Abstract
A 76-year-old male cigarette smoker presented with a 2-week history of cough and haemoptysis. Chest CT on admission revealed multiple new lung nodules concerning for malignancy. CT-guided biopsy of the nodule in left lower lobe was attempted in prone oblique position for tissue diagnosis. Local anaesthetic (lidocaine) was administered using a 25-gauge (1.5-inch) needle to anaesthetise the skin and subcutaneous tissue. This was followed by insertion of a 25-gauge (3.5-inch) Whitacre needle to anaesthetise deeper tissues and parietal pleura. Due to patient’s coughing and proximity of the nodule to the diaphragm, the circumstances were judged to be too risky for a needle biopsy. Therefore, it was decided to biopsy another nodule in the left lung that was visible on the same CT section. During this portion of the procedure, the patient became hypoxic and developed pulseless electrical activity arrest. Cardiopulmonary resuscitation was unsuccessful and the efforts ceased after 45 min. Subsequent review of CT scan revealed air in the left ventricle.
Keywords: healthcare improvement and patient safety, adult intensive care, respiratory medicine
Background
Air embolism is a rare but potentially fatal complication during lung biopsies. It can lead to neurological complications and haemodynamic collapse. In the literature, all reported cases of air embolism were caused by needles 18 gauge or larger. We present a case of air embolism in the left ventricle following local anaesthetisation of the pleura using a smaller 25-gauge needle.
We believe that the case sheds light on a potentially fatal adverse outcome using smaller sized needle, that is not commonly encountered in clinical practice. The size of the needle may not matter when the possibility of air embolism is considered.
Case presentation
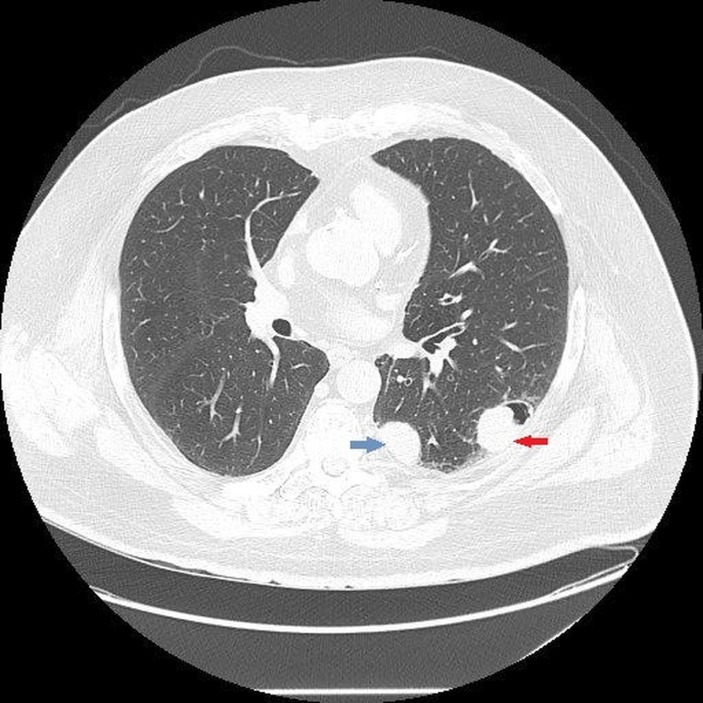
The patient was a 76-year-old man with a 30 pack-year history of cigarette smoking who presented with 2 weeks of cough, haemoptysis and intermittent haematuria. Medical history was significant for hypertension and chronic obstructive pulmonary disease with bronchitis. The patient denied any family history of malignancy. Chest CT on admission was performed which revealed emphysema and multiple nodules in both lungs concerning for metastatic cancer, the largest one measuring 4.2×2.6 cm (figure 1). Initial investigations including infectious workup: sputum gram stain, Acid-Fast Bacilli (AFB) stain, sputum culture and blood cultures, immunological workup and purified protein derivative (PPD), were all negative. Abdominal and pelvic CT scan with contrast was performed for evaluation of haematuria and to possibly locate the primary tumour. Imaging was notable for a mass along the left urinary bladder wall likely the primary tumour.
