Abstract
A 31-year-old man with a mitral bioprosthetic valve presented with recent worsening of exertional dyspnoea 7 years after the mitral valve replacement. Evaluation revealed an increased gradient across the thickened mitral bioprosthetic valve leaflets. Marked eosinophilia was present and was considered as a putative cause for bioprosthetic valve thrombosis. The treatment with systemic corticosteroids and oral anticoagulation led to complete resolution of symptoms with significant decrease in mitral bioprosthetic valve gradient and leaflet thinning. The case is reported to highlight the fact that eosinophilia may cause reversible bioprosthetic valve thrombosis.
Keywords: valvar diseases, cardiovascular medicine
Background
Bioprosthetic valve thrombosis is uncommon but under-recognised. The annual incidence is reported to be around 1.46% in a large retrospective series.1 With the increasing utilisation of aortic bioprosthetic valves in transcatheter aortic valve implantation procedures, bioprosthetic valve thrombosis has become more relevant and is being carefully studied.2–4 We report a patient with mitral bioprosthetic valve thrombosis due to eosinophilia that responded to treatment with corticosteroids and anticoagulation. To the best of our knowledge, reversible bioprosthetic valve thrombosis due to eosinophilia has not been previously described.
Case presentation
A 31-year-old man underwent mitral valve replacement in 2004 with a #29 bileaflet mechanical prosthetic valve for severe rheumatic calcific mitral stenosis. A year later, he developed prosthetic heart valve thrombosis which was successfully treated with thrombolysis. Two years later, he developed a second episode of prosthetic valve thrombosis. Thrombolysis was attempted again with partial success only. Subsequently, he underwent redo-mitral valve replacement with a #25 Hancock bioprosthetic valve in 2009 in view of residual gradient across the mechanical valve and recurrent prosthetic valve thrombosis.
He remained asymptomatic for the next 7 years with regular follow-up. The last follow-up echocardiogram showed normally functioning bioprosthetic valve with no signs of degeneration and a mean diastolic pressure gradient of 4 mm Hg across the bioprosthesis. Left ventricular function was normal. About a year after the last follow-up visit, he started to experience gradually progressive dyspnoea on exertion. He was in New York Heart Association class III at presentation. On examination, a mid-diastolic murmur was noted at apex.
Investigations
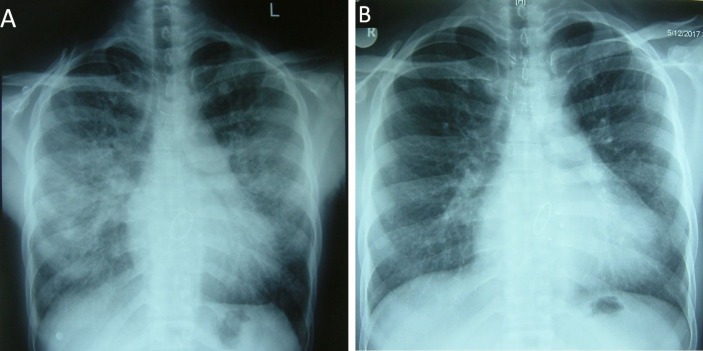
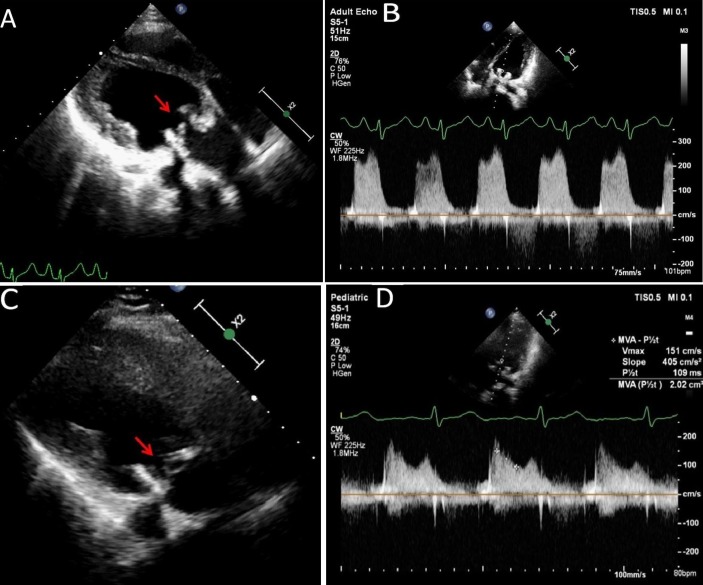
The chest X-ray (figure 1A) showed normal cardiothoracic ratio with left atrial enlargement and severe pulmonary venous hypertension. Echocardiography revealed increased thickness of the bioprosthetic valve leaflets (figure 2A (arrow); video 1) with restricted mobility. The mean diastolic pressure gradient across the mitral bioprosthesis had increased from 4 to 12 mm Hg (figure 2B). There was global left ventricular hypokinesia with ejection fraction of 35% with mild ventricular dilation.
