Abstract
Nervous necrosis virus (NNV), also known as betanodavirus, has been recently implicated in mass mortalities of cultured marine fish. An effective vaccine is urgently needed to protect fish against this virus. However, parenteral immunization methods are very stressful. Individual immunization for thousands of fish is very labor intensive and expensive. Therefore, we expressed NNV coat protein in tobacco chloroplasts and used it as an oral vaccine to induce immunities in fish followed by challenges with NNV. Our results revealed that mice (IgG and IgA) and fish (IgM) immunized with the oral vaccine developed significantly higher antibody titers against the NNV coat protein. Fish were partially protected against viral challenge. Taken together, our results demonstrated that a plant-based vaccine could effectively induce immune response and protect groupers against NNV. The present method could be used to develop oral fish vaccine in the future.
Keywords: betanodavirus, nervous necrosis virus, oral vaccine, tobacco
Betanodavirus (family Nodaviridae) causes viral nervous necrosis (VNN). It has emerged as a major constraint to the culture and sea ranching of marine fish in almost all parts of the world. More than 30 species of marine fish have been affected by VNN during the seedling period and the culturing process [7, 14]. Grouper nervous necrosis virus causes severe mortality to larvae and juveniles, resulting in significant economic losses in aquaculture [12]. NNV contains two segments of (+) single-stranded RNA: RNA1 (~3.1 kb) and RNA2 (~1.4 kb). The RNA1 and RNA2 of striped jack nervous necrosis virus (SJNNV) encode RNA-dependent RNA polymerase and major coat protein, respectively [18, 20]. Four genotypes of VNN (designated as SJNNV, tiger puffer nervous necrosis virus, red-spotted grouper nervous necrosis virus, and barfin flounder nervous necrosis virus isolated from a variety of diseased fish) have been identified as betanodaviruses based on similarities in partial RNA2 sequences encoding the C-terminal halves of coat protein [16]. Vaccination against NNV infection in fish is a promising way to effectively control NNV and reduce economic losses. Vaccines consisting of recombinant coat protein expressed in Escherichia coli [9, 27], virus-like particles [28] expressed in baculovirus expression system, and inactivated virus [18, 19, 31] have been reported to provide partial protective immunity to several fish species against NNV. However, parenteral immunization methods are very stressful. Individual immunization for thousands of fish is very labor-intensive, time-consuming and expensive. Several expression systems have been used to produce recombinant proteins, including bacterial, yeast, insect and mammalian cells. Plant-based expression systems have received a lot of attention as an alternative platform to produce recombinant proteins due to their relatively low cost, easy scale-up, efficient storage, and risk-free animal pathogen contamination [30]. Moreover, plants have the potential as edible vaccines. This is one of the greatest advantages of plant-based expression systems [3]. Target genes can be introduced into plant cells using transient expression system. Target genes are normally inserted into nuclear genome or chloroplast genome during transgenic plant transformation [21]. Plant cells contain a large number of chloroplasts per cell. And in chloroplast, there are some grana, which contain many genome copies. Therefore, each chloroplast contains about 100 genomes, offering plant cells enormous capacity to accumulate target proteins [2, 6, 13]. The objective of this study was to express NNV coat protein in transgenic tobacco chloroplasts and evaluate its efficacy as an oral vaccine candidate in mice and fish (grouper).
MATERIALS AND METHODS
Coat protein gene cloning and E. coli expression
Standard DNA, RNA and protein manipulations were carried out as described previously [1]. Total RNA was extracted from brain samples collected from seven-band grouper (Hyporthodus septemfasciatus, previously known as Epinephelus septemfasciatus) aseptically using TRIzol® reagent (Invitrogen, Carlsbad, CA, U.S.A.) according to the manufacturer’s protocol. After reverse transcription (RT) using Reverse Transcriptase SuperScript®II (Invitrogen), polymerase chain reaction (PCR) was conducted to amplify coat protein gene. Primers used for coat protein gene amplification were designed based on the sequences of a relatively conserved region of the Sevenband grouper nervous necrosis virus strain (GenBank accession no. AY324870). The following primers were used: coat-F (5′-ATATCTCGAGATGGAGACCCACTTGTATGG-3′) and coat-R (5′-ACACAAGCTT TTGGGCGACCGTGTAGCCGG-3′, underlined sequences indicating restriction enzyme sites). Amplified PCR product was cloned into pGEM®-T Easy (Promega, Madison, WI, U.S.A.), digested with restriction enzymes (XhoI+HindIII), and subcloned into pRSET prokaryotic expression vector (Invitrogen) digested with the same two restriction enzymes. The sequence of the insert was verified with ABI PRISM® 3100 Genetic Analyzer (Applied Biosystems, Foster City, CA, U.S.A.). Generated construct (pRSET-NNV) was transformed into E. coli BL21 (DE3) pLysS (Invitrogen) host cells. Recombinant protein (rCoat) was expressed after induction with IPTG (final concentration at 1 mM) and purified using a Probond™ purification system (Invitrogen) according to the manufacturer’s instructions. Purified rCoat was used to produce rabbit polyclonal antibody as described previously [8].