Figure 1.
CT chest with intravenous contrast showing nodules in the left lung. Largest nodule measuring 4.2×2.6 cm (red arrow) and the second largest nodule measuring 3.1×2.5 cm (blue arrow).
Urine cytology showed atypical urothelial cells suspicious for urothelial carcinoma.
CT-guided percutaneous core needle biopsy (PCNB) was planned to establish the histopathological diagnosis and staging of malignancy. One of the peripheral pulmonary nodules in the posterior aspect of the left lower lobe was chosen for PCNB.
Patient was placed in prone oblique position for the biopsy, and limited CT scan was done to locate the nodules. A skin marker was placed by the CT technologist and an initial CT scan was performed to confirm the location of nodule in relation to the BB marker. Using aseptic precaution, 3–4 mL of local anaesthetic (lidocaine) was infiltrated into the skin and subcutaneous tissue using 25-gauge needle (1.5 inches long). Following this, a 25-gauge Whitacre needle (3.5 inches long) was attached to the syringe after removing the stylet. The syringe contained local anaesthetic, air from the syringe was removed by tapping on the syringe and pushing on the plunger until lidocaine drops were seen coming out at the tip. The needle was then inserted in the skin and used to anaesthetise deeper tissues and parietal pleura with additional 3–4 mL of lidocaine. Intermittent negative pressure was applied while advancing the needle to avoid injecting the anaesthetic in a blood vessel. No air or blood was aspirated. Interventional radiologist selected the Whitacre needle based on his extensive clinical experience with its use.
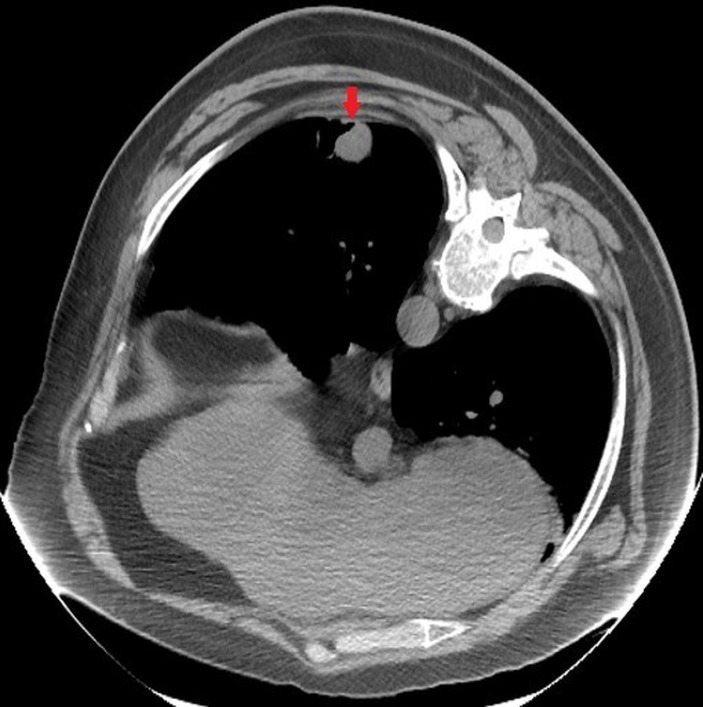
