Abstract
A 42-year-old, otherwise healthy, woman presented with persistent left-sided chest pain. A chest X-ray revealed a large opacity in the left hemithorax which prompted further investigation with an MRI. MRI revealed a large left apical mass occupying approximately two-thirds of the left hemithorax. The mass was investigated further with a CT with contrast which did not reveal any vascular involvement or invasion into adjacent structures. The patient successfully underwent tumour resection via left thoracotomy. The tumour was removed in its entirety. Grossly, the tumour was a 23×10×10 cm, well encapsulated, ovoid, fibrous nodule. Histopathology revealed ganglion cells, nerve fibres and Schwann cells in a mucous matrix consistent with ganglioneuroma. Postoperative course was unremarkable.
Keywords: cancer intervention, radiology, cardiothoracic surgery, surgical oncology, pathology
Background
Thoracic ganglioneuromas (TGs) are exceedingly rare and there are very few cases reported in the literature. TGs present a significant diagnostic challenge given the rarity of the pathology. They frequently present as incidental radiographic findings in young healthy patients. TGs have a characteristic appearance on imaging; however, the findings are subtle and in order to be detected, clinicians must have an awareness of the radiographic findings associated with TGs.1 Furthermore, proper diagnosis of intrathoracic pathology is crucial given the broad differential diagnosis and potential for involvement of cardiovascular and pulmonary structures.
Here, we present a case of a TG. We elaborate on the diagnostic and therapeutic challenges that TGs pose and share our strategy for management from initial presentation to postoperative follow-up. On review of our current literature, this case represents the largest TG reported to date. We detail patient presentation, imaging, surgical management, pathology, outcome and follow-up in an effort to increase awareness and proper care of this rare and challenging entity.
