Abstract
Objective
In Vietnam, where 58% of prevalent HIV cases are attributed to PWID, we evaluated whether a multi-level intervention could improve care outcomes and increase survival.
Methods
We enrolled 455 HIV-infected male PWID from 32 communes in Thai Nguyen Province. Communes were randomized to a community stigma reduction intervention or standard of care and then within each commune, to an individual enhanced counseling intervention or standard of care, resulting in four arms: Arm 1 (standard of care); Arm 2 (community intervention alone); Arm 3 (individual intervention alone); and Arm 4 (community + individual interventions). Follow-up was conducted at 6, 12, 18, and 24 months to assess survival.
Results
Overall mortality was 23% (n = 103/455) over two years. There were no losses to follow-up for the mortality endpoint. Survival at 24-months was different across arms: Arm 4 (87%) vs Arm 1 (82%) vs Arm 2 (68%) vs Arm 3 (73%); log-rank test for comparison among arms: p=0.001. Among those with CD4 cell count <200 cells/mm3 and not on antiretroviral therapy (ART) at baseline (n=162), survival at 24 months was higher in Arm 4 (84%) compared to other arms (Arm 1: 61%; Arm 2: 50%; Arm 3: 53%; p-value=0.002). Overall, Arm 4 (community + individual interventions), increased uptake of ART compared to Arms 1, 2, and 3.
Conclusion
This multi-level behavioral intervention appeared to increase survival of HIV-infected participants over a two-year period. Relative to the standard of care, the greatest intervention effect was among those with lower CD4 cell counts.
Keywords: Vietnam, HIV, Behavioral Intervention, Stigma, Mortality, Intravenous Drug Use
Introduction
People who inject drugs (PWID) are at increased risk of mortality compared to their non-injecting peers, primarily due to blood borne infections and drug overdose.1,2 For HIV-infected PWID, crude mortality rates are 3 times higher than for HIV-uninfected PWID, underscoring the impact of delayed diagnosis and entry into antiretroviral therapy.3 HIV-infected PWID are less likely to be engaged in the HIV care continuum4 than other key populations. Globally, only 36% of PWID have received an HIV test and know their result,4 and approximately 4% of HIV-infected PWID receive antiretroviral therapy (ART).5 PWID initiate care at a later state of infection6,7 and experience poorer outcomes when ART initiation is delayed.8,9
In Vietnam, the HIV epidemic is concentrated primarily among PWID, who currently account for an estimated 58% of reported infections.10 As in many countries, injecting drug use is both criminalized and stigmatized in Vietnam. PWID encounter discrimination in health care settings11 and HIV stigma and social isolation present considerable barriers to access to care and support for HIV.12,13 We hypothesized that a multi-level intervention that combined an individual level component that provides support, risk-reduction skills, and resilience to stigma with a community-level component that aimed to reduce HIV and PWID-related stigma in the community would improve access to ART and reduce mortality compared to each component alone or the standard of care.
Methods
The study was approved by the ethical review committees at the Johns Hopkins Bloomberg School of Public Health and the Thai Nguyen Center for Preventive Medicine.
The study was conducted in Thai Nguyen, a mountainous province in northeast Vietnam located 50 miles from the capital, Hanoi. Thai Nguyen is close to the China border and has a tradition of opium cultivation and use. In 2009, at the start of this study, there were an estimated 6418 PWID14 in the province and a total of 7 outpatient antiretroviral treatment clinics and no MMT clinics. At the time of the study, ART eligibility criteria were having a CD4 cell count <200 cells/mm3 with HIV stage 1 and 2. PWID face severe social marginalization within families and communities,15,16 and they may be subject to compulsory detoxification and incarceration and are discriminated against in health care settings.11 As a result, they are difficult to reach for intervention and care and treatment programs17 and are underrepresented in ART clinics. The province consists of 180 communes and the average population in each commune is approximately 10,000.
Study design
Our multi-level intervention was evaluated using a four-arm randomized controlled trial: Arm 1: a standard of care condition; Arm 2: community-level stigma reduction programs; Arm 3: individual-level HIV post-test counseling and skill-building support groups; and Arm 4: both community and individual level activities. Details of the trial and our primary outcomes are reported elsewhere.18
Randomization
The study design was as follows: first, out of the 180 communes in Thai Nguyen, the 32 communes with the largest number of people who inject drugs were selected. The 32 communes were then partitioned into 9 groups (2 groups with 2 communes each, and 7 groups with 4 communes each) (Table 1), so that within each group the communes were similar in number of drug users and population. Within each group, a random half of the communes were selected to receive the community level stigma reduction and the remaining communes were selected to receive the standard of care. Within each commune, regardless of stigma reduction assignment, a random half of index participants were assigned to receive enhanced post-test HIV counseling and skill building and the other half received the standard of care.
Table 1.
