, and diets often caused wild fluctuations in blood glucose levels. The increased comfort with aggressive insulin dosing helped patients keep their A1C (glycated hemoglobin) levels lower, to the overall benefit of their health.
I was always going high overnight, and I didn't know it, because I wasn't waking up in the middle of the night to test myself. When I saw that … consistently happening, then I could start taking action to address that. And that in itself allowed me to improve my A1C fairly significantly.
For [my son,] I'm making adjustments sometimes on a daily basis, based on what his blood sugar is doing. For me, having [continuous glucose monitoring], honestly, I couldn't do it without it, because I'm able to get that data reporting just by plugging it in, and I can see what the trends are, and I can make adjustments on the fly so easily.
I think every parent tries for a good A1C. And so it's allowed us to improve significantly, I believe, with continuous glucose monitoring, because I make changes more frequently, and I can see trends more easily, and so we have been able to get better control. So I think, like, when he was diagnosed he was 9.1, and his last one was 6.8, which is good.
With [continuous glucose monitoring,] I can be far more aggressive with [dosing] to bring down her high blood sugar. Like, if I talk to people, if they don't have a continuous glucose monitoring device, I would never suggest that to them, because too much insulin can kill them. Without a [continuous glucose monitoring device], I would never risk it, ever.
A number of parents of children with type 1 diabetes reported that prior to continuous glucose monitoring, they had kept their child's blood glucose deliberately high, in fear of hypoglycemic events. Parents knew that this was putting their child at risk for long-term complications, but felt it was safer than risking low blood glucose levels in the short term. Continuous glucose monitoring helped to alleviate this risk, allowing better management decisions and better blood glucose control:
You are shooting in the dark without continuous glucose monitoring. You cannot make educated choices about treatment without continuous glucose monitoring … Without a continuous glucose monitoring device, you kind of have to run them higher.
Like, that 5.5 [mmol/L], is that a steady 5.5 and she's going to be OK, or is that a 5.5 and she's dropping quickly? You just don't know off that one piece of information. Whereas with [continuous glucose monitoring], you have the trends and the directions, and it allows you to adjust your reactions to suit it.
So I said, “Do you want me to wake you up? I can keep on checking and wake you up.” She says, “No, I want to sleep, because sleep is precious.” So she just says, “Let me run myself high so I don't have to worry, and I can actually sleep through the night and I'll wake up in the morning.” And it breaks my heart for me to hear my 17-year-old say that.
Commonly, both patients and parents commented that more information was helpful in managing the diabetes in the short and long term. Continuous glucose monitoring provided that information. It allowed for data tracking and uploading to computers, which was useful for seeing longer-term trends and make adjustments:
When I was seeing my A1C dropping, it was making me really happy, and I was glad to be on [continuous glucose monitoring]. But at the same time, I do always think about the long term and the best systems I can use … to make sure that I have the best control I have now, because I know it's going to affect later on. So seeing that it was impacting my A1C right now, I knew that even that was beneficial for the future.
But we knew, we said, “We've researched it, this is what we want, like, we can cope better with the more information we have.” It's the unknown that makes us nervous.
From a safety perspective, parents of children with diabetes were almost universal in their praise for continuous glucose monitoring. And while adult patients were less effusive, they also emphasized the safety benefit. This was even more pronounced for those using the Nightscout feature. The overwhelming fear of a hypoglycemic event was mostly mitigated by continuous glucose monitoring; alarms would sound if the blood glucose decreased to a set level, alerting individuals to remedy the situation. This had a large impact on nighttime diabetes management.
This safety net brought incredible relief to a majority of those interviewed and was often described as life-changing. For children or adult patients with nocturnal (nighttime) hypoglycemia or hypoglycemia unawareness (inability to “feel” a low blood glucose), continuous glucose monitoring alleviated the fear of a sudden hypoglycemic event. Several patients had experienced an extreme blood glucose event and lamented the fact that they had not had access to continuous glucose monitoring at the time:
I mean, the first year before continuous glucose monitoring, I just didn't sleep. It was just constantly finger poking him and, really, this sense [that] I just had no control over this disease. I had absolutely no idea when he was going to go low. I didn't know when he was going to go high.
He does not feel a low blood sugar. And that's dangerous and deadly, especially at night, when he will not wake up from a low blood sugar. This [continuous glucose monitoring device] has saved his life numerous times. If we don't have [continuous glucose monitoring], I'm poking him every hour just to see where his blood sugar is at.
I wasn't wearing a sensor, because my money was going to [my daughter] for her to wear a sensor, because there's only so much money … And I was not wearing the sensor, and my pump got disconnected in the middle of the night and I went into diabetic ketoacidosis, and that is a life-threatening situation, and it was pretty horrendous and pretty awful. I mean, unfortunately, I know somebody who died in that situation. But all I was thinking of as I was vomiting the next morning is, “If I'd only had a sensor on, this wouldn't have happened, because the sensor would have woken me up when my blood sugar went high.”
For adult patients, the continuous glucose monitoring also provided safety by alerting them to potential hypoglycemic events when family or friends were not present:
Also, now I'm separated, so I live by myself, which is a big worry for me, because if you have someone there with you, especially a spouse that's in the same room, they can wake you or see signs of something going on. But when you're by yourself, it's a big worry.
Concerns With the Use of Continuous Glucose Monitoring
A minority of patients and families expressed concerns about using continuous glucose monitoring, or reasons for not using it. These reasons included “alarm fatigue” and overwhelming data. Additionally, patients reported that older models were more inaccurate, potentially leading to a loss of trust in the displayed results. Some simply used continuous glucose monitoring as a targeted tool for a limited amount of time. Removing the device or choosing to not use continuous glucose monitoring was much more common among adults than among parents of children with type 1 diabetes, although numbers in both groups were relatively low in this study. Having managed diabetes for many years without continuous glucose monitoring, some adults were likely to contemplate ceasing to use the device:
There's been some times when I've actually taken the continuous glucose monitoring device off for a couple of weeks. I've just had enough, because you're trying to sleep, and it's telling you you're low and … it's about a 20-minute delay.
So even though you may have brought your blood sugar up, it's still going, “You're low!” and it's like, “Oh, shut the hell up.”
But some of the drawbacks to it, too, were sometimes that information gave us more cause for anxiety. You know, like, you tend to micromanage things more.
Yeah, well, because of the cost, really … and because I've been diabetic for so long, I kind of get into a routine, so I wasn't as quick to see the benefits.
A few parents of children with type 1 diabetes spoke of an initial hesitation and the challenges of inserting the continuous glucose monitoring device and having their child accept it. It became a new device to carry and be responsible for, and occasionally this was seen to be too much of a burden. Some people got over such hesitation quickly, but for others it was a long-standing concern. Parents of teenagers were especially concerned about their child's dedication to managing their diabetes and whether continuous glucose monitoring was seen as a hassle:
For me, I needed a couple of months to kind of get over the fact that it'd be a thing on him all the time, which is pretty common. It's a common obstacle for parents to get over.
So, it was a lot for him, and it was actually [my son] that asked to stop wearing it. Because he found it to be bulky, cumbersome, and all of the stuff that he had to carry for us to be able to see what his data was. We tried the Nightscout, and it was just too much stuff for him to have to carry around and be accountable for.
We have a very delicate balance with these kids to keep them normal, and the last thing I want is a child rebelling against their [continuous glucose monitoring device] and diabetes diagnosis, because when they refuse to take insulin because they hate diabetes that much, it becomes very dangerous. You can't miss doses, you know what I mean?
Discussion
Patient engagement surrounding the topic of continuous glucose monitoring was robust. We interviewed many adults with type 1 diabetes and parents of children with type 1 diabetes. Additionally, we held two focus groups, allowing for the discussion of themes and perspectives related to continuous glucose monitoring. Patients who had direct experience with a continuous glucose monitoring device were able to compare their experiences with those of usual care, such as finger pricks and a blood glucose meter.
Those interviewed were overwhelmingly supportive of continuous glucose monitoring and the many benefits it provides for the management of type 1 diabetes. Both adults and parents of children with type 1 diabetes reported the great impact the disease had on their daily activities and quality of life. They emphasized the positive effects of continuous glucose monitoring, including social, emotional, and medical and safety benefits.
We did not discuss the specific benefits of particular brands of devices as part of patient engagement for this topic. However, a number of patients spoke of the increased benefit they received from a particular device because of its unofficial feature known as Nightscout. This feature allowed wireless transmission of blood glucose data to multiple receivers, which patients and parents felt provided greater independence and increased safety.
While most of those interviewed were positively inclined toward continuous glucose monitoring, upon further probing some identified concerns or challenges related to using the devices. They reported hearing these concerns from friends or connections through social media or support groups. Such concerns included inaccurate readings (especially with older generations of devices), hesitation to adopt a new technology, difficulty understanding the data provided by the devices, and slow acceptance from the health care system.
However, nearly all of those interviewed felt that these concerns were minor and were overshadowed by the many benefits of continuous glucose monitoring. Many patients stated that continuous glucose monitoring was an essential part of their diabetes management, and they would not consider managing their diabetes without it.
Conclusions
Adult patients and parents of children with type 1 diabetes reported very positive experiences with continuous glucose monitoring. Patients perceived that these devices provided important social, emotional, and medical and safety benefits in managing type 1 diabetes, especially in children.
The high ongoing cost of continuous glucose monitoring devices was seen as the greatest barrier to their widespread use.
CONCLUSIONS OF THIS HEALTH TECHNOLOGY ASSESSMENT
Continuous glucose monitoring was more effective than self-monitoring of blood glucose in managing type 1 diabetes for some outcomes, such as time spent in target glucose range and time spent outside the target glucose range (quality of evidence: moderate). We obtained similar findings for severe hypoglycemic events, although the findings were less certain because the quality of the evidence was low.
Compared with self-monitoring of blood glucose, the costs of continuous glucose monitoring were higher, with relatively small increases in observed health benefits. Publicly funding continuous glucose monitoring for the type 1 diabetes population in Ontario would result in additional costs to the health system over the next 5 years.
Adult patients and parents of children with type 1 diabetes reported very positive experiences with continuous glucose monitoring. The high ongoing cost of continuous glucose monitoring devices was seen as the greatest barrier to their widespread use.
Acknowledgments
The medical editors were Susan Harrison, Jeanne McKane, and Kara Stahl; others involved in the development and production of this report were Merissa Mohamed, Claude Soulodre, Kellee Kaulback, Alison Clement, Ana Laing, Andrée Mitchell, Vivian Ng, Sarah McDowell, Anil Thota, Nancy Sikich, and Irfan Dhalla.
We are grateful to Tony Chetty and Margaret Lawson for providing expert consultative advice on earlier drafts of the report. The statements, conclusions, and views expressed in this report do not necessarily represent the views of the experts consulted.
ABBREVIATIONS
- A1C
Glycated hemoglobin
- GRADE
Grading of Recommendations Assessment, Development, and Evaluation
- ICER
Incremental cost-effectiveness ratio
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- QALY
Quality-adjusted life-year
- RoBANS
Risk of Bias Assessment Tool for Non-randomized Studies
APPENDICES
Appendix 1: Literature Search Strategies
Clinical Evidence Search
Clinical Literature Search—Continuous Glucose Monitoring
Search requested by: Stacey Vandersluis
Search date: January 24, 2017
Librarian: Corinne Holubowich
Databases searched: Ovid MEDLINE, Embase, Cochrane Database of Systematic Reviews, Database of Abstracts of Reviews of Effects, CRD Health Technology Assessment Database, Cochrane Central Register of Controlled Trials, NHS Economic Evaluation Database, and CINAHL
Database: EBM Reviews - Cochrane Central Register of Controlled Trials <November 2016>, EBM Reviews - Cochrane Database of Systematic Reviews <2005 to January 18, 2017>, EBM Reviews - Database of Abstracts of Reviews of Effects <1st Quarter 2015>, EBM Reviews - Health Technology Assessment <4th Quarter 2016>, EBM Reviews - NHS Economic Evaluation Database <1st Quarter 2015>, Embase <1980 to 2017 Week 04<, Epub Ahead of Print, In-Process & Other Non-Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) <1946 to Present>
Search Strategy:
-
1
exp Diabetes Mellitus, Type 1/ (176236)
-
2
((diabet* adj3 (typ* 1 or typ* i or type1 or typei or typ* one or brittl* or juvenil* or pediatric or paediatric or early or keto* or labil* or acidos* or autoimmun* or auto immun* or sudden onset or young onset)) or (insulin* adj2 depend*) or insulindepend* or dm1 or dm 1 or dmt1 or dm t1 or t1dm or t1 dm or t1d or iddm).ti,ab,kf. (232927)
-
3
Diabetic Ketoacidosis/ (15987)
-
4
(ketoacidosis or acidoketosis or keto acidosis or ketoacidemia or ketosis).ti,ab,kf. (20760)
-
5
Hypoglycemia/ (95050)
-
6
Diabetes Mellitus/ (672266)
-
7
5 and 6 (25348)
-
8
((hypoglyc?em* or ((low or lower or decreas* or deficien* or insufficien* or reduce* or reduction* or fluctuat* or fallen or falling or threshold or safe) adj3 (glucose* or sugar* or hba1c or hb a1 or hba1 or a1c or h?emoglob* or glycoh?emoglob*))) adj5 (diabet* or IDDM or DM)).ti,ab,kf. (20611)
-
9
or/1–4,7–8 (326151)
-
10
Blood Glucose Self-Monitoring/ (21053)
-
11
(continu* or uninterrupt* or ongoing or looped or interminable).ti,ab,kf. (2393790)
-
12
10 and 11 (4475)
-
13
((continu* or uninterrupt* or ongoing or looped or interminable) adj4 (blood glucose or blood sugar*) adj2 (self monitor* or home monitor*)).ti,ab,kf. (104)
-
14
((continu* or uninterrupt* or ongoing or looped or interminable) adj4 SMBG).ti,ab,kf. (128)
-
15
(CGM or CGMS or continuous glucose monitor* or continuous glucose sensor*).ti,ab,kf. (9984)
-
16
((medtronic adj3 (paradigm* or glucose monitor* or glucose sensor* or CGM or insulin pump* or LGS or 630G)) or veo or veotm or minimed or dexcom or g4 platinum or g5 platinum).ti,ab,kf. (1787)
-
17
((flash or novel) adj2 glucose adj2 (monitor* or sensor or sensing)).ti,ab,kf. (182)
-
18
((integrat* adj2 (pump or pumps or infusion*)) or (sensor adj3 (pump or pumps or therap* or infusion*)) or (sensor augment* adj2 pump*) or low glucose suspend*).ti,ab,kf. (1538)
-
19
(closed loop adj2 (pump* or deliver* or infus* or therap* or treatment* or system* or sensor* or control* or monitor* or hybrid*)).ti,ab,kf. (7164)
-
20
Pancreas, Artificial/ (2671)
-
21
(artificial adj3 (pancreas or beta cell*)).ti,ab,kf. (3436)
-
22
or/12–21 (22784)
-
23
9 and 22 (9180)
-
24
exp Animals/ not Humans/ (16218972)
-
25
23 not 24 (5561)
-
26
Case Reports/ or Comment.pt. or Editorial.pt. or Letter.pt. or Congresses.pt. (5047347)
-
27
25 not 26 (5308)
-
28
limit 27 to english language [Limit not valid in CDSR,DARE; records were retained] (4886)
-
29
limit 28 to yr=“2010 -Current” [Limit not valid in DARE; records were retained] (2863)
-
30
29 use ppez,cctr,coch,dare,clhta,cleed (2282)
-
31
exp insulin dependent diabetes mellitus/ (176079)
-
32
((diabet* adj3 (typ* 1 or typ* i or type1 or typei or typ* one or brittl* or juvenil* or pediatric or paediatric or early or keto* or labil* or acidos* or autoimmun* or auto immun* or sudden onset or young onset)) or (insulin* adj2 depend*) or insulindepend* or dm1 or dm 1 or dmt1 or dm t1 or t1dm or t1 dm or t1d or iddm).tw,kw. (239526)
-
33