Plant expression
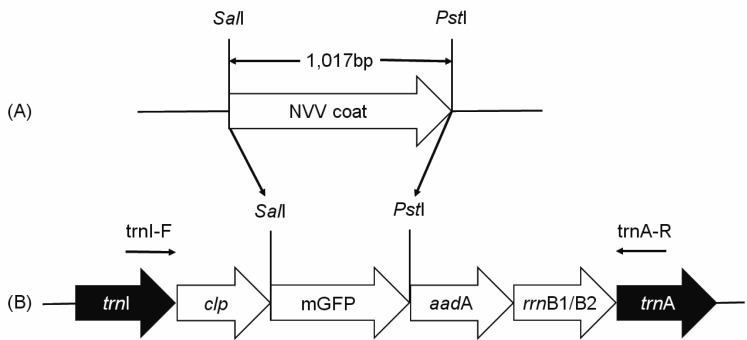
The coat protein gene was codon-optimized according to tobacco codon usage data at Kazusa DNA Research Institute (http://www. kazusa.or.jp/codon) for efficient expression in tobacco plastids. Optimized gene was synthesized by overlap extension PCR as described previously [25] and subsequently cloned into pGEM®-T Easy to create cloning vector pGEM-NNVOpt (Fig. 1A). To produce a chloroplast transformation vector, coat protein gene was excised from pGEM-NNVOpt using SalI/PstI restriction sites and subcloned into the SalI/PstI site of TIA::RclpGAH containing clp promoter of rice and rrnB1/B2 terminator of E. coli to generate TIA::RclpSynNNV (named after underlined terms: trnItrnArice clp promoter synthetic NNV) construct (Fig. 1B). The trnI-trnA loci in the inverted repeat regions were used to allow homologous insertion into the chloroplast genome. Tobacco (Nicotiana tabacum L. cultivar Samsun) chloroplast transformation was performed as described previously [10]. Primary shoots were maintained for 3 to 5 generations on selective medium containing 500 µg ml−1 spectinomycin. Positive transgenic shoots (T0) were screened by PCR. Shoots of 12 independent lines were transferred to Murashige and Skoog (MS) basal medium [15] containing 2% sucrose, transferred to soil, and grown in a growth chamber (800–1,600 mol m−2 s−1, 25–35°C) with a 16 hr light/8 hr dark cycle. All analyses were carried out using T1 transgenic plants germinated from seeds of T0 plant.
Fig. 1.
Diagram of the chloroplast transformation vector. (A) pGEM-NNVOpt cloning vector harboring the codon-optimized coat protein gene; (B) Transformation vector TIA::RclpGAH. The vector consisted of two flanking regions (trnI and trnA) of which the sequences were the same as those of integration targets in the chloroplast genome and the coat protein gene driven by rice clp promoter. aadA: aminoglycoside 3′-adenylyltransferase; rrnB1/B2: terminator. Arrows indicating primers used to confirm the integration of coat protein gene and homoplastomy of transgenic plants.
Verification PCR and RT-PCR analyses
Genomic DNA was extracted from the leaves of both transformed and untransformed plants using genomic DNA extraction buffer (200 mM Tris-HCl pH7.5, 250 mM NaCl, 25 mM EDTA, and 0.5% SDS). Vector specific primers trnI-F (5′-ATC TCT CGA GCA CAG GTT TA-3′) and trnA-R (5′-TTC TTG ACA GCC CAT CTT T-3′) were used to confirm the integration of coat protein gene and homoplastomy of transformants. Total RNA was extracted from the leaves of both transformed and untransformed plants using Plant Total RNA Prep Kit (GeneAll, Seoul, Korea). First-strand cDNA synthesis was performed using 5 µg of DNase I-treated total RNA and First Strand cDNA Synthesis Kit (Takara Bio, Kusatsu, Japan) containing reverse transcriptase according to the manufacturer’s protocol. PCR was performed using actin primers (actin-F: 5′-TGGACTCTGGTGATGGTGTC-3′; actin-R: 5′-CCTCCAATCCAAACACTGTA-3′) and NNV primers (NNV-F: 5′- GGTGAGAAGAAATTGGCAAAAC-3′; NNV-R: 5′- GACGAGGCTGCTCATCAGAGTA).
Western blot analysis
Leaves were homogenized in protein extraction buffer (50 mM Tris-HCl pH 7.5, 500 mM NaCl, 10% glycerol, 1 mM DTT, 5 mM EDTA, 0.025% SDS, and 2 mM PMSF). Supernatants were collected from samples after centrifugation at 13,500 ×g for 20 min and subjected to protein concentration measurements using BCA [26] according to the manufacturer’s manual (Pierce™ BCA Protein Assay Kit, Pierce, Rockford, IL, U.S.A.). Protein samples were boiled for 5 min before loading. Protein samples (20 µg per lane) were subjected to 12% sodium dodecyl sulfate (SDS) polyacrylamide gel electrophoresis (PAGE) and transferred to polyvinylidene difluoride (PVDF) membranes using a semi-dry transfer apparatus (Bio-Rad, Hercules, CA, U.S.A.). After protein transfer, membranes were blocked with 5% skim milk for 2 hr at room temperature followed by incubation at room temperature overnight with a rabbit anti-NNV primary antibody (produced using rCoat as described in Materials & Methods) diluted at 1:5,000 in 5% skim milk. After washing three times with PBS containing 0.1% Tween 20 (PBST), membranes were then incubated with horseradish peroxidase (HRP)-conjugated goat anti-rabbit secondary antibody (Pierce, diluted at 1:10,000 in 5% skim milk) at room temperature for 6 hr. Chemiluminescent image signal was produced by adding SuperSignal WestPico (Thermo Scientific, Rockford, IL, U.S.A.) and documented with ChemiDoc XRS (Bio-Rad).