Distribution of index participants in the matched communes and trial arms
| Matched Communes Group ID |
Commune ID | Community Intervention |
Individual Intervention |
Trial Arm Code* |
Number of Participants |
% of Total Participants |
|---|---|---|---|---|---|---|
| 1 | 2 | No | No | 1 | 5 | 1.1 |
| Yes | 3 | 5 | 1.1 | |||
| 1 | Yes | No | 2 | 8 | 1.76 | |
| Yes | 4 | 10 | 2.2 | |||
| 2 | 4 | No | No | 1 | 7 | 1.54 |
| Yes | 3 | 8 | 1.76 | |||
| 14 | No | 1 | 7 | 1.54 | ||
| Yes | 3 | 8 | 1.76 | |||
| 3 | Yes | No | 2 | 8 | 1.76 | |
| Yes | 4 | 8 | 1.76 | |||
| 13 | No | 2 | 4 | 0.88 | ||
| Yes | 4 | 3 | 0.66 | |||
| 3 | 6 | No | No | 1 | 10 | 2.2 |
| Yes | 3 | 7 | 1.54 | |||
| 12 | No | 1 | 6 | 1.32 | ||
| Yes | 3 | 8 | 1.76 | |||
| 5 | Yes | No | 2 | 5 | 1.1 | |
| Yes | 4 | 5 | 1.1 | |||
| 11 | No | 2 | 6 | 1.32 | ||
| Yes | 4 | 4 | 0.88 | |||
| 4 | 8 | No | No | 1 | 3 | 0.66 |
| Yes | 3 | 2 | 0.44 | |||
| 16 | No | 1 | 1 | 0.22 | ||
| Yes | 3 | 3 | 0.66 | |||
| 7 | Yes | No | 2 | 27 | 5.93 | |
| Yes | 4 | 32 | 7.03 | |||
| 15 | No | 2 | 5 | 1.1 | ||
| Yes | 4 | 1 | 0.22 | |||
| 5 | 10 | No | No | 1 | 3 | 0.66 |
| Yes | 3 | 4 | 0.88 | |||
| 20 | Yes | 3 | 3 | 0.66 | ||
| 9 | Yes | No | 2 | 20 | 4.4 | |
| Yes | 4 | 24 | 5.27 | |||
| 25 | No | 2 | 8 | 1.76 | ||
| Yes | 4 | 2 | 0.44 | |||
| 6 | 18 | No | Yes | 3 | 1 | 0.22 |
| 26 | No | 1 | 5 | 1.1 | ||
| Yes | 3 | 2 | 0.44 | |||
| 17 | Yes | No | 2 | 2 | 0.44 | |
| Yes | 4 | 1 | 0.22 | |||
| 27 | No | 2 | 5 | 1.1 | ||
| Yes | 4 | 5 | 1.1 | |||
| 7 | 28 | No | No | 1 | 6 | 1.32 |
| Yes | 3 | 6 | 1.32 | |||
| 30 | No | 1 | 2 | 0.44 | ||
| Yes | 3 | 5 | 1.1 | |||
| 19 | Yes | No | 2 | 12 | 2.64 | |
| Yes | 4 | 10 | 2.2 | |||
| 29 | No | 2 | 13 | 2.86 | ||
| Yes | 4 | 13 | 2.86 | |||
| 8 | 22 | No | No | 1 | 4 | 0.88 |
| Yes | 3 | 5 | 1.1 | |||
| 24 | No | 1 | 25 | 5.49 | ||
| Yes | 3 | 22 | 4.84 | |||
| 21 | Yes | No | 2 | 9 | 1.98 | |
| Yes | 4 | 8 | 1.76 | |||
| 23 | No | 2 | 6 | 1.32 | ||
| Yes | 4 | 4 | 0.88 | |||
| 9 | 32 | No | No | 1 | 5 | 1.1 |
| Yes | 3 | 6 | 1.32 | |||
| 31 | Yes | No | 2 | 1 | 0.22 | |
| Yes | 4 | 2 | 0.44 | |||
| Total | 455 | 100.00 | ||||
Trial Arm Code:
1=Standard of care; 2=Community intervention alone;
3=Individual intervention alone; 4=Community + Individual intervention
Participants
From July 2009–January 2011, index participants were recruited by a team of seven recruiters who themselves were former or current drug users. Using a snowball sampling technique, recruiters approached their current or former drug networks in a private place and distributed brochures and answered questions about the study. They then accompanied or referred subjects who were interested in participating to the project office to be screened for eligibility. Because almost all (97%) of PWID in Thai Nguyen are male (and female PWID typically have different risk factors19,20), our study focused on male drug injectors. To be eligible for our study, individuals had to have an HIV-positive diagnosis confirmed through testing by our study, be able and willing to bring in an injecting network member for screening, be male, be 18 years of age or older, have had sex in the previous six months, have injected drugs in the previous six months and have planned to be a resident in Thai Nguyen for the next 24 months. Individuals who were unwilling to provide locator information or were currently participating in other HIV interventions were excluded from the trial. Subsequently, recruited network partners were not included in this analysis as they were HIV-uninfected (as per study design).
Procedures
Participants received HIV testing and counseling and a face-to-face interview using a structured questionnaire at the screening visit. Two rapid HIV antibody tests were run simultaneously (Determine: Abbot Laboratories, Abbott Park, IL and Bioline: SD, Toronto Canada)21 and discordant results were resolved through a third rapid assay (HIV Rapid Test: ACON, San Diego, CA). Results were provided at the screening visit, but we enrolled participants at a later visit so that individuals would have the opportunity to process the results of their tests.
Index participants were asked to provide blood specimens at the 6, 12, 18 and 24 months follow-up visits in order to assess the stage of their HIV disease. Follow-up interviews, approximately an hour in length, were administered face-to-face in a private room at the project office by trained interviewers. Participants were reimbursed 75,000 Vietnamese Dong, equivalent to $3.50 USD, at each visit and 5000 Vietnamese Dong ($0.23 USD) for each kilometer traveled.
