Abstract
Okayama University-type retinal prosthesis (OURePTM) is a photoelectric dye-coupled polyethylene film which generates electric potential in response to light and stimulates nearby neurons. This study aims to test surgical feasibility for subretinal film implantation and to examine functional durability of films in subretinal space. Dye-coupled films were implanted subretinally by vitrectomy in the right eye of normal white rabbits: 8 rabbits for 1 month and 8 rabbits for 6 months. The implanted films were removed by vitrectomy in 4 of these 8 rabbits in 1-month or 6-month implantation group. The films were also implanted in 4 rhodopsin-transgenic retinal dystrophic rabbits. Visual evoked potential was measured before film implantation as well as 1 or 6 months after film implantation, or 1 month after film removal. The films were successfully implanted in subretinal space of retinal detachment induced by subretinal fluid injection with a 38G polyimide tip. The retina was reattached by fluid-air exchange in vitreous cavity, retinal laser coagulation, and silicone oil injection. The ratios of P2 amplitudes of visual evoked potential in the implanted right eye over control left eye did not show significant changes between pre-implantation and post-implantation or post-removal (paired t-test). In Kelvin probe measurements, 4 pieces each of removed films which were implanted for 1 or 6 months showed proportional increase of surface electric potential in response to increasing light intensity. The film implantation was safe and implanted films were capable of responding to light.
Keywords: dye-coupled thin film retinal prosthesis, rabbit, retinal dystrophy (retinitis pigmentosa), visual evoked potential, vitrectomy
Blind patients with hereditary retinal diseases, such as retinitis pigmentosa, have dead photoreceptor cells, but the other retinal neurons, which send axons to the brain, remain alive [11]. The basic concept of retinal prostheses is to stimulate surviving retinal neurons such as ganglion cells and bipolar cells with artificial devices and to exploit the function of these living neurons, and finally to send messages to the brain, following artificial stimulation in response to light [14, 19].
Okayama University-type retinal prosthesis (OURePTM) is a novel type of retinal prostheses, so called photoelectric dye-coupled thin film retinal prosthesis [1,2,3, 12, 15, 20,21,22,23]. Stable photoelectric dye molecules with absorption spectrum of visible light [10, 18] were chemically coupled to polyethylene film surface. The dye-coupled film generates electric potential in response to light and stimulates nearby neuronal cells to induce action potential. The dye-coupled film, implanted in subretinal space, serves as a light-receiver and an electric potential-generator, and thus, replaces the function of dead photoreceptor cells in retinal dystrophy [14, 19] to send signals to the brain through living retinal bipolar cells, ganglion cells and their axons as optic nerve fibers [11].
The main stream of retinal prosthesis utilizes a multielectrode array [7]. Camera-captured image is disintegrated to 60 pixels, and the electric current, corresponding to grayscale tone in each pixel, is outputted from 60 electrodes to stimulate retinal living neurons in Argus II Retinal Prosthesis System (Second Sight Medical Products, Inc., Sylmar, CA, U.S.A.). Sophisticated techniques are required to implant surgically the multielectrode arrays. In contrast, the dye-coupled film is a soft and thin sheet which would be rolled up and inserted in subretinal space by routine vitrectomy [16, 17].
In our previous study, the dye-coupled films were implanted subretinally in canine eyes and were also removed by vitrectomy in order to examine the feasibility of surgical techniques [17]. In this study, the dye-coupled films were implanted in normal rabbits’ eyes to be monitored with visual evoked potential. The films were also implanted in retinal dystrophic eyes of rhodopsin-transgenic rabbits to test whether surgical techniques would work in the condition of dystrophic thin retina. In addition, spectrophotometric absorbance and light-evoked surface electric potential was examined on the dye-coupled films which were implanted for 1 or 6 months in normal eyes of rabbits and then were removed by vitrectomy.
MATERIALS AND METHODS
Preparation of dye-coupled polyethylene film
Thin films were made from polyethylene powder and exposed to fuming nitric acid to introduce carboxyl moieties on the film surface. Photoelectric dye molecules, 2-[2-[4-(dibutylamino) phenyl]ethenyl]-3-carboxymethylbenzothiazolium bromide (NK-5962, Hayashibara, Inc., Okayama, Japan), were coupled to carboxyl moieties of the polyethylene film surface via ethylenediamine, as described previously [3, 21, 23]. The fuming nitric acid-treated only polyethylene film and the photoelectric dye-coupled polyethylene film were designated as the plain film and the dye-coupled film, respectively. Films were manufactured in quality management system at a clean-room facility in Okayama University Incubator.
Animals
Normal male white rabbits (Kbl: NZW) and transgenic male white rabbits with rhodopsin P347L mutation (Kbl:NZW, specific pathogen-free, Kitayama Labes Co., Ina, Japan) [5, 6, 8, 9] were used in this study. A pilot study used 3 normal male white rabbits at the age of 31–32 weeks and 2 rhodopsin transgenic rabbits at the age of 13 weeks to test surgical feasibility of dye-coupled film implantation as well as recording for electroretinography and visual evoked potential. In pivotal studies, dye-coupled films were implanted for 1 month in 8 normal rabbits and for 6 months in 8 normal rabbits at the age of 14–16 weeks or 28–29 weeks. Of these, dye-coupled films were removed in 4 rabbits with the 1-month implantation and in 4 rabbits with the 6-month implantation. In addition, dye-coupled films were implanted for 1 month in 4 rhodopsin-transgenic rabbits at the age of 14 weeks. This study was approved by the Animal Care and Use Committee in Okayama University and also by the Committee at Ina Research, Inc., based on the Animal Welfare and Management Act in Japan.
All surgeries were done by T. M. only in the right eye. The dye-coupled films, removed by vitrectomy, were analyzed for spectrophotometric absorbance and light-evoked surface electric potential as described later. In the initial plan, electroretinography was scheduled to be recorded immediately after removal of silicone oil in vitreous cavity since silicone oil was a non-conducting material to inhibit electric recording with a contact lens electrode placed on the corneal surface. In 1-month implantation study, removal of silicone oil resulted in immediate severe inflammation with massive fibrin deposition in the anterior chamber, and electroretinography was not recorded accurately. Therefore, in 6-month implantation study, removal of silicone oil was not done and electroretinography was not recorded.
All animals were sacrificed with bleeding after overdose of intravenous thiopental (Ravonal, Mitsubishi Tanabe Pharma, Osaka, Japan), and the eyes were enucleated. After the cornea and iris were removed by circumferential incision of the eye ball, the posterior segment was cut meridionally to view the entire retina. The posterior segment was then fixed with phosphate-buffered 1% formaldehyde and 2.5% glutaraldehyde, stored in 10% neutral-pH formalin, and embedded in paraffin. Paraffin sections were cut and stained with hematoxylin and eosin for pathological examinations.
Surgical procedures
Rabbits were anesthetized with a mixture (1.5 ml/kg body weight) of intramuscular ketamine (50 mg/kg, Ketamine 5%, Fujita Pharm, Tokyo, Japan) and xylasine (10 mg/kg, Celactal 2%, Bayer Animal Health, Tokyo, Japan). Mydriasis in the right eye was induced by 0.5% tropicamide and 0.5% phenylephrine eye drops (Mydrin-P, Santen Pharmaceutical, Osaka, Japan) on the day of surgery. Subcutaneous injection of meloxicam (0.2 mg/0.04 ml/kg of body weight, Metacam 0.5%, Boehringer Ingelheim, Ingelheim am Rhein, Germany) was given once daily for three days after the surgery as a non-steroidal anti-inflammatory drug. Subcutaneous enrofloxacin (5 mg/kg body weight, Baytril 2.5%, Bayer Animal Health) was given once daily for 5 days. Postoperative instillation of 0.5% levofloxacin (Cravit, Santen Pharmaceutical) and 0.1% betamethasone (Rinderon, Shionogi & Co., Osaka, Japan) twice daily as well as 1% atropine (Nitten, Nagoya, Japan) once daily was continued for postoperative one month.