Case presentation
Patient is a 42-year-old female with hypothyroidism who presented with a chief complaint of left-sided chest pain. She denied any fevers, chills, weight loss, night sweats, oropharyngeal disease, lymphadenopathy, cough, haemoptysis, angina and history of similar chest pain. She denied any history of smoking or family history of lung cancer. Her vital signs were normal and her oxygen saturation was 99% on room air. Physical examination was notable for decreased breath sounds to auscultation on the left.
Investigations
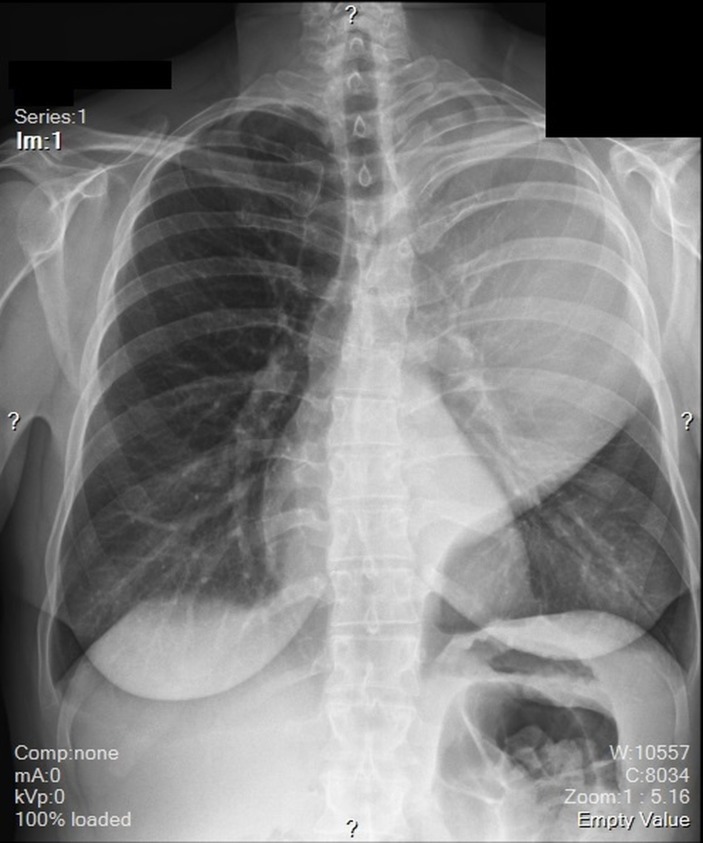
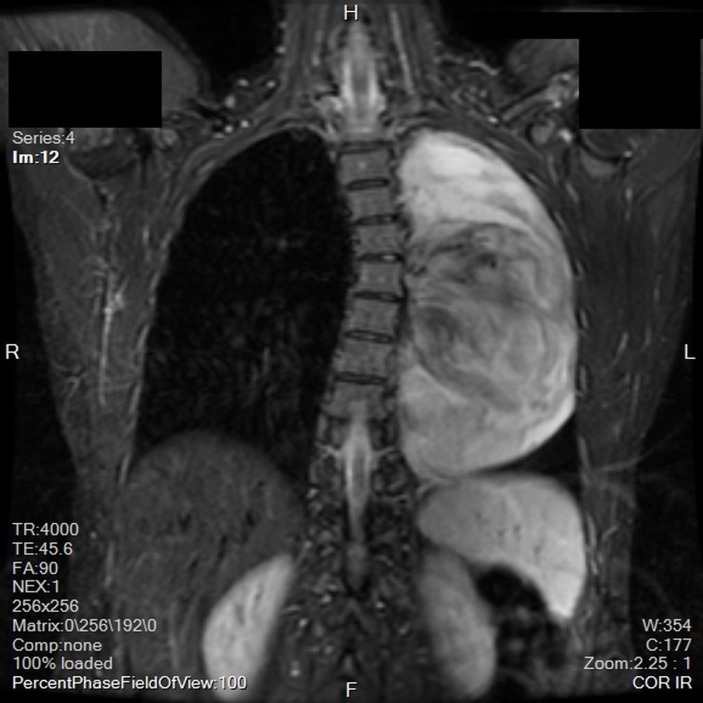
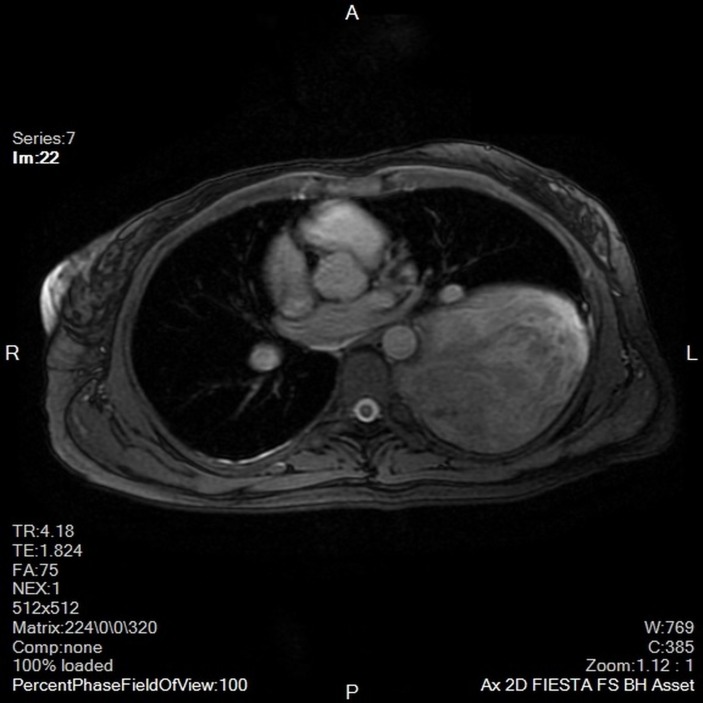
A chest X-ray revealed a large opacity in the left hemithorax (figure 1) which prompted further investigation with MRI. MRI revealed a large, smoothly marginated, left apical mass occupying approximately two-thirds of the left hemithorax. It was heterogeneous in nature with a mix of low and mid enhancement in T2 phase and low T1 enhancement. There was mass effect on the left main stem bronchus, displacing it anteriorly. There were no signs of thoracic spine invasion (figures 2 and 3). CT with contrast was then obtained which showed no vascular involvement. The mass appeared hypodense and mildly heterogeneous. There was no evidence of invasion into lung parenchyma, mediastinum or the chest wall. The mass itself appeared well circumscribed and pushed away the parenchyma leading to a mild rightward mediastinal shift (figure 4).
Figure 1.
Chest radiograph taken at initial presentation revealing an opacity in the superior two-thirds of the left hemithorax.
Figure 2.
Coronal view MRI displaying the heterogeneity of the mass.
Figure 3.