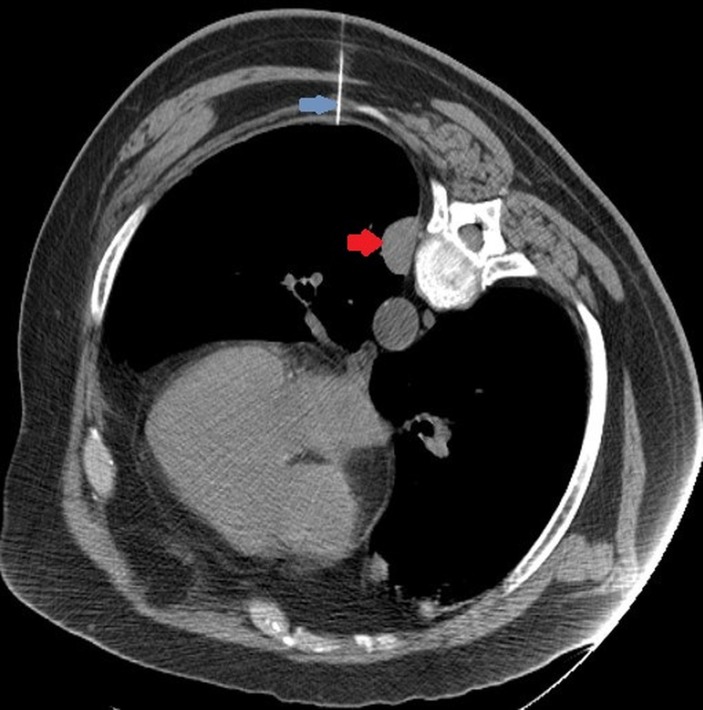
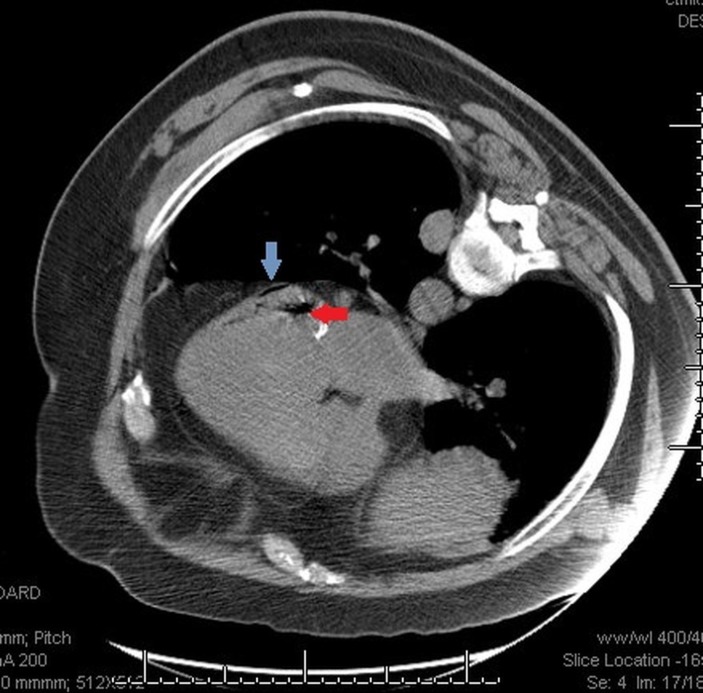
Another CT image using Smart step technique was done to evaluate the location of 25G Whitacre needle in relation to the nodule. However, due to patient’s paroxysmal cough and proximity of the selected nodule to the diaphragm, this nodule was judged to be too risky for PCNB. Decision was made to biopsy another nodule (3.1×2.5 cm) that was visible in the same CT section (figures 2 and 3). Immediately after withdrawing the needle, significant change in patient’s appearance was noted. Patient became restless and cyanotic with pulse oximetry showing drop in oxygen saturation to 72%. Patient was placed on supplemental oxygen through nasal cannula, and the procedure was aborted. Patient’s oxygen saturation improved to high 80s. A stat CT scan was done to rule out pneumothorax. A code blue was called while the CT scan was being performed. After the CT scan was completed, the patient was moved to the cart from CT table by the nursing staff in the interventional radiology suite. Code team arrived as the patient was being transferred to the gurney. The patient was started on 100% fractional inspired oxygen (FiO2) through Ventimask by the code team. Unfortunately, he developed worsening hypoxia leading to pulseless electrical activity (PEA) arrest. Cardiopulmonary resuscitation (CPR) was initiated immediately. Chest compressions were started along with bag mask ventilation (ratio of 30:2). While resuscitation efforts were under way, CT chest images were reviewed by radiologist and depicted air in the left ventricle and possibly coronary artery (figure 4).
