Abstract
Light-emitting firefly luciferin analogues contain electron-donating groups in the 6′-position, but the scope of known 6′-substitution remains narrow. A two-step route to a broad range of 6′-substituted luciferin analogues was developed to fill this void and enable more extensive study of the 6′-functionality. This chemistry allowed direct access to “caged” amide and bright azetidine analogues, but also revealed thioether inhibitors and unexpectedly luminogenic aryl amine derivatives.
Graphical abstract

The natural substrate for firefly luciferase, D-luciferin, contains an electron-donating hydroxyl group in the 6′ position that is crucial for bioluminescence (Figure 1).1 Synthetic analogues containing amine or alkylamine donors are also luminogenic and can offer advantages for in vivo imaging.2,3 However, to date most firefly luciferin substrates and “caged” sensors have been synthesized from 6-amino or 6-hydroxy benzothiazoles, placing inherent restrictions on the nature of the electron-donating 6′-functionality.1,4–10 As such, only a relatively narrow range of 6′-substituted luciferin analogues are known, limiting our understanding of the chemistry of bioluminescence and ability to take full advantage of this imaging modality. In order to further investigate the role of this vital component, we explored the derivatization of 6-fluoro and 6-bromo-2-cyano-benzothiazoles via nucleophilic aromatic substitution and Buchwald-Hartwig amination, respectively. We found that this chemistry allowed access to new classes of luciferase substrates, inhibitors, and their precursors, with immediate applications for biocompatible chemistry and bioluminescence imaging.
Figure 1.
Firefly luciferase substrates all have hydroxy, amino, or alkylamino electron donors. Examples include D-luciferin, 6′-aminoluciferin, CycLuc1, and AkaLumine.
We envisioned that 6-halo-2-cyanobenzothiazoles could be modified at the 6-position by nucleophilic aromatic substitution or palladium catalysis.11 One concern was that the activated nitrile is prone to react with nucleophiles. Indeed, fear of this possibility initially dissuaded us from exploring this route, in favor of reductive alkylation and other synthetic strategies.5,12 Nonetheless, this chemistry could offer access to a wide variety of analogues not readily accessible by other approaches.
We first synthesized 6-fluoro-2-cyanobenzothiazole 3 (Scheme S1) and performed SNAr reactions with a variety of cyclic secondary amines that could be challenging or tedious to synthesize by the conventional N-alkylation approach (Scheme 1). Of particular interest, azetidine-substituted fluorophores have been reported to have higher quantum yields than those of analogous dyes.13 Although the desired 6-substituted-2-cyano-benzothiazoles 4–9 could be isolated in most cases, the yields from the SNAr route were low (<20%; Scheme 1). The reaction with azetidine was particularly problematic (2% yield). Primary amines and weakly nucleophilic amines such as thiomorpholine dioxide failed to give any desired product. However, displacement with methanethiol was successful (48% yield), giving access to a new class of analogue that was expected to be fluorescent.14,15 The corresponding sulfoxide and sulfone could be prepared by oxidation with Oxone and mCPBA, respectively (Scheme 1). The thiophenol analogue could also be prepared, albeit in low yield (13%).
Scheme 1.

Nucleophilic aromatic substitution of 6-fluoro-2-cyanobenzothiazole with secondary amines and thiols.
We next turned to Buchwald-Hartwig amination of 14, synthesized from 6-bromo-2-chlorobenzothiazole by heating with KCN in DMSO, or alternatively in higher yield at room temperature using DABCO as catalyst (Scheme S2).16 Palladium-catalyzed amination using xantphos as ligand17,18 allowed synthesis of the morpholine analogue in 71% yield vs 12% for the SNAr reaction (Scheme 2). Additionally, the thiomorpholine dioxide analogue was obtained in 74% yield, and the azetidine in 33% yield (low, but vastly improved over 2%). Boc-piperazine was similarly accessed, where the Boc group could be retained or later removed with TFA.
Scheme 2.

Buchwald-Hartwig substitution of 6-bromo-2-cyanobenzothiazole with a wide variety of partners.
