Abstract
The advent of disease evaluation by means of multi-slice spiral computed tomography (MSCT) and magnetic resonance imaging (MRI) represents a continually emerging role in the evaluation of various diseases; however, its role is yet to be adequately defined. Thus, the aim of the study was to compare the diagnostic value of MSCT and MRI in the diagnosis of peritoneal metastasis in primary ovarian carcinoma. Between January 2013 and December 2015, MSCT or MRI data were collected from 42 patients who had been previously diagnosed with peritoneal metastasis of ovarian carcinoma at the First Affiliated Hospital of Kunming Medical University. The tumor location, size, edge, and shape were all evaluated independently by three qualified imaging physicians using a double-blind method to confirm whether the patients were indeed suffering from peritoneal metastasis, as well as to rank the metastatic lesions recorded on a five-point scale. It was hypothesized that MRI and MSCT were comparable in the evaluation of ovarian carcinoma. Therefore, a receiver operating characteristics (ROC) curve was used to analyze the results and also to directly compare the respective diagnostic values of MSCT and MRI. In total, 165 metastatic lesions were confirmed by means of surgical operation. MSCT revealed 131 metastatic lesions, while MRI confirmed 154 metastatic lesions. The metastatic sites were primarily located on the subphrenic, epiploon, and gastrocolic ligaments and were further confirmed by either MRI or CT. In regard to MSCT, the most common site of underdiagnoses was in the vicinity of the uterus–rectum–fossa. MRI displayed a high detection rate in every site. The omission diagnostic rate of MSCT and MRI were 20.61% and 6.67%, respectively, while the accuracy rates were 79.39% and 93.33%, respectively. The obtained results revealed that the MSCT value of area under the ROC curve was smaller than that for MRI. Our findings provided evidence asserting that MRI, in comparison to MSCT, was more accurate in diagnosing peritoneal metastasis in patients with ovarian carcinoma.
Keywords: multi-slice spiral computed tomography, magnetic resonance imaging, ovarian carcinoma, peritoneal metastasis, accuracy, omission diagnostic rate, ROC curves
Introduction
The incidence and prevalence of ovarian carcinoma continues to grow at an alarming rate. At present, it represents the sixth most common malignancy in females. Ovarian carcinoma is a cancer that manifests itself in an ovary, occurring in abnormal cells that can invade or spread to other parts of the body.1,2 Previous reports previously in 2012 indicated that ovarian carcinoma affected approximately 239,000 women, resulting in 152,000 deaths worldwide.1 Approximately 20% of patients with stage I and stage II ovarian carcinoma may suffer from a recurrence within a 5-year period. Additionally, the 5-year survival rate of patients in stage III and stage IV remain at extremely low figures.3,4 Many risk factors have been associated ovarian carcinoma including hormones, genetics, as well as environmental factors.5,6 While the majority of ovarian carcinoma recurrences present within the abdomen, peritoneal metastasis remains one of the leading causes of morbidity and mortality for ovarian carcinoma.7 Patients with ovarian carcinoma with peritoneal metastasis generally have poor prognosis, which consequently results in the use of fewer therapeutic approaches.8,9 Early stage ovarian carcinoma symptoms of peritoneal metastasis are generally nonspecific, which subsequently allows for frequent misdiagnoses as well as underdiagnoses. Thus, ovarian carcinoma is rarely diagnosed correctly until it spreads and advances to later stages.
Magnetic resonance imaging (MRI) is a medical imaging technique widely used in radiology to image the physiological processes and anatomy of the body in disease and health, through the application of field gradients, strong magnetic fields, and radio waves to construct images of the body.10,11 MRI plays an important role in the diagnosis and treatment of many human diseases, as well as in reducing the risks involved with exposure to ionizing radiation.12,13 However, MRI may, in certain situations, be unfavorable for patients, due to its time-consuming, claustrophobia-exacerbating, and expensive nature.14 Computed tomography (CT) scans are relatively associated with patient exposure to high radiation; however, it remains an important contributory tool for population doses.15 Multi-slice spiral CT (MSCT) was devised in order to improve the diagnoses of diseases by X-rays and medical ultrasonography, and has since had a progressively wider use in screening for disease as well as for purposes of preventive medicine.16 The radiation used in MSCT scans can destroy body cells and DNA molecules, leading to cancer.17,18 Both MSCT and MRI have been widely used in the diagnosis of human diseases and a variety of cancers.19,20 However, the diagnostic values of MSCT and MRI with regard to peritoneal metastasis in patients with primary ovarian carcinoma is still largely debated.21 Therefore, this study was conducted using MSCT and MRI, retrospectively analyzed, and compared the imaging features of 42 pathologically confirmed patients with ovarian carcinoma with peritoneal metastasis, in order to explore the diagnostic value of both MSCT and MRI in relation to peritoneal metastasis in primary ovarian carcinoma.
