Abstract
Major depressive disorder (MDD) is a complex illness caused by both genetic and environmental factors. Antidepressant resistance also has a genetic component. To date, however, very few genes have been identified for major depression or antidepressant resistance. In this study, we investigated whether outbred heterogeneous stock (HS) rats would be a suitable model to uncover the genetics of depression and its connection to antidepressant resistance. The Wistar Kyoto (WKY) rat, one of the eight founders of the HS, is a recognized animal model of juvenile depression and is resistant to fluoxetine antidepressant treatment. We therefore hypothesized that adolescent HS rats would exhibit variation in both despair-like behavior and response to fluoxetine treatment. We assessed heritability of despair-like behavior and response to sub-acute fluoxetine using a modified forced swim test (FST) in 4-week-old HS rats. We also tested whether blood transcript levels previously identified as depression biomarkers in adolescent human subjects are differentially expressed in HS rats with high vs. low FST immobility. We demonstrate heritability of despair-like behavior in 4-week-old HS rats and show that many HS rats are resistant to fluoxetine treatment. In addition, blood transcript levels of Amfr, Cdr2 and Kiaa1539, genes previously identified in human adolescents with MDD, are differentially expressed between HS rats with high vs. low immobility. These data demonstrate that FST despair-like behavior will be amenable to genetic fine-mapping in adolescent HS rats. The overlap between human and HS blood biomarkers suggest that these studies may translate to depression in humans.
Keywords: Adolescence, antidepressant resistance, blood transcript levels, depression biomarkers, forced swim test, major depression, outbred rats, QTL mapping, RNA expression, Wistar Kyoto
Major depressive disorder (MDD) is a debilitating disease that affects 6.7% of the adult population and 11.4% of adolescents (https://www.nimh.nih.gov/health/statistics/prevalence/index.shtml). Hallmarks of MDD include a sense of hopelessness and despair and are frequently coupled with changes in sleep and appetite, inability to concentrate and low energy. Adolescent depression is of particular concern, as it is a common and impairing condition that often develops into chronic or recurrent MDD (Brent et al. 2008). It is known that MDD is affected by both the environment and genetics. Early childhood trauma and stress are the largest environmental factors known to contribute to MDD risk and heritability of MDD is around 30–40% (Sullivan et al. 2000; Kendler et al. 2006) but this increases to 60–80% for childhood or adolescent MDD (Wigg et al. 2009). Despite this, genome-wide association studies (GWAS) have had relatively limited success identifying genetic loci associated with disease (Converge Consortium 2015; Hyde et al. 2016; Power et al. 2016). One of the reasons that GWAS has had limited success is because MDD itself is a heterogenous disease (Verduijn et al. 2016), at times occurring as a secondary disease to other chronic illnesses. Other reasons include the relatively small contribution of each genetic locus, as well as the complex interaction between genes and the environment (Levinson et al. 2014). This is further complicated by the fact that neither environment nor genetics can be well-controlled in human studies.
Response to antidepressant treatment varies among individuals and to-date, there are no direct and efficient ways to identify which drug will work in which patient. As a result, patients often need to try multiple antidepressants before finding a drug that works for them. The approved treatments for adolescent depression include selective serotonin reuptake inhibitors (SSRIs), but a large percentage of adolescents with MDD do not respond to them. A meta-analysis has suggested that fluoxetine has only about 20% more efficacy than placebo for pediatric MDD (Bridge et al. 2007). It is known that response to antidepressant treatment is also genetic (Fabbri et al. 2014). Similar to GWAS for MDD, however, GWAS for antidepressant response have had limited success, likely due to under-powered sample sizes (Biernacka et al. 2015). Identifying the genetics of antidepressant resistance, particularly in the highly vulnerable adolescent population, is therefore of major interest.
The study of despair-like behavior in animal models has the potential to circumvent some of the challenges faced by studying this disease in humans, namely disease heterogeneity and environmental influences. One of the most widely used measures of despair-like behavior in the rat is the Forced Swim Test (FST) (Overstreet 2012; Slattery & Cryan 2012; Barkus 2013). Animals with high levels of immobility in the FST are said to exhibit high levels of despair. The WKY rat is a recognized animal model of depression, with high FST immobility (Armario et al. 1995; Lahmame & Armario 1996; Lopez-Rubalcava & Lucki 2000; Rittenhouse et al. 2002; Solberg et al. 2003; Solberg et al. 2004), altered sucrose preference (D’Souza & Sadananda 2016), increased response to stress (Courvoisier et al. 1996; Gilad & Shiller 1989; Solberg et al. 2006), altered sleep and circadian patterns (Dugovic et al. 2000; Solberg et al. 2001), and passive coping in multiple other tests including defensive burying (Pare 1989; Pare 1994; Ahmadiyeh et al. 2003). Because WKY rats demonstrate despair-like behavior at an early age, they are regarded as a model for juvenile depression (Malkesman & Weller 2009). In addition, the WKY rat does not respond to treatment with fluoxetine and has been suggested as a model of antidepressant resistance (; Lahmame et al. 1997; Lahmame & Armario 1996; Griebel et al. 1999; Willner & Belzung 2015).
Other inbred strains, including F344 and BN rats show low levels of immobility in the FST (Lahmame & Armario 1996; Solberg et al. 2003) and previous work in our laboratory used a WKY × F344F2 intercross to demonstrate that FST behaviors are heritable (Solberg et al. 2003). We conducted a quantitative trait locus (QTL) analysis on FST behavior, stress response and other behaviors (Ahmadiyeh et al. 2003; Solberg et al. 2004; Ahmadiyeh et al. 2005; Baum et al. 2005; Baum et al. 2006; Solberg et al. 2006; Solberg Woods et al. 2009), identifying seven loci for immobility and seven loci for climbing. One of the FST loci confirmed a previous QTL identified for immobility in the tail suspension test, a test for behavioral despair in mice (Yoshikawa et al. 2002). To date, however, none of the underlying genes for these loci have been identified. One of the drawbacks of QTL studies in F2 intercross models is that the identified QTL are usually very large (40–60 Mb) and include several hundred genes. An alternative approach is to use outbred heterogeneous stock (HS) rats. HS rats are created by combining eight inbred strains together and then maintaining the colony in a way that minimizes inbreeding (Solberg Woods 2014). After 50–60 generations of breeding, the distance between recombination events decreases, allowing one to fine-map QTL to only a few megabytes (Solberg Woods et al. 2010, 2012; Baud et al. 2013). The HS rat colony was created by the NIH in the 1980s using the following inbred strains: ACI/N, BN/N, BUF/N, F344/N, M520/N, MR/N, WKY/N, WN/N (Hansen & Spuhler 1984). Because the WKY rat is a founder of the HS rat colony, and BN and F344 founder strains exhibit low immobility and do respond to sub-acute antidepressant treatment (Lahmame & Armario 1996), alleles that drive despair-like behavior and antidepressant resistance should segregate in the HS population. Although previous work has shown that adult HS rats tend to exhibit high levels of despair-like behavior in the FST (Diaz-Moran et al. 2012), to-date, FST behavior has not been assessed in adolescent HS rats, nor has the colony been used to fine-map genetic loci for despair-like behavior in the FST.