diabetic ketoacidosis/ (15987)
-
34
(ketoacidosis or acidoketosis or keto acidosis or ketoacidemia or ketosis).tw,kw. (21100)
-
35
hypoglycemia/ (95050)
-
36
diabetes mellitus/ (672266)
-
37
35 and 36 (25348)
-
38
((hypoglyc?em* or ((low or lower or decreas* or deficien* or insufficien* or reduce* or reduction* or fluctuat* or fallen or falling or threshold or safe) adj3 (glucose* or sugar* or hba1c or hb a1 or hba1 or a1c or h?emoglob* or glycoh?emoglob*))) adj5 (diabet* or IDDM or DM)).tw,kw. (21910)
-
39
or/31–34,37–38 (332387)
-
40
blood glucose monitoring/ (21276)
-
41
((continu* or uninterrupt* or ongoing or looped or interminable) adj5 (self* or home*)).tw,kw,dv. (16003)
-
42
40 and 41 (116)
-
43
((continu* or uninterrupt* or ongoing or looped or interminable) adj4 (blood glucose or blood sugar*) adj2 (self monitor* or home monitor*)).tw,kw,dv. (106)
-
44
((continu* or uninterrupt* or ongoing or looped or interminable) adj4 SMBG).tw,kw,dv. (132)
-
45
(CGM or CGMS or continuous glucose monitor* or continuous glucose sensor*).tw,kw,dv. (10250)
-
46
((medtronic adj3 (paradigm* or glucose monitor* or glucose sensor* or CGM or insulin pump* or LGS or 630G)) or veo or veotm or minimed or dexcom or g4 platinum or g5 platinum).tw,kw,dv. (2387)
-
47
((flash or novel) adj2 glucose adj2 (monitor* or sensor or sensing)).tw,kw,dv. (184)
-
48
((integrat* adj2 (pump or pumps or infusion*)) or (sensor adj3 (pump or pumps or therap* or infusion*)) or (sensor augment* adj2 pump*) or low glucose suspend*).tw,kw,dv. (1589)
-
49
(closed loop adj2 (pump* or deliver* or infus* or therap* or treatment* or system* or sensor* or control* or monitor* or hybrid*)).tw,kw,dv. (7269)
-
50
artificial pancreas/ (2671)
-
51
(artificial adj3 (pancreas or beta cell*)).tw,kw,dv. (3492)
-
52
or/42–51 (21829)
-
53
39 and 52 (8862)
-
54
(exp animal/ or nonhuman/) not exp human/ (10585394)
-
55
53 not 54 (8708)
-
56
Case Report/ or Comment/ or Editorial/ or Letter/ or conference abstract.pt. (9318976)
-
57
55 not 56 (6317)
-
58
limit 57 to english language [Limit not valid in CDSR,DARE; records were retained] (5819)
-
59
limit 58 to yr=“2010 -Current” [Limit not valid in DARE; records were retained] (4070)
-
60
59 use emez (1754)
-
61
30 or 60 (4036)
-
62
61 use ppez (1766)
-
63
61 use emez (1754)
-
64
61 use coch (2)
-
65
61 use cctr (493)
-
66
61 use clhta (10)
-
67
61 use cleed (4)
-
68
61 use dare (7)
-
69
remove duplicates from 61 (2194)
CINAHL
| # | Query | Results |
| S1 | (MH “Diabetes Mellitus, Type 1+”) | 17,154 |
| S2 | ((diabet* N3 (typ* 1 or typ* i or type1 or typei or typ* one or brittl* or juvenil* or pediatric or paediatric or early or keto* or labil* or acidos* or autoimmun* or auto immun* or sudden onset or young onset)) or (insulin* N2 depend*) or insulindepend* or dm1 or dm 1 or dmt1 or dm t1 or t1dm or t1 dm or t1d or iddm) | 26,754 |
| S3 | (MH “Diabetic Ketoacidosis”) | 1,653 |
| S4 | (ketoacidosis or acidoketosis or keto acidosis or ketoacidemia or ketosis) | 2,266 |
| S5 | (MH “Hypoglycemia”) | 6,680 |
| S6 | (MH “Diabetes Mellitus”) | 46,253 |
| S7 | S5 AND S6 | 1,343 |
| S8 | ((hypoglyc#em* or ((low or lower or decreas* or deficien* or insufficien* or reduce* or reduction* or fluctuat* or fallen or falling or threshold or safe) N3 (glucose* or sugar* or hba1c or hb a1 or hba1 or a1c or h#emoglob* or glycoh#emoglob*))) N5 (diabet* or IDDM or DM)) | 4,751 |
| S9 | S1 OR S2 OR S3 OR S4 OR S7 OR S8 | 30,991 |
| S10 | (MH “Blood Glucose Self-Monitoring”) | 2,779 |
| S11 | (continu* or uninterrupt* or ongoing or looped or interminable) | 272,534 |
| S12 | S10 AND S11 | 537 |
| S13 | ((continu* or uninterrupt* or ongoing or looped or interminable) N4 (blood glucose or blood sugar*) N2 (self monitor* or home monitor*)) | 26 |
| S14 | ((continu* or uninterrupt* or ongoing or looped or interminable) N4 SMBG) | 25 |
| S15 | (CGM or CGMS or continuous glucose monitor* or continuous glucose sensor*) | 1,176 |
| S16 | ((medtronic N3 (paradigm* or glucose monitor* or glucose sensor* or CGM or insulin pump* or LGS or 630G)) or veo or veotm or minimed or dexcom or g4 platinum or g5 platinum) | 205 |
| S17 | ((flash or novel) N2 glucose N2 (monitor* or sensor or sensing)) | 17 |
| S18 | ((integrat* N2 (pump or pumps or infusion*)) or (sensor N3 (pump or pumps or therap* or infusion*)) or (sensor augment* N2 pump*) or low glucose suspend*) | 199 |
| S19 | (closed loop N2 (pump* or deliver* or infus* or therap* or treatment* or system* or sensor* or control* or monitor* or hybrid*)) | 393 |
| S20 | (artificial N3 (pancreas or beta cell*)) | 197 |
| S21 | S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 | 1,971 |
| S22 | S9 AND S21 | 1,031 |
| S23 | (MH “Animals+”) OR (MH “Rodents+”) | 123,580 |
| S24 | S22 NOT S23 | 1,021 |
| S25 | PT Case Study or Commentary or Editorial or Letter or Proceedings | 389,044 |
| S26 | S24 NOT S25 | 982 |
| S27 | S24 NOT S25 Limiters - English Language | 979 |
| S28 | S24 NOT S25 Limiters - Published Date: 20100101-20171231; English Language | 681 |
Economic Evidence Search
Search requested by: Sandjar Djalalov
Librarians: Corinne Holubowich
Economic Evaluation and Cost Effectiveness Search
Search date: January 25, 2017
Databases searched: Ovid MEDLINE, Embase, Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, Database of Abstracts of Reviews of Effects (DARE), Centre for Reviews and Dissemination (CRD) Health Technology Assessment Database, National Health Service (NHS) Economic Evaluation Database and Cumulative Index to Nursing and Allied Health Literature (CINAHL)
Database: EBM Reviews - Cochrane Central Register of Controlled Trials <November 2016>, EBM Reviews - Cochrane Database of Systematic Reviews <2005 to January 18, 2017>, EBM Reviews - Database of Abstracts of Reviews of Effects <1st Quarter 2015>, EBM Reviews - Health Technology Assessment <4th Quarter 2016>, EBM Reviews - NHS Economic Evaluation Database <1st Quarter 2015>, Embase <1980 to 2017 Week 04>, Epub Ahead of Print, In-Process & Other Non-Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) <1946 to Present>
Search Strategy:
-
1
exp Diabetes Mellitus, Type 1/ (176240)
-
2
((diabet* adj3 (typ* 1 or typ* i or type1 or typei or typ* one or brittl* or juvenil* or pediatric or paediatric or early or keto* or labil* or acidos* or autoimmun* or auto immun* or sudden onset or young onset)) or (insulin* adj2 depend*) or insulindepend* or dm1 or dm 1 or dmt1 or dm t1 or t1dm or t1 dm or t1d or iddm).ti,ab,kf. (232943)
-
3
Diabetic Ketoacidosis/ (15987)
-
4
(ketoacidosis or acidoketosis or keto acidosis or ketoacidemia or ketosis).ti,ab,kf. (20764)
-
5
Hypoglycemia/ (95050)
-
6
Diabetes Mellitus/ (672276)
-
7
5 and 6 (25348)
-
8
((hypoglyc?em* or ((low or lower or decreas* or deficien* or insufficien* or reduce* or reduction* or fluctuat* or fallen or falling or threshold or safe) adj3 (glucose* or sugar* or hba1c or hb a1 or hba1 or a1c or h?emoglob* or glycoh?emoglob*))) adj5 (diabet* or IDDM or DM)).ti,ab,kf. (20615)
-
9
or/1–4,7–8 (326173)
-
10
Blood Glucose Self-Monitoring/ (21053)
-
11
(continu* or uninterrupt* or ongoing or looped or interminable).ti,ab,kf. (2394149)
-
12
10 and 11 (4475)
-
13
((continu* or uninterrupt* or ongoing or looped or interminable) adj4 (blood glucose or blood sugar*) adj2 (self monitor* or home monitor*)).ti,ab,kf. (103)
-
14
((continu* or uninterrupt* or ongoing or looped or interminable) adj4 SMBG).ti,ab,kf. (127)
-
15
(CGM or CGMS or continuous glucose monitor* or continuous glucose sensor*).ti,ab,kf. (9984)
-
16
((medtronic adj3 (paradigm* or glucose monitor* or glucose sensor* or CGM or insulin pump* or LGS or 630G)) or veo or veotm or minimed or dexcom or g4 platinum or g5 platinum).ti,ab,kf. (1788)
-
17
((flash or novel) adj2 glucose adj2 (monitor* or sensor or sensing)).ti,ab,kf. (182)
-
18
((integrat* adj2 (pump or pumps or infusion*)) or (sensor adj3 (pump or pumps or therap* or infusion*)) or (sensor augment* adj2 pump*) or low glucose suspend*).ti,ab,kf. (1538)
-
19
(closed loop adj2 (pump* or deliver* or infus* or therap* or treatment* or system* or sensor* or control* or monitor* or hybrid*)).ti,ab,kf. (7164)
-
20
Pancreas, Artificial/ (2671)
-
21
(artificial adj3 (pancreas or beta cell*)).ti,ab,kf. (3436)
-
22
or/12–21 (22784)
-
23
9 and 22 (9179)
-
24
economics/ (255645)
-
25
economics, medical/ or economics, pharmaceutical/ or exp economics, hospital/ or economics, nursing/ or economics, dental/ (787307)
-
26
economics.fs. (426430)
-
27
(econom* or price or prices or pricing or priced or discount* or expenditure* or budget* or pharmacoeconomic* or pharmaco-economic*).tw. (762124)
-
28
exp “costs and cost analysis”/ (555558)
-
29
cost*.ti. (255725)
-
30
cost effective*.tw. (277253)
-
31
(cost* adj2 (util* or efficacy* or benefit* or minimi* or analy* or saving* or estimate* or allocation or control or sharing or instrument* or technolog*)).ab. (174616)
-
32
models, economic/ (167652)
-
33
markov chains/ or monte carlo method/ (72304)
-
34
(decision adj1 (tree* or analy* or model*)).tw. (37669)
-
35
(markov or markow or monte carlo).tw. (113113)
-
36
quality-adjusted life years/ (34238)
-
37
(QOLY or QOLYs or HRQOL or HRQOLs or QALY or QALYs or QALE or QALEs).tw. (58790)
-
38
((adjusted adj (quality or life)) or (willing* adj2 pay) or sensitivity analys*s).tw. (111268)
-
39
or/24–38 (2463705)
-
40
23 and 39 (636)
-
41
40 use ppez,cctr,coch,dare,clhta (186)
-
42
23 use cleed (4)
-
43
or/41–42 (190)
-
44
limit 43 to english language [Limit not valid in CDSR,DARE; records were retained] (187)
-
45
limit 44 to yr=“2010 -Current” [Limit not valid in DARE; records were retained] (143)
-
46
exp insulin dependent diabetes mellitus/ (176083)
-
47
((diabet* adj3 (typ* 1 or typ* i or type1 or typei or typ* one or brittl* or juvenil* or pediatric or paediatric or early or keto* or labil* or acidos* or autoimmun* or auto immun* or sudden onset or young onset)) or (insulin* adj2 depend*) or insulindepend* or dm1 or dm 1 or dmt1 or dm t1 or t1dm or t1 dm or t1d or iddm).tw,kw. (239545)
-
48
diabetic ketoacidosis/ (15987)
-
49
(ketoacidosis or acidoketosis or keto acidosis or ketoacidemia or ketosis).tw,kw. (21104)
-
50
hypoglycemia/ (95050)
-
51
diabetes mellitus/ (672276)
-
52
50 and 51 (25348)
-
53
((hypoglyc?em* or ((low or lower or decreas* or deficien* or insufficien* or reduce* or reduction* or fluctuat* or fallen or falling or threshold or safe) adj3 (glucose* or sugar* or hba1c or hb a1 or hba1 or a1c or h?emoglob* or glycoh?emoglob*))) adj5 (diabet* or IDDM or DM)).tw,kw. (21914)
-
54
or/46–49,52–53 (332412)
-
55
blood glucose monitoring/ (21276)
-
56
((continu* or uninterrupt* or ongoing or looped or interminable) adj5 (self* or home*)).tw,kw,dv. (16009)
-
57
55 and 56 (116)
-
58
((continu* or uninterrupt* or ongoing or looped or interminable) adj4 (blood glucose or blood sugar*) adj2 (self monitor* or home monitor*)).tw,kw,dv. (105)
-
59
((continu* or uninterrupt* or ongoing or looped or interminable) adj4 SMBG).tw,kw,dv. (131)
-
60
(CGM or CGMS or continuous glucose monitor* or continuous glucose sensor*).tw,kw,dv. (10250)
-
61
((medtronic adj3 (paradigm* or glucose monitor* or glucose sensor* or CGM or insulin pump* or LGS or 630G)) or veo or veotm or minimed or dexcom or g4 platinum or g5 platinum).tw,kw,dv. (2388)
-
62
((flash or novel) adj2 glucose adj2 (monitor* or sensor or sensing)).tw,kw,dv. (184)
-
63
((integrat* adj2 (pump or pumps or infusion*)) or (sensor adj3 (pump or pumps or therap* or infusion*)) or (sensor augment* adj2 pump*) or low glucose suspend*).tw,kw,dv. (1589)
-
64
(closed loop adj2 (pump* or deliver* or infus* or therap* or treatment* or system* or sensor* or control* or monitor* or hybrid*)).tw,kw,dv. (7269)
-
65
artificial pancreas/ (2671)
-
66
(artificial adj3 (pancreas or beta cell*)).tw,kw,dv. (3492)
-
67
or/57–66 (21829)
-
68
54 and 67 (8861)
-
69
Economics/ (255645)
-
70
Health Economics/ or exp Pharmacoeconomics/ (223136)
-
71
Economic Aspect/ or exp Economic Evaluation/ (436072)
-
72
(econom* or price or prices or pricing or priced or discount* or expenditure* or budget* or pharmacoeconomic* or pharmaco-economic*).tw. (762124)
-
73
exp “Cost”/ (555558)
-
74
cost*.ti. (255725)
-
75
cost effective*.tw. (277253)
-
76
(cost* adj2 (util* or efficacy* or benefit* or minimi* or analy* or saving* or estimate* or allocation or control or sharing or instrument* or technolog*)).ab. (174616)
-
77
Monte Carlo Method/ (58378)
-
78
(decision adj1 (tree* or analy* or model*)).tw. (37669)
-
79
(markov or markow or monte carlo).tw. (113113)
-
80
Quality-Adjusted Life Years/ (34238)
-
81
(QOLY or QOLYs or HRQOL or HRQOLs or QALY or QALYs or QALE or QALEs).tw. (58790)
-
82
((adjusted adj (quality or life)) or (willing* adj2 pay) or sensitivity analys*s).tw. (111268)
-
83
or/69–82 (2039296)
-
84
68 and 83 (475)
-
85
limit 84 to english language [Limit not valid in CDSR,DARE; records were retained] (461)
-
86
limit 85 to yr=“2010 -Current” [Limit not valid in DARE; records were retained] (354)
-
87
86 use emez (220)
-
88
45 or 87 (363)
-
89
88 use ppez (110)
-
90
88 use emez (220)
-
91
88 use coch (2)
-
92
88 use cctr (23)
-
93
88 use clhta (1)
-
94
88 use cleed (4)
-
95
remove duplicates from 88 (253)
CINAHL
| # | Query Results | |
| S1 | (MH “Diabetes Mellitus, Type 1+”) | 17,153 |
| S2 | ((diabet* N3 (typ* 1 or typ* i or type1 or typei or typ* one or brittl* or juvenil* or pediatric or paediatric or early or keto* or labil* or acidos* or autoimmun* or auto immun* or sudden onset or young onset)) or (insulin* N2 depend*) or insulindepend* or dm1 or dm 1 or dmt1 or dm t1 or t1dm or t1 dm or t1d or iddm) | 26,773 |
| S3 | (MH “Diabetic Ketoacidosis”) | 1,653 |
| S4 | (ketoacidosis or acidoketosis or keto acidosis or ketoacidemia or ketosis) | 2,266 |
| S5 | (MH “Hypoglycemia”) | 6,679 |
| S6 | (MH “Diabetes Mellitus”) | 46,256 |
| S7 | S5 AND S6 | 1,343 |
| S8 | ((hypoglyc#em* or ((low or lower or decreas* or deficien* or insufficien* or reduce* or reduction* or fluctuat* or fallen or falling or threshold or safe) N3 (glucose* or sugar* or hba1c or hb a1 or hba1 or a1c or h#emoglob* or glycoh#emoglob*))) N5 (diabet* or IDDM or DM)) | 4,756 |
| S9 | S1 OR S2 OR S3 OR S4 OR S7 OR S8 | 31,012 |
| S10 | (MH “Blood Glucose Self-Monitoring”) | 2,778 |
| S11 | (continu* or uninterrupt* or ongoing or looped or interminable) | 272,487 |
| S12 | S10 AND S11 | 537 |
| S13 | ((continu* or uninterrupt* or ongoing or looped or interminable) N4 (blood glucose or blood sugar*) N2 (self monitor* or home monitor*)) | 27 |
| S14 | ((continu* or uninterrupt* or ongoing or looped or interminable) N4 SMBG) | 26 |
| S15 | (CGM or CGMS or continuous glucose monitor* or continuous glucose sensor*) | 1,179 |
| S16 | ((medtronic N3 (paradigm* or glucose monitor* or glucose sensor* or CGM or insulin pump* or LGS or 630G)) or veo or veotm or minimed or dexcom or g4 platinum or g5 platinum) | 205 |
| S17 | ((flash or novel) N2 glucose N2 (monitor* or sensor or sensing)) | 17 |
| S18 | ((integrat* N2 (pump or pumps or infusion*)) or (sensor N3 (pump or pumps or therap* or infusion*)) or (sensor augment* N2 pump*) or low glucose suspend*) | 199 |
| S19 | (closed loop N2 (pump* or deliver* or infus* or therap* or treatment* or system* or sensor* or control* or monitor* or hybrid*)) | 393 |
| S20 | (artificial N3 (pancreas or beta cell*)) | 197 |
| S21 | S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 | 1,974 |
| S22 | S9 AND S21 | 1,034 |
| S23 | (MH “Economics”) | 10,992 |
| S24 | (MH “Economic Aspects of Illness”) | 6,584 |
| S25 | (MH “Economic Value of Life”) | 518 |
| S26 | MH “Economics, Dental” | 104 |
| S27 | MH “Economics, Pharmaceutical” | 1,760 |
| S28 | MW “ec” | 140,414 |
| S29 | (econom* or price or prices or pricing or priced or discount* or expenditure* or budget* or pharmacoeconomic* or pharmaco-economic*) | 210,045 |
| S30 | (MH “Costs and Cost Analysis+”) | 83,883 |
| S31 | TI cost* | 39,344 |
| S32 | (cost effective*) | 26,695 |
| S33 | AB (cost* N2 (util* or efficacy* or benefit* or minimi* or analy* or saving* or estimate* or allocation or control or sharing or instrument* or technolog*)) | 17,578 |
| S34 | (decision N1 (tree* or analy* or model*)) | 4,861 |
| S35 | (markov or markow or monte carlo) | 3,005 |
| S36 | (MH “Quality-Adjusted Life Years”) | 2,571 |
| S37 | (QOLY or QOLYs or HRQOL or HRQOLs or QALY or QALYs or QALE or QALEs) | 5,656 |
| S38 | ((adjusted N1 (quality or life)) or (willing* N2 pay) or sensitivity analys?s) | 10,819 |
| S39 | S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 | 279,380 |
| S40 | S22 AND S39 | 66 |
| S41 | S22 AND S39 | |
| Limiters - English Language | 66 | |
| S42 | S22 AND S39 | |
| Limiters - Published Date: 20100101-20171231; English Language | 44 |
Health State Utility Value Search
Search date: January 30, 2017
Database: Ovid MEDLINE(R) Epub Ahead of Print, In-Process & Other Non-Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) <1946 to Present>
Search Strategy:
-
1
exp Diabetes Mellitus, Type 1/ (67682)
-
2
((diabet* adj3 (typ* 1 or typ* i or type1 or typei or typ* one or brittl* or juvenil* or pediatric or paediatric or early or keto* or labil* or acidos* or autoimmun* or auto immun* or sudden onset or young onset)) or (insulin* adj2 depend*) or insulindepend* or dm1 or dm 1 or dmt1 or dm t1 or t1dm or t1 dm or t1d or iddm).ti,ab,kf. (91144)
-
3
Diabetic Ketoacidosis/ (5695)
-
4
(ketoacidosis or acidoketosis or keto acidosis or ketoacidemia or ketosis).ti,ab,kf. (8451)
-
5
Hypoglycemia/ (24398)
-
6
Diabetes Mellitus/ (103774)
-
7
5 and 6 (2167)
-
8
((hypoglyc?em* or ((low or lower or decreas* or deficien* or insufficien* or reduce* or reduction* or fluctuat* or fallen or falling or threshold or safe) adj3 (glucose* or sugar* or hba1c or hb a1 or hba1 or a1c or h?emoglob* or glycoh?emoglob*))) adj5 (diabet* or IDDM or DM)).ti,ab,kf. (7240)
-
9
or/1–4,7–8 (123756)
-
10
Blood Glucose Self-Monitoring/ (5246)
-
11
(continu* or uninterrupt* or ongoing or looped or interminable).ti,ab,kf. (963628)
-
12
10 and 11 (1335)
-
13
((continu* or uninterrupt* or ongoing or looped or interminable) adj4 (blood glucose or blood sugar*) adj2 (self monitor* or home monitor*)).ti,ab,kf. (37)
