Abstract
Background
The complexity of treatment regimens, costs and pill burden decrease the medication adherence and contribute to shortfall in cardiovascular preventive drug coverage. The polypill, a fixed dose combination pill of established drugs is expected to increase adherence and reduce the costs whilst preventing the major cardiovascular events (MCVE).
Design and methods
The PolyIran trial is a pragmatic cluster randomized trial nested within Golestan Cohort Study (GCS). Subjects were randomized to either non-pharmacologic preventive interventions alone (minimal care arm) or together with a polypill (polypill arm) comprising hydrochlorothiazide, aspirin, atorvastatin and either enalapril or valsartan. This study benefits from the infrastructure of the primary health care system in Iran and the interventions are delivered by the local auxiliary health workers (Behvarz) to the participants. The primary outcome of the study is the occurrence of first MCVE within five years defined as non-fatal and fatal myocardial infarction, unstable angina, sudden death, heart failure, coronary artery revascularization procedures and non-fatal and fatal stroke.
Trial status
From February 2011 to April 2013, 8410 individuals (236 clusters) attended the eligibility assessment. Of those, 3421 in polypill arm and 3417 in minimal care arm were eligible. The study is ongoing.
Conclusion
The infrastructure of GCS and the primary health care system in Iran enabled the conduct of this pragmatic large-scale trial. If the polypill strategy proves effective, it may be implemented to prevent cardiovascular disease in developing countries.
Keywords: Cardiovascular diseases, Primary prevention, Secondary prevention, Polypill
Introduction
Coronary artery disease (CAD) mortality is anticipated to increase twofold from 1990 to 2020, and 82% of the increase will occur in low and middle income countries (LMIC)1, 2. Nevertheless, widespread healthy lifestyle improvement to prevent CAD is difficult to maintain. Even though the preventive effectiveness of antiplatelet, lipid-lowering and blood pressure-lowering agents has been demonstrated3, 4, the complexity of treatment regimens and pill burden decrease the adherence to preventive medications and contribute to the shortfall in preventive drug coverage 5,6.
The polypill concept, a fixed dose combination pill of established generic drugs may simplify the treatment regimen, reducing the cost whilst preventing up to 88% of heart attacks and 80% of strokes7. A recent analysis suggested that a polypill could prevent 28,500 deaths from CAD and 12,700 deaths from stroke annually in Iran8. However these estimates are based on studies of individual drug treatments, as yet, no randomized controlled trial has evaluated the effectiveness of the polypill in reducing CVD-related mortality. Since the effectiveness of the polypill’s separate components is well established for secondary prevention of CVD, it is likely that the polypill will also be effective 7, 9, 10. For the primary prevention of CVD, several short-term studies have been conducted on surrogate markers and demonstrated the safety and effectiveness of polypill with different formulations at improving blood pressure and lipid levels 9, 11–14. However, there is a concern that the effectiveness of polypill in reducing CVD-related events in the real world setting will be lower than in the setting of strictly controlled trials.
We previously investigated the effects of a polypill on blood pressure and lipid levels in a predominantly rural population 11 demonstrating the feasibility of conducting a large scale intervention trial to investigate the effects on major cardiovascular events (MCVE).
We subsequently designed the fully-powered main phase study with five years of follow up to assess the effectiveness and safety of a four component polypill (aspirin, atorvastatin, hydrochlorothiazide and either enalapril or valsartan) alongside advice on lifestyle modification for primary and secondary prevention of CVD compared to advice on lifestyle modification alone: the PolyIran trial. The polypill tablets are produced at a cost of only 5 cents (US) per pill.
This study is a pragmatic cluster randomised controlled trial nested within the Golestan Cohort Study (GCS)15. The study makes use of the infrastructure of GCS and the established rural primary health care system and addresses the value of the polypill in a real world setting16, 17.