Buoyed by the success of this approach, we then sought access to entire classes of 6′-modifications heretofore unknown in luciferin analogues, in order to more broadly explore the range of electron-donating groups (EDGs) that could be accommodated in luciferin substrates or caged sensors. For example, no 6′-arylamino luciferin analogues have been reported. Excitingly, Buchwald-Hartwig amination with xantphos allowed ready access to a wide variety of 6-arylamino derivatives (Scheme 2, 17–30). Furthermore, secondary and tertiary “caged” 6-amide analogs could also be synthesized directly in good yields under these conditions (31–35).18 Although simple 6′-amidoluciferins are all potential sensors for amidases, only the 6′-acetamide has been previously described.19 We extended this chemistry to carbamates and ureas such as 2-oxazolidone 39, benzyl carbamate 36, dimethylurea 37, and trimethylurea 38. The thiophenol derivative 13 could be prepared in improved yield. Primary amines could also be coupled (40–42), enabling the direct synthesis of derivatives that previously required functional group protection (42).
The new 6-substituted nitriles were all readily converted into their respective luciferin analogues 3a-41a by reaction with D-cysteine (Schemes S1–2). However, it should be noted that these nitriles are also of direct interest for their mild biocompatible condensation with N-terminal cysteines and related aminothiols.20–23
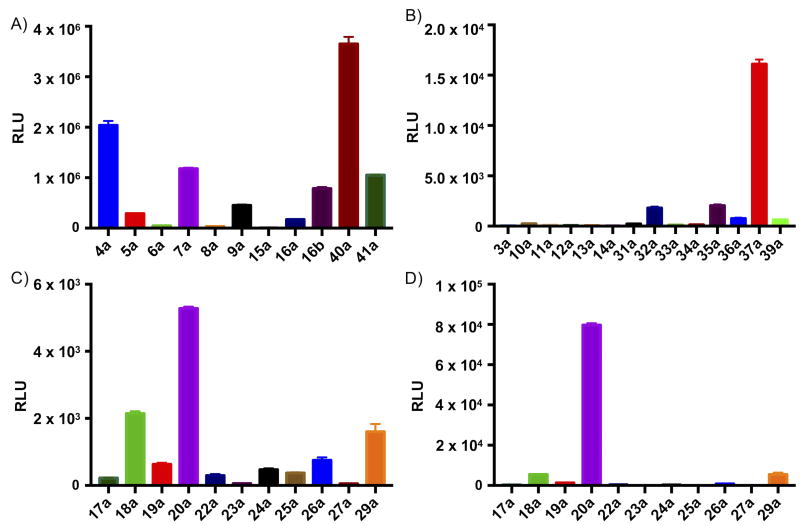
A set of 36 new luciferin analogues was then evaluated in burst bioluminescence assays with purified firefly luciferase (Figures 2; S1–2; Table S1).12,24 This assay was used to determine whether the new analogues have the capacity for light emission. Unsurprisingly, monoalkyl amine 40a was the brightest, while azetidine 4a led the new cyclic secondary amines (Figure 2a, 3). Although disparate structures such as azepane 7a,25 thiomorpholine 9a, piperazine 16b and trifluoroethylamine 41a were all good emitters, the thiomorpholine dioxide analogue 15a was unexpectedly only weakly luminescent (Figures 2a; S1–2), despite its fluorescence (Table S2). Potentially, accommodation of the bulky sulfone group requires twisting of the amine within the enzyme pocket, lowering the quantum yield. Such differences could be exploited for the development of orthogonal luciferase substrates24,26–28 and for the creation and modulation of selective luminogenic reporters.8
Figure 2.
Bioluminescence emission from WT luciferase with A) alkylamino luciferins; B) putative “caged” luciferins; C) arylamino luciferins; D) arylamino luciferins incubated with R218K mutant luciferase. Note differences in scale between panels.
Figure 3.

Newly-synthesized luminogenic substrates.25
In contrast to alkylamines, the presence of arylamine EDGs in dyes is generally associated with negligible fluorescence.11,15,29,30 However, fluorescence can be observed in rigid, viscous, or nonpolar environments (Table S2).15,31–33 We suspected that the luciferase active site might serve as a suitably rigid environment for luminescence. Although most of the aniline analogues were essentially non-luminescent, the subset of ortho-substituted anilines, expected to experience hindered rotation, was indeed emissive: 2,6-dimethylaniline 20a, indoline 29a and 2-ethylaniline 18a (Figure 2c, 3). Furthermore, a substantial increase in luminescence for 20a was observed with a mutant luciferase that is known to better accommodate many synthetic luciferins (Figure 2d, S2), suggesting that further enhancement of 6′-arylamine luminescence is possible by alteration of the substrate pocket.