In addition to QTL mapping with the WKY rat, our laboratory has also exploited the fact that the WKY strain is not completely inbred, and generated two fully inbred strains with opposite behavior in the FST test: the ‘depressed’ Wistar Kyoto More Immobile (WMI), and the ‘non-depressed’ control strain, the WKY Less Immobile (WLI) (Andrus et al. 2012; Will et al. 2003). Similar to WKY rats, the WMI strain does not respond to fluoxetine, while tricyclic antidepressants and MAOIs normalize their immobility behavior in the FST (Will et al. 2003). Creation of the WMI strain allowed us to identify differentially expressed transcripts in the hippocampus and amygdala between WMI and WLI and compare them to those obtained from several rat strains in a chronic stress model of depression (Andrus et al. 2012). Differentially expressed transcripts were also obtained from the blood of these models, 26 of which were selected for overlapping with those in the brain and/or for their high expression in human blood (Pajer et al. 2012). We carried out an unbiased analysis of these 26 transcripts in blood from 15- to 19-year old humans with early onset MDD (Pajer et al. 2012). Blood levels of 11 transcripts differentiated participants with Early Onset MDD from the non-depressed group and a partially overlapping panel of 18 transcripts distinguished between those adolescents who had anxiety disorder co-morbid with MDD from those that had MDD without anxiety (Pajer et al. 2012). In the current work, we hypothesized that at least some of these transcripts would be differentially expressed in HS rats with high vs. low immobility in the FST.
In the current paper, we confirm that adolescent HS rats exhibit high phenotypic variation in despair-like behavior and show that both swimming and immobility behaviors are heritable, demonstrating that HS rats should provide an ideal model to fine-map genetic loci for despair-like behavior. We further demonstrate that most HS rats are resistant to sub-acute treatment with fluoxetine. Finally, we show that transcript levels of three genes (Amfr, Cdr2 and Kiaa1539), have lower expression levels in HS rats with high immobility relative to those with low immobility, similar to what has previously been found in the above mentioned animal models and humans with MDD (Pajer et al. 2012; Redei et al. 2014). Taken together, these data demonstrate that despair-like behavior will be amenable to genetic fine-mapping in the HS rat and that these studies will likely translate to humans with MDD.
Materials and methods
Animals
Heterogeneous stock (HS) (NMcwi:HS) rats were maintained at the Medical College of Wisconsin (MCW). All protocols were approved by MCW IACUC (PHS Assurance number A3102–01). We maintained 64 breeder pairs using a random breeding strategy, ensuring that we did not mate two animals that are closely related. Two hundred and sixty-three HS rats were used in the studies described below. Rats for the following studies were weaned at 3 weeks of age, with the FST being conducted 1 week later at 4 weeks of age. Rats were housed 4–8 per cage prior to the FST. Rats were housed in micro-isolation cages in a conventional facility using autoclaved bedding (sani-chips from PJ Murphy). They had ad libitum access to autoclaved Teklad 5010 diet (Harlan Laboratories, Madison, WI) and were provided reverse osmosis water chlorinated to 2–3 ppm. The HS rat colony is positive for Helicobacter spp. but otherwise are pathogen free. All studies were conducted during the light phase. Rats were taken from several different breeder pairs to ensure genetic diversity within the population.
Original FST protocol
In the initial study, we determined if a 1-day FST could replace the original 2-day FST protocol. A 1-day FST has previously been used to test behavior in adolescent rats (Mehta et al. 2013), but to our knowledge, a direct comparison between behavior in day 1 and day 2 has not been conducted. To test this, we conducted the 2-day FST protocol, video-recording and scoring behavior during both day 1 and day 2. Seventy-two animals (48 males, 24 females) 4 weeks of age were placed into a 7 in. × 10.5 in. beaker filled with water at 25°C for 15 min on day 1 and 5 min on day 2. This size beaker is frequently used when testing FST in mice and we expected it would be sufficiently large to test adolescent rats. In each trial, two animals were placed side by side with males on the left and females on the right. A divider was placed in between the beakers so the animals could not see each other and influence behavior during the test. All tests were conducted between 0800 and 1300 h.
Tests were recorded using a digital video camera. The recorded videos were then visually scored using a time sampling technique in which every 5 seconds (60 times total) the animal was marked as floating, swimming, climbing or diving (Detke et al. 1995). Floating (immobility) was defined as a general lack of motion consisting of only small movements to keep the head above water. Swimming was interpreted as larger movements, more than necessary to keep the head above water, which displaced water within the tank. Climbing was classified as vigorous movements of the forepaws in and out of the water, typically against the wall of tank. Diving was characterized as an animal’s full submersion. The first 5 min of day 1, last 5 min of day 1 and 5 min of day 2 were visually scored for all animals, and behaviors during these time points were then correlated.
Modified FST protocol
The strong correlations between day 1 and day 2 (see results) led us to conduct a modified FST for all subsequent studies. The modified FST consisted of a single 6 min FST on day 1 similar to Mehta et al. (2013)). The last 5-min of this test were scored for immobility, climbing, swimming and diving behaviors as described above. Upon learning that some adolescent rats were able to hold themselves up during the FST, all subsequent tests were conducted in a larger beaker (11.4 in. × 17 in.). We assessed despair-like behavior in 50 male and 50 female 4-week old HS rats (all from different parents) using the modified 1-day FST.
Antidepressant study
At 4 weeks of age, a separate group of 91 HS male rats were subjected to the 1-day 6-min FST. The test was video-recorded and baseline immobility, climbing, swimming and diving were scored as described above. A second 6-min FST was performed 7 days later. This second test was preceded by sub-acute intra-peritoneal injections with either saline or fluoxetine. The injections were performed 23.5, 5 and 1 h before the swim test, as previously described (Lopez-Rubalcava & Lucki 2000; Cryan et al. 2005; Slattery & Cryan 2012). The fluoxetine group received 10 mg/kg of fluoxetine (Letco Medical, Decatur, AL, USA) in a volume of 2 ml/kg. The saline group received the same volume of a saline solution.
Heritability estimates
Heritability estimates were calculated for baseline FST behaviors (swimming, immobility, climbing) on 191 HS rats from the two studies above (modified FST protocol and antidepressant study). Potentially confounding variables (sex, experimenter, scorer and date) were regressed out and heritability was calculated using the residuals. Heritability estimates were also calculated for response to the antidepressant using 91 HS rats; the effect of treatment (fluoxetine or saline) was regressed out prior to estimating heritability.
It is known that HS rats are genetically related. Relatedness can be calculated from a pedigree or estimated from genetic marker data. For our data, genetic marker data is not available, and so relationship matrices were obtained from the HS pedigree using QTLRel (Cheng et al. 2011). A random effect model was fitted to estimate variance components with respect to polygenic and environmental variation
| (1) |
where y = (y1, y2, ···, yn)′ is a vector of phenotypic values, μ = (μ, μ, ···, μ)′ denotes intercept, represents random polygenic effect with G = (gij) being the additive genetic matrix (i.e. kinship matrix), and ε ~ N(0, Iσ2) is the residual effect. Heritability was estimated by
Where and σ̂2 are maximum likelihood estimates (MLE) of the variance components (equation 1). Variance of the estimated heritability was then estimated using the delta method (Green & William 2000).