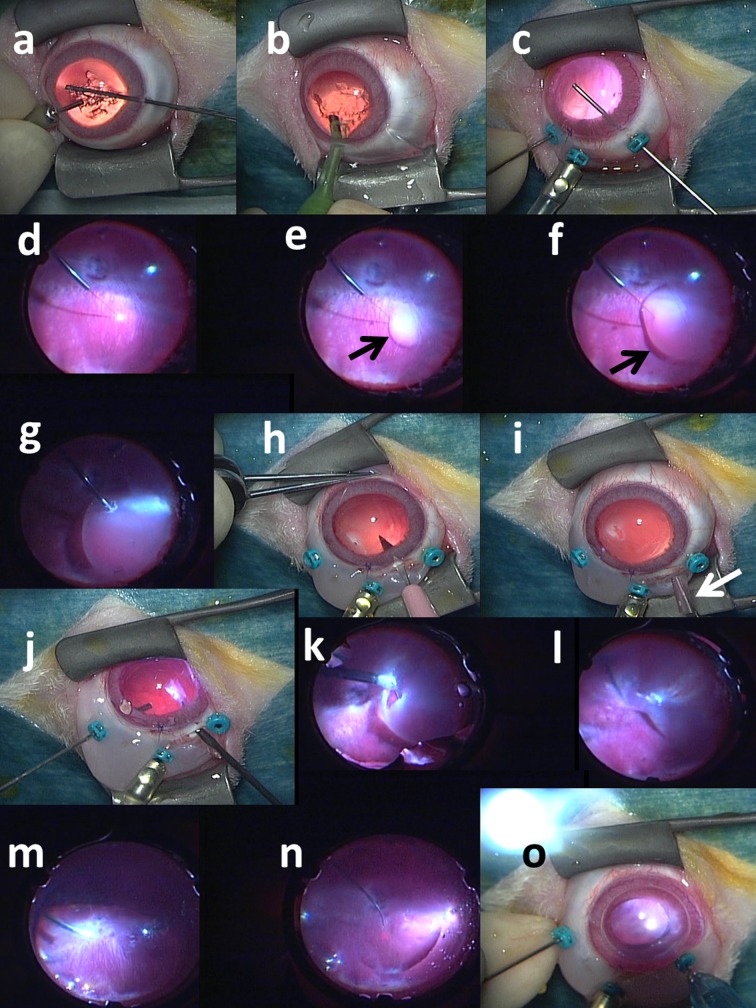
After disinfection with 10% povidone iodine (Negmin Solution, Pfizer Japan, Tokyo) on the haired skin around the eye and then with 40-time saline-diluted povidone iodine on the ocular surface, the rabbit’s head was positioned on the left side down, and covered with a surgical drape. Topical anesthesia was further obtained with 4% lidocaine (Xylocaine Ophthalmic Solution, AstraZeneka, London, U.K.). The surgery was done under a surgical microscope (OPMI VISU150, Carl Zeiss Meditec, Tokyo, Japan) with a surgical machine (Constellation Vision System, Alcon Laboratories, Inc., Fort Worth, TX, U.S.A.). Anterior capsulectomy (Fig. 1a) was done with a 25-gauge vitreous cutter under irrigation with a 25-gauge infusion cannula through two side ports which were made at the corneal limbus with a 20-gauge knife (V-Lance Knife, Alcon), as done in congenital cataract surgery in human eyes [13]. Phacoemulsification and aspiration of the lens in the capsular bag (Fig. 1b) was done through a 2.4 mm-wide corneal incision made on the superior side with a disposable knife (Safety Knife, Kai Medical, Seki, Japan). The corneal incision was sutured with 8–0 Vicryl (polyglactin 910) suture (Ethicon, Johnson & Johnson, New Brunswick, NJ, U.S.A.). Three 25-gauge trocars were inserted into the vitreous through the conjunctiva and sclera 2.5 mm from the limbus on the superior to temporal side within 120 degrees of meridian (Fig. 1c). The presence of a large vascularized nictitating membrane on the nasal side of the conjunctiva limited the surgical area used for placing trocars.
Fig. 1.
Surgical procedures to implant retinal prosthesis, OURePTM, in right eye of rhodopsin-transgenic white rabbit. a) Lens anterior capsule is cut with 25G vitreous cutter under irrigation with 25G infusion cannula in anterior chamber. b) Lens nucleus and cortex is aspirated with phaco-tip from corneal incision. c) Three 25G trocars are inserted over conjunctiva through sclera into vitreous at 2.5 mm from corneal limbus: a middle trocar is connected with infusion cannula, and the other two trocars are used for vitreous cutter and light guide. Posterior capsule is cut with vitreous cutter. d) After vitreous gel has been cut, subretinal fluid infusion is started with 38G tip. e) Bleb retinal detachment (arrow) is made by 38G tip infusion of BSS-Plus solution. f) Bleb (arrow) is enlarged with further infusion. g) Retinal tear is made by retinal coagulation with 25G bipolar diathermy. h) Scleral incision is made with 22.5° knife after conjunctival incision. i) Rolled-up dye-coupled film (arrow) is inserted through scleral incision with 20G subretinal forceps. j) Rolled-up film is inserted into vitreous with 20G subretinal forceps. k) Rolled-up film is inserted into subretinal space through retinal tear with 20G subretinal forceps. l) Film is now under detachment retina. m) Fluid-air exchange in vitreous cavity is accomplished with 25G vitreous cutter in aspiration mode to reattach the retina. n) Laser photocoagulation is applied around retinal tear. o) Silicone oil is injected in vitreous cavity with 25G tip. Finally, scleral and conjunctival incision is sutured and trocars are removed (not shown).
The wide-field fundus was viewed with a +128-diopter front lens by Resight 500 fundus viewing system (Carl Zeiss Meditec). Posterior capsulectomy (Fig. 1c) and core vitrectomy was done under irrigation with a 25-gauge cannula placed at the middle trocar on the superior side. Retinal detachment (Fig. 1d, 1e and 1f) was then made by infusing irrigation solution (BSS-Plus Intraocular Irrigating Solution, Alcon) into the subretinal space with a 38-gauge polyimide tip (PolyTip Cannula 25G/38G, MedOne Surgical, Inc., Sarasota, FL, U.S.A.) attached to a 10-ml syringe for the viscous fluid control (VFC) system at the setting of low intraocular pressure. A retinotomy (Fig. 1g) was made by 25-gauge diathermy (Grieshaber Diathermy Probe DSP 25Ga, Alcon) at the edge of retinal detachment. After conjunctival incision, a 3 mm-wide scleral incision (Fig. 1h) was placed with a microsurgery knife (Straight/Stab 22.5°, Kai Medical) 2 mm posteriorly in parallel with the corneal limbus, and wound hemostasis was done with a wet-field hemostatic eraser bipolar instrument (Beaver-Visitec International, Inc., Waltham, MA, U.S.A.). A rolled-up sheet of the dye-coupled film in 5 × 5 mm square size (Fig. 1i) was grasped with a 20-gauge subretinal forceps (Synergetics 39.21S, Bausch+Lomb Retina, St. Louis, MO, U.S.A.), and inserted into the vitreous (Fig. 1j) and then under the detached retina through a retinotomy (Fig. 1k and 1l). The scleral incision for film insertion was sutured with 8-0 Vicryl. The subretinal fluid was aspirated with a vitreous cutter, and then fluid in vitreous cavity was exchanged with air to reattach the retina (Fig. 1m). Laser photocoagulation (Fig. 1n) was applied around the retinal tear caused by retinotomy, and silicone oil (polydimethylsiloxane, Silikon 1000, Alcon) was infused into vitreous cavity (Fig. 1o) by the VFC syringe. Trocars were removed, and the conjunctiva was sutured with 8-0 Vicryl.