Subjects and Methods
Overview
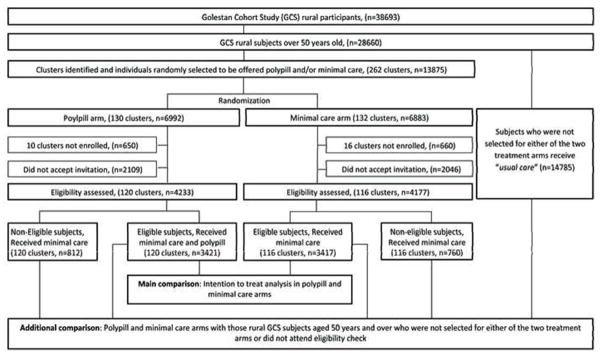
The PolyIran study is a two arm pragmatic cluster randomized trial nested within the GCS. The GCS was launched in January 2004 and was to investigate the epidemiology of esophageal cancer in subjects 40–75 years old in Golestan province, Iran. It includes 50,045 participants, 20% enrolled from Gonbad city and 80% from rural areas: the villages in the regions of Gonbad, Aq-Qala and Kalaleh. Further details are described elsewhere 15. GCS participants who lived in rural areas and were aged 50 years and over at the beginning of the trial constituted the sampling frame for the PolyIran study. From this sampling frame, individuals were selected using a simple stratified random selection procedure in proportion to the number of eligible inhabitants in each village. Selected subjects were invited to participate in the study. The sample selection took into account the predicted rate of 33% declining to participate based on the experience of the pilot study. These random samples from each village constituted the clusters. Villages (i.e. clusters) were then randomly allocated to a package of non-pharmacologic preventive interventions either alone (minimal care arm) or together with a once daily polypill (polypill arm). Accordingly, all subjects within a cluster were randomized to receive the same intervention. The outcomes of the study will be compared between these two intervention arms. An additional comparison will be made between these two arms and the remaining GCS subjects who lived in rural areas, were aged 50 years and over and were not selected for either of the two intervention arms (usual care arm). The usual care arm neither receives minimal care from the PolyIran study team nor polypill but they receive usual care as currently provided by the public and private sectors. The trial design is presented in figure 1.
Figure 1.
The trial Profile
Study setting and enrolment of participants
This study is being conducted in rural areas near three main cities within the GCS, Gonbad, AqQala and Kalaleh. The study benefits from the established infrastructure of the primary health care system in Iran 16, 17, consisting of health houses in villages, each staffed by auxiliary health workers locally called “Behvarz”. The Behvarz in each village is originally in charge of vaccination programs, family planning, reporting births, deaths, major communicable diseases and first-contact primary health care. Each group of health houses is staffed by a family physician and other professional health care providers.
The selected individuals were invited by the Behvarz to attend their local health house. They were assessed by the trial enrolment team consisting of a physician, a registrar and a laboratory assistant. After obtaining informed consent, the study physician completed a questionnaire on current medications, past medical history, family history and other factors relevant to eligibility and CVD risk. Two seated blood pressures, 1 minute apart after 5 minutes rest and a blood pressure after 1 min standing were measured by the Behvarz under the supervision and stringent training of the study physician. A blood sample after 8 hours of fasting was taken to assess complete blood count, lipid profile, serum urea, creatinine, glomerular filtration rate, liver enzymes and fasting blood sugar (Pars Azmoon Inc., Tehran, Iran). Eligibility of the subjects was then assessed by the study physician.
Eligibility criteria
The eligibility criteria are outlined in table 1. Participants were included whether or not they had a history of cardiovascular disease, with the exception of a history of stroke which was an exclusion criterion. The exclusion criteria were only applied to the minimal care and polypill arms. Those subjects receiving usual care did not attend the initial assessment and thus were not evaluated for exclusion criteria.
Table 1.
Eligibility criteria: All subjects in polypill arm, minimal care arm and those receiving usual care were assessed for inclusion criteria, but the exclusion criteria were only applied to minimal care and polypill arms.
|
|
Interventions
All subjects in minimal care arm whether or not they meet the exclusion criteria receive a package of non-pharmacologic interventions delivered by the Behvarz through face to face interview. This is supplemented by short text messages (SMS) twice monthly and a well designed pictorial pamphlet. The contents include educational training about a healthy lifestyle, including a healthy food intake with low salt, sugar and fat content, exercise, weight control and abstinence from smoking and opium. Alongside this, participants have biannual blood pressure measurement to identify hypertensive subjects; these participants receive education about the impact of hypertension on CVD and are referred to their local family physicians for blood pressure control.