Blood collection and RNA analysis
After 3 weeks of the antidepressant or saline FST, animals were euthanized by decapitation. Trunk blood was collected from 29 rats into PAXgene RNA tubes (PreAnalytix, Qiagen Germantown, Maryland) for RNA analysis. Rats were chosen based on FST score: low immobility during weeks 1 and 2 (≤20; 12 rats), or moderate or high immobility during weeks 1 and 2 (>20, 10 rats), or low immobility during week 1, but moderate or high immobility during week 2 (7 rats). Half of the rats from each group received fluoxetine and half received saline treatment during week 2.
RNA was extracted from blood using the PAXgene Blood RNA Kit 50 v2 (PreAnalytix, Qiagen). cDNA was synthesized as described previously (Mehta-Raghavan et al. 2016). ABI 7900HT real time cycler was used to amplify 5 ng cDNA using SYBR green reaction mix (ABI, Carlsbad, CA, USA). Primers were designed using the default settings in ABI’s Primer Express software (version 3.0, PE Applied Biosystems Foster City, CA) to generate primers that amplify 80–150 bp products. The primer pairs used for each gene are listed in Table S1, Supporting Information.
qPCR reactions were performed in triplicate and reached threshold amplification within 35 PCR cycles. Transcript levels were determined relative to GAPDH (commercially available from ABI, Foster City, CA, USA) and to a calibrator using the ΔΔCT method. For each gene, qPCR was carried out on one plate.
Statistical analysis
Pearson correlation was used to determine relationship between day 1 and day 2 behavior in the FST. Pearson correlation was also used to determine correlations between immobility, climbing, diving and swimming behavior in the FST. An ANOVA was used to determine if there were significant differences in FST behavior between males and females. A Wilcoxon rank sum test was used to determine if the change in immobility between week 1 and week 2 FST was different between the saline and fluoxetine groups. The Fligner-Killeen test of homogeneity of variances was used to detect if variation between the saline and fluoxetine groups differed. To determine if genes are differentially expressed by immobility or antidepressant treatment, we fit a linear model that included week 1 immobility (as two levels categorical variable: low vs. high immobility), week 2 immobility (as a continuous numeric variable) and antidepressant treatment (fluoxetine or saline).
Results
Immobility in the 1-day FST is highly correlated with the standard 2-day FST protocol
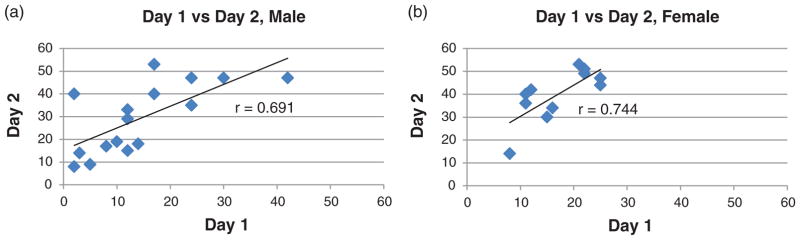
After the first experiment, we observed that several of the adolescent rats (32 males and 13 females) were able to hold themselves up in the beaker by their tail and forepaws. All of these animals were removed prior to analysis such that correlations were run on only 27 rats (16 males and 11 females). Despite having to remove many of the animals from this initial test, there is still a strong correlation between immobility during the first 5-min of day 1 and immobility on day 2 (males: r = 0.691, P = 0.003; females: r = 0.744, P = 0.008; see Fig. 1). Immobility during the last 5-min of day 1 also correlates with day 2 immobility in males, although to a lesser extent (males: r = 0.536, P = 0.030), while no correlation is found in females (r = 0.440, P = 0.180).
Figure 1. Correlation between the first 5 min of day 1 and day 2 of the original FST protocol in both males (a) and females (b).
Immobility score for day 1 (x-axis) and day 2 (y-axis) are shown. Results indicate a very strong positive correlation between day 1 and day 2 in both male (r = 0.691, P = 0.003) and female (r = 0.744, P = 0.008) HS rats. n = 16 males and 11 females.
HS rats exhibit large variation in despair-like behavior in the 1-day FST
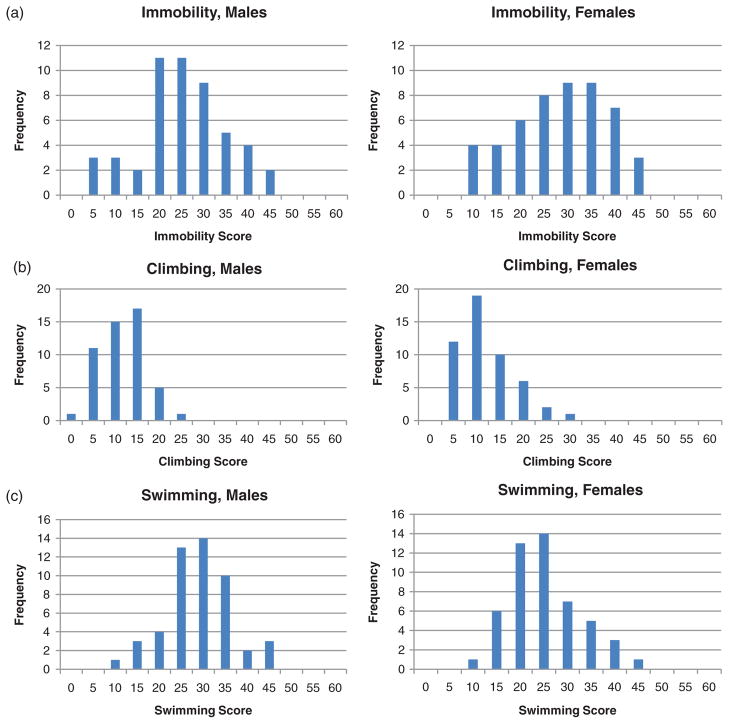
Both male and female adolescent rats exhibit large variation for immobility, swimming and climbing behaviors (see Fig. 2). Despite similar ranges for swimming, males exhibit significantly more swimming than females (F(1,99) = 5.17, P = 0.025). Other than swimming, there are no differences in behavior between males and females. Immobility negatively correlates with swimming and climbing behaviors in both males (r = −0.85 and −0.64, P = 5.27e-15 and 4.82e-07, respectively) and females (r = −0.81 and −0.64, P = 6.39e-13 and 6.24e-07, respectively). Diving shows no correlation with any of the other behaviors (possibly because there were so few animals that showed diving behavior). Climbing and swimming are not correlated in either sex.
Figure 2. Frequency histograms of behavior in the FST in male and female 4-week-old HS rats.
Immobility (a), climbing (b) and swimming (c) behaviors are shown. n = 50 for both males and females.