The intervention is described in detail elsewhere.18 Briefly, men randomized to the individual level standard of care arm received pre-test and post-test HIV and sexually transmitted diseases counseling and appropriate referrals. Men randomized to the individual level intervention arm received the following services: 1) two additional individual HIV posttest counseling sessions that included discussion about coping with stigma, social support, partner testing, and disclosure; 2) two small group sessions with other participants that focused on HIV knowledge, injecting and sexual risk reduction, and skill-building for coping with HIV infection and also provided social support through shared experiences of being an HIV-infected PWID. Participants were also offered an optional “person important to me” (PIM) session which focused on how the PIM could best support the participant in coping with HIV and reducing HIV risk behaviors. The individual level intervention was delivered over 5 weeks and builds on an overall staged sequential approach to behavior change for HIV-infected PWID.
Communes randomized to the standard of care arm received standard messages on HIV through village weekly public loudspeakers and educational pamphlets that were already being provided by community health stations.
Communes randomized to the intervention arm received community-wide programs that aimed to reduce community HIV and injection drug-related stigma by correcting misconceptions about HIV transmission, de-linking people living with HIV from “social evils” and promoting positive messages on HIV and drug use in the community. Specifically, two 2-session video-screenings were held in the intervention communes. These videos were developed by our team in collaboration with Johns Hopkins University’s Center for Communication Programs and were based on formative research conducted prior to the trial. The video was piloted with focus groups of community members in the province prior to use. Each video presentation was followed by a question-answer session on HIV with a trained facilitator. On average, approximately 44 community members attended the video screening. The program was supplemented by community outreach that took place throughout the study period. Three teams of community mobilization volunteers disseminated HIV information and answered questions through six rounds of a combination of one-on-one and group discussions in the community.18
Outcomes
During baseline and all follow-up visits index participants completed a face-to-face survey with an interviewer in a private room at the study office. Surveys collected information on age, education, marital status, employment status, sexual risk behaviors, injecting behaviors, and HIV and injection drug use-related stigma.
Additionally at each follow-up visit, each index participant was asked to provide blood specimens to assess CD4 cell count, and received a follow-up physical examination by the study physician.
Antiretroviral treatment
At each visit, participants were asked if they used ART in the six months prior to the interview. After the interview, they received a follow-up physical examination by the study physician where the physician asked about ART use in prior six months. A kappa statistic was calculated and indicated strong agreement (85.5%) between the two reports of ART status. For analyses, we used the physician-reported ART status; if physician-reported ART status was missing then self-reported ART status was used.
Mortality
All participants who missed a follow-up visit were contacted through study outreach workers, using contact information collected at baseline. During tracing procedures, family members of participants informed the study team if a participant died. We conducted a verbal autopsy during which we established date and reported cause of death. For analysis, “time of death” is the first scheduled follow-up visit after death.
Statistical analysis
Mortality and hazard of death rates were estimated by the Kaplan-Meier estimator and Nelson-Aalen estimator for each arm separately. Hazard rates across arms were compared using the log-rank statistic, whose significance level was obtained from its exact distribution over the permutation of the matched-cluster treatment assignment process22 as defined by the design above.
Mortality information was complete for all participants regardless of completion of visits, and so mortality analysis required no adjustment. Baseline antiretroviral treatment and CD4 cell count data were provided for 443 (97%) participants. For the analysis of hazard of death for participants who were not on ART throughout the study, at least one of the antiretroviral reports was not available for 194 participants. Antiretroviral treatment, whenever observed, was monotone across visits (either no use throughout, or complete uses, or initiating treatment) for 97% of participants. Missing treatment data were addressed with the technique of multiple imputation,23 which has recently been considered by a National Academy of Sciences report as among the best for treating missing data.24
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Table 2 describes baseline demographics and injecting and sex behaviors by arm. The mean age of all participants was 35.2 years, 47% were married and 70% were working full time. There were no significant differences between arms with respect to any demographic or risk characteristics.
Table 2.