All participants in polypill arm whether or not they meet the exclusion criteria receive the same lifestyle education as the minimal care arm but in addition, participants who met the eligibility criteria are prescribed polypill. Two formulations of polypill tablet are used in this study (Alborz Darou Pharmaceutical Company, Tehran, Iran). Participants are first prescribed polypill 1 which consists of hydrochlorothiazide 12.5 mg, aspirin 81 mg, atorvastatin 20 mg and enalapril 5 mg. Those who develop cough during follow-up are switched by the study physician to polypill 2, which substitutes enalapril 5 mg with valsartan 40 mg. Polypill tablets are provided free of charge and administered once daily with the dose timing left to the discretion of the participant. At the time of enrolment, if the subject was already taking aspirin or statin, it was discontinued by the study physician or the dosage was reduced and replaced by the polypill. In the case of antihypertensive medications, the study physician decided to discontinue or decrease the dose of antihypertensives, preferably those in the same class as polypill components. Subjects (or a literate family member for illiterate participants) in the polypill arm are also sent text massages twice monthly to improve medication adherence.
Subjects aged 50 years and over in rural areas of GCS who were not selected for either of the two treatment arms, receive usual care offered by the public and private sector in Iran (usual care arm).
All subjects may receive any treatment judged necessary by their physician. A record will be kept of any additional medication prescribed in minimal care and polypill arms.
Follow-ups
Follow-ups are scheduled to occur at month 1, 2, 3, 6 and then every 6 months in the polypill arm and at month 3, 6 and then every 6 months in minimal care arm.
Follow-up visits are carried out by Behvarz until 5 years after the date of enrolment. At followup visits, participants are offered minimal care, polypill tablets are dispensed and pill counts undertaken (in the polypill arm). Participants are interviewed to maintain study participation and to assess for the presence of symptoms which might be adverse events. Subjects who report symptoms are identified by the Behvarz and are visited by the study physician. These subjects are referred to the local family physicians as required at the study physician’s discretion.
Behvarzs are provided with regular educational training just before each follow-up visit about standard blood pressure measurement, healthy life style with respect to CVD risk, possible adverse drug reactions and other issues relevant to the follow-up protocol. Family physicians and Behvarzs of the study region also attend the annual lectures by the principal investigator (RM) about modifiable CVD risk factors.
Subjects receiving usual care are not subject to the regular follow-ups described above.
Outcomes
The primary outcome is the occurrence of MCVE within five years of enrolment. MCVE includes either hospitalization for acute coronary syndrome (non-fatal myocardial infarction and unstable angina), fatal myocardial infarction, sudden death, heart failure, coronary artery revascularization procedures, non-fatal and fatal stroke. For subjects with more than one MCVE, the first event will be included in the primary outcome analysis.
Secondary outcomes are the components of the primary outcome considered individually, noncardiovascular causes of death (including neoplastic, respiratory, hepatic, renal and other medical causes), adherence to the polypill (based on pill count) and changes in blood pressure and lowdensity lipoprotein (LDL) cholesterol during the trial. Secondary comparisons also include the effects of the interventions on MCVE in the following subcategories: 1) In the presence or absence of pre-existing CVD (secondary and primary prevention groups, respectively), 2) In the presence or absence of pre-existing hypertension and/or diabetes mellitus, 3) In males and females, 4) In those aged ≤ 65 and > 65 years at baseline, 5) Serum cholesterol level above and below the median value at baseline, 6) Smokers and non-smokers at baseline, 7) Among subjects subdivided with respect to the size of the reduction in blood pressure during the trial. Exploratory analysis may be carried out in the context of evidence from other studies, with due allowance made for their exploratory nature.