FST behaviors are heritable in HS rats
Estimated heritability for baseline swimming and immobility were 0.358 (SD = 0.236) and 0.520 (SD = 0.240), respectively. We do not report heritability for climbing and response to antidepressant because the delta method did not provide an appropriate standard deviation due to boundary constraints.
Most adolescent HS rats are resistant to sub-acute treatment with fluoxetine
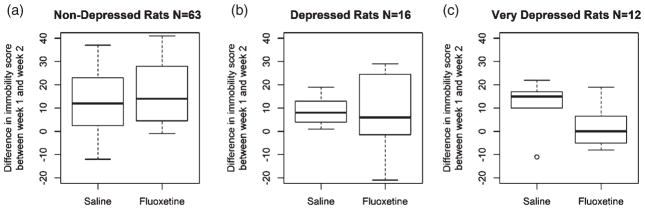
As expected, most animals increase immobility between week 1 and week 2 (Mezadri et al. 2011). We find that this is the case whether the animals received saline or fluoxetine, as the difference in immobility between week 1 and week 2 does not differ between saline and fluoxetine groups (W = 945.5, P = 0.591). We also considered a model with separate terms for time (week 1 or week 2) and drug and their interaction between time and drug; this model did not yield a significant interaction for any of dependent measures, indicating no effect of treatment with fluoxetine. In order to further explore these data the animals were divided into the following categories based on week 1 immobility scores: low immobility (<20), moderate immobility (20 < immobility < 30) and high immobility (>30). Using these criteria, 63 rats have low immobility (31 given saline, 32 given fluoxetine), 16 rats have moderate immobility (9 given saline and 7 given fluoxetine) and 12 rats have high immobility (5 given saline and 7 given fluoxetine). In the low immobility group, there is not a significant difference in change in immobility from week 1 to week 2 between the saline and fluoxetine groups (W = 410, P = 0.239; Fig. 3). In the moderate immobility group, although there is still no difference between the fluoxetine and saline groups (W = 28, P = 0.750), there is a trend toward increased variability in the fluoxetine group (χ2 = 3.566, P = 0.059; Fig. 3). In the high immobility animals, the fluoxetine group appears to have decreased immobility relative to the saline group, although this difference is not statistically significant (W = 25, P = 0.255), likely because of the small number of animals and relatively high variability in this group (Fig. 3). These data indicate that fluoxetine has little or no effect in HS rats with low immobility, while fluoxetine may have a variable effect in the moderate and high immobility groups, although more animals are needed to substantiate this claim.
Figure 3. Difference in immobility during FST between week 1 and week 2 in male HS rats given sub-acute injections of either saline or fluoxetine.
Rats are separated into (a) low immobility (< 20, n = 63), (b) moderate immobility (30 > immobility >20, n = 16) and (c) high immobility (> 30, n = 12). Week 1 represents baseline immobility values and week 2 represents response to either saline or fluoxetine. In the box plot, the middle line shows the mean of the difference, with the surrounding box representing the 25th and 75th percentiles. The maxima and minima are represented by the uppermost and lowermost lines, respectively.
Expression levels of Amfr, Cdr2 and Kiaa1593 are differentially expressed in HS rats with high vs. low immobility
We determined expression levels of 18 genes in the blood of 29 HS rats with varying degrees of immobility in the FST. The genes chosen were previously identified to be differentially expressed between rat models of depression and/or depressed vs. non-depressed human adolescents (Pajer et al. 2012). To determine if genes were differentially expressed by immobility or antidepressant treatment, we fit a linear model that included week 1 immobility (low vs. high immobility), week 2 immobility (as a continuous numeric variable) and antidepressant treatment (fluoxetine or saline). In the initial model, week 1 immobility was not a significant factor for any of the genes, so it was removed from the final model. After including only week 2 immobility and antidepressant treatment, we found that three genes (Amfr, Cdr2 and Kiaa1539) reach unadjusted levels of significance for differential expression during week 2 (P = 0.004, 0.016 and 0.006, respectively) and Dgka was significantly different in the antidepressant treated vs. non-treated groups (P = 0.030). Use of the Benjamini–Hochberg procedure showed that Amfr, Cdr2 and Kiaa1539, but not Dgka, remain significant after adjusting for multiple comparisons at 10% FDR. Expression levels of all three genes are lower in HS rats with high immobility, similar to what is seen in human adolescents with major depression (Pajer et al. 2012; Redei et al. 2014).
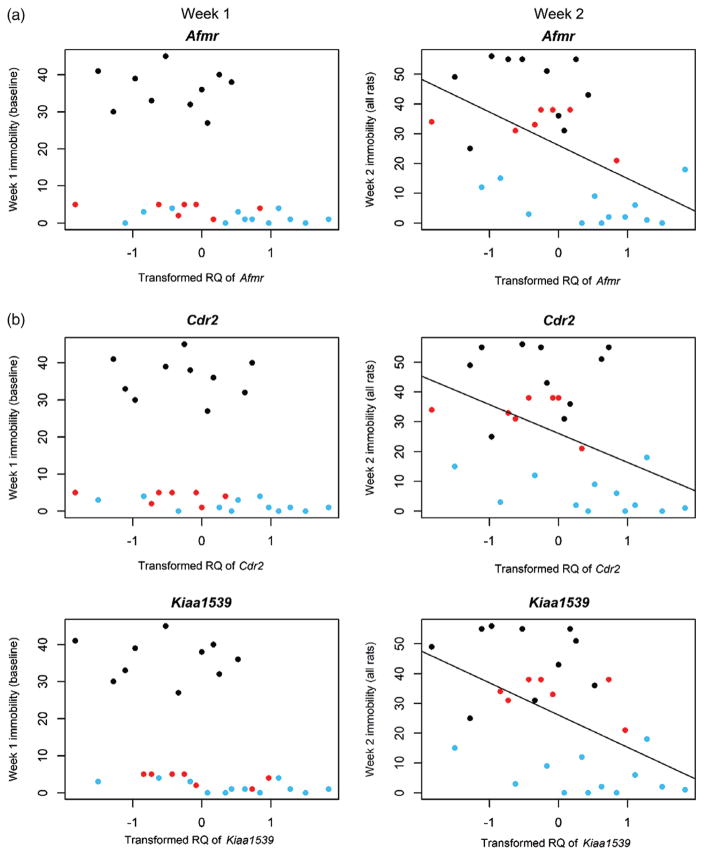
To visualize the data, we plotted RQ expression levels against immobility score for both week 1 and week 2 (see Fig. 4). We note that several of the HS rats that originally had low immobility scores in week 1 increased to moderate or high immobility in week 2, while others remained low during both weeks 1 and 2. Interestingly, RQ levels of Amfr, Cdr2 and Kiaa1593 appear highest in animals that have low immobility during both week 1 and week 2. To determine if this difference is statistically significant, we ran a Wilcoxon rank sum test between animals with low immobility in week 1 and 2 (blue dots in Fig. 4) vs. those that had low immobility in week 1 and moderate/high immobility in week 2 (red dots in Fig. 4). We found that the difference was statistically significant for Cdr2 (P = 0.028) and approached statistical significance for Amfr (P = 0.083), but not for Kiaa1539 (P = 0.142). These data indicate that expression levels of Amfr and Cdr2 may predict which animals will be protected from developing high immobility during the week 2 FST, although larger number of animals are needed to confirm this.