-
14
((continu* or uninterrupt* or ongoing or looped or interminable) adj4 SMBG).ti,ab,kf. (39)
-
15
(CGM or CGMS or continuous glucose monitor* or continuous glucose sensor*).ti,ab,kf. (3161)
-
16
((medtronic adj3 (paradigm* or glucose monitor* or glucose sensor* or CGM or insulin pump* or LGS or 630G)) or veo or veotm or minimed or dexcom or g4 platinum or g5 platinum).ti,ab,kf. (459)
-
17
((flash or novel) adj2 glucose adj2 (monitor* or sensor or sensing)).ti,ab,kf. (72)
-
18
((integrat* adj2 (pump or pumps or infusion*)) or (sensor adj3 (pump or pumps or therap* or infusion*)) or (sensor augment* adj2 pump*) or low glucose suspend*).ti,ab,kf. (458)
-
19
(closed loop adj2 (pump* or deliver* or infus* or therap* or treatment* or system* or sensor* or control* or monitor* or hybrid*)).ti,ab,kf. (3028)
-
20
Pancreas, Artificial/ (556)
-
21
(artificial adj3 (pancreas or beta cell*)).ti,ab,kf. (1331)
-
22
or/12–21 (7943)
-
23
9 and 22 (2604)
-
24
Quality-Adjusted Life Years/ (9025)
-
25
(quality adjusted or adjusted life year*).tw. (11674)
-
26
(qaly* or qald* or qale* or qtime*).tw. (7579)
-
27
(illness state$1 or health state$1).tw. (5032)
-
28
(hui or hui1 or hui2 or hui3).tw. (1169)
-
29
(multiattribute* or multi attribute*).tw. (678)
-
30
(utility adj3 (score$1 or valu* or health* or cost* or measure* or disease* or mean or gain or gains or index*)).tw. (10653)
-
31
utilities.tw. (5404)
-
32
(eq-5d or eq5d or eq-5 or eq5 or euro qual or euroqual or euro qual5d or euroqual5d or euro qol or euroqol or euro qol5d or euroqol5d or euro quol or euroquol or euro quol5d or euroquol5d or eur qol or eurqol or eur qol5d or eurqol5d or euro?qul or eur?qul5d or euro* quality of life or European qol).tw. (7232)
-
33
(euro* adj3 (5 d or 5d or 5 dimension* or 5dimension* or 5 domain* or 5domain*)).tw. (2442)
-
34
(sf36* or sf 36* or sf thirtysix or sf thirty six).tw. (17901)
-
35
(time trade off$1 or time tradeoff$1 or tto or timetradeoff$1).tw. (1529)
-
36
((qol or hrqol or quality of life).ti. or *quality of life/) and ((qol or hrqol* or quality of life) adj2 (increas* or decreas* or improve* or declin* or reduc* or high* or low* or effect or effects of worse or score or scores or change$1 or impact$1 or impacted or deteriorate$)).ab. (23194)
-
37
Cost-Benefit Analysis/ and (cost effectiveness ratio* and (perspective* or life expectanc*)).tw. (2420)
-
38
*quality of life/ and (quality of life or qol).ti. (42116)
-
39
quality of life/ and ((quality of life or qol) adj3 (improve* or chang*)).tw. (18236)
-
40
quality of life/ and ((quality of life or qol) adj (score$1 or measure$1)).tw. (9109)
-
41
quality of life/ and health-related quality of life.tw. (22860)
-
42
quality of life/ and ec.fs. (8369)
-
43
quality of life/ and (health adj3 status).tw. (7018)
-
44
(quality of life or qol).tw. and cost-benefit analysis/ (9308)
-
45
models, economic/ (7973)
-
46
or/24–45 (121614)
-
47
23 and 46 (51)
-
48
limit 47 to english language (50)
Grey Literature
Performed on:
January 12–27, 2017
Websites searched:
HTA Database Canadian Repository, Alberta Health Technologies Decision Process reviews, Canadian Agency for Drugs and Technologies in Health (CADTH), Institut national d'excellence en santé et en services sociaux (INESSS), Institute of Health Economics (IHE), McGill University Health Centre Health Technology Assessment Unit, National Institute for Health and Care Excellence (NICE), Agency for Healthcare Research and Quality (AHRQ) Evidence-based Practice Centers, Australian Government Medical Services Advisory Committee, Centers for Medicare & Medicaid Services Technology Assessments, Institute for Clinical and Economic Review, Ireland Health Information and Quality Authority Health Technology Assessments, Washington State Health Care Authority Health Technology Reviews, ClinicalTrials.gov, Tuft's Cost-Effectiveness Analysis Registry
Keywords used: continuous glucose monitor, continuous glucose monitors, CGM, minimed, Medtronic, dexcom, platinum, paradigm, closed loop, artificial pancreas, integrated pump, sensor augment, sensor augmented
Results: 8
35 clinical trials not counted in PRISMA
Appendix 2: Clinical Evidence Quality Assessment
Table A1:
GRADE Evidence Profile for Comparison of Continuous Glucose Monitoring and Usual Care
| Number of Studies (Design) | Risk of Biasa | Inconsistency | Indirectness | Imprecision | Publication Bias | Upgrade Considerations | Quality |
|---|---|---|---|---|---|---|---|
| Time-Related Glucose Variability—Time Spent in Target Glycemic Range | |||||||
| 2 (RCTs, adults)24,39 | Serious limitations (−1) | No serious limitations | No serious limitations | No serious limitations | Undetected | None | ⊕⊕⊕ Moderate |
| 1 (observational, adults)43 | Serious limitations (−2) | No serious limitations | No serious limitations | Serious limitationsb (−1) | Undetected | None | ⊕ Very low |
| Time-Related Glucose Variability—Time Spent Outside of Target Glycemic Range | |||||||
| 4 (RCTs, 24,27,33,39 adults) | Serious limitations (−1) | No serious limitationsc | No serious limitations | No serious limitations | Undetected | None | ⊕⊕⊕ Moderate |
| Hypoglycemia | |||||||
| 4 (RCTs, 25,27,30,38 adults) | Serious limitations (−1) | Serious limitations (−1) | No serious limitations | No serious limitations | Undetected | None | ⊕⊕ Low |
| 2 (observational, adults)42,43 | Serious limitations (−3) | No serious limitations | No serious limitations | No serious limitationsd | Undetected | None | ⊕ Very low |
| 3 (RCTs, children)25,26,37 | Serious limitations (−3) | No serious limitationsc | No serious limitations | No serious limitations | Undetected | None | ⊕ Very low |
| Hypoglycemia—Severe Hypoglycemic Events | |||||||
| 3 (RCTs, adults)32,33,39 | Serious limitations (−1) | Serious limitationse (−1) | No serious limitations | No serious limitationsd | Undetected | None | ⊕⊕ Low |
| A1C Levels—Change From Baseline | |||||||
| 8 (RCTs, adults)24,25,27,30,31,35,38,39 | Serious limitations (−1) | No serious limitations | No serious limitations | No serious limitationsd | Undetected | None | ⊕⊕⊕ Moderate |
| 4 (observational, adults)40–43 | Serious limitations (−2) | Serious limitationsf (−1) | No serious limitations | No serious limitationsd | Undetected | None | ⊕ Very low |
| 4 (RCTs, children)25,26,29,34 | Serious limitations (−1) | Serious limitationsf (−1) | No serious limitations | No serious limitations | Undetected | None | ⊕⊕ Low |
| User Satisfaction | |||||||
| 7 (RCTs, adults)24,27,28,30–32,36 | Serious limitations (−1) | Serious limitationsf (−1) | No serious limitations | No serious limitationsd | Undetected | None | ⊕⊕ Low |
| 1 (observational, adults)42 | Serious limitations (−2) | No serious limitations | No serious limitations | Serious limitationsb (−1) | Undetected | None | ⊕ Very low |
| 4 (RCTs, children)28,29,34,36 | Serious limitations (−1) | Serious limitationsf (−1) | No serious limitations | No limitations | Undetected | None | ⊕⊕ Low |
Abbreviations: RCT, randomized controlled trial.
Some studies had wide confidence intervals.
Trend in results was inconsistent in favouring intervention or control groups; however, results were not statistically significant.
Some studies did not find significant results or had large confidence intervals; however, this was likely owing to smaller sample sizes in individual studies. The body of evidence was substantive.
Results were inconsistent in favouring intervention or control groups.
Inconsistency in results; some studies favoured the intervention, and others favoured the control group.
Table A2:
Risk of Bias Among Randomized Controlled Trials for Comparison of Continuous Glucose Monitoring and Usual Carea
| Selection Biasb | Performance Bias | Detection Bias | Attrition Bias | Reporting Bias | Other Bias | |
|---|---|---|---|---|---|---|
| Beck et al, 201724 | N | Y | Y | N | U | Y |
| Bergenstal et al, 201025 | N | Y | Y | N | N | Yc |
| Bukara-Radujkovic et al, 201126 | N | Y | Y | N | U | N |
| Hermanides et al, 201127 | N | Y | Y | N | U | N |
| Hommel et al, 201428 | Y | Y | Y | N | N | Y |
| Kordonouri et al, 201229 | N | Y | N | N | U | U |
| Langeland et al, 201230 | N | Y | Y | N | U | N |
| Lind et al, 201731 | N | Y | Y | N | N | N |
| Little et al, 201432 | N | Y | Y | N | N | Yc |
| Ly et al, 201333 | N | Y | Y | N | U | N |
| Olivier et al, 201434 | N | Y | U | Y | U | Ud |
| Rosenlund et al, 201535 | N | Y | Y | N | U | N |
| Rubin and Peyrot, 201236 | N | Y | Y | N | U | Y |
| Slover et al, 201337 | N | Y | Y | N | U | Yc |
| Tumminia et al, 201538 | N | Y | Y | N | U | Yc |
| van Beers et al, 201639 | N | Y | Y | N | U | Y |
Abbreviations: Y, yes or high risk of bias likely; N, no or low risk of bias detected; U, unclear risk of bias.
Risk of bias assessed using the Cochrane Risk of Bias Tool.22
The term selection bias refers to confounding in Table A3.
Bias in the analytic approach.
109 of 141 patients were excluded owing to preference for a different device from that included in the study.
Table A3:
Risk of Bias Among Observational Studies for Comparison of Continuous Glucose Monitoring and Usual Carea
| Selection Bias | Performance Bias on Exposure | Detection Bias | Attrition Bias | Reporting Bias | Confounding | Other Biases | |
|---|---|---|---|---|---|---|---|
| McQueen et al, 201440 | N | N | Y | N | U | Y | U |
| Quiros et al, 201541 | Y | N | Y | N | U | Y | U |
| Radermecker et al, 201042 | Y | N | Y | Y | U | Y | U |
| Soupal et al, 201643 | N | N | Y | N | U | Y | Yb |
Abbreviations: Y, yes or high risk of bias likely; N, no or low risk of bias detected; U, unclear risk of bias.
Using the Risk of Bias Assessment Tool for Non-randomized Studies (RoBANS) tool.21
Bias in the analytic approach.
Appendix 3: Results of Applicability Checklist for Studies Included in the Economic Evidence Review
Table A4:
Assessment of the Cost-Effectiveness of Continuous Glucose Monitoring
| Objective: To assess the cost-effectiveness of continuous glucose monitoring | |||||
|---|---|---|---|---|---|
| Author, Year | Is the study population similar to the question? | Are the interventions similar to the question? | Is the health care system in which the study was conducted sufficiently similar to the current Ontario context? | Was/were the perspective(s) clearly stated, and what were they? | Are estimates of relative treatment effect from the best available source? |
| Huang et al, 201057 | Yes; adults aged ≥ 25 years | Partially; study did not consider insulin treatment | No; US health system | Yes | Yes |
| Kamble et al, 201252 | Partially; patient mean age 41.3 years; clinical experts suggest assessing younger patients with a shorter disease history | Yes | No; US health system | Yes | Yes |
| McQueen et al, 201151 | Partially; patient mean age 40; clinical experts suggest assessing younger patients | Partially; insulin infusion therapy not specified | No; US health system | Yes | Yes |
| Riemsma et al, 201658 | Partially; patients with a 27-year history of diabetes and a mean age of 42 years; clinical experts suggest assessing younger patients with a shorter disease history | Yes | Partially; UK health system | Yes | Yes |
| Roze et al, 201554 | Yes; patient mean age 27 years | Partially; comparator arm used SMPG | No; Sweden health system | Yes | Yes |
| Roze et al, 201653 | Yes; patient mean age 27 years | Yes | Partially; UK health system | Yes | Yes |
| Roze et al, 201655 | Yes; patient mean age 27 years | Yes | No; France health system | Yes | Yes |
| Roze et al, 201756 | No; patient population not specified | Yes | No; Denmark health system | Yes | Yes |
| Author, Year | Are all future costs and outcomes discounted? (If yes, at what rate?) | Is the value of health effects expressed in terms of quality-adjusted life-years? | Are costs and outcomes from other sectors fully and appropriately measured and valued? | Overall judgment (directly applicable/partially applicable/ not applicable) |
|---|---|---|---|---|
| Huang et al, 201057 | Yes; both costs and outcomes discounted at 3% | Yes | Yes | Partially applicable |
| Kamble et al, 201252 | Yes; both costs and outcomes discounted at 3% | Yes | Yes | Partially applicable |
| McQueen et al, 201151 | Yes; both costs and outcomes discounted at 3% | Yes | Yes | Partially applicable |
| Riemsma et al, 201658 | Yes; costs discounted at 1.5%, outcomes discounted at 3.5% | Yes | Yes | Partially applicable |
| Roze et al, 201554 | Yes; both costs and outcomes discounted at 3% | Yes | Yes | Partially applicable |
| Roze et al, 201653 | Yes; costs discounted at 1.5%, outcomes discounted at 3.5% | Yes | Yes | Partially applicable |
| Roze et al, 201655 | Yes; both costs and outcomes discounted at 4% | Yes | Yes | Partially applicable |
| Roze et al, 201756 | Yes; both costs and outcomes discounted at 3% | Yes | Yes | Partially applicable |
Abbreviations: CGM, continuous glucose monitoring; SMBG, self-monitoring of blood glucose.
Appendix 4: Primary Economic Analysis, Included Interventions
Table A5:
Reasons for Including Four Interventions in the Primary Economic Analysis
| Case | Suggested Reference for Base Case | Reason for Inclusion |
|---|---|---|
| For A1C Change From Baseline | ||
| SMBG plus multiple daily injections vs. standalone CGM plus multiple daily injections | Lind et al, 201731 |
|
| SMBG plus multiple daily injections vs. sensor-augmented pump | Bergenstal et al, 201025 |
|
| SMBG + insulin pump vs. sensor-augmented pump | Quiros et al, 201541 |
|
| SMBG + insulin pump vs. standalone CGM plus insulin pump | Tumminia et al, 201538 |
|
| For Severe Hypoglycemic Events | ||
| All cases | Bergenstal et al, 201025 |
|
Abbreviations: A1C, glycated hemoglobin; CGM, continuous glucose monitoring; SMBG, self-monitoring of blood glucose.
Appendix 5: Primary Economic Analysis, Risk Reduction Estimation
We assumed that the reduction in glycated hemoglobin (A1C) levels resulting from continuous glucose monitoring would be similar to that resulting from intensive treatment. However, the Diabetes Control and Complications Trial reported absolute risk reduction, which “does not involve an explicit comparison to the control group as in the relative risk reduction and thus does not confound the effect size with the baseline risk.”128 We used relative risk reduction (RRR), which determines how much a treatment reduces the risk of incidence relative to a control group that does not receive treatment. We then determined relative risk (RR) and applied this to the model (Table A6). The formula for relative risk reduction was RRR = 1 – RR. The formula for risk reduction was RR = 1 - RRR.
Table A6:
Relative Risk Associated With a 1% Reduction in A1C
| Complication | RR | 1% Reduction in A1C | Source | |
|---|---|---|---|---|
| RR | RR (%) | |||
| Retinopathy | 0.462 | 0.029 | 2.9 | DCCT, 199364 |
| Nephropathy | 0.611 | 0.038 | 3.8 | DCCT, 199364 |
| Neuropathy | 0.390 | 0.025 | 2.5 | Martin et al, 201482 |
| Cardiovascular disease | 0.643 | 0.040 | 4.0 | DCCT, 200976 |
| Severe hypoglycemiaa | 1.13 | 0.061 | 6.1 | DCCT, 200976 |
Abbreviations: A1C, glycated hemoglobin; DCCT, Diabetes Control and Complications Trial; RR, relative risk.
The risk of severe hypoglycemia increases as A1C is reduced.
-
1.
Obtain the relative change in A1C level (%) associated with continuous glucose monitoring. Use the change in A1C level associated with intensive treatment (15.9%; the difference between intensive versus conventional treatments after 6.5 years of follow-up from the 1993 Diabetes Control and Complications Trial64).
-
2.
Obtain the relative risk for long-term complications (i.e., retinopathy, nephropathy, neuropathy, cardiovascular disease) from the 1993 Diabetes Control and Complications Trial,64 the 2009 Diabetes Control and Complications Trial,76 and the 2014 study conducted by Martin et al.82 (RR = cumulative incidences of intensive arm divided by cumulative incidences of treatment arm).
-
3.
Calculate relative risk reduction (RRR = 1 - RR; details in Tables A7, A8, and A9).
-
4.
Calculate the relative risk reduction associated with a decrease in complications resulting from a 1% reduction in A1C level.
-
5.
Calculate relative risk reduction (RRR = change in A1C level associated with continuous glucose monitoring multiplied by the relative risk reduction resulting from a 1% reduction in A1C).
-
6.
Determine relative risk (RR = 1 – RRR) and apply it to the model. (A sample calculation of risk reduction resulting from changes in A1C level in the GOLD31 and DIAMOND24 trials is presented in Table A9.)
Table A7:
Relationship Between Percentage Relative Change From Baseline A1C and Relative Risk of Diabetes Complications
| % Relative Change From Baseline A1C | RR, Retinopathy | RR, Nephropathy | RR, Neuropathy | RR, Cardiovascular Disease |
|---|---|---|---|---|
| 10 | 0.709 | 0.781 | 0.676 | 0.797 |
| 9 | 0.734 | 0.800 | 0.703 | 0.815 |
| 8 | 0.760 | 0.820 | 0.731 | 0.834 |
| 7 | 0.786 | 0.841 | 0.761 | 0.853 |
| 6 | 0.814 | 0.862 | 0.791 | 0.873 |
| 5 | 0.842 | 0.884 | 0.822 | 0.893 |
| 4 | 0.872 | 0.906 | 0.855 | 0.913 |
| 3 | 0.902 | 0.928 | 0.889 | 0.934 |
| 2 | 0.934 | 0.952 | 0.925 | 0.956 |
| 1 | 0.966 | 0.976 | 0.962 | 0.978 |
Abbreviation: A1C, glycated hemoglobin; RR, relative risk.
Table A8:
Relative Risk Reduction and Relative Risk Associated With a 1% Reduction in A1C Level
| Complication | RR | RRR | 1% Reduction in A1C | Source | |||
|---|---|---|---|---|---|---|---|
| RR | RRR | RR (%) | RRR (%) | ||||
| Retinopathy | 0.462 | 0.538 | 0.025 | 0.029 | 2.5 | 2.9 | DCCT, 199364 |
| Nephropathy | 0.611 | 0.389 | 0.033 | 0.021 | 3.3 | 2.1 | DCCT, 199364 |
| Neuropathy | 0.390 | 0.610 | 0.021 | 0.033 | 2.1 | 3.3 | DCCT, 199364 |
| Cardiovascular disease | 0.643 | 0.357 | 0.034 | 0.019 | 3.4 | 1.9 | Martin et al, 201482 |
| Severe hypoglycemiaa | 1.13 | −0.13 | 0.061 | −0.007 | 6.1 | −0.7 | DCCT, 200976 |
Abbreviations: A1C, glycated hemoglobin; DCCT, Diabetes Control and Complications Trial; RR, relative risk; RRR, relative risk reduction.
The risk of severe hypoglycemia increases as A1C is reduced.