Causes of death are currently ascertained for all GCS participants blinded to allocation arm of the PolyIran trial 15, 18. Personnel in charge of outcome ascertainment act independent of PolyIran trial team. Briefly, all GCS participants are contacted by telephone annually inquiring about health status and any admission to hospital or outpatient CVD clinics. If the participant is not reached after seven telephone calls made in a 2-week period, friends or the Behvarzs are contacted. Using this protocol, the follow-up success rate so far is over 99% 18. On learning of a death or possible non-fatal CVD event, a general practitioner visits the home of the subject and completes a detailed verbal autopsy by interviewing the next of kin of the dead person or completes a questionnaire on hospitalisation or outpatient CVD clinic visit by interviewing the patient. All relevant medical documents are also captured in an electronic database from hospitals or patients’ house. Two separate internists then review all documents and ascertain the outcome on the basis of standardized criteria. In case of discordance, outcome ascertainment is being done by reviewing all documents within a panel of expert cardiologists and neurologists.
Randomization
Cluster (i.e. village) randomization was used in this study to avoid issues of contamination which would likely arise due to sharing of medicines.
Randomization was stratified by the three districts, Gonbad, Aq-Qala and Kalaleh with the village as the unit of randomization. A balanced randomization algorithm was used 19. Balancing was implemented using block sizes of 20 and balancing over cluster size or natural log of the cluster size (depending on the skewness within strata). Randomization was undertaken at a fixed point in time (January 2011) by statisticians at the University of Birmingham, UK, independent of the local study team.
Allocation concealment and blinding
All selected subjects within villages were invited to participate in the trial. For the first 48 clusters enrolled, the day that the enrolment team visited the villages to undertake baseline assessment coincided with the day that the first polypill tablets were dispensed. Thus the enrolment team was aware of the allocation of clusters. An interim analysis at this point (48 clusters, 2,115 participants) demonstrated that the proportion of subjects who met the exclusion criteria differed between the polypill and minimal care arms, presumably due to preferential behavior of the enrolling physician applying the exclusion criteria more stringently to the polypill arm. Accordingly the decision was made to blind the enrolment team for the reminder of the study. Polypill tablets were since then provided by the Behvarz a few days after enrolment of the participants and the allocation of the clusters was thus concealed from the enrolment team members.
Due to pragmatic nature of this study, the participants, investigators and staff offering the interventions are not blinded. However, personnel responsible for ascertainment of the primary and most of the secondary outcomes are blinded to the allocation of participants, being members of the GCS follow-up group rather than the trial team. Blood pressure measurement and pill count are undertaken by the Behvarzs who are not blinded.
Sample size justification
This is a pragmatic study, nested within an existing cohort study, subject to funding constraints and so the study sample size is fixed by design. At the time of designing the study it was anticipated that the total sample size would consist of 7,224 individuals (3,612 per arm), spread over 262 clusters with average size of 28 and coefficient of variation of 0.9.
Given the MCVE rate of 0.0171 per year in the GCS (unpublished data) we anticipated the risk of an event to be approximately 0.077 over 5 years. A clinically important effect size of 0.65 translates into a reduction of 35% in the event rate. We anticipate a 48% drop-out rate (20% in the first year and 10% annually from the second year). Given that the trial is nested within an existing cohort study, even if the subjects do not continue to take polypill or minimal care or do not comply with the PolyIran trial follow-ups, it will be still possible to collect primary outcome data for the majority of these individuals in the context of GCS follow-up and so we have allowed for a 20% loss to follow-up rate only. Estimates of the intra-cluster correlation (ICC) are limited, but unpublished data suggest that it is between 0.007 and 0.038.
With the available 262 clusters, with an average size of 28 individuals (22 after drop out), and using the upper estimate of the ICC of 0.038, coefficient of variation of cluster size of 0.9 (which translates to an estimated design effect of 2.85), the study has approximately 80% power (at 5% significance) to detect a relative risk of 0.62. Using the lower estimate of the ICC this effect size would be 0.73. Sample size calculations were carried out using the clustersampsi function in Stata 20.