Animals
All animal experiments complied with the guidelines to the care and use of experimental animals of Canadian council on animal care and were approved by the animal care committee of the Chonnam National University, Republic of Korea.
Mouse immunizations
A total of 21 6-week-old ICR female mice were divided into four groups as shown in Table 1. Mice in group 2 were intraperitoneally (IP) primed with 5 µg of purified rCoat expressed in E. coli. Group 3 received an additional boost injection two weeks after priming for group 2. Freund’s complete adjuvant was used for priming while Freund’s incomplete adjuvant was used for boosting. Mice in groups 1–3 received plant-derived rCoat orally (1.5 µg of rCoat for groups 1-1, 2-1, 3-1; 3 µg of rCoat for groups 1-2, 2-2, 3-2). The desired concentration of rCoat in formulae was adjusted by mixing transformants with wild-type leaf powders.
Table 1. Immunization design, methods, and trial number in mouse.
| Groups | Immunization methods | Formulae | Number |
|---|---|---|---|
| 1–1 | Feda)-only | 90% normal diet+10% leaf powderb) | 3 |
| 1–2 | 80% normal diet+20% leaf powder | 3 | |
| 2–1 | Priming+fed | 90% normal diet+10% leaf powder | 3 |
| 2–2 | 80% normal diet+20% leaf powder | 3 | |
| 3–1 | Priming+boosting+fed | 90% normal diet+10% leaf powder | 3 |
| 3–2 | 80% normal diet+20% leaf powder | 3 | |
| 4 | Negative control | Normal diet | 3 |
a) Oral immunization (fed) every Monday at 2-week intervals for a total of four times; b) Lyophilized transformant tobacco leaf powder (10% powder containing 1.5 µg of coat protein, while 3.0 µg for 20% powder).
Mouse antibody titrations
As controls, blood samples were collected from tail veins before experiments. One day before each immunization, sequential blood samples were collected. Mice were sacrificed to obtain blood samples and small-intestine lavage at 13 days after the final immunization. The presence of IgA (lavage) and IgG (serum) against rCoat was determined using enzyme-linked immunosorbent assay (ELISA) as described previously [24]. Briefly, rCoat from E. coli (1 µg well−1 90 µl−1) was absorbed onto 96-well flat-bottom microtiter plates (Maxisorp™; Nunc, Roskilde, Denmark) and blocked with 90 µl PBS in 1% skim milk at 37°C for 1 hr. Plates were then washed three times with PBST and incubated with 90 µl of sample (lavage or serum diluted 1:100 in PBS) at 37°C for 1 hr. Plates were washed and 90 µl of goat anti-mouse IgA or IgG HRP conjugate (Pierce, diluted 1:500 in PBS) was added. After 1 hr of incubation at 37°C, plates were washed and incubated with 90 µl of substrate (0.1 M citric acid buffer, pH 4.0, 2.2% ABTS stock solution, and H2O2) at room temperature for 15 min. Plates were then developed at room temperature in the dark for 5 min. Absorbance at 405 nm was measured using an ELISA reader. Results were expressed as mean ± standard deviation of the optical density values. Non-immunized diet-only mouse sample was used as negative control.
Grouper immunizations
Before experiments, the presence (infection) of NNV in nervous tissues from five randomly-selected fish was screened using NNV-specific RT-PCR method as described previously [16]. A total of 75 3-month-old seven-band grouper were divided into five groups as shown in Table 2. Fish in groups 2 and 3 received plant-derived rCoat orally by feeding at a concentration of 5 µg (group 2) or 10 µg (group 3). Fish in groups 4 and 5 were IP primed with 5 µg of purified rCoat expressed in E. coli. Montanide™ adjuvant (Seppic, Fairfield, NJ, U.S.A.) was used to prepare the priming injection. After injections, fish in groups 4 and 5 were fed with plant-derived rCoat.
Table 2. Immunization design, methods, and trial number in grouper.
| Groups | Immunization methods | Formulae | Number |
|---|---|---|---|
| 1 | Negative control | Normal diet | 15 |
| 2 | Feda)-only | 90% normal diet+10% leaf powderb) | 15 |
| 3 | 80% normal diet+20% leaf powder | 15 | |
| 4 | Priming+fed | 90% normal diet+10% leaf powder | 15 |
| 5 | 80% normal diet+20% leaf powder | 15 | |
a) Oral immunization (fed) every Monday at 2-week intervals for a total of 4 times; b) Lyophilized transformant tobacco leaf powder (10% powder containing 5 µg of coat protein, while 10 µg for 20% powder).
Grouper antibody titrations
At six days after the final immunization, blood samples were collected from caudal veins of five randomly selected fish. The presence of IgM against rCoat was screened by ELISA as described above. Fish sera (diluted 1:100 in PBS) were used as primary antibody and mouse anti-grouper IgM (diluted 1:10, Aquatic Diagnostics, Stirling, Scotland) was used as secondary antibody. Goat anti-mouse IgG HRP (1:500, Pierce) was used as tertiary antibody.