At surgery to remove dye-coupled films implanted in the subretinal space, three 25-gauge trocars were placed and retinal detachment was induced by infusing irrigation fluid into the subretinal space by a 38-gauge tip. A retinotomy was made by diathermy and a subretinal dye-coupled film was grasped with a 25-gauge forceps (Grieshaber Revolution DSP 25Ga ILM Forceps, Alcon) and brought to the vitreous cavity. The film was brought out of the eye ball with a 25-gauge forceps through a newly made 3-mm-wide scleral incision 2 mm posterior to the corneal limbus. The retina was reattached by fluid-air exchange, laser photocoagulation, and silicone oil infusion. The films were immersed in distilled water for functional analyses. Microscopic surgical view was recorded with a digital processor miniature 3CCD color camera (THD-311, Ikegami Tsushinki Co., Tokyo, Japan).
Electroretinography and visual evoked potential
Rabbits were sedated with intramuscular injection of the mixture of medetomidine (0.1 mg/0.1 ml to 0.2 mg/0.2 ml/kg of body weight, Domitor, Nippon Zenyaku Kogyo Co., Koriyama, Japan) and midazolam (0.75 mg/0.15 ml to 1.5 mg/0.3 ml/kg of body weight, Dormicum, Astellas Pharma Inc., Tokyo, Japan). Mydriasis was obtained with 0.5% tropicamide and 0.5% phenylephrine eye drops (Mydrin-P), and dark adaptation was achieved for at least 40 min. After ocular surface anesthesia with 0.4% bupivacaine eye drops (Benoxil, Santen Pharmaceutical), a contact lens electrode with white light-emitting diode (LED) was placed on the corneal surface with hydroxyethylcellulose gel (Scopisol, Senju Pharmaceutical, Osaka, Japan), a reference electrode was put at cranial front, and a ground clip was placed along the tail. Maximal response to standard flash (5,623 cd/m2, 3 msec) was recorded (White LED Visual Stimulator LS-W and White LED electrode, Mayo Corp., Aichi, Japan, Dual BioAmp ML135 and A/D Converter ML820 PowerLab 2/25, ADInstruments Japan, Nagoya). After the measurements, sedation was reversed with intramuscular injection of atipamezole (1 mg/kg of body weight, Antisedan, Nippon Zenyaku Kogyo Co.).
Visual evoked potential was measured with subcutaneous needle electrodes placed on the parietal center for recording, on the dorsum of nose for reference, and along the right auricle for ground by LE-4000 (Tomey Co., Nagoya, Japan). After dark adaptation and mydriasis for at least 15 min, flash light (LE-4000) stimuli in the intensity of 30 cd × s/m2 (100,000 cd/m2 × 0.3 msec) at the frequency of 2 Hz were given to the right eye and then to the left eye. The amplitude and latency of P2 and the latency of N2 in the 128 summation of recordings were used for statistical analysis, based on the International Society for Clinical Electrophysiology of Vision (ISCEV) standards for clinical visual evoked potentials in humans. P2 amplitude and latency as well as N2 latency were automatically calculated after the measurement by LE-4000.
Spectrophotometry
Absorbance spectra of dye-coupled films were measured by an ultraviolet and visible light spectrophotometer with an integrating sphere unit (V-750 and PIV-756, JASCO Corp., Tokyo, Japan). Plain polyethylene films were used to obtain baseline absorbance. Absorbance was measured in the wavelength ranging from 300 to 800 nm at 1 nm spectral bandwidth. Maximum absorbance values around 500 nm were used for comparison between the implanted film and the non-implanted same lot.
Light-evoked surface electric potential
Light-evoked surface electric potential on dye-coupled films in the dry condition was measured by the scanning Kelvin Probe system (SKP5050, KP Technology, Ltd., Highlands and Islands, U.K.) in the surface photovoltage mode. The entire measuring system was placed in a humidity-controlling box and kept at low humidity. The dye-coupled film was fixated on a sample device. The capacitance between the probe and the sample was changed by oscillating the probe. The surface potential of the sample was measured by adjusting the bias to set electrostatic attractive force at zero. The distance between the probe and the sample was kept constant by setting the gradient at 200. To confirm the measuring system to be stable, work function at a single point on the sample was measured repeatedly 100 times until standard deviation of work function became 10 or less. Only after a waiting time to obtain the stability, the surface potential was measured at changing light intensity with a light source (Surface Photovoltage Spectroscopy SPS040, KP Technology). Surface potential changes in response to increase of light intensity as well as the surface potential in light intensity of dial setting at 2,500 (300 lux equivalent) was used as the outcome.
RESULTS
Surgical feasibility
The lens of rabbits at younger ages was soft enough to be aspirated by an ultrasound tip without phacoemulsification (Fig. 1b). The vitreous cavity in rabbits was smaller in space, compared with human eyes. Bleb retinal detachment could be made successfully in dystrophic thin retina as done in the normal retina by puncture with an irrigating 38-gauge tip (Fig. 1d, 1e and 1f) in the lower part of the retina to avoid the medullary rays. A major complication was dislodgment of 25G trocars which were placed over the conjunctiva through the sclera because the rabbits’ sclera is thinner compared with human sclera. A surgical assistant monitored the position of the trocars and pushed the trocars with a forceps to avoid the dislodgment during the surgery.
Electroretinography and visual evoked potential
Electroretinogrpahy was recorded in both eyes of all rabbits before film implantation in the study. The amplitudes of a-wave and b-wave before surgery in 4 normal rabbits for 1-month implantation were 172.6 ± 44.8 µV of a-wave and 460.6 ± 122.1 µV of b-wave in the right eyes with surgery and 178.7 ± 48.2 µV of a-wave and 488.4 ± 136.0 µV of b-wave in the left eyes with no intervention, described as mean ± standard deviation (n=4). In contrast, the amplitudes of a-wave and b-wave were reduced in 4 rhodopsin-transgenic rabbits before surgery: 65.9 ± 6.5µV of a-wave and 234.0 ± 39.9 µV of b-wave in the right eyes with surgery and 67.4 ± 11.8 µV of a-wave and 246.0 ± 78.3 µV of b-wave in the left eyes with no intervention, as mean ± standard deviation.
P2 amplitude, P2 and N2 latency of visual evoked potential in the right eyes with dye-coupled film implantation did not change significantly between pre-implantation and 1-month or 6-month implantation in normal rabbits (n=4 for each group, paired t-test, Table 1). P2 amplitude, P2 and N2 latency of visual evoked potential in the left eyes of these rabbits as control also did not show significant change (n=4 for each group, paired t-test, Table 1). The ratio of P2 amplitude of the right eye over the left eye did not show significant change in 1-month or 6-month implantation (n=4 for each group, paired t-test, Table 1).
