Abstract
Individuals affected by Prader-Willi syndrome (PWS) may show increased risk for coronary artery disease (CAD), which probably relates, at least, with high burden of cardiovascular risk factors.
A 27-year-old man with PWS, obesity, hypertension, diabetes mellitus and dyslipidaemia attended the emergency department with complaints of flu-like condition and chest pain. The ECG revealed a mild ST-segment elevation in inferior leads, followed by positive myocardial necrosis biomarkers. Attending to the high cardiovascular risk profile, ST-segment elevation in inferior territory and wall motion abnormalities, a coronary angiogram was performed. The latter showed a three-vessel CAD, 60% stenosis in midanterior descending artery, total occlusion (100%) of the obtuse marginal artery and 99% stenosis with high thrombi burden in the proximal right coronary artery.
The present case report emphasises the plausibility of premature CAD in patients with PWS, a possible underdiagnosed feature of this condition.
Keywords: ischaemic heart disease, genetics, endocrinology, interventional cardiology, metabolic disorders
Background
Prader-Willi syndrome (PWS) is a complex genetic disorder with a prevalence range of 1:10 000–1:30 000 births1 and the first one related to imprinting defect in humans.2 In individuals with individuals commonly have obesity3 and may present hypogonadism4 and growth hormone deficiency (GHD).5 6 Consequently, they appear to be at high risk of vascular disease due to obesity-related cardiovascular risk factors, such as hypertension, diabetes mellitus type 2 or obstructive sleep apnoea syndrome.4 6 7
Only three case reports have been published about premature coronary artery disease (CAD) in living subjects with PWS.8–10 We describe a case of an acute coronary syndrome (ACS) and three-vessel CAD in a 27-year-old patient with PWS. This report adds relevant information regarding this association, which should not be undervalued when dealing with young patients with PWS.
Case presentation
The diagnosis of PWS was made when the patient was 12 years old, after a Fluorescence In Situ Hybridisation test showed a 46, XY, del (15) (q11.2q13.1) (SNRPN-) karyotype. Consanguinity is present in his family. At the same age, he already had several characteristics that are present in PWS, such as low stature, morbid obesity with a body mass index (BMI) of 41.5 kg/m2, hypogonadism (follicle-stimulating hormone 0.9 mIU/mL, luteinising hormone 0.0 mIU/mL, total testosterone 0.6 mmol/L), hyperphagia and cognitive deficit. At 14 years of age, he was diagnosed with dyslipidaemia (total cholesterol 6.1 mmol/L, high-density lipoprotein cholesterol 1.1 mmol/L, low-density lipoprotein cholesterol 3.9 mmol/L, triglycerides 2.5 mmol/L), diabetes mellitus type 2 and hypertension. Growth hormone treatment was never performed.
The patient attended the emergency room with complaints of precordial stabbing pain, with no accompanying radiation, diaphoresis or nausea. No relationship was stated between pain and deep inspiration, forward leaning, lying position or exertion. The pain duration could not be pointed, but it was not sustained. Also, he already had a similar episode the night before admission and stated recent history of flu-like condition. His medication was metformin 1000 mg three times a day, vildagliptin 50 mg once daily, ramipril 10 mg once daily and nifedipin 30 mg two times daily.