Viral isolation and challenge
Virus isolation was conducted using published method [7]. NNV was prepared in striped snakehead fry (SSN-1, ATCC, Rockville, MD, U.S.A.) cell line [5]. Brain samples positive in PCR assay were homogenized, centrifuged, filtered, and added into 24-well tissue culture plates containing SSN-1 cells at 60–70% confluency. After TCID50 calibration (data not shown), 1 ml of viral culture (corresponding to 103.25 TCID ml−1) was injected intraperitoneally. Fish were monitored for 60 days. Dead and moribund fish were collected for PCR to detect viral infection using NNV-F and NNV-R primers as described above.
Statistical analysis
Differences between groups were identified by one-way analysis of variance (ANOVA). Specific group differences were determined with Dunnett’s multiple comparison test using GraphPad Instat 3.05 Software (GraphPad, La Jolla, CA, U.S.A.). Statistical significance was considered when P value was less than 0.05.
RESULTS
rCoat expression in E. coli and tobacco
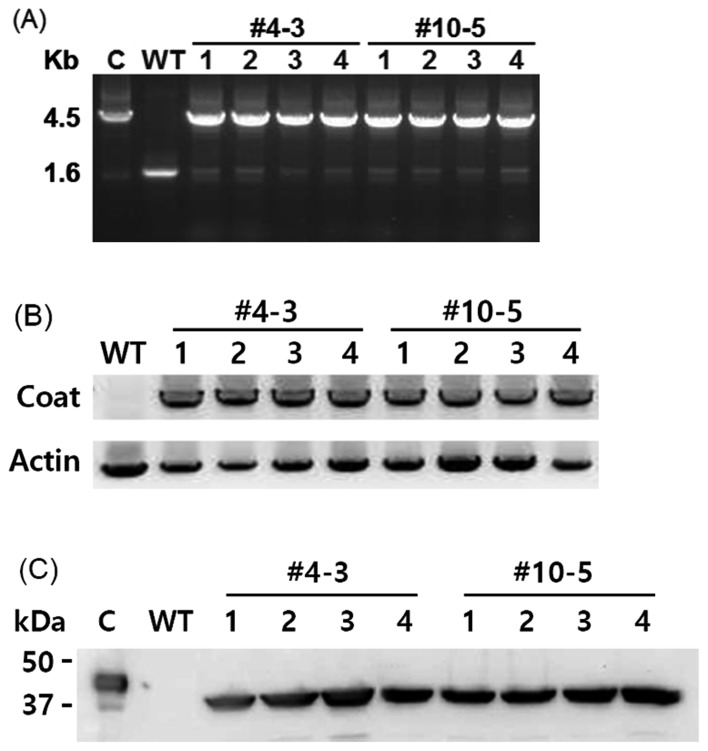
The sequence of the insert was confirmed to be coat protein gene because it shared 99.7% (1,013 of 1,017 bp) sequence similarities with seven band grouper nervous necrosis virus (sequence data not shown). rCoat (37-kDa) was produced in E. coli, purified, and used to produce rabbit polyclonal antibody (data not shown). All transgenic plants matured normally to T0 and T1 generations without any morphological abnormality. More than 10 T0 transgenic plants were obtained. The presence of coat protein gene was verified by PCR using primers specific to rice clp promoter and coat protein gene. Two independent T1 transgenic plants were selected for further analyses. PCR analysis was performed using primers specific to the trnI and trnA regions to confirm site-specific integration of coat protein gene into the chloroplast genome. All T1 transgenic plants generated a band at size of 4.5 kb, whereas wild-type plants had band size of 1.6 kb size (Fig. 2A). PCR analysis also reflected the levels of homoplastomy based on different intensities of the two DNA bands. Higher intensity of the upper DNA bands compared to the lower DNA bands indicated that all T1 transgenic plant were homoplastomous.
Fig. 2.
Expression of coat protein in tobacco chloroplasts. (A) Genomic DNA PCR analysis of randomly selected transgenic T1 plants (#4-3 and #10-5). C: coat protein gene in the expression vector; WT: wild-type; (B) RT-PCR analysis of transgenic T1 plants. WT: wild-type; (C) Western blot analysis of transgenic T1 plants. C: rCoat protein purified from E. coli; WT: wild-type. PCR and RT-PCR products were seen on agarose gels under UV illumination and the mages were acquired and analyzed using KODAK gel logic imaging system (Fig 2A: Carestream Health, New York, NY, U.S.A.) or ImageQuantTM LAS 4000 (Fig 2B: GE Healthcare Bio-Science AB, Uppsala, Sweden).
Gene expression (at both transcriptional and translational) was determined for all T1 transgenic plants. The transcription of coat protein gene was analyzed by RT-PCR using gene-specific primers. As expected, the transcription of coat protein gene in all T1 transgenic plants was confirmed (Fig. 2B). Western blot analysis with a polyclonal antibody against rCoat confirmed the presence of a 37-kDa protein in all T1 transgenic plants (Fig. 2C). Since rCoat from E. coli was a His-tagged fusion protein (approx. 4-kDa), the detected protein band in the control lane was larger in size than that in transgenic plants.