Table 1. P2 amplitude, P2 latency and N2 latency of visual evoked potential in normal rabbits with 1-month or 6-month dye-coupled film implantation and removal, and in rhodopsin-transgenic rabbits with 1-month dye-coupled film implantation.
| P2 amplitude in right eye (surgery) Mean ± SD (µV) |
P2 amplitude in left eye (control)
Mean ± SD (µV) |
Right eye/Left eye ratio of P2 amplitude | P2 latency in right eye (surgery)
Mean ± SD (msec) |
P2 latency in left eye (control)
Mean ± SD (msec) |
N2 latency in right eye (surgery)
Mean ± SD (msec) |
N2 latency in left eye (control) Mean ± SD (msec) |
|
|---|---|---|---|---|---|---|---|
| Normal rabbits with 1-month implantation | |||||||
| Pre-implantation | 2.51 ± 0.97 (n=4) | 2.18 ± 0.93 (n=4) | 1.21 ± 0.29 (n=4) | 90.2 ± 13.8 (n=4) | 84.5 ± 2.0 (n=4) | 75.5 ± 12.8 (n=4) | 69.5 ± 3.6 (n=4) |
| 1-month implantation | 1.55 ± 1.39 (n=4) | 2.18 ± 1.09 (n=4) | 1.20 ± 0.80 (n=4) | 89.2 ± 10.8 (n=4) | 92.5 ± 26.0 (n=4) | 79.2 ± 10.0 (n=4) | 65.2 ± 1.2 (n=4) |
| Paired t-test P value | P=0.0736 | P>0.9999 | P=0.9853 | P=0.8571 | P=0.5742 | P=0.3622 | P=0.1203 |
| Normal rabbits with 1-month implantation and removal | |||||||
| Pre-implantation | 4.67 ± 1.77 (n=4) | 4.30 ± 1.46 (n=4) | 1.16 ± 0.45 (n=4) | 90.0 ± 10.4 (n=4) | 86.0 ± 9.8 (n=4) | 63.7 ± 1.5 (n=4) | 64.7 ± 5.1 (n=4) |
| 1 month after removal | 1.55 ± 1.86 (n=3) | 3.63 ± 1.72 (n=3) | 0.79 ± 0.59 (n=3) | 83.3 ± 8.0 (n=3) | 97.6 ± 7.0 (n=3) | 74.3 ± 6.0 (n=3) | 74.0 ± 4.0 (n=3) |
| Paired t-test P value | P=0.1282 | P=0.3472 | P=0.4136 | P=0.8171 | P=0.1567 | P=0.1009 | P=0.0128 |
| Normal rabbits with 6-month implantation | |||||||
| Pre-implantation | 2.23 ± 0.20 (n=4) | 1.75 ± 0.80 (n=4) | 1.41 ± 0.42 (n=4) | 82.5 ± 1.9 (n=4) | 83.7 ± 2.9 (n=4) | 68.0 ± 5.7 (n=4) | 68.0 ± 6.1 (n=4) |
| 6-month implantation | 1.67 ± 0.88 (n=4) | 2.06 ± 0.39 (n=4) | 0.79 ± 0.38 (n=4) | 100.5 ± 22.4 (n=4) | 87.0 ± 11.4 (n=4) | 72.5 ± 12.7 (n=4) | 69.7 ± 12.5 (n=4) |
| Paired t-test P value | P=0.2805 | P=0.4908 | P=0.2119 | P=0.2110 | P=0.5222 | P=0.5934 | P=0.8310 |
| Normal rabbits with 6-month implantation and removal | |||||||
| Pre-implantation | 4.63 ± 2.83 (n=4) | 3.26 ± 1.24 (n=4) | 1.31 ± 0.63 (n=4) | 83.2 ± 4.9 (n=4) | 83.2 ± 2.7 (n=4) | 65.2 ± 3.8 (n=4) | 68.2 ± 3.5 (n=4) |
| 6 month implantation | 0.81 ± 0.33 (n=4) | 2.63 ± 0.53 (n=4) | 0.32 ± 0.16 (n=4) | 85.5 ± 4.5 (n=4) | 78.5 ± 4.7 (n=4) | 74.2 ± 5.8 (n=4) | 65.7 ± 4.5 (n=4) |
| 1 month after removal | 1.36 ± 0.38 (n=3) | 1.95 ± 0.48 (n=3) | 0.78 ± 0.48 (n=3) | 108.2 ± 30.5 (n=3) | 78.5 ± 2.6 (n=3) | 76.2 ± 9.2 (n=3) | 64.0 ± 2.1 (n=3) |
| Paired t-test P value (pre vs 6 month) | P=0.0668 | P=0.4486 | P=0.0595 | P=0.6289 | P=0.1169 | P=0.1432 | P=0.5696 |
| Paired t-test P value (pre vs removal) | P=0.2523 | P=0.2105 | P=0.4376 | P=0.2189 | P=0.0192 | P=0.1269 | P=0.1828 |
| Rhodopsin-transgenic rabbits with 1-month implantation | |||||||
| Pre-implantation | 1.46 ± 0.61 (n=4) | 1.86 ± 0.85 (n=4) | 0.86 ± 0.31 (n=4) | 78.7 ± 2.3 (n=4) | 83.0 ± 7.4 (n=4) | 69.0 ± 1.8 (n=4) | 72.7 ± 9.1 (n=4) |
| 1-month implantation | 0.86 ± 0.56 (n=4) | 1.67 ± 1.15 (n=4) | 0.82 ± 0.63 (n=4) | 96.5 ± 18.6 (n=4) | 95.7 ± 11.0 (n=4) | 73.2 ± 6.3 (n=4) | 80.5 ± 9.0 (n=4) |
| Paired t-test P value | P=0.0469 | P=0.8633 | P=0.9358 | P=0.1196 | P=0.1704 | P=0.3000 | P=0.1134 |
SD, standard deviation; µV, microvolt.
In 4 normal rabbits with 1-month film implantation and film removal, P2 amplitude, P2 and N2 latency of visual evoked potential in the right eyes with surgeries did not change significantly between pre-implantation and 1 month after film removal (n=3 due to death of 1 rabbit, paired t-test, Table 1). P2 amplitude, P2 and N2 latency of visual evoked potential in the left eyes of these rabbits as control also did not show significant change (n=3, paired t-test, Table 1). In 4 normal rabbits with 6-month film implantation and film removal, visual evoked potential was measured at 3 time points: pre-implantation, 6-month implantation, and 1 month after surgical removal of 6-month implanted films. In statistical analysis by repeat-measure analysis of variance (ANOVA), P2 amplitude showed significant change in the time course (P=0.0270), but did not show significant change between the right eyes with surgeries and the left eyes with no intervention (P=0.2152). P2 amplitude, P2 and N2 latency in the right eyes with surgery and in the left eyes with no intervention did not show significant change between pre-implantation and 6-month implantation or between pre-implantation and 1-month after film removal (n=4 for 6-month implantation, n=3 for film removal due to loss of 1 rabbit, paired t-test, Table 1).