Table A9:
Calculation of Risk Reduction Owing to a Decrease in A1C Level
| Intervention: CGM + MDI vs. SMBG + | ||||
|---|---|---|---|---|
| MDI | GOLDa | DIAMONDb | ||
| Steps | ||||
| 1. Calculate % change relative to baseline | 5.15 | 10.47 | ||
| 2. Obtain RR of complications from DCCT | Retinopathy: 0.462 | Nephropathy: 0.611 | Neuropathy: 0.390 | CVD: 0.643 |
| 3. Calculate RRR from retinopathy (DCCT): RRR = 1 - RR | 0.538 | 0.389 | 0.610 | 0.357 |
| 4. Calculate RRR change owing to 1% reduction in A1C level (15.9% A1C reduction from DCCT) | 0.034 | 0.024 | 0.038 | 0.022 |
| GOLD | ||||
| 5. Calculate RRR for intervention | 0.174 | 0.126 | 0.197 | 0.116 |
| 6. Calculate RR: RR = 1 - RRR | 0.826 | 0.874 | 0.803 | 0.884 |
| DIAMOND | ||||
| 5. Calculate RRR for intervention | 0.354 | 0.256 | 0.401 | 0.235 |
| 6. Calculate RR: RR = 1 - RRR | 0.646 | 0.744 | 0.599 | 0.765 |
| Average RR (GOLD and DIAMOND) | 0.736 | 0.809 | 0.701 | 0.825 |
Abbreviations: A1C, glycated hemoglobin; CGM, continuous glucose monitoring; CVD, cardiovascular disease; DCCT, Diabetes Control and Complications Trial; MDI, multiple daily injections; SMBG, self-monitoring of blood glucose; RR, relative risk; RRR relative risk reduction.
The GOLD study31 was a randomized controlled trial conducted in Sweden with a 26-week duration. The mean age of study participants was 44 years, and the mean duration of diabetes duration was 22 years.
The DIAMOND study24 was randomized controlled trial conducted in the United States with a 24-week duration. The mean age of study participants was 48 years, and the mean duration of diabetes was 19 years.
Appendix 6: Primary Economic Analysis, Cost Parameters
Table A10:
| Year | Age, y | |||
|---|---|---|---|---|
| 27–59 | 60–69 | 70–79 | 80+ | |
| 1 | 0.14 | 0.245 | 0.2 | 0.44 |
| 2 | 0.205 | 0.21 | 0.25 | 0.425 |
| 3 | 0.019 | 0.145 | 0.175 | 0.305 |
| 4 | 0.034 | 0.06 | 0.19 | 0.31 |
| 5 | 0.185 | 0.125 | 0.17 | 0.145 |
| 6 | 0.125 | 0.13 | 0.32 | 0.275 |
| 7 | 0.125 | 0.14 | 0.135 | 0.515 |
| 8 | 0.075 | 0.065 | 0.13 | 0.61 |
| 9 | 0.107 | 0.075 | 0.265 | 0.57 |
| 10 | 0.16 | 0.135 | 0.245 | 0.57 |
Used for cardiovascular disease alone, and for cardiovascular disease combined with diabetes complications.
Table A11:
Annual and Daily Cost of Multiple Daily Insulin Injections for Type 1 Diabetes— Canadian Clinical Practice
| Treatment | Daily Treatment Cost Without Test Strips |
|---|---|
| Insulin NPH (insulin isophane) | $1.95 |
| Biphasic human insulin | $3.81 |
| Long-acting insulin analogue | $3.04 |
| Biphasic insulin analogue | $4.34 |
| Average daily cost | $3.29 |
| Annual costa | $1,348 |
Annual costs inflated to 2017 Canadian dollars.
Source: McIntosh et al, 2011.129
Table A12:
Annual Cost of Insulin Pump Therapy
| We obtained an average daily insulin use of 42 units from 731 insulin pumps130 |
| People with diabetes using an insulin pump use 200-unit cartridges, so one insulin cartridge lasts approximately 4.5 days, or 81 cartridges (365/4.5 = 81) per year |
| Humalog (insulin lispro) is provided in 3 × 5 mL = 15 mL packs (1 mL insulin = 100 units; 15 mL = 1,500 units). One pack of Humalog equals 7.5 cartridges (1,500/200) Humalog costs $67.99 per pack131 |
| The annual cost of Humalog with an insulin pump is $734 ([81/7.5] × $67.99). |
Table A13:
Daily and Annual Costs of Diabetes Treatment Suppliesa
| Technology | Strips | Needles | Syringes | Total Annual Cost | Total Daily Cost | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Quantity, units | Unit Price | Cost | Quantity, units | Unit Price | Cost | Quantity, units | Unit Price | Cost | |||
| SAP (Dexcom G4 Platinum + Animas Vibe) | 4 | $0.73 | $2.92 | $2.92 | $1,066 | ||||||
| SAP (Dexcom G5 Mobile + Animas Vibe) | 2 | $0.73 | $1.46 | $1.46 | $533 | ||||||
| SAP with LGS (MiniMed Veo with LGS) | 4 | $0.73 | $2.92 | $2.92 | $1,066 | ||||||
| Standalone CGM + MDI (Dexcom G4 Platinum) | 6 | $0.73 | $4.38 | 4 | $0.10 | $0.40 | 4 | $0.40 | $1.60 | $6.38 | $2,329 |
| Standalone CGM + MDI (Dexcom G5 Mobile) | 2 | $0.73 | $1.46 | 4 | $0.10 | $0.40 | 4 | $0.40 | $1.60 | $3.46 | $1,263 |
| SMBG + insulin pump (Animas Vibe or MiniMed Veo) | 6 | $0.73 | $4.38 | $4.38 | $1,599 | ||||||
| SMBG + MDI | 6 | $0.73 | $4.38 | 4 | $0.10 | $0.40 | 4 | $0.40 | $1.60 | $6.38 | $2,329 |
Abbreviations: CGM, continuous glucose monitoring; LGS, low-glucose suspend; MDI, multiple daily injections; SAP, sensor-augmented pump; SMBG, self-monitoring of blood glucose.
All costs are presented in 2017 Canadian dollars.
Table A14:
Annual Costs of Continuous Glucose Monitoring and Insulin Pumpa
| Product | Annual Cost | Description |
|---|---|---|
| Standalone CGM | ||
| Dexcom G4 Platinum | ||
| Receiver | $700 | 1 unit ($700 per unit) |
| Transmitters | $1,300 | 2 units ($650 per unit, replaced every 6 months) |
| Sensors | $4,420 | 52 units ($85 per unit, replaced every 7 days) |
| Total | $6,420 | |
| Dexcom G5 Mobile | ||
| Transmitters | $1,556 | 4 units ($389 per unit, replaced every 90 days) |
| Sensors | $4,420 | 52 units ($85 per unit, replaced every 7 days) |
| Total | $5,976 | |
| Standalone Insulin Pump | ||
| Insulin pump | $1,260 | 4-year warranty and 1-year extended warranty to meet Assistive Devices Program funding period of 5 years |
| Insulin pump infusion sets | $2,494 | $20.50 per set every 3 days |
| Insulin pump reservoirs | $517 | Assumed average use of reservoir: 4.5 days |
| Insulin pump batteries | $19 | Energizer lithium AA battery |
| Total | $4,290 | |
| CGM With Insulin Pump | ||
| Insulin pump (Animas Vibe) | $4,290 | |
| Transmitters | $1,300 | 2 units ($650 per unit, replaced every 6 months) |
| Sensors | $4,420 | 52 units ($85 per unit, replaced every 7 days) |
| Total: CGM (Dexcom G4 Platinum) | $5,720 | |
| Total: Insulin pump and CGM | $10,140 | |
| SAP With LGS | ||
| Insulin pump (MiniMed Veo) | $4,290 | |
| Sensors | $3,120 | 52 units ($60 per unit, replaced every 7 days)b |
| Total: CGM | $3,120 | |
| Total: Insulin pump and CGM | $7,410 | |
Abbreviations: CGM, continuous glucose monitoring; MDI, multiple daily injections; SMBG, self-monitoring of blood glucose; LGS, low glucose suspend; SAP, sensor-augmented pump.
All costs are presented in 2017 Canadian dollars.
According to Medtronic, the average retail price for a CGM sensor is between $50 and $60. We have assumed a price of $60.
Table A15:
Costs of Different Diabetes Treatment Technologiesa
| Intervention | Brands (Approved in Canada) | Diabetes Treatment Supplies | Insulin Treatment | CGM | Insulin Pump | Total Cost |
|---|---|---|---|---|---|---|
| SAP | Dexcom G4 Platinum + Animas Vibe | $1,066 | $734 | $5,720 | $4,290 | $11,811 |
| SAP | Dexcom G5 Mobile + Animas Vibe or MiniMed VEO | $533 | $734 | $5,976 | $4,290 | $11,534 |
| SAP with LGS | MiniMed Veo with LGS | $1,066 | $734 | $3,120 | $4,290 | $9,211 |
| CGM + MDI | Dexcom G4 Platinum | $2,329 | $1,348 | $6,420 | — | $10,097 |
| CGM + MDI | Dexcom G5 Mobile | $1,263 | $1,348 | $5,976 | — | $8,587 |
| SMBG + insulin pump | Animas Vibe and MiniMed Veo | $1,599 | $734 | — | $4,484 | $6,817 |
| SMBG + MDI | $2,329 | $1,348 | — | — | $3,677 |
Abbreviations: CGM, continuous glucose monitoring; LGS, low-glucose suspend; MDI, multiple daily injections; SAP, sensor-augmented pump; SMBG, self-monitoring of blood glucose.
All costs are presented in 2017 Canadian dollars.
Appendix 7: Primary Economic Analysis, Sensitivity Analyses
Figure A1: Continuous Glucose Monitoring With Sensor-Augmented Pump Versus Self-Monitoring of Blood Glucose Plus Insulin Pump.
Abbreviations: CGM, continuous glucose monitoring; CSII, continuous subcutaneous insulin infusion (insulin pump); CVD, cardiovascular disease; ICER, incremental cost-effectiveness ratio; LEA, lower-extremity amputation; QALY, quality-adjusted life-year; RR, relative risk; SAP, sensor-augmented pump; SMBG, self-monitoring of blood glucose.
Figure A2: Continuous Glucose Monitoring Plus Insulin Pump Versus Self-Monitoring of Blood Glucose Plus Insulin Pump.
Abbreviations: CGM, continuous glucose monitoring; CSII, continuous subcutaneous insulin infusion (insulin pump); CVD, cardiovascular disease; ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life-year; LEA, lower-extremity amputation; RR, relative risk.
Figure A3: Continuous Glucose Monitoring With Sensor-Augmented Pump Versus Self-Monitoring of Blood Glucose With Multiple Daily Injections.
Abbreviations: CGM, continuous glucose monitoring; CSII, continuous subcutaneous insulin infusion (insulin pump); CVD, cardiovascular disease; ICER, incremental cost-effectiveness ratio; LEA, lower-extremity amputation; MDI, multiple daily injections; QALY, quality-adjusted life-year; RR, relative risk; SAP, sensor-augmented pump; SMBG, self-monitoring of blood glucose.
Figure A4: Cost-Effectiveness Acceptability Curves: Continuous Glucose Monitoring Interventions.
Abbreviations: CGM, continuous glucose monitoring; CSII, continuous subcutaneous insulin infusion (insulin pump); MDI, multiple daily injections; QALY, quality-adjusted life-year; SAP, sensor-augmented pump; SMBG, self-monitoring of blood glucose.
Appendix 8: Budget Impact Analysis, Additional Calculations
Table A16:
Government Expenses for the $170 Annual Grant to Purchase Needles and Syringes for People With Type 1 Diabetes Age 65 Years and Older in Ontario
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | |
|---|---|---|---|---|---|
| Ontario Population With Type 1 Diabetes | |||||
| Number of people age 65+ | 16,196 | 17,201 | 18,271 | 19,371 | 20,527 |
| Number of people using MDI (83%) | 13,442 | 14,276 | 15,165 | 16,078 | 17,037 |
| Total grant amounta | $2,285,201 | $2,426,998 | $2,578,000 | $2,733,296 | $2,896,324 |
| Population With Hypoglycemia Unawareness | |||||
| Number of people age 65+ | 4,049 | 4,300 | 4,568 | 4,843 | 5,132 |
| Number of people using MDI (83%) | 3,361 | 3,569 | 3,791 | 4,020 | 4,259 |
| Total grant amounta | $571,300 | $606,749 | $644,500 | $683,324 | $724,081 |
| Population Using Continuous Glucose Monitoring | |||||
| Number of people age 65+ | 489 | 594 | 732 | 885 | 1011 |
| Number of people using MDI (40%) | 196 | 238 | 293 | 354 | 405 |
| Total grant amounta | $33,259 | $40,419 | $49,774 | $60,170 | $68,767 |
Abbreviations: MDI, multiple daily injections.
$170 per person per year.
Table A17:
Government Expenses for the $920 Annual Grant to Purchase of Blood Glucose Test Strips Through the Ontario Monitoring for Health Program
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | |
|---|---|---|---|---|---|
| Ontario Population With Type 1 Diabetes | |||||
| Number of people aged 25–64 years | 50,937 | 52,487 | 53,987 | 55,437 | 56,779 |
| Number of people without private or ODB funding for type 1 diabetes–related expenses (17%) | 8,659 | 8,923 | 9,178 | 9,424 | 9,652 |
| Cost of strips fundeda | $7,966,607 | $8,208,969 | $8,443,618 | $8,670,420 | $8,880,254 |
| Population With Hypoglycemia Unawareness | |||||
| Number of people aged 25–64 years | 12,734 | 13,122 | 13,497 | 13,859 | 14,195 |
| Number of people without private or ODB funding for type 1 diabetes–related expenses (17%) | 2,165 | 2,231 | 2,294 | 2,356 | 2,413 |
| Cost of strips fundeda | $1,991,652 | $2,052,242 | $2,110,905 | $2,167,605 | $2,220,064 |
| Population Using Continuous Glucose Monitoring | |||||
| Number of people aged 25–64 years | 106 | 128 | 158 | 191 | 218 |
| Number of people without private or ODB funding for type 1 diabetes–related expenses (17%) | 18 | 22 | 27 | 32 | 37 |
| Cost of strips fundeda | $16,523 | $20,080 | $24,728 | $29,892 | $34,163 |
Abbreviation: ODB, Ontario Drug Benefit program.
Funding for 75% of the cost of blood glucose test strips, to a maximum of $920.
Table A18:
Estimated Number of Current Users of Continuous Glucose Monitoring in Ontario by Age Group
| Age Group | Standalone CGM + MDI | Standalone CGM + Insulin Pump | Total Standalone CGM | SAP | SAP With LGS | Total SAP With or Without LGS | Total CGM Users |
|---|---|---|---|---|---|---|---|
| ≤ 24 years, 28% | 121 | 99 | 220 | 98 | 264 | 362 | 581 |
| 25–64 years, 54% | 237 | 193 | 430 | 191 | 515 | 706 | 1,135 |
| ≥ 65 years, 18% | 78 | 64 | 142 | 63 | 170 | 233 | 375 |
Abbreviations: CGM, continuous glucose monitoring; LGS, low-glucose suspend; MDI, multiple daily injections; SAP, sensor-augmented pump.
Table A19:
Medtronic Projection of Sensor-Augmented Pump and Low-Glucose Suspend Use in People With Type 1 Diabetes in Ontario
| Assumption | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 | |
|---|---|---|---|---|---|---|---|---|
| Ontario pump users | 4% year-over-year increase | 14,400 | 15,000 | 15,600 | 16,224 | 16,873 | 17,548 | 18,250 |
| Ontario SAP users | 4% year-over-year increase | 9,600 | 10,000 | 10,400 | 10,816 | 11,249 | 11,699 | 12,167 |
| Percentage of SAP users using a CGM sensor | Percentage use will plateau around 30% after a gradual increase over 5 years | 13% | 13% | 17% | 20% | 24% | 28% | 30% |
| SAP users using a CGM sensor | — | 1,248 | 1,300 | 1,768 | 2,163 | 2,700 | 3.276 | 3,650 |
| SAP users with LGS functionality | 90% of SAP users are using both SAP and LGS devices | 8,640 | 9,000 | 9,360 | 9,734 | 10,124 | 10,529 | 10,950 |
| Percentage of SAP and LGS users using a CGM sensor | Percentage use will plateau around 30% after a gradual increase over 5 years | 13% | 13% | 17% | 20% | 24% | 28% | 30% |
| SAP and LGS users using a CGM sensor | — | 1,123 | 1,170 | 1,591 | 1,947 | 2,430 | 2,948 | 3,285 |
Abbreviations: CGM, continuous glucose monitoring; LGS, low-glucose suspend; SAP, sensor-augmented pump.
Table A20:
Ontario Drug Benefit Plan Funding for People With Type 1 Diabetes
| Age Group | Supplies Covered |
|---|---|
| People Taking Multiple Daily Injections | |
| Children and youth ≤ 24 years of age | Insulin and blood glucose test strips |
| Adults 25–64 years of agea | Blood glucose test strips to a maximum of $920 |
| Insulin not funded | |
| Adults ≥ 65 years of age | Most types of insulin and blood glucose test strips |
| Annual grant of $170, paid once per year, for the purchase of needles and syringes used to inject insulin | |
| People Using an Insulin Pump | |
| All ages | 100% of the cost of an insulin pump listed with the program, which must be sold to the person at the Assistive Devices Program–approved price of $6,300 |
| Funding for insulin pump can be renewed every 5 years if the pump is no longer in good working order | |
| Annual grant of $2,400 for related supplies, paid in 4 equal payments of $600 directly to the patient or their legal agent; grant to be used only for pump-related supplies and must be renewed yearly | |
| Children and youth ≤ 24 years of age | Insulin and blood glucose test strips |
| Adults 25–64 years of agea | Most types of insulin and blood glucose test strips |
| Adults ≥ 65 years of age | Most types of insulin and blood glucose test strips |
Assuming 17% of population without private drug funding and who do not qualify for Ontario Drug Benefit Plan funding.
Source: Canadian Diabetes Association.114
Table A21:
Net Budget Impact of Funding Continuous Glucose Monitoring in Ontario by Age Group—Reference Case, Conservative Projection of a 20% Annual Increasea
| Intervention | Total Budget Impact | ||||
|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | |
| All Ages | |||||
| CGM | $14,192,322 | $17,835,103 | $22,441,206 | $27,412,678 | $31,857,150 |
| SMBG | $5,701,743 | $7,953,809 | $10,498,720 | $13,226,191 | $15,621,231 |
| NBI | $8,490,579 | $9,881,295 | $11,942,486 | $14,186,487 | $16,235,919 |
| ≤24 Years; Full Funding for Blood Glucose Test Strips (Annual Cost $1,243) | |||||
| CGM | $3,803,296 | $4,794,113 | $6,031,616 | $7,372,929 | $8,558,233 |
| SMBG | $1,392,221 | $1,976,076 | $2,637,262 | $3,339,882 | $3,938,578 |
| NBI | $2,411,076 | $2,818,038 | $3,394,353 | $4,033,047 | $4,619,655 |
| 25–64 Years; Blood Glucose Test Strips Covered to a Maximum of $920 | |||||
| CGM | $7,946,862 | $9,995,260 | $12,536,319 | $15,305,231 | $17,800,809 |
| SMBG | $3,235,553 | $4,488,736 | $5,903,659 | $7,424,545 | $8,773,875 |
| NBI | $4,711,309 | $5,506,524 | $6,632,660 | $7,880,685 | $9,026,934 |
| ≥65 Years; Full Funding for Blood Glucose Test Strips + $170 Annual Grant for Syringes and Needles | |||||
| CGM | $2,442,164 | $3,045,730 | $3,873,271 | $4,734,518 | $5,498,108 |
| SMBG | $1,073,970 | $1,488,997 | $1,957,798 | $2,461,763 | $2,908,778 |
| NBI | $1,368,194 | $1,556,732 | $1,915,473 | $2,272,755 | $2,589,330 |
Abbreviations: CGM, continuous glucose monitoring; NBI, net budget impact; SMBG, self-monitoring of blood glucose.