Materials and methods
Ethical statement
The study was approved by the Institutional Review Board of the First Affiliated Hospital of Kunming Medical University. Written informed consent documentation was signed by all study participants.
Study subjects
Fifty-one patients diagnosed with ovarian carcinoma, who were yet to undergo radiotherapy, chemotherapy, and any other adjuvant therapies at the First Affiliated Hospital of Kunming Medical University, were enrolled into the study between January 2013 and December 2015. Forty-two patients were confirmed to have peritoneal metastasis by means of both surgical operation and pathology. The included patients underwent MSCT and MRI scanning 1 week prior to surgery.
Multi-slice spiral CT scanning
A lightspeed 16-slices spiral CT scanner (GE Health Care, MA, USA) was employed for patient examination purposes, at 120 kV as well as 300 mA. Slice thickness was 0.5 mm, and pitch was 0.375 mm. The scanning region was in the vicinity of the lower abdomen, including the entire pelvic region, until 14.0–20.0 cm below the iliac spine. A whole-abdominal dual-phase enhanced scanning was performed following plain scanning, and a non-ionic contrast agent (1.5–2.0 mL/kg iohexol; 300 mgI/mL, Guangzhou Schering Pharmaceutical Ltd., Guangzhou, People’s Republic of China) was injected with a CT injector system (Medrad Vistron CT injection system, Medrad Inc., Indianola, PA, USA) via the vena brachialis at a flow rate between 2.5–3 mL/s. In regard to the delay time of CT scanner, there were a delay of 25–30 s at the arterial phase and a delay of 55–65 s at the venous phase. All CT data were transferred to the Advantage workstation, followed by multi-planar reconstructions (MPR) for tumor metastasis observation.
MRI scanning
All MRI experiments were conducted using a 1.5-T HDXT MRI scanner (GE Health Care, MA, USA). A phased-array body coil was used for abdominal scanning. The scanning parameters of the MRI scanner were as follows: T1-weighted imaging (T1WI)/turbo-spin-echo (TSE) [repetition time (TR): 520 ms; echo time (TE): 12 ms]; T2-weighted imaging (T2WI)/TSE (TR: 1,300 ms; TE: 92 ms). Furthermore, a fat-suppressed technique was used in all of the examinations conducted. The scanning parameters of the diffusion-weighted imaging (DWI) included: TR: 4,100 ms; TE: 80 ms; matrix: 256×160; slice thickness: 6 mm, slice gap: 2 mm. The scanning parameters of the dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) were as follows: TR: 4.4~4.8 ms; TE: 1.8–2.2 ms; TI: 5.0 ms; bandwidth: 62.5 Hz; flip angle: 15°; Fov: 36–40 cm; matrix: 256×192; NEX: 0.71–0.74; slice thickness: 3 mm, slice gap: 1 mm. A contrast agent (0.1–0.2 mmol/kg gadolinium diethylene triaminepenta acetate (Gd-DTPA); Guangzhou Schering Pharmaceutical Ltd., Guangzhou, People’s Republic of China) was injected intravenously at a flow rate of 3.5 mL/s, and the injection time was about approximately 6 s. Next, an enhanced scan of the axle location, coronal, and arrow was undertaken. The signal collecting time was 6, 60, and 120 s, respectively. The total time was approximately 240 s.
Imaging analysis
Both the MSCT and MRI results were analyzed and recorded by three qualified imaging physicians via the double-blind method. The main observations included the unilateral and bilateral ovarian tumor, tumor size (the maximum of diameter axial), the edge, the shape, the number of housing allocation, the signal (low and high signals were divided by the signal strength near the myometrium), and the degree of enhancement. The analyzed sites included the subphrenic, subhepatic, paracolonic gutter, epiploon, and gastrocolic ligaments; the mesenterium, uterus–rectum–fossa, and uterine and ovarian ligament were all evaluated in order to confirm the occurrence of peritoneal metastasis. Evaluation criteria were determined by the involvement and infiltration of the primary sites of ovarian carcinoma, peritoneal metastasis, as well as the patient’s respective organs. Results were compared with both the surgical and pathological findings in order to investigate the MSCT and MRI scanning indicators of peritoneal metastasis in ovarian carcinoma. The metastatic lesions were scored on a five-point scale: no represented 1 point; 2 points for possibly not; 3 points for not sure/uncertainty; 4 points for possible; and, finally, 5 points for yes.