In rhodopsin-transgenic rabbits with retinal dystrophy, P2 amplitude in the right eyes with 1-month film implantation show significant decrease compared with pre-implantation (n=4, P=0.0469, paired t-test, Table 1) while P2 amplitude in the left eyes with no intervention showed no significant change (n=4, P=0.8633). However, the ratio of P2 amplitude of the right eye over the left eye did not show significant change between pre-implantation and 1-month implantation (n=4, P=0.9358, paired t-test, Table 1). P2 and N2 latency in the right eyes with surgery and in the left eyes with no intervention did not show significant change between pre-implantation and 1-month after implantation (Table 1).
Pathology
Gross anatomy of enucleated eyes with 1-month or 6-month film implantation showed no marked proliferation (Fig. 2a). The enucleated eyes were cut meridionally to take photographs before fixation. This process resulted in flowing-out of dye-coupled film under the retina, together with silicone oil in vitreous cavity. Microscopic observation disclosed no infiltration with inflammatory cells or no proliferation. In the eyes of normal rabbits with 1-month film implantation, photoreceptor outer segments in the retina were present but shortened in length (Fig. 2b). In the eyes of rhodopsin-transgenic rabbits with 1-month film implantation, photoreceptor outer segments were almost lost (Fig. 2c). In the eyes of normal rabbits with 6-month film implantation, retinal outer nuclear layer with photoreceptor outer segments was lost (Fig. 2d).
Fig. 2.
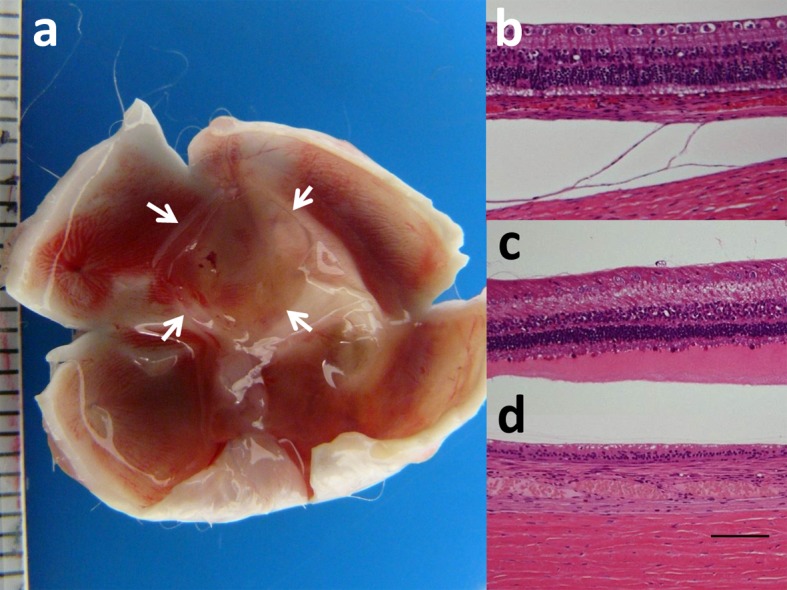
Macroscopic view of unfixed and dissected eye with subretinal square dye-coupled film (5 × 5 mm, arrows) in 1-month implantation (a). Light microscopic sections of the retina of the posterior pole near film implantation in normal rabbit with 1-month film implantation (b), rhodopsin-transgenic rabbit with 1-month film implantation (c), and normal rabbit with 1 month observation after removal of 6-month implanted film (d). The photoreceptors are at bottom of photographs. Separation between the choroid and sclera is artifact (b). Photoreceptor outer segments are relatively maintained in normal rabbit (b) while outer segments are shortened with eosin-stained serous fluid in rhodopsin-transgenic rabbit (c). Loss of retinal outer nuclear layer is noted 1 month after removal of 6-month implanted film in normal rabbit (d). Hematoxylin-eosin stain. Scale in ruler is 1 mm in a. Scale bar=100 µm in b, c and d.
Absorbance and light-evoked surface electric potential
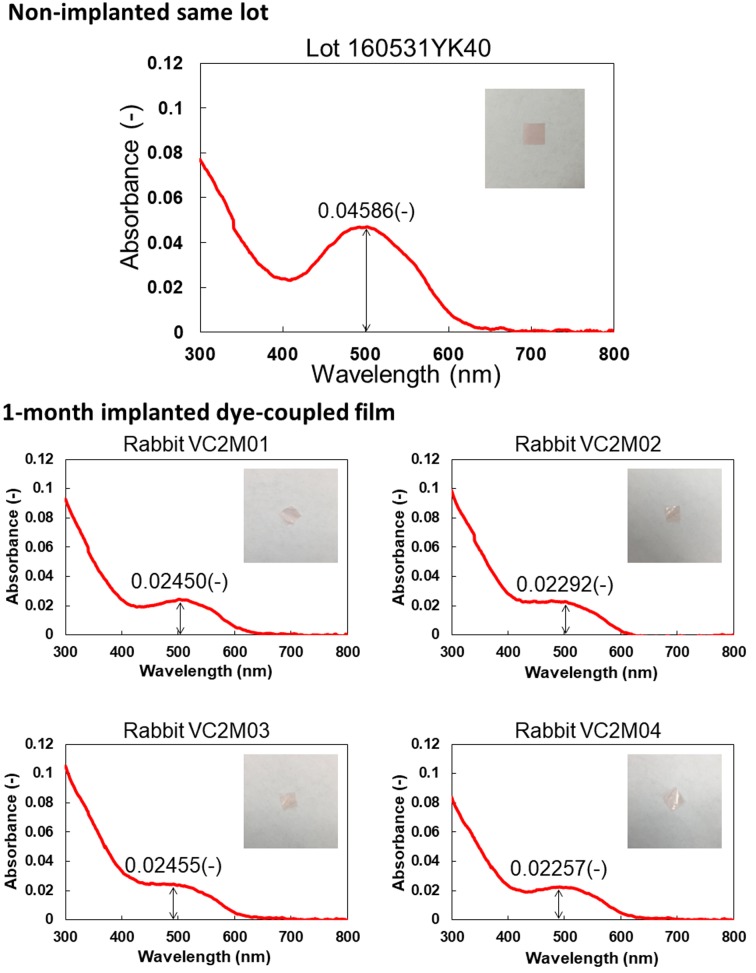
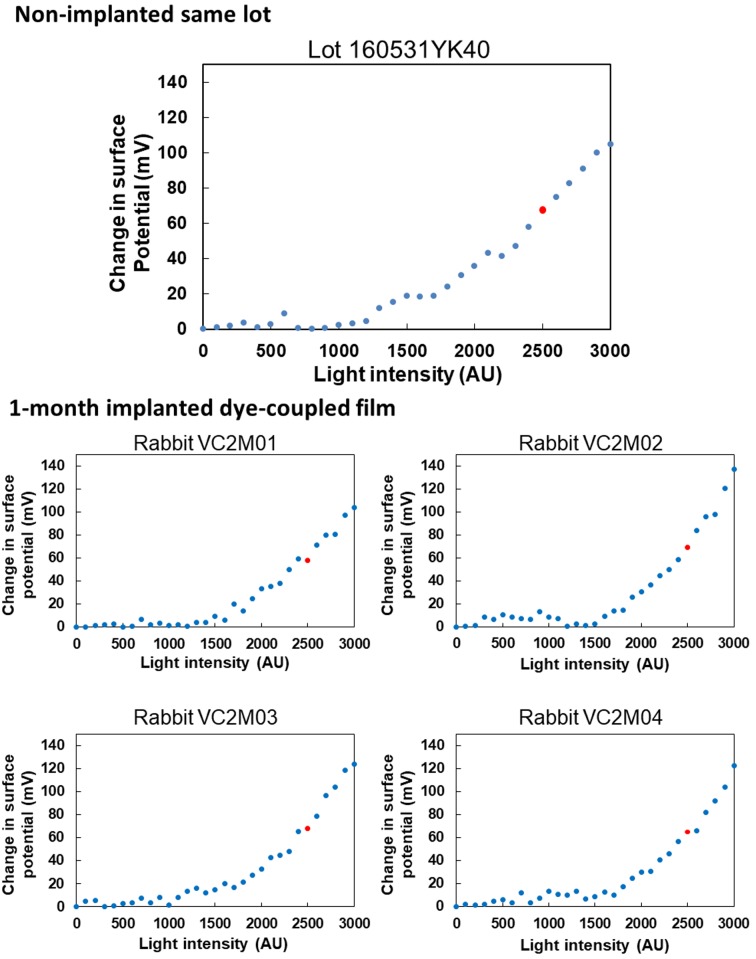
Figures 3 and 4 show spectrophotometric absorbance and light-evoked surface electric potential, respectively, of dye-coupled films which were implanted for 1 month and removed by vitrectomy. The mean of maximum absorbance around 500 nm of 4 pieces of 1-month implanted films was 0.02363, with the range from 0.02257 to 0.02455. The percentage of the mean absorbance, relative to the non-implanted same lot film (0.04586), was 51.5%. The mean of surface potential at light intensity of 2500, equivalent to 300 lux, of 4 pieces of 1-month implanted films was 65 mV, with the range from 58 to 69.3 mV. The percentage of the mean surface potential, relative to the non-implanted same lot film (67.6 mV), was 96.2%.
