Abstract
Background
Bipolar voltage mapping, as part of atrial fibrillation (AF) ablation, is traditionally performed in a point-by-point (PBP) approach using single-tip ablation catheters. Alternative techniques for fibrosis-delineation include fast-anatomical mapping (FAM) with multi-electrode circular catheters, and late gadolinium-enhanced magnetic-resonance imaging (LGE-MRI). The correlation between PBP, FAM, and LGE-MRI fibrosis assessment is unknown.
Objective
In this study, we examined AF substrate using different modalities (PBP, FAM, and LGE-MRI mapping) in patients presenting for an AF ablation.
Methods
LGE-MRI was performed pre-ablation in 26 patients (73% males, age 63±8years). Local image-intensity ratio (IIR) was used to normalize myocardial intensities. PBP- and FAM-voltage maps were acquired, in sinus rhythm, prior to ablation and co-registered to LGE-MRI.
Results
Mean bipolar voltage for all 19,087 FAM voltage points was 0.88±1.27mV and average IIR was 1.08±0.18. In an adjusted mixed-effects model, each unit increase in local IIR was associated with 57% decrease in bipolar voltage (p<0.0001). IIR of >0.74 corresponded to bipolar voltage <0.5 mV. A total of 1554 PBP-mapping points were matched to the nearest FAM-point. In an adjusted mixed-effects model, log-FAM bipolar voltage was significantly associated with log-PBP bipolar voltage (ß=0.36, p<0.0001). At low-voltages, FAM-mapping distribution was shifted to the left compared to PBP-mapping; at intermediate voltages, FAM and PBP voltages were overlapping; and at high voltages, FAM exceeded PBP-voltages.
Conclusion
LGE-MRI, FAM and PBP-mapping show good correlation in delineating electro-anatomical AF substrate. Each approach has fundamental technical characteristics, the awareness of which allows proper assessment of atrial fibrosis.
Keywords: Atrial Fibrillation, Magnetic Resonance Imaging, Voltage Mapping, Substrate, Fibrosis
INTRODUCTION
Pulmonary vein isolation (PVI) by radiofrequency ablation (RF) has emerged as an effective treatment of AF by interrupting signal conduction arising from ectopic triggers in the pulmonary veins to the left atrial (LA) myocardium (1). Atrial myocardial substrate including areas with low bipolar voltage, shortened effective refractory periods, and conduction heterogeneity all likely play a role in AF perpetuation (2–4). These regional disparities in myocardial properties are thought to support re-entry and triggered activity in the atrium (5). Assessment of atrial fibrosis burden is increasingly used for prognostication and to inform ablation strategies (9–11).
Initial studies examining low-voltage substrate and its correlation with late-gadolinium enhancement magnetic resonance imaging (LGE-MRI) used a point-by-point (PBP) mapping approach using ablation catheters (9, 10). Although this approach offers the advantage of confirming tissue contact using force-sensing catheters, it is time-consuming. An alternative is fast-anatomical mapping (FAM) using decapolar circular-mapping catheters (CARTO LASSO 2515 Variable Loop Eco Nav, Biosense Webster, Diamond Bar, CA) with smaller electrode size that can acquire highly detailed (>1000 points) voltage maps in a significantly shorter time. However, the correlation of LA-fibrosis detected with LGE-MRI, PBP- and FAM-mapping is unknown. Voltage correlation between these techniques has not been established despite the use, in clinical practice, of common voltage thresholds (0.2-0.5mV) to define diseased myocardium irrespective of the mapping technique. In this study, we sought to examine and compare AF substrate characteristics using different modalities (LGE-MRI, FAM and PBP-mapping) in patients presenting for an AF ablation.
METHODS
PATIENT POPULATION
Twenty-six patients with symptomatic, drug-refractory paroxysmal or persistent AF referred for AF ablation underwent pre-procedural MRI between June 2016 and December 2016. The Johns Hopkins Institutional Review Board approved the study and all patients provided written informed consent for both the ablation procedure as well as inclusion in medical research at the time of the procedure.
MRI ACQUISITION
Images were obtained using a 1.5 Tesla MRI scanner (Avanto, Siemens, Erlangen, Germany) and a 6-channel phased array body coil in combination with a 6-channel spine matrix coil. Contrast enhanced three-dimensional fast low angle shot MR angiography sequences, obtained immediately following intravenous administration of 0.1mmol/kg of contrast, were used to define LA and pulmonary vein (PV) anatomy (echo time 0.8ms, repetition time 2.2ms, in-plane resolution 1.4 × 1.4mm, slice thickness 1.4mm). To optimize image quality, patients with persistent AF were kept on anti-arrhythmic medications and/or referred for cardioversion prior to MRI. LGE-MRI scans were acquired within a range of 15-25 (mean 18.8±2.4) minutes following 0.2mmol/kg gadolinium injection (gadopentetate dimeglumine; Bayer Healthcare Pharmaceuticals, Montville, NJ) using a fat-saturated 3D IR-prepared fast spoiled gradient recalled echo sequence with respiratory navigation and ECG-gating, echo time of 1.52ms, repetition time of 3.8ms, in-plane resolution of 1.3 × 1.3, slice thickness of 2.0 mm, and flip angle of 10 degrees. Trigger time for 3D LGE-MRI images was optimized to acquire data during LA diastole. The optimal inversion time (TI) was identified with a TI scout scan (median 270ms, range 240-290ms) to maximize nulling of LA myocardium. A parallel imaging technique, Generalized Auto-calibrating Partially Parallel Acquisition (reduction factor 2), was used.
IMAGE ANALYSIS
Pre-ablation LGE-MRI images were processed off-line on QMass MR software (version 7.4, Medis, Leiden, The Netherlands). Epicardial and endocardial contours were manually drawn around LA myocardium. The reference point was placed at the anterior base of the LA septum, and the LA myocardium in each axial plane was divided into 25 sectors as seen in Figure 1. The image intensity ratio (IIR), a previously described LGE-MRI technique that normalizes mean myocardial intensities by mean intensity of the entire blood pool, was calculated for each sector. The methodology for calculation of the IIR and its association with regional voltage by electro-anatomic mapping were previously validated (9, 11). Inter- and intra-observer variability of our LA analysis was previously assessed and found to be excellent (9).
Figure 1.