Figure 1.
Chest radiograph at presentation (A) and after treatment with corticosteroids and anticoagulation (B) for 2 months.
Figure 2.
Echocardiogram recorded at presentation shows increased thickness of the bioprosthetic valve leaflets (A) (arrow). The pressure gradient across the mitral bioprosthesis is increased significantly (B). Echocardiogram recorded after the treatment with corticosteroids and anticoagulation (C,D). There is remarkable thinning of the bioprosthetic valve leaflets (C) (arrow). The pressure gradient across the mitral bioprosthesis has decreased significantly (D). MVA, mitral valve area.
Video 1.
Echocardiogram recorded at presentation shows increased thickness of the bioprosthetic valve leaflets with restricted mobility.
Routine blood investigations showed marked eosinophilia (77%) with an absolute eosinophil count of 25 400/mm3 in multiple samples. In view of the marked eosinophilia and the echocardiographic picture, the possibility of bioprosthetic valve thrombosis was considered. The left ventricular systolic dysfunction was attributed to the cardiomyopathic effect of hypereosinophilia.
Evaluation was done for a secondary cause of eosinophilia. Peripheral blood smear examination did not reveal microfilariae. Stool for ova and cysts showed no parasites. Bone marrow biopsy showed no blast cells.
The previous records were analysed. However, the episodes of prosthetic valve thrombosis involving the mechanical valves in 2005 and 2007 were not associated with eosinophilia.
Differential diagnosis
A diagnosis of idiopathic hypereosinophilic syndrome leading to bioprosthetic valve thrombosis was made.
Treatment
The patient was accordingly treated with diethylcarbamazine for 21 days and oral steroids which were tapered over 6 months. Oral anticoagulation with vitamin K antagonists was initiated.
Outcome and follow-up
On follow-up at 4 months, there was significant improvement in symptoms and the patient was in functional class I. The differential leucocyte count showed 1% eosinophils with an absolute eosinophil count of 140/mm3 only. Repeat chest X-ray (figure 1B) showed resolution of pulmonary venous hypertension. Echocardiogram showed normal thickness and mobility of bioprosthetic valve leaflets (figure 2C (arrow); video 2) with a remarkable decrease in mean diastolic pressure gradient across the bioprosthetic valve (figure 2D) and normalisation of left ventricular function.
Video 2.
Echocardiogram recorded after the treatment with corticosteroids and anticoagulation. There is remarkable thinning of the bioprosthetic valve leaflets.