Axial view MRI. This demonstrates that the mass is abutting the lateral border of the aorta and the posterior border of the pulmonary artery but still able to visualise a distinct tissue plane indicating that there is not likely a tumour invasion into the vasculature.
Figure 4.
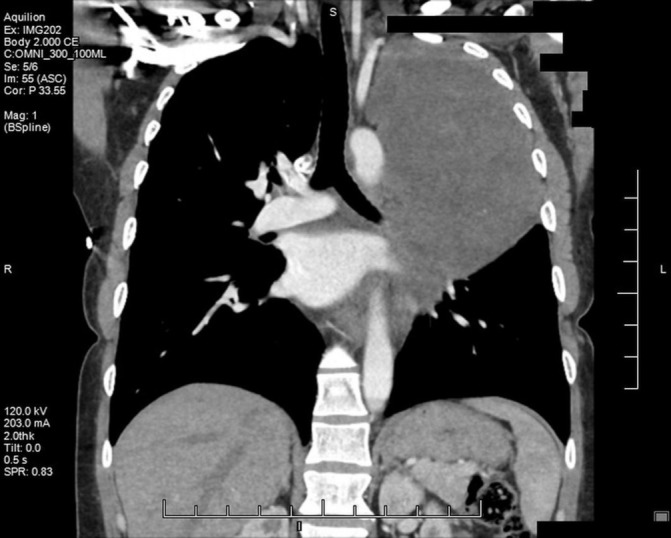
Coronal CT with contrast detailing the relationship of mass to the thoracic aorta, left pulmonary artery and left mainstem bronchus.
Differential diagnosis
Radiographic findings were consistent with benign mesenchymal neoplasm. Differential diagnosis included ganglioneuroma, neurilemmoma (Schwannoma), neurofibroma, paraganglioma and neuroblastoma.
Treatment
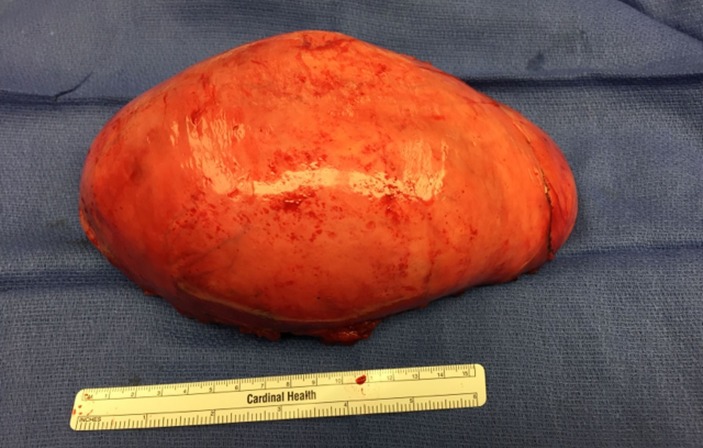
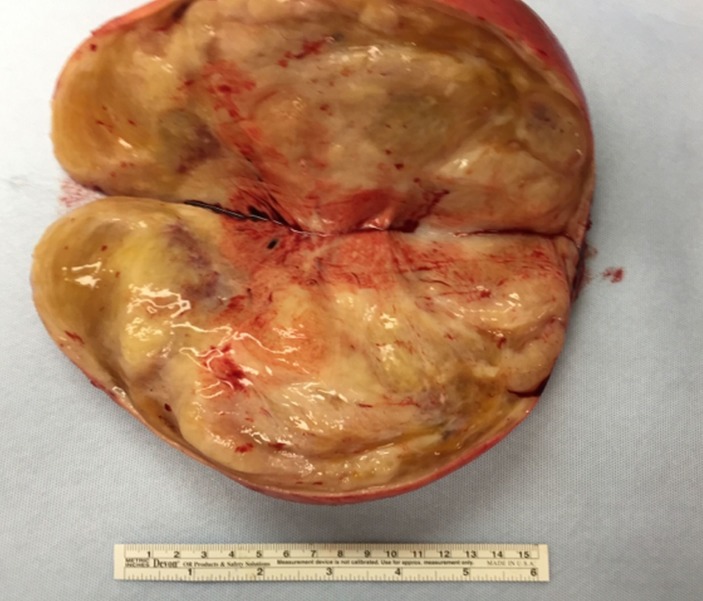
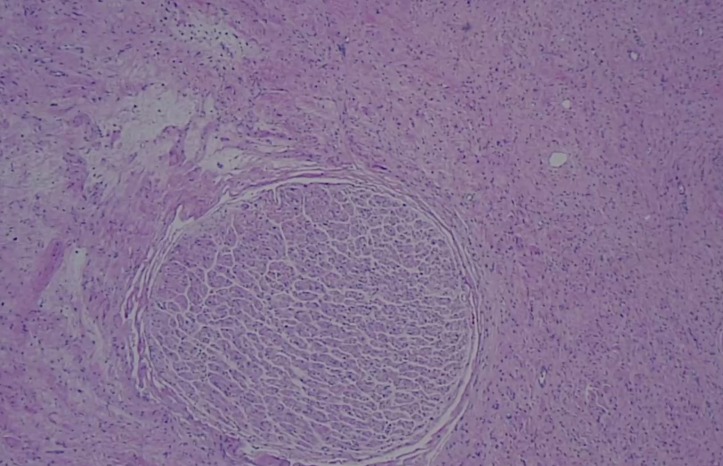
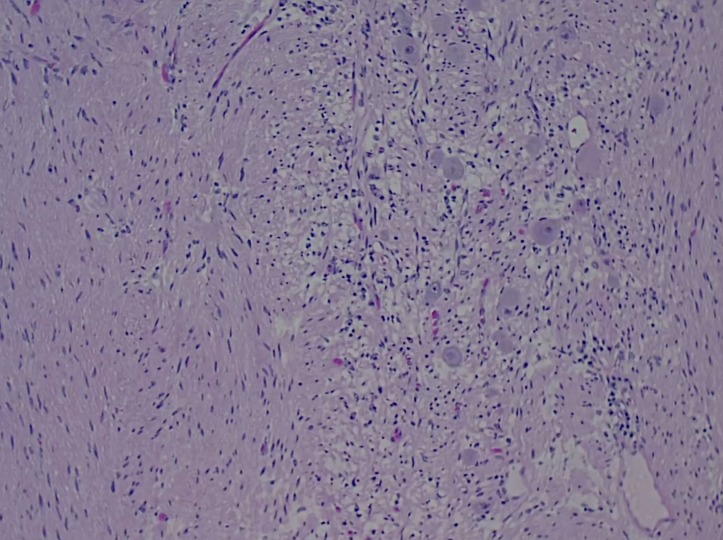
Given the size of the mass, potential for local destruction, continued growth and malignant transformation, the patient elected to undergo surgical resection. The patient was taken to the operating room, general anaesthesia was induced and an endotrachial tube was placed. The patient was placed in the right lateral decubitus position and a left-sided thoracotomy was performed. The mass was found to extend deeply into the posterior superior sulcus with dense posterior and medial adhesions. The left mainstem bronchus and the pulmonary vasculature were displaced anteriorly