Mouse antibody titrations
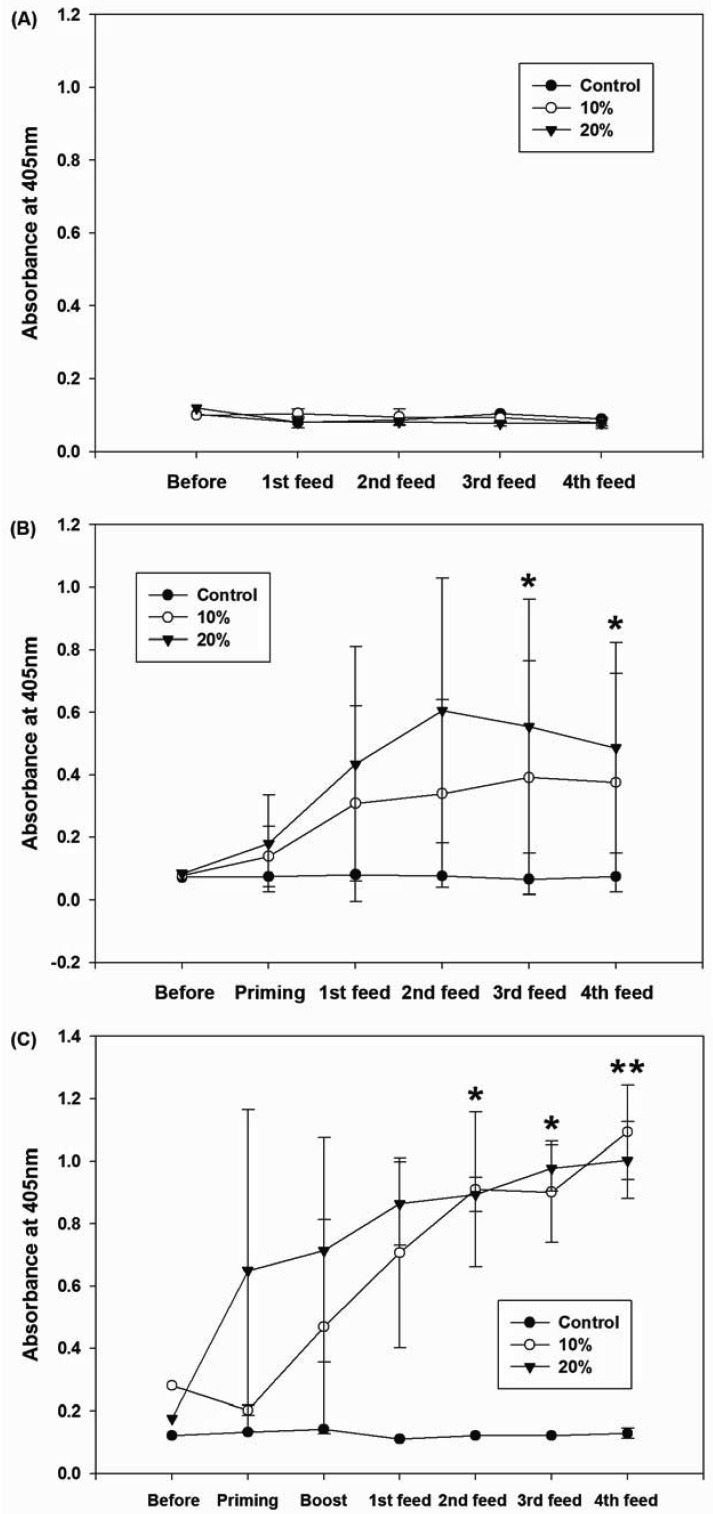
A significant (P<0.05) increase in IgA level was found in the 3 µg of rCoat-administrated group (Fig. 3). The 1.5 µg of rCoat-administrated group tended to have higher IgA level than that of the control. However, the difference was not significant. No significant increase in IgG titer was observed in the fed-only group (Fig. 4A). However, a significantly higher IgG titer was maintained after antigen recognition by priming (Fig. 4B, *P<0.05). The IgG titer was also significantly increased in boosted group (Fig. 4C, *P<0.05 and **P<0.01).
Fig. 3.
Results of mouse IgA titration using small intestine lavage samples. A significant increase (*P<0.05) in the IgA titer was observed in the 20%-administered group. 1: Non-immunized diet-only controls; 2-3: 10–20%-administered groups. Group 4 was used as a negative control as described in Table 1.
Fig. 4.
Changes in mouse IgG titers (serum samples) after immunizations. (A) No changes were observed in fed-only group; (B) primed + fed group; (C) primed + boosted + fed group. Significant increases (*P<0.05, **P<0.01) in IgG titers were observed in primed groups (B and C). Group 4 was used as a negative control as described in Table 1.