Interestingly, not all “caged” analogs are completely dark (Figure 2b; Figures S1–2). In particular, the dimethylurea derivative 37a was luminescent and brighter than 15a (Figures S1–2). Several carboxamide analogues also had weak emission (>1,000-fold weaker than D-luciferin, but still >10-fold over background). In contrast, all of the new thio analogues are dark, despite the fluorescence of the S-methyl analogue 10a (Table S2).14,15 Indeed, thioethers 10a and 13a are potent luciferase inhibitors, as are the non-emissive aryl amines (Figure S3). On the other hand, the thiomethyl sulfoxide 11a and sulfone 12a are only weakly inhibitory, suggesting a potential strategy for constructing bioluminescent sensors of S-oxidation based on relief of luciferase inhibition.
Finally, we evaluated the new panel of luciferin analogues (Figure 3) as substrates for luciferase in live Chinese hamster ovary (CHO) cells (Figure 4, Figure S4). Unlike the in vitro assay, this requires the luciferin analogue to cross the cell membrane. The brightest of the new substrates in this context was the allylamine 40a (Figure S5). None of the new dialkylamines was brighter than CycLuc2, but the azetidine, azepane, and thiomorpholine analogues 4a, 7a, and 9a yielded higher photon flux than D-luciferin or the 6′-dimethylamino analogue.5 The polar morpholine and piperazine analogues 8a and 16b are substantially weaker emitters in cells, perhaps reflective of their relatively poor permeability across the cell membrane, and in the case of piperazine, a higher Km than the more lipophilic aminoluciferins (Table S3). In cells expressing the R218K luciferase, bioluminescence emission from D-luciferin could be exceeded by many of the alkyl aminoluciferins. Impressively, this includes the unconventional 2,6-dimethylaniline derivative 20a, even though it is a relatively weak emitter.
Figure 4.
Comparison of bioluminescence from selected luciferin analogues (10 μM) in live CHO cells expressing A) wild-type firefly luciferase or B) R218K mutant luciferase. Note log scale.
In summary, we have developed a two-step synthesis of luciferin analogues from a common intermediate. The essential 6′-donating group can be easily modified, enabling investigation of its role and supporting the development of many new substrates, inhibitors, and potential probes. Novel 6′-aniline analogues can be inhibitors or luminescent substrates, depending on the nature of the aryl substituent, and also hold potential as sensors of oxidative species.7,30 Furthermore, numerous other “caged” luciferins can be directly accessed, and importantly not all are dark. This work thus pushes the boundaries of what can be considered a luciferin and suggests new avenues to exploit this light-emitting chemistry for detecting and imaging chemical reactivity and biological processes.
Supplementary Material
Acknowledgments
This work was supported by the US National Institutes of Health (DA039961 and EB020243). S.T.A. was supported by F31GM016586.
Footnotes
Experimental procedures, supplemental tables and figures, and compound spectra. The Supporting Information is available free of charge on the ACS Publications website.
References
- 1.Adams ST, Miller SC. Curr Opin Chem Biol. 2014;21:112. doi: 10.1016/j.cbpa.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Evans MS, Chaurette JP, Adams ST, Jr, Reddy GR, Paley MA, Aronin N, Prescher JA, Miller SC. Nat Methods. 2014;11:393. doi: 10.1038/nmeth.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuchimaru T, Iwano S, Kiyama M, Mitsumata S, Kadonosono T, Niwa H, Maki S, Kizaka-Kondoh S. Nat Commun. 2016;7:11856. doi: 10.1038/ncomms11856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woodroofe CC, Shultz JW, Wood MG, Osterman J, Cali JJ, Daily WJ, Meisenheimer PL, Klaubert DH. Biochemistry. 2008;47:10383. doi: 10.1021/bi800505u. [DOI] [PubMed] [Google Scholar]
- 5.Reddy GR, Thompson WC, Miller SC. J Am Chem Soc. 2010;132:13586. doi: 10.1021/ja104525m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jathoul AP, Grounds H, Anderson JC, Pule MA. Angew Chem Int Ed Engl. 2014;53:13059. doi: 10.1002/anie.201405955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takakura H, Kojima R, Kamiya M, Kobayashi E, Komatsu T, Ueno T, Terai T, Hanaoka K, Nagano T, Urano Y. J Am Chem Soc. 2015;137:4010. doi: 10.1021/ja511014w. [DOI] [PubMed] [Google Scholar]