Baseline characteristics of enrolled index participants (N=455), by trial arm
| Baseline Characteristic | Total N=455 |
Standard of Care (Arm 1) n=89 |
Community Intervention Alone (Arm 2) n=139 |
Individual Intervention Alone (Arm 3) n=95 |
Community + Individual Intervention (Arm 4) n=132 |
p-value* | |
|---|---|---|---|---|---|---|---|
| Age, in years: Mean (SD) | 35.2 (6.3) | 33.7 (5.0) | 35.4 (6.4) | 35.7 (6.5) | 35.7 (6.7) | 0.12 | |
| Years of education: Mean (SD) | 8.6 (2.9) | 8.1 (3.3) | 8.9 (2.8) | 8.5 (2.5) | 8.5 (3.1) | 0.44 | |
| Marital status: % | |||||||
| Single (never married) | 38 | 37 | 44 | 31 | 39 | 0.51 | |
| Married | 47 | 53 | 44 | 49 | 45 | ||
| Widowed/ divorced/ separated | 14 | 10 | 12 | 20 | 15 | ||
| Employment status: % | |||||||
| Work full-time (≥30 h/w) | 70 | 73 | 65 | 75 | 67 | 0.53 | |
| Work part-time (<30 h/w) | 18 | 11 | 21 | 18 | 20 | ||
| Unemployed/unable to work/other | 13 | 16 | 14 | 7 | 13 | ||
| Income per month (Vietnamese Dong, in millions): Mean (SD) |
1.8 (1.2) | 1.6 (0.9) | 1.7 (1.1) | 1.9 (1.4) | 1.9 (1.2) | 0.12 | |
| Religion: % No religion | 96 | 98 | 94 | 99 | 95 | 0.49 | |
| Ethnic group: % Kinh (ethnic Vietnamese) | 87 | 84 | 86 | 89 | 86 | 0.27 | |
| % Who have ever spent a night on the street, in a park, alley, or abandoned building, in the last 3 months? |
12 | 9 | 15 | 8 | 14 | 0.71 | |
| % Who injected daily, past 3 months | 54 | 40 | 48 | 43 | 48 | 0.48 | |
| % Who ever overdosed on drugs in lifetime? | 18 | 16 | 24 | 16 | 15 | 0.47 | |
| % Who were ever in drug treatment in lifetime | 31 | 24 | 33 | 29 | 36 | 0.54 | |
| % Who had ever been tested for HIV prior to baseline visit |
41 | 47 | 40 | 43 | 36 | 0.84 | |
| In general, how would you say your health is? | |||||||
| % Reporting poor health | 30 | 34 | 32 | 23 | 30 | 0.67 | |
| % Who have ever been incarcerated (put in prison, jail, or detention center) in lifetime |
36 | 34 | 43 | 27 | 35 | 0.09 | |
| In your opinion, are you stigmatized by your community because of your drug use? |
|||||||
| % Reporting yes | 75 | 74 | 75 | 74 | 76 | 0.98 | |
| CD4 cell count, cells/mm3: Mean (SD) | 272 (182) | 264 (161) | 254 (188) | 277 (176) | 292 (193) | 0.12 | |
| % Who were on ART | 13 | 18 | 13 | 15 | 8 | 0.013 | |
| % Who had at least 1 opportunistic infection | 58 | 61 | 57 | 53 | 60 | 0.63 | |
P-values are based on Wald Chi-squares with 3 df, accounting for clustering on commune matched groups.
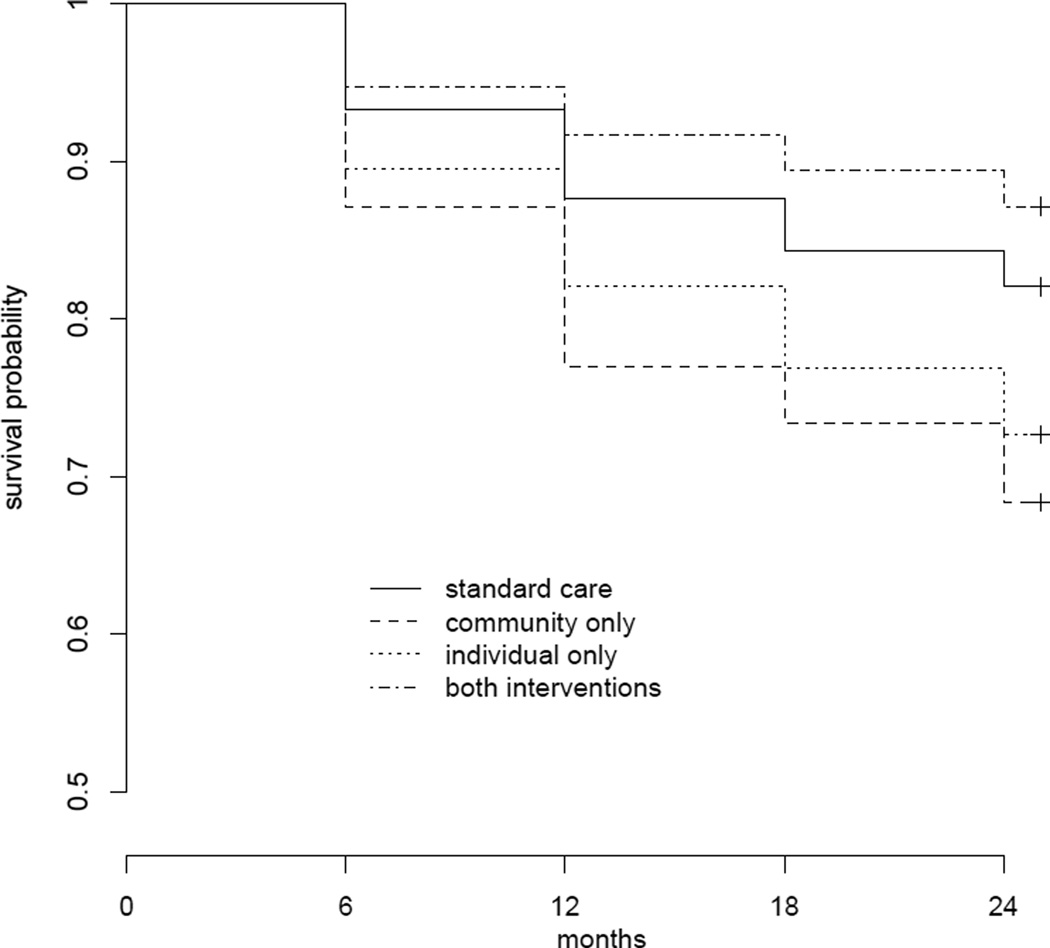
The overall mortality was 23% (n = 103/455) over a two-year period. Causes of mortality were HIV-related [TB, bacterial infections, malignant neoplasm, unspecified malignancy, wasting syndromes, or other infections stemming from HIV] (72%), drug overdose (11%), suicide (5%), and injury (3%). There was no loss to follow-up for the mortality endpoint. There is a statistically significant difference between Arm 1 (standard of care) versus Arm 2 (community alone) versus Arm 3 (individual alone) versus Arm 4 (community + individual) (p = 0.001) (Figure 1; Table 3a).