Protective effects of immunization in grouper
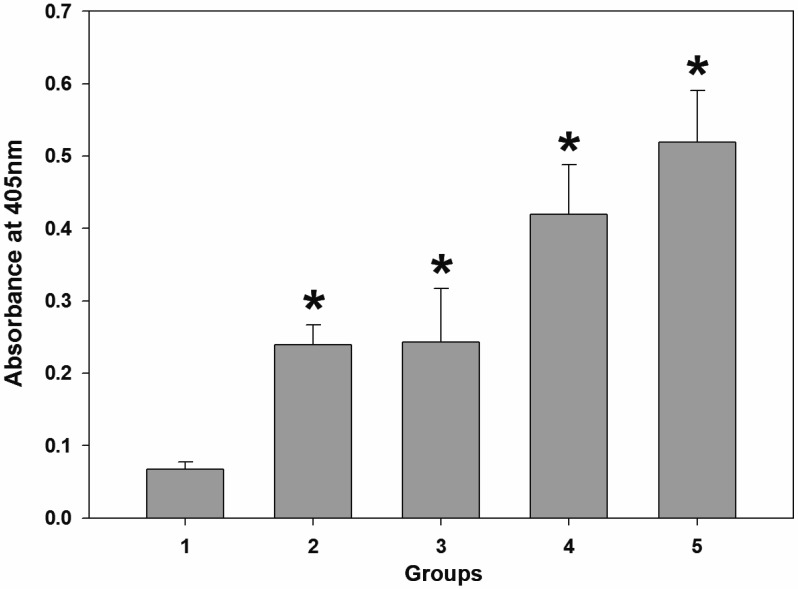
Grouper IgM titer was significantly increased in all experimental groups (Fig. 5, *P<0.0001). Some fish showed abnormal swimming behavior such as circling and spinal curvature after viral challenge without corneal opacity. After NVV challenge, lower mortality rate was observed in the immunized groups (group 2, 3, 4 and 5) as summarized in Table 3. PCR assay for viral infection confirmed that the dead and moribund fish were infected with NNV. However, no specific PCR bands were observed in survivors (data not shown).
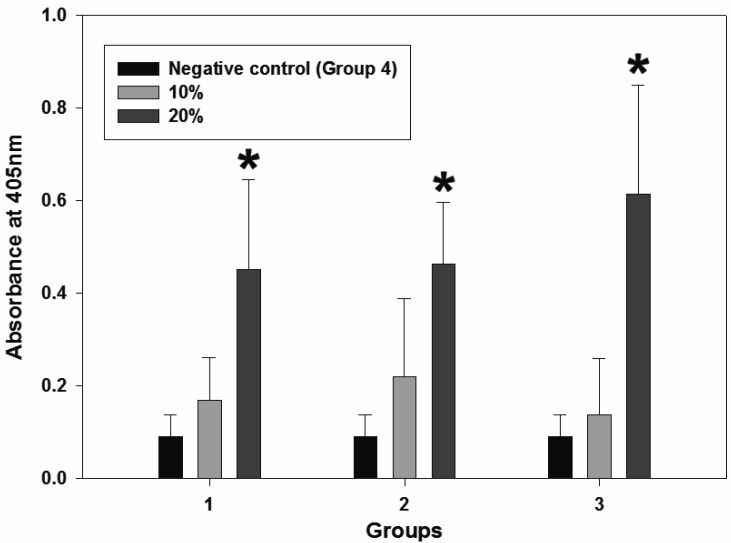
Fig. 5.
Result of grouper IgM titration using serum samples. Significant increases (*P<0.0001) in IgM titers were observed in all experimental groups (2–5). 2–3: 10–20% fed-only groups; 4–5: primed + fed groups. Group 1 (normal diet) was used as a negative control as described in Table 2.
Table 3. Cumulative mortality after NNV challenge with 60 days of observation.
| Group | Cumulative mortality rate (%) |
|---|---|
| 1 | 93.3 |
| 2 | 60a) |
| 3 | 73.3b) |
| 4 | 60a) |
| 5 | 53.3c) |
Two-sided P-values over group 1 were a) 0.0810, b) 0.3295 and c) 0.0352 based on Fisher’s exact test.
DISCUSSION
Transgenic plants have been increasingly used to express recombinant antigens and produce experimental immunogens because of their low cost of production. In addition, antigens can be administrated parenterally as well as orally [4]. Here, we report the sufficient and stable expression of an animal viral antigen in a plant (tobacco chloroplasts) as possible oral vaccine. Gene insertion into the chloroplast genome was accomplished by homologous recombination. A typical chloroplast vector carries both promoter and terminator isolated from the same plant species. However, such combination can promote another homologous recombination between the terminator and the corresponding sequence in the original chloroplast genome of the host plant in addition to the first homologous recombination, which can result in low transformation efficiency [11]. Therefore, we used TIA::RclpGAH vector containing exogenous promoter and terminator pairs with low homology to the chloroplast genome sequences in tobacco to construct a NNV virus coat protein expression vector. The clp promoter and rrnB1/B2 terminator were derived from rice and E. coli, respectively. The intergenic region between trnI and trnA genes was chosen as an insertion site for the target gene in the plastid genome (Fig. 1).
It has been reported that mouse has better and more advanced immune systems than fish [29]. And it has been known that oral immunization can induce both systemic and humoral immunity in mice [17]. Based on those, we performed immunization studies (IgA and IgG induction) in mouse model prior to immunizing fish to check whether such plant-based immunization could induce immune responses in mouse. Prior to the main experiments, mice were fed with wild-type tobacco (cultivar Samsun, which is Nicotine-free) powder up to 30% (v/v) to check the side effects such as intestinal problems. Fortunately, no inflammatory signs were found histologically (data not shown). After immunization, significant increases in IgA (Fig. 3) and IgG (Fig. 4) were observed in mouse. Therefore, we proceeded the immunization in fish. Few details are known about the immune system of fish, particularly in teleosts [22]. Until recently, teleost fish B cells are thought to be able to express IgM and IgD, with IgM as the only Ig responding to pathogens both in systemic and mucosal compartments [23]. However, a third teleost immunoglobulin class, IgT/IgZ, was discovered in 2005 as the prevalent immunoglobulin during gut mucosal immune response [22, 23]. In rainbow trout, IgT Ab is considered as a mucosal antibody in teleost fish [32]. However, characterization of the responsible class of Igs for systemic and mucosal immunity has not been fully studied in grouper. In general, most carnivorous marine fish including groupers do not eat plant or plant containing feed. However, in our previous [25] and preliminary study (data not shown), fish fed with plant (up to 30% v/v) had no side-effect such as intestinal inflammation and/or decreased growth rate. They did not even show reluctance toward plant feed. In our experiments, the IgM titer in the grouper serum was significantly higher (P<0.0001) in the immunized groups with partially protection against viral challenge. It is currently unclear whether another Ig class might be involved in this protective response.