Assumes government funding for insulin, insulin pump, and blood glucose test strips.
Table A22:
Net Budget Impact of Funding Continuous Glucose Monitoring in Ontario by Age Group—Reference Case, Entire Population With Hypoglycemia Unawarenessa
| Intervention | Total Budget Impact | ||||
|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | |
| All Ages | |||||
| CGM | $120,487,478 | $131,981,755 | $140,758,669 | $147,524,997 | $152,658,439 |
| SMBG | $41,693,554 | $51,518,633 | $59,307,520 | $65,546,416 | $70,408,816 |
| NBI | $78,793,925 | $80,463,123 | $81,451,149 | $81,978,581 | $82,249,622 |
| ≤24 Years; Full Funding for Blood Glucose Test Strips (Annual Cost $1,243) | |||||
| CGM | $31,013,678 | $33,825,196 | $35,827,008 | $37,269,166 | $38,302,048 |
| SMBG | $7,537,092 | $10,055,012 | $11,965,768 | $13,425,453 | $14,500,223 |
| NBI | $23,476,586 | $23,770,184 | $23,861,240 | $23,843,714 | $23,801,825 |
| 25–64 Years; Blood Glucose Test Strips Covered to a Maximum of $920 | |||||
| CGM | $70,927,409 | $77,170,104 | $81,860,181 | $85,381,041 | $87,872,712 |
| SMBG | $25,456,301 | $30,761,244 | $34,910,874 | $38,175,589 | $40,624,409 |
| NBI | $45,471,108 | $46,408,861 | $46,949,308 | $47,205,451 | $47,248,303 |
| ≥65 Years; Full Funding for Blood Glucose Test Strips + $170 Annual Grant for Syringes and Needles | |||||
| CGM | $18,546,392 | $20,986,455 | $23,071,480 | $24,874,790 | $26,483,678 |
| SMBG | $8,700,160 | $10,702,377 | $12,430,878 | $13,945,374 | $15,284,185 |
| NBI | $9,846,231 | $10,284,078 | $10,640,602 | $10,929,416 | $11,199,494 |
Abbreviations: CGM, continuous glucose monitoring; NBI, net budget impact; SMBG, self-monitoring of blood glucose.
Assumes government funding for insulin, insulin pump, and blood glucose test strips.
Table A23:
Net Budget Impact of Funding Continuous Glucose Monitoring in Ontario by Age Group—Reference Case, Entire Type 1 Diabetes Populationa
| Intervention | Total Budget Impact | |||||
|---|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | ||
| All Ages | ||||||
| CGM | $481,949,914 | $527,927,021 | $563,034,675 | $590,099,986 | $610,633,755 | |
| SMBG | $166,774,215 | $206,074,531 | $237,230,078 | $262,185,663 | $281,753,568 | |
| NBI | $315,175,698 | $321,852,490 | $325,804,597 | $327,914,323 | $328,880,187 | |
| ≤24 Years; Full Funding for Blood Glucose Test Strips (Annual Cost $1,243) | ||||||
| CGM | $124,054,711 | $135,300,783 | $143,308,031 | $149,076,666 | $153,208,192 | |
| SMBG | $30,148,369 | $40,220,049 | $47,863,071 | $53,701,811 | $58,033,759 | |
| NBI | $93,906,342 | $95,080,735 | $95,444,960 | $95,374,854 | $95,174,433 | |
| 25–64 Years; Blood Glucose Test Strips Covered to a Maximum of $920 | ||||||
| CGM | $283,709,635 | $308,680,418 | $327,440,726 | $341,524,162 | $351,490,850 | |
| SMBG | $101,825,204 | $123,044,975 | $139,643,495 | $152,702,357 | $162,561,861 | |
| NBI | $181,884,430 | $185,635,443 | $187,797,231 | $188,821,805 | $188,928,989 | |
| ≥65 Years; Full Funding for Blood Glucose Test Strips + $170 Annual Grant for Syringes and Needles | ||||||
| CGM | $74,185,567 | $83,945,820 | $92,285,919 | $99,499,158 | $105,934,714 | |
| SMBG | $34,800,642 | $42,809,507 | $49,723,513 | $55,781,494 | $61,157,948 | |
| NBI | $39,384,926 | $41,136,313 | $42,562,406 | $43,717,664 | $44,776,765 | |
Abbreviations: CGM, continuous glucose monitoring; NBI, net budget impact; SMBG, self-monitoring of blood glucose.
Assumes government funding for insulin, insulin pump, and blood glucose test strips.
Table A24:
Net Budget Impact of Funding Continuous Glucose Monitoring in Ontario by Age Group—Scenario 2, Conservative Projection of a 20% Annual Increasea
| Intervention | Total Budget Impact | ||||
|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | |
| All Ages | |||||
| CGM | $11,751,030 | $14,879,176 | $18,809,532 | $23,072,200 | $26,932,164 |
| SMBG | $5,492,916 | $7,666,296 | $10,192,975 | $12,859,486 | $15,185,252 |
| NBI | $6,258,114 | $7,212,880 | $8,616,558 | $10,212,713 | $11,746,912 |
| ≤24 Years; Full Funding for Blood Glucose Test Strips (Annual Cost $1,243) | |||||
| CGM | $3,130,910 | $3,980,027 | $5,031,197 | $6,177,277 | $7,202,217 |
| SMBG | $1,392,221 | $1,976,076 | $2,637,262 | $3,339,882 | $3,938,578 |
| NBI | $1,738,689 | $2,003,952 | $2,393,935 | $2,837,394 | $3,263,640 |
| 25–64 Years; Blood Glucose Test Strips Covered to a Maximum of $920 | |||||
| CGM | $6,633,000 | $8,404,513 | $10,581,474 | $12,968,892 | $15,151,118 |
| SMBG | $3,235,553 | $4,488,736 | $5,903,659 | $7,424,545 | $8,773,875 |
| NBI | $3,397,447 | $3,915,777 | $4,677,815 | $5,544,347 | $6,377,243 |
| ≥65 Years; Full Funding for Blood Glucose Test Strips + $170 Annual Grant for Syringes and Needles | |||||
| CGM | $1,987,120 | $2,494,636 | $3,196,861 | $3,926,031 | $4,578,829 |
| SMBG | $865,142 | $1,201,485 | $1,652,053 | $2,095,059 | $2,472,800 |
| NBI | $1,121,977 | $1,293,151 | $1,544,808 | $1,830,972 | $2,106,029 |
Abbreviations: CGM, continuous glucose monitoring; NBI, net budget impact; SMBG, self-monitoring of blood glucose.
Assumes full government funding for insulin, insulin pump, and blood glucose test strips, as well as 75% CGM sensor funding.
Table A25:
Net Budget Impact of Funding Continuous Glucose Monitoring in Ontario by Age Group—Scenario 2, Entire Population With Hypoglycemia Unawarenessa
| Intervention | Total Budget Impact | ||||
|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | |
| All Ages | |||||
| CGM | $99,261,412 | $110,217,741 | $118,338,523 | $124,780,315 | $129,734,734 |
| SMBG | $37,075,088 | $46,587,198 | $54,041,932 | $59,937,590 | $64,439,296 |
| NBI | $62,186,324 | $63,630,543 | $64,296,591 | $64,842,725 | $65,295,437 |
| ≤24 Years; Full Funding for Blood Glucose Test Strips (Annual Cost $1,243) | |||||
| CGM | $25,036,832 | $27,776,999 | $29,654,330 | $31,071,725 | $32,113,835 |
| SMBG | $7,537,092 | $10,055,012 | $11,965,768 | $13,425,453 | $14,500,223 |
| NBI | $17,499,740 | $17,721,986 | $17,688,562 | $17,646,273 | $17,613,612 |
| 25–64 Years; Blood Glucose Test Strips Covered to a Maximum of $920 | |||||
| CGM | $59,354,621 | $65,362,015 | $69,715,189 | $73,109,997 | $75,584,696 |
| SMBG | $25,456,301 | $30,761,244 | $34,910,874 | $38,175,589 | $40,624,409 |
| NBI | $33,898,320 | $34,600,771 | $34,804,315 | $34,934,408 | $34,960,287 |
| ≥65 Years; Full Funding for Blood Glucose Test Strips + $170 Annual Grant for Syringes and Needles | |||||
| CGM | $14,869,960 | $17,078,727 | $18,969,004 | $20,598,593 | $22,036,203 |
| SMBG | $4,081,695 | $5,770,942 | $7,165,291 | $8,336,548 | $9,314,665 |
| NBI | $10,788,265 | $11,307,785 | $11,803,714 | $12,262,045 | $12,721,538 |
Abbreviations: CGM, continuous glucose monitoring; NBI, net budget impact; SMBG, self-monitoring of blood glucose.
Assumes full government funding for insulin, insulin pump, and blood glucose test strips, as well as 75% CGM sensor funding.
Table A26:
Net Budget Impact of Funding Continuous Glucose Monitoring in Ontario by Age Group—Scenario 2, Entire Type 1 Diabetes Populationa
| Intervention | Total Budget Impact ($) | ||||
|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | |
| All Ages | |||||
| CGM | $397,045,650 | $440,870,963 | $473,354,092 | $499,121,262 | $518,938,935 |
| SMBG | $148,300,352 | $186,348,792 | $216,167,728 | $239,750,361 | $257,875,488 |
| NBI | $248,745,298 | $254,522,171 | $257,186,365 | $259,370,901 | $261,063,447 |
| ≤24 Years; Full Funding for Blood Glucose Test Strips (Annual Cost $1,243) | |||||
| CGM | $100,147,329 | $111,107,994 | $118,617,319 | $124,286,902 | $128,455,340 |
| SMBG | $30,148,369 | $40,220,049 | $47,863,071 | $53,701,811 | $58,033,759 |
| NBI | $69,998,960 | $70,887,946 | $70,754,249 | $70,585,090 | $70,421,580 |
| 25–64 Years; Blood Glucose Test Strips Covered to a Maximum of $920 | |||||
| CGM | $237,418,483 | $261,448,061 | $278,860,755 | $292,439,988 | $302,338,784 |
| SMBG | $101,825,204 | $123,044,975 | $139,643,495 | $152,702,357 | $162,561,861 |
| NBI | $135,593,278 | $138,403,086 | $139,217,260 | $139,737,631 | $139,776,924 |
| ≥65 Years; Full Funding for Blood Glucose Test Strips + $170 Annual Grant for Syringes and Needles | |||||
| CGM | $59,479,838 | $68,314,908 | $75,876,018 | $82,394,372 | $88,144,811 |
| SMBG | $16,326,778 | $23,083,769 | $28,661,162 | $33,346,192 | $37,279,868 |
| NBI | $43,153,060 | $45,231,139 | $47,214,856 | $49,048,180 | $50,864,943 |
Abbreviations: CGM, continuous glucose monitoring; NBI, net budget impact; SMBG, self-monitoring of blood glucose.
Assumes full government funding for insulin, insulin pump, and blood glucose test strips, as well as 75% CGM sensor funding.
Table A27:
Net Budget Impact of Funding Continuous Glucose Monitoring in Ontario by Age Group—Scenario 2, Population With Hypoglycemia Unawareness Using a Conservative Projectiona
| Intervention | Total Budget Impact | ||||
|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | |
| All Ages | |||||
| CGM | $2,937,757 | $3,719,794 | $4,702,383 | $5,768,050 | $6,733,041 |
| SMBG | $1,373,229 | $1,916,574 | $2,548,244 | $3,214,872 | $3,796,313 |
| NBI | $1,564,529 | $1,803,220 | $2,154,139 | $2,553,178 | $2,936,728 |
| ≤24 Years; Full Funding for Blood Glucose Test Strips (Annual Cost $1,243) | |||||
| CGM | $782,727 | $995,007 | $1,257,799 | $1,544,319 | $1,800,554 |
| SMBG | $348,055 | $494,019 | $659,316 | $834,971 | $984,644 |
| NBI | $434,672 | $500,988 | $598,484 | $709,349 | $815,910 |
| 25–64 Years; Blood Glucose Test Strips Covered to a Maximum of $920 | |||||
| CGM | $1,658,250 | $2,101,128 | $2,645,369 | $3,242,223 | $3,787,780 |
| SMBG | $808,888 | $1,122,184 | $1,475,915 | $1,856,136 | $2,193,469 |
| NBI | $849,362 | $978,944 | $1,169,454 | $1,386,087 | $1,594,311 |
| ≥65 Years; Full Funding for Blood Glucose Test Strips + $170 Annual Grant for Syringes and Needles | |||||
| CGM | $496,780 | $623,659 | $799,215 | $981,508 | $1,144,707 |
| SMBG | $216,286 | $300,371 | $413,013 | $523,765 | $618,200 |
| NBI | $280,494 | $323,288 | $386,202 | $457,743 | $526,507 |
Abbreviations: CGM, continuous glucose monitoring; NBI, net budget impact; SMBG, self-monitoring of blood glucose.
Assumes full government funding for insulin, insulin pump, and blood glucose test strips, as well as 75% CGM sensor funding
Table A28:
Continuous Glucose Monitoring Device Cost Reduction Scenarios
| Intervention | CGM Device Cost | Diabetes Treatment | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CGM Cost | CGM Cost (75% Sensor Funding) | 10% Reduction | 20% Reduction | 30% Reduction | Base Case | 75% Sensor Funding | 10% CGM Cost Reduction | 20% CGM Cost Reduction | 30% CGM Cost Reduction | |
| CGM + MDI (average) | $6,198 | $4,743 | $5,578 | $4,958 | $4,339 | $9,342 | $8,237 | $8,408 | $7,474 | $6,539 |
| CGM + insulin pump (average) | $5,848 | $5,093 | $5,263 | $4,678 | $4,094 | $11,673 | $10,567 | $10,505 | $9,338 | $8,171 |
| SAP (LGS) | $3,120 | $2,340 | $2,808 | $2,496 | $2,184 | $9,211 | $8,431 | $8,290 | $7,369 | $6,448 |
Abbreviations: CGM, continuous glucose monitoring; LGS, low-glucose suspend; MDI, multiple daily injections; SAP, sensor-augmented pump.
Table A29:
Net Budget Impact of Funding Continuous Glucose Monitoring in Ontario—Scenario 3, Conservative Projection of a 20% Annual Increase, Device Cost Reduction by 30%, 20%, and 10%
| Intervention | Total Budget Impact | ||||
|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | |
| CGM Device Cost | |||||
| CGM | $24,229,379 | $29,075,255 | $34,890,306 | $41,868,368 | $50,242,041 |
| SMBG | $10,565,739 | $12,678,887 | $15,214,665 | $18,257,598 | $21,909,117 |
| NBI | $13,663,640 | $16,396,368 | $19,675,642 | $23,610,770 | $28,332,924 |
| CMG Device Cost + 75% Funding of Sensor Costs | |||||
| CGM | $21,962,668 | $26,355,201 | $31,626,241 | $37,951,490 | $45,541,787 |
| SMBG | $10,565,739 | $12,678,887 | $15,214,665 | $18,257,598 | $21,909,117 |
| NBI | $11,396,928 | $13,676,314 | $16,411,577 | $19,693,892 | $23,632,670 |
| CGM Device Cost Reduction of 30% | |||||
| CGM | $16,960,566 | $20,352,679 | $24,423,214 | $29,307,857 | $35,169,429 |
| SMBG | $10,565,739 | $12,678,887 | $15,214,665 | $18,257,598 | $21,909,117 |
| NBI | $6,394,826 | $7,673,791 | $9,208,550 | $11,050,260 | $13,260,312 |
| CGM Device Cost Reduction of 20% | |||||
| CGM | $19,383,504 | $23,260,204 | $27,912,245 | $33,494,694 | $40,193,633 |
| SMBG | $10,565,739 | $12,678,887 | $15,214,665 | $18,257,598 | $21,909,117 |
| NBI | $8,817,764 | $10,581,317 | $12,697,580 | $15,237,096 | $18,284,516 |
| CGM Device Cost Reduction of 10% | |||||
| CGM | $21,806,441 | $26,167,730 | $31,401,276 | $37,681,531 | $45,217,837 |
| SMBG | $10,565,739 | $12,678,887 | $15,214,665 | $18,257,598 | $21,909,117 |
| NBI | $11,240,702 | $13,488,843 | $16,186,611 | $19,423,933 | $23,308,720 |
Abbreviations: CGM, continuous glucose monitoring; NBI, net budget impact; SMBG, self-monitoring of blood glucose.
Table A30:
Net Budget Impact of Funding Continuous Glucose Monitoring in Ontario—Scenario 3, Entire Population With Hypoglycemia Unawareness, Device Cost Reduction by 30%, 20%, and 10%
| Intervention | Total Budget Impact | ||||
|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | |
| CGM Device Cost | |||||
| CGM | $219,832,134 | $227,215,875 | $234,583,188 | $241,925,675 | $249,234,764 |
| SMBG | $98,136,450 | $101,511,186 | $104,899,097 | $108,297,686 | $111,704,418 |
| NBI | $121,695,684 | $125,704,689 | $129,684,091 | $133,627,989 | $137,530,346 |
| CMG Device Cost + 75% Funding of Sensor Costs | |||||
| CGM | $198,111,455 | $204,765,640 | $211,405,020 | $218,022,026 | $224,608,936 |
| SMBG | $98,136,450 | $101,511,186 | $104,899,097 | $108,297,686 | $111,704,418 |
| NBI | $99,975,005 | $103,254,454 | $106,505,923 | $109,724,341 | $112,904,518 |
| CGM Device Cost Reduction of 30% | |||||
| CGM | $153,882,494 | $159,051,113 | $164,208,231 | $169,347,972 | $174,464,335 |
| SMBG | $98,136,450 | $101,511,186 | $104,899,097 | $108,297,686 | $111,704,418 |
| NBI | $55,746,044 | $57,539,926 | $59,309,134 | $61,050,287 | $62,759,917 |
| CGM Device Cost Reduction of 20% | |||||
| CGM | $175,865,707 | $181,772,700 | $187,666,550 | $193,540,540 | $199,387,811 |
| SMBG | $98,136,450 | $101,511,186 | $104,899,097 | $108,297,686 | $111,704,418 |
| NBI | $77,729,257 | $80,261,514 | $82,767,453 | $85,242,854 | $87,683,393 |
| CGM Device Cost Reduction of 10% | |||||
| CGM | $197,848,921 | $204,494,288 | $211,124,869 | $217,733,107 | $224,311,288 |
| SMBG | $98,136,450 | $101,511,186 | $104,899,097 | $108,297,686 | $111,704,418 |
| NBI | $99,712,471 | $102,983,102 | $106,225,772 | $109,435,422 | $112,606,870 |
Abbreviations: CGM, continuous glucose monitoring; NBI, net budget impact; SMBG, self-monitoring of blood glucose.