by the mass. The medial border of the tumour was found to be abutting, and partially encompassing, the thoracic aorta. The medial border of the tumour was then dissected free from pleural attachments. The inferior border was dissected free from the patient’s fourth to sixth ribs. There was no violation of the capsule of the tumour and it was resected in its entirety. Grossly, the tumour was a 23×10×10 cm, well encapsulated, ovoid, fibrous nodule (figures 5 and 6). Histopathology revealed ganglion cells, nerve fibres and Schwann cells in a mucous matrix (figures 7 and 8) and the diagnosis of thoracic ganglioneuroma was made.
Figure 5.
Gross specimen of the 1220 g, well-encapsulated fibrous nodule.
Figure 6.
Cut surface of the specimen revealing a slightly lobulated, fibrotic mass with focal myxomatous areas.
Figure 7.
Low power (10×) microscopic image of the specimen revealing a nerve fascicle within a heterogeneous stroma.
Figure 8.
High power (40×) microscopic image revealing ganglion cells and Schwann cells.
Outcome and follow-up
The patient tolerated the procedure well and had an uneventful postoperative course. The patient was monitored in the intensive care unit briefly and discharged home on postoperative day 4. One week after discharge, the patient followed up as an outpatient and was asymptomatic aside from mild surgical site pain, well controlled with non-steroidal anti-inflammatory drugs.