Discussion
Bioprosthetic valve thrombosis is a rare but potentially life threatening condition that tends to occur significantly earlier than structural valve degeneration. It is difficult to ascertain the exact incidence and prevalence of bioprosthetic valve thrombosis as this entity has not been systematically reported in most large prospective series. In the largest series of bioprosthetic valve thrombosis, the incidence was estimated at 1.46% per year and the prevalence was reported to be around 11.6%.1 In this series, the rates of bioprosthetic valve thrombosis were largely comparable across all valve locations. However, despite its rarity, it can be life threatening with high early mortality.5
Oral anticoagulation with vitamin K antagonists is recommended for all patients with bioprosthetic heart valves for 3–6 months after implantation in view of the increased risk for thrombosis during this period. However, it is not well recognised that bioprosthetic valve thrombosis may occur later as well. In fact, the incidence of bioprosthetic valve thrombosis peaks around 13–24 months after the implantation.6
Bioprosthetic valve thrombosis is under-recognised. A diagnostic model has been suggested for the diagnosis of bioprosthetic valve thrombosis that includes five features.1 A >50% increase in mean echo-Doppler gradient from baseline within 5 years, increased cusp thickness, abnormal cusp mobility, paroxysmal atrial fibrillation and subtherapeutic international normalised ratio together were found to predict bioprosthetic valve thrombosis with 76% sensitivity, 93% specificity, 85% positive predictive value and 89% negative predictive value. These criteria need to be validated in other studies.
Since bioprosthetic valve thrombosis tends to occur significantly earlier than structural valve degeneration, all patients presenting with bioprosthetic valve dysfunction <5 years after implantation should be meticulously evaluated for valve thrombosis with the aforementioned criteria. The differential diagnoses for increased gradient across the bioprosthetic valve include early prosthetic degeneration, pannus formation, patient–prosthesis mismatch and high cardiac output state. Prosthetic valve degeneration usually presents later and involves wear and tear, fracture, calcification, leaflet tear, stent creep or suture line disruption of valve components.
Transthoracic echocardiogram alone as a first line screening examination has been found to detect only 13% of bioprosthetic valve thrombosis cases.7 So, transoesophageal echocardiography is recommended whenever the index of suspicion is high. Of late, volume rendered four-dimensional (4D) CT imaging has been evaluated in a number of studies to detect subclinical bioprosthetic valve thrombosis in patients receiving transcatheter aortic valve replacement.8 The 4D CT is exquisitely sensitive in assessment of bioprosthetic valve leaflet motion and detects valve thrombosis even before an increase in gradient across the valve. Therefore, in cases where the index of suspicion is high but the echocardiogram remains inconclusive 4D CT may be used to diagnose bioprosthetic valve thrombosis.
The specific risk factors for bioprosthetic valve thrombosis have not been studied systematically since the condition itself is under-recognised. However, it may be inferred that patients with hypercoaguability, a history of thromboembolic disorder, concomitant atrial fibrillation and severe left ventricular dysfunction are at increased risk. In these subsets, long-term anticoagulation has been recommended by the current American and European guidelines. Other proposed risk factors include stented design and small bioprosthetic valve size.
Eosinophilia is a rare cause of thrombophilia. Less than 10 cases of thrombosis of mechanical heart valves associated with eosinophilia have been reported.9 10 To the best of our knowledge no case of bioprosthetic valve thrombosis secondary to eosinophilia has been reported previously.
Idiopathic hypereosinophilic syndrome is associated with a high incidence of thromboembolic disorders.11 The primary underlying mechanism is platelet hyperactivation secondary to endothelial damage due to the major basic protein and increased biosynthesis of platelet activating factor in activated eosinophils. The products released during degranulation of eosinophils are highly thrombogenic. The specific granules of eosinophils contain high levels of tissue factor which stimulates fibrin formation. The major eosinophil basic protein binds to and inhibits the capacity of thrombomodulin to activate protein C resulting in an enhanced activity of the thrombotic cascade.
Several recent reports have compared an initial approach with vitamin K antagonists with surgery or thrombolysis as initial management for bioprosthetic valve thrombosis. In the largest retrospective series,1 no difference was found between the two approaches with respect to decrease in mean gradients, embolic events, strokes, deaths and bleeding. In view of the significant risks associated with surgery or thrombolysis, anticoagulation is considered reasonable as the first line treatment for bioprosthetic valve thrombosis in patients who are haemodynamically stable. Clearly, surgery or thrombolysis remains the treatment of choice in patients who are haemodynamically unstable bioprosthetic valve thrombosis. Surgery is preferred in those with large mobile thrombi and high embolic risk.
In summary, we describe a case of reversible bioprosthetic valve thrombosis due to eosinophilia. A wider appreciation of this entity seems warranted.
Learning points.