Figure 1.
Kaplan-Meier plots of survival probability across the study follow-up for the four trial arms
Table 3.
| a. Differences in cumulative all-cause mortality among all index participants, over 24 months, across trial arms | |||||
|---|---|---|---|---|---|
| n | 6 months % (n) died |
12 months Cumulative % (n) died |
18 months Cumulative % (n) died |
24 months Cumulative % (n) died |
|
| Arm 1: Standard of care | 89 | 7% (6) | 12% (11) | 16% (14) | 18% (16) |
| Arm 2: Community alone | 139 | 13% (18) | 23% (32) | 27% (37) | 32% (44) |
| Arm 3: Individual alone | 95 | 11% (10) | 18% (17) | 23% (22) | 27% (26) |
| Arm 4: Community + Individual | 132 | 5% (7) | 8% (11) | 10% (14) | 13% (17) |
| Comparison: log rank (df), p-value | |||||
| Any difference between arms | 16.4 (3), 0.001 | ||||
| b. Differences in cumulative all-cause mortality among index participants who had CD4 cell count <200 cells/mm3 and were not on ART at baseline, over 24 months, across trial arms | |||||
|---|---|---|---|---|---|
| n | 6 months % (n) died |
12 months Cumulative % (n) died |
18 months Cumulative % (n) died |
24 months Cumulative % (n) died |
|
| Arm 1: Standard of care | 31 | 19% (6) | 26% (8) | 32% (10) | 39% (12) |
| Arm 2: Community alone | 56 | 25% (14) | 39% (22) | 45% (25) | 50% (28) |
| Arm 3: Individual alone | 30 | 20% (6) | 32% (10) | 43% (13) | 47% (14) |
| Arm 4: Community + Individual | 45 | 7% (3) | 9% (4) | 11% (5) | 16% (7) |
| Comparison: log rank (df), p-value | |||||
| Any difference between arms | 11.6 (3), 0.002 | ||||
| Arm 1 vs. Arm 4 | 4.9 (1), 0.008 | ||||
| Arm 1 vs. Arms 2 and 3 | 0.9 (1), 0.296 | ||||
In order to understand the factors driving this difference, we looked at mortality across arms within three strata: those on ART at baseline, those eligible for ART (CD4 cell count <200 cells/mm3) but not on ART at baseline, and those not eligible and not on ART at baseline. We see no difference in mortality across arms for those on ART or those not eligible and not on ART. However, Arm 4 (community + individual) has statistically significantly lower mortality than Arm 1 (standard of care) for those eligible but not on ART at baseline (p=0.008) (Table 3b). In summary, for participants who are eligible but not on ART at baseline, the combined intervention (Arm 4 community + individual) increases survival compared to the standard of care arm.
Tables 4 and 5 investigate whether the mortality difference across arms in Tables 3a and 3b is accounted for by higher uptake of ART (Table 4) and/or if the mortality difference across arms exists also among those not taking ART (Table 5). In Table 4, the 38% transition probability tabulated for Arm 1 (standard of care) at 6 months is the fraction of individuals who start ART at 6 months among those who were alive at that visit and had not been taking ART up to the 6 month visit, among individuals who have CD4 cell count <200 cells/mm3 at baseline. Transition probabilities were not statistically significantly different based on a comparison of all four arms (p=0.158), although Arm 4 (community + individual) was statistically significantly different in comparison to the other three arms (p=0.033).
Table 4.
Differences in the transition probability of initiating ART among index participants who had CD4 cell count <200 cells/mm3 and were not on ART at baseline, over 24 months, across trial arms
| 6 months % Initiated ART (n) |
12 months % Initiated ART (n) |
18 months % Initiated ART (n) |
24 months % Initiated ART (n) |
|
|---|---|---|---|---|
| Arm 1: Standard of care | 38% (10) | 32% (4) | 32% (2) | 68% (3) |
| Arm 2: Community alone | 50% (21) | 43% (6) | 9% (1) | 20% (1) |
| Arm 3: Individual alone | 33% (8) | 46% (6) | 20% (1) | 40% (1) |
| Arm 4: Community + Individual | 59% (25) | 44% (7) | 60% (6) | 43% (1) |
| Comparison: log rank (df), p-value | ||||
| Any difference between arms | 5.19 (3), 0.158 | |||
| Arm 4 vs. Other Arms | 4.56 (1), 0.033 | |||
Table 5.
Hazard of death at each follow-up visit among index participants who had not been on ART prior to that visit and had CD4 cell count <200 cells/mm3 at baseline, by trial arm
| 6 months | 12 months | 18 months | 24 months | |
|---|---|---|---|---|
| Arm 1: Standard of care | 19% (6) | 10% (2) | 21% (2) | 12% (1) |
| Arm 2: Community alone | 25% (14) | 29% (6) | 23% (2) | 50% (3) |
| Arm 3: Individual alone | 20% (6) | 25% (4) | 24% (2) | 25% (1) |
| Arm 4: Community + Individual | 7% (3) | 0% (0) | 0% (0) | 26% (1) |
| Comparison: log rank (df), p-value | ||||
| Any difference between arms | 11.1 (3), 0.001 | |||
In Table 5, the percent tabulated at each visit (e.g., 24% for Arm 3 at 18 months) among those not on ART prior to that visit and with baseline CD4 cell count <200 cells/mm3, is the fraction of the individuals who died since the prior (12 month) visit among all individuals in the stratum who were alive and not on treatment at the previous visit. Hazard rates of death accumulating information across all visits are lower for Arm 4 (community + individual) compared to the other arms (p=0.001 for comparison among all arms) for individuals who were not on ART.