Fig. 3.
Spectrophotometric absorbance spectra (4 bottom panels) of 4 pieces of dye-coupled films which have been implanted for 1 month in subretinal space of rabbits’ eyes and removed by vitrectomy. Top panel shows absorbance spectrum of the non-implanted same lot. Insets are photographs of films. Values in each panel represent maximum absorbance around the wavelength of 500 nm.
Fig. 4.
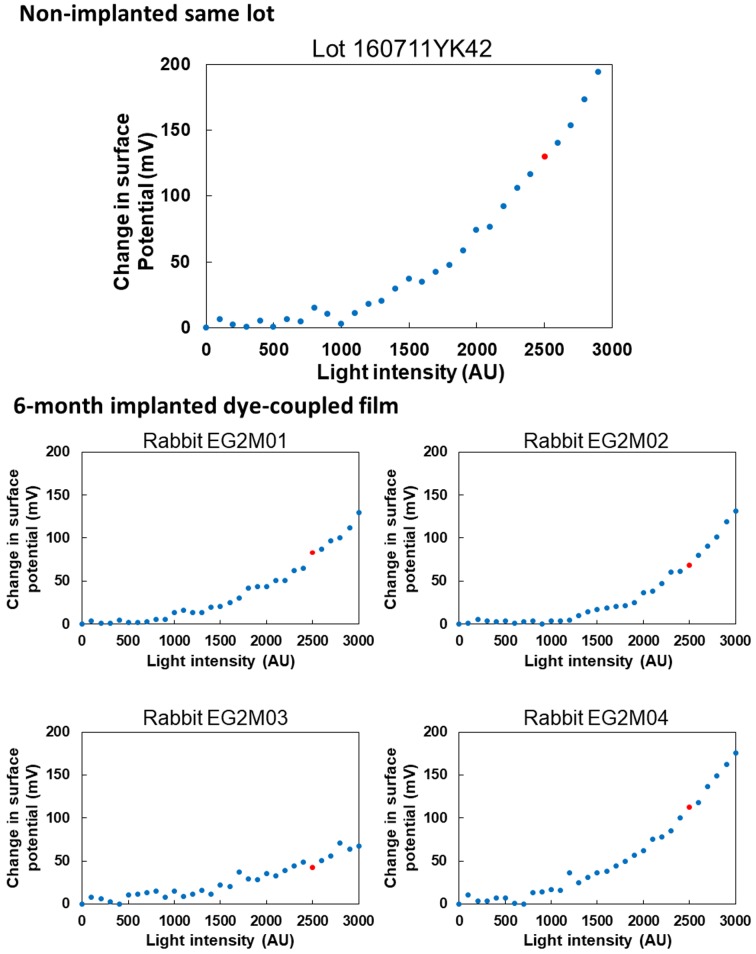
Surface electric potential in response to increasing light intensity on 4 pieces of dye-coupled films which have been implanted for 1 month in subretinal space of rabbits’ eyes and removed by vitrectomy. Top panel shows surface electric potential on the non-implanted same lot. Red dots on each panel represent electric potential at 2,500 arbitrary unit (AU) of light intensity which corresponds to 300 lux.
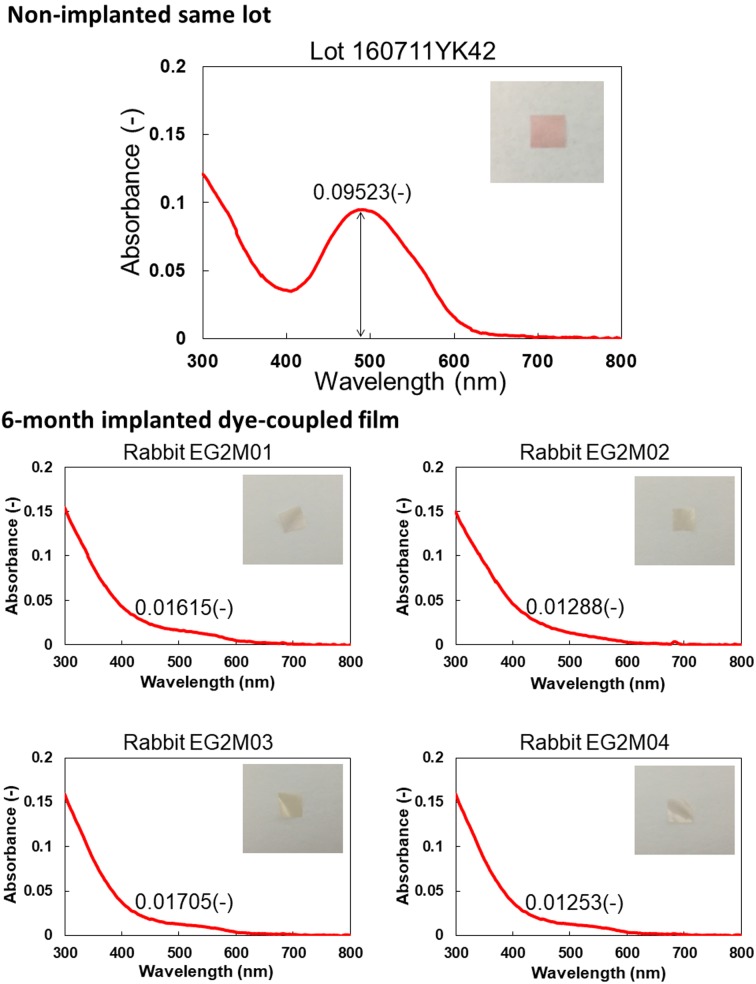
Figures 5 and 6 show spectrophotometric absorbance and light-evoked surface electric potential, respectively, of dye-coupled films which were implanted for 6 months and removed by vitrectomy. The mean of maximum absorbance around 500 nm of 4 pieces of 6-month implanted films was 0.01465, with the range from 0.01253 to 0.01705. The percentage of the mean absorbance, relative to the non-implanted same lot film (0.09523), was 15.4%. The mean of surface potential at light intensity of 2,500, equivalent to 300 lux, of 4 pieces of 6-month implanted films was 76 mV, with the range from 43 to 112.5 mV. The percentage of the mean surface potential, relative to the non-implanted same lot film (129.8 mV), was 59.1%.