Bioprosthetic valve thrombosis is an under-recognised entity with a peak incidence around 13–24 months after implantation.
-
Predictors of bioprosthetic valve thrombosis include
>50% increase in mean echo-Doppler gradient from baseline within 5 years
increased cusp thickness
abnormal cusp mobility
paroxysmal atrial fibrillation
subtherapeutic international normalised ratio.
Eosinophilia is a thrombophilic condition which is known to be associated with mechanical heart valve thrombosis. In this report, we have described a case of reversible bioprosthetic valve thrombosis due to eosinophilia.
Footnotes
Contributors: SSK has contributed to the diagnosis and treatment of the patient. SD and NR have contributed to the planning and reporting of the case under the guidance of SSK.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Egbe AC, Pislaru SV, Pellikka PA, et al. Bioprosthetic Valve Thrombosis Versus Structural Failure: Clinical and Echocardiographic Predictors. J Am Coll Cardiol 2015;66:2285–94. 10.1016/j.jacc.2015.09.022 [DOI] [PubMed] [Google Scholar]
- 2.Chakravarty T, Søndergaard L, Friedman J, et al. Subclinical leaflet thrombosis in surgical and transcatheter bioprosthetic aortic valves: an observational study. Lancet 2017;389:2383–92. 10.1016/S0140-6736(17)30757-2 [DOI] [PubMed] [Google Scholar]
- 3.Makkar RR, Fontana G, Søndergaard L. Possible Subclinical Leaflet Thrombosis in Bioprosthetic Aortic Valves. N Engl J Med 2016;374:1591–2. 10.1056/NEJMc1600179 [DOI] [PubMed] [Google Scholar]
- 4.Rafiq S, Steinbrüchel DA, Lilleør NB, et al. Antithrombotic therapy after bioprosthetic aortic valve implantation: Warfarin versus aspirin, a randomized controlled trial. Thromb Res 2017;150:104–10. 10.1016/j.thromres.2016.11.021 [DOI] [PubMed] [Google Scholar]
- 5.Hammermeister KE, Sethi GK, Henderson WG, et al. A Comparison of Outcomes in Men 11 Years after Heart-Valve Replacement with a Mechanical Valve or Bioprosthesis. N Engl J Med Overseas Ed 1993;328:1289–96. 10.1056/NEJM199305063281801 [DOI] [PubMed] [Google Scholar]
- 6.Pislaru SV, Hussain I, Pellikka PA, et al. Misconceptions, diagnostic challenges and treatment opportunities in bioprosthetic valve thrombosis: lessons from a case series. Eur J Cardiothorac Surg 2015;47:725–32. 10.1093/ejcts/ezu201 [DOI] [PubMed] [Google Scholar]
- 7.Daniel WG, Mügge A, Grote J, et al. Comparison of transthoracic and transesophageal echocardiography for detection of abnormalities of prosthetic and bioprosthetic valves in the mitral and aortic positions. Am J Cardiol 1993;71:210–5. 10.1016/0002-9149(93)90740-4 [DOI] [PubMed] [Google Scholar]
- 8.Puri R, Auffret V, Rodés-Cabau J. Bioprosthetic Valve Thrombosis. J Am Coll Cardiol 2017;69:2193–211. 10.1016/j.jacc.2017.02.051 [DOI] [PubMed] [Google Scholar]
- 9.Awasthy N, Bhat Y, Radhakrishnan S, et al. Recurrent stuck mitral valve: eosinophilia an unusual pathology. Pediatr Cardiol 2015;36:692–3. 10.1007/s00246-015-1102-z [DOI] [PubMed] [Google Scholar]
- 10.Zakhama L, Slama I, Boussabah E, et al. Recurrent native and prosthetic mitral valve thrombosis in idiopathic hypereosinophilic syndrome. J Heart Valve Dis 2014;23:168–70. [PubMed] [Google Scholar]
- 11.Ogbogu PU, Rosing DR, Horne MK. Cardiovascular manifestations of hypereosinophilic syndromes. Immunol Allergy Clin North Am 2007;27:457–75. 10.1016/j.iac.2007.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]