Table A31:
Net Budget Impact of Funding Continuous Glucose Monitoring in Ontario—Scenario 3, Entire Type 1 Diabetes Population, Device Cost Reduction by 30%, 20%, and 10%
| Intervention | Total Budget Impact | ||||
|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | |
| CGM Device Cost | |||||
| CGM | $879,328,537 | $908,863,502 | $938,332,751 | $967,702,698 | $996,939,057 |
| SMBG | $392,545,801 | $406,044,745 | $419,596,388 | $433,190,742 | $447,178,667 |
| NBI | $486,782,736 | $502,818,757 | $518,736,363 | $534,511,956 | $549,760,390 |
| CMG Device Cost + 75% Funding of Sensor Costs | |||||
| CGM | $792,445,820 | $819,062,561 | $845,620,078 | $872,088,106 | $898,435,744 |
| SMBG | $392,545,801 | $406,044,745 | $419,596,388 | $433,190,742 | $447,178,667 |
| NBI | $399,900,019 | $413,017,815 | $426,023,690 | $438,897,364 | $451,257,077 |
| CGM Device Cost Reduction of 30% | |||||
| CGM | $615,529,976 | $636,204,451 | $656,832,926 | $677,391,889 | $697,857,340 |
| SMBG | $392,545,801 | $406,044,745 | $419,596,388 | $433,190,742 | $447,178,667 |
| NBI | $222,984,175 | $230,159,706 | $237,236,538 | $244,201,146 | $250,678,673 |
| CGM Device Cost Reduction of 20% | |||||
| CGM | $703,462,830 | $727,090,802 | $750,666,201 | $774,162,159 | $797,551,245 |
| SMBG | $392,545,801 | $406,044,745 | $419,596,388 | $433,190,742 | $447,178,667 |
| NBI | $310,917,029 | $321,046,056 | $331,069,813 | $340,971,416 | $350,372,578 |
| CGM Device Cost Reduction of 10% | |||||
| CGM | $791,395,683 | $817,977,152 | $844,499,476 | $870,932,428 | $897,245,151 |
| SMBG | $392,545,801 | $406,044,745 | $419,596,388 | $433,190,742 | $447,178,667 |
| NBI | $398,849,882 | $411,932,406 | $424,903,088 | $437,741,686 | $450,066,484 |
Abbreviations: CGM, continuous glucose monitoring; NBI, net budget impact; SMBG, self-monitoring of blood glucose.
Table A32:
Net Budget Impact of Funding Continuous Glucose Monitoring in Ontario—Scenario 4, Device Cost Reduction by 30%, 20%, and 10%, Plus Government Fundinga
| Intervention | Total Budget Impact | ||||
|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | |
| Conservative Projection of a 20% Annual Increase | |||||
| CGM Device Cost Reduction of 30% | |||||
| CGM | $7,463,670 | $8,955,523 | $10,744,428 | $12,892,552 | $15,477,008 |
| SMBG | $5,531,782 | $6,638,139 | $7,965,767 | $9,558,920 | $11,470,704 |
| NBI | $1,931,887 | $2,317,384 | $2,778,662 | $3,333,632 | $4,006,304 |
| CMG Device Cost Reduction of 20% | |||||
| CGM | $8,591,500 | $10,308,920 | $12,368,505 | $14,841,444 | $17,815,678 |
| SMBG | $5,531,782 | $6,638,139 | $7,965,767 | $9,558,920 | $11,470,704 |
| NBI | $3,059,718 | $3,670,781 | $4,402,738 | $5,282,524 | $6,344,974 |
| CGM Device Cost Reduction of 10% | |||||
| CGM | $9,719,331 | $11,662,317 | $13,992,581 | $16,790,336 | $20,154,348 |
| SMBG | $5,531,782 | $6,638,139 | $7,965,767 | $9,558,920 | $11,470,704 |
| NBI | $4,187,549 | $5,024,178 | $6,026,815 | $7,231,416 | $8,683,644 |
| Entire Population With Hypoglycemia Unawareness | |||||
| CGM Device Cost Reduction of 30% | |||||
| CGM | $74,689,992 | $77,196,635 | $79,699,062 | $82,195,855 | $84,683,452 |
| SMBG | $51,548,728 | $53,261,896 | $54,966,445 | $56,660,112 | $58,340,589 |
| NBI | $23,141,265 | $23,934,738 | $24,732,618 | $25,535,742 | $26,342,862 |
| CGM Device Cost Reduction of 20% | |||||
| CGM | $87,021,085 | $89,941,905 | $92,857,588 | $95,766,244 | $98,663,832 |
| SMBG | $51,548,728 | $53,261,896 | $54,966,445 | $56,660,112 | $58,340,589 |
| NBI | $35,472,357 | $36,680,008 | $37,891,144 | $39,106,132 | $40,323,242 |
| CGM Device Cost Reduction of 10% | |||||
| CGM | $99,352,177 | $102,687,175 | $106,016,115 | $109,336,634 | $112,644,212 |
| SMBG | $51,548,728 | $53,261,896 | $54,966,445 | $56,660,112 | $58,340,589 |
| NBI | $47,803,449 | $49,425,278 | $51,049,670 | $52,676,522 | $54,303,623 |
| Entire Type 1 Diabetes Population | |||||
| CGM Device Cost Reduction of 30% | |||||
| CGM | $298,759,970 | $308,786,539 | $318,796,249 | $328,783,418 | $338,733,806 |
| SMBG | $206,194,912 | $213,047,586 | $219,865,778 | $226,640,450 | $233,447,017 |
| NBI | $92,565,058 | $95,738,953 | $98,930,471 | $102,142,968 | $105,286,789 |
| CGM Device Cost Reduction of 20% | |||||
| CGM | $348,084,338 | $359,767,619 | $371,430,354 | $383,064,978 | $394,655,327 |
| SMBG | $206,194,912 | $213,047,586 | $219,865,778 | $226,640,450 | $233,447,017 |
| NBI | $141,889,427 | $146,720,033 | $151,564,575 | $156,424,528 | $161,208,310 |
| CGM Device Cost Reduction of 10% | |||||
| CGM | $397,408,707 | $410,748,698 | $424,064,458 | $437,346,537 | $450,576,848 |
| SMBG | $206,194,912 | $213,047,586 | $219,865,778 | $226,640,450 | $233,447,017 |
| NBI | $191,213,795 | $197,701,112 | $204,198,680 | $210,706,087 | $217,129,831 |
Abbreviations: CGM, continuous glucose monitoring; NBI, net budget impact; SMBG, self-monitoring of blood glucose.
Assumes full government funding for insulin treatment, insulin pump, and blood glucose test strips, as well as 75% CGM sensor funding.
Table A33:
Net Budget Impact of Funding Continuous Glucose Monitoring in Ontario—Scenario 5, Manufacturer's Scenario With an Annual Increase in Adoption of 40%
| Intervention | Total Budget Impact | ||||
|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | |
| Reference Casea | |||||
| CGM | $15,332,591 | $20,637,518 | $28,070,987 | $37,349,650 | $48,228,466 |
| SMBG | $6,005,165 | $8,767,523 | $12,192,126 | $16,294,145 | $20,772,757 |
| NBI | $9,327,425 | $11,869,995 | $15,878,861 | $21,055,505 | $27,455,709 |
| All Direct Medical Costs | |||||
| CGM | $27,788,637 | $37,017,152 | $49,038,530 | $63,905,655 | $80,450,278 |
| SMBG | $12,107,734 | $16,675,345 | $22,510,807 | $29,610,087 | $37,415,877 |
| NBI | $15,680,903 | $20,341,806 | $26,527,723 | $34,295,568 | $43,034,401 |
| Government Funding of Insulin, Insulin Pump, and Blood Glucose Test Strips + 75% of CGM Sensor Costs | |||||
| CGM | $12,714,256 | $17,239,259 | $23,557,815 | $31,465,902 | $40,777,115 |
| SMBG | $5,767,077 | $8,407,082 | $11,740,884 | $15,671,920 | $19,917,114 |
| NBI | $6,947,180 | $8,832,176 | $11,816,931 | $15,793,982 | $20,860,001 |
| CGM Device Cost Reduction of 30% | |||||
| CGM | $19,452,046 | $25,221,182 | $33,189,610 | $43,216,206 | $54,468,371 |
| SMBG | $12,107,734 | $15,637,058 | $20,507,344 | $26,588,295 | $33,282,780 |
| NBI | $7,344,312 | $9,584,124 | $12,682,266 | $16,627,911 | $21,185,591 |
| CGM Device Cost Reduction of 20% | |||||
| CGM | $22,230,910 | $28,824,208 | $37,930,983 | $49,389,950 | $62,249,567 |
| SMBG | $12,107,734 | $15,637,058 | $20,507,344 | $26,588,295 | $33,282,780 |
| NBI | $10,123,176 | $13,187,150 | $17,423,639 | $22,801,655 | $28,966,786 |
| CGM Device Cost Reduction of 10% | |||||
| CGM | $25,009,774 | $32,427,234 | $42,672,356 | $55,563,693 | $70,030,762 |
| SMBG | $12,107,734 | $15,637,058 | $20,507,344 | $26,588,295 | $33,282,780 |
| NBI | $12,902,039 | $16,790,176 | $22,165,012 | $28,975,399 | $36,747,982 |
| CGM Device Cost Reduction of 30% + Government Funding of Insulin, Insulin Pump, and Blood Glucose Test Strips + 75% of CGM Sensor Costs | |||||
| CGM | $8,526,961 | $11,200,526 | $14,903,805 | $19,674,577 | $25,333,014 |
| SMBG | $5,779,420 | $7,409,960 | $9,656,020 | $12,418,114 | $15,341,921 |
| NBI | $2,747,541 | $3,790,566 | $5,247,785 | $7,256,462 | $9,991,093 |
| CGM Device Cost Reduction of 20% + Government Funding of Insulin, Insulin Pump, and Blood Glucose Test Strips + 75% of CGM Sensor Costs | |||||
| CGM | $9,825,054 | $12,911,595 | $17,187,316 | $22,699,861 | $29,249,668 |
| SMBG | $6,339,107 | $8,186,914 | $10,736,794 | $13,920,527 | $17,425,481 |
| NBI | $3,485,947 | $4,724,680 | $6,450,522 | $8,779,334 | $11,824,186 |
| CGM Device Cost Reduction of 10% + Government Funding of Insulin, Insulin Pump, and Blood Glucose Test Strips + 75% of CGM Sensor Costs | |||||
| CGM | $11,123,147 | $14,622,663 | $19,470,827 | $25,725,145 | $33,166,321 |
| SMBG | $6,339,107 | $8,186,914 | $10,736,794 | $13,920,527 | $17,425,481 |
| NBI | $4,784,040 | $6,435,749 | $8,734,034 | $11,804,618 | $15,740,840 |
Abbreviations: CGM, continuous glucose monitoring; NBI, net budget impact; SMBG, self-monitoring of blood glucose.
Government funding of insulin treatment, insulin pump and blood glucose test strips.
Table A34:
Net Budget Impact of Funding Continuous Glucose Monitoring in Ontario—Scenario 5, Manufacturer's Scenario With an Annual Increase in CGM Adoption of 40%, Entire Type 1 Diabetes Population and Population With Hypoglycemia Unawareness
| Intervention | Total Budget Impact | ||||
|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | |
| Reference Case | |||||
| Funding Continuous Glucose Monitoring in the Entire Type 1 Diabetes Population | |||||
| CGM | $481,949,914 | $527,927,021 | $563,034,675 | $590,099,986 | $610,633,755 |
| SMBG | $166,774,215 | $206,074,531 | $237,230,078 | $262,185,663 | $281,753,568 |
| NBI | $315,175,698 | $321,852,490 | $325,804,597 | $327,914,323 | $328,880,187 |
| Funding Continuous Glucose Monitoring in the Entire Population With Hypoglycemia Unawareness | |||||
| CGM | $120,487,478 | $131,981,755 | $140,758,669 | $147,524,997 | $152,658,439 |
| SMBG | $41,693,554 | $51,518,633 | $59,307,520 | $65,546,416 | $70,408,816 |
| NBI | $78,793,925 | $80,463,123 | $81,451,149 | $81,978,581 | $82,249,622 |
| All Direct Medical Cost | |||||
| Funding Continuous Glucose Monitoring in the Entire Type 1 Diabetes Population | |||||
| CGM | $879,328,537 | $940,384,046 | $982,740,880 | $1,015,543,322 | $1,040,991,872 |
| SMBG | $392,545,801 | $439,784,653 | $476,011,572 | $504,717,816 | $527,683,516 |
| NBI | $486,782,736 | $500,599,393 | $506,729,308 | $510,825,506 | $513,308,356 |
| Funding Continuous Glucose Monitoring in the Entire Population With Hypoglycemia Unawareness | |||||
| CGM | $219,832,134 | $235,096,011 | $245,685,220 | $253,885,830 | $260,247,968 |
| SMBG | $98,136,450 | $109,946,163 | $119,002,893 | $126,179,454 | $131,823,378 |
| NBI | $121,695,684 | $125,149,848 | $126,682,327 | $127,706,376 | $128,424,590 |
| Government Funding of Insulin, Insulin Pump, and Blood Glucose Test Strips + 75% of CGM Sensor Costs Funding Continuous Glucose Monitoring in the Entire Type 1 Diabetes Population | |||||
| CGM | $397,045,650 | $440,870,963 | $473,354,092 | $499,121,262 | $518,940,535 |
| SMBG | $148,300,352 | $186,348,792 | $216,167,728 | $239,750,361 | $257,875,960 |
| NBI | $248,745,298 | $254,522,171 | $257,186,365 | $259,370,901 | $261,064,575 |
| Funding Continuous Glucose Monitoring in the Entire Population With Hypoglycemia Unawareness | |||||
| CGM | $99,261,412 | $110,217,741 | $118,338,523 | $124,780,315 | $129,735,134 |
| SMBG | $37,075,088 | $46,587,198 | $54,041,932 | $59,937,590 | $64,439,414 |
| NBI | $62,186,324 | $63,630,543 | $64,296,591 | $64,842,725 | $65,295,719 |
| CGM Device Cost Reduction of 30% | |||||
| Funding Continuous Glucose Monitoring in the Entire Type 1 Diabetes Population | |||||
| CGM | $615,529,976 | $636,204,451 | $656,832,926 | $677,391,889 | $697,857,340 |
| SMBG | $392,545,801 | $406,044,745 | $419,596,388 | $433,190,742 | $447,178,667 |
| NBI | $222,984,175 | $230,159,706 | $237,236,538 | $244,201,146 | $250,678,673 |
| Funding Continuous Glucose Monitoring in the Entire Population With Hypoglycemia Unawareness | |||||
| CGM | $153,882,494 | $159,051,113 | $164,208,231 | $169,347,972 | $174,464,335 |
| SMBG | $98,136,450 | $101,511,186 | $104,899,097 | $108,297,686 | $111,704,418 |
| NBI | $55,746,044 | $57,539,926 | $59,309,134 | $61,050,287 | $62,759,917 |
| CGM Device Cost Reduction of 20% | |||||
| Funding Continuous Glucose Monitoring in the Entire Type 1 Diabetes Population | |||||
| CGM | $703,462,830 | $727,090,802 | $750,666,201 | $774,162,159 | $797,551,245 |
| SMBG | $392,545,801 | $406,044,745 | $419,596,388 | $433,190,742 | $447,178,667 |
| NBI | $310,917,029 | $321,046,056 | $331,069,813 | $340,971,416 | $350,372,578 |
| Funding Continuous Glucose Monitoring in the Entire Population With Hypoglycemia Unawareness | |||||
| CGM | $175,865,707 | $181,772,700 | $187,666,550 | $193,540,540 | $199,387,811 |
| SMBG | $98,136,450 | $101,511,186 | $104,899,097 | $108,297,686 | $111,704,418 |
| NBI | $77,729,257 | $80,261,514 | $82,767,453 | $85,242,854 | $87,683,393 |
| CGM Device Cost Reduction of 10% | |||||
| Funding Continuous Glucose Monitoring in the Entire Type 1 Diabetes Population | |||||
| CGM | $791,395,683 | $817,977,152 | $844,499,476 | $870,932,428 | $897,245,151 |
| SMBG | $392,545,801 | $406,044,745 | $419,596,388 | $433,190,742 | $447,178,667 |
| NBI | $398,849,882 | $411,932,406 | $424,903,088 | $437,741,686 | $450,066,484 |
| Funding Continuous Glucose Monitoring in the Entire Population With Hypoglycemia Unawareness | |||||
| CGM | $197,848,921 | $204,494,288 | $211,124,869 | $217,733,107 | $224,311,288 |
| SMBG | $98,136,450 | $101,511,186 | $104,899,097 | $108,297,686 | $111,704,418 |
| NBI | $99,712,471 | $102,983,102 | $106,225,772 | $109,435,422 | $112,606,870 |
| CGM Device Cost Reduction of 30% + Government Funding of Insulin, Insulin Pump, and Blood Glucose Test Strips + 75% of CGM Sensor Costs | |||||
| Funding Continuous Glucose Monitoring in the Entire Type 1 Diabetes Population | |||||
| CGM | $298,759,970 | $308,786,539 | $318,796,249 | $328,783,418 | $338,733,806 |
| SMBG | $159,684,304 | $164,966,568 | $170,223,295 | $175,452,951 | $180,730,177 |
| NBI | $139,075,666 | $143,819,971 | $148,572,954 | $153,330,467 | $158,003,629 |
| Funding Continuous Glucose Monitoring in the Entire Population With Hypoglycemia Unawareness | |||||
| CGM | $74,689,992 | $77,196,635 | $79,699,062 | $82,195,855 | $84,683,452 |
| SMBG | $39,921,076 | $41,241,642 | $42,555,824 | $43,863,238 | $45,161,379 |
| NBI | $34,768,917 | $35,954,993 | $37,143,238 | $38,332,617 | $39,522,072 |
| CGM Device Cost Reduction of 20% + Government Funding of Insulin, Insulin Pump, and Blood Glucose Test Strips + 75% of CGM Sensor Costs | |||||
| Funding Continuous Glucose Monitoring in the Entire Type 1 Diabetes Population | |||||
| CGM | $348,084,338 | $359,767,619 | $371,430,354 | $383,064,978 | $394,655,327 |
| SMBG | $206,194,912 | $213,047,586 | $219,865,778 | $226,640,450 | $233,447,017 |
| NBI | $141,889,427 | $146,720,033 | $151,564,575 | $156,424,528 | $161,208,310 |
| Funding Continuous Glucose Monitoring in the Entire Population With Hypoglycemia Unawareness | |||||
| CGM | $87,021,085 | $89,941,905 | $92,857,588 | $95,766,244 | $98,663,832 |
| SMBG | $51,548,728 | $53,261,896 | $54,966,445 | $56,660,112 | $58,340,589 |
| NBI | $35,472,357 | $36,680,008 | $37,891,144 | $39,106,132 | $40,323,242 |
| CGM Device Cost Reduction of 10% + Government Funding of Insulin, Insulin Pump, and Blood Glucose Test Strips + 75% of CGM Sensor Costs | |||||
| Funding Continuous Glucose Monitoring in the Entire Type 1 Diabetes Population | |||||
| CGM | $397,408,707 | $410,748,698 | $424,064,458 | $437,346,537 | $450,576,848 |
| SMBG | $206,194,912 | $213,047,586 | $219,865,778 | $226,640,450 | $233,447,017 |
| NBI | $191,213,795 | $197,701,112 | $204,198,680 | $210,706,087 | $217,129,831 |
| Funding Continuous Glucose Monitoring in the Entire Population With Hypoglycemia Unawareness | |||||
| CGM | $99,352,177 | $102,687,175 | $106,016,115 | $109,336,634 | $112,644,212 |
| SMBG | $51,548,728 | $53,261,896 | $54,966,445 | $56,660,112 | $58,340,589 |
| NBI | $47,803,449 | $49,425,278 | $51,049,670 | $52,676,522 | $54,303,623 |
Abbreviations: CGM, continuous glucose monitoring; SMBG, self-monitoring of blood glucose; NBI, net budget impact.
Appendix 9: Public and Patient Engagement—Interview Materials
Figure A5: Call for Participation.
Figure A6: Interview Guide.
Author contributions
This report was developed by a multidisciplinary team from Health Quality Ontario. The clinical epidemiologists were Stacey Vandersluis and Conrad Kabali, the health economists were Sandjar Djalalov and Olga Gajic-Veljanoski, the patient, caregiver, and public engagement specialist was David Wells, and the medical librarian was Corinne Holubowich.