Fig. 5.
Spectrophotometric absorbance spectra (4 bottom panels) of 4 pieces of dye-coupled films which have been implanted for 6 months in subretinal space of rabbits’ eyes and removed by vitrectomy. Top panel shows absorbance spectrum of the non-implanted same lot. Insets are photographs of films. Values in each panel represent maximum absorbance around the wavelength of 500 nm.
Fig. 6.
Surface electric potential in response to increasing light intensity on 4 pieces of dye-coupled films which have been implanted for 6 months in subretinal space of rabbits’ eyes and removed by vitrectomy. Top panel shows surface electric potential on the non-implanted same lot. Red dots on each panel represent electric potential at 2,500 arbitrary unit (AU) of light intensity which corresponds to 300 lux.
DISCUSSION
The goals of this study are two folds: the first to test surgical feasibility of subretinal film implantation by vitrectomy in rabbits, and the second to test functional durability of the implanted films. In the previous study [17], beagle dogs were used for dye-coupled film implantation. The canine study demonstrated technical feasibility of film implantation and 5-month functional durability of the implanted films. In the present study, rabbits were used for film implantation since rabbits have been more frequently used as an animal model for ophthalmic surgeries [4]. In addition, visual evoked potential can be easily recorded in rabbits with thin bony skull, in contrast with difficulty in the recording in dogs with thick bony skull.
We could successfully implant dye-coupled films in square size of 5 × 5 mm in subretinal space of rabbits’ eyes by vitrectomy even though the film size was rather larger compared with smaller size of rabbits’ eyes, relative to human eyes. The dye-coupled films which had been implanted in subretinal space of the eyes were scheduled to be removed 1 or 6 months after implantation, and to be submitted to functional analyses. Base on a technological limitation at that time, the films in this square size were required for fixation on the sample device to measure light-evoked surface electric potential by Kelvin probe.
We also tested to implant dye-coupled films in rhodopsin-transgenic rabbits which developed retinal dystrophy. Retinal prosthesis is scheduled to be applied in patients with retinitis pigmentosa who have thin degenerative retina. Therefore, we thought that technical feasibility of implantation surgery should be tested in an animal model with thin degenerative retina. We could successfully induce retinal detachment by infusing solution with a 38-gauge tip under the thin degenerative retina in rhodopsin-transgenic rabbits.
In our previous study using dogs [17], light-evoked surface electric potential could be measured by Kelvin probe only on one piece of dye-coupled films which were implanted in subretinal space for 5 months and then removed by vitrectomy. Surface electric potential could not be measured on the other 4 pieces of dye-coupled films which were implanted either for 3 or for 5 months [17]. The Kelvin probe system has been renovated since then to control humidity in the environment to measure surface electric potential of dye-coupled films repeatedly at a constant level. In the present study, light-evoked surface electric potential could be measured on all pieces of 1-month or 6-month implanted dye-coupled films.
In addition, we measured absorbance of dye-coupled films with a new type of spectrophotometry with an integrating sphere in the present study. In the previous study with dogs [17], absorbance of one piece of implanted films with surface wrinkling could not be measured with a preceding type of spectrophotometry. With spectrophotometry with an integrating sphere unit, absorbance of all the implanted films was measured successfully in the present study.
It should be noted that surface electric potential on 4 pieces of 1-month implanted dye-coupled films was basically at the same level as that of the non-implanted same lot. In contrast, spectrophotometric absorbance of 1-month implanted films was reduced to about the half of absorbance of the non-implanted same lot. This discrepancy between the absorbance and surface electric potential would be explained by the presence of non-covalently bound dye molecules which were attached to the film surface by ionic binding. Non-covalently bound dye molecules would contribute to absorption but would not contribute to the generation of surface electric potential.
The range of surface electric potential on 4 pieces of 6-month implanted films was large while surface electric potential generated on the non-implanted same lot was rather high. Therefore, mean percentage of electric potential of 6-month implanted films was reduced to 60%, relative to the potential of the non-implanted same lot, but actual values of potential were comparable to values for 1-month implanted films. The absorbance of 6-month implanted films was further reduced to 15% of the non-implanted same lot, but was not in parallel with light-evoked surface electric potential. In other words, the discrepancy between the absorbance and surface electric potential was further widened in the 6-month implanted films. It should be emphasized that absorbance and surface electric potential did not change in parallel with each other among 4 pieces of dye-coupled films which were implanted not only for 6 months but also for 1 month. A method for measuring absorbance of films has to be further refined in future to assess accurately the dye-coupled films.
We initially planned to assess safety of dye-coupled film implantation by electroretinography and visual evoked potential. Visual evoked potential is recorded from the skull in response to repeat flashing light to the eyes. The presence of silicone oil in the vitreous did not influence the recording of visual evoked potential. In contrast, electroretinography records retinal electric activity in response to light via the vitreous with a contact lens electrode placed on the corneal surface. The presence of silicone oil, a non-conductive material, in the vitreous prevents electroretinographic recording. We, therefore, planned to remove silicone oil just before electroretinographic recording in the studies of 1-month and 6-month film implantation. However, the procedure of silicone oil removal by vitrectomy led to so early and so severe intraocular inflammation as massive fibrin deposition in the anterior chamber, and resulted in no recording of electroretinography in 1-month implantation study.
We, therefore, used visual evoked potential as an indicator to assess safety of dye-coupled film implantation in rabbits. The amplitude and latency of visual evoked potential did not show significant change in the right eyes with film implantation and in the left eyes with no intervention for 1 and 6 months in normal rabbits. In addition, the ratio of amplitudes of the right eye over the left eye did not show significant change in the time course of 1 and 6 months in normal rabbits. Furthermore, no significant change in the right eye/left eye ratio of amplitudes was noted at 1 month after surgical removal of 1- or 6-month implanted films. These facts suggest the safety of film implantation and film removal by vitrectomy in rabbits. No significant change in the right eye/left eye ratio of amplitudes of visual evoked potential was noted also in rhodopsin-transgenic rabbits with retinal dystrophy. The presence of visual evoked potential in rhodopsin-transgenic rabbits suggests that these rabbits at this stage of the age would not be suitable for a model of retinal dystrophy with no vision.
In conclusion, this study proved surgical feasibility of subretinal implantation and removal as well as 6-month functional durability of retinal prosthesis, OURePTM, in rabbits. The filing of a first-in-human clinical trial for OURePTM is now negotiated at Pharmaceuticals and Medical Devices Agency (PMDA) in Japan.
Acknowledgments
This study was supported by a grant for the Translational Research Network Program from the Japan Agency for Medical Research and Development (AMED). The authors declare that they have no conflict of interest. We thank Chie Matsuo, DDS, PhD, for statistical analysis and preparation of figures.
