Abstract
Background
Schizophrenia (SCZ) and psychotic bipolar disorder (PBD) share considerable overlap in clinical features, genetic risk factors and co-occurrence among relatives. The common and unique functional cerebral deficits in these disorders, and in unaffected relatives, remain to be identified.
Method
A total of 59 healthy controls, 37 SCZ and 57 PBD probands and their unaffected first-degree relatives (38 and 28, respectively) were studied using resting-state functional magnetic resonance imaging (rfMRI). Regional cerebral function was evaluated by measuring the amplitude of low-frequency fluctuations (ALFF). Areas with ALFF alterations were used as seeds in whole-brain functional connectivity analysis. We then tested whether abnormalities identified in probands were present in unaffected relatives.
Results
SCZ and PBD probands both demonstrated regional hypoactivity in the orbital frontal cortex and cingulate gyrus, as well as abnormal connectivity within striatal-thalamo-cortical networks. SCZ probands showed greater and more widely distributed ALFF alterations including the thalamus and bilateral parahippocampal gyri. Increased parahippocampal ALFF was related to positive symptoms and cognitive deficit. PBD patients showed uniquely increased functional connectivity between the thalamus and bilateral insula. Only PBD relatives showed abnormal connectivity within striatal-thalamo-cortical networks seen in both proband groups.
Conclusions
The present findings reveal a common pattern of deficits in frontostriatal circuitry across SCZ and PBD, and unique regional and functional connectivity abnormalities that distinguish them. The abnormal network connectivity in PBD relatives that was present in both proband groups may reflect genetic susceptibility associated with risk for psychosis, but within-family associations of this measure were not high.
Keywords: Bipolar disorder, brain function, magnetic resonance imaging, relatives, resting state, schizophrenia
Introduction
Schizophrenia (SCZ) and bipolar disorder (BD) are severe and debilitating psychiatric disorders. Numerous overlaps between these disorders, especially for bipolar patients with a history of psychosis (psychotic bipolar disorder; PBD), have been reported in clinical features (Lichtenstein et al. 2009), brain structure (Ellison-Wright & Bullmore, 2010), brain function (Chai et al. 2011) and genetic risk factors (Lichtenstein et al. 2009). Furthermore, there are significant rates of both disorders in relatives of probands with either disorder (Lichtenstein et al. 2009; Ivleva et al. 2010). Clarifying the common and distinct neural substrates of these two clinical syndromes remains a major focus of psychiatric research.
Previous neuroimaging studies of SCZ and BD have revealed similar functional deficits, notably hypoactivity in the orbital frontal cortex (Kruger et al. 2006; Lui et al. 2010), dorsal prefrontal cortex (Whalley et al. 2004; Houenou et al. 2012) and anterior cingulate cortex (Yucel et al. 2007; Wessa & Linke, 2009). However, illness-specific deficits have been reported as well, especially hypoactivity of the thalamus in SCZ (Andreasen et al. 1996). The few studies directly comparing functional alterations in PBD and SCZ (Meda et al. 2012; Filippi et al. 2013) have yielded inconsistent results. For example, using language tasks, patients with PBD have shown higher (McIntosh et al. 2008) and lower (Costafreda et al. 2011) activation in the dorsal prefrontal cortex.
Besides regional functional deficits, neural network deficits have also been studied in PBD and SCZ. Using ‘resting-state’ functional magnetic resonance imaging (rfMRI), a non-invasive method that can be used to evaluate regional brain function as well as functional connectivity (FC) within brain networks (Lui et al. 2009a), Meda et al. (2012) found shared abnormal connectivity in fronto/occipital and anterior default mode/prefrontal circuitry in both patient groups, as well as distinct network alterations in each disorder. Ongur et al. (2010) also reported both shared and distinct abnormalities in the default mode network (DMN). Liu et al. (2014) reported a dorsal versus ventral prefrontal cortex differentiation in amygdala/prefrontal neural system abnormalities between SCZ and BD. Khadka et al. (2013), applying independent component analysis on the dataset used by Meda et al. (2012), reported fronto/occipital, frontal/thalamic/basal ganglia and sensorimotor network abnormalities shared by both probands and relatives. Chai et al. (2011) found distinct FC patterns between the medial prefrontal cortex and the insula/ventral lateral prefrontal cortex that distinguished BD from SCZ. These findings suggest similar and unique FC alterations in SCZ and BD. However, none of these studies explored the integrity of regional function of the brain at rest, or whether regions with altered resting function have abnormal patterns of FC with other brain areas.
The amplitude of low-frequency fluctuations (ALFF) in the blood oxygenation level-dependent (BOLD) signal is one way to explore regional neural function, and has been correlated with local field potential activity (Logothetis et al. 2001). Other research correlating the ALFF with reaction time found that the degree of signal change in the BOLD fMRI signal is related to the speed of visuomotor response time. Thus, the ALFF appears to be a biologically significant parameter for assessing regional brain function (Mohamed et al. 2004). Kiviniemi et al. (2000) reported the ALFF in the visual cortex peaking at 0.034 Hz, suggesting that activity in this range is related to regional spontaneous neuronal activity. Such a conclusion was supported by later studies of healthy individuals (Yang et al. 2007) and children with attention deficit/hyperactivity disorder (Zang et al. 2007).
The objective of the present study was to use rfMRI to investigate both regional and network brain function during resting-state studies of SCZ and PBD probands and their first-degree relatives. Distinct from the prior study by Meda et al. (2012), the present study evaluated regional abnormalities of SCZ and PBD, and the FC of identified regions with regional ALFF abnormalities across the brain using a seed approach. Relative to more exploratory approaches such as graph-based network analysis and the fully inductive connectivity modeling approach used by Meda et al. (2012), our connectivity analysis used a hypothesis-driven approach aimed at determining whether regions with abnormal ALFF have altered FC with other brain areas. Analyses proceeded in three steps. First, we identified shared and distinctive regional deficits in SCZ and PBD probands; second, we determined whether brain areas with regional dysfunction had altered FC with other brain areas; and finally we determined whether functional abnormalities seen in probands were present in their first-degree relatives and related to cognitive and symptom measures.
Method
Participants
The study was approved by the University of Illinois at Chicago Institutional Review Board, and all participants provided written informed consent. The scans were acquired as part of the Bipolar and Schizophrenia Network for Intermediate Phenotypes (B-SNIP) Consortium study. Subjects were interviewed with the Structured Clinical Interview for DSM-IV (SCID) and consensus diagnoses were made using all available information. A full description of subject ascertainment procedures and clinical characteristics of the sample is available elsewhere (Tamminga et al. 2013). The following exclusion criteria applied to all groups: history of significant neurological or systemic illness, positive urine drug screen for common drugs of abuse on the day of testing, diagnosis of substance abuse in the prior 30 days or substance dependence in the prior 6 months, pregnancy, and head translation or rotation movement during scanning >1.5 mm. All relatives included in this report were free of Axis I psychopathology in order to study familial alterations unrelated to illness manifestation, and were not taking psychoactive medications. In total, 59 controls, 37 SCZ and 57 PBD probands, and 38 SCZ relatives and 28 PBD relatives were included in the study (Table 1). All patients were clinically stable for 1 month before testing. All patients were receiving stable medication treatment for at least 1 month before testing. Of the subjects, five SCZ and 16 PBD probands were not receiving antipsychotic medication; 38 PBD probands were taking mood stabilizers or lithium. The mean chlorpromazine equivalent daily dose of SCZ and PBD probands was 483 (s.d.=386) and 236 (s.d. =249) mg, respectively. Symptom severity and functioning were quantified using the Positive and Negative Symptom Scale (PANSS), the Young Mania Research Scale, the Montgomery–Åsberg Depression Rating Scale and the Global Assessment of Functioning Scale (GAF). Cognitive function was evaluated using the Brief Assessment of Cognition in Schizophrenia (BACS; Hill et al. 2013). Relatives were assessed for DSM-IV-TR cluster A (psychosis spectrum) personality disorders using the Structured Interview for Disorders of Personality. Only three SCZ and five PBD relatives met those diagnostic criteria, and they did not differ significantly from other relatives on any MRI measure.