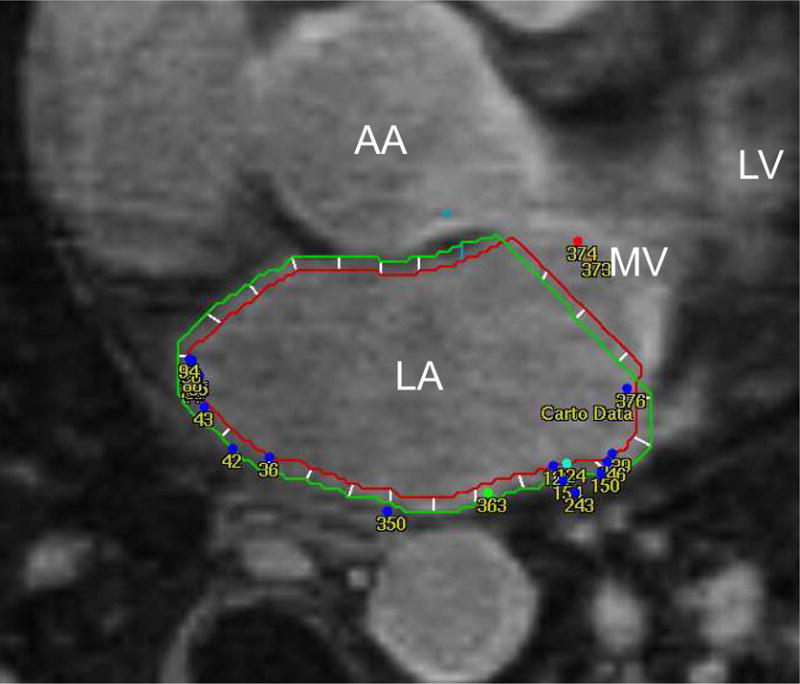
Endo- and epicardial contours are manually drawn on axial LGE-MRI images. Fast-anatomical-mapping points are co-registered and projected to LGE-MRI. Bipolar voltage of each point is correlated to the image intensity ratio of the nearest segment. LA: left atrium; LGE-MRI: late gadolinium enhanced magnetic resonance imaging; MV: mitral valve; LV: left ventricle; AA: ascending aorta.
MAPPING AND ABLATION PROCEDURE
A double trans-atrial septal puncture was performed under fluoroscopic guidance. Intravenous heparin was administered to achieve an activated clotting time >350 seconds. With hemodynamic and electrocardiographic monitoring, a 3.5-mm irrigated tip with 2-mm inter-electrode spacing ablation catheter (Thermocool Smarttouch, Biosense Webster, Diamond Bar, CA) and a decapolar circular mapping catheter, with 25-15 loop diameter, 8mm inter-electrode spacing (LASSO 2515 Variable Loop Eco Nav, Biosense Webster, Diamond Bar, CA) were advanced under fluoroscopic guidance to the LA. In patients presenting in AF on the day of the procedure, a cardioversion was performed prior to image registration and electro-anatomical mapping to minimize patient movement and registration errors. If sinus rhythm could not be restored, patients were excluded from analysis. Mapping was performed with an equal distribution of points using a fill-threshold of 15 mm excluding PV ostia. A target of >100 PBP-mapping points and >1,000 FAM-mapping points was set by consensus at study onset but the extent of mapping was ultimately left to the operator’s discretion. In 19 (73%) patients, PBP mapping was performed initially with the ThermoCool-SmartTouch ablation catheter and the CARTO3 mapping system (Biosense Webster, Inc, Diamond Bar, CA). In those patients, target contact force>10 g was adopted when obtaining points in order to ensure adequate tissue–catheter contact. The decision to undergo point-by-point mapping was left to the operator’s discretion. LA magnetic resonance angiography (MRA) image was registered to the voltage map by using standard landmark image registration techniques. A detailed FAM map of the entire LA was also created with the LassoNav catheter and superimposed on pre-existing MR images. An internal point filter was used to limit data acquisition to within 5 mm from the anatomical shell in order to reduce mapping in the blood pool. ConfiDENSE module, which utilizes impedance measurement to determine tissue proximity for each electrode, was used to assess electrode-tissue contact during FAM. Intra-cardiac echocardiography, orthogonal fluoroscopy, and electrogram characteristics were also utilized to monitor for adequate contact.
After voltage mapping was complete, circumferential lesions were applied surrounding the PV. If conduction from PV to LA persisted despite PVI, additional lesions were delivered along the original ablation line at sites of earliest activation on the circular mapping catheter. Entrance block into the pulmonary veins was confirmed in all patients as the primary procedural endpoint. To prevent short-term recurrences of AF, pre-procedural antiarrhythmic medications were continued for at least 3 months. No immediate postoperative complications were reported.
ABLATION MAP REGISTRATION
FAM-derived voltage maps were registered offline to pre-ablation LGE images using MASS Research Software (Leiden University Medical Center, Leiden, The Netherlands). Mapping points were extracted from the CARTO workstation and imported into LGE-MR images using the registration matrix defined during the procedure (Figure 1). An intermediate registration step, performed on ParaView (Kitware, Clifton Park, NY), was needed to reconcile the CARTO coordinate system to the MR coordinate system. Mean IIR of the neighboring sector to each mapping point was recorded to evaluate the correlation between bipolar voltage and signal intensity.
FAM and PBP VOLTAGE COMPARISON
Each of the points acquired during FAM and PBP mapping have coordinates (x, y, z). For each PBP point, the distances between this point and all FAM points were calculated using a custom written python script. PBP points were matched in a 1:1 ratio with FAM points using the shortest orthogonal distance.
STATISTICAL ANALYSIS
Continuous variables were expressed as mean ± SD. Categorical variables were expressed as number (percentage). The unadjusted relationship between IIR and bipolar voltage was initially explored with Scatter-plots. Due to the skewed nature of bipolar voltage measures, log-transformation was utilized to accommodate modeling within a linear normal framework. A Generalized Estimating Equations (GEE) marginal model with exchangeable working correlation structure was utilized to examine the association between bipolar voltage and IIR. The model was clustered by patient, and adjusted for age, LA end-diastolic area, gender, BMI, CHA2DS2-VASc score, AF type, AF duration, left ventricular ejection fraction (LV-EF), and history of previous ablation. Similarly, the correlation between the log transformed PBP voltage and FAM voltage was assessed using an adjusted, patient-clustered GEE marginal model with exchangeable working correlation structure. The receiver-operator characteristic was used to assess the sensitivity and specificity of FAM voltage with PBP-derived fibrosis. Two-sided P values less than 0.05 were considered statistically significant. Statistical analysis was performed using STATA software (version 13, StataCorp, College Station, TX).
RESULTS
PATIENT CHARACTERISTICS
We prospectively enrolled 26 patients (19[73%] males, mean age of 63.1±7.9 years)). Table 1 summarizes the baseline characteristics of patients. Fourteen (54%) patients had paroxysmal AF, and 9(35%) were undergoing their first ablation procedure. Mean BMI was 27.2±3.9 kg/m2 and the median CHA2DS2-VASc score was 2 (IQR 1–2). Patients with persistent-AF had LV-EF and higher rates of pre-mapping cardioversion than those with paroxysmal-AF; there were no other statistically significant differences between the two patient groups.
Table 1.
Baseline Patient Characteristics
| Total | Paroxysmal AF | Persistent AF | p-value (Paroxysmal vs. Persistent) | |
|---|---|---|---|---|
| Patients, n | 26 | 14 | 12 | |
| Age, years | 63.1±7.9 | 62.3±7.3 | 64.1±8.8 | 0.575 |
| Male Gender | 19(73%) | 10(71%) | 9(75%) | 0.838 |
| Caucasian | 22(85%) | 12(86%) | 10(83%) | 0.867 |
| BMI, kg/m2 | 27.2±3.9 | 26.3±4.9 | 28.3±2.0 | 0.199 |
| Hypertension | 17(65%) | 10(71%) | 7(58%) | 0.484 |
| Diabetes Mellitus | 1(4%) | 0(0%) | 1(8%) | 0.271 |
| Coronary/Vascular Disease | 4(15%) | 2(14%) | 2(17%) | 0.867 |
| Congestive Heart Failure | 1(4%) | 0(0%) | 1 (8%) | 0.271 |
| Prior Thromboembolism | 2(8%) | 2(14%) | 0(0%) | 0.173 |
| Obstructive Sleep Apnea | 3(12%) | 2(14%) | 1(8%) | 0.636 |
| CHA2DS2-VASc score | 1.8±1.1 | 1.9±1.0 | 1.8±1.2 | 0.957 |
| 0 | 3(8%) | 1(7%) | 1(8%) | 0.918 |
| 1 | 6(8%) | 4(29%) | 2(17%) | |
| 2 | 11(8%) | 6(43%) | 5(42%) | |
| 3 | 4(8%) | 2(14%) | 2(16%) | |
| 4 | 2(8%) | 1(7%) | 1(8%) | |
| 5 | 0(0%) | 0(0%) | 0(0%) | |
| 6 | 0(0%) | 0(0%) | 0(0%) | |
| AF Duration, years | 4.8±4.3 | 4.6±3.8 | 5.0±4.9 | 0.813 |
| Prior Ablations | 0.7±0.6 | 0.6±0.5 | 0.8±0.7 | 0.434 |
| 0 | 9(35%) | 5(36%) | 4(33%) | 0.276 |
| 1 | 15(58%) | 9(75%) | 6(50%) | |
| 2 | 2(7%) | 0(0%) | 2(17%) | |
| Prior Ablation Strategy | ||||
| Radiofrequency Ablation | 11(42%) | 7(50%) | 4(33%) | 0.565 |
| Cryoballoon Ablation | 6(23%) | 2(14%) | 4(33%) | |
| Smoking | 0.532 | |||
| Never | 12(46%) | 7(50%) | 5(42%) | |
| Prior | 13(50%) | 7(50%) | 6(50%) | |
| Current | 1(4%) | 0(0%) | 1(8%) | |
| DCV pre-mapping | 7(27%) | 0(0%) | 7(58%) | 0.0008 |
| LVEF, (%) | 61.0±9.4 | 65.9±6.1 | 54.8±9.4 | 0.002 |
| LA end-systolic area, cm2 | 26.8±8.4 | 24.5±5.9 | 29.3±10.1 | 0.157 |
AF: atrial fibrillation; BMI: body mass index; DCV: direct-current cardioversion; LA: left atrium; LVEF: left ventricular ejection fraction. Data are presented as mean ± SD and n (%).
IMAGE ANALYSIS
MRI studies were performed 1[0;6] (median[IQR]) days prior to the procedure. Image-intensity in arbitrary units (au) and IIR were measured for each sector. The relationship of signal intensity versus IIR and the distribution of each measure are plotted in Supplemental Figure 1. While image-intensity and IIR measures have a linear relationship within each patient, the slope of this association varies across patients with a range of 7.79 to 75.62 au. In contrast to the right-skewed image-intensity distribution, IIR displayed a normal distribution (Figure 2).
Figure 2.
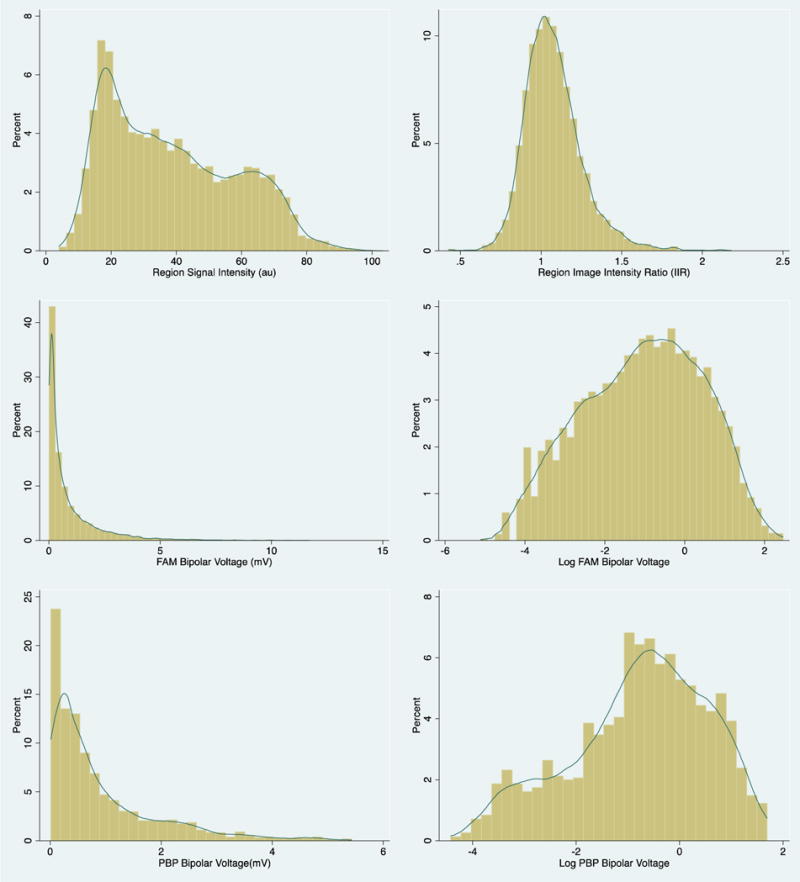
Histograms of distribution of signal intensity and image intensity ratio (top panels) as well as fast-anatomical-mapping and point-by-point bipolar voltage and its log transformation (mid and bottom panels).
FAM-VOLTAGE-IIR CORRELATION
Seven of the 26 patients (26%) presented in AF at the beginning of the procedure and required cardioversion prior to mapping. A mean of 734 (min 397; max 1089) FAM-points was acquired per patient and a total of 19,087 points were registered to LGE-MRI images. The average bipolar voltage was 0.88±1.27mV and the average IIR was 1.08±0.18. Local IIR was strongly associated with bipolar voltage; however, the relationship was more linear with log-transformation of bipolar voltage measures (Figure 2). Using a GEE-model, clustered by patient and adjusted for age, gender, CHA2DS2-VASc score, AF-type, AF-duration, previous ablations, LA end-diastolic area and LV-EF, each unit increase in local IIR was associated with 57% decrease in LA bipolar voltage (Table 2, p<0.0001). Based upon unadjusted analyses, local IIR thresholds of >0.74 corresponded to bipolar voltage <0.5 mV.
Table 2.
Results of the generalized estimating equation model.
| Log FAM Bipolar Voltage (mV) | Univariate | Multivariate | ||
|---|---|---|---|---|
|
| ||||
| Coeff | p-value | Coeff | p-value | |
| IIR (per unit) | −0.90 | < 0.0001 | −0.85 | <0.0001 |
| Age (per year) | −0.03 | 0.054 | −0.01 | 0.661 |
| Male Sex | −0.60 | 0.019 | −0.53 | 0.014 |
| BMI (per kg/m2) | 0.06 | 0.036 | 0.07 | 0.004 |
| CHA2DS2-VASc score (per unit) | −0.02 | 0.162 | −0.13 | 0.187 |
| Persistent AF | −0.14 | 0.565 | 0.02 | 0.911 |
| Repeat Ablation | −0.53 | 0.027 | −0.43 | 0.026 |
| AF duration (per year) | −0.05 | 0.076 | −0.06 | 0.012 |
| LA area (per cm2) | −0.02 | 0.162 | −0.001 | 0.952 |
| LVEF (per unit %) | 0.01 | 0.415 | 0.01 | 0.308 |
AF: atrial fibrillation; BMI: body mass index; FAM: fast anatomical mapping; IIR: image intensity ratio; LA: left atrium; LVEF: left ventricular ejection fraction.
We performed a stratified analysis in patients with prior ablations vs first-time ablation. The findings are summarized in Suppl. Table 1 & 2. In brief, mean bipolar voltage was 1.20mV in ablation-naïve patients and 0.78mV in previously ablated patients. The adjusted relationship between IIR and FAM-voltage was significantly negative in both groups. We also performed a subset analysis of 4 patients with the least percentage of low-voltage mapping points (characterized by voltage ≤0.5 mV). All 4 patients had paroxysmal AF with a mean bipolar voltage of 1.84mV and an average proportion of low voltage points of 8.7±4.1%. As seen in Suppl. Table 3, the adjusted relationship between IIR and FAM-measured bipolar voltage was significantly negative. It was similarly negative upon stratifying mapping points into ‘normal’ voltage (>0.5mV) and ‘low’ voltage (≤0.5 mV) (Suppl. Table 4 & 5).
FAM and PBP-VOLTAGE COMPARISON
Serial PBP- and FAM-mapping were performed in 19 patients. A mean of 82 points per patient were acquired using PBP technique and a total of 1554 points were matched in a 1:1 ratio to the nearest FAM-EAM point. In the matched 1554 voltage-point pairs, mean PBP voltage was 0.91±0.99mV and corresponding mean FAM voltage was 1.20±1.36mV. 4 Distribution of PBP-voltage and its log-transform are displayed in Figure 2. Scatter plot of voltage measurements correlating PBP-EAM and FAM-EAM is displayed in Supplemental Figure 2. Using an adjusted, patient-clustered GEE model, log-FAM bipolar voltage was significantly associated with log-PBP bipolar voltage (ß=0.36, p<0.0001), as shown in Table 3. Individual log-PBP vs log-FAM-voltage linear relationships are demonstrated in Suppl. Figure 3. At low-voltages (<40th percentile), FAM-mapping distribution was shifted to the left compared to PBP-mapping (Table 4). On FAM, 20th and 40th percentiles were 0.08mV, 0.25mV, respectively compared to 0.18mV and 0.42mV on PBP. At intermediate voltages (40th-80th percentiles), FAM and PBP-voltages were overlapping (60th and 80th percentiles on FAM were 0.69mV and 1.52mV respectively vs. 0.73mV and 1.54mV on PBP). At high voltages (>80th percentile), FAM-voltage exceeded PBP (99th percentile was 6.26mV on FAM vs. 4.65mV on PBP). ROC analysis of FAM-voltage predicting low-voltage (<0.5 mV) on PBP-mapping showed an area under the curve (AUC) of 0.75 (Supplemental Figure 4). The FAM voltage with the highest sensitivity and specificity was 0.58 mV (63% sensitivity, 77% specificity).
Table 3.
Results of the generalized estimating equation model.
| Log Bipolar PBP Voltage (mV) | Univariate | Multivariate | ||
|---|---|---|---|---|
|
| ||||
| Coeff | p-value | Coeff | p-value | |
| Log Bipolar FAM Voltage (mV) | 0.38 | < 0.0001 | 0.36 | <0.0001 |
| Age (per year) | 0.02 | 0.382 | 0.02 | 0.366 |
| Male Sex | −0.29 | 0.426 | −0.45 | 0.042 |
| BMI (per kg/m2) | 0.04 | 0.356 | −0.01 | 0.661 |
| CHA2DS2-VASc score (per unit) | −0.12 | 0.428 | −0.17 | 0.244 |
| Persistent AF | −0.25 | 0.422 | −0.08 | 0.767 |
| Repeat Ablation | −0.72 | 0.004 | −0.62 | 0.033 |
| AF duration (per year) | −0.01 | 0.935 | 0.02 | 0.518 |
| LA area (per cm2) | −0.01 | 0.797 | 0.01 | 0.490 |
| LVEF (per unit %) | 0.02 | 0.170 | 0.01 | 0.510 |
AF: atrial fibrillation; BMI: body mass index; FAM: fast anatomical mapping; PBP: point-by-point; LA: left atrium; LVEF: left ventricular ejection fraction.
Table 4.
Correlations of voltage thresholds between voltage-mapping modalities.
| Percentiles | 5th | [95% CI] | 20th | [95% CI] | 40th | [95% CI] | 60th | [95% CI] | 80th | [95% CI] | 99th | [95% CI] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FAM Voltage | 0.03 | [0.02–0.03] | 0.09 | [0.09–0.10] | 0.28 | [0.27–0.29] | 0.69 | [0.67–0.71] | 1.52 | [1.50–1.54] | 6.26 | [5.99–6.45] |
| PBP Voltage | 0.04 | [0.03–0.04] | 0.18 | [0.16–0.20] | 0.42 | [0.39–0.45] | 0.73 | [0.68–0.79] | 1.54 | 1.41–1.67] | 4.65 | [4.27–4.79] |
IIR: image intensity ratio; FAM: fast anatomical mapping; PBP: point-by-point.
Figure 3 compares IIR maps, FAM- and PBP-voltage maps in an ablation-naive patient with minimal baseline fibrosis, an ablation-naïve patient with posterior wall fibrosis, and a patient with a history of prior AF-radiofrequency ablation. The figure demonstrates good correlation of LA fibrotic substrate between the three modalities. Similarly, Suppl. Figure 5 demonstrates an anterior view of IIR and FAM-voltage maps in two patients.
Figure 3.
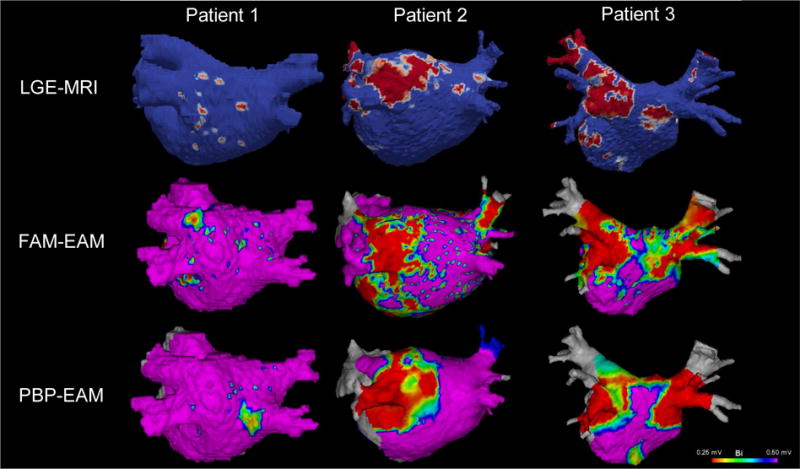
This illustration compares IIR maps (Top Panel), FAM voltage maps (Middle Panel), and PBP voltage maps (Bottom Panel) in an ablation-naive patient with minimal baseline fibrosis (patient 1), an ablation-naïve patient with posterior wall fibrosis (patient 2), and a patient with a history of prior radiofrequency ablation for AF (patient 3). The figure demonstrates good correlation between the three mapping modalities. Of note, no voltage mapping was done in PV ostia and this area was excluded from any multimodal correlation analysis. Indeed, the thin myocardial sleeves in that area render voltage and MRI less suited for myocardial substrate mapping.
DISCUSSION
SUMMARY
In this study, we sought to compare LA substrate using different modalities (LGE-MRI, FAM-, and PBP-mapping) in patients presenting for an AF ablation. The major findings are (1) voltage acquired by FAM using decapolar circular (LASSO 2515 Variable Loop Eco Nav, Biosense Webster, Diamond Bar, CA) mapping catheter with small electrode size correlates with IIR on LGE-MRI; and (2) voltage acquired by FAM correlates with voltage on PBP-mapping. These findings might have significant impact on the interpretation of substrate-mapping in ablation strategies targeting extra-pulmonary vein LA remodeling.
FAM vs IIR CORRELATION
A variety of novel AF substrate-mapping approaches have been introduced in recent years including dominant-frequency analysis (6, 7), Shannon entropy (12), complex-fractionated electrogram activity (CFAE) (3), focal impulse and rotor modulation (8), bipolar electrogram amplitude (13), and LGE-MRI (9, 11, 14). Prior studies have shown poor correlation between various techniques (15–18), with the exception of bipolar voltage which correlates well with LGE-MRI (9, 10). In a landmark study, Oakes et al. found a positive correlation (r2=0.61) between the extent of LGE on MRI with low-voltage zones area (10). Kapa et al. projected EAM points, acquired in sinus rhythm, onto LGE-MRI and found that mean bipolar voltage in areas of MRI-fibrosis was significantly lower than in non-fibrosis areas (19). In a study by Khurram et al. (9), IIR was used to normalize signal intensities, and intra-cardiac mapping points were registered to LGE-images. In a multivariable model, each unit increase in IIR was associated with 91.3% decrease in bipolar LA voltage (p<0.0001). In the above-mentioned studies, voltage maps were created using PBP-acquisition (with or without force-sensing). In our investigation, we used a multipolar circular mapping catheter with small electrodes (~1mm) to acquire a detailed (>1000 points) voltage maps of the LA. In a semi-automated fashion, we were able to merge mapping points onto LGE images and correlate bipolar voltage with neighboring IIR on MRI. Using an adjusted patient-clustered GEE model, each unit increase in local IIR was associated with 57% decrease in local bipolar LA voltage, demonstrating a good correlation between FAM-voltage and IIR in mapping AF substrate.
The distinction between ablation-induced scar and AF-related interstitial fibrosis is an important one to make. On the microscopic level, these represent different pathological processes; necrosis and replacement fibrosis in ablation-related scar and interstitial, diffuse collagen deposition in native AF-related fibrosis. Our group has recently established the distinct characteristics of these processes on LGE-MRI(20). Regions with ablation-induced scar exhibit increased contrast uptake (higher IIR), likely signifying higher scar density, as compared with regions with native interstitial fibrosis. For the above reasons, we performed a stratified analysis in patients with prior ablations vs first-time ablation and found an inverse relationship between IIR and FAM-measured bipolar voltage in both groups.
These findings further demonstrate the utility of MRI in mapping abnormal myocardium as well as guiding AF ablation. In particular, long-term procedural outcomes of patients with persistent AF (PsAF) remain dismal (~50%)(21) and is attributed to extensive atrial fibrotic remodeling not targeted by PVI(10, 22–24). This is supported by the DECAAF study that shows that pre-ablation fibrotic tissue is a major predictor of ablation outcome (22). The efficacy of targeting atrial fibrosis tissue during persistent AF ablation will be assessed in the much-awaited DECAAF II study (Clinicaltrials.gov ID: NCT02529319).
FAM vs PBP CORRELATION
Several studies have shown smaller low-voltage areas and higher mean regional bipolar voltages on FAM compared to PBP mapping (25–27). In line with previous studies, mean PBP bipolar voltage in our study was 0.91±0.99mV and mean FAM voltage was 1.20±1.36mV. Log-FAM bipolar voltage was significantly associated with log-PBP bipolar voltage (ß=0.36, p<0.0001). Several factors might influence bipolar voltage amplitude such as catheter characteristics (electrode size, inter-electrode spacing), wavefront of activation, electrode-wall contact force and angle (26, 28, 29). In fact, our linear ablation catheters had 3.5-mm distal electrodes with inter-electrode spacing of ~5mm whereas multipolar circular catheters used had 20 poles, 1-mm electrode size, and 2-6-2mm inter-electrode spacing. Smaller electrode sizes allow for increased mapping resolution and a greater ability to discriminate between surviving myocardial fibers and fibrosis; at the same time, smaller electrode size may increase susceptibility of the electrodes to far-field sensing. This was seen on the voltage distribution of the two modalities. At low-voltages (<40th percentile), FAM-mapping distribution was shifted to the left compared to PBP-mapping which may reflect increased capacity by FAM to detect fibrotic fibers interspaced with normal myocytes. Whereas at high voltages (>80th percentile), FAM voltages exceeded PBP (99th percentile was 6.26mV on FAM vs. 4.65mV on PBP) which may be due to far-field sensing. Moreover, although adequate tissue contact during mapping with the PBP catheter was confirmed by contact force measurement, such data were not available for FAM catheters, which could account for some of the voltage differences.
Voltage mapping in the LA is increasingly used to guide AF ablation the accuracy of which relies heavily on the accurate identification of LA fibrosis (3, 30–33). Our work raises the awareness of the differences in substrate delineation between various mapping techniques and catheters available that would influence the interpretation of mapped substrate. Based in part on our observations in the current study, our current clinical practice is to use 0.25-0.5mV as a voltage range indicative of abnormal fibrotic tissue and <0.25mV as consistent with scar when performing either FAM or PBP-mapping; and in cases in which LGE-MRI data is used for scar assessment, we use an IIR value of > 1.22 based on equations derived from our work. An important caveat, of course, is that data is lacking about how these parameters correlate with scar assessment based on actual tissue evaluation.
LIMITATIONS
There are a number of limitations to the study. The gold standard in assessing atrial substrate in AF is tissue evaluation. In patients with AF, the muscle bundles were surrounded by thick connective tissue fibres. These thick fibres were also interspaced between the single muscle cells (34). The resolution of PBP or FAM maps using bipolar electrodes is very poor compared to this gold standard, regardless of the mapping density. The fill-threshold utilized by the mapping systems compounds the problem by interpolating areas that are not directly touched by the catheter. Although LGE-MRI characterizes the entirety of atrial tissue within each axial slice, the thickness of the slices adds error and interpolation as well. Given the limitations of both EAM and IIR methodologies, in addition to the fact that IIR has not been validated against tissue samples as of yet, the results of this study should be interpreted as two methods in relative agreement rather that against a gold standard. This study includes a relatively small cohort of patients who were heterogeneous with regards to prior ablations. However, such heterogeneity did not modify the association between IIR and voltage and demonstrated the wide applicability of LGE-MRI in detecting abnormal LA myocardium. Voltage can be affected by tissue contact. Though it cannot be completely ruled out that diminished bipolar voltage on FAM was measured as a consequence of reduced catheter contact (since FAM catheters are not capable of force sensing), it is unlikely to account for the differences between catheters: 1) every attempt was made to ensure adequate contact by means of splaying of the electrodes in contact with the myocardium on fluoroscopy; 2) points were only accepted if they were within 5 mm from the anatomical shell; and 3) patient-clustered association of IIR with voltage suggest that, on average, the measures represent LA myocardial tissue characteristics. Our results may also be affected by positional errors when registering ablation points to the MR-based segmentation; however, the extent of positional errors appears to be very low based upon prior validation studies of our technique(35). For the PBP vs. FAM correlation, distances between PBP and FAM-points were calculated using the coordinates extracted from CARTO. As such, our results are contingent on having an accurate 3D registration of PBP and FAM-EAM and would be affected by patient movement in-between the two mapping modalities (as seen on individual graphs in Fig. 3).
CONCLUSION
LGE-MRI, FAM and PBP-mapping show a good degree of correlation for delineating fibrosis-based AF substrate. Each approach has fundamental technical characteristics, the awareness of which allows proper assessment of electro-anatomical AF substrate and potentially avoidance of unnecessary ablation.
Supplementary Material
CONDENSED ABSTRACT.
In this study, we examine the correlation of atrial fibrillation (AF) mapping by (1) point-by-point (PBP) mapping using linear-ablation catheters, (2) fast-anatomical mapping (FAM) with multi-electrode circular catheters, and (3) late gadolinium-enhanced magnetic-resonance imaging (LGE-MRI) in 26 patients presenting for AF ablation. LGE-MRI, FAM and PBP-mapping show good correlation in delineating electro-anatomical AF substrate such that MRI intensity was inversely associated with FAM-voltage and FAM- was directly associated with PBP-bipolar voltage. Each approach has fundamental technical characteristics, the awareness of which allows proper assessment of atrial substrate by adjustment of fibrosis-defining thresholds across various modalities.
CLINICAL COMPETENCIES.
Substrate modification, targeting low-voltage areas and/or enhancing areas on LGE-MRI, has been proposed as an individualized ablation approach. As seen in our study, differences in catheter used for LA substrate mapping may explain the variable results of fibrosis-based ablation strategies (31). Our findings reflect the importance of adjusting voltage and/or IIR thresholds when examining AF substrate using different modalities.
TRANSLATIONAL OUTLOOK.
Intra-cardiac electrograms and LGE-MRI can delineate fibrosis in the LA of AF patients and this study has shown good correlation between the two modalities. However, histological confirmation remains of paramount necessity to establish sensitivity and specificity of these less invasive tools compared to a gold standard. Moreover, a study on healthy volunteers without AF is needed in order to validate the associations between the various modalities in structurally normal atria.
Acknowledgments
Funding: The study was funded by the National Institutes of Health (grant nos. K23HL089333 and R01HL116280) as well as by a Biosense Webster grant to Dr Nazarian; the Roz and Marvin H. Weiner and Family Foundation; the Dr. Francis P. Chiaramonte Foundation; Marilyn and Christian Poindexter; and the Norbert and Louise Grunwald Cardiac Arrhythmia Research Fund.
Dr Nazarian is principal investigator for research funding to Johns Hopkins University from Biosense Webster and has served as a scientific advisor to Biosense Webster, CardioSolv, and St. Jude Medical, Inc.
ABBREVIATIONS
- PVI
Pulmonary Vein-Isolation
- RF
Radio-Frequency
- AF
Atrial Fibrillation
- LA
Left Atrium
- LGE-MRI
Late-Gadolinium Enhancement Magnetic-Resonance Imaging
- FAM
Fast Anatomical Mapping
- PBP
Point-by-Point
- EAM
Electro-Anatomical Mapping
- IIR
Image-Intensity Ratio
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The other authors report no conflicts.
References
- 1.Calkins H, Kuck KH, Cappato R, et al. 2012 HRS/EHRA/ECAS Expert Consensus Statement on Catheter and Surgical Ablation of Atrial Fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. Europace. 2012;14:528–606. doi: 10.1093/europace/eus027. [DOI] [PubMed] [Google Scholar]
- 2.Wijffels MC, Kirchhof CJ, Dorland R, Allessie MA. Atrial fibrillation begets atrial fibrillation. A study in awake chronically instrumented goats. Circulation. 1995;92:1954–68. doi: 10.1161/01.cir.92.7.1954. [DOI] [PubMed] [Google Scholar]
- 3.Nademanee K, McKenzie J, Kosar E, et al. A new approach for catheter ablation of atrial fibrillation: mapping of the electrophysiologic substrate. J Am Coll Cardiol. 2004;43:2044–2053. doi: 10.1016/j.jacc.2003.12.054. [DOI] [PubMed] [Google Scholar]
- 4.Miyamoto K, Tsuchiya T, Narita S, et al. Bipolar electrogram amplitudes in the left atrium are related to local conduction velocity in patients with atrial fibrillation. Europace. 2009;11:1597–605. doi: 10.1093/europace/eup352. [DOI] [PubMed] [Google Scholar]
- 5.Morillo CA, Klein GJ, Jones DL, Guiraudon CM. Chronic rapid atrial pacing. Structural, functional, and electrophysiological characteristics of a new model of sustained atrial fibrillation. Circulation. 1995;91:1588–95. doi: 10.1161/01.cir.91.5.1588. [DOI] [PubMed] [Google Scholar]
- 6.Jarman JWE, Wong T, Kojodjojo P, et al. Spatiotemporal Behavior of High Dominant Frequency During Paroxysmal and Persistent Atrial Fibrillation in the Human Left Atrium. Circ Arrhythmia Electrophysiol. 2012;5:650–658. doi: 10.1161/CIRCEP.111.967992. [DOI] [PubMed] [Google Scholar]
- 7.Guillem MS, Climent AM, Millet J, et al. Noninvasive Localization of Maximal Frequency Sites of Atrial Fibrillation by Body Surface Potential Mapping. Circ Arrhythmia Electrophysiol. 2013;6:294–301. doi: 10.1161/CIRCEP.112.000167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Narayan SM, Krummen DE, Shivkumar K, Clopton P, Rappel W-J, Miller JM. Treatment of Atrial Fibrillation by the Ablation of Localized Sources. J Am Coll Cardiol. 2012;60:628–636. doi: 10.1016/j.jacc.2012.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khurram IM, Beinart R, Zipunnikov V, Dewire J, Yarmohammadi H, Sasaki T, Spragg DD, Marine JE, Berger RD, Halperin HR, Calkins H, Zimmerman SLNS. Magnetic resonance image intensity ratio, a normalized measure to enable interpatient comparability of left atrial fibrosis. Heart Rhythm. 2014;11:85–92. doi: 10.1016/j.hrthm.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oakes RS, Badger TJ, Kholmovski EG, et al. Detection and Quantification of Left Atrial Structural Remodeling With Delayed-Enhancement Magnetic Resonance Imaging in Patients With Atrial Fibrillation. Circulation. 2009;119 doi: 10.1161/CIRCULATIONAHA.108.811877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khurram IM, Habibi M, Gucuk Ipek E, et al. Left Atrial LGE and Arrhythmia Recurrence Following Pulmonary Vein Isolation for Paroxysmal and Persistent AF. JACC Cardiovasc Imaging. 2016;9:142–8. doi: 10.1016/j.jcmg.2015.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ganesan AN, Kuklik P, Lau DH, et al. Bipolar Electrogram Shannon Entropy at Sites of Rotational ActivationClinical Perspective. Circ Arrhythmia Electrophysiol. 2013;6 doi: 10.1161/CIRCEP.112.976654. [DOI] [PubMed] [Google Scholar]
- 13.Verma A, Wazni OM, Marrouche NF, et al. Pre-existent left atrial scarring in patients undergoing pulmonary vein antrum isolation. J Am Coll Cardiol. 2005;45:285–292. doi: 10.1016/j.jacc.2004.10.035. [DOI] [PubMed] [Google Scholar]
- 14.Oakes RS, Badger TJ, Kholmovski EG, et al. Detection and quantification of left atrial structural remodeling with delayed-enhancement magnetic resonance imaging in patients with atrial fibrillation. Circulation. 2009;119:1758–67. doi: 10.1161/CIRCULATIONAHA.108.811877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chrispin J, Gucuk Ipek E, Zahid S, et al. Lack of regional association between atrial late gadolinium enhancement on cardiac magnetic resonance and atrial fibrillation rotors. Heart Rhythm. 2016;13:654–660. doi: 10.1016/j.hrthm.2015.11.011. [DOI] [PubMed] [Google Scholar]
- 16.Ghoraani B, Dalvi R, Gizurarson S, et al. Localized rotational activation in the left atrium during human atrial fibrillation: Relationship to complex fractionated atrial electrograms and low-voltage zones. Heart Rhythm. 2013;10:1830–1838. doi: 10.1016/j.hrthm.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 17.Viles-Gonzalez JF, Gomes JA, Miller MA, et al. Areas with complex fractionated atrial electrograms recorded after pulmonary vein isolation represent normal voltage and conduction velocity in sinus rhythm. Europace. 2013;15:339–346. doi: 10.1093/europace/eus321. [DOI] [PubMed] [Google Scholar]
- 18.Schade A, Nentwich K, Costello-Boerrigter LC, et al. Spatial Relationship of Focal Impulses, Rotors and Low Voltage Zones in Patients With Persistent Atrial Fibrillation. J Cardiovasc Electrophysiol. 2016;27:507–514. doi: 10.1111/jce.12913. [DOI] [PubMed] [Google Scholar]
- 19.Kapa S, Desjardins B, Callans DJ, Marchlinski FE, Dixit S. Contact Electroanatomic Mapping Derived Voltage Criteria for Characterizing Left Atrial Scar in Patients Undergoing Ablation for Atrial Fibrillation. J Cardiovasc Electrophysiol. 2014;25:1044–1052. doi: 10.1111/jce.12452. [DOI] [PubMed] [Google Scholar]
- 20.Fukumoto K, Habibi M, Gucuk Ipek E, et al. Comparison of preexisting and ablation-induced late gadolinium enhancement on left atrial magnetic resonance imaging. Heart Rhythm. 2015;12:668–672. doi: 10.1016/j.hrthm.2014.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verma A, Jiang C, Betts TR, et al. Approaches to Catheter Ablation for Persistent Atrial Fibrillation. N Engl J Med. 2015;372:1812–1822. doi: 10.1056/NEJMoa1408288. [DOI] [PubMed] [Google Scholar]
- 22.Marrouche NF, Wilber D, Hindricks G, et al. Association of atrial tissue fibrosis identified by delayed enhancement MRI and atrial fibrillation catheter ablation: the DECAAF study. JAMA. 2014;311:498–506. doi: 10.1001/jama.2014.3. [DOI] [PubMed] [Google Scholar]
- 23.Cochet H, Mouries A, Nivet H, et al. Age, Atrial Fibrillation, and Structural Heart Disease Are the Main Determinants of Left Atrial Fibrosis Detected by Delayed-Enhanced Magnetic Resonance Imaging in a General Cardiology Population. J Cardiovasc Electrophysiol. 2015;26:484–492. doi: 10.1111/jce.12651. [DOI] [PubMed] [Google Scholar]
- 24.Xu J, Cui G, Esmailian F, et al. Atrial Extracellular Matrix Remodeling and the Maintenance of Atrial Fibrillation. Circulation. 2004;109:363–368. doi: 10.1161/01.CIR.0000109495.02213.52. [DOI] [PubMed] [Google Scholar]
- 25.Huemer M, Qaiyumi D, Attanasio P, et al. Does the extent of left atrial arrhythmogenic substrate depend on the electroanatomical mapping technique: impact of pulmonary vein mapping catheter vs. ablation catheter. Europace. 2016:euw185. doi: 10.1093/europace/euw185. [DOI] [PubMed] [Google Scholar]
- 26.Anter E, Tschabrunn CM, Josephson ME. High-resolution mapping of scar-related atrial arrhythmias using smaller electrodes with closer interelectrode spacing. Circ Arrhythm Electrophysiol. 2015;8:537–45. doi: 10.1161/CIRCEP.114.002737. [DOI] [PubMed] [Google Scholar]
- 27.Liang JJ, Elafros MA, Muser D, et al. Comparison of Left Atrial Bipolar Voltage and Scar Using Multielectrode Fast Automated Mapping versus Point-by-Point Contact Electroanatomic Mapping in Patients With Atrial Fibrillation Undergoing Repeat Ablation. J Cardiovasc Electrophysiol. 2017;28:280–288. doi: 10.1111/jce.13151. [DOI] [PubMed] [Google Scholar]
- 28.Stinnett-Donnelly JM, Thompson N, Habel N, et al. Effects of electrode size and spacing on the resolution of intracardiac electrograms. Coron Artery Dis. 2012;23:126–32. doi: 10.1097/MCA.0b013e3283507a9b. [DOI] [PubMed] [Google Scholar]
- 29.Ullah W, Hunter RJ, Baker V, et al. Impact of Catheter Contact Force on Human Left Atrial Electrogram Characteristics in Sinus Rhythm and Atrial Fibrillation. Circ Arrhythm Electrophysiol. 2015;8:1030–9. doi: 10.1161/CIRCEP.114.002483. [DOI] [PubMed] [Google Scholar]
- 30.Schreiber D, Rieger A, Moser F, Kottkamp H. Catheter ablation of atrial fibrillation with box isolation of fibrotic areas: Lessons on fibrosis distribution and extent, clinical characteristics, and their impact on long-term outcome. J Cardiovasc Electrophysiol. 2017 doi: 10.1111/jce.13278. [DOI] [PubMed] [Google Scholar]
- 31.Blandino A, Bianchi F, Grossi S, et al. Left Atrial Substrate Modification Targeting Low-Voltage Areas for Catheter Ablation of Atrial Fibrillation: A Systematic Review and Meta-Analysis. Pacing Clin Electrophysiol. 2017;40:199–212. doi: 10.1111/pace.13015. [DOI] [PubMed] [Google Scholar]
- 32.Kottkamp H, Berg J, Bender R, Rieger A, Schreiber D. Box Isolation of Fibrotic Areas (BIFA): A Patient-Tailored Substrate Modification Approach for Ablation of Atrial Fibrillation. J Cardiovasc Electrophysiol. 2016;27:22–30. doi: 10.1111/jce.12870. [DOI] [PubMed] [Google Scholar]
- 33.Jadidi AS, Lehrmann H, Keyl C, et al. Ablation of Persistent Atrial Fibrillation Targeting Low-Voltage Areas With Selective Activation Characteristics. Circ Arrhythmia Electrophysiol. 2016;9 doi: 10.1161/CIRCEP.115.002962. [DOI] [PubMed] [Google Scholar]
- 34.Boldt A, Wetzel U, Lauschke J, et al. Fibrosis in left atrial tissue of patients with atrial fibrillation with and without underlying mitral valve disease. Heart. 2004;90:400–405. doi: 10.1136/hrt.2003.015347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dong J, Calkins H, Solomon SB, et al. Integrated electroanatomic mapping with three-dimensional computed tomographic images for real-time guided ablations. Circulation. 2006;113:186–94. doi: 10.1161/CIRCULATIONAHA.105.565200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