Discussion
Despite the high mortality rates of HIV-infected PWID, few interventions have focused on improving health outcomes in this population,25,26 and to our knowledge, this is the first study to look at the effect of a multi-level intervention on preventing mortality among HIV-infected PWID. The multi-level behavioral intervention appeared to increase survival over a two-year period. Relative to the standard of care, the greatest intervention effect was among those with lower CD4 cell counts, where starting antiretroviral treatment is most advantageous.
Specifically, Table 3a shows an overall effect of the interventions on mortality for all index participants comparing all 4 arms (Arm 1 versus Arm 2 versus Arm 3 versus Arm 4). We then conducted further analysis to understand the differences between arms. We found that among those who had baseline CD4 cell count ≥200 cells/mm3 (and therefore did not meet eligibility criteria for ART at the time), there was not a statistically significant difference in mortality between arms (logrank = 7.1, 3 df, p-value = 0.077, data not shown). Furthermore, among those who had a CD4 cell count <200 cells/mm3 and were on ART at baseline, there were no statistically significant differences in mortality between arms (logrank = 0.11, 3 df, p-value = 0.99, data not shown).
Among those with a CD4 cell count below 200 cells/mm3 and who were not on ART at baseline, the higher mortality in Arm 2 (community alone) and Arm 3 (individual alone) is not significant compared to Arm 1 (standard of care), whereas the difference between Arm 1 (standard of care) and Arm 4 (community + individual) is statistically significant. Although not statistically significant, Arms 2 and 3 did have a higher mortality than the standard of care arm. This may be because individuals in Arm 2 (community alone) and Arm 3 (individual alone) perceived they were receiving care and/or support, but did not have either sufficient skills and/or community support to obtain and maintain HIV medical care. The perception that they received valuable services from the intervention may have made them less motivated to seek medical care.
Globally, drug users have poor engagement in every step of the HIV care continuum. In our study, those in the community + individual arm (Arm 4) were more likely to initiate ART, and subsequent to ART initiation there was no difference in mortality among arms. This finding is similar to other studies that show that initiation of ART is associated with survival both among HIV-infected PWID and people who live with HIV more generally.27–30 Uptake of ART in the combined community + individual intervention group occurred more rapidly with increased probability of initiating ART within the initial six months. In addition, the results in Table 5 suggest that the combined intervention lowered mortality through pathways other than ART for those with CD4 cell count <200 cells/mm3 at baseline. Specifically, among those who were ART-free prior to a particular visit, participants in the community + individual arm also had a significantly lower mortality rate over the 24-month period of observation. Similar to the uptake of HIV treatment, this difference was observed even in the first six months suggesting that the intervention had an almost immediate effect on survival. Our findings indicate that enhanced individualized counseling and small group support sessions should be conducted within a broader supportive community environment. The synergistic effect of individual and community level programs may have enhanced self-efficacy and response efficacy for participants to navigate the HIV care system while simultaneously alleviating fear both of being exposed as HIV-infected and of subsequent stigmatization.
Our intervention addressed HIV risk, coping, stigma, and social support at the individual and community levels among highly marginalized HIV-infected PWID who have poor physical health, are more likely to experience social disadvantage, and are more likely to engage in risky behaviors. In order to understand other possible pathways, in addition to antiretroviral therapy, through which mortality decreased in the community + individual arm, we conducted sub-analyses to assess potential mediators between intervention arm and mortality, including self-reported overdose, social support, symptoms of depression, visits to HIV providers, and physician-reported presence of opportunistic infections. None of the variables we assessed were statistically significant mediators, suggesting that variables affecting mortality excluding antiretroviral treatment uptake, may not have been adequately measured in our study. For example, participants in the community + individual arm may have had more self-efficacy or community support and been more likely to see a general physician for opportunistic infections. In addition, participants in the community + individual arm may have reduced risk-taking behaviors and thereby reduced HIV and/or violence/injury-related mortality.
The study was conducted in a context where study conditions could not be tightly controlled for self-reporting bias, attrition, secular trends, historic factors, and contamination. Our previous randomized controlled trial investigated the possible presence of these conditions and did not find evidence that they influenced results.31 However, the chance that any of these factors may be operating cannot be ruled out. Given the high mobility of this population, 44% of participants missed at least one of the four follow-up visits, which is a limitation to our mediation analyses. Incarceration among this population was not uncommon. Overall 158 participants dropped out of the study. Of those, 37 (23%) were incarcerated, while 103 died and 18 dropped out for other reasons. A total of 58 participants were incarcerated during the study period, and of those, 37 (63.7%) dropped out of the study due to incarceration. However, the rate of incarceration did not differ by study arm and is therefore is unlikely to have affected the association between the interventions and mortality. Overall mortality includes mortality that may not be HIV or drug related (in this study, 12%) and therefore might not have been in the path that can be affected by the intervention. Methadone Maintenance Therapy (MMT) was introduced to Thai Nguyen on March 2012. We do not have data on how many participants enrolled in MMT during the last year of the study. MMT may have reduced mortality by preventing drug overdose; however, similar to incarceration, we would not expect there to be a difference in enrollment into MMT by study arm. In addition, several potential mediators, including overdose, social support, and symptoms of depression, were self-reported and social desirability bias may have contributed to our results.