Discussion
On review of current literature, this is the largest TG ever reported in adult and/or paediatric patients.2–4 There are very few cases of TGs in the literature. TGs are benign entities and are generally discovered as incidental findings.5 However, as evidenced by this case, they are capable of growing to impressive sizes and becoming symptomatic, manifested by pain and compression of adjacent organs. Thoracic ganglioneuroma represent a significant diagnostic challenge given the broad differential diagnosis of intrathoracic masses. Furthermore, the radiographic distinctions between ganglioneuromas and other diagnoses are subtle and require skilled radiologists and imaging technicians.1 5
Although TGs are generally incidental radiographic findings, they may present with chest discomfort due to mass effect as seen in our patient. TGs have also been reported to present with symptoms mimicking spondylodiscitis.6 There is also a documented case of a TG causing hydrops fetalis in a neonate.7
Radiology plays an important role in initial workup. A recent review of the documented cases helped to characterise the radiographic findings which define TGs. On CT imaging, TGs appear as hypodense lesions which are homogeneous or slightly heterogeneous. TGs may show calcifications, potentially leading to misdiagnosis as dermoid cyst; however, no calcifications were observed in this case. The histopathology of TGs explains their MRI findings. The mucous matrix appears hypodense on CT and hyperintense on T2 MRI. The nerve cell bodies and fibres account for the areas of increased density on CT as well as the heterogeneous hypointense areas seen on T2 MRI. T1 MRI may reveal enhancement of the edge of the TG due to the capsule; however, dense adhesions, as seen in this case, may obscure the capsule. MRI will also reveal progressive enhancement in the delay phase.1
Transthoracic needle biopsy has a sensitivity of 90% for intrathoracic pathology.8 Microscopically, they appear as nerve fibres in wave-like patterns with scattered mature ganglion cells. Immunohistochemical staining with S-100, Vim, neuron-specific enolase, neuron filament protein, myelin basic protein will frequently be positive and reveal a nerve origin of the tissue.1 Biopsy provides valuable information which guides decision-making in many cases of intrathoracic pathology. However, given that our patient was symptomatic and that the lesion had potential for continued growth, the results of a biopsy would not have altered management strategy and therefore biopsy not performed. Furthermore, the TG seen in our patient exhibited many of the classic CT and MRI findings of TGs which decreased the likelihood that biopsy would lead to any variation in the treatment strategy. Biopsy also has potential complications including the creation of a pneumothorax or the seeding of the pleura and biopsy tract.9 10 Biopsy is less likely to yield a diagnosis in heterogeneous lesions such as TGs.
Surgery is the definitive treatment for TGs.11 Given the sizes that TGs reach (table 1) and their locations in the thorax, they can present considerable challenges during the perioperative period. Due to the size of the TG in this case, thoracotomy was necessary if the mass was to be removed ‘en bloc.’ Given the proximity of the mass to the aorta, left pulmonary artery and left mainstem bronchus, a thoracotomy was the safest approach because it would allow for timely intervention in the event of an operative complication. Removal of large intrathoracic bodies may precipitate fluid shifts and flash pulmonary oedema. Re-expansion pulmonary oedema can occur in patients after decompression of a chronically compressed lung.12 13 Careful monitoring is necessitated in the postoperative period given the high mortality of this phenomenon. Surgeons must also exercise caution not to disrupt the sympathetic trunk which can lead to various complications including Harlequinn syndrome.14
Table 1.
Table of all reported thoracic ganglioneuromas dimensions
| Author | Age | Sex | Dimensions | Volume |
| Lambdin et al | 42 | F | 23×10×10 cm | 2300 cm3 |
| Guan et al1 | 8 | M | 21×15×18 cm | 5670 cm3 |
| Guan et al1 | 18 | F | 13×11.5×6.9 cm | 1032 cm3 |
| Huang et al3 | 12 | F | 12×12×12 cm | 1728 cm3 |
| Kizildag et al15 | 19 | F | 9×6×3.5 cm | 189 cm3 |
| Guan et al1 | 14 | M | 7×5×3 cm | 105 cm3 |
| Guan et al1 | 22 | M | 6.7×4.5×2.5 cm | 75 cm3 |
| Guan et al1 | 53 | M | 3.2×2.0×2.0 cm | 13 cm3 |
Learning points.
Thoracic ganglioneuromas are rare but clinicians should be aware of their existence and should include them in the differential diagnosis when appropriate.