In conclusion, we developed a plant-based fish oral vaccine against NNV infection with some promising results. This plant-based antigen expression system can be used to produce desired antigen molecules with many benefits. It could be used as a model to develop oral vaccines for animals.
Acknowledgments
This study was part of the project titled “Fishery Commercialization Technology Development Program” funded by the Ministry of Oceans and Fisheries, Republic of Korea.
REFERENCES
- 1.Ausubel F. M., Brent R., Kingston R. E., Moore D. D., Seidman J. G., Smith J. A., Struhl K.2002. Short protocols in molecular biology. 5th ed. John Wiley & Sons, New York. [Google Scholar]
- 2.Cardi T., Lenzi P., Maliga P.2010. Chloroplasts as expression platforms for plant-produced vaccines. Expert Rev. Vaccines 9: 893–911. doi: 10.1586/erv.10.78 [DOI] [PubMed] [Google Scholar]
- 3.Coku A.2007. Molecular Farming of Vaccines from Transgenic Plants. Basic Biotechnol. eJournal 3: 110–116. [Google Scholar]
- 4.Dus Santos M. J., Carrillo C., Ardila F., Ríos R. D., Franzone P., Piccone M. E., Wigdorovitz A., Borca M. V.2005. Development of transgenic alfalfa plants containing the foot and mouth disease virus structural polyprotein gene P1 and its utilization as an experimental immunogen. Vaccine 23: 1838–1843. doi: 10.1016/j.vaccine.2004.11.014 [DOI] [PubMed] [Google Scholar]
- 5.Frerichs G. N., Rodger H. D., Peric Z.1996. Cell culture isolation of piscine neuropathy nodavirus from juvenile sea bass, Dicentrarchus labrax. J. Gen. Virol. 77: 2067–2071. doi: 10.1099/0022-1317-77-9-2067 [DOI] [PubMed] [Google Scholar]
- 6.Gao M., Li Y., Xue X., Wang X., Long J.2012. Stable plastid transformation for high-level recombinant protein expression: promises and challenges. J. Biomed. Biotechnol. 2012: 158232. doi: 10.1155/2012/158232 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.Gomez D. K., Baeck G. W., Kim J. H., Choresca C. H., Jr, Park S. C.2008. Molecular detection of betanodavirus in wild marine fish populations in Korea. J. Vet. Diagn. Invest. 20: 38–44. doi: 10.1177/104063870802000107 [DOI] [PubMed] [Google Scholar]
- 8.Greenfield E. A.2014. Antibodies, A laboratory manual, 2nd ed., Cold Spring Harbor Laboratory, New York. [Google Scholar]
- 9.Húsgağ S., Grotmol S., Hjeltnes B. K., Rødseth O. M., Biering E.2001. Immune response to a recombinant capsid protein of striped jack nervous necrosis virus (SJNNV) in turbot Scophthalmus maximus and Atlantic halibut Hippoglossus hippoglossus, and evaluation of a vaccine against SJNNV. Dis. Aquat. Organ. 45: 33–44. doi: 10.3354/dao045033 [DOI] [PubMed] [Google Scholar]
- 10.Jeong S. W., Jeong W. J., Woo J. W., Choi D. W., Park Y. I., Liu J. R.2004. Dicistronic expression of the green fluorescent protein and antibiotic resistance genes in the plastid for selection and tracking of plastid-transformed cells in tobacco. Plant Cell Rep. 22: 747–751. doi: 10.1007/s00299-003-0740-4 [DOI] [PubMed] [Google Scholar]
- 11.Liu J. R., Chung H. J., Jeong W. J., Min S. R., Park J. Y.2011. Plastid transformation system to prevent the intramolecular recombination of transgene. US Patent No US 7,888,562 B2.