Statistical analysis plan
Primary analyses of outcomes comparing the polypill group and minimal care group will be by intention to treat and all subjects who met the eligibility criteria will be included. In the comparison with those receiving usual care, we will include all randomly selected participants in the polypill and minimal care arms in the analysis (i.e. would not exclude those ineligible). As randomisation was at the village (cluster) level, appropriate statistical methods to account for the clustering effect will be used in the analysis. Analysis of outcomes will be for five-year followup stage. An interim analysis will be also carried out after 2 years of the enrolment of the last participant and the blinded results would be given to the data monitoring committee (DMC). DMC members meet with 6 months regular intervals and also travel to the study field and continuously monitor the trial in terms of any harm or protocol deviation. There is limited reason to suspect any harm (as each component is in use independently) and it is also unlikely that the effect will be so large for clear differences to be observed so early, however, DMC would consider the data for possible early termination of the study due to efficacy, futility or harm.
The null hypothesis (no difference) for the primary outcome would be tested using a random effects Cox model with time to the primary outcome and censoring those who are lost to followup or who die from other causes.
The primary analysis will be unadjusted. Secondary analysis will adjust for baseline covariates including age, sex, diabetes mellitus, blood pressure, history of MCVE.
Null hypotheses for secondary outcomes would take a similar form to that for the primary outcome. Secondary outcomes are either binary (e.g. non-cardiovascular mortality), or continuous (e.g. LDL cholesterol), and therefore either logistic or linear link functions within a generalised linear model with random effect (with transformations where appropriate to accommodate any non-normality) will be used.
All model assumptions will be checked, goodness of fit explored and alternative models considered if necessary. All outcomes will be considered significant at the 5% level.
The significance of subgroup effects will be assessed by test of interactions of covariates and the treatment effect. The study will have low power to detect all but the largest differences.
We will also look at differences in outcomes by subgroup of fidelity of uptake (broadly classified as low, medium or high) and, whether fidelity is related to baseline measures.
Ethical considerations
The study protocol was approved by the ethics committee of Digestive Diseases Research Institute, Tehran University of Medical Sciences to conform to the ethical guidelines of Declaration of Helsinki and International Conference for Harmonization Guidelines for Good Clinical Practice. The protocol was registered at ClinicalTrials.gov (ClinicalTrials.gov ID: NCT01271985)
Individual informed consent, by the nature of the study, was obtained from subjects in polypill and minimal care arms after randomization for logistical reasons. Other subjects receiving usual care have already consented to provide observational data at the outset of GCS.
Trial status
Figure 1 shows the trial profile. In total, 13,875 individuals in 262 clusters were randomly selected. Between February 2011 and April 2013, 8,410 individuals (236 clusters) of the selected sample attended the eligibility assessment visit and 3,421 in polypill arm and 3,417 in minimal care arm met the eligibility criteria. We were not able to enroll 26 clusters (10 clusters in polypill arm and 16 clusters in minimal care arm) due to difficulties accessing the villages by road. Post hoc calculations albeit showed that any loss in power should be minimal. The two treatment arms were well balanced with respect to rate of exclusion (19.2% in polypill arm versus 18.2% in minimal care arm). Table 2 outlines the reasons for which subjects were not eligible at the baseline visit. Median cluster size in polypill and minimal care arms was 27 (interquartile range, 15.0–45.5) and 29.5 (15.0–48.5), respectively.
Table 2.