KEY MESSAGES
What Is This Health Technology Assessment About?
Type 1 diabetes is a condition in which the pancreas produces little or no insulin. Insulin is a hormone that helps the body's cells use glucose (a type of sugar) for energy. Without insulin, glucose builds up in the blood and can cause serious damage to the body. People with type 1 diabetes must take insulin via injection or an insulin pump, and they should monitor their blood glucose levels several times a day.
Most people with type 1 diabetes use a blood glucose meter to check their blood glucose levels. They prick their finger to obtain a drop of blood, and they apply the blood to a test strip inserted into the meter. This is called self-monitoring of blood glucose. Continuous glucose monitoring is another way to measure blood glucose. It measures a person's blood glucose levels every few minutes via a sensor inserted under the skin.
This health technology assessment evaluates how effective continuous glucose monitoring is for people with type 1 diabetes, if it is good value for money, and the preferences and values of people living with type 1 diabetes and/or their caregivers.
What Did This Health Technology Assessment Find?
People who used continuous glucose monitoring spent more time in the target blood glucose range, less time out of range, and had fewer severe low blood glucose episodes than people who used self-monitoring of blood glucose.
Compared with self-monitoring of blood glucose, the costs of continuous glucose monitoring were higher, with only small increases in health benefits. Publicly funding continuous glucose monitoring for people with type 1 diabetes in Ontario would result in additional costs to the health system over the next 5 years.
Adult patients and parents of children with type 1 diabetes reported very positive experiences with continuous glucose monitoring, but the high cost of using the devices was a barrier to their widespread use.
Contributor Information
Health Quality Ontario:
Stacey Vandersluis, Conrad Kabali, Sandjar Djalalov, Olga Gajic-Veljanoski, David Wells, and Corinne Holubowich
About Health Quality Ontario
Health Quality Ontario is the provincial advisor on the quality of health care. We are motivated by a single-minded purpose: Better health for all Ontarians.
Who We Are
We are a scientifically rigorous group with diverse areas of expertise. We strive for complete objectivity, and look at things from a vantage point that allows us to see the forest and the trees. We work in partnership with health care providers and organizations across the system, and engage with patients themselves, to help initiate substantial and sustainable change to the province's complex health system.
What We Do
We define the meaning of quality as it pertains to health care, and provide strategic advice so all the parts of the system can improve. We also analyze virtually all aspects of Ontario's health care. This includes looking at the overall health of Ontarians, how well different areas of the system are working together, and most importantly, patient experience. We then produce comprehensive, objective reports based on data, facts and the voice of patients, caregivers and those who work each day in the health system. As well, we make recommendations on how to improve care using the best evidence. Finally, we support large scale quality improvements by working with our partners to facilitate ways for health care providers to learn from each other and share innovative approaches
Why It Matters
We recognize that, as a system, we have much to be proud of, but also that it often falls short of being the best it can be. Plus certain vulnerable segments of the population are not receiving acceptable levels of attention. Our intent at Health Quality Ontario is to continuously improve the quality of health care in this province regardless of who you are or where you live. We are driven by the desire to make the system better, and by the inarguable fact that better has no limit.
REFERENCES
- (1).Canadian Diabetes Association. Diabetes Statistics in Canada [Internet]. Toronto (ON): The Association; 2016. [cited 2016 Dec 7]. Available from: http://www.diabetes.ca/how-you-can-help/advocate/why-federal-leadership-is-essential/diabetes-statistics-in-canada [Google Scholar]
- (2).JDRF. Type 1 diabetes: about T1D [Internet]. Toronto (ON): JDRF; 2016. [cited 2016 Dec 7]. Available from: http://www.jdrf.ca/news-and-media/fact-sheets/type-1-diabetes/ [Google Scholar]
- (3).Canadian Diabetes Association. Types of diabetes [Internet]. Toronto (ON): The Association; 2016. [cited 2016 Nov 10]. Available from: http://www.diabetes.ca/about-diabetes/types-of-diabetes#sthash.wQM6BPDc.dpuf [Google Scholar]
- (4).Rosella LC, Lebenbaum M, Fitzpatrick T, O'Reilly D, Wang J, Booth GL, et al. Impact of diabetes on healthcare costs in a population-based cohort: a cost analysis. Diabet Med. 2015;33(3):395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Maahs DM, West NA, Lawrence JM, Mayer-Davis EJ. Epidemiology of type 1 diabetes. Endocrinol Metab Clin North Am. 2010;39(3):481–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Canadian Diabetes Association Clinical Practice Guidelines Expert Committee. Canadian Diabetes Association 2013 clinical practice guidelines for the prevention and management of diabetes in Canada. Can J Diabetes. 2013;37 Suppl 1:S1–S212. [DOI] [PubMed] [Google Scholar]
- (7).Spero D. Is continuous glucose monitoring worth it? [Internet]. San Francisco (CA): Diabetes Self-Management; 2011. [cited 2016 Dec 9]. Available from: http://www.diabetesselfmanagement.com/blog/is-continuous-glucose-monitoring-worth-it/ [Google Scholar]
- (8).National Institute of Diabetes and Digestive and Kidney Diseases. Continuous glucose monitoring [Internet]. Bethesda (MD): The Institute; 2008. [cited 2016 Dec 9]. Available from: http://www.niddk.nih.gov/health-information/diabetes/manage-monitoring-diabetes/continuous-glucose-monitoring [Google Scholar]
- (9).Heo YJ, Shibata H, Okitsu T, Kawanishi T, Takeuchi S. Long-term in vivo glucose monitoring using fluorescent hydrogel fibers. Proc Natl Acad Sci U S A. 2011;108(33):13399–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Health Canada. Medical devices active licence listing [Internet]. Ottawa (ON): Government of Canada; c2016 - [cited 2016 Dec 9]. Available from: http://www.canada.ca/en/health-canada/services/drugs-health-products/medical-devices/licences/medical-devices-active-licence-listing.html [Google Scholar]
- (11).Ontario Disability Support Program [Internet]. Toronto (ON): Ministry of Community and Social Services; c2012–14 [updated 2015 Dec 31; cited 2017 Oct 4]. Available from: http://www.mcss.gov.on.ca/en/mcss/programs/social/odsp/ [Google Scholar]
- (12).Government of Ontario. Insulin pumps and diabetes supplies [Internet]. Toronto (ON): Queen's Printer for Ontario; [cited 2016 Dec 9]. Available from: http://www.ontario.ca/page/insulin-pumps-and-diabetes-supplies [Google Scholar]
- (13).National Institute for Health and Care Excellence. Type 1 diabetes in adults: diagnosis and management [Internet]. London (UK): The Institute; 2015. [cited 2016 Dec]. Available from: http://www.nice.org.uk/guidance/ng17/resources/type-1-diabetes-in-adults-diagnosis-and-management-1837276469701 [PubMed] [Google Scholar]
- (14).National Institute for Health and Care Excellence. Diabetes (type 1 and type 2) in children and young people: diagnosis and management [Internet]. London (UK): The Institute; 2015. [cited 2016 Dec]. Available from: http://www.nice.org.uk/guidance/ng18/resources/diabetes-type-1-and-type-2-in-children-and-young-people-diagnosis-and-management-1837278149317 [PubMed] [Google Scholar]
- (15).Intergrated Diabetes Services. CGM insurance coverage [Internet]. Wynnewood (PA): Integrated Diabetes Services; 2017. [cited 2017 May 24]. Available from: http://integrateddiabetes.com/cgm-insurance-coverage/ [Google Scholar]
- (16).McGowan J, Sampson M, Salzwedel DM, Cogo E, Foerster V, Lefebvre C. PRESS peer review of electronic search strategies: 2015 guideline statement. J Clin Epidemiol. 75:40–6. [DOI] [PubMed] [Google Scholar]
- (17).O'Neill J, Tabish H, Welch V, Petticrew M, Pottie K, Clarke M, et al. Applying an equity lens to interventions: using PROGRESS ensures consideration of socially stratifying factors to illuminate inequities in health. J Clin Epidemiol. 2014;67(1):56–64. [DOI] [PubMed] [Google Scholar]
- (18).Nordic Cochrane Centre. Review Manager (RevMan) [Computer program]. Version 5.3. Copenhagen (Denmark): The Cochrane Collaboration; 2014. [Google Scholar]
- (19).Guyatt GH, Oxman AD, Schünemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol. 2011;64(4):380–2. [DOI] [PubMed] [Google Scholar]
- (20).Murad MH, Mustafa RA, Schunemann HJ, Sultan S, Santesso N. Rating the certainty in evidence in the absence of a single estimate of effect. Evid Based Med. 2017;22(3):85–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Park J, Lee Y, Seo H, Jang B, Son H, Kim S, Shin S, Hahn S. Risk of bias assessment tool for non-randomized studies (RoBANS): development and validation of a new instrument. In: Abstracts of the 19th Cochrane Colloquium; Oct 19–22, 2011; Madrid (Spain). Wiley; 2011.
- (22).Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions, version 5.1.0 [updated 2011 Mar]. The Cochrane Collaboration; 2011. Available from www.handbook.cochrane.org.
- (23).Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Beck RW, Riddlesworth T, Ruedy K, Ahmann A, Bergenstal R, Haller S, et al. Effect of continuous glucose monitoring on glycemic control in adults with type 1 diabetes using insulin injections: the DIAMOND randomized clinical trial. J Am Med Assoc. 2017;317(4):371–8. [DOI] [PubMed] [Google Scholar]
- (25).Bergenstal RM, Tamborlane WV, Ahmann A, Buse JB, Dailey G, Davis SN, et al. Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes. N Engl J Med. 2010;363(4):311–20. [DOI] [PubMed] [Google Scholar]
- (26).Bukara-Radujkovic G, Zdravkovic D, Lakic S. Short-term use of continuous glucose monitoring system adds to glycemic control in young type 1 diabetes mellitus patients in the long run: a clinical trial. Vojnosanit Pregl. 2011;68(8):650–4. [DOI] [PubMed] [Google Scholar]
- (27).Hermanides J, Norgaard K, Bruttomesso D, Mathieu C, Frid A, Dayan CM, et al. Sensor-augmented pump therapy lowers HbA1c in suboptimally controlled type1 diabetes: a randomized controlled trial. Diabet Med. 2011;28(10):1158–67. [DOI] [PubMed] [Google Scholar]
- (28).Hommel E, Olsen B, Battelino T, Conget I, Schutz-Fuhrmann I, Hoogma R, et al. Impact of continuous glucose monitoring on quality of life, treatment satisfaction, and use of medical care resources: analyses from the SWITCH study. Acta Diabetol. 2014;51(5):845–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Kordonouri O, Hartmann R, Pankowska E, Rami B, Kapellen T, Coutant R, et al. Sensor augmented pump therapy from onset of type 1 diabetes: late follow-up results of the Pediatric Onset Study. Pediatr Diabetes. 2012;13(7):515–8. [DOI] [PubMed] [Google Scholar]
- (30).Langeland LBL, Salvesen O, Selle H, Carlsen SM, Fougner KJ. Short-term continuous glucose monitoring: effects on glucose and treatment satisfaction in patients with type 1 diabetes mellitus: a randomized controlled trial. Int J Clin Pract. 2012;66(8):741–7. [DOI] [PubMed] [Google Scholar]
- (31).Lind M, Polonsky W, Hirsch IB, Heise T, Bolinder J, Dahlqvist S, et al. Continuous glucose monitoring vs conventional therapy for glycemic control in adults with type 1 diabetes treated with multiple daily insulin injections: the GOLD randomized clinical trial. J Am Med Assoc. 2017;317(4):379–87. [DOI] [PubMed] [Google Scholar]
- (32).Little SA, Leelarathna L, Walkinshaw E, Tan HK, Chapple O, Lubina-Solomon A, et al. Recovery of hypoglycemia awareness in long-standing type 1 diabetes: a multicenter 2 × 2 factorial randomized controlled trial comparing insulin pump with multiple daily injections and continuous with conventional glucose self-monitoring (HypoCOMPaSS). Diabetes Care. 2014;37(8):2114–22. [DOI] [PubMed] [Google Scholar]
- (33).Ly TT, Nicholas JA, Retterath A, Lim EM, Davis EA, Jones TW. Effect of sensor-augmented insulin pump therapy and automated insulin suspension vs standard insulin pump therapy on hypoglycemia in patients with type 1 diabetes: a randomized clinical trial. J Am Med Assoc. 2013;310(12):1240–7. [DOI] [PubMed] [Google Scholar]
- (34).Olivier P, Lawson ML, Huot C, Richardson C, Nakhla M, Romain J. Lessons learned from a pilot RCT of simultaneous versus delayed initiation of continuous glucose monitoring in children and adolescents with type 1 diabetes starting insulin pump therapy. J Diabetes Sci Technol. 2014;8(3):523–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Rosenlund S, Hansen TW, Rossing P, Andersen S. Effect of sensor-augmented pump treatment versus multiple daily injections on albuminuria: a 1-year randomized study. J Clin Endocrinol Metab. 2015;100(11):4181–8. [DOI] [PubMed] [Google Scholar]
- (36).Rubin RR, Peyrot M. Health-related quality of life and treatment satisfaction in the sensor-augmented pump therapy for A1C reduction 3 (STAR 3) trial. Diabetes Technol Ther. 2012;14(2):143–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Slover RH, Welsh JB, Criego A, Weinzimer SA, Willi SM, Wood MA, et al. Effectiveness of sensor-augmented pump therapy in children and adolescents with type 1 diabetes in the STAR 3 study. Pediatr Diabetes. 2012;13(1):6–11. [DOI] [PubMed] [Google Scholar]
- (38).Tumminia A, Crimi S, Sciacca L, Buscema M, Frittitta L, Squatrito S, et al. Efficacy of real-time continuous glucose monitoring on glycaemic control and glucose variability in type 1 diabetic patients treated with either insulin pumps or multiple insulin injection therapy: a randomized controlled crossover trial. Diabetes Metab Res Rev. 2015;31(1):61–8. [DOI] [PubMed] [Google Scholar]
- (39).Van Beers CA, DeVries JH, Kleijer SJ, Smits MM, Geelhoed-Duijvestijn PH, Kramer MH, et al. Continuous glucose monitoring for patients with type 1 diabetes and impaired awareness of hypoglycaemia (IN CONTROL): a randomised, open-label, crossover trial. Lancet Diabetes Endocrinol. 2016;4(11):893–902. [DOI] [PubMed] [Google Scholar]
- (40).McQueen RB, Ellis SL, Maahs DM, Anderson HD, Nair KV, Campbell JD. Frequency of continuous glucose monitoring use and change in hemoglobin A1C for adults with type 1 diabetes in a clinical practice setting. Endocr Pract. 2014;20(10):1007–15. [DOI] [PubMed] [Google Scholar]
- (41).Quiros C, Gimenez M, Orois A, Conget I. Metabolic control after years of completing a clinical trial on sensor-augmented pump therapy. Endocrinol Nutr. 2015;62(9):447–50. [DOI] [PubMed] [Google Scholar]
- (42).Radermecker RP, Remy AS, Scheen AJ, Bringer J, Renard E. Continuous glucose monitoring reduces both hypoglycaemia and HbA1c in hypoglycaemia-prone type 1 diabetic patients treated with a portable pump. Diabetes Metab. 2010;36(5):409–13. [DOI] [PubMed] [Google Scholar]
- (43).Soupal J, Petruzelkova L, Flekac M, Pelcl T, Matoulek M, Dankova M, et al. Comparison of different treatment modalities for type 1 diabetes, including sensor-augmented insulin regimens, in 52 weeks of follow-up: a COMISAIR study. Diabetes Technol Ther. 2016;18(9):532–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Aschner PJ, Ruiz AJ. Metabolic memory for vascular disease in diabetes. Diabetes Technol Ther. 2012;14 Suppl 1:S68–74. [DOI] [PubMed] [Google Scholar]
- (45).Chamberlain JJ, Dopita D, Gilgen E, Neuman A. Impact of frequent and persistent use of continuous glucose monitoring (CGM) on hypoglycemia fear, frequency of emergency medical treatment, and SMBG frequency after one year. J Diabetes Sci Technol. 2015;10(2):383–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Giani E, Snelgrove R, Volkening LK, Laffel LM. Continuous glucose monitoring (CGM) adherence in youth with type 1 diabetes: associations with biomedical and psychosocial variables. J Diabetes Sci Technol. 2017;11(3):476–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Halford J, Harris C. Determining clinical and psychological benefits and barriers with continuous glucose monitoring therapy. Diabetes Technol Ther. 2010;12(3):201–5. [DOI] [PubMed] [Google Scholar]
- (48).Picard S, Hanaire H, Baillot-Rudoni S, Gilbert-Bonnemaison E, Not D, Reznik Y, et al. Evaluation of the adherence to continuous glucose monitoring in the management of type 1 diabetes patients on sensor-augmented pump therapy: the SENLOCOR study. Diabetes Technol Ther. 2016;18(3):127–35. [DOI] [PubMed] [Google Scholar]
- (49).Scaramuzza AE, Iafusco D, Rabbone I, Bonfanti R, Lombardo F, Schiaffini R, et al. Use of integrated real-time continuous glucose monitoring/insulin pump system in children and adolescents with type 1 diabetes: a 3-year follow-up study. Diabetes Technol Ther. 2011;13(2):99–103. [DOI] [PubMed] [Google Scholar]
- (50).Glanville J, Arber M, Veale T, Garcia S. Sensitivity of a search filter designed to identify studies reporting health state utility values. Paper presented at: Mosaic, 2016 Annual Conference of the Medical Library Association; 2016 May 13–18; Toronto, ON: Medical Library Association.