REFERENCES
- 1.Alamusi M., Matsuo T., Hosoya O., Uchida T.2017. Visual evoked potential in RCS rats with Okayama University-type retinal prosthesis (OUReP™) implantation. J. Artif. Organs 20: 158–165. doi: 10.1007/s10047-016-0943-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alamusi M., Matsuo T., Hosoya O., Tsutsui K. M., Uchida T.2015. Vision maintenance and retinal apoptosis reduction in RCS rats with Okayama University-type retinal prosthesis (OUReP™) implantation. J. Artif. Organs 18: 264–271. doi: 10.1007/s10047-015-0825-1 [DOI] [PubMed] [Google Scholar]
- 3.Alamusi M., Matsuo T., Hosoya O., Tsutsui K. M., Uchida T.2013. Behavior tests and immunohistochemical retinal response analyses in RCS rats with subretinal implantation of Okayama-University-type retinal prosthesis. J. Artif. Organs 16: 343–351. doi: 10.1007/s10047-013-0697-1 [DOI] [PubMed] [Google Scholar]
- 4.Al-Nawaiseh S., Thieltges F., Liu Z., Strack C., Brinken R., Braun N., Wolschendorf M., Maminishkis A., Eter N., Stanzel B. V.2016. A step by step protocol for subretinal surgery in rabbits. J. Vis. Exp. 115: 53927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asakawa K., Ishikawa H., Uga S., Mashimo K., Kondo M., Terasaki H.2016. Histopathological changes of inner retina, optic disc, and optic nerve in rabbit with advanced retinitis pigmentosa. Neuroophthalmology 40: 286–291. doi: 10.1080/01658107.2016.1229339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hirota R., Kondo M., Ueno S., Sakai T., Koyasu T., Terasaki H.2012. Photoreceptor and post-photoreceptoral contributions to photopic ERG a-wave in rhodopsin P347L transgenic rabbits. Invest. Ophthalmol. Vis. Sci. 53: 1467–1472. doi: 10.1167/iovs.11-9006 [DOI] [PubMed] [Google Scholar]
- 7.Humayun M. S., Dorn J. D., da Cruz L., Dagnelie G., Sahel J. A., Stanga P. E., Cideciyan A. V., Duncan J. L., Eliott D., Filley E., Ho A. C., Santos A., Safran A. B., Arditi A., Del Priore L. V., Greenberg R. J., Argus II Study Group 2012. Interim results from the international trial of Second Sight’s visual prosthesis. Ophthalmology 119: 779–788. doi: 10.1016/j.ophtha.2011.09.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones B. W., Kondo M., Terasaki H., Watt C. B., Rapp K., Anderson J., Lin Y., Shaw M. V., Yang J. H., Marc R. E.2011. Retinal remodeling in the Tg P347L rabbit, a large-eye model of retinal degeneration. J. Comp. Neurol. 519: 2713–2733. doi: 10.1002/cne.22703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kondo M., Sakai T., Komeima K., Kurimoto Y., Ueno S., Nishizawa Y., Usukura J., Fujikado T., Tano Y., Terasaki H.2009. Generation of a transgenic rabbit model of retinal degeneration. Invest. Ophthalmol. Vis. Sci. 50: 1371–1377. doi: 10.1167/iovs.08-2863 [DOI] [PubMed] [Google Scholar]
- 10.Liu S., Matsuo T., Hosoya O., Uchida T.2017. Photoelectric dye used for Okayama University-type retinal prosthesis reduces the apoptosis of photoreceptor cells. J. Ocul. Pharmacol. Ther. 33: 149–160. doi: 10.1089/jop.2016.0093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loewenstein J. I., Montezuma S. R., Rizzo J. F., 3rd.2004. Outer retinal degeneration: an electronic retinal prosthesis as a treatment strategy. Arch. Ophthalmol. 122: 587–596. doi: 10.1001/archopht.122.4.587 [DOI] [PubMed] [Google Scholar]
- 12.Matsuo T.2003. A simple method for screening photoelectric dyes towards their use for retinal prostheses. Acta Med. Okayama 57: 257–260. [DOI] [PubMed] [Google Scholar]
- 13.Matsuo T.2014. Intraocular lens implantation in unilateral congenital cataract with minimal levels of persistent fetal vasculature in the first 18 months of life. Springerplus 3: 361. doi: 10.1186/2193-1801-3-361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsuo T., Morimoto N.2007. Visual acuity and perimacular retinal layers detected by optical coherence tomography in patients with retinitis pigmentosa. Br. J. Ophthalmol. 91: 888–890. doi: 10.1136/bjo.2007.114538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsuo T., Dan-oh Y., Suga S. (Inventors). 2006. Agent for inducing receptor potential. Assignee: Okayama University. United States Patent. Patent No.: US 7,101,533 B2. Date of Patent: Sep. 5, 2006.
- 16.Matsuo T., Uchida T., Takarabe K.2009. Safety, efficacy, and quality control of a photoelectric dye-based retinal prosthesis (Okayama University-type retinal prosthesis) as a medical device. J. Artif. Organs 12: 213–225. doi: 10.1007/s10047-009-0471-6 [DOI] [PubMed] [Google Scholar]
- 17.Matsuo T., Uchida T., Nitta M., Yamashita K., Takei S., Ido D., Tanaka M., Oguchi M., Furukawa T.2017. Subretinal implantation of Okayama University-type retinal prosthesis (OURePTM) in canine eyes by vitrectomy. J. Vet. Med. Sci. 79: 1939–1946. doi: 10.1292/jvms.17-0450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okamoto K., Matsuo T., Tamaki T., Uji A., Ohtsuki H.2008. Short-term biological safety of a photoelectric dye used as a component of retinal prostheses. J. Artif. Organs 11: 45–51. doi: 10.1007/s10047-008-0403-x [DOI] [PubMed] [Google Scholar]
- 19.Tamaki M., Matsuo T.2011. Optical coherence tomographic parameters as objective signs for visual acuity in patients with retinitis pigmentosa, future candidates for retinal prostheses. J. Artif. Organs 14: 140–150. Erratum 14: 385. [DOI] [PubMed] [Google Scholar]
- 20.Tamaki T., Matsuo T., Hosoya O., Tsutsui K. M., Uchida T., Okamoto K., Uji A., Ohtsuki H.2008. Glial reaction to photoelectric dye-based retinal prostheses implanted in the subretinal space of rats. J. Artif. Organs 11: 38–44. doi: 10.1007/s10047-007-0398-8 [DOI] [PubMed] [Google Scholar]
- 21.Uchida T., Ishimaru S., Shimamura K., Uji A., Matsuo T., Ohtsuki H.2005. Immobilization of photoelectric dye on the polyethylene film surface. Mem. Fac. Eng. Okayama Univ. 39: 16–20. [Google Scholar]
- 22.Uji A., Matsuo T., Uchida T., Shimamura K., Ohtsuki H.2006. Intracellular calcium response and adhesiveness of chick embryonic retinal neurons to photoelectric dye-coupled polyethylene films as prototypes of retinal prostheses. Artif. Organs 30: 695–703. doi: 10.1111/j.1525-1594.2006.00286.x [DOI] [PubMed] [Google Scholar]
- 23.Uji A., Matsuo T., Ishimaru S., Kajiura A., Shimamura K., Ohtsuki H., Dan-oh Y., Suga S.2005. Photoelectric dye-coupled polyethylene film as a prototype of retinal prostheses. Artif. Organs 29: 53–57. doi: 10.1111/j.1525-1594.2004.29010.x [DOI] [PubMed] [Google Scholar]