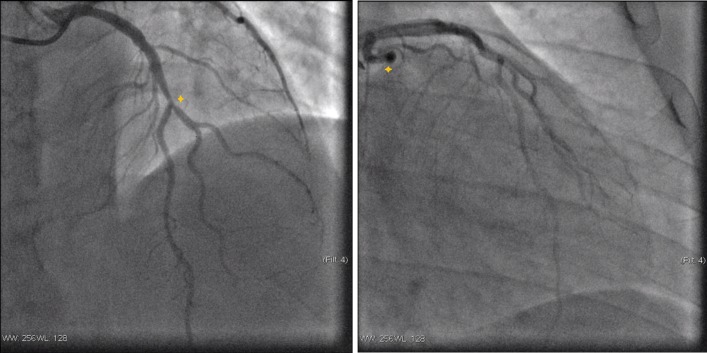
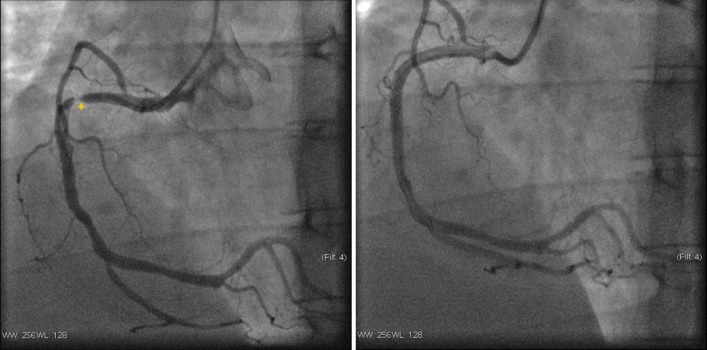
His heart rate at admission was 99 beats per minute with a blood pressure of 159/110 mm Hg. Cardiac auscultation was rhythmic, with no murmurs or pericardial rub and pulmonary auscultation was also normal. His current BMI was 31.3 kg/m2. Chest radiography showed no abnormalities. The ECG performed during pain episode revealed a mild (<1 mm) ST-segment elevation in III and aVF and ST-segment depression in I and aVL. The highest serum troponin I was 3.93 ng/mL (cut-off for myocardial infarction: 0.120 ng/mL). Echocardiogram revealed a mildly depressed left ventricle systolic function, with hypokinetic midbasal segments of the inferior, posterior and lateral walls, as well as mild mitral regurgitation, without pericardial effusion. The patient was admitted in the coronary intensive care unit with the diagnostic hypothesis of ACS versus myopericarditis. The coronary angiogram showed a right coronary dominance, three-vessel CAD, with 60% stenosis in midleft anterior descending artery (figure 1A), a total occlusion (100%) in the obtuse marginal artery (figure 1B) and 99% stenosis with high thrombi burden in the proximal right coronary artery (figure 2A). A direct drug-eluting stent was successfully placed in the right coronary artery (figure 2B).
Figure 1.
Coronary angiograms of the left coronary (left to right). (A) Stenosis in midleft anterior descending artery; (B) total occlusion of the obtuse marginal artery.
Figure 2.
Coronary angiogram of the right coronary (left to right). (A) Severe occlusion in proximal right coronary artery; (B) right coronary artery treated with a drug-eluting stent.
Differential diagnosis
The reported clinical history did not seem a case of a typical angina. Regarding the patient’s age, stabbing chest pain, past flu-like condition and ECG changes, we considered the diagnosis of myopericarditis. However, attending to the mild ST-segment elevation in inferior leads, elevated troponin I and wall motion abnormalities, our main differential diagnosis remained between myopericarditis and ACS. Given the cardiovascular profile and work-up findings, we decided to perform a coronary angiogram, which confirmed three-vessel CAD, with an obvious culprit lesion in the right coronary artery.
Outcome and follow-up
After angioplasty, the patient did not have additional complaints. At discharge, an attention call was made regarding weight loss and calorie restriction. Also, acetylsalicylic acid, clopidogrel, carvedilol and atorvastatin were added to his medication. Two months after the event, he attended the cardiology follow-up appointment. He reported no chest pain or tiredness and confirmed compliance to the medication; however, he did not significantly lose weight (91 kg).
Discussion
This case describes an ST-segment elevation ACS in a 27-year-old man with PWS and medical history of obesity, diabetes, dyslipidaemia and hypertension for more than a decade, whose coronary angiogram showed three-vessel CAD, including an apparently chronic occlusion of the obtuse marginal artery. It is true that our initial diagnostic hypothesis was myopericarditis, supported by the patient’s age, stabbing chest pain, past flu-like syndrome and ECG changes. However, an urgent (<24 hours) coronary angiogram should always be considered, because premature coronary atherosclerosis may take place in young PWS individuals.