Thoracic ganglioneuromas present diagnostic challenges but MRI provides valuable information which aids clinical decision-making.
Thoracic ganglioneuromas have the potential to cause compressive symptoms and destruction of parenchyma if they grow to a significant size.
Large intrathoracic masses require a high level of care during the perioperative time period to guard against fluid shifts and haemodynamic instability.
Footnotes
Contributors: JTL is the primary guarantor, primary author and editor and performed planning and literature review. KBL is the coauthor who assisted in drafting, reviewing and editing of the case. GT is the coauthor and corresponding author who identified and managed the case as well as provided scientific mentorship and review/editing of the report. CP is the coauthor who identified and managed the case as well as provided scientific mentorship and review/editing of the report.
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Guan YB, Zhang WD, Zeng QS, et al. CT and MRI findings of thoracic ganglioneuroma. Br J Radiol 2012;85:e365–e372. 10.1259/bjr/53395088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hayat J, Ahmed R, Alizai S, et al. Giant ganglioneuroma of the posterior mediastinum. Interact Cardiovasc Thorac Surg 2011;13:344–5. 10.1510/icvts.2011.267393 [DOI] [PubMed] [Google Scholar]
- 3.Huang Y, Liu L, Li Q, et al. Giant ganglioneuroma of thoracic spine: a case report and review of literature. J Korean Neurosurg Soc 2017;60:371–4. 10.3340/jkns.2015.0708.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang J, Li J, Shrestha R, et al. Giant cervicothoracic ganglioneuroma. Neurol India 2011;59:465–6. 10.4103/0028-3886.82735 [DOI] [PubMed] [Google Scholar]
- 5.Pavlus JD, Carter BW, Tolley MD, et al. Imaging of thoracic neurogenic tumors. AJR Am J Roentgenol 2016;207:552–61. 10.2214/AJR.16.16018 [DOI] [PubMed] [Google Scholar]
- 6.Mogharrabi M, Azarhoush R, Javadi H, et al. Extradural ganglioneuroma with T1–T2 involvement mimicking spondylodiscitis: a case report and a review of the literature. Nuclear Medicine Review 2016;19(B):3–4. 10.5603/NMR.2016.0026 [DOI] [PubMed] [Google Scholar]
- 7.Singh P, Jodicke C, Swanson T, et al. Thoracic ganglioneuromas resulting in nonimmune hydrops fetalis. AJP Rep 2014;4:49–54. 10.1055/s-0034-1371751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schreiber G, McCrory DC. Performance characteristics of different modalities for diagnosis of suspected lung cancer: summary of published evidence. Chest 2003;123(1 Suppl):115S–28. [DOI] [PubMed] [Google Scholar]
- 9.Birchard K. Transthoracic needle biopsy. Semin Intervent Radiol 2011;28:087–97. 10.1055/s-0031-1273943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boskovic T, Stanic J, Pena-Karan S, et al. Pneumothorax after transthoracic needle biopsy of lung lesions under CT guidance. J Thorac Dis 2014;6(Suppl 1):S99–S107. 10.3978/j.issn.2072-1439.2013.12.08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fraga JC, Aydogdu B, Aufieri R, et al. SurgIcal treatment for pediatric mediastinal neurogenic tumors. Ann Thorac Surg 2010;90:413–8. 10.1016/j.athoracsur.2010.04.086 [DOI] [PubMed] [Google Scholar]
- 12.Mahfood S, Hix WR, Aaron BL, et al. Reexpansion pulmonary edema. Ann Thorac Surg 1988;45:340–5. 10.1016/S0003-4975(10)62480-0 [DOI] [PubMed] [Google Scholar]
- 13.Sohara Y. Reexpansion pulmonary edema. Ann Thorac Cardiovasc Surg 2008;14:205–9. [PubMed] [Google Scholar]
- 14.Jeon YJ, Son J, Cho JH. Harlequin syndrome following resection of mediastinal ganglioneuroma. Korean J Thorac Cardiovasc Surg 2017;50:130–2. 10.5090/kjtcs.2017.50.2.130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kızıldağ B, Alar T, Karatağ O, et al. A case of posterior mediastinal ganglioneuroma: the importance of preoperative multiplanar radiological imaging. Balkan Med J 2013;30:126–8. 10.5152/balkanmedj.2012.099 [DOI] [PMC free article] [PubMed] [Google Scholar]