Statistical analysis
Statistical analyses were conducted using the SPSS 21.0 (IBM Corp. Armonk, NY, USA). Measurement data were presented as mean ± standard deviations; t-tests were used for comparisons between the diagnostic values of MSCT and MRI. The categorical data were analyzed by means of a χ2 test or Fisher’s exact test. The receiver operating characteristics (ROC) curves were used to analyze the results of MSCT and MRI, and calculate the value of area under the ROC curve (AUC) in order to compare the differences between the various examination methods. All statistical tests were two-sided probability tests. P<0.05 was considered to be statistically significant.
Results
Surgical and pathological findings of peritoneal metastasis of ovarian carcinoma
Diagnoses of 42 patients with ovarian carcinoma were confirmed by means of surgical operation and histopathology. Ages ranged between 31 and 82 years, with an average age of 50.81±15.79 years, while the average body mass index (BMI) was 23.42±3.94. There were 29 premenopausal and 13 postmenopausal patients. Clinical manifestations of these patients included abnormal menstruation (eight patients), abdominal pain and distension (21 patients), and other abnormal conditions determined during physical examination (13 patients). Among the 42 patients, 22 were diagnosed with papillary cystic adenocarcinoma, six patients had serous adenocarcinoma, seven patients had papillary adenocarcinoma, four patients had moderately differentiated adenocarcinoma, two patients had poorly differentiated adenocarcinoma, and one patient had endometrioid adenocarcinoma. The sites and diameter size of the 165 metastatic lesions that were confirmed by surgical operation and histopathology are illustrated in Table 1.
Table 1.
Sites and diameter size of metastatic lesions confirmed by surgical operation and pathology
| Metastatic sites | Diameter size <5 mm | Diameter size ≥5 mm | Average diameter |
|---|---|---|---|
| Subphrenic | 15 | 13 | 5.23±1.55 |
| Subhepatic | 2 | 8 | 5.57±1.07 |
| Paracolonic gutter | 9 | 5 | 4.95±1.79 |
| Epiploon and gastrocolic ligament | 7 | 19 | 5.74±1.69 |
| Mesenterium | 0 | 15 | 6.31±0.99 |
| Uterus–rectum–fossa | 10 | 20 | 5.51±1.53 |
| Uterine and ovarian ligament | 2 | 7 | 6.00±1.43 |
| Other | 15 | 18 | 5.15±1.89 |
| Total | 60 | 105 | 5.48±1.61 |
MSCT imaging features in peritoneal metastasis of ovarian carcinoma
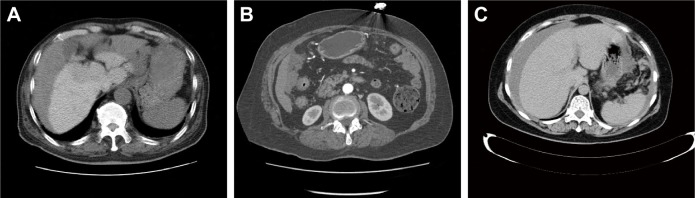
One hundred and sixty-five metastatic lesions were confirmed during surgery in 42 cases of ovarian carcinoma. MSCT confirmed 131 metastatic sites, including 51 cases with diameter less than 5 mm and 80 cases with diameter greater than 5 mm. The average diameter was 5.44±1.68 mm, while the maximum diameter of the metastatic site was 10.92 mm. The area and size of metastatic sites and the relation between metastasis and adjacent ascites on MSCT scans are depicted in Table 2. Among the 131 metastatic sites, 103 metastatic sites presented in combination with ascites. Metastatic sites were primarily located on the subphrenic (Figure 1A), epiploon, and gastrocolic ligaments (Figure 1B), and in the uterus–rectum–fossa region (Figure 1C). However, 34 metastatic lesions were undetected by MSCT, nine of which had a diameter less than 5 mm, and 25 with a diameter more than or equal to 5 mm. The most common site of MSCT underdiagnoses was in the uterus–rectum–fossa region.
Table 2.
Comparison of the metastatic sites between surgical operation and MSCT scans
| Metastatic sites | MSCT (positive)
|
MSCT (negative)
|
||||
|---|---|---|---|---|---|---|
| Diameter size <5 mm | Diameter size ≥5 mm | Ascites | Diameter size <5 mm | Diameter size ≥5 mm | Ascites | |
| Subphrenic | 15 | 12 | 25 | 0 | 1 | 0 |
| Subhepatic | 2 | 7 | 9 | 0 | 1 | 0 |
| Paracolonic gutter | 8 | 4 | 12 | 1 | 1 | 1 |
| Epiploon and gastrocolic ligament | 6 | 16 | 17 | 1 | 3 | 1 |
| Mesenterium | 0 | 11 | 8 | 0 | 4 | 1 |
| Uterus–rectum–fossa | 9 | 16 | 21 | 1 | 4 | 2 |
| Uterine and ovarian ligament | 2 | 5 | 6 | 0 | 2 | 2 |
| Other | 9 | 9 | 5 | 6 | 9 | 4 |
| Total | 51 | 80 | 103 | 9 | 25 | 11 |
Abbreviation: MSCT, multi-slice spiral computed tomography.