Figure 2.
CT chest with intravenous contrast showing the identified lung nodule (red arrow) after positioning the patient for biopsy.
Figure 3.
CT section with lidocaine needle (blue arrow) inserted to administer local anaesthetic prior to the biopsy; however, the nodule is not visible any more, likely due to patient’s breathing and cough. Note the second nodule (red arrow) visible in the CT section.
Figure 4.
CT chest showing air in the left ventricle (red arrow) and in coronary artery (blue arrow).
During the code, patient was started on intravenous fluid bolus. One milligram of intravenous epinephrine was given. Patient remained pulseless after first round of CPR (2 min). Chest compression and ventilation was continued. Another 1 mg of epinephrine given after 3–4 min. Endotracheal (ET) intubation was performed, and the ET tube was connected to mechanical ventilator (FiO2100%) after confirming the position with an end-tidal CO2 detector. Patient had a ROSC after 15 min of continued CPR. Meanwhile, triple lumen central line catheter was placed in left femoral vein, and patient was started on norepinephrine drip along with intravenous fluid boluses. Unfortunately, patient lost pulse again after 5 min. Resuscitation efforts were continued for another 25–30 min using the standard Advanced cardiac life support (ACLS) protocol but remained unsuccessful. Resuscitation efforts were ceased after 45 min.
Investigations
Due to ongoing CPR with repeated chest compression, bedside echocardiogram could not be done successfully. CT images were reviewed by the radiologist while CPR was being performed and were significant for air in the left ventricle.
Patient’s family agreed to autopsy which was performed and was significant for air in the left ventricle.
Differential diagnosis
Common causes of PEA arrest are listed below:
hypovolaemia
hypoxia
metabolic or respiratory acidosis
hypothermia
hypoglycaemia
pulmonary embolism
myocardial infarction
cardiac tamponade.
Outcome and follow-up
Patient became hypoxic due to air embolism which lead to PEA arrest and death.
Discussion
According to the literature review, risk factors associated with systemic air embolism following PCNB are broadly categorised into patient factors and procedural factors. Patient-related risk factors include pre-existing emphysema, breathing characteristics, cough during the procedure and patient non-adherence to the instructions during the procedure like minimising movement and withholding breath. Common procedure-related risk factors are improper patient positioning, longer needle path through ventilated lung and coaxial biopsy system.
Most of the patient factors are beyond our control. Regardless, procedural factors should be taken into account to minimise the risk or air embolism. Proper positioning is pivotal. Patient should be placed in prone oblique/lateral or supine position to minimise movement during the procedure. Seating position should be avoided as it increases the risk of air travelling to cranial circulation in the event of an air embolism.1 A retrospective observational study found full prone position to be a risk factor for air embolism.2 Ishii et al have argued that patient position does not have any significant impact on the development of air embolism. However, this study had limitations as the patients were placed in supine and prone oblique position for the procedure; these two positions are associated with lower risk of air embolism.3 We do not believe that poor positioning was the cause of air embolism in our patient as he was placed in the prone oblique position. Prone oblique position also offers the advantage of compressing the ipsilateral (operative) lung causing hypoinflation and theoretically reduces the motion of the hemithorax and diaphragm on the operative side.4
In terms of the mechanisms that lead to the development of air embolism, air can either enter the systemic circulation if the needle tip comes in contact with pulmonary vein when the stylet is removed creating communication between the atmosphere and the pulmonary vessel.5 This is unlikely in our patient, since the Whitacre needle was already attached to the syringe and no blood was aspirated with negative pressure while advancing the needle.