Reasons for which subjects did not meet the eligibility criteria, n (%)
| Polypill arm, n=812, 19.2% | Minimal care arm, n=760, 18.2% | |
|---|---|---|
| Hypersensitivity to one of components of polypill | 12(0.28) | 7(0.17) |
| History of angioedema | 1(0.02) | 1(0.02) |
| History of gastrointestinal bleeding or peptic ulcer disease in last 3 months | 13(0.31) | 12(0.29) |
| History of stroke | 37(0.87) | 58(1.39) |
| Pregnancy or lactation | - | - |
| Bleeding disorders such as hemophilia | 1(0.02) | 1(0.02) |
| Regular anticoagulant use | 25(0.59) | 16(0.38) |
| Alcohol consumption | 2(0.05) | 1(0.02) |
| Advanced liver disease | 25(0.59) | 18(0.43) |
| Uncontrolled seizures | 10(0.24) | 14(0.34) |
| Severe asthma | 108(2.55) | 143(3.42) |
| History of gout | 2(0.05) | 1(0.02) |
| Serum creatinine > 2 mg/dL | 27(0.64) | 31(0.74) |
| Glomerular filtration rate < 30 ml/min | 52(1.23) | 64(1.53) |
| Hemoglobin<10 mg/dL in females and <11 mg/dL in males | 210(4.96) | 191(4.57) |
| Systolic blood pressure <90 mmHg and diastolic blood pressure < 60 mmHg | 91(2.15) | 37(0.89) |
| Medical/psychiatric comorbidities | 305(7.21) | 309(7.4) |
| Unavailability of the subjects | 28(0.66) | 2(0.05) |
Note: Each subject may meet more than one exclusion criterion
Subjects aged 50 years and over who were not selected for random allocation in polypill and minimal care arms (14,785), receive usual care.
Among subjects who met the eligibility criteria, history of CAD, myocardial infarction and heart failure was detected in 375(11.0%), 45(1.3%) and 12(0.3%) of those in polypill arm and 333(9.7%), 51(1.5%) and 9(0.3%) in minimal care arm, respectively. Overall, 386(11.3%) of the participants in polypill arm and 346(10.1%) in minimal care arm are available for assessment of the outcomes for secondary prevention and 3035(88.7%) and 3071(89.9%) for primary prevention of MCVE, respectively.
Discussion
In LMIC, high drug costs, make them prohibitively expensive as preventive measures 5. On the other hand, dose complexity and the number of pills used per day are inversely related to adherence and contribute to the shortfall in prevention coverage 5, 6. These drugs could however best be provided as a low cost polypill to improve the availability and affordability of the preventive drugs and adherence of the subjects 7, 8.
To date, the duration of the trials on polypill concept were short and mostly focused on LDL-cholesterol and blood pressure as the primary outcome 9, 11–14. We therefore designed the present fully powered large scale trial with 5 years duration in rural population in Iran to directly assess the value of polypill compared to life-style modification on CVD-related mortality and morbidity. A strength of this study is that it comprises subjects with or without history of CVD and therefore would address the effectiveness of polypill in both primary and secondary prevention.
This study is being conducted within an established cohort study and its infrastructure 15 which allows for considerable economies of scope. It would also allow us to compare the individuals in polypill and minimal care arms with those receiving usual care. This additional comparison may help to assess the value of recommendations of a healthy life-style and blood pressure screening compared to no intervention in primary health care setting.
In recent years, there has been much interest and debate over who the polypill should be offered to. As about 96% of CVD related mortality occur in people aged 55 and over, some experts advocate offering the polypill to all individuals above 55 years of age 7, whilst others recommend that polypill strategy should be implemented based on individual risk assessment 10, 21. There is still controversy around using age as the only inclusion criteria to prescribe polypill and we hope to address this question in this trial.
Moreover, despite previous clinical trials in the literature, an important question is, however, whether polypill can prevent CVD in a real world setting or not. Conventional explanatory trials are conducted in strictly controlled settings which might limit the generalizability of their findings in the usual care setting. We therefore, used a pragmatic design to evaluate the effectiveness of polypill in as realistic a setting as possible utilizing the established efficient primary health-care system 16, 17, 22 consisted of health houses in all villages staffed by auxiliary health personnel (Behvarz). Being raised up from the same village that Behvarz work, results in trust-based interactions with rural communities and a self-sacrificing impetus to serve rural people. The Behvarz system has survived as an essential component of the rural health care system in Iran and has been identified as a key for progress in the health of Iran’s rural population. Recently, attempts were made to integrate the programs for prevention of NCD in Behvarz system 23. Though challenging, the integration seems possible and potentially effective. We believe that conducting the present trial would allow us to assess the capabilities of the primary health care system for prevention of CVD.