Figure 1.
The main manifestations of peritoneal metastasis of ovarian carcinoma detected by MSCT scans.
Notes: (A) Stage III ovarian carcinoma (the right side): multiple metastatic sites were presented in subphrenic, with a maximum diameter of about 8.08 mm. (B) Stage III ovarian carcinoma (the right side): metastasis in epiploon, similar to biscuits, with a maximum diameter of approximately 10.92 mm. (C) Stage III ovarian carcinoma (the right side): calcified metastasis presented in the uterus–rectum–fossa, with a diameter of approximately 7.82 mm.
Abbreviation: MSCT, multi-slice spiral computed tomography.
MRI features in peritoneal metastasis of ovarian carcinoma
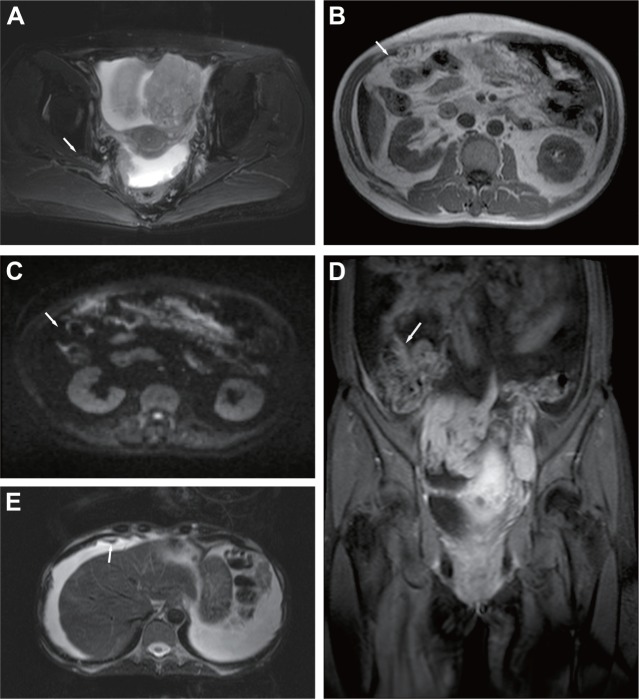
One hundred and fifty-four metastatic sites were confirmed by means of MRI, with 54 cases and 165 lesions during surgery, with a diameter less than 5 mm and 100 with a diameter greater than 5 mm. The average diameter was 5.51±1.62 mm and the maximum diameter of the metastatic site was 10.92 mm. Both the location and size of the metastatic sites, as well as the relationship of metastasis and adjacent ascites on MRI scans are displayed in Table 3. The main manifestations of peritoneal metastatic sites detected by means of MRI included irregular linear thickening of the peritoneum (Figure 2A), fouling thickening or biscuit-like formations of the epiploon (Figure 2B and C), the smudged appearances of the mesentery (Figure 2D), as well as plaques and nodular changes in the abdominal soft tissue (Figure 2E). Among the 154 metastatic sites, 108 presented in combination with ascites. The main metastatic sites that were confirmed by MRI were the same as those detected by MSCT. In total, 11 metastatic sites were undetected by MRI – six cases with diameter less than 5 mm, and five with diameter greater than or equal to 5 mm. MRI exhibited a high detection rate in every site.
Table 3.
Comparison of the detection of metastatic sites between surgical operation and MRI
| Metastatic sites | MRI (positive)
|
MRI (negative)
|
||||
|---|---|---|---|---|---|---|
| Diameter size <5 mm | Diameter size ≥5 mm | Ascites | Diameter size <5 mm | Diameter size ≥5 mm | Ascites | |
| Subphrenic | 12 | 11 | 22 | 3 | 2 | 3 |
| Subhepatic | 1 | 8 | 8 | 1 | 0 | 1 |
| Paracolonic gutter | 9 | 5 | 13 | 0 | 0 | 0 |
| Epiploon and gastrocolic ligament | 6 | 19 | 17 | 1 | 0 | 1 |
| Mesenterium | 0 | 14 | 9 | 0 | 1 | 0 |
| Uterus–rectum–fossa | 10 | 20 | 23 | 0 | 0 | 0 |
| Uterine and ovarian ligament | 2 | 7 | 8 | 0 | 0 | 0 |
| Other | 14 | 16 | 8 | 1 | 2 | 1 |
| Total | 54 | 100 | 108 | 6 | 5 | 6 |
Abbreviation: MRI, magnetic resonance imaging.
Figure 2.