Figure 4. Scatter plots showing relationship between immobility score and normalized RQ levels of (a) Amfr, (b) Cdr2 and (c) Kiaa1539.
Data are shown for all animals during both week 1 and week 2. Animals are divided into three groups based on week 1 and week 2 immobility scores: low immobility both weeks (blue), high immobility both weeks (black), low immobility week 1 and moderate/high immobility week 2 (red). Results indicate that high RQ levels of Amfr and Cdr2 may predict which animals continue to have low immobility during week 2.
Discussion
This work demonstrates that male and female adolescent HS rats show a large degree of variability in immobility, climbing and swimming behaviors during a single 6-min FST and that both swimming and immobility are heritable in this population. We also show that, unexpectedly, many of the HS rats are unresponsive to treatment with fluoxetine. Finally, with a very small sample size, we were able to demonstrate that three genes previously known to be differentially expressed in the blood of rat models of depression and humans with MDD are differentially expressed in HS rats with low vs. high immobility. These studies strongly indicate that the HS rat model will be useful for identifying the underlying genetics of despair-like behavior and that these studies may prove useful for understanding MDD in humans.
We initially demonstrate that behavior during the first FST day is strongly correlated with behavior during the second day of testing during the original FST protocol. A single day of testing has previously been used in adolescent rats to avoid the stress of a 15 min test for these young rats (Mehta et al. 2013). Previous studies have also shown that immobility tends to increase after repeated FST (Mezadri et al. 2011). To our knowledge, however, this is the first study to directly test the relationship between behavior during the first day of testing and the second day of testing. Similar to Mezadri et al. (2011), we see that immobility increases during the second day of testing. Despite this increase, however, the correlation between the first 5-min of testing on day 1 and day 2 behavior is very strong, indicating that day 1 behavior is representative of behavior on day 2 and a single day of testing can be used in adolescent rats.
We demonstrate that immobility and swimming behaviors after a single 6-min FST are heritable in the adolescent HS population. We show that both male and female HS rats exhibit large variation in FST behavior, particularly immobility. The variation in behavior is similar to previous studies (Diaz-Moran et al. 2012) and much greater than what we have seen in an F2 intercross between WKY and F344 rats, where most of the animals had immobility scores less than 10 (highly skewed toward F344 behavior) and only a few had scores greater than 30 (Solberg et al. 2003). Immobility scores in the HS rats tend to be normally distributed, with the majority of rats showing immobility scores around 25 and several showing scores greater than 30. These data indicate that the HS rat will likely prove a much better model for genetic mapping of this trait than the WKY × F344 F2 intercross. Variation in climbing behavior in the HS rat is similar to what we had previously seen in the WKY × F344 F2 intercross, with the exception that the data are more normally distributed in the HS. Other than a slight increase in swimming behavior in male HS rats, we do not see any differences between adolescent males and females in FST behavior. As expected, immobility is strongly inversely correlated with both climbing and swimming behavior in the HS rat (Solberg et al. 2003).
We were surprised to find that most adolescent HS rats do not respond to sub-acute treatment with fluoxetine and due to high variability, we were unable to determine if fluoxetine response is heritable in this population. We found that most animals from both the saline and fluoxetine groups increased immobility during the second week of testing, which was expected for the saline group (Mezadri et al. 2011), but not for the fluoxetine group. While we expected that at least some of the rats within the fluoxetine group would increase immobility, demonstrating resistance to fluoxetine, it was unexpected that most of the animals in the fluoxetine group appeared resistant to the drug. We started to see decreases in immobility behavior in the fluoxetine group only in the rats with high immobility scores (>30), although due to the low number of animals, these numbers were not statistically different from the saline group. These data suggest that the testing of antidepressant drugs in animals that do not exhibit despair-like behavior may have limited meaning compared to testing drugs in despair models. Previous studies have assessed response to antidepressants, including fluoxetine, in several different strains. These studies demonstrate that the WKY rat is unresponsive to fluoxetine and 8-OH-DPAT (Lahmame & Armario 1996; Lopez-Rubalcava & Lucki 2000) as well as imipramine (Lahmame et al. 1997), with conflicting findings for desipramine (Lahmame & Armario 1996, Lopez-Rubalcava & Lucki 2000). Our own work has shown that the WMI, a sub-strain created from the WKY is also unresponsive to fluoxetine (Will et al. 2003). It is important to recognize, however, that fluoxetine metabolism may differ in adolescent rats relative to adult rats. In fact, previous studies in Sprague Dawley (Iniguez et al. 2010) and Wistar Unilever (Homberg et al. 2011) rats demonstrate an opposite or decreased responsiveness to fluoxetine in adolescent rats relative to adults. It is also possible that the adolescent HS rat population requires a higher dose of fluoxetine to elicit a response to the drug.
We found that Amfr, Cdr2 and Kiaa1539 have significantly lower transcript abundance levels in the blood of HS rats with moderate or high FST immobility relative to those with low immobility, similar to what has been found in human adolescents with MDD (Pajer et al. 2012; Redei et al. 2014). Although a trend of decreased expression of these three genes is noted during week 1, expression levels reach statistical significance only during week 2, suggesting that these markers reflect either the learning response or the response to the antidepressant treatment. Interestingly, we note that Amfr and Cdr2 show the highest expression levels in HS rats that consistently have low immobility even during the second FST, indicating that high expression levels of these genes may predict which animals are protected from developing high immobility over time. Importantly, these transcript levels were measured in the blood several weeks after the FST and are therefore likely to be trait markers of FST immobility.
All three genes were initially identified in the chronic stress rat model (Pajer et al. 2012). Amfr, and Cdr2 are also differentially expressed between MDD and non-depressed adolescent subjects as well as between adolescents with MDD-alone vs. those with MDD and anxiety disorder (Pajer et al. 2012). In contrast, Kiaa1539 was differentially expressed only between MDD-alone and MDD with anxiety indicating this gene may be specific to adolescent MDD comorbid with anxiety (Pajer et al. 2012). Interestingly, in a separate study, Kiaa1539 was differentially expressed in adult subjects with MDD relative to non-depressed adults even after successful cognitive behavioral therapy indicating this gene may be a trait marker for depression (Redei et al. 2014). Importantly, this study and previous work in rat models and humans demonstrate a negative relationship between blood transcript levels of these genes and depression (Pajer et al. 2012; Redei et al. 2014), such that those with depression (or high levels of immobility in the rat) exhibit lower expression levels of these three genes. In a recent study both Amfr and Cdr2 were among eight genes whose expression were found to be highly predictive of MDD in humans (Yu et al. 2016).