Table 1.
Demographic and clinical characteristics of patients with SCZ and PBD, their first-degree relatives and healthy comparison subjects
| Patients with SCZ (n=37) |
Patients with PBD (n=57) |
Relatives of SCZ (n=38) |
Relatives of PBD (n=28) |
Healthy comparison (n=59) |
|
|---|---|---|---|---|---|
| Female, n (%) | 15 (40.5) | 39 (68.4) | 29 (76.3) | 17 (60.7) | 33 (55.9) |
| Handedness: right, n (%) | 32 (86.5) | 46 (80.7) | 33 (86.8) | 27 (96.4) | 53 (89.8) |
| Age, years | 36 (14) | 34 (13) | 36 (12) | 37 (15) | 38 (17) |
| Education, years | 14 (3) | 14 (3) | 15 (2) | 14 (2) | 14 (3) |
| Illness duration, years | 14.81 (11.99) | 16.89 (12.71) | |||
| PANSS scores | |||||
| Total | 71.2 (15.50) | 58.75 (13.67) | |||
| Negative | 18.31 (6.53) | 13.35 (3.98) | |||
| Positive | 18.2 (4.77) | 13.51 (4.52) | |||
| General | 34.69 (7.39) | 31.89 (8.39) | |||
| Montgomery–Åsberg Depression Rating Scale total score | 11.11 (8.75) | 11.33 (9.26) | |||
| Young Mania Rating Scale total score | 6.17 (4.73) | 5.58 (5.73) | |||
| Global assessment function | 45.69 (8.24) | 56.44 (13.67) |
Data are given as mean (standard deviation) unless otherwise indicated.
SCZ, Schizophrenia; PBD, psychotic bipolar disorder; PANSS, Positive and Negative Symptom Scale.
Data acquisition and preprocessing
Subjects underwent scanning using a GE Signa EXCITE 3.0 Tesla MR imaging system and an eight-channel phased array head coil (GE, USA). All the imaging data were collected at the Chicago B-SNIP site. Preprocessing and statistical analysis of functional images were carried out using SPM8 (Wellcome Department of Imaging Neuroscience, UK; http://www.fil.ion.ucl.ac.uk). Details of the MRI protocol and data preprocessing are presented in the online Supplementary material.
ALFF calculation
Measurement of the ALFF (0.01–0.08 Hz) of the BOLD signal, which is considered to be physiologically meaningful and related to regional spontaneous neural activity (Cordes et al. 2001), was used to identify regional cerebral function. The ALFF was calculated using REST software (Resting-State fMRI Data Analysis Toolkit V1.8; http://restfmri.net/forum/rest), using methods similar to those in an earlier study (Zang et al. 2007). After bandpass filtering (0.01–0.08 HZ) and linear-trend removal, the time series were transformed to the frequency domain using fast Fourier transformation (parameters: taper percentage =0, fast Fourier transform length =shortest), and the power spectrum obtained. Since the power of a given frequency is proportional to the square of the amplitude, power was square root transformed and then averaged across 0.01–0.08 Hz to yield a measure of the ALFF from each voxel. The ALFF of each voxel was then divided by the global mean ALFF value of the individual to standardize data across subjects.
FC analysis
FC was examined using a seed voxel correlation approach (Friston, 1994; Horwitz et al. 1998) which utilized as six seeds areas where altered regional function (ALFF) was demonstrated in the proband groups: four from the comparison between all the patients (combined SCZ and PBD) and controls, and two additional regions identified from the comparison of SCZ probands and controls. This was done to focus the analysis on determining the functional circuitry implication of identified regional dysfunctions.
The entire volume of altered ALFF clusters with altered regional function was averaged and used as a seed for FC analysis between the region with altered ALFF values and the remainder of the brain. The first step in the analysis was to extract a reference time series by obtaining the average of the time series of voxels within each cluster-defined region of interest defined by their showing a significant abnormality of the ALFF. Correlations were then computed between the time series of the seed reference and all brain voxels outside the seed region after removing voxels with high correlation with cerebrospinal fluid or white matter, which are thought to be associated with cardiac- or respiratory-induced variations, or with low correlation with gray matter. Parameters representing head motion were used as covariates. Finally, correlation coefficients relating the time series of the seed to each voxel outside the seed region were transformed to Z values using the Fisher r-to-Z transformation in order to improve normality before averaging correlations across participants.
Statistical analysis
To identify differences and similarities across SCZ and PBD in brain function, both regional measurements (ALFF maps) and FC measurements (Z maps) were analysed. Voxel-based comparison of ALFF maps from patient and control groups was performed using a one-way analysis of variance (ANOVA) and pairwise t tests in SPM8 (http://www.fil.ion.ecl.ac.uk/spm). Connectivity of regions with identified abnormality was examined as discussed above. Briefly, connectivity was examined from six seed areas, then six ANOVAs and eighteen pairwise t tests (three t tests for pairwise group comparison for each seed) were used to compare Z value maps among SCZ and PBD probands and controls. Next, similar analyses were performed to determine whether the SCZ and PBD relative groups shared regional and connectivity abnormalities demonstrated by their respective proband group (i.e. a one-way ANOVA and pairwise t test to compare the ALFF maps, six ANOVAs and 18 pairwise t tests to compare Z value maps). In all image analyses, a threshold of p=0.05 after AlphaSim correction was used, an approach which utilizes Monte Carlo simulations to correct for multiple comparisons. In our analyses, the probability of a false-positive detection for the study was set to p<0.05 using a minimum cluster size of 10 contiguous voxels significant individually at a threshold of pπ.001 (http://afni.nimh.nih.gov/pub/dist/doc/manual/AlphaSim.pdf). The AlphSim calculation was conducted using REST software (http://restfmri.net/forum/index.php), which integrated the true smoothness kernel estimated based on the statistical map and mask file.
Correlations between mean ALFF values in regions with identified abnormalities and clinical variables (PANSS, GAF and BACS scores) were examined separately in the two patient groups. Correlation between the probands and their relatives in mean ALFF values of all voxels in regions with abnormal ALFF in proband groups were computed separately in SCZ and PBD groups to explore within-family consistency (familiality) of effects.
Age, handedness, years of education and head motion were not significantly different among the proband and control groups. Sex ratios were significantly different among SCZ, PBD and control groups, and therefore sex was used as a covariate in the analyses of probands and controls. There were no significant differences in age, sex, handedness, years of education or head motion among the SCZ relatives, PBD relatives and controls (p>0.05, two-tailed).
All procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Results
Regional cerebral function
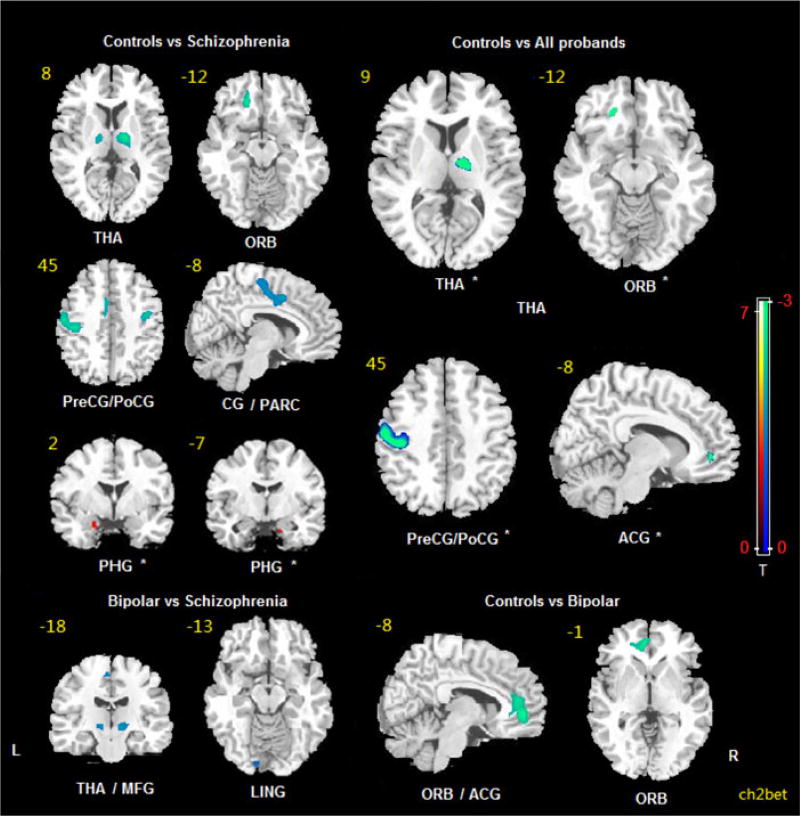
The ANOVA comparing patient groups and controls showed significant differences of the ALFF in the right thalamus, left precentral/postcentral gyrus, left orbital frontal cortex and left anterior cingulate cortex (Table 2 and Fig. 1). Relative to the control group, the SCZ probands showed a significantly decreased ALFF in the bilateral precentral/postcentral gyrus, bilateral thalamus, left orbital frontal cortex, left cingulate gyrus (extending to the left paracentral lobule) and an increased ALFF in the bilateral parahippocampal gyri. PBD probands relative to controls showed a decreased ALFF in the left orbital frontal cortex and left anterior cingulate. Thus there were four areas of altered ALFF detected in the patient compared with control analysis (right thalamus, left pre/postcentral gyrus, left orbitofrontal cortex, left anterior cingulate cortex) and two additional parahippocampal gyri findings in the SCZ group relative to controls. These six areas were used as seeds in connectivity analyses.
Table 2.
The location of regions where ALFF values were altered in the whole-brain analysis
| Location | MNI: peak voxel
|
Cluster size |
p | Mean ALFF value (s.d.)
|
||||
|---|---|---|---|---|---|---|---|---|
| x | y | z | Controls | Bipolar | Schizophrenia | |||
| ANOVA analyses of patient and control groups | ||||||||
| Left precentral/postcentral gyrus# | −42 | −21 | 60 | 162 | # | 0.98 (0.29) | 0.83 (0.21) | 0.73 (0.17) |
| Right thalamus# | 18 | −12 | 9 | 71 | # | 0.78 (0.15) | 0.74 (0.14) | 0.65 (0.15) |
| Left orbital frontal cortex# | −18 | 36 | −15 | 20 | # | 0.60 (0.14) | 0.52 (0.12) | 0.19 (0.12) |
| Left anterior cingulate cortex# | −6 | 45 | −3 | 12 | # | 1.23 (0.34) | 1.00 (0.29) | 1.10 (0.25) |
| Control group>bipolar group | ||||||||
| Left orbital frontal cortex/anterior cingulate cortex | −6 | 45 | −3 | 98 | # | 1.08 (0.24) | 0.90 (0.20) | |
| Control group>schizophrenia group | ||||||||
| Left precentral/postcentral gyrus | −42 | −21 | 60 | 20 | # | 0.96 (0.25) | 0.75 (0.17) | |
| Right precentral/postcentral gyrus | 39 | −12 | 42 | 30 | # | 0.81 (0.26) | 0.66 (0.11) | |
| Right thalamus | 18 | −12 | 9 | 107 | # | 0.80 (0.15) | 0.67 (0.15) | |
| Left thalamus | −9 | −15 | −6 | 40 | # | 0.76 (0.14) | 0.66 (0.14) | |
| Left cingulated gyrus/paracentral lobule | −6 | 3 | 42 | 72 | # | 0.90 (0.19) | 0.74 (0.12) | |
| Left orbital frontal cortex | −18 | 33 | −15 | 35 | # | 0.60 (0.13) | 0.49 (0.11) | |
| Schizophrenia group>control group | ||||||||
| Left parahippocampus# | −18 | 0 | −27 | 24 | # | 0.74 (0.28) | 0.98 (0.42) | |
| Right parahippocampus# | 18 | −8 | −29 | 11 | # | 0.75 (0.18) | 0.94 (0.32) | |
ALFF, Amplitude of low-frequency fluctuations; MNI, Montreal Neurological Institute; s.d., standard deviation; ANOVA, analysis of variance.
p<0.001.
Fig. 1.
Comparisons of the amplitude of low-frequency fluctuations (ALFF) among schizophrenia probands, psychotic bipolar probands and normal controls (p<0.05, AlphaSim corrected). The blue areas indicate lower ALFF in the latter group; the red areas indicate higher ALFF. The slice location is marked in the upper-left. The color bars on the right indicate the T values from comparisons of the two groups. * Areas used as seed regions. THA, Thalamus; ORB, orbital frontal cortex; PreCG, precentral gyrus; PoCG, postcentral gyrus; CG, cingulate cortex; PARC, paracentral lobe; PHG, parahippocampus; ACG, anterior cingulate cortex; L, left; MFG, middle frontal gyrus; LING, lingual gyrus; R, right.
Direct comparison between the proband groups showed a lower ALFF in SCZ than PBD in the bilateral thalamus, left medial frontal gyrus and left lingual gyrus. Details about localization of these findings are presented in Table 2. Neither PBD relatives nor SCZ relatives showed significant ALFF abnormalities relative to controls.
Exploratory correlational analyses were conducted for heuristic purposes between ALFF values and clinical measures conducted separately for each group without correction for multiple comparison. In SCZ patients, the ALFF in the right parahippocampal gyrus was correlated with positive symptoms (r = 0.37, p=0.029) and poorer BACS total scores were correlated with ALFF values in the left parahippocampal gyrus (r =−0.40, p=0.015) (see online Supplementary Tables S1–S5). Thus, the increases in parahippocampal ALFF values in the SCZ group were related to more severe positive symptoms and greater cognitive impairment. No associations with clinical measures were seen in PBD. Dose of antipsychotic treatment, and anticonvulsant use in PBD, were unrelated to identified brain abnormalities. The mean within-family intercorrelations of ALFF values were not significant in any region with ALFF alterations in either proband group, suggesting that regional resting-state abnormalities were not highly familial.
Neural network function
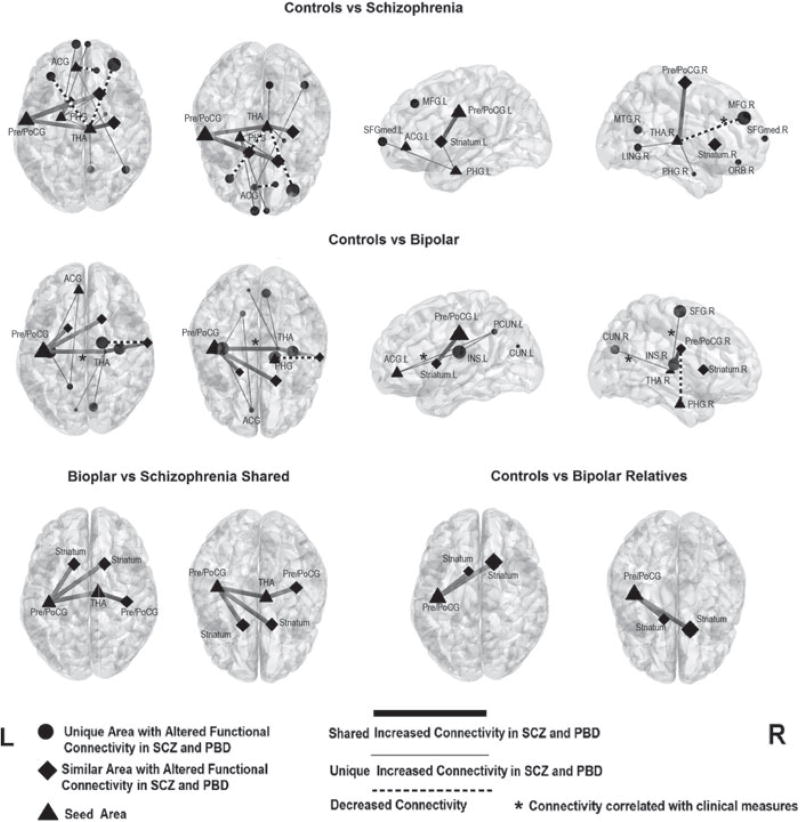
Multiple regions had altered FC with the six seed areas described above (Table 3 and Fig. 2). Relative to controls, both SCZ and PBD proband groups had increased FC: (1) between the left precentral/postcentral gyri with the bilateral striatum; and (2) between the bilateral precentral/postcentral gyri and the right thalamus. Disorder-specific alterations in connectivity were also identified. In SCZ, increased FC was seen: (1) between the right thalamus and both the right lingual gyrus and bilateral parahippocampus; (2) between the left parahippocampus and both the right striatum and bilateral medial superior frontal gyrus; and (3) between the left anterior cingulate cortex and the right middle temporal gyrus. Decreased FC in SCZ was observed: (1) between the right thalamus and the bilateral middle frontal gyrus; and (2) between the left anterior cingulate cortex and the right orbital frontal cortex. In PBD, disorder-specific increased FC was observed: (1) between the right thalamus and a large contiguous volume of the cortex including the left insula, left pre- and postcentral gyri and the right superior frontal gyrus; (2) between the right thalamus and bilateral cuneus; and (3) between the left anterior cingulate cortex and left precuneus (all p<0.05 using AlphaSim to correct for multiple comparisons). Decreased FC in PBD was identified between the right parahippocampus and the right pre- and postcentral gyri. Differences in FC between SCZ and PBD were not significant.
Table 3.
Significant alterations of functional connectivity with the six identified seed regions in probands with schizophrenia and psychotic bipolar disorder
| Seed area | Area with altered functional connectivity |
pa | MNI (peak voxel)
|
Cluster size |
||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Schizophrenia group>control group | ||||||
| Left precentral/postcentral | Right striatum | 0.024 | 18 | 18 | 3 | 59 |
| Left striatum | 0.005 | −9 | 10 | −2 | 99 | |
| Right thalamus | <0.001 | 9 | −18 | 10 | 284 | |
| Right thalamus | Bilateral precentral/postcentral gyri | <0.001 | −57 | −9 | 33 | 1767 |
| Right parahippocampus | 0.004 | 24 | −3 | −27 | 111 | |
| Left parahippocampus | 0.031 | −21 | −6 | −27 | 54 | |
| Right lingual gyrus | 0.021 | 9 | −60 | 0 | 13 | |
| Left parahippocampus | Right striatum | 0.003 | 18 | 0 | 21 | 106 |
| Bilateral medial superior frontal gyri | 0.036 | −6 | 69 | 3 | 47 | |
| Left anterior cingulate cortex | Right middle temporal gyrus | 0.049 | 45 | −60 | 18 | 45 |
| Control group>schizophrenia group | ||||||
| Right thalamus | Right middle frontal gyrus | 0.043 | 33 | 48 | 30 | 46 |
| Left middle frontal gyrus | 0.039 | −36 | 30 | 51 | 48 | |
| Left anterior cingulate cortex | Right orbital frontal cortex | 0.038 | 12 | 51 | −24 | 51 |
| Bipolar group>control group | ||||||
| Left precentral/postcentral | Right striatum | 0.002 | 18 | 15 | 6 | 130 |
| Left striatum | 0.002 | −15 | 3 | 3 | 136 | |
| Right thalamus | <0.001 | 15 | −12 | 6 | 275 | |
| Right thalamus | Right precentral/postcentral gyri | 0.002 | 63 | −9 | 27 | 128 |
| Left precentral/postcentral gyri/left insula/right superior frontal gyrus | <0.001 | −36 | −18 | 18 | 1831 | |
| Right insula | <0.001 | 36 | −15 | 12 | 199 | |
| Bilateral cuneus | 0.012 | −9 | −78 | 24 | 76 | |
| Left anterior cingulate cortex | Left precuneus | 0.045 | −7 | −54 | 40 | 45 |
| Control group>bipolar group | ||||||
| Right parahippocampus | Right precentral/postcentral gyri | 0.035 | 12 | −33 | 75 | 47 |
| Bipolar relatives group>control group | ||||||
| Left precentral/postcentral | Left striatum | 0.026 | −12 | 12 | 3 | 59 |
| Right striatum | <0.001 | 12 | 6 | 6 | 198 | |
| Schizophrenia relatives group>control group | ||||||
| No significant results | ||||||
MNI, Montreal Neurological Institute.
p Value was corrected with Monte Carlo simulations in AlphaSim.
Fig. 2.
Comparisons of functional connectivity among schizophrenia (SCZ) probands, psychotic bipolar disorder (PBD) probands, PBD relatives and controls (p<0.05, AlphaSim corrected). Dot, rhombus and triangle locations identify the location of peak group differences within clusters, and the size indicates the T value from the statistics analysis. * Connectivity correlated with clinical measures (detailed in online Supplementary Tables S1–S5). ACG, Anterior cingulate cortex; PHG, parahippocampus; PreCG, precentral gyrus; PoCG, postcentral gyrus; THA, thalamus; MFG, middle frontal gyrus; L, left; SFGmed, superior frontal gyrus (medial); MTG, middle temporal gyrus; R, right; LING, lingual gyrus; ORB, orbital frontal cortex; PCUN, precuneus; INS, insula; CUN, cuneus.
In SCZ probands, those with decreased connectivity between the thalamus and the right middle frontal gyrus had lower GAF scores (r =0.39, p=0.018). In PBD probands, the increased relationship between the thalamus and a contiguous region including the left precentral/postcentral gyrus/left insula/right superior frontal gyrus was correlated with negative symptom severity (r =0.27, p=0.044) and BACS scores (r = 0.40, p= 0.002), the increased functional relationship between the thalamus and bilateral cuneus was correlated with BACS scores (r =0.40, p=0.002), and increased FC between the left anterior cingulate and left precuneus was correlated with negative symptoms (r =0.36, p=0.006) (see online Supplementary Tables S1–S5).
FC was examined in the relative groups using the areas with regional deficits identified in the analysis of probands as seeds. Similar to both proband groups, PBD relatives had increased network connectivity relative to controls between the left precentral/postcentral gyrus and bilateral caudate. Correlations of connectivity values between probands and their relatives were not significant, suggesting limited familiality. SCZ relatives showed no significant FC alterations paralleling those seen in the SCZ probands. No significant correlations were identified between the ALFF of seed regions and connectivity strength (p>0.05) in either probands or relatives.
Discussion
Using rfMRI, the present study directly compared regional brain function (ALFF) and FC within widely distributed brain networks in SCZ and PBD probands. Though SCZ and PBD probands shared regional and FC deficits within striatal-thalamo-cortical networks, SCZ showed more and greater regional functional deficits and more nodes with abnormal network connectivity. However, there were no significant differences in FC between SCZ and PBD probands when the groups were compared directly. Second, we investigated the unaffected relatives of the probands to determine whether they expressed abnormalities observed in their proband relatives in order to assess their potential as endophenotypes for family genetic risk. We observed one such effect: an alteration in frontostriatal circuitry in PBD relatives that was identified in both proband groups. SCZ and PBD probands showed similar reductions of the ALFF in the left orbital frontal cortex and left cingulate gyrus. Deficits in these regions have been demonstrated in prior structural/anatomical studies of SCZ (Wang et al. 2007; Nakamura et al. 2008) and PBD (Bora et al. 2010). Independent studies of PBD (Rolls, 1996) and SCZ (Gur et al. 2000) have revealed relationships between deficits of the orbitofrontal and cingulate cortex with abnormalities of cognition, behavior and emotion. Our findings thus suggest a role of hypoactivity of the orbital frontal cortex and cingulate gyrus in pathogenesis across psychotic disorders, including both PBD and SCZ.
SCZ probands showed more extensive regional functional deficits than PBD probands. SCZ showed reduced function in the left precentral and postcentral gyri, in the bilateral thalamus and in the left paracentral lobule, as well as an increased rather than decreased ALFF in the parahippocampus that was related to more severe cognitive deficit and positive symptoms. Previous studies have found hippocampal system hyperactivity in SCZ related to positive symptoms (Medoff et al. 2001). Additionally, it is note-worthy that even relative to the PBD probands, the SCZ probands indicated more severe and widespread regional functional deficits within thalamo-cortical networks. These findings provide important new direct evidence to support the model that thalamo-cortical network abnormalities are important in the pathogenesis of psychotic disorders, and that they are greater in psychotic disorders with more persistent psychotic symptoms and greater cognitive deficits as are typically more pronounced in SCZ than PBD.
Consistent with the regional function findings, we also identified both overlapping and unique abnormalities of FC within striatal-thalamo-cortical networks in SCZ and PBD probands. These findings in broad terms parallel those observed in a recent rfMRI study using an exploratory whole-brain FC analysis method in a different sample (Khadka et al. 2013). Using seed-based correlation analysis, the current study identified areas with regional dysfunction, and then evaluated the FC of those regions with other brain areas. Compared with data-driven methods, this hypothesis-driven approach for examining FC provides a direct assessment of functional correlates at the network level of abnormalities seen in regional brain function (Shehzad et al. 2009; Cole et al. 2010). Additionally, correction for multiple comparison to control type I error can be less stringent by limiting the number of statistical tests to only those areas with demonstrated regional dysfunction (Poldrack, 2007), enhancing statistical power and robustness of statistical results. This approach has been useful in previous fMRI studies of both healthy individuals and several neuropsychiatric disorders (Horwitz et al. 1998; Lui et al. 2009a, b, 2011).
Both proband groups showed increased FC between the basal ganglia and both motor and somatosensory cortical areas, which are important components of the striatal-thalamo-cortical loops. Consistent with our findings, previous task-based fMRI studies of SCZ (Siegel et al. 1993) and PBD (Blumberg et al. 2003) demonstrated abnormality in these areas. Also, previous findings have shown that striatal-thalamocortical networks have changed after antipsychotic treatment in both SCZ (Keedy et al. 2009; Lui et al. 2010) and PBD (Phillips et al. 2008), and the alterations is relation to clinical improvement.
Though SCZ and PBD probands showed overlapping abnormalities in FC, unique circuitry dysfunctions of each disorder were also prominent. Multiple lines of evidence from functional and structural imaging studies and postmortem work (Minzenberg et al. 2009; Welsh et al. 2010) support a core role of thalamo-cortical systems in SCZ, the dysfunction of which may account for a range of its clinical and cognitive symptoms (Kyriakopoulos et al. 2009). Of note, the thalamo-cortical alterations were somewhat different in PBD, including an increased FC between the thalamus and bilateral insula that was associated with more severe negative symptoms. Previous studies have implicated a role for insular abnormalities in BD, which is part of the paralimbic cortex that plays a critical role in emotion processing (Wang et al. 2011). Increased connectivity of the parahippocampus with the thalamus and striatum was seen only in SCZ probands.
It should be noted that some altered connectivity in SCZ and PBD belonged to the same general networks, but differed in detail. For example, though both SCZ and PBD showed increased connectivity between the thalamus and visual cortex, SCZ probands showed abnormal connectivity with the lingual gyrus, while in PBD it involved the cuneus and precuneus. Further, though both SCZ and PBD showed abnormal connectivity between the right thalamus and prefrontal areas, some abnormalities were in opposite directions although related to clinical dimensions. Finally, we observed that both SCZ and PBD probands showed abnormal connectivity within the DMN but involving different areas. A recent study also suggested that different DMN alterations distinguish SCZ and PBD from each other and from healthy controls (Calhoun et al. 2011).
We also examined unaffected SCZ and PBD relatives to ascertain if they showed similar changes to those seen in their proband relatives. Neither SCZ nor PBD relatives showed parallel regional functional alterations in the ALFF seen in their proband relatives, and a whole-brain analysis of ALFF values failed to identify abnormalities relative to controls in either relative group. This suggests that some functional brain alterations seen in the patients reflect physiological alterations related to a history of psychosis in patients, its treatment, rather than manifestations of common familial/genetic risk factors. Even though our patients were clinically and pharmacologically stable, this possibility is raised by findings indicating that some resting-state functional changes in SCZ can change after antipsychotic treatment and clinical remission (Lui et al. 2010). It is also possible that by excluding relatives with any Axis I DSM-IV disorder to identify familial characteristics unrelated to illness expression we may have excluded relatives more likely to manifest brain alterations of interest. Last, it remains possible that some additional abnormalities, group differences or trends in family members might be detected with larger samples.
One finding in the PBD relatives of particular interest was the increased connectivity between the precentral/postcentral gyrus and bilateral striatum, an alteration that was seen in both SCZ and PBD probands. This suggests that PBD relatives may more often, or with greater penetrance than in SCZ, show alterations in this circuitry, and that it may represent a useful endophenotype for tracking risk. This would be consistent with the fact that some familial endophenotypes for psychosis, such as saccadic and pursuit eye movement abnormalities, are heavily dependent upon the integrity of striatal–precentral connectivity (Sweeney et al. 1996, 1998). However, certain negative findings from the present study fail to support this line of inference: (1) SCZ relatives did not show this finding; (2) this finding was not increased in the small group of relatives with psychosis spectrum personality disorders; and (3) within-family correlation of the abnormality was not observed. However, we note again that some effects of interest might not be identified due to our sample size or confounds related to illness chronicity, medication and possible progressive cerebral changes in probands. Future studies testing untreated first-episode patients could help clarify familiality effects. As the B-SNIP study is the first large parallel study of these relative groups with these measures, more work is needed to validate this and other resting-state abnormalities as endophenotypes for psychotic disorders. Further validation of methods for combining resting-state data from sites with different MRI scanners, especially for connectivity analysis, will benefit this effort.
The strategy used in the present study, which was relatively novel in the parallel recruitment of SCZ and PBD probands and their relatives, was also novel in its data-analytic approach. This latter aspect of our study brings both strengths and limitations. To proceed in a focused hypothesis-driven manner, we examined whole-brain resting physiology to define regions of dysfunction in SCZ and PBD, then tested for potential impact of those deficits on the integration of those regions in functional brain circuitry, and whether regional and connectivity alterations identified in probands were present in unaffected relatives. Thus, analyses of connectivity in probands and of regional function and connectivity in relatives were focused on areas where patients demonstrated regional abnormalities. While this approach reduced the risk of falsepositive findings and increased the power to detect effects of interest, it does not rule out the possibility of other connectivity abnormalities or compensations in the unaffected relatives. Other factors should be considered when interpreting the present results. First, medications used to treat the SCZ and PBD were not equivalent. While treatments were stable, and correlational analyses of MRI data and medication treatment were not significant, medication treatments may have influenced regional and neural network function measurements in our study (Phillips et al. 2008; Lui et al. 2010), perhaps differentially in the two patient groups. Second, although we examined relationships between fMRI data and current clinical symptoms, an important remaining question is the relationship between brain function and course-of-illness factors that distinguish BPD and SCZ, such as the persistence of psychosis and the prominence of affective features during acute episodes over the course of illness. Future studies with such data will further clarify the relationships of abnormal brain function and dimensions of illness presentation across the disorders, and the extent to which there is a continuum of such effects in schizo-affective patients between SCZ and PBD. Also, generalization to other psychotic disorders remains to be determined.
Overall, SCZ and PBD probands showed similar regional deficits in prefrontal cortex and parallel abnormalities of FC within striatal-thalamo-cortical networks, but different regional and neural network deficits as well. The overlapping findings present in both disorders might account for some clinical similarities across them. The pattern of both overlapping and distinctive patterns of abnormalities in SCZ and PBD probands suggests a model for rapprochement between continuum views of psychosis and views that the disorders are fully distinct. Future studies combining brain measurements with behavioral data over the course of illness can provide a better understanding of similarities and differences between SCZ and PBD and how to best define them. Better understanding of these boundaries and relationships is crucial not only to develop better models of the pathophysiology of psychosis, but for improving and bringing greater objectivity to clinical diagnosis and treatment planning.
Supplementary Material
Acknowledgments
This work was supported by National Institute of Mental Health grants (no. MH078113, MH077945, MH077852, MH077851, MH077862, MH072767 and MH083888) and the National Natural Science Foundation (China; grants no. 81222018, 81371527, 81030027, 81227002 and 81220108013). C.A.T. has received support from Intracellular Therapies (ITI, Inc.), PureTech Ventures, Eli Lilly, Sunovion, Astellas, Merck (ad hoc consulting), International Congress on Schizophrenia Research (unpaid volunteer),National Alliance on Mental Illness (unpaid volunteer), the American Psychiatric Association (Deputy Editor) and Finnegan Henderson Farabow Garrett & Dunner, LLP. S.K. has received support from Sunovion and GlaxoSmithKline. J.A.S. has received support from Takeda, Pfizer, BMS, Eli Lilly and Janssen. R.S.K. has received support from Allon, AstraZeneca, the Department of Veterans’ Affairs, Feinstein Institute for Medical Research, GlaxoSmithKline, the National Institute of Mental Health, Novartis, Psychogenics, Research Foundation for Mental Hygiene, Inc. and Singapore National Medical Research Council. R.S.K. also receives royalties from the BACS and Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) testing batteries and is a shareholder in NeuroCog Trials, Inc., Durham, NC.
Footnotes
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S003329171400110X.
Declaration of Interest
None.
References
- Andreasen NC, O’Leary DS, Cizadlo T, Arndt S, Rezai K, Ponto LL, Watkins GL, Hichwa RD. Schizophrenia and cognitive dysmetria: a positron-emission tomography study of dysfunctional prefrontal-thalamic-cerebellar circuitry. Proceedings of the National Academy of Sciences USA. 1996;93:9985–9990. doi: 10.1073/pnas.93.18.9985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg HP, Martin A, Kaufman J, Leung HC, Skudlarski P, Lacadie C, Fulbright RK, Gore JC, Charney DS, Krystal JH, Peterson BS. Frontostriatal abnormalities in adolescents with bipolar disorder: preliminary observations from functional MRI. American Journal of Psychiatry. 2003;160:1345–1347. doi: 10.1176/appi.ajp.160.7.1345. [DOI] [PubMed] [Google Scholar]
- Bora E, Fornito A, Yucel M, Pantelis C. Voxelwise meta-analysis of gray matter abnormalities in bipolar disorder. Biological Psychiatry. 2010;67:1097–1105. doi: 10.1016/j.biopsych.2010.01.020. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Sui J, Kiehl K, Turner J, Allen E, Pearlson G. Exploring the psychosis functional connectome: aberrant intrinsic networks in schizophrenia and bipolar disorder. Frontiers in Psychiatry. 2011;2:75. doi: 10.3389/fpsyt.2011.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai XJ, Whitfield-Gabrieli S, Shinn AK, Gabrieli JD, Nieto Castanon A, McCarthy JM, Cohen BM, Ongur D. Abnormal medial prefrontal cortex resting-state connectivity in bipolar disorder and schizophrenia. Neuropsychopharmacology. 2011;36:2009–2017. doi: 10.1038/npp.2011.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole DM, Smith SM, Beckmann CF. Advances and pitfalls in the analysis and interpretation of resting-state FMRI data. Frontiers in Systems Neuroscience. 2010;4:8. doi: 10.3389/fnsys.2010.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordes D, Haughton VM, Arfanakis K, Carew JD, Turski PA, Moritz CH, Quigley MA, Meyerand ME. Frequencies contributing to functional connectivity in the cerebral cortex in “resting-state” data. American Journal of Neuroradiology. 2001;22:1326–1333. [PMC free article] [PubMed] [Google Scholar]
- Costafreda SG, Fu CH, Picchioni M, Toulopoulou T, McDonald C, Kravariti E, Walshe M, Prata D, Murray RM, McGuire PK. Pattern of neural responses to verbal fluency shows diagnostic specificity for schizophrenia and bipolar disorder. BMC Psychiatry. 2011;11:18. doi: 10.1186/1471-244X-11-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison-Wright I, Bullmore E. Anatomy of bipolar disorder and schizophrenia: a meta-analysis. Schizophrenia Research. 2010;117:1–12. doi: 10.1016/j.schres.2009.12.022. [DOI] [PubMed] [Google Scholar]
- Filippi M, Canu E, Gasparotti R, Agosta F, Valsecchi P, Lodoli G, Galluzzo A, Comi G, Sacchetti E. Patterns of brain structural changes in first-contact, antipsychotic drug-naive patients with schizophrenia. American Journal of Neuroradiology. 2013;35:30–37. doi: 10.3174/ajnr.A3583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ. Functional and effective connectivity in neuroimaging: a synthesis. Human Brain Mapping. 1994;2:56–78. [Google Scholar]
- Gur RE, Cowell PE, Latshaw A, Turetsky BI, Grossman RI, Arnold SE, Bilker WB, Gur RC. Reduced dorsal and orbital prefrontal gray matter volumes in schizophrenia. Archives of General Psychiatry. 2000;57:761–768. doi: 10.1001/archpsyc.57.8.761. [DOI] [PubMed] [Google Scholar]
- Hill SK, Reilly JL, Keefe RS, Gold JM, Bishop JR, Gershon ES, Tamminga CA, Pearlson GD, Keshavan MS, Sweeney JA. Neuropsychological impairments in schizophrenia and psychotic bipolar disorder: findings from the Bipolar and Schizophrenia Network on Intermediate Phenotypes (B-SNIP) Study. American Journal of Psychiatry. 2013;170:1275–1284. doi: 10.1176/appi.ajp.2013.12101298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz B, Rumsey JM, Donohue BC. Functional connectivity of the angular gyrus in normal reading and dyslexia. Proceedings of the National Academy of Sciences USA. 1998;95:8939–8944. doi: 10.1073/pnas.95.15.8939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houenou J, d’Albis MA, Vederine FE, Henry C, Leboyer M, Wessa M. Neuroimaging biomarkers in bipolar disorder. Frontiers in Bioscience. 2012;4:593–606. doi: 10.2741/e402. [DOI] [PubMed] [Google Scholar]
- Ivleva EI, Morris DW, Moates AF, Suppes T, Thaker GK, Tamminga CA. Genetics and intermediate phenotypes of the schizophrenia–bipolar disorder boundary. Neuroscience and Biobehavioral Reviews. 2010;34:897–921. doi: 10.1016/j.neubiorev.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keedy SK, Rosen C, Khine T, Rajarethinam R, Janicak PG, Sweeney JA. An fMRI study of visual attention and sensorimotor function before and after antipsychotic treatment in first-episode schizophrenia. Psychiatry Research. 2009;172:16–23. doi: 10.1016/j.pscychresns.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khadka S, Meda SA, Stevens MC, Glahn DC, Calhoun VD, Sweeney JA, Tamminga CA, Keshavan MS, O’Neil K, Schretlen D, Pearlson GD. Is aberrant functional connectivity a psychosis endophenotype? A resting state functional magnetic resonance imaging study. Biological Psychiatry. 2013;74:458–466. doi: 10.1016/j.biopsych.2013.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiviniemi V, Jauhiainen J, Tervonen O, Paakko E, Oikarinen J, Vainionpaa V, Rantala H, Biswal B. Slow vasomotor fluctuation in fMRI of anesthetized child brain. Magnetic Resonance in Medicine. 2000;44:373–378. doi: 10.1002/1522-2594(200009)44:3<373::aid-mrm5>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Kruger S, Alda M, Young LT, Goldapple K, Parikh S, Mayberg HS. Risk and resilience markers in bipolar disorder: brain responses to emotional challenge in bipolar patients and their healthy siblings. American Journal of Psychiatry. 2006;163:257–264. doi: 10.1176/appi.ajp.163.2.257. [DOI] [PubMed] [Google Scholar]
- Kyriakopoulos M, Perez-Iglesias R, Woolley JB, Kanaan RA, Vyas NS, Barker GJ, Frangou S, McGuire PK. Effect of age at onset of schizophrenia on white matter abnormalities. British Journal of Psychiatry. 2009;195:346–353. doi: 10.1192/bjp.bp.108.055376. [DOI] [PubMed] [Google Scholar]
- Lichtenstein P, Yip BH, Bjork C, Pawitan Y, Cannon TD, Sullivan PF, Hultman CM. Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: a population-based study. Lancet. 2009;373:234–239. doi: 10.1016/S0140-6736(09)60072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Tang Y, Womer F, Fan G, Lu T, Driesen N, Ren L, Wang Y, He Y, Blumberg HP, Xu K, Wang F. Differentiating patterns of amygdala-frontal functional connectivity in schizophrenia and bipolar disorder. Schizophrenia Bulletin. 2014;40:469–477. doi: 10.1093/schbul/sbt044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412:150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- Lui S, Deng W, Huang X, Jiang L, Ma X, Chen H, Zhang T, Li X, Li D, Zou L, Tang H, Zhou XJ, Mechelli A, Collier DA, Sweeney JA, Li T, Gong Q. Association of cerebral deficits with clinical symptoms in antipsychotic-naive first-episode schizophrenia: an optimized voxel-based morphometry and resting state functional connectivity study. American Journal of Psychiatry. 2009a;166:196–205. doi: 10.1176/appi.ajp.2008.08020183. [DOI] [PubMed] [Google Scholar]