The main manifestations of peritoneal metastasis of ovarian carcinoma detected by MRI.
Notes: (A) Stage II bilateral ovarian carcinoma: the arrow indicates the irregular thickening of the peritoneum. (B) Stage III ovarian carcinoma; the arrow indicates caked thickening in epiploon. (C) A high signal of DWIBS; the arrow indicates caked thickening in epiploon. (D) Stage III ovarian carcinoma: the arrow indicates the smudged appearances of the mesentery of the small intestine, combined with multiple lymphadenectasis. (E) Stage III ovarian carcinoma: the arrow indicates the right diaphragmatic peritoneum is presented in nodules and plaques.
Abbreviations: DWIBS, diffusion-weighted whole-body imaging with background body signal suppression; MRI, magnetic resonance imaging.
Diagnostic value of MSCT and MRI in peritoneal metastasis of ovarian carcinoma
Results of MSCT and MRI in relation to the diagnoses of peritoneal metastasis in ovarian carcinoma are shown in Table 4. The omission diagnosis rate of MSCT and MRI were 20.61% and 6.67%, respectively, while the rates of accuracy were 79.39% and 93.33%, respectively (both P<0.05). The ROC curves were used to analyze the results of MSCT and MRI (Figure 3). The result showed that the AUC of MSCT was smaller than that of MRI (P<0.05). This result suggested that MRI had a stronger diagnostic rate with regard to peritoneal metastasis of ovarian carcinoma when compared with that of MSCT.
Table 4.
Comparison of omission diagnosis rate and accuracy between MSCT and MRI
| Metastatic sites | Omission diagnosis rate (%)
|
Accuracy (%)
|
||
|---|---|---|---|---|
| MSCT | MRI | MSCT | MRI | |
| Subphrenic | 0.61 | 3.03 | 99.39 | 96.97 |
| Subhepatic | 0.61 | 0.61 | 99.39 | 99.39 |
| Paracolonic gutter | 1.21 | 0.00 | 98.79 | 100.00 |
| Epiploon and gastrocolic ligament | 2.42 | 0.61 | 97.58 | 99.39 |
| Mesenterium | 2.42 | 0.61 | 97.58 | 99.39 |
| Uterus–rectum–fossa | 3.03 | 0.00 | 96.97 | 100.00 |
| Uterine and ovarian ligament | 1.21 | 0.00 | 98.79 | 100.00 |
| Other | 9.09 | 1.82 | 90.91 | 98.18 |
| Total | 20.61 | 6.67* | 79.39 | 93.33* |
Note:
Indicating the comparison of the results on the same MSCT scan, P<0.05.
Abbreviations: MSCT, multi-slice spiral computed tomography; MRI, magnetic resonance imaging.
Figure 3.
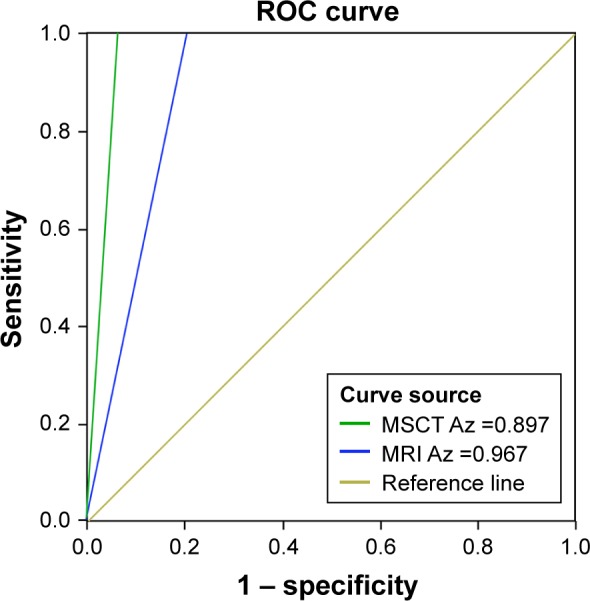
The receiver operating characteristics (ROC) curve on peritoneal metastatic lesions of patients with ovarian carcinoma that were detected by multi-slice spiral computed tomography (MSCT) and magnetic resonance imaging (MRI).
Abbreviation: Az, area under the curve.
Discussion
During this study, we retrospectively analyzed and compared the MSCT and MRI features of patients with ovarian carcinoma with peritoneal metastasis. Our results revealed that 165 metastatic lesions were confirmed by surgery, 131 metastatic lesions confirmed by MSCT, while 154 metastatic lesions were confirmed by MRI. The omission diagnosis rate of MSCT was observed to be higher than that of MRI in addition to exhibiting a superior accuracy rate. These results suggest that MRI has a better diagnosis rate in diagnosing peritoneal metastasis of ovarian carcinoma in comparison with MSCT.
Peritoneal metastasis is commonly observed in the stomach, gallbladder, pancreas, lung, intestinal, uterus, and ovary.22–24 Peritoneal metastasis has been highlighted due to its widespread recurrence pattern for many malignancies that have a particularly high recurrence rate, which may lead to a decline in the quality of life and worsening prognoses.9 The most common findings of metastatic sites are generally represented by ascites; greater omentum and mesenteric involvement; serosal and parietal peritoneal implants, with liver metastasis; and/or lymphadenopathies as accessory findings.25 Based on our research, previous studies have mostly indicated that MSCT still remains the most important and valuable imaging method among certain diseases.26,27 A previous study reported the main advantage of MSCT to be the rapid scanning speed as well as its wide scanning coverage.28 On the other hand, a previous study has demonstrated the sensitivity of peritoneal metastasis detection in relation to CT techniques as being largely limited in patients with advanced stages of gastric cancer.29 Mabille et al also indicated CT has a lower detection rate for peritoneal metastases and is not sufficient to diagnose metastasis of soft tissues.30 Compared with the MSCT, MRI is used in clinics and hospitals for diagnosing human diseases, which may clearly present the soft tissues and pelvic cavity of the human body.31,32 In our study, the main metastatic sites were located on the subphrenic, epiploon, and the gastrocolic ligaments, and were detected by MRI and MSCT. Meanwhile, we detected that the omission diagnosis rate of MSCT was approximately 20%, with the most common site of MSCT underdiagnoses being the uterus–rectum–fossa region.
Both MSCT and MRI can diagnose peritoneal metastasis in patients with ovarian carcinoma; however, our findings suggested that the MRI technique maintains superior accuracy over that of MSCT in diagnosing peritoneal metastasis of ovarian carcinoma. A previous study demonstrates the accuracy of MRI in the detection of ovarian carcinoma as being between 78% and 88%, and the accuracy of CT is between 53% and 92%.33 Peritoneal metastasis, with larger tumor diameters, can be detected by MRI, but may be missed on CT scans, suggesting that MRI may be better than CT in evaluating peritoneal metastasis in various cancers.34,35 In the present study, both MSCT and MRI presented false negatives in their respective diagnoses of peritoneal metastasis in patients with ovarian carcinoma. A total of 34 metastatic lesions were undetected by MSCT and 12 metastatic lesions were undetected by MRI. In MSCT imaging features, there were nine metastatic lesions with a diameter less than 5 mm that had been underdiagnosed, and 25 with metastatic lesions having a diameter more than or equal to 5 mm were underdiagnosed. The accuracy of MSCT was significantly lower than that of MRI, and the results of the ROC curve revealed the AUC of MSCT as being smaller than that of MRI. This result suggested that, when compared with MSCT, MRI has a better diagnosis rate in diagnosing peritoneal metastasis of ovarian carcinoma. However, the high accuracy of both MSCT and MRI can be drawn mainly down to the small sample size and cohort studies; thus, larger sample sizes are required to confirm our findings.
Conclusion
Both MSCT and MRI are capable of diagnosing peritoneal metastasis in patients with ovarian carcinoma; however, MRI possesses superior diagnostic value in identification of peritoneal metastasis in patients with ovarian carcinoma. Moreover, the accuracy of MRI is also higher than that of MSCT, which may represent a noninvasive, reliable, and effective method for the diagnosis of peritoneal metastasis in patients with ovarian carcinoma.
Acknowledgments
The authors would like to acknowledge the helpful comments on this paper received from our reviewers.
Footnotes
Author contributions
All authors contributed toward data analysis, drafting and critically revising the paper, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Piek JM, van Diest PJ, Verheijen RH. Ovarian carcinogenesis: an alternative hypothesis. Adv Exp Med Biol. 2008;622:79–87. doi: 10.1007/978-0-387-68969-2_7. [DOI] [PubMed] [Google Scholar]
- 2.Shaaban A, Rezvani M. Ovarian cancer: detection and radiologic staging. Clin Obstet Gynecol. 2009;52(1):73–93. doi: 10.1097/GRF.0b013e3181961625. [DOI] [PubMed] [Google Scholar]
- 3.Kumar A, Le N, Tinker AV, Santos JL, Parsons C, Hoskins PJ. Early-stage endometrioid ovarian carcinoma: population-based outcomes in British Columbia. Int J Gynecol Cancer. 2014;24(8):1401–1405. doi: 10.1097/IGC.0000000000000230. [DOI] [PubMed] [Google Scholar]
- 4.Arikan SK, Kasap B, Yetimalar H, Yildiz A, Sakarya DK, Tatar S. Impact of prognostic factors on survival rates in patients with ovarian carcinoma. Asian Pac J Cancer Prev. 2014;15(15):6087–6094. doi: 10.7314/apjcp.2014.15.15.6087. [DOI] [PubMed] [Google Scholar]
- 5.Gong TT, Wu QJ, Vogtmann E, Lin B, Wang YL. Age at menarche and risk of ovarian cancer: a meta-analysis of epidemiological studies. Int J Cancer. 2013;132(12):2894–2900. doi: 10.1002/ijc.27952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hunn J, Rodriguez GC. Ovarian cancer: etiology, risk factors, and epidemiology. Clin Obstet Gynecol. 2012;55(1):3–23. doi: 10.1097/GRF.0b013e31824b4611. [DOI] [PubMed] [Google Scholar]
- 7.Werner ME, Karve S, Sukumar R, et al. Folate-targeted nanoparticle delivery of chemo- and radiotherapeutics for the treatment of ovarian cancer peritoneal metastasis. Biomaterials. 2011;32(33):8548–8554. doi: 10.1016/j.biomaterials.2011.07.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cannistra SA. Cancer of the ovary. N Engl J Med. 2004;351(24):2519–2529. doi: 10.1056/NEJMra041842. [DOI] [PubMed] [Google Scholar]
- 9.Bakkar R, Gershenson D, Fox P, Vu K, Zenali M, Silva E. Stage IIIC ovarian/peritoneal serous carcinoma: a heterogeneous group of patients with different prognoses. Int J Gynecol Pathol. 2014;33(3):302–308. doi: 10.1097/PGP.0b013e3182988dfd. [DOI] [PubMed] [Google Scholar]
- 10.Hollingworth W, Todd CJ, Bell MI, et al. The diagnostic and therapeutic impact of MRI: an observational multi-centre study. Clin Radiol. 2000;55(11):825–831. doi: 10.1053/crad.2000.0546. [DOI] [PubMed] [Google Scholar]
- 11.Charles-Edwards EM, deSouza NM. Diffusion-weighted magnetic resonance imaging and its application to cancer. Cancer Imaging. 2006;6:135–143. doi: 10.1102/1470-7330.2006.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Semelka RC, Armao DM, Elias J, Jr, Huda W. Imaging strategies to reduce the risk of radiation in CT studies, including selective substitution with MRI. J Magn Reson Imaging. 2007;25(5):900–909. doi: 10.1002/jmri.20895. [DOI] [PubMed] [Google Scholar]
- 13.Bernardin L, Douglas NH, Collins DJ, et al. Diffusion-weighted magnetic resonance imaging for assessment of lung lesions: repeatability of the apparent diffusion coefficient measurement. Eur Radiol. 2014;24(2):502–511. doi: 10.1007/s00330-013-3048-y. [DOI] [PubMed] [Google Scholar]
- 14.Pasquinelli F, Belli G, Mazzoni LN, et al. MR-diffusion imaging in assessing chronic liver diseases: does a clinical role exist? Radiol Med. 2012;117(2):242–253. doi: 10.1007/s11547-011-0730-5. [DOI] [PubMed] [Google Scholar]
- 15.Mc Laughlin PD, O’Connor OJ, O’Neill SB, Shanahan F, Maher MM. Minimization of radiation exposure due to computed tomography in inflammatory bowel disease. ISRN Gastroenterol. 2012;2012:790279. doi: 10.5402/2012/790279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liang X, Jacobs R, Hassan B, et al. A comparative evaluation of Cone Beam Computed Tomography (CBCT) and Multi-Slice CT (MSCT) Part I. On subjective image quality. Eur J Radiol. 2010;75(2):265–269. doi: 10.1016/j.ejrad.2009.03.042. [DOI] [PubMed] [Google Scholar]
- 17.Brenner DJ, Hall EJ. Computed tomography – an increasing source of radiation exposure. N Engl J Med. 2007;357(22):2277–2284. doi: 10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]
- 18.Mathews JD, Forsythe AV, Brady Z, et al. Cancer risk in 680,000 people exposed to computed tomography scans in childhood or adolescence: data linkage study of 11 million Australians. BMJ. 2013;346:f2360. doi: 10.1136/bmj.f2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tian YT, Wang CF, Shan Y, et al. Prospective evaluation of ultra-sonography, multi-slice spiral CT, endoscopic ultrasonography, and magnetic resonance imaging in assessment of TNM staging and assessment of resectability in pancreatic carcinoma. Zhonghua Yi Xue Za Zhi. 2008;88(40):2829–2832. Chinese. [PubMed] [Google Scholar]
- 20.Xu J, Shen J, Ding Y, et al. The clinical value of combined use of MR imaging and multi-slice spiral CT in limb salvage surgery for orthopaedic oncology patients: initial experience in nine patients. Radiol Oncol. 2012;46(3):189–197. doi: 10.2478/v10019-012-0020-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmidt S, Meuli RA, Achtari C, Prior JO. Peritoneal carcinomatosis in primary ovarian cancer staging: comparison between MDCT, MRI, and 18F-FDG PET/CT. Clin Nucl Med. 2015;40(5):371–377. doi: 10.1097/RLU.0000000000000768. [DOI] [PubMed] [Google Scholar]
- 22.Wang Z, Chen L. Present status of treatment in peritoneal metastasis of gastric cancer. Zhonghua Wei Chang Wai Ke Za Zhi. 2015;18(2):194–197. Chinese. [PubMed] [Google Scholar]
- 23.Lin CC, Liang HP, Lee HS, et al. Clinical manifestations and survival of hepatocellular carcinoma patients with peritoneal metastasis. J Gastroenterol Hepatol. 2009;24(5):815–820. doi: 10.1111/j.1440-1746.2009.05848.x. [DOI] [PubMed] [Google Scholar]
- 24.Kuniyasu H, Oue N, Sasahira T, et al. Reg IV enhances peritoneal metastasis in gastric carcinomas. Cell Prolif. 2009;42(1):110–121. doi: 10.1111/j.1365-2184.2008.00577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Sio I, Castellano L, Terracciano F, Vitale LM, Niosi M, Loguercio C. Peritoneal metastasis. In: Maconi G, Porro GB, editors. Ultrasound of the Gastrointestinal Tract. Berlin: Springer; 2014. pp. 187–193. [Google Scholar]
- 26.Plattner T, Thali MJ, Yen K, et al. Virtopsy-postmortem multislice computed tomography (MSCT) and magnetic resonance imaging (MRI) in a fatal scuba diving incident. J Forensic Sci. 2003;48(6):1347–1355. [PubMed] [Google Scholar]
- 27.de Graaf FR, Schuijf JD, van Werkhoven JM, et al. Abstract 5817.5: Assessment of global left ventricular function and volumes with 320-slice MSCT: a comparison with 2D echocardiography. Circulation. 2008;118(Suppl 18):S_1010. [Google Scholar]
- 28.Li BG, Ma DQ, Xian ZY, et al. The value of multislice spiral CT features of cavitary walls in differentiating between peripheral lung cancer cavities and single pulmonary tuberculous thick-walled cavities. Br J Radiol. 2012;85(1010):147–152. doi: 10.1259/bjr/79051309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim SJ, Kim HH, Kim YH, et al. Peritoneal metastasis: detection with 16- or 64-detector row CT in patients undergoing surgery for gastric cancer. Radiology. 2009;253(2):407–415. doi: 10.1148/radiol.2532082272. [DOI] [PubMed] [Google Scholar]
- 30.Mabille M, Vanel D, Albiter M, et al. Follow-up of hepatic and peritoneal metastases of gastrointestinal tumors (GIST) under Imatinib therapy requires different criteria of radiological evaluation (size is not everything!!!) Eur J Radiol. 2009;69(2):204–208. doi: 10.1016/j.ejrad.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 31.Nayak TK, Garmestani K, Milenic DE, Brechbiel MW. PET and MRI of metastatic peritoneal and pulmonary colorectal cancer in mice with human epidermal growth factor receptor 1-targeted 89Zr-labeled panitumumab. J Nucl Med. 2012;53(1):113–120. doi: 10.2967/jnumed.111.094169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kyriazi S, Kaye SB, deSouza NM. Imaging ovarian cancer and peritoneal metastases – current and emerging techniques. Nat Rev Clin Oncol. 2010;7(7):381–393. doi: 10.1038/nrclinonc.2010.47. [DOI] [PubMed] [Google Scholar]
- 33.Forstner R. Radiological staging of ovarian cancer: imaging findings and contribution of CT and MRI. Eur Radiol. 2007;17(12):3223–3235. doi: 10.1007/s00330-007-0736-5. [DOI] [PubMed] [Google Scholar]
- 34.Semelka RC, Lawrence PH, Shoenut JP, Heywood M, Kroeker MA, Lotocki R. Primary ovarian cancer: prospective comparison of contrast-enhanced CT and pre-and postcontrast, fat-suppressed MR imaging, with histologic correlation. J Magn Reson Imaging. 1993;3(1):99–106. doi: 10.1002/jmri.1880030117. [DOI] [PubMed] [Google Scholar]
- 35.Coakley FV, Choi PH, Gougoutas CA, et al. Peritoneal metastases: detection with spiral CT in patients with ovarian cancer. Radiology. 2002;223(2):495–499. doi: 10.1148/radiol.2232011081. [DOI] [PubMed] [Google Scholar]