Abstract
Antrodia camphorata and Panax ginseng are well-known medicinal plants in Taiwan folk and traditional Chinese medicine, which have been reported for multifunctional bioactivities. However, there is limited evidence that a fixed combination formula of these two plant extracts is effective for the exercise improvement or anti-fatigue. We aimed to evaluate the potential beneficial effects of the mix formulation of these two herbal medicines (AG formulation) on fatigue and ergogenic functions following physiological challenge. Male Institute of Cancer Research (ICR) mice from four groups (n=10 per group) were orally administered AG formulation for 4 weeks at 0.984, 2.952 and 5.904 g/kg/day, which were designated the Vehicle, AG-1X, AG-3X and AG-6X groups, respectively. The anti-fatigue activity and exercise performance were evaluated using exhaustive swimming time, forelimb grip strength, and levels of serum lactate, ammonia, glucose, blood urea nitrogen (BUN) and creatine kinase (CK) after a swimming exercise. The exhaustive swimming time of the 1X, 3X or 6X AG group was significantly longer than that of the Vehicle group, and the forelimb grip strength of the 1X, 3X or 6X AG group was also significantly higher than that of the Vehicle group. AG supplementation also produced decreases in serum lactate, ammonia, BUN and CK activity after the swimming test, as well as increases in glucose. Therefore, the AG complex could be a potential formulation with an anti-fatigue pharmacological effect.
Keywords: exercise performance, fatigue, lactate
Fatigue is one of the most commonly physiological reactions, which has some symptoms such as a feeling of exhaustion, tiredness, weariness or lack of energy. Fatigue in long-term may cause aging, depression, human immunodeficiency virus (HIV) infection, cancers, multiple sclerosis and Parkinson’s disease [15]. Thus, many researchers were interested in herbal medicines, natural compounds or sports equipment technology to postpone fatigue and accelerate the elimination of fatigue-related metabolites [2, 12]. Up to date, many phytocompounds, such as triterpenoid [26], ginsenosides [13], polysaccharides [18], flavonoids [25] and peptides [23], have been studied as supplements to improve fatigue symptoms.
Antrodia camphorata has been frequently used in traditional Chinese medicine to treat many disorders, such as the treatment of food and drug intoxication, diarrhea, abdominal pain, hypertension, skin itching and cancer [16]. In our previous studies [2], it has been found that the crude extracts of A. camphorata fruiting bodies contains a wide variety of triterpenoid compounds, as well as enhances exercise performance and reduces muscle fatigue physiological indexes. Panax ginseng is traditionally used as a medicine for debility, ageing, stress, diabetes, insomnia, antitