Abstract
Enterotoxigenic Escherichia coli (ETEC) is primary pathogenic bacteria of piglet diarrhea, over two thirds of piglets diarrhea caused by ETEC are resulted from STa-producing ETEC strains. This experiment was conducted to construct the recombinant E. coli expressing STa and study the injury and mechanism of recombinant E. coli expressing STa on 7 days old piglets colon. Twenty-four 7 days old piglets were allotted to four treatments: control group, STa group (2 × 109 CFU E. coli LMG194-STa), LMG194 group (2 × 109 CFU E. coli LMG194) and K88 group (2 × 109 CFU E. coli K88). The result showed that E. coli infection significantly increased diarrhea rates; changed DAO activity in plasma and colon; damaged colonic mucosal morphology including crypt depth, number of globet cells, density of lymphocytes and lamina propria cell density; substantially reduced antioxidant capacity by altering activities of GSH-Px, SOD, and TNOS and productions of MDA and H2O2; obviously decreased AQP3, AQP4 and KCNJ13 protein expression levels; substantially altered the gene expression levels of inflammatory cytokines. Conclusively, STa group had the biggest effect on these indices in four treatment groups. These results suggested that the recombinant strain expressed STa can induce piglets diarrhea and colonic morphological and funtional damage by altering expression of proteins connect to transportation function and genes associated with intestinal injury and inflammatory cytokines.
Keywords: colon, heat-stable enterotoxin A, piglet, recombinant escherichia coli
Piglet diarrhea is one of the most challenging problems what pig breeding industry has faced at home and abroad, which causes a huge economic loss annually. Enterotoxigenic Escherichia coli (ETEC) is the primary pathogenic bacteria of piglet diarrhea, whose pathogenicity depends on the co-effect of adhesion and enterotoxin [10]. Among the six recognized diarrheagenic classifications of Escherichia coli (E. coli), ETEC is the most ordinary etiologic agent [29].
ETEC isolates secrete two primary toxins (either one or both): the heat-stable (ST) and the heat-labile (LT) enterotoxin [23]. ST enterotoxin is a low-molecular-weight peptide and has no immunogenicity, while LT consists essentially of one A and five B subunits, which is a hexameric protein with strong immunogenicity [22]. There are two varieties of STs, which can be clearly characterised by structure and function: soluble in carbinol, resistant to proteolytic enzyme and binding of guanylyl cyclase-C-STa (also referred to as STI); insoluble in carbinol and sensitive to proteolytic enzyme-STb (STII) [19]. Over two thirds of piglets diarrhea caused by ETEC are resulted from STa-producing ETEC strains [25], so the most heated studies of ETEC are frequently focused on STa [31].
In general, ETEC induces piglets diarrhea and intestinal damage via simultaneous effects of several enterotoxins. Therefore, it is extremely difficult to research the effect of each enterotoxin respectively. In order to study the mechanism of STa and evaluate the effects of drugs or nutrients to the piglet diarrhea caused by STa, the host strant E. coli LMG194 and the plasmid-pBAD202/D-topo were utilized in this research to construct a recombinant E. coli strain named LMG194-STa, which expressed heat-stable enterotoxin A (STa). Furthermore, LMG194-STa rebombinant strain was utilized to investigate its effects as well as mechanism on colonic injury, transport activity and antioxidant capacity of 7 days old piglets.
MATERIALS AND METHODS
Construction and verification of recombinant E. coli strain LMG194-STa
STa gene estA cloned and expressed in nonpathogenic E. coli strain LMG194. Enterotoxin clone DH5a-STa with estA gene of ETEC was presented by agriculture and agri-food center of Canada (AAFC) was cultivated at 37°C in Luria-Bertani (LB) broth or agar. The estA gene was amplified from the DH5a-STa by PCR using the primers 5′- CACCATGAAAAAGCTAATGTT-3′ and 5′- ATAACATCCAGCACAGGCA-3′ and DNA polymerase (Takara, Dalian, China) according to the supplier’s instructions. The PCR product was examined by gel electrophoresis and sequence. The STa fragments were purified from agarose gels using PCR cleanup and gel extraction kit (Takara). The plasmid pBAD-STa was constructed according to the manual of the pBAD202 Directional TOPO® Expression Kit (Invitrogen, Carlsbad, CA, U.S.A.) and verified by enzyme digestion. The recombinant E. coli LMG194 which expression STa of ETEC was constructed by transferring the recombinant plasmid pBAD-STa into the E. coli LMG194 component cell and cultivated on LB agar with kanamycin (30 µg/ml) according the manual of the pBAD202 Directional TOPO® Expression Kit (Invitrogen). The positive clones were cultivated in LB broth with kanamycin (30 µg/ml) and checked by PCR using the primer previously used.
The results showed that 219 bp fragments got when the estA gene was amplified by PCR with the DH5a-STa as template (Fig. 1A). Nucleic acid sequence analysis demonstrated that the fragment was 100% homologous to the estA gene of ETEC. The verification of the recombinant strain shown that the same size fragments got when the estA gene was amplified by PCR with the positive clones as template. The plasmid was extracted from recombinant strain LMG194-STa and identified by enzyme digestion, the result showed the correct size (393+216 bp) fragments got (Fig. 1B). These results means that the recombinant strain expressed STa constructed correctly, named LMG194-STa.
Fig 1.
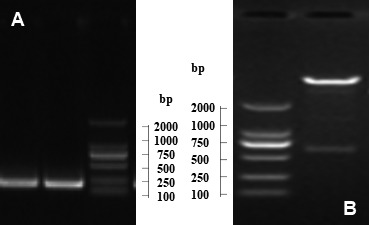
Construction of the recombinant E. coli LMG194-STa. A) Amplification of STa by PCR (216 bp) M: DL2000 Marker. B) Verification of pBAD-STa by enzyme digestion (393+216 bp) M: DL2000 Marker.
Animals experimental
Approval of animal use protocol for this experiment was authorized by the Animal Care and Use Committee of Hubei Province. Twenty-four healthy crossbred (duroc × landrace × yorkshire) 7-day-old piglets were assigned to 4 groups at random: (1) control group, 6 piglets were fed with commercially available milk-substitutes (artificial milk); (2) STa group, 6 piglets were fed with artificial milk and challenged with 2 × 109 CFU recombinant E. coli LMG194-STa; (3) LMG194 group, 6 piglets were fed with artificial milk and challenged with 2 × 109 CFU E. coli LMG194; and (4) K88 group, 6 piglets were fed with artificial milk and challenged with 2 × 109 CFU E. coli K88. The challenge doses and duration were determined by a preliminary experiment before. ETEC strains K88 was used as positive control (State Key Laboratory of Agricultural Microbiology, Wuhan, China) and LMG194 was used as negative control.
The basal diet in this experiment used commercially available artificial milk (Anyou Feed Technology Co., Wuhan, China), and the nutrition-allocated proportion of the milk-substitutes is presented in Table 1. Each piglet was individually housed in a 1.10 × 1.20 m2 steel metabolic cage with six replicate cages in each group. The challenge of E. coli started on day 5, 1 × 109 CFU twice a day (in morning and evening). Piglets were killed by jugular puncture, and blood and colon samples were collected on day 7. In the whole process of the experiment the diarrhea was observed and diarrhea rate was calculated.
Table 1. Nutrition-allocated proportion of milk-substitutes.
| Composition | Contents | Composition | Contents |
|---|---|---|---|
| Crude Protein /% | ≥20.0 | Crude Fiber /% | ≤0.3 |
| Ether Extract /% | ≤10.0 | Calcium /% | 0.4–1.1 |
| Crude Ash /% | ≤9.0 | Total Phosphorus /% | ≥0.3 |
| Water /% | ≤10.0 | Lysine /% | ≥1.4 |
Blood and colon samples collection
Blood samples (anterior vena cava) were centrifuged at 3,000 rpm for 10 min at 4°C to acquire plasma, and that was stored at −80°C. After mesentery was separated, 5 to 10 cm colon were fixed in 10% formalin solution for assessments of colonic mucosal morphology, the colonic mucosa was rapidly collected, froze in liquid nitrogen and stored at −80°C until analyses.
Assessments of colonic mucosal morphology
Morphological analysis of colonic mucosa was performed on hematoxylin and eosin (H&E)-stained sections. Morphological examination was carried out using a light microscope (American Optical Co., New York, NY, U.S.A.). The crypt depth, number of globet cells and lymphocytes, and density of lymphocytes and lamina propria cell were measured and calculated by Leica Application Suite image analysis software (Leica, Wetzlar, Germany).
Analysis of colonic barrier function and antioxidant capacity
Activity of diamine oxidase (DAO) in colon, which is a biomarker of intestinal injury, is used as an assessment of colonic barrier function. DAO activity in colonic mucosa and plasma was analyzed using commercial enzyme kits (Jiancheng Bioengineering Institute, Nanjing, China) by spectrophotometry, which was described by Hosoda et al. [14].
Activities of superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), and total nitric oxide synthase (TNOS), as well as productions of malondialdehyde (MDA) and hydrogen peroxide (H2O2) in colonic samples, which are used as an assessment of colonic antioxidant capacity, were analyzed by utilizing commercial enzyme kits (Jiancheng Bioengineering Institute, Nanjing, China). Each colon sample was carried out in triplicate.
Analysis of colonic transport function
The expression levels of three protein, aquaporin 3 (AQP3), aquaporin 4 (AQP4), and potassium inwardly-rectifying channel subfamily J member 13 (KCNJ13), which are used as assessment of colonic transport function, were proformed by western bloting [32]. The primary antibodies: AQP3, AQP4 and KNCJ13 (rabbit monoclonal antibodies, 1:1,000 in dilution buffer; Cell Signaling Technology, Inc., Danvers, MA, U.S.A.), β-actin (mouse monoclonal antibody, 1:2,000 in dilution buffer; Sigma-Aldrich Inc., St. Louis, MO, U.S.A.). The secondary antibody: anti-rabbit (mouse monoclonal antibody, 1:5,000 in dilution buffer; Zhongshan Golden Bridge Biological Technology Co., Ltd., Beijing, China). Blots were carried out by utilizing a chemiluminescence kit (Amersham Biosciences, Uppsala, Sweden) and image forming system (Alpha Innotech, New York, NY, U.S.A.).
Analysis of gene expression levels
The gene expression levels in colonic mucosa samples: villin, I-FABP and MMP3 which are associated with intestinal injury, as well as IL-4, CCL-2, CXCL9, IFN-γ, HSPH1 and VNN1 which are associated with inflammatory cytokines of the intestine, were quantitated by the method of real-time PCR [15]. The real-time PCR was carried out with primers (Table 2) of these genes as well as reference gene ribosomal protein L 4 (RPL4) and the SYBR® Premix Ex TaqTM (Takara, Dalian, China) on 7,500 Fast Real-Time PCR System (Foster City, CA, U.S.A.). Data was analyzed by the 2-ΔCt method [30].
Table 2. Primers for Real-Time PCR analysis.
| Gene | Primers | Gene code |
|---|---|---|
| RPL4 | 5′-GAGAAACCGTCGCCGAAT-3′ | XM_005659862 |
| 5′-GCCCACCAGGAGCAAGTT-3′ | ||
| Villin | 5′-TATTATTGGTGTTCGTGCTA-3′ | YMD14268 |
| 5′-TCTGGAGGAATAGGATACTAA-3′ | ||
| MMP3 | 5′-GATGTTGGTTACTTCAGCAC-3′ | YMD14303 |
| 5′-ATCATTATGTCAGCCTCTCC-3′ | ||
| I-FABP | 5′-AGATAGACCGCAATGAGA-3′ | YMD14286 |
| 5′-TCCTTCTTGTGTAATTATCATCAGT-3′ | ||
| IL-4 | 5′-AGGAGCCACACGTGCTTGA-3′ | M77120 |
| 5′-TTGCCAAGCTGTTGAGATTCC-3′ | ||
| IFN-γ | 5′-TCTGGGAAACTGAATGACTTCG-3′ | YMD14356 |
| 5′-GACTTCTCTTCCGCTTTCTTAGGTT-3′ | ||
| CCL2 | 5′-CATAAGCCACCTGGACAAGAAAA-3′ | NM-214214 |
| 5′-GGGTATTTAGGGCAAGTTAGAAGGA-3′ | ||
| CXCL9 | 5′-CTTGCTTTTGGGTATCATCTTCCT-3′ | NM_001114289 |
| 5′-TCATCCTTTGGCTGGTGTTG-3′ | ||
| HSPH-1 | 5′-ATGAGCACGGCTTCATTTCC-3′ | NM_001007518 |
| 5′-GGGCTTTTCCGACTTTCCA-3′ | ||
| VNN1 | 5′-ATGGATGTGTCGCTGGTCTTT-3′ | NM_004666 |
| 5′-CACAACTGCTTGCCTTTACGAG-3′ |
Statistical analysis
Data were analyzed using one-way analysis of variance to analysis, expressed as mean values ± SEM. All experimental data was analysed using SPSS (Version 17.0). A P-value of <0.05 was considered statistically significant.
RESULTS
The rate of piglet diarrhea
The diarrhea rate was listed in Table 3. Compare to the diarrhea rate with different group, there is no significant difference before challenge (P>0.05), but the diarrhea rate of LMG194-STa group and K88 group increased sharply and was much higher than the LMG194 group and control group after challenge (P<0.05). However, there is no significant difference between the LMG194-STa group and K88 group (P>0.05).
Table 3. The rate of piglet diarrhea.
| Items | Control group | STa group | LMG194 group | K88 group |
|---|---|---|---|---|
| Before challenge (%) | 22.2 | 5.6 | 5.6 | 11.1 |
| After challenge (%) | 25.0a) | 91.7b) | 33.3a) | 91.7b) |
Data are mean ± SEM, n=6. a, b) Different letters differ significant (P<0.05).
Colonic mucosal morphology
Colonic mucosal morphology was showed in Fig. 2 and Table 4. Relative to the control group, both STa and K88 group significant increased the depth of crypt, the number of globet cells and lamina propria cell density in colonic mucosa (P<0.05). Three treatments all reduced the density of lymphocyte (P<0.05), and at the same time, STa group had more serious impacts than LMG194 group particularly.
Fig 2.
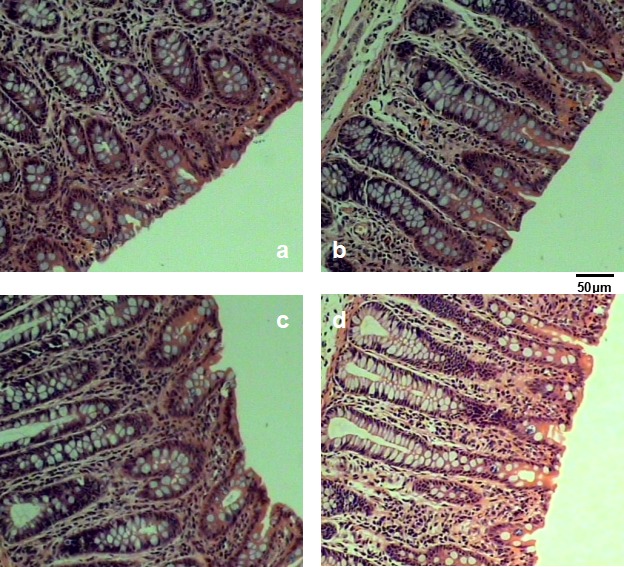
Colonic mucosal morphology. a) control group, b) STa group, c) LMG194 group and d) K88 group.
Table 4. Index of colonic mucosal morphology.
| Items | Control group | STa group | LMG194 group | K88 group |
|---|---|---|---|---|
| Depth of crypt (µm) | 411.3 ± 52.4b) | 679.3 ± 41.6a) | 517.6 ± 52.3b) | 716.2 ± 61.1a) |
| Number of goblet cells | 7.0 ± 1.0b) | 17.0 ± 1.7a) | 9.5 ± 1.5b) | 16.5 ± 2.2a) |
| Number of lymphocytes | 59.5 ± 5.8 | 58.8 ± 6.2 | 57.3 ± 7.7 | 58.3 ± 6.7 |
| Lamina propria cell density (/mm2) | 527.4 ± 54.3a) | 674.4 ± 43.8c) | 550.2 ± 58.9a) | 694.7 ± 24.8b) |
| Density of lymphocyte (/mm2) | 711.9 ± 61.7b) | 546.7 ± 55.9a) | 613.5 ± 48.1a) | 595.5 ± 56.7a) |
Data are mean ± SEM, n=6. a–c) Different letters differ significant (P<0.05).
DAO level and gene expression associated with intestinal integrity
The result showed that, both STa group and LMG194 group had a higher DAO activity in plasma (P<0.05), and a lower activity of that in colonic mucosa (P<0.05) than the control group (Table 5). Nevertheless, STa group had a bigger impact than LMG194 group.
Table 5. DAO activities in plasma and colon and antioxidant capacity in colon.
| Items | Control group | STa group | LMG194 group | K88 group |
|---|---|---|---|---|
| DAO in plasma (U/ml) | 5.26 ± 1.05a) | 16.42 ± 1.49d) | 10.48 ± 2.99b) | 13.48 ± 1.60c) |
| DAO in colon (U/ml) | 0.74 ± 0.16a) | 0.52 ± 0.09b) | 0.59 ± 0.12b) | 0.68 ± 0.13ab) |
| SOD (U/mg) | 36.52 ± 4.41ab) | 31.16 ± 5.42c) | 33.11 ± 5.23bc) | 38.32 ± 4.61a) |
| GSH-Px (U/mg) | 197.29 ± 50.58a) | 140.85 ± 22.52b) | 132.15 ± 17.89b) | 136.98 ± 23.26b) |
| H2O2 (nmol/mg) | 9.34 ± 2.64b) | 12.93 ± 2.51a) | 10.57 ± 2.19ab) | 8.73 ± 1.65b) |
| MDA (nmol/mg) | 15.97 ± 5.91a) | 21.90 ± 5.42b) | 20.54 ± 5.62b) | 13.52 ± 4.78a) |
| TNOS (U/mg) | 1.59 ± 0.36a) | 0.91 ± 0.28b) | 0.81 ± 0.15b) | 0.87 ± 0.27b) |
a–d) Different letters differ significant (P<0.05).
The mRNA levels of these three genes in STa group were lower (P<0.05) than that in control group. Similarly, villin and MMP3 in K88 group were lower (P<0.05) than in control group, but LMG194 group had a contrary effect on MMP3 and I-FABP (Fig. 4).
Fig 4.
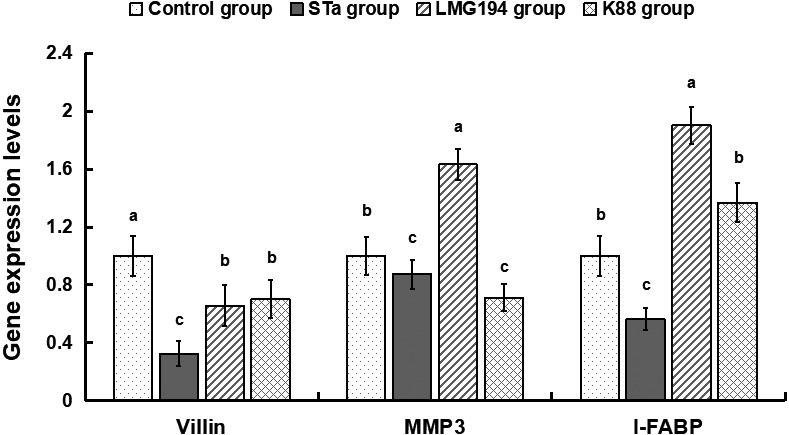
Gene expression levels of villin, I-FABP and MMP3 in colonic mucosa.
Transport function
Relative to the control group, STa group as well as LMG194 group significantly decreased AQP3, AQP4 and KCNJ13 expression (P<0.05) in colon (Fig. 3). K88 group also significantly reduced AQP3 expression (P<0.05) in colon.
Fig. 3.
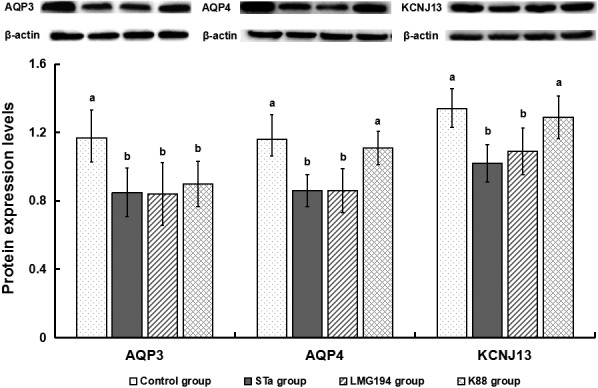
Protein expression levels of AQP3, AQP4 and KCNJ13 in colonic mucosa. Data are mean ± SEM, n=6. a-c Different letters differ significant (P<0.05), the same as below.
Antioxidant capacity and inflammatory cytokines
Relative to the control group, STa group noticeably decreased the activities of SOD, GSH-Px, and TNOS (P<0.05), and raised the productions of MDA and H2O2 (P<0.05) in colon (Table 5). At the same time, LMG194 group as well as K88 group decreased the activities of GSH-Px, TNOS and iNOS (P<0.05).
Relative to the control group, LMG194-STa increased expression of CCL-2 and HSPH-1 gene and decreased expression of IL-4, IFN-γ, CXCL-9 and VNN1 gene (Fig. 5). Moreover, other two group except IL-4 in LMG194 and VNN1 in K88 had the same effect as STa group.
Fig. 5.
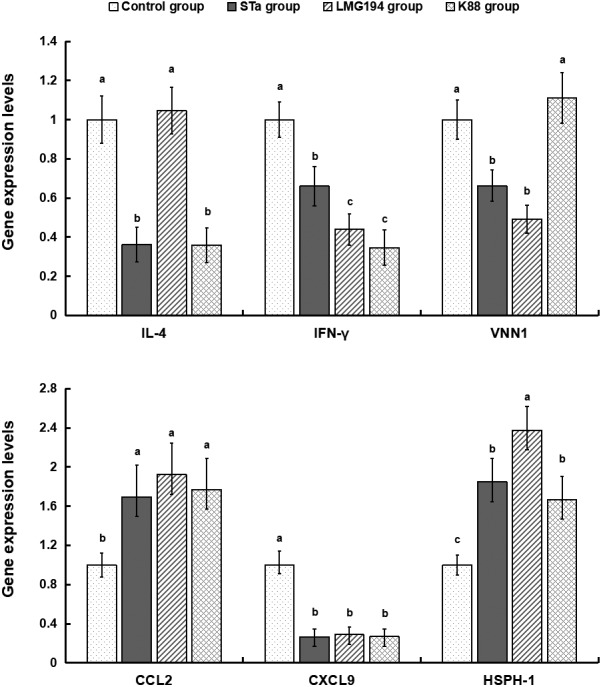
Gene expression levels of inflammatory cytokines in colonic mucosa.
DISCUSSION
The large intestine (also called the colon) is very important segment in vertebrates, which is one portion of the eventual section of digestion, and also a large tunnel that convoys waste from the body. The function of colon mainly consist of absorption, serection, digestion and excretion [11]. There are plenty of caliciform cells in colon, which can serecte alkaline liquid to protect mucosa and help excretion. Colon has no digestion function in itself, but bacteria which live in colon have that. Moreover, by means of peristalsis, colon eliminates waste after nutrients are removed from it [26].
During the experiment, the diarrhea rate of LMG194-STa group and K88 group increased sharply and was much higher than the LMG194 group and control group, but there is no significant difference between the LMG194-STa group and K88 group, it indicated that E. coli LMG194-STa and K88 had similar effects on diarrhea which was greater than E. coli LMG194. Of note, the diarrheal rate is 22.2% in the control group before the inoculation, and the group inoculated with the host strain alone showed a significant increase of diarrhea rate, this condition might be mainly caused by weaning prematurely and stress reaction during inoculating.
The elongation of crypts caused by bacteria or viruses may have symbolized a irritation of the intestinal repairation process, which was obvious as a compensatory pathway for the wastage of crypts in the proximal mucosa concomitant injury to the gut [7]. The mucus layer covering the epithelia is secreted by the caliciform cells, which facilitates the removal of alimentary canal contents. In addition, it affords the first defendant line against physical and chemical injury caused by intake food, bacteria and the bacteria products [17]. In this study, both STa and K88 group significant increased the depth of crypt, the number of globet cells and lamina propria cell density in colonic mucosa (P<0.05). Three treatments all reduced the density of lymphocyte, this result showed that these three kind of E. coli could simultaneously induce colonic mucosal damage. Particularly, recombinant E. coli LMG194-STa and E. coli K88 had a bigger impact than E. coli LMG194.
DAO activity is frequently used as a noninvasive biomarker of alterations in the function and structure of intestinal mucosa [2, 16]. Under certain conditions, cells in intestinal mucosa experience necroses, and slough off into the enteric entocoele, resulting in a decline of DAO levels in intestinal mucosa and an increase of DAO levels in circulation [18]. The data in this research, which STa group and LMG194 group relative to the control group had a higher activity of DAO in plasma and lower that in colonic mucosa, implied that both recombinant E. coli LMG194-STa and E. coli LMG194 could reduce the colonic barrier function. Moreover, recombinant E. coli LMG194-STa had a greater influence than E. coli LMG194.
The injury and slow growth as well as the barrier function reducing of colonic mucosa were probably associated with some genes such as villin, I-FABP and MMP3. Villin is one kind of actin binding protein and a marker of villus cell differentiation, which conduce to prop up the microfilaments of the microvilli of the mucosal villus [6]. I-FABP is located mainly in the enterocytes of the small intestine, and is released into the blood stream after intestinal ischemia and cell disruption [4]. MMP3 as well as their inhibitors (TIMPs) play a crucial role in the repairation of extracorpuscular matrix homeostasis, which is expressed at high levels in the intestine of clinical IBD and celiac diseases [5]. In this research, mRNA levels of these three genes in STa group were simultaneously less than that in control group, these data showed that LMG194-STa could obviously caused the damage of the colon.
AQP3 and AQP4 are two of the most important water channel protein which regulating the water homeostasis in the central nervous system [1]. All function of them is to afford fast water transport as well as support self-balanced within the CNS [20]. KCNJ13 is an ATP-dependent kalium channel that transports kalium out of cells, which plays a very considerable role in kalium homeostasis [12, 13]. In this study, STa group as well as LMG194 group remarkably decreased AQP3, AQP4 and KCNJ13 expression in colon, it revealed that recombinant E. coli LMG194-STa and E. coli LMG194 could substantially reduce colonic transport function.
Oxidant stress reflects the unbalance between the systematic phenomenon of reactive oxygen species and the capacity of biosystem to readily detoxicate the reactive intermediaries or to renovate the resulting injury [8, 21]. Whereas, cells protect themselves from hydroxyl radicals and other oxygenants by antioxidant enzymes, including SOD, GSH-Px and CAT [27, 28]. MDA can induce noxious stress in cells and constitute homopolar protein adducts known as advanced lipoxidation end-products (ALEs), which is usually utilized as a marker to evaluate the oxidant stress levels in an biosome [9]. NOS catalyzes the production of NO, helps modulate insulin secretion, vascular and airway tone, and is actively involved in neural and angiogenesis development [15]. In this research, STa group significantly reduced the activities of SOD, GSH-Px and TNOS, and increased the productions of H2O2 and MDA in colon. This result indicated that recombinant E. coli LMG194-STa can induce oxidative stress, and reduce the colonic antioxidant capacity. Futhermore, LMG194 group as well as K88 group also decreased the activities of GSH-Px and TNOS, it showed that E. coli LMG194 and E. coli K88 could reduce the colonic antioxidant capacity as well.
Oxidative stress has been implicated in the development of many chronic inflammatory disorders, such as enteritis, myocarditis and thyroiditis [3]. Antioxidant defense systems may be impaired as a consequence of excessive oxidative stress, and inflammatory responses can be partially mediated by oxidative stress [24]. The genes associated with inflammatory cytokines of the intestine such as interleukin 4 (IL-4), chemokine ligand 2 (CCL-2), chemokine ligand 9 (CXCL9), Interferon gamma (IFN-γ), heat shock protein h 1 (HSPH1), vanin 1 (VNN1) were altered when the piglets challenged by E. coli. In present study we found that relative to the control group, LMG194-STa noticeably increased expression of CCL-2 and HSPH-1 gene and decreased expression of IL-4, IFN-γ, CXCL-9 and VNN1 gene. Moreover, LMG194 gourp as well as K88 group had similar effect on inflammatory cytokines. These results of these genes related to inflammatory cytokines declared that E. coli infection caused intestinal inflammatory reaction. Accordingly, these genes might be the potential inducement of oxidative stress.
In this research, ETEC strains K88 was used as positive control which was confirmed by PCR genotyping as genes expressing K88 fimbrial antigen. Results showed that E. coli K88 had analogous impact to LMG194-STa on piglet diarrhea, colonic injury and inflammatory reaction; but less influence on colonic antioxidant capacity than LMG194-STa. Therefore, the similarities and difference between E. coli LMG194-STa and K88 has become a new topic worthy of inquiry.
In conclusion, the recombinant E. coli expressing heat-stable enterotoxin A (LMG194-STa) constructed correctly, and it can induce colonic mucosal damage, decrease barrier, immune and transport function and antioxidant capacity of seven days old piglets. A further mechanistic study revealed that the injury, dysfunction and oxidative stress of colon were induced by altering expression of proteins connected with transportation function and genes associated with injury and inflammatory cytokines when piglets challenged by E. coli.
CONFLICT OF INTEREST
We declare that we have no conflict of interest.
Acknowledgments
This work was supported by Hubei Provincial Technology and Innovation Program (2017AHB062), National Key Research and Development Program of China (2017YFD0500505), National Natural Science Foundation of China (Grant No. 31302089) and Agriculture and Agri-Food Canada A-base project (J-001391).
REFERENCES
- 1.Agúndez J. A., Ayuso P., Cornejo-García J. A., Blanca M., Torres M. J., Doña I., Salas M., Blanca-López N., Canto G., Rondon C., Campo P., Laguna J. J., Fernández J., Martínez C., García-Martín E.2012. The diamine oxidase gene is associated with hypersensitivity response to non-steroidal anti-inflammatory drugs. PLOS ONE 7: e47571. doi: 10.1371/journal.pone.0047571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arnspang E. C., Sundbye S., Nelson W. J., Nejsum L. N.2013. Aquaporin-3 and aquaporin-4 are sorted differently and separately in the trans-Golgi network. PLOS ONE 8: e73977. doi: 10.1371/journal.pone.0073977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Besnard P., Niot I., Poirier H., Clément L., Bernard A.2002. New insights into the fatty acid-binding protein (FABP) family in the small intestine. Mol. Cell. Biochem. 239: 139–147. doi: 10.1023/A:1020505512364 [DOI] [PubMed] [Google Scholar]
- 4.Bhuvarahamurthy V., Kristiansen G. O., Johannsen M., Loening S. A., Schnorr D., Jung K., Staack A.2006. In situ gene expression and localization of metalloproteinases MMP1, MMP2, MMP3, MMP9, and their inhibitors TIMP1 and TIMP2 in human renal cell carcinoma. Oncol. Rep. 15: 1379–1384. [PubMed] [Google Scholar]
- 5.Boutten A., Goven D., Artaud-Macari E., Bonay M.2011. [Protective role of Nrf2 in the lungs against oxidative airway diseases]. Med. Sci. (Paris) 27: 966–972. doi: 10.1051/medsci/20112711012 [DOI] [PubMed] [Google Scholar]
- 6.Chantret I., Barbat A., Dussaulx E., Brattain M. G., Zweibaum A.1988. Epithelial polarity, villin expression, and enterocytic differentiation of cultured human colon carcinoma cells: a survey of twenty cell lines. Cancer Res. 48: 1936–1942. [PubMed] [Google Scholar]
- 7.Del Rio D., Stewart A. J., Pellegrini N.2005. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr. Metab. Cardiovasc. Dis. 15: 316–328. doi: 10.1016/j.numecd.2005.05.003 [DOI] [PubMed] [Google Scholar]
- 8.Doke N.1983. Involvement of superoxide anion generation in the hypersensitive response of potato tuber tissues to infection with an incompatible race of Phytophthora infestans, and to the hyphal wall components. Physiol. Plant Pathol. 23: 345–357. doi: 10.1016/0048-4059(83)90019-X [DOI] [Google Scholar]
- 9.Drozdowski L., Thomson A. B.2006. Intestinal mucosal adaptation. World J. Gastroenterol. 12: 4614–4627. doi: 10.3748/wjg.v12.i29.4614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fasina Y. O., Moran E. T., Ashwell C. M., Conner D. E.2007. Effect of dietary gelatin supplementation on the expression of selected enterocyte genes, intestinal development and early chick performance. Int. J. Poult. Sci. 6: 944–951. doi: 10.3923/ijps.2007.944.951 [DOI] [Google Scholar]
- 11.Fu W. X., Liu Y., Lu X., Niu X. Y., Ding X. D., Liu J. F., Zhang Q.2012. A genome-wide association study identifies two novel promising candidate genes affecting Escherichia coli F4ab/F4ac susceptibility in swine. PLoS One 7: e32127. doi: 10.1371/journal.pone.0032127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hames C. A. C., Hopkin S. P.2010. The structure and function of the digestive system of terrestrial isopods. J. Zool. (Lond.) 217: 599–627. doi: 10.1111/j.1469-7998.1989.tb02513.x [DOI] [Google Scholar]
- 13.Hejtmancik J. F., Jiao X., Li A., Sergeev Y. V., Ding X., Sharma A. K., Chan C. C., Medina I., Edwards A. O.2008. Mutations in KCNJ13 cause autosomal-dominant snowflake vitreoretinal degeneration. Am. J. Hum. Genet. 82: 174–180. doi: 10.1016/j.ajhg.2007.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hosoda N., Nishi M., Nakagawa M., Hiramatsu Y., Hioki K., Yamamoto M.1989. Structural and functional alterations in the gut of parenterally or enterally fed rats. J. Surg. Res. 47: 129–133. doi: 10.1016/0022-4804(89)90076-0 [DOI] [PubMed] [Google Scholar]
- 15.Hou Y., Wang L., Ding B., Liu Y., Zhu H., Liu J., Li Y., Wu X., Yin Y., Wu G.2010. Dietary α-ketoglutarate supplementation ameliorates intestinal injury in lipopolysaccharide-challenged piglets. Amino Acids 39: 555–564. doi: 10.1007/s00726-010-0473-y [DOI] [PubMed] [Google Scholar]