Hypogonadism and GHD are known to be important players in premature cardiovascular disease development.11–13 Hyperphagia and obesity are also determinant, as they convey a metabolic profile that favours atherosclerosis. Obesity is generally associated with cardiovascular risk factors, such as hypertension, dyslipidaemia or diabetes mellitus type 2. It is also known that obesity-related complications are major risk factors to morbidity and mortality in adults with PWS.14 Therefore, long-term weight loss is encouraged and a main target in the treatment of PWS.14 15
The presence of premature three-vessel CAD in a 26-year-old patient with PWS was first described in 1987 by Lamb et al.9 Originally, no connection was established between PWS and CAD beyond high prevalence of cardiovascular risk factors. However, in 2007, a case–control study16 between individuals with PWS and a control group showed a significantly elevated high sensibility C reactive protein and abnormal microcirculatory responses in the first group. Marzullo et al17 also described that obese individuals with PWS differ from obese controls by a healthier metabolic profile.
Previous cases of premature CAD in patients with PWS are summarised in table 1.
Table 1.
Summary of previous premature CAD reports in patients with PWS
| Report | Age | Gender | BMI (kg/m2) | CV risk factors | Complaint | Coronarography |
| Lamb and Johnson9 | 26 | Male | 37.3 | DM2, dyslipidaemia | Lower limb oedema | Totally occluded pRCA and pLCA |
| Page et al8 | 28 | Female | 55.8 (at 15 yo) | Smoking | Constricting chest pain and breathlessness | Severe LAD stenosis |
| Jabbar et al10 | 36 | Male | 48 | DM2, dyslipidaemia, CKD, OSAS | Breathlessness and peripheral oedema | Occluded dLCA, severe LAD and RCA stenosis |
BMI, body mass index; CAD, coronary artery disease; CKD, chronic kidney disease; CV, cardiovascular; dLCA, distal left circumflex artery; DM2, diabetes mellitus type 2; LAD, left anterior descending artery; OSAS, obstructive sleep apnoea syndrome; pLCA, proximal left circumflex artery; pRCA, proximal right coronary artery; PWS, Prader-Willi syndrome; yo, years old.
From the four cases available, only the case reported by Page et al8 revealed typical chest pain as initial complaint. This could indicate that, in these individuals, CAD is either silent or with atypical presentation, which may lead to underdiagnosis. Consequently, clinicians should not rely on typical cardiac ischaemia symptoms to further investigate its presence. We also contemplate the possibility raised by Page et al, of an unrecognised premature CAD as a trait in patients with PWS, as well as the feasible relationship between q11.1–q11.2 region deletion in chromosome 15 and its implication in premature CAD development.
This report outlines the importance of premature CAD in PWS, a possible underdiagnosed feature that should be bore in mind when dealing with these patients.
Learning points.
Coronary artery disease may be present even in young patients with Prader-Willi syndrome due to high burden of cardiovascular risk factors.
Clinicians should be aware of typical cardiac ischaemia symptoms not being essential to further investigation of these patients.
Coronary angiogram should not be delayed if myocardial ischaemia is likely, even at a young age.
Modification of risk factors and lifestyle changes (such as controlling hyperphagia) are a cornerstone in slowing down atherosclerosis.
Acknowledgments
We thank Dr. José Aguiar, head of Cardiology Dpt., Dr. Hélder Gonçalves, head of Paediactrics Dpt., Dr. Claudiu Guz and Dr. Carolina Marques for their support and availability.
Footnotes
Competing interests: None declared.