Although highly conserved across species, very little is known about Kiaa1539 and it remains an uncharacterized protein. There is much more known about the other two potential biomarkers. The autocrine motility factor receptor (Amfr) encodes an endoplasmic reticulum membrane-anchored ubiquitin ligase, which might be protective by enhancing the removal of accumulated neurodegenerative disease proteins, such as mutant huntingtin, SOD1 and ataxin-3 (Ying et al. 2009; Yang et al. 2010). Increased Amfr can also enhance learning and memory in the central nervous system (Yang et al. 2012) suggesting that a decrease of Amfr expression, as we found in subjects with MDD, and in HS rats with moderate/high immobility, might make the organism more vulnerable to neurodegeneration. Cdr2, the cytoplasmic cerebellar degeneration-related protein 2 antigen harbors a helix-leucine zipper motif and interacts specifically with c-Myc (Okano et al. 1999). CDR2 can inhibit NFκB-dependent transcription in neurons (Sakai et al. 2001) and is proposed to regulate the nuclear helix–loop–helix leucine zipper protein MRGX, which has been implicated in cell growth, DNA repair, cell aging and apoptosis.
Given the recurrent nature of adolescent depression, and the fact that relatively few adolescents respond to treatment with fluoxetine, identification of the underlying genetic basis of this disorder, as well as the genetics of antidepressant resistance is of major interest. Our results demonstrate that despair-like behavior is heritable in the HS population. We also show that most adolescent HS rats are unresponsive to fluoxetine. Although we saw some increased variability in rats with moderate or high immobility, our numbers were insufficient to demonstrate statistical significance or to determine if fluoxetine response is heritable in this population. These data indicate that despair-like behavior in the FST, but not necessarily fluoxetine response, will be amenable to genetic fine-mapping using the adolescent HS rat model. We also find that blood-based expression of three genes previously identified in rat models of depression and in adolescent subjects with major depression are differentially expressed in HS rats with low vs. moderate or high immobility. Concordance of differentially expressed genes between rat and human models indicate that information gained from the HS rat will likely improve our understanding of human depression, particularly in the adolescent population.
Supplementary Material
Box-plots demonstrating variability in FST behavior in 4-week-old (a) male and (b) female HS rats. In the box plot, the middle line shows the mean, with the surrounding box representing the 25th and 75th percentiles. The maxima and minima are represented by the uppermost and lowermost lines, respectively. n = 50 for both males and females.
Quantitative RT-PCR primer sequences. F, Forward; R, Reverse.
Acknowledgments
The study was supported by National Institute on Drug Abuse (grant no. P50 DA037844) (A.A.P., L.S.W.) and Davee Foundation (E.E.R.). The authors have declared no conflicting interests.
Footnotes
Additional supporting information may be found in the online version of this article at the publisher’s web-site:
References
- Ahmadiyeh N, Churchill GA, Shimomura K, Solberg LC, Takahashi JS, Redei EE. X-linked and lineage-dependent inheritance of coping responses to stress. Mamm Genome. 2003;14:748–757. doi: 10.1007/s00335-003-2292-x. [DOI] [PubMed] [Google Scholar]
- Ahmadiyeh N, Churchill GA, Solberg LC, Baum AE, Shimomura K, Takahashi JS, Redei EE. Lineage is an epi-genetic modifier of QTL influencing behavioral coping with stress. Behav Genet. 2005;35:189–198. doi: 10.1007/s10519-004-1018-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrus BM, Blizinsky K, Vedell PT, Dennis K, Shukla PK, Schaffer DJ, Radulovic J, Churchill GA, Redei EE. Gene expression patterns in the hippocampus and amygdala of endogenous depression and chronic stress models. Mol Psychiatry. 2012;17:49–61. doi: 10.1038/mp.2010.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armario A, Gavalda A, Marti J. Comparison of the behavioural and endocrine response to forced swimming stress in five inbred strains of rats. Psychoneuroendocrinology. 1995;20:879–890. doi: 10.1016/0306-4530(95)00018-6. [DOI] [PubMed] [Google Scholar]
- Barkus C. Genetic mouse models of depression. Curr Top Behav Neurosci. 2013;14:55–78. doi: 10.1007/7854_2012_224. [DOI] [PubMed] [Google Scholar]
- Baud A, Hermsen R, Guryev V, et al. Combined sequence-based and genetic mapping analysis of complex traits in outbred rats. Nat Genet. 2013;45:767–775. doi: 10.1038/ng.2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum AE, Solberg LC, Churchill GA, Ahmadiyeh N, Takahashi JS, Redei EE. Test- and behavior-specific genetic factors affect WKY hypoactivity in tests of emotionality. Behav Brain Res. 2006;169:220–230. doi: 10.1016/j.bbr.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum AE, Solberg LC, Kopp P, Ahmadiyeh N, Churchill G, Takahashi JS, Jameson JL, Redei EE. Quantitative trait loci associated with elevated thyroid-stimulating hormone in the Wistar-Kyoto rat. Endocrinology. 2005;146:870–878. doi: 10.1210/en.2004-0948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biernacka JM, Sangkuhl K, Jenkins G, et al. The International SSRI Pharmacogenomics Consortium (ISPC): a genome-wide association study of antidepressant treatment response. Transl Psychiatry. 2015;5:e553. doi: 10.1038/tp.2015.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brent D, Emslie G, Clarke G, et al. Switching to another SSRI or to venlafaxine with or without cognitive behavioral therapy for adolescents with SSRI-resistant depression: the TORDIA randomized controlled trial. JAMA. 2008;299:901–913. doi: 10.1001/jama.299.8.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridge JA, Iyengar S, Salary CB, Barbe RP, Birmaher B, Pincus HA, Ren L, Brent DA. Clinical response and risk for reported suicidal ideation and suicide attempts in pediatric antidepressant treatment: a meta-analysis of randomized controlled trials. JAMA. 2007;297:1683–1696. doi: 10.1001/jama.297.15.1683. [DOI] [PubMed] [Google Scholar]