Despite these limitations, our study demonstrates that a multi-level intervention may be effective in increasing survival among HIV-infected PWID. The intervention was well attended by index participants, with 83% attending all sessions at each level. It is important to note that since the completion of the study, there has been a dramatic change in the availability of ART and MMT. ART eligibility guidelines in Vietnam changed in December 2014, to include ART for HIV-infected individuals in mountainous regions and high risk groups (including PWID), regardless of CD4 cell count, and among all other HIV-infected persons, ART for those with CD4 cell count <500 cells/mm3. There are currently 3546 patients on ART in 10 ART clinics in Thai Nguyen province. In addition, the first MMT clinic opened in Thai Nguyen in 2011, during our study. There are currently 6 MMT clinics in Thai Nguyen, with 1729 patients on MMT. Increased access to ART and MMT should in theory, compound the effect of our intervention. In order understand the applicability of this intervention to other settings, this intervention may need to be evaluated in settings where social norms may play a less influential role on individual behaviors. This intervention is relatively intense, requiring two individual post-test counseling sessions, two small support group sessions, and a community-wide video and discussions. To assess the scalability of this program, it may be necessary to consider the cost-effectiveness of this intervention and to explore the minimum intervention dose needed to reduce mortality. Overall, our results suggest the importance of intervening on social and individual factors simultaneously.
Acknowledgments
This study was funded by the National Institute on Drug Abuse (1R01-DA022962). CZ was partly supported by the Johns Hopkins University Center for AIDS Research (P30AI094189).
Footnotes
Contributions: VFG and CF prepared the first draft. VFG finalized the draft based on comments from other authors. VFG, CF, NLM, CAL, DDC and VMQ conceived of the study and provided overall guidance. CF and TS performed final statistical analyses. All other authors contributed to data collection, interpreted data, reviewed results, provided guidance on methodology and reviewed the manuscript.
References
- 1.Darke S, Degenhardt L, Mattick RP. Mortality amongst illicit drug users. Cambridge: Cambridge University Press; 2006. [Google Scholar]
- 2.May MT, Justice AC, Birnie K, et al. Injection drug use and Hepatitis C as risk factors for mortality in HIV-infected individuals: the Antiretroviral Therapy Cohort Collaboration. J Acquir Immune Defic Syndr. 2015 doi: 10.1097/QAI.0000000000000603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mathers BM, Degenhardt L, Bucello C, et al. Mortality among people who inject drugs: a systematic review and meta-analysis. Bull World Health Organ. 2013;91(2):102–123. doi: 10.2471/BLT.12.108282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.UNODC. [Accessed on: April 2, 2015];United Nations Office on Drugs and Crime, World Drug Report 2014 (United Nations publication, Sales No. E.14.XI.7) 2014 Available at: http://www.unodc.org/documents/wdr2014/World_Drug_Report_2014_web.pdf.
- 5.Mathers BM, Degenhardt L, Ali H, et al. HIV prevention, treatment, and care services for people who inject drugs: a systematic review of global, regional, and national coverage. Lancet. 2010;375(9719):1014–1028. doi: 10.1016/S0140-6736(10)60232-2. [DOI] [PubMed] [Google Scholar]
- 6.Celentano DD, Galai N, Sethi AK, et al. Time to initiating highly active antiretroviral therapy among HIV-infected injection drug users. AIDS. 2001;15(13):1707–1715. doi: 10.1097/00002030-200109070-00015. [DOI] [PubMed] [Google Scholar]
- 7.Wang C, Vlahov D, Galai N, et al. Mortality in HIV-seropositive versus -seronegative persons in the era of highly active antiretroviral therapy: implications for when to initiate therapy. J Infect Dis. 2004;190(6):1046–1054. doi: 10.1086/422848. [DOI] [PubMed] [Google Scholar]
- 8.Celentano DD, Lucas G. Optimizing treatment outcomes in HIV-infected patients with substance abuse issues. Clin Infect Dis. 2007;45(Suppl 4):S318–S323. doi: 10.1086/522557. [DOI] [PubMed] [Google Scholar]
- 9.Rodriguez-Arenas MA, Jarrin I, del Amo J, et al. Delay in the initiation of HAART, poorer virological response, and higher mortality among HIV-infected injecting drug users in Spain. AIDS Res Hum Retroviruses. 2006;22(8):715–723. doi: 10.1089/aid.2006.22.715. [DOI] [PubMed] [Google Scholar]
- 10.Petersen Z, Myers B, van Hout MC, et al. Availability of HIV prevention and treatment services for people who inject drugs: findings from 21 countries. Harm Reduct J. 2013;10:13. doi: 10.1186/1477-7517-10-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khoat DV, Hong LD, An CQ, et al. A situational analysis of HIV/AIDS-related discrimination in Hanoi, Vietnam. AIDS Care. 2005;17(Suppl 2):S181–S193. doi: 10.1080/09540120500119940. [DOI] [PubMed] [Google Scholar]