Patient consent: Guardian consent obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Goldstone AP, Holland AJ, Hauffa BP, et al. Recommendations for the diagnosis and management of Prader-Willi syndrome. J Clin Endocrinol Metab 2008;93:4183–97. 10.1210/jc.2008-0649 [DOI] [PubMed] [Google Scholar]
- 2.Nicholls RD, Knoll JH, Butler MG, et al. Genetic imprinting suggested by maternal heterodisomy in nondeletion Prader-Willi syndrome. Nature 1989;342:281–5. 10.1038/342281a0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hurren BJ, Flack NA. Prader-Willi syndrome: a spectrum of anatomical and clinical features. Clin Anat 2016;29:590–605. 10.1002/ca.22686 [DOI] [PubMed] [Google Scholar]
- 4.Cassidy SB, Schwartz S, Miller JL, et al. Prader-Willi syndrome. Genet Med 2012;14:10–26. 10.1038/gim.0b013e31822bead0 [DOI] [PubMed] [Google Scholar]
- 5.Sode-Carlsen R, Farholt S, Rabben KF, et al. Growth hormone treatment in adults with Prader-Willi syndrome: the Scandinavian study. Endocrine 2012;41:191–9. 10.1007/s12020-011-9560-4 [DOI] [PubMed] [Google Scholar]
- 6.Burman P, Ritzén EM, Lindgren AC. Endocrine dysfunction in Prader-Willi syndrome: a review with special reference to GH. Endocr Rev 2001;22:787–99. 10.1210/edrv.22.6.0447 [DOI] [PubMed] [Google Scholar]
- 7.Greenswag LR. Adults with Prader-Willi syndrome: a survey of 232 cases. Dev Med Child Neurol 1987;29:145–52. 10.1111/j.1469-8749.1987.tb02129.x [DOI] [PubMed] [Google Scholar]
- 8.Page SR, Nussey SS, Haywood GA, et al. Premature coronary artery disease and the Prader-Willi syndrome. Postgrad Med J 1990;66:232–4. 10.1136/pgmj.66.773.232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lamb AS, Johnson WM. Premature coronary artery atherosclerosis in a patient with Prader-Willi syndrome. Am J Med Genet 1987;28:873–80. 10.1002/ajmg.1320280412 [DOI] [PubMed] [Google Scholar]
- 10.Jabbar A, Khan JN, Singh A, et al. ’A one-sided affair': unilateral pulmonary oedema and the role of cardiac MRI in diagnosing premature coronary artery disease in a patient with Prader-Willi syndrome. BMJ Case Rep 2013;2013:bcr2013008692 10.1136/bcr-2013-008692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu PY, Death AK, Handelsman DJ. Androgens and cardiovascular disease. Endocr Rev 2003;24:313–40. 10.1210/er.2003-0005 [DOI] [PubMed] [Google Scholar]
- 12.de Boer H, Blok GJ, Van der Veen EA. Clinical aspects of growth hormone deficiency in adults. Endocr Rev 1995;16:63–86. 10.1210/er.16.1.63 [DOI] [PubMed] [Google Scholar]
- 13.Rosén T, Bengtsson BA. Premature mortality due to cardiovascular disease in hypopituitarism. Lancet 1990;336:285–8. 10.1016/0140-6736(90)91812-O [DOI] [PubMed] [Google Scholar]
- 14.Smith A, Loughnan G, Steinbeck K. Death in adults with Prader-Willi syndrome may be correlated with maternal uniparental disomy. J Med Genet 2003;40:e63–. 10.1136/jmg.40.5.e63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schrander-Stumpel CT, Curfs LM, Sastrowijoto P, et al. Prader-Willi syndrome: causes of death in an international series of 27 cases. Am J Med Genet A 2004;124A:333–8. 10.1002/ajmg.a.20371 [DOI] [PubMed] [Google Scholar]
- 16.Patel S, Harmer JA, Loughnan G, et al. Characteristics of cardiac and vascular structure and function in Prader-Willi syndrome. Clin Endocrinol 2007;66:771–7. 10.1111/j.1365-2265.2007.02808.x [DOI] [PubMed] [Google Scholar]
- 17.Marzullo P, Marcassa C, Campini R, et al. The impact of growth hormone/insulin-like growth factor-I axis and nocturnal breathing disorders on cardiovascular features of adult patients with Prader-Willi syndrome. J Clin Endocrinol Metab 2005;90:5639–46. 10.1210/jc.2005-0829 [DOI] [PubMed] [Google Scholar]