- 16.Hou Y., Yao K., Wang L., Ding B., Fu D., Liu Y., Zhu H., Liu J., Li Y., Kang P., Yin Y., Wu G.2011. Effects of α-ketoglutarate on energy status in the intestinal mucosa of weaned piglets chronically challenged with lipopolysaccharide. Br. J. Nutr. 106: 357–363. doi: 10.1017/S0007114511000249 [DOI] [PubMed] [Google Scholar]
- 17.Jiang H. B., Ichikawa Y.1999. Neuronal nitric oxide synthase catalyzes the reduction of 7-ethoxyresorufin. Life Sci. 65: 1257–1264. doi: 10.1016/S0024-3205(99)00361-6 [DOI] [PubMed] [Google Scholar]
- 18.Kim Y. S., Ho S. B.2010. Intestinal goblet cells and mucins in health and disease: recent insights and progress. Curr. Gastroenterol. Rep. 12: 319–330. doi: 10.1007/s11894-010-0131-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kitanaka J., Kitanaka N., Tsujimura T., Terada N., Takemura M.2002. Expression of diamine oxidase (histaminase) in guinea-pig tissues. Eur. J. Pharmacol. 437: 179–185. doi: 10.1016/S0014-2999(02)01302-X [DOI] [PubMed] [Google Scholar]
- 20.Li J. Y., Lu Y., Hu S., Sun D., Yao Y. M.2002. Preventive effect of glutamine on intestinal barrier dysfunction induced by severe trauma. World J. Gastroenterol. 8: 168–171. doi: 10.3748/wjg.v8.i1.168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu M., Ruan X., Zhang C., Lawson S. R., Knudsen D. E., Nataro J. P., Robertson D. C., Zhang W.2011. Heat-labile- and heat-stable-toxoid fusions (LTR₁₉₂G-STaP₁₃F) of human enterotoxigenic Escherichia coli elicit neutralizing antitoxin antibodies. Infect. Immun. 79: 4002–4009. doi: 10.1128/IAI.00165-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oklinski M. K., Skowronski M. T., Skowronska A., Rützler M., Nørgaard K., Nieland J. D., Kwon T. H., Nielsen S.2016. Aquaporins in the Spinal Cord. Int. J. Mol. Sci. 17: 2050. doi: 10.3390/ijms17122050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qadri F., Svennerholm A. M., Faruque A. S. G., Sack R. B.2005. Enterotoxigenic Escherichia coli in developing countries: epidemiology, microbiology, clinical features, treatment, and prevention. Clin. Microbiol. Rev. 18: 465–483. doi: 10.1128/CMR.18.3.465-483.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qi H., Liu X. L.2016. Research progress of expression systems of Escherichia coli and Yeast. J. Anhui Agri. Sci. 44: 4–6. [Google Scholar]
- 25.Ringseis R., Piwek N., Eder K.2007. Oxidized fat induces oxidative stress but has no effect on NF-kappaB-mediated proinflammatory gene transcription in porcine intestinal epithelial cells. Inflamm. Res. 56: 118–125. doi: 10.1007/s00011-006-6122-y [DOI] [PubMed] [Google Scholar]
- 26.Rocha L. B., Ozaki C. Y., Horton D. S., Menezes C. A., Silva A., Fernandes I., Magnoli F. C., Vaz T. M., Guth B. E., Piazza R. M.2013. Different assay conditions for detecting the production and release of heat-labile and heat-stable toxins in enterotoxigenic Escherichia coli isolates. Toxins (Basel) 5: 2384–2402. doi: 10.3390/toxins5122384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Su P., Gong G. L.2017. Research progress on optimizing the expression of exogenous proteins in Escherichia coil. Biotechnol. Bull. 33: 16–23. [Google Scholar]
- 28.Svennerholm A. M., Lundgren A.2012. Recent progress toward an enterotoxigenic Escherichia coli vaccine. Expert Rev. Vaccines 11: 495–507. doi: 10.1586/erv.12.12 [DOI] [PubMed] [Google Scholar]
- 29.Uzar E., Ozay R., Evliyaoglu O., Aktas A., Ulkay M. B., Uyar M. E., Ersoy A., Burakgazi A. Z., Turkay C., Ilhan A.2012. Hydroxycloroquine-induced oxidative stress on sciatic nerve and muscle tissue of rats: a stereological and biochemical study. Hum. Exp. Toxicol. 31: 1066–1073. doi: 10.1177/0960327111433183 [DOI] [PubMed] [Google Scholar]
- 30.Wang M. Z., Ding L. Y., Wang J. F., Yu L. H., Wang H. R.2012. Dietary effects of n-6:n-3 polyunsaturated fatty acid ratios on the antioxidant status of the liver in goslings. J. Anim. Feed Sci. 21: 372–382. doi: 10.22358/jafs/66094/2012 [DOI] [Google Scholar]
- 31.Wang R. R., Ma Y. P.2010. Progress in development of recombinant human Enterotoxigenic Escherichia coli vaccine. Chin. J. Biol. 23: 544–548. [Google Scholar]
- 32.Yao K., Yin Y. L., Chu W., Liu Z., Deng D., Li T., Huang R., Zhang J., Tan B., Wang W., Wu G.2008. Dietary arginine supplementation increases mTOR signaling activity in skeletal muscle of neonatal pigs. J. Nutr. 138: 867–872. [DOI] [PubMed] [Google Scholar]