- Lui S, Huang X, Chen L, Tang H, Zhang T, Li X, Li D, Kuang W, Chan RC, Mechelli A, Sweeney JA, Gong Q. High-field MRI reveals an acute impact on brain function in survivors of the magnitude 8.0 earthquake in China. Proceedings of the National Academy of Sciences USA. 2009b;106:15412–15417. doi: 10.1073/pnas.0812751106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui S, Li T, Deng W, Jiang L, Wu Q, Tang H, Yue Q, Huang X, Chan RC, Collier DA, Meda SA, Pearlson G, Mechelli A, Sweeney JA, Gong Q. Short-term effects of antipsychotic treatment on cerebral function in drug-naive first-episode schizophrenia revealed by “resting state” functional magnetic resonance imaging. Archives of General Psychiatry. 2010;67:783–792. doi: 10.1001/archgenpsychiatry.2010.84. [DOI] [PubMed] [Google Scholar]
- Lui S, Wu Q, Qiu L, Yang X, Kuang W, Chan RC, Huang X, Kemp GJ, Mechelli A, Gong Q. Resting-state functional connectivity in treatment-resistant depression. American Journal of Psychiatry. 2011;168:642–648. doi: 10.1176/appi.ajp.2010.10101419. [DOI] [PubMed] [Google Scholar]
- McIntosh AM, Whalley HC, McKirdy J, Hall J, Sussmann JE, Shankar P, Johnstone EC, Lawrie SM. Prefrontal function and activation in bipolar disorder and schizophrenia. American Journal of Psychiatry. 2008;165:378–384. doi: 10.1176/appi.ajp.2007.07020365. [DOI] [PubMed] [Google Scholar]
- Meda SA, Gill A, Stevens MC, Lorenzoni RP, Glahn DC, Calhoun VD, Sweeney JA, Tamminga CA, Keshavan MS, Thaker G, Pearlson GD. Differences in resting-state functional magnetic resonance imaging functional network connectivity between schizophrenia and psychotic bipolar probands and their unaffected first-degree relatives. Biological Psychiatry. 2012;71:881–889. doi: 10.1016/j.biopsych.2012.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medoff DR, Holcomb HH, Lahti AC, Tamminga CA. Probing the human hippocampus using rCBF: contrasts in schizophrenia. Hippocampus. 2001;11:543–550. doi: 10.1002/hipo.1070. [DOI] [PubMed] [Google Scholar]
- Minzenberg MJ, Laird AR, Thelen S, Carter CS, Glahn DC. Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Archives of General Psychiatry. 2009;66:811–822. doi: 10.1001/archgenpsychiatry.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed MA, Yousem DM, Tekes A, Browner N, Calhoun VD. Correlation between the amplitude of cortical activation and reaction time: a functional MRI study. American Journal of Roentgenology. 2004;183:759–765. doi: 10.2214/ajr.183.3.1830759. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Nestor PG, Levitt JJ, Cohen AS, Kawashima T, Shenton ME, McCarley RW. Orbitofrontal volume deficit in schizophrenia and thought disorder. Brain. 2008;131:180–195. doi: 10.1093/brain/awm265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongur D, Lundy M, Greenhouse I, Shinn AK, Menon V, Cohen BM, Renshaw PF. Default mode network abnormalities in bipolar disorder and schizophrenia. Psychiatry Research. 2010;183:59–68. doi: 10.1016/j.pscychresns.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, Travis MJ, Fagiolini A, Kupfer DJ. Medication effects in neuroimaging studies of bipolar disorder. American Journal of Psychiatry. 2008;165:313–320. doi: 10.1176/appi.ajp.2007.07071066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA. Region of interest analysis for fMRI. Social Cognitive and Affective Neuroscience. 2007;2:67–70. doi: 10.1093/scan/nsm006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET. The orbitofrontal cortex. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 1996;351:1433–1444. doi: 10.1098/rstb.1996.0128. [DOI] [PubMed] [Google Scholar]
- Shehzad Z, Kelly AM, Reiss PT, Gee DG, Gotimer K, Uddin LQ, Lee SH, Margulies DS, Roy AK, Biswal BB, Petkova E, Castellanos FX, Milham MP. The resting brain: unconstrained yet reliable. Cerebral Cortex. 2009;19:2209–2229. doi: 10.1093/cercor/bhn256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel BV, Jr, Buchsbaum MS, Bunney WE, Jr, Gottschalk LA, Haier RJ, Lohr JB, Lottenberg S, Najafi A, Nuechterlein KH, Potkin SG, et al. Cortical–striatal–thalamic circuits and brain glucose metabolic activity in 70 unmedicated male schizophrenic patients. American Journal of Psychiatry. 1993;150:1325–1336. doi: 10.1176/ajp.150.9.1325. [DOI] [PubMed] [Google Scholar]
- Sweeney JA, Luna B, Srinivasagam NM, Keshavan MS, Schooler NR, Haas GL, Carl JR. Eye tracking abnormalities in schizophrenia: evidence for dysfunction in the frontal eye fields. Biological Psychiatry. 1998;44:698–708. doi: 10.1016/s0006-3223(98)00035-3. [DOI] [PubMed] [Google Scholar]
- Sweeney JA, Mintun MA, Kwee S, Wiseman MB, Brown DL, Rosenberg DR, Carl JR. Positron emission tomography study of voluntary saccadic eye movements and spatial working memory. Journal of Neurophysiology. 1996;75:454–468. doi: 10.1152/jn.1996.75.1.454. [DOI] [PubMed] [Google Scholar]
- Tamminga CA, Ivleva EI, Keshavan MS, Pearlson GD, Clementz BA, Witte B, Morris DW, Bishop J, Thaker GK, Sweeney JA. Clinical phenotypes of psychosis in the Bipolar and Schizophrenia Network on Intermediate Phenotypes (B-SNIP) American Journal of Psychiatry. 2013;170:1263–1274. doi: 10.1176/appi.ajp.2013.12101339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Kalmar JH, Womer FY, Edmiston EE, Chepenik LG, Chen R, Spencer L, Blumberg HP. Olfactocentric paralimbic cortex morphology in adolescents with bipolar disorder. Brain. 2011;134:2005–2012. doi: 10.1093/brain/awr124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Hosakere M, Trein JC, Miller A, Ratnanather JT, Barch DM, Thompson PA, Qiu A, Gado MH, Miller MI, Csernansky JG. Abnormalities of cingulate gyrus neuroanatomy in schizophrenia. Schizophrenia Research. 2007;93:66–78. doi: 10.1016/j.schres.2007.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh RC, Chen AC, Taylor SF. Low-frequency BOLD fluctuations demonstrate altered thalamocortical connectivity in schizophrenia. Schizophrenia Bulletin. 2010;36:713–722. doi: 10.1093/schbul/sbn145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessa M, Linke J. Emotional processing in bipolar disorder: behavioural and neuroimaging findings. International Review of Psychiatry. 2009;21:357–367. doi: 10.1080/09540260902962156. [DOI] [PubMed] [Google Scholar]
- Whalley HC, Simonotto E, Flett S, Marshall I, Ebmeier KP, Owens DG, Goddard NH, Johnstone EC, Lawrie SM. fMRI correlates of state and trait effects in subjects at genetically enhanced risk of schizophrenia. Brain. 2004;127:478–490. doi: 10.1093/brain/awh070. [DOI] [PubMed] [Google Scholar]
- Yang H, Long XY, Yang Y, Yan H, Zhu CZ, Zhou XP, Zang YF, Gong QY. Amplitude of low frequency fluctuation within visual areas revealed by resting-state functional MRI. Neuroimage. 2007;36:144–52. doi: 10.1016/j.neuroimage.2007.01.054. [DOI] [PubMed] [Google Scholar]
- Yucel M, Brewer WJ, Harrison BJ, Fornito A, O’Keefe GJ, Olver J, Scott AM, Egan GF, Velakoulis D, McGorry PD, Pantelis C. Anterior cingulate activation in antipsychotic-naive first-episodeschizophrenia. Acta Psychiatrica Scandinavica. 2007;115:155–158. doi: 10.1111/j.1600-0447.2006.00902.x. [DOI] [PubMed] [Google Scholar]
- Zang YF, He Y, Zhu CZ, Cao QJ, Sui MQ, Liang M, Tian LX, Jiang TZ, Wang YF. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain and Development. 2007;29:83–91. doi: 10.1016/j.braindev.2006.07.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.