While traversing the lung parenchyma, the needle can also create a bronchovenous fistula, making it possible for intrabronchial air to enter pulmonary circulation via the fistula. In our patient, the biopsy needle was never introduced. However, we hypothesised that the Whitacre needle may have accidentally punctured the pleura and entered the lung causing a fistulous connection between the bronchus and pulmonary vein and coupled with negative intrathoracic pressure generated by patient’s coughing likely resulted in left ventricle air embolism. Also, we cannot completely rule out the possibility of some air being left in the syringe despite the radiologists attempt to remove air prior to injecting the lidocaine. Literature review shows reported cases of air embolism after core lung biopsy but to our knowledge, this is the first reported case of air embolism using the smaller 25-gauge needle. Smaller needle sizes have also been quoted to reduce the risk of air embolism6 but this case highlights that the risk of air embolism is independent of needle size. Precautions to prevent air embolism during lung biopsy should be taken throughout the procedure.
Immediate resuscitation efforts with use of 100% oxygen should be reinstated if haemodynamic collapse occurs due to air embolism. Placing the patient in the left lateral decubitus (Durant’s manoeuvre) helps prevent further air entrainment. If CPR is required, placing the patient in a supine and head-down position is found to be helpful.7 Use of Trendelenburg’s position as a favourable placement after development of air embolism is controversial.8 After revival, hyperbaric oxygen therapy has proven to be beneficial in cases of systemic air embolism.7
Learning points.
Air embolism can occur with the use of smaller 25-gauge needle commonly used for administering subcutaneous/intradermal local anaesthetics.
Precautionary measures to prevent air embolism should be followed while administering local anaesthetic prior to biopsy.
Pulseless electrical activity (PEA) arrest is most commonly preceded by hypoxia and prolonged hypotension.
Management of PEA arrest secondary to air embolism include immediate cardiopulmonary resuscitation, administration of 100% oxygen, intravenous fluids and Durant’s manoeuvre. After ROSC, hyperbaric oxygen therapy is useful.
Footnotes
Contributors: FK contributed towards conceptualisation and drafting the article. SR provided description for the images and assisted in draft revision. RAR provided insight regarding the integrity of the paper. SG provided final revision of the draft and its intellectual content.
Competing interests: None declared.
Patient consent: Next of kin consent obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Manhire A, Charig M, Clelland C, et al. Guidelines for radiologically guided lung biopsy. Thorax 2003;58:920–36. 10.1136/thorax.58.11.920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Freund MC, Petersen J, Goder KC, et al. Systemic air embolism during percutaneous core needle biopsy of the lung: frequency and risk factors. BMC Pulm Med 2012;12:2 10.1186/1471-2466-12-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ishii H, Hiraki T, Gobara H, et al. Risk factors for systemic air embolism as a complication of percutaneous CT-guided lung biopsy: multicenter case-control study. Cardiovasc Intervent Radiol 2014;37:1312–20. 10.1007/s00270-013-0808-7 [DOI] [PubMed] [Google Scholar]
- 4.Rott G, Boecker F. Influenceable and avoidable risk factors for systemic air embolism due to percutaneous ct-guided lung biopsy: patient positioning and coaxial biopsy technique-case report, systematic literature review, and a technical note. Radiol Res Pract 2014;2014:1–8. 10.1155/2014/349062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mokhlesi B, Ansaarie I, Bader M, et al. Coronary artery air embolism complicating a CT-guided transthoracic needle biopsy of the lung. Chest 2002;121:993–6. 10.1378/chest.121.3.993 [DOI] [PubMed] [Google Scholar]
- 6.Geraghty PR, Kee ST, McFarlane G, et al. CT-guided transthoracic needle aspiration biopsy of pulmonary nodules: needle size and pneumothorax rate. Radiology 2003;229:475–81. 10.1148/radiol.2291020499 [DOI] [PubMed] [Google Scholar]
- 7.Mirski MA, Lele AV, Fitzsimmons L, et al. Diagnosis and treatment of vascular air embolism. Anesthesiology 2007;106:164–77. 10.1097/00000542-200701000-00026 [DOI] [PubMed] [Google Scholar]
- 8.Shaikh N, Ummunisa F. Acute management of vascular air embolism. J Emerg Trauma Shock 2009;2:180–5. 10.4103/0974-2700.55330 [DOI] [PMC free article] [PubMed] [Google Scholar]