- 12.Liu W., Hsu C. H., Chang C. Y., Chen H. H., Lin C. S.2006. Immune response against grouper nervous necrosis virus by vaccination of virus-like particles. Vaccine 24: 6282–6287. doi: 10.1016/j.vaccine.2006.05.073 [DOI] [PubMed] [Google Scholar]
- 13.Maliga P., Bock R.2011. Plastid biotechnology: food, fuel, and medicine for the 21st century. Plant Physiol. 155: 1501–1510. doi: 10.1104/pp.110.170969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Munday B. L., Kwang J., Moody N.2002. Betanodavirus infections of teleost fish: a review. J. Fish Dis. 25: 127–142. doi: 10.1046/j.1365-2761.2002.00350.x [DOI] [Google Scholar]
- 15.Murashige T., Skoog F.1962. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 15: 473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x [DOI] [Google Scholar]
- 16.Nishizawa T., Furuhashi M., Nagai T., Nakai T., Muroga K.1997. Genomic classification of fish nodaviruses by molecular phylogenetic analysis of the coat protein gene. Appl. Environ. Microbiol. 63: 1633–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oliveira A. F., Cardoso S. A., Almeida F. B., de Oliveira L. L., Pitondo-Silva A., Soares S. G., Hanna E. S.2012. Oral immunization with attenuated Salmonella vaccine expressing Escherichia coli O157:H7 intimin gamma triggers both systemic and mucosal humoral immunity in mice. Microbiol. Immunol. 56: 513–522. doi: 10.1111/j.1348-0421.2012.00477.x [DOI] [PubMed] [Google Scholar]
- 18.Pakingking R., Jr, Bautista N. B., de Jesus-Ayson E. G., Reyes O.2010. Protective immunity against viral nervous necrosis (VNN) in brown-marbled grouper (Epinephelus fuscogutattus) following vaccination with inactivated betanodavirus. Fish Shellfish Immunol. 28: 525–533. doi: 10.1016/j.fsi.2009.12.004 [DOI] [PubMed] [Google Scholar]
- 19.Pakingking R., Jr, Seron R., dela Peña L., Mori K., Yamashita H., Nakai T.2009. Immune responses of Asian sea bass, Lates calcarifer Bloch, against an inactivated betanodavirus vaccine. J. Fish Dis. 32: 457–463. doi: 10.1111/j.1365-2761.2009.01040.x [DOI] [PubMed] [Google Scholar]
- 20.Ransangan J., Manin B. O.2012. Genome analysis of Betanodavirus from cultured marine fish species in Malaysia. Vet. Microbiol. 156: 16–44. doi: 10.1016/j.vetmic.2011.10.002 [DOI] [PubMed] [Google Scholar]
- 21.Rigano M. M., Manna C., Giulini A., Vitale A., Cardi T.2009. Plants as biofactories for the production of subunit vaccines against bio-security-related bacteria and viruses. Vaccine 27: 3463–3466. doi: 10.1016/j.vaccine.2009.01.120 [DOI] [PubMed] [Google Scholar]
- 22.Rombout J. H., Abelli L., Picchietti S., Scapigliati G., Kiron V.2011. Teleost intestinal immunology. Fish Shellfish Immunol. 31: 616–626. doi: 10.1016/j.fsi.2010.09.001 [DOI] [PubMed] [Google Scholar]
- 23.Salinas I., Zhang Y. A., Sunyer J. O.2011. Mucosal immunoglobulins and B cells of teleost fish. Dev. Comp. Immunol. 35: 1346–1365. doi: 10.1016/j.dci.2011.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seo J. Y., Chung H. J., Kim T. J.2013. Codon-optimized expression of fish iridovirus capsid protein in yeast and its application as an oral vaccine candidate. J. Fish Dis. 36: 763–768. doi: 10.1111/jfd.12037 [DOI] [PubMed] [Google Scholar]
- 25.Shin Y. J., Kwon T. H., Seo J. Y., Kim T. J.2013. Oral immunization of fish against iridovirus infection using recombinant antigen produced from rice callus. Vaccine 31: 5210–5215. doi: 10.1016/j.vaccine.2013.08.085 [DOI] [PubMed] [Google Scholar]
- 26.Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C.1985. Measurement of protein using bicinchoninic acid. Anal. Biochem. 150: 76–85. doi: 10.1016/0003-2697(85)90442-7 [DOI] [PubMed] [Google Scholar]
- 27.Tanaka S., Mori K., Arimoto M., Iwamoto T., Nakai T.2001. Protective immunity of sevenband grouper, Epinephelus septemfasciatus Thunberg, against experimental viral nervous necrosis. J. Fish Dis. 24: 15–22. doi: 10.1046/j.1365-2761.2001.00259.x [DOI] [Google Scholar]
- 28.Thiéry R., Cozien J., Cabon J., Lamour F., Baud M., Schneemann A.2006. Induction of a protective immune response against viral nervous necrosis in the European sea bass Dicentrarchus labrax by using betanodavirus virus-like particles. J. Virol. 80: 10201–10207. doi: 10.1128/JVI.01098-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tizard I. R.2012. Evolution of the immune system. pp. 477–493. In: Veterinary Immunology, 9th ed. (Tizard, I. R. ed.) Elsevier, St. Louis. [Google Scholar]
- 30.Twyman R. M., Schillberg S., Fischer R.2012. The production of vaccines and therapeutic antibodies in plants. pp. 145–159. In: Molecular Farming in Plants: Recent Advances and Future Prospects, (Wang, A. and Ma, S. eds.), Springer, Amsterdam. [Google Scholar]
- 31.Yamashita H., Fujita Y., Kawakami H., Nakai T.2005. The efficacy of inactivated virus vaccine against viral nervous necrosis (VNN). Fish Pathol. 40: 15–21. doi: 10.3147/jsfp.40.15 [DOI] [Google Scholar]
- 32.Zhang Y. A., Salinas I., Li J., Parra D., Bjork S., Xu Z., LaPatra S. E., Bartholomew J., Sunyer J. O.2010. IgT, a primitive immunoglobulin class specialized in mucosal immunity. Nat. Immunol. 11: 827–835. doi: 10.1038/ni.1913 [DOI] [PMC free article] [PubMed] [Google Scholar]