In the present study, similar to the real world setting, we do not offer placebo and the participants and care providers are not blinded with regard to the allocated interventions. Such a design would estimate the adherence to the polypill in general population more realistically than conventional explanatory trials 24. However, lack of blinding in the first months of recruitment resulted in imbalance in the rate of exclusion between the polypill and minimal care arms. The enrolling physician was more conservative for the polypill arm and excluded more subjects. This necessitated the concealment of allocation from the enrolment team for the remainder of the study and the balancing was then achieved. But the participants and staff offering the interventions are still not blinded.
There is also a potential for “Hawthorne effect” in this study favouring those not receiving polypill in an unblinded design, in which Behvarzs may improve the level of minimal care. However, the cluster randomization is expected to militate against this effect as the people covered by each Behvarz have the similar allocation and so the Behvarz may not change the behavior on specific individuals in response to lack of an additional intervention.
Cluster randomisation at the level of villages minimizes the risk of contamination through pill sharing. The only disadvantage with cluster randomization is that it affects the power of the study. However we took the effect of clustering into account while calculating the sample size of the study.
In the present study, despite the usual care setting in many countries, subjects receive the treatment free of charge which might unrealistically improve the adherence to treatment. However, rural subjects in Iran are not currently charged for most of the components of the primary health care provided by Behvarz. Considering the very low cost for manufacturing polypill, this study would therefore reflect the real impact, if the polypill and minimal care were to be made available at no cost or very low cost to the end-users.
The results of the study are likely to be generalizable to rural but not urban population in Iran. Other limitation regarding generalizability is that the ethnicity of the participants in GCS and thus in PolyIran trial are mostly Turkmen which should be considered while extrapolating the results of the study to other ethnic populations.
If the present study proves the feasibility and effectiveness of primary and secondary prevention of CVD by the polypill on top of the advice of lifestyle modification, this would provide important evidence for an effective strategy to prevent CVD in third world countries.
Acknowledgments
Funding: The study was supported by the Digestive Disease Research Institute and Alborz Darou and Barekat Research Funding Agencies.
Footnotes
Conflict of interest disclosure: No conflict of interest exists in relation to the submitted manuscript.
References
- 1.Lim SS, Vos T, Flaxman AD, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2013;380:2224–60. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Non-communicable diseases (NCD) WHO; 2012. Available from: http://www.who.int/gho/ncd/en/index.html. [Google Scholar]
- 3.Law M, Wald N, Morris J. Lowering blood pressure to prevent myocardial infarction and stroke: a new preventive strategy. Health Technol Assess. 2003;7:1–94. doi: 10.3310/hta7310. [DOI] [PubMed] [Google Scholar]
- 4.Antithrombotic Trialists’Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324:71–86. doi: 10.1136/bmj.324.7329.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mendis S, Fukino K, Cameron A, et al. The availability and affordability of selected essential medicines for chronic diseases in six low-and middle-income countries. Bull World Health Organ. 2007;85:279–88. doi: 10.2471/BLT.06.033647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adherence to long-term therapies-Evidence for action. WHO; 2003. Available from: http://www.who.int/chp/knowledge/publications/adherence_full_report.pdf. [PubMed] [Google Scholar]