- (51).McQueen RB, Ellis SL, Campbell JD, Nair KV, Sullivan PW. Cost-effectiveness of continuous glucose monitoring and intensive insulin therapy for type 1 diabetes. Cost Eff Resour Alloc. 2011;9:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Kamble S, Schulman KA, Reed SD. Cost-effectiveness of sensor-augmented pump therapy in adults with type 1 diabetes in the United States. Value Health. 2012;15(5):632–8. [DOI] [PubMed] [Google Scholar]
- (53).Roze S, Smith-Palmer J, Valentine WJ, Cook M, Jethwa M, de Portu S, et al. Long-term health economic benefits of sensor-augmented pump therapy vs continuous subcutaneous insulin infusion alone in type 1 diabetes: a UK perspective. J Med Econ. 2016;19(3):236–42. [DOI] [PubMed] [Google Scholar]
- (54).Roze S, Saunders R, Brandt AS, de Portu S, Papo NL, Jendle J. Health-economic analysis of real-time continuous glucose monitoring in people with type 1 diabetes. Diabet Med. 2015;32(5):618–26. [DOI] [PubMed] [Google Scholar]
- (55).Roze S, Smith-Palmer J, Valentine W, Payet V, de Portu S, Papo N, et al. Cost-effectiveness of sensor-augmented pump therapy with low glucose suspend versus standard insulin pump therapy in two different patient populations with type 1 diabetes in France. Diabetes Technol Ther. 2016;18(2):75–84. [DOI] [PubMed] [Google Scholar]
- (56).Roze S, de Portu S, Smith-Palmer J, Delbaere A, Valentine W, Ridderstrale M. Cost-effectiveness of sensor-augmented pump therapy versus standard insulin pump therapy in patients with type 1 diabetes in Denmark. Diabetes Res Clin Pract. 2017;128:6–14. [DOI] [PubMed] [Google Scholar]
- (57).Huang ES, O'Grady M, Basu A, Winn A, John P, Lee J, et al. The cost-effectiveness of continuous glucose monitoring in type 1 diabetes. Diabetes Care. 2010;33(6):1269–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Riemsma R, Corro Ramos I, Birnie R, Buyukkaramikli N, Armstrong N, Ryder S, et al. Integrated sensor-augmented pump therapy systems (the MiniMed ParadigmTM Veo system and the VibeTM and G4 PLATINUM CGM [continuous glucose monitoring] system) for managing blood glucose levels in type 1 diabetes: a systematic review and economic evaluation. Health Technol Assess. 2016;20(17):v–xxxi, 1–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Vigersky RA. The benefits, limitations, and cost-effectiveness of advanced technologies in the management of patients with diabetes mellitus. J Diabetes Sci Technol. 2015;9(2):320–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Ly TT, Brnabic AJM, Eggleston A, Kolivos A, McBride ME, Schrover R, et al. A cost-effectiveness analysis of sensor-augmented insulin pump therapy and automated insulin suspension versus standard pump therapy for hypoglycemic unaware patients with type 1 diabetes. Value Health. 2014;17(5):561–9. [DOI] [PubMed] [Google Scholar]
- (61).Bronstone A, Graham C. The potential cost implications of averting severe hypoglycemic events requiring hospitalization in high-risk adults with type 1 diabetes using real-time continuous glucose monitoring. J Diabetes Sci Technol. 2016;10(4):905–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Yeaw J, Lee WC, Aagren M, Christensen T. Cost of self-monitoring of blood glucose in the United States among patients on an insulin regimen for diabetes. J Manag Care Pharm. 2012;18(1):21–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Kamble S, Weinfurt KP, Schulman KA, Reed SD. Patient time costs associated with sensor-augmented insulin pump therapy for type 1 diabetes: results from the STAR 3 randomized trial. Med Decis Making. 2013;33(2):215–24. [DOI] [PubMed] [Google Scholar]
- (64).Diabetes Control and Complications Trial Research Group, Nathan DM, Genuth S, Lachin J, Cleary P, Crofford O, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–86. [DOI] [PubMed] [Google Scholar]
- (65).Palmer AJ, Roze S, Valentine WJ, Minshall ME, Foos V, Lurati FM, et al. The CORE diabetes model: projecting long-term clinical outcomes, costs and cost-effectiveness of interventions in diabetes mellitus (types 1 and 2) to support clinical and reimbursement decision-making. Curr Med Res Opin. 2004;20 Suppl 1:S5–26. [DOI] [PubMed] [Google Scholar]
- (66).Klein BE, Klein R, McBride PE, Cruickshanks KJ, Palta M, Knudtson MD, et al. Cardiovascular disease, mortality, and retinal microvascular characteristics in type 1 diabetes: Wisconsin epidemiologic study of diabetic retinopathy. Arch Intern Med. 2004;164(17):1917–24. [DOI] [PubMed] [Google Scholar]
- (67).JDRF CGM Study Group. JDRF randomized clinical trial to assess the efficacy of real-time continuous glucose monitoring in the management of type 1 diabetes: research design and methods. Diabetes Technol Ther. 2008;10(4):310–21. [DOI] [PubMed] [Google Scholar]
- (68).Tamborlane WV, Beck RW, Bode BW, Buckingham B, Chase HP, Clemons R, et al. Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med. 2008;359(14):1464–76. [DOI] [PubMed] [Google Scholar]
- (69).Beck RW, Hirsch IB, Laffel L, Tamborlane WV, Bode BW, Buckingham B, et al. The effect of continuous glucose monitoring in well-controlled type 1 diabetes. Diabetes Care. 2009;32(8):1378–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (70).Eastman RC, Javitt JC, Herman WH, Dasbach EJ, Zbrozek AS, Dong F, et al. Model of complications of NIDDM. I. Model construction and assumptions. Diabetes Care. 1997;20(5):725–34. [DOI] [PubMed] [Google Scholar]
- (71).Pickup JC, Freeman SC, Sutton AJ. Glycaemic control in type 1 diabetes during real time continuous glucose monitoring compared with self monitoring of blood glucose: meta-analysis of randomised controlled trials using individual patient data. Br Med J (Clin Res Ed). 2011;343:d3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (72).Persson U, Glenngard AH, Hjortsberg C. Estimating the willingness to pay for a QALY in Sweden: a pilot study. In: Scalone L, Mantovani LG, editors. 25th Scientific Plenary Meeting of the EuroQol Group Proceedings. Baveno (Italy): EuroQol Group; 2008. p. 121–32.
- (73).Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS)–explanation and elaboration: a report of the ISPOR Health Economic Evaluation Publication Guidelines Good Reporting Practices Task Force. Value Health. 2013;16(2):231–50. [DOI] [PubMed] [Google Scholar]
- (74).Nathan DM. The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: overview. Diabetes Care. 2014;37(1):9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (75).Paulden M, Galvanni V, Chakraborty S, Kudinga B, McCabe C. Discounting and the evaluation of health care programs [Internet]. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2015. [cited 2016 Dec]. Available from: http://www.cadth.ca/sites/default/files/pdf/CP0008_Economic_Evaluation_Guidelines_Discount_Rate_Report.pdf [Google Scholar]
- (76).Nathan DM, Zinman B, Cleary PA, Backlund JY, Genuth S, Miller R, et al. Modern-day clinical course of type 1 diabetes mellitus after 30 years’ duration: the diabetes control and complications trial/epidemiology of diabetes interventions and complications and Pittsburgh epidemiology of diabetes complications experience (1983–2005). Arch Intern Med. 2009;169(14):1307–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (77).Scheiman M, Scheiman M, Whittaker S, Freeman PB, Sokol-McKay DA. Low vision rehabilitation: a practical guide for occupational therapists. Thorofare (NJ): Slack; 2007. [Google Scholar]
- (78).Early Treatment Diabetic Retinopathy Study Research Group. Early photocoagulation for diabetic retinopathy. ETDRS report number 9. Ophthalmology. 1991;98 Suppl 5:766–85. [PubMed]
- (79).Jonasson JM, Ye W, Sparen P, Apelqvist J, Nyren O, Brismar K. Risks of nontraumatic lower-extremity amputations in patients with type 1 diabetes: a population-based cohort study in Sweden. Diabetes Care. 2008;31(8):1536–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (80).Wu SY, Sainfort F, Tomar RH, Tollios JL, Fryback DG, Klein R, et al. Development and application of a model to estimate the impact of type 1 diabetes on health-related quality of life. Diabetes Care. 1998;21(5):725–31. [DOI] [PubMed] [Google Scholar]
- (81).Hoerger TJ, Harris R, Hicks KA, Donahue K, Sorensen S, Engelgau M. Screening for type 2 diabetes mellitus: a cost-effectiveness analysis. Ann Intern Med. 2004;140(9):689–99. [DOI] [PubMed] [Google Scholar]
- (82).Martin CL, Albers JW, Pop-Busui R. Neuropathy and related findings in the diabetes control and complications trial/epidemiology of diabetes interventions and complications study. Diabetes Care. 2014;37(1):31–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (83).Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. Br Med J (Clin Res Ed). 2000;321(7258):405–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (84).Orchard TJ, Nathan DM, Zinman B, Cleary P, Brillon D, Backlund JY, et al. Association between 7 years of intensive treatment of type 1 diabetes and long-term mortality. J Am Med Assoc. 2015;313(1):45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (85).Vamos EP, Bottle A, Majeed A, Millett C. Trends in lower extremity amputations in people with and without diabetes in England, 1996–2005. Diabetes Res Clin Pract. 2010;87(2):275–82. [DOI] [PubMed] [Google Scholar]
- (86).Wolowacz S, Pearson I, Shannon P, Chubb B, Gundgaard J, Davies M, et al. Development and validation of a cost-utility model for type 1 diabetes mellitus. Diabet Med. 2015;32(8):1023–35. [DOI] [PubMed] [Google Scholar]
- (87).Statistics Canada. Table 102-0552: Deaths and mortality rate (age standardization using 1991 population), by selected grouped causes and sex, Canada, provinces and territories, annual [Internet]. Ottawa (ON): Statistics Canada; c2017. - [cited 2017 Apr]. Available from: http://www5.statcan.gc.ca/cansim/a26?lang=eng&id=1020552 [Google Scholar]
- (88).Clarke P, Gray A, Holman R. Estimating utility values for health states of type 2 diabetic patients using the EQ-5D (UKPDS 62). Med Decis Making. 2002;22(4):340–9. [DOI] [PubMed] [Google Scholar]
- (89).Currie CJ, Poole CD, Woehl A, Morgan CL, Cawley S, Rousculp MD, et al. The health-related utility and health-related quality of life of hospital-treated subjects with type 1 or type 2 diabetes with particular reference to differing severity of peripheral neuropathy. Diabetologia. 2006;49(10):2272–80. [DOI] [PubMed] [Google Scholar]
- (90).Currie CJ, Morgan CL, Poole CD, Sharplin P, Lammert M, McEwan P. Multivariate models of health-related utility and the fear of hypoglycaemia in people with diabetes. Curr Med Res Opin. 2006;22(8):1523–34. [DOI] [PubMed] [Google Scholar]
- (91).Tengs TO, Wallace A. One thousand health-related quality-of-life estimates. Med Care. 2000;38(6):583–637. [DOI] [PubMed] [Google Scholar]
- (92).Sullivan PW, Ghushchyan V. Preference-based EQ-5D index scores for chronic conditions in the United States. Med Decis Making. 2006;26(4):410–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (93).Willis WD, Diago-Cabezudo JI, Madec-Hily A, Aslam A. Medical resource use, disturbance of daily life and burden of hypoglycemia in insulin-treated patients with diabetes: results from a European online survey. Expert Rev Pharmacoecon Outcomes Res. 2013;13(1):123–30. [DOI] [PubMed] [Google Scholar]
- (94).Diago-Cabezudo JI, Madec-Hily A, Aslam A. Perceptions of hypoglycemia and self-monitoring of blood glucose in insulin-treated diabetes patients: results from a European online survey. Diabetes Manag (Lond). 2013;3(1):8. [DOI] [PubMed] [Google Scholar]
- (95).O'Brien JA, Patrick AR, Caro JJ. Cost of managing complications resulting from type 2 diabetes mellitus in Canada. BMC Health Serv Res. 2003;3(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (96).O'Reilly D, Hopkins R, Blackhouse G, Clarke P, Hux H, Tarride J, et al. Long-term cost-utility analysis of a multidisciplinary primary care diabetes management program in Ontario. Can J Diabetes. 2007;1(3):205–14. [Google Scholar]
- (97).McQueen RB, Breton MD, Ott M, Koa H, Beamer B, Campbell JD. Economic value of improved accuracy for self-monitoring of blood glucose devices for type 1 diabetes in Canada. J Diabetes Sci Technol. 2015;10(2):366–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (98).Canadian Agency for Drugs and Technologies in Health. Optimal therapy report: second-line therapy for patients with diabetes inadequately controlled on metformin: a systematic review and cost-effectiveness analysis [Internet]. Ottawa (ON): The Agency; 2010. [cited 2016 Dec]. Available from: http://www.cadth.ca/media/pdf/C1110_SR_Report_final_e.pdf [Google Scholar]
- (99).Briggs A, Sculpher M, Claxton K. Decision modelling for health economic evaluation. Oxford: Oxford University Press; 2006. [Google Scholar]
- (100).Drummond MF, Sculpher MJ, Torrance GW, O'Brien BJ, Stoddart GL. Methods for the economic evaluation of health care programmes. 3rd ed. Oxford: Oxford University Press; 2005. [Google Scholar]
- (101).Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353(25):643–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (102).Vexiau P, Mavros P, Krishnarajah G, Lyu R, Yin D. Hypoglycaemia in patients with type 2 diabetes treated with a combination of metformin and sulphonylurea therapy in France. Diabetes Obes Metab. 2008;10 Suppl 1:16–24. [DOI] [PubMed] [Google Scholar]
- (103).Marrett E, Stargardt T, Mavros P, Alexander CM. Patient-reported outcomes in a survey of patients treated with oral antihyperglycaemic medications: associations with hypoglycaemia and weight gain. Diabetes Obes Metab. 2009;11(12):1138–44. [DOI] [PubMed] [Google Scholar]
- (104).Choudhary P, Geddes J, Freeman JV, Emery CJ, Heller SR, Frier BM. Frequency of biochemical hypoglycaemia in adults with type 1 diabetes with and without impaired awareness of hypoglycaemia: no identifiable differences using continuous glucose monitoring. Diabet Med. 2010;27(6):666–72. [DOI] [PubMed] [Google Scholar]
- (105).Geddes J, Schopman JE, Zammitt NN, Frier BM. Prevalence of impaired awareness of hypoglycaemia in adults with type 1 diabetes. Diabet Med. 2008;25(4):501–4. [DOI] [PubMed] [Google Scholar]
- (106).Gandhi GY, Kovalaske M, Kudva Y, Walsh K, Elamin MB, Beers M, et al. Efficacy of continuous glucose monitoring in improving glycemic control and reducing hypoglycemia: a systematic review and meta-analysis of randomized trials. J Diabetes Sci Technol. 2011;5(4):952–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (107).Office of the Premier. Free prescription medications for children and youth through OHIP+ [Internet]. Toronto (ON): Queen's Printer for Ontario; 2017. Apr 28 [cited 2017 May]. Available from: http://news.ontario.ca/opo/en/2017/04/free-prescription-medications-for-children-and-youth-through-ohip.html [Google Scholar]
- (108).Sullivan SD, Mauskopf JA, Augustovski F, Jaime Caro J, Lee KM, Minchin M, et al. Budget impact analysis-principles of good practice: report of the ISPOR 2012 Budget Impact Analysis Good Practice II Task Force. Value Health. 2014;17(1):5–14. [DOI] [PubMed] [Google Scholar]
- (109).Canadian Diabetes Association. Diabetes charter for Canada: diabetes in Ontario [Internet]. Toronto (ON): The Association; 2016. [cited 2016 Dec]. Available from: http://www.diabetes.ca/getmedia/73485544-2d42-4fd8-a834-b80b13ed231e/diabetes-charter-backgrounder-on-2016-09.pdf [Google Scholar]
- (110).Statistics Canada. Table 052-0005: Projected population, by projection scenario, age and sex, as of July 1, Canada, provinces and territories, annual (persons × 1,000) [Internet]. Ottawa (ON): Statistics Canada; c2014 - [cited 2017 May 24]. Available from: http://www5.statcan.gc.ca/cansim/a26?lang=eng&id=520005 [Google Scholar]
- (111).Dexcom Canada Inc. Budget impact analysis of introducing Dexcom RT-CGM in type 1 diabetes patients with hypoglycemia unawareness in Ontario, Canada (forthcoming). Burnaby (BC): Dexcom Canada Inc.; 2017
- (112).Gomez AM, Alfonso-Cristancho R, Orozco JJ, Lynch PM, Prieto D, Saunders R, et al. Clinical and economic benefits of integrated pump/CGM technology therapy in patients with type 1 diabetes in Colombia. Endocrinol Nutr. 2016;63(9):466–74. [DOI] [PubMed] [Google Scholar]
- (113).Ly TT, Gallego PH, Davis EA, Jones TW. Impaired awareness of hypoglycemia in a population-based sample of children and adolescents with type 1 diabetes. Diabetes Care. 2009;32(10):1802–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (114).Canadian Diabetes Association. Financial assistance programs for people living with diabetes: Ontario [Internet]. Toronto (ON): The Association; 2016. [cited 2017 May 17]. Available from: http://www.diabetes.ca/getmedia/4873c9cc-1105-4c11-9e90-230ea52a9c60/ontario-financial-assistance-programs.pdf.aspx [Google Scholar]
- (115).Bronstone A, Chaugule S, Graham C, Forte L. Does real-time continuous glucose monitoring reduce short-term medical costs for patients with type 1 diabetes and hypoglycemia unawareness in Ontario, Canada? Presentation at: Canadian Association for Health Services and Policy Research Conference; 2017 May 24–26; Toronto, ON.
- (116).Barham L. Public and patient involvement at the UK National Institute for Health and Clinical Excellence. Patient. 2011;4(1):1–10. [DOI] [PubMed] [Google Scholar]
- (117).Messina J, Grainger DL. A pilot study to identify areas for further improvements in patient and public involvement in health technology assessments for medicines. Patient. 2012;5(3):199–211. [DOI] [PubMed] [Google Scholar]
- (118).OHTAC Public Engagement Subcommittee. Public engagement for health technology assessment at Health Quality Ontario—final report from the Ontario Health Technology Advisory Committee Public Engagement Subcommittee [Internet]. Toronto (ON): Queen's Printer for Ontario; 2015. [cited 2017 Jan]. Available from: http://www.hqontario.ca/Portals/0/documents/evidence/special-reports/report-subcommittee-20150407-en.pdf [Google Scholar]
- (119).Kvale S. Interviews: an introduction to qualitative research interviewing. Thousand Oaks (CA): Sage Publications; 1996. [Google Scholar]
- (120).Kuzel AJ. Sampling in qualitative inquiry. In: Miller WL, Crabtree BF, editors. Doing qualitative research. Thousand Oaks (CA): Sage Publications; 1999. p. 33–45. [Google Scholar]
- (121).Morse J. Emerging from the data: cognitive processes of analysis in qualitative research. In: Morse J, editor. Critical issues in qualitative research methods Thousand Oaks (CA): Sage Publications; 1994. p. 23–41. [Google Scholar]
- (122).Patton MQ. Qualitative research and evaluation methods. 3rd ed. Thousand Oaks (CA): Sage Publications; 2002. [Google Scholar]
- (123).Strauss AL, Corbin JM. Basics of qualitative research: techniques and procedures of developing a grounded theory. 2nd ed. Thousand Oaks (CA): Sage Publications; 1998. [Google Scholar]
- (124).Health Technology Assessment International Interest Group on Patient and Citizen Involvement in HTA. Introduction to health technology assessment [Internet]. Edmonton (AB): Health Technology Assessment International; 2015. [cited 2017 Jan]. Available from: http://www.htai.org/fileadmin/HTAiFiles/ISG/PatientInvolvement/v2files/Resource/PCI SG-Resource-Intro_to_HTA__KFacey__Jun13.pdf [Google Scholar]
- (125).Strauss AL, Corbin JM. Grounded theory research: procedures, canons, and evaluative criteria. Qual Sociol. 1990;13(1):3–21. [Google Scholar]
- (126).Strauss AL, Corbin JM. Grounded theory methodology: an overview. In: Denzin NK, Lincoln YS, editors. Handbook of qualitative research. Thousand Oaks (CA): Sage Publications; 1994. p. 273–85. [Google Scholar]
- (127).Radermecker RP, Scheen AJ. Management of blood glucose in patients with stroke. Diabetes Metab. 2010;36 Suppl 3:S94–9. [DOI] [PubMed] [Google Scholar]
- (128).Sackett DL, Richardson WS, Rosenberg W, Haynes RB. Measures of effect size of an intervention. In: Evidence-based medicine: how to practice and teach EBM. New York: Churchill Livingston; 1997. [Google Scholar]
- (129).McIntosh B, Cameron C, Singh SR, Yu C, Ahuja T, Welton NJ, et al. Second-line therapy in patients with type 2 diabetes inadequately controlled with metformin monotherapy: a systematic review and mixed-treatment comparison meta-analysis. Open Med. 2011;5(1):e35–48. [PMC free article] [PubMed] [Google Scholar]
- (130).All about insulin pumpers [Internet]. Milpitas (CA): Insulin Pumpers; c1997–2017 [cited 2017 Apr 22]. Available from: http://www.insulin-pumpers.org/about.shtml [Google Scholar]
- (131).Humalog insulin lispro injection in cartridges [Internet]. Toronto (ON): Diabetes Source; c2013. [cited 2017 Apr 22]. Available from: http://diabetessource.ca/product/humalog-insulin-lispro-injection-cartridges/ [Google Scholar]