- 8.Mofford DM, Adams ST, Reddy GSKK, Reddy GR, Miller SC. J Am Chem Soc. 2015;137:8684. doi: 10.1021/jacs.5b04357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heffern MC, Park HM, Au-Yeung HY, Van de Bittner GC, Ackerman CM, Stahl A, Chang CJ. Proc Natl Acad Sci U S A. 2016;113:14219. doi: 10.1073/pnas.1613628113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steinhardt RC, Rathbun CM, Krull BT, Yu JM, Yang Y, Nguyen BD, Kwon J, McCutcheon DC, Jones KA, Furche F, Prescher JA. Chembiochem. 2017;18:96. doi: 10.1002/cbic.201600564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grimm JB, Lavis LD. Org Lett. 2011;13:6354. doi: 10.1021/ol202618t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mofford DM, Reddy GR, Miller SC. J Am Chem Soc. 2014;136:13277. doi: 10.1021/ja505795s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grimm JB, English BP, Chen J, Slaughter JP, Zhang Z, Revyakin A, Patel R, Macklin JJ, Normanno D, Singer RH, Lionnet T, Lavis LD. Nat Methods. 2015;12:244. doi: 10.1038/nmeth.3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barr BK, Holewinski RJ. Biochemistry. 2002;41:4447. doi: 10.1021/bi015854q. [DOI] [PubMed] [Google Scholar]
- 15.Granzhan A, Ihmels H, Viola G. J Am Chem Soc. 2007;129:1254. doi: 10.1021/ja0668872. [DOI] [PubMed] [Google Scholar]
- 16.Hauser JR, Beard HA, Bayana ME, Jolley KE, Warriner SL, Bon RS. Beilstein J Org Chem. 2016;12:2019. doi: 10.3762/bjoc.12.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kranenburg M, van der Burgt YEM, Kamer PCJ, van Leeuwen PWNM, Goubitz K, Fraanje J. Organometallics. 1995;14:3081. [Google Scholar]
- 18.Yin J, Buchwald SL. J Am Chem Soc. 2002;124:6043. doi: 10.1021/ja012610k. [DOI] [PubMed] [Google Scholar]
- 19.White EH, Worther H, Seliger HH, McElroy WD. J Am Chem Soc. 1966;88:2015. [Google Scholar]
- 20.Ren H, Xiao F, Zhan K, Kim YP, Xie H, Xia Z, Rao J. Angew Chem Int Ed. 2009;48:9658. doi: 10.1002/anie.200903627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van de Bittner GC, Bertozzi CR, Chang CJ. J Am Chem Soc. 2013;135:1783. doi: 10.1021/ja309078t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Godinat A, Park HM, Miller SC, Cheng K, Hanahan D, Sanman LE, Bogyo M, Yu A, Nikitin GF, Stahl A, Dubikovskaya EA. ACS Chem Biol. 2013;8:987. doi: 10.1021/cb3007314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramil CP, An P, Yu Z, Lin Q. J Am Chem Soc. 2016;138:5499. doi: 10.1021/jacs.6b00982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harwood KR, Mofford DM, Reddy GR, Miller SC. Chem Biol. 2011;18:1649. doi: 10.1016/j.chembiol.2011.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Synthesis of 5a–8a has been reported via N-alkylation: Kakiuchi M, Ito S, Kiyama M, Goto F, Matsuhashi T, Yamaji M, Maki S, Hirano T. Chem Lett. 2017;46:1090.
- 26.Mofford DM, Reddy GR, Miller SC. Proc Natl Acad Sci. 2014;111:4443. doi: 10.1073/pnas.1319300111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adams ST, Mofford DM, Reddy GSKK, Miller SC. Angew Chem Int Ed. 2016;55:4943. doi: 10.1002/anie.201511350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jones KA, Porterfield WB, Rathbun CM, McCutcheon DC, Paley MA, Prescher JA. J Am Chem Soc. 2017;139:2351. doi: 10.1021/jacs.6b11737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kitamura N, Fukagawa T, Kohtani S, Kitoh S, Kunimoto KK, Nakagaki R. J Photochem Photobiol Chem. 2007;188:378. [Google Scholar]
- 30.Peng T, Chen X, Gao L, Zhang T, Wang W, Shen J, Yang D. Chem Sci. 2016;7:5407. doi: 10.1039/c6sc00012f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen C-T, Chiang C-L, Lin Y-C, Chan L-H, Huang Tsai Z-W, Chen C-T. Org Lett. 2003;5:1261. doi: 10.1021/ol034268h. [DOI] [PubMed] [Google Scholar]
- 32.Kosower EM. Acc Chem Res. 1982;15:259. [Google Scholar]
- 33.Ren W, Ji A, Karmach O, Carter DG, Martins-Green MM, Ai H. Analyst. 2016;141:3679. doi: 10.1039/c5an01860a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.