- Cheng R, Abney M, Palmer AA, Skol AD. QTLRel: an R package for genome-wide association studies in which relatedness is a concern. BMC Genet. 2011;12:66. doi: 10.1186/1471-2156-12-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Converge Consortium. Sparse whole-genome sequencing identifies two loci for major depressive disorder. Nature. 2015;523:588–591. doi: 10.1038/nature14659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courvoisier H, Moisan MP, Sarrieau A, Hendley ED, Mormede P. Behavioral and neuroendocrine reactivity to stress in the WKHA/WKY inbred rat strains: a multifactorial and genetic analysis. Brain Res. 1996;743:77–85. doi: 10.1016/s0006-8993(96)01023-2. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Valentino RJ, Lucki I. Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test. Neurosci Biobehav Rev. 2005;29:547–569. doi: 10.1016/j.neubiorev.2005.03.008. [DOI] [PubMed] [Google Scholar]
- D’Souza D, Sadananda M. Anxiety- and depressive-like profiles during early- and mid-adolescence in the female Wistar Kyoto rat. Int J Dev Neurosci. 2016;56:18–26. doi: 10.1016/j.ijdevneu.2016.11.003. [DOI] [PubMed] [Google Scholar]
- Detke MJ, Rickels M, Lucki I. Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology (Berl) 1995;121:66–72. doi: 10.1007/BF02245592. [DOI] [PubMed] [Google Scholar]
- Diaz-Moran S, Palencia M, Mont-Cardona C, Canete T, Blazquez G, Martinez-Membrives E, Lopez-Aumatell R, Tobena A, Fernandez-Teruel A. Coping style and stress hormone responses in genetically heterogeneous rats: comparison with the Roman rat strains. Behav Brain Res. 2012;228:203–210. doi: 10.1016/j.bbr.2011.12.002. [DOI] [PubMed] [Google Scholar]
- Dugovic C, Solberg LC, Redei E, Van Reeth O, Turek FW. Sleep in the Wistar-Kyoto rat, a putative genetic animal model for depression. Neuroreport. 2000;11:627–631. doi: 10.1097/00001756-200002280-00038. [DOI] [PubMed] [Google Scholar]
- Fabbri C, Porcelli S, Serretti A. From pharmacogenetics to pharmacogenomics: the way toward the personalization of antidepressant treatment. Can J Psychiatry. 2014;59:62–75. doi: 10.1177/070674371405900202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilad GM, Shiller I. Differences in open-field behavior and in learning tasks between two rat strains differing in their reactivity to stressors. Behav Brain Res. 1989;32:89–93. doi: 10.1016/s0166-4328(89)80076-2. [DOI] [PubMed] [Google Scholar]
- Green, William H. Econometric Analysis. Prentice Hall; New York, NY: 2000. [Google Scholar]
- Griebel G, Cohen C, Perrault G, Sanger DJ. Behavioral effects of acute and chronic fluoxetine in Wistar-Kyoto rats. Physiol Behav. 1999;67:315–320. doi: 10.1016/s0031-9384(98)00298-4. [DOI] [PubMed] [Google Scholar]
- Hansen C, Spuhler K. Development of the National Institutes of Health genetically heterogeneous rat stock. Alcohol Clin Exp Res. 1984;8:477–479. doi: 10.1111/j.1530-0277.1984.tb05706.x. [DOI] [PubMed] [Google Scholar]
- Homberg JR, Olivier JD, Blom T, Arentsen T, Van Brunschot C, Schipper P, Korte-Bouws G, Van Luijtelaar G, Reneman L. Fluoxetine exerts age-dependent effects on behavior and amygdala neuroplasticity in the rat. PLoS One. 2011;6:e16646. doi: 10.1371/journal.pone.0016646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde CL, Nagle MW, Tian C, Chen X, Paciga SA, Wendland JR, Tung JY, Hinds DA, Perlis RH, Winslow AR. Identification of 15 genetic loci associated with risk of major depression in individuals of European descent. Nat Genet. 2016;48:1031–1036. doi: 10.1038/ng.3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iniguez SD, Warren BL, Bolanos-Guzman CA. Short- and long-term functional consequences of fluoxetine exposure during adolescence in male rats. Biol Psychiatry. 2010;67:1057–1066. doi: 10.1016/j.biopsych.2009.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Gatz M, Gardner CO, Pedersen NL. A Swedish national twin study of lifetime major depression. Am J Psychiatry. 2006;163:109–114. doi: 10.1176/appi.ajp.163.1.109. [DOI] [PubMed] [Google Scholar]
- Lahmame A, Armario A. Differential responsiveness of inbred strains of rats to antidepressants in the forced swimming test: are Wistar Kyoto rats an animal model of subsensitivity to antidepressants? Psychopharmacology (Berl) 1996;123:191–198. doi: 10.1007/BF02246177. [DOI] [PubMed] [Google Scholar]
- Lahmame A, Del Arco C, Pazos A, Yritia M, Armario A. Are Wistar-Kyoto rats a genetic animal model of depression resistant to antidepressants? Eur J Pharmacol. 1997;337:115–123. doi: 10.1016/s0014-2999(97)01276-4. [DOI] [PubMed] [Google Scholar]
- Levinson DF, Mostafavi S, Milaneschi Y, Rivera M, Ripke S, Wray NR, Sullivan PF. Genetic studies of major depressive disorder: why are there no genome-wide association study findings and what can we do about it? Biol Psychiatry. 2014;76:510–512. doi: 10.1016/j.biopsych.2014.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Rubalcava C, Lucki I. Strain differences in the behavioral effects of antidepressant drugs in the rat forced swimming test. Neuropsychopharmacology. 2000;22:191–199. doi: 10.1016/S0893-133X(99)00100-1. [DOI] [PubMed] [Google Scholar]
- Malkesman O, Weller A. Two different putative genetic animal models of childhood depression – a review. Prog Neurobiol. 2009;88:153–169. doi: 10.1016/j.pneurobio.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Mehta-Raghavan NS, Wert SL, Morley C, Graf EN, Redei EE. Nature and nurture: environmental influences on a genetic rat model of depression. Transl Psychiatry. 2016;6:e770. doi: 10.1038/tp.2016.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta NS, Wang L, Redei EE. Sex differences in depressive, anxious behaviors and hippocampal transcript levels in a genetic rat model. Genes Brain Behav. 2013;12:695–704. doi: 10.1111/gbb.12063. [DOI] [PubMed] [Google Scholar]
- Mezadri TJ, Batista GM, Portes AC, Marino-Neto J, Lino-De-Oliveira C. Repeated rat-forced swim test: reducing the number of animals to evaluate gradual effects of antidepressants. J Neurosci Methods. 2011;195:200–205. doi: 10.1016/j.jneumeth.2010.12.015. [DOI] [PubMed] [Google Scholar]
- Okano HJ, Park WY, Corradi JP, Darnell RB. The cytoplasmic Purkinje onconeural antigen cdr2 down-regulates c-Myc function: implications for neuronal and tumor cell survival. Genes Dev. 1999;13:2087–2097. doi: 10.1101/gad.13.16.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet DH. Modeling depression in animal models. Methods Mol Biol. 2012;829:125–144. doi: 10.1007/978-1-61779-458-2_7. [DOI] [PubMed] [Google Scholar]
- Pajer K, Andrus BM, Gardner W, Lourie A, Strange B, Campo J, Bridge J, Blizinsky K, Dennis K, Vedell P, Churchill GA, Redei EE. Discovery of blood transcriptomic markers for depression in animal models and pilot validation in subjects with early-onset major depression. Transl Psychiatry. 2012;2:e101. doi: 10.1038/tp.2012.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pare WP. Stress ulcer susceptibility and depression in Wistar Kyoto (WKY) rats. Physiol Behav. 1989;46:993–998. doi: 10.1016/0031-9384(89)90203-5. [DOI] [PubMed] [Google Scholar]