- 12.Rudolph AE, Davis WW, Quan VM, et al. Perceptions of community- and family-level injection drug user (IDU)- and HIV-related stigma, disclosure decisions and experiences with layered stigma among HIV-positive IDUs in Vietnam. AIDS Care. 2012;24(2):239–244. doi: 10.1080/09540121.2011.596517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salter ML, Go VF, Minh NL, et al. Influence of Perceived Secondary Stigma and Family on the Response to HIV Infection Among Injection Drug Users in Vietnam. AIDS Educ Prev. 2010;22(6):558–570. doi: 10.1521/aeap.2010.22.6.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thai Nguyen Provincial AIDS Center, Division of Social Evils Control and Prevention. Quarterly Report of the Statistics Office of Thai Nguyen Province, Thai Nguyen Provincial AIDS Center and the Division of Social Evils Control and Prevention, Department of Labor, Invalids and Social Affairs of Thai Nguyen. 2007. [Google Scholar]
- 15.Hong KT, Anh NTV, Oanh KTH, et al. Understanding and challenging stigma toward injecting drug users and HIV in Vietnam: Toolkit for Action. Washington, DC: International Center for Research on Women; 2011. [Google Scholar]
- 16.UN Country Team. Reduction of HIV/AIDS related employment discrimination in Viet Nam, Discussion Paper No. 5 Ha Noi. 2005. [Google Scholar]
- 17.Nguyen TH, Nguyen TL, Trinh QH. HIV/AIDS epidemics in Vietnam: evolution and responses. AIDS Educ Prev. 2004;16(3 Suppl A):137–154. doi: 10.1521/aeap.16.3.5.137.35527. [DOI] [PubMed] [Google Scholar]
- 18.Go VF, Frangakis C, Minh NL, et al. Efficacy of a Multi-level Intervention to Reduce Injecting and Sexual Risk Behaviors among HIV-Infected People Who Inject Drugs in Vietnam: A Four-Arm Randomized Controlled Trial. PLoS One. 2015;10(5):e0125909. doi: 10.1371/journal.pone.0125909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Do K, Minichiello V, Hussain R. HIV risks among injecting drug users in Vietnam: a review of the research evidence. Curr HIV Res. 2012;10(6):479–486. doi: 10.2174/157016212802429767. [DOI] [PubMed] [Google Scholar]
- 20.Khuat OT, Morrow M, Nguyen TN, et al. Social context, diversity and risk among women who inject drugs in Vietnam: descriptive findings from a cross-sectional survey. Harm Reduct J. 2015;12:35. doi: 10.1186/s12954-015-0067-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koblavi-Deme S, Maurice C, Yavo D, et al. Sensitivity and specificity of human immunodeficiency virus rapid serologic assays and testing algorithms in an antenatal clinic in Abidjan, Ivory Coast. J Clin Microbiol. 2001;39(5):1808–1812. doi: 10.1128/JCM.39.5.1808-1812.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu Z, Frangakis CE, Louis TA, et al. Estimation of treatment effects in matched-pair cluster randomized trials by calibrating covariate imbalance between clusters. Biometrics. 2014;70(4):1014–1022. doi: 10.1111/biom.12214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rubin DB. Multiple Imputation for Nonresponse in Surveys. John Wiley & Sons; 2009. [Google Scholar]
- 24.Little RJ, D'Agostino R, Cohen ML, et al. The prevention and treatment of missing data in clinical trials. N Engl J Med. 2012;367(14):1355–1360. doi: 10.1056/NEJMsr1203730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang N, Sun X, Yin L, et al. Meta-Analysis of Interventions for Reducing Number of Sexual Partners and Drug and Alcohol Abuse among People Living with HIV/AIDS. J AIDS Clin Res. 2013;4 doi: 10.4172/2155-6113.1000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Purcell DW, Latka MH, Metsch LR, et al. Results from a randomized controlled trial of a peer-mentoring intervention to reduce HIV transmission and increase access to care and adherence to HIV medications among HIV-seropositive injection drug users. J Acquir Immune Defic Syndr. 2007;46(Suppl 2):S35–S47. doi: 10.1097/QAI.0b013e31815767c4. [DOI] [PubMed] [Google Scholar]
- 27.Hammer SM, Eron JJ, Jr, Reiss P, et al. Antiretroviral treatment of adult HIV infection: 2008 recommendations of the International AIDS Society-USA panel. JAMA. 2008;300(5):555–570. doi: 10.1001/jama.300.5.555. [DOI] [PubMed] [Google Scholar]
- 28.Hogg RS, Heath KV, Yip B, et al. Improved survival among HIV-infected individuals following initiation of antiretroviral therapy. JAMA. 1998;279(6):450–454. doi: 10.1001/jama.279.6.450. [DOI] [PubMed] [Google Scholar]
- 29.Sterne JA, May M, Costagliola D, et al. Timing of initiation of antiretroviral therapy in AIDS-free HIV-1-infected patients: a collaborative analysis of 18 HIV cohort studies. Lancet. 2009;373(9672):1352–1363. doi: 10.1016/S0140-6736(09)60612-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wood E, Hogg RS, Lima VD, et al. Highly active antiretroviral therapy and survival in HIV-infected injection drug users. JAMA. 2008;300(5):550–554. doi: 10.1001/jama.300.5.550. [DOI] [PubMed] [Google Scholar]
- 31.Go VF, Frangakis C, Le Minh N, et al. Effects of an HIV peer prevention intervention on sexual and injecting risk behaviors among injecting drug users and their risk partners in Thai Nguyen, Vietnam: a randomized controlled trial. Soc Sci Med. 2013;96:154–164. doi: 10.1016/j.socscimed.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]