- 7.Wald NJ, Law MR. A strategy to reduce cardiovascular disease by more than 80% BMJ. 2003;326:1419. doi: 10.1136/bmj.326.7404.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sepanlou SG, Farzadfar F, Jafari E, Danaei G. Cardiovascular Disease Prevention Using Fixed Dose Pharmaco-therapy in Iran: Updated Meta-Analyses and Mortality Estimation. Arch Iran Med. 2012;15:531–37. [PubMed] [Google Scholar]
- 9.Indian Polycap Study (TIPS) Yusuf S, Pais P, et al. Effects of a polypill (Polycap) on risk factors in middle-aged individuals without cardiovascular disease (TIPS): a phase II, double-blind, randomised trial. Lancet. 2009;373:1341–51. doi: 10.1016/S0140-6736(09)60611-5. [DOI] [PubMed] [Google Scholar]
- 10.Rastegarpanah M, Malekzadeh F, Thomas GN, et al. A new horizon in primary prevention of cardiovascular disease, can we prevent heart attack by” heart polypill”? Arch Iran Med. 2008;11:306–13. [PubMed] [Google Scholar]
- 11.Malekzadeh F, Marshall T, Pourshams A, et al. A pilot double−blind randomised placebo−controlled trial of the effects of fixed-dose combination therapy (‘polypill’) on cardiovascular risk factors. Int J Clin Pract. 2010;64:1220–27. doi: 10.1111/j.1742-1241.2010.02412.x. [DOI] [PubMed] [Google Scholar]
- 12.Soliman EZ, Mendis S, Dissanayake WP, et al. A Polypill for primary prevention of cardiovascular disease: A feasibility study of the World Health Organization. Trials. 2011;12:3. doi: 10.1186/1745-6215-12-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wald DS, Morris JK, Wald NJ. Randomized polypill crossover trial in people aged 50 and over. PLoS One. 2012;7:e41297. doi: 10.1371/journal.pone.0041297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thom S, Field J, Poulter N, et al. Use of a Multidrug Pill In Reducing cardiovascular Events (UMPIRE): rationale and design of a randomised controlled trial of a cardiovascular preventive polypill based strategy in India and Europe. Eur J Prev Cardiol. 2014;21:252–61. doi: 10.1177/2047487312463278. [DOI] [PubMed] [Google Scholar]
- 15.Pourshams A, Khademi H, Malekshah AF, et al. Cohort profile: the Golestan Cohort Study—a prospective study of oesophageal cancer in northern Iran. Int J Epidemiol. 2010;39:52–59. doi: 10.1093/ije/dyp161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farzadfar F, Murray CJ, Gakidou E, et al. Effectiveness of diabetes and hypertension management by rural primary health-care workers (Behvarz workers) in Iran: a nationally representative observational study. Lancet. 2012;379:47–54. doi: 10.1016/S0140-6736(11)61349-4. [DOI] [PubMed] [Google Scholar]
- 17.Javanparast S, Baum F, Labonte R, et al. Community health workers’ perspectives on their contribution to rural health and well-being in Iran. AM J Public Health. 2011;101:2287–92. doi: 10.2105/AJPH.2011.300355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khademi H, Malekzadeh R, Pourshams A, et al. Opium use and mortality in Golestan Cohort Study: prospective cohort study of 50 000 adults in Iran. BMJ. 2012;344:e2502. doi: 10.1136/bmj.e2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carter BR, Hood K. Balance algorithm for cluster randomized trials. BMC Med Res Methodol. 2008;8:65. doi: 10.1186/1471-2288-8-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hemming K, Marsh J. A menu-driven facility for sample-size calculations in cluster randomized controlled trials. Stata Journal. 2013;13:114–35. [Google Scholar]
- 21.Reddy KS. The preventive polypill--much promise, insufficient evidence. N Engl J Med. 2007;356:212. doi: 10.1056/NEJMp068219. [DOI] [PubMed] [Google Scholar]
- 22.Javanparast S, Baum F, Labonte R, et al. A policy review of the community health worker programme in Iran. J Public Health Policy. 2011;32:263–76. doi: 10.1057/jphp.2011.7. [DOI] [PubMed] [Google Scholar]
- 23.Rabiei K, Kelishadi R, Sarrafzadegan N, et al. Process evaluation of a community-based program for prevention and control of non-communicable disease in a developing country: The Isfahan Healthy Heart Program, Iran. BMC Public Health. 2009;9:57. doi: 10.1186/1471-2458-9-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ware JH, Hamel MB. Pragmatic trials–guides to better patient care. N Engl J Med. 2011;364:1685–87. doi: 10.1056/NEJMp1103502. [DOI] [PubMed] [Google Scholar]