- Pare WP. Open field, learned helplessness, conditioned defensive burying, and forced-swim tests in WKY rats. Physiol Behav. 1994;55:433–439. doi: 10.1016/0031-9384(94)90097-3. [DOI] [PubMed] [Google Scholar]
- Power RA, Tansey KE, Buttenschon HN, et al. Genome-wide association for major depression through age at onset stratification: major depressive disorder working group of the Psychiatric Genomics Consortium. Biol Psychiatry. 2016;81(4):325–335. doi: 10.1016/j.biopsych.2016.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redei EE, Andrus BM, Kwasny MJ, Seok J, Cai X, Ho J, Mohr DC. Blood transcriptomic biomarkers in adult primary care patients with major depressive disorder undergoing cognitive behavioral therapy. Transl Psychiatry. 2014:e442, 4. doi: 10.1038/tp.2014.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittenhouse PA, Lopez-Rubalcava C, Stanwood GD, Lucki I. Amplified behavioral and endocrine responses to forced swim stress in the Wistar-Kyoto rat. Psychoneuroendocrinology. 2002;27:303–318. doi: 10.1016/s0306-4530(01)00052-x. [DOI] [PubMed] [Google Scholar]
- Sakai K, Kitagawa Y, LI Y, Shirakawa T, Hirose G. Suppression of the transcriptional activity and DNA binding of nuclear factor-kappa B by a paraneoplastic cerebellar degeneration-associated antigen. J Neuroimmunol. 2001;119:10–15. doi: 10.1016/s0165-5728(01)00368-x. [DOI] [PubMed] [Google Scholar]
- Slattery DA, Cryan JF. Using the rat forced swim test to assess antidepressant-like activity in rodents. Nat Protoc. 2012;7:1009–1014. doi: 10.1038/nprot.2012.044. [DOI] [PubMed] [Google Scholar]
- Solberg LC, Ahmadiyeh N, Baum AE, Vitaterna MH, Takahashi JS, Turek FW, Redei EE. Depressive-like behavior and stress reactivity are independent traits in a Wistar Kyoto × Fisher 344 cross. Mol Psychiatry. 2003;8:423–433. doi: 10.1038/sj.mp.4001255. [DOI] [PubMed] [Google Scholar]
- Solberg LC, Baum AE, Ahmadiyeh N, Shimomura K, Li R, Turek FW, Churchill GA, Takahashi JS, Redei EE. Sex-and lineage-specific inheritance of depression-like behavior in the rat. Mamm Genome. 2004;15:648–662. doi: 10.1007/s00335-004-2326-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solberg LC, Baum AE, Ahmadiyeh N, Shimomura K, LI R, Turek FW, Takahashi JS, Churchill GA, Redei EE. Genetic analysis of the stress-responsive adrenocortical axis. Physiol Genomics. 2006;27:362–369. doi: 10.1152/physiolgenomics.00052.2006. [DOI] [PubMed] [Google Scholar]
- Solberg LC, Olson SL, Turek FW, Redei E. Altered hormone levels and circadian rhythm of activity in the WKY rat, a putative animal model of depression. Am J Physiol Regul Integr Comp Physiol. 2001;281:R786–R794. doi: 10.1152/ajpregu.2001.281.3.R786. [DOI] [PubMed] [Google Scholar]
- Solberg Woods LC. QTL mapping in outbred populations: successes and challenges. Physiol Genomics. 2014;46:81–90. doi: 10.1152/physiolgenomics.00127.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solberg Woods LC, Ahmadiyeh N, Baum A, Shimomura K, LI Q, Steiner DF, Turek FW, Takahashi JS, Churchill GA, Redei EE. Identification of genetic loci involved in diabetes using a rat model of depression. Mamm Genome. 2009;20:486–497. doi: 10.1007/s00335-009-9211-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solberg Woods LC, Holl K, Tschannen M, Valdar W. Fine-mapping a locus for glucose tolerance using heterogeneous stock rats. Physiol Genomics. 2010;41:102–108. doi: 10.1152/physiolgenomics.00178.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solberg Woods LC, Holl KL, Oreper D, Xie Y, Tsaih SW, Valdar W. Fine-mapping diabetes-related traits, including insulin resistance, in heterogeneous stock rats. Physiol Genomics. 2012;44:1013–1026. doi: 10.1152/physiolgenomics.00040.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PF, Neale MC, Kendler KS. Genetic epidemiology of major depression: review and meta-analysis. Am J Psychiatry. 2000;157:1552–1562. doi: 10.1176/appi.ajp.157.10.1552. [DOI] [PubMed] [Google Scholar]
- Verduijn J, Milaneschi Y, Peyrot WJ, Hottenga JJ, Abdellaoui A, De Geus EJ, Smit JH, Breen G, Lewis CM, Boomsma DI, Beekman AT, Penninx BW. Using clinical characteristics to identify which patients with major depressive disorder have a higher genetic load for three psychiatric disorders. Biol Psychiatry. 2016;81(4):316–324. doi: 10.1016/j.biopsych.2016.05.024. [DOI] [PubMed] [Google Scholar]
- Wigg K, Feng Y, Gomez L, et al. Genome scan in sibling pairs with juvenile-onset mood disorders: evidence for linkage to 13q and Xq. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:638–646. doi: 10.1002/ajmg.b.30883. [DOI] [PubMed] [Google Scholar]
- Will CC, Aird F, Redei EE. Selectively bred Wistar-Kyoto rats: an animal model of depression and hyper-responsiveness to antidepressants. Mol Psychiatry. 2003;8:925–932. doi: 10.1038/sj.mp.4001345. [DOI] [PubMed] [Google Scholar]
- Willner P, Belzung C. Treatment-resistant depression: are animal models of depression fit for purpose? Psychopharmacology (Berl) 2015;232:3473–3495. doi: 10.1007/s00213-015-4034-7. [DOI] [PubMed] [Google Scholar]
- Yang H, Liu C, Zhong Y, Luo S, Monteiro MJ, Fang S. Huntingtin interacts with the cue domain of gp78 and inhibits gp78 binding to ubiquitin and p97/VCP. PLoS One. 2010;5:e8905. doi: 10.1371/journal.pone.0008905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Cheng XR, Zhang GR, Zhou WX, Zhang YX. Autocrine motility factor receptor is involved in the process of learning and memory in the central nervous system. Behav Brain Res. 2012;229:412–418. doi: 10.1016/j.bbr.2012.01.043. [DOI] [PubMed] [Google Scholar]
- Ying Z, Wang H, Fan H, Zhu X, Zhou J, Fei E, Wang G. Gp78, an ER associated E3, promotes SOD1 and ataxin-3 degradation. Hum Mol Genet. 2009;18:4268–4281. doi: 10.1093/hmg/ddp380. [DOI] [PubMed] [Google Scholar]
- Yoshikawa T, Watanabe A, Ishitsuka Y, Nakaya A, Nakatani N. Identification of multiple genetic loci linked to the propensity for “behavioral despair” in mice. Genome Res. 2002;12:357–366. doi: 10.1101/gr.222602. [DOI] [PubMed] [Google Scholar]
- Yu JS, Xue AY, Redei EE, Bagheri N. A support vector machine model provides an accurate transcript-level-based diagnostic for major depressive disorder. Transl Psychiatry. 2016;6:e931. doi: 10.1038/tp.2016.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Box-plots demonstrating variability in FST behavior in 4-week-old (a) male and (b) female HS rats. In the box plot, the middle line shows the mean, with the surrounding box representing the 25th and 75th percentiles. The maxima and minima are represented by the uppermost and lowermost lines, respectively. n = 50 for both males and females.
Quantitative RT-PCR primer sequences. F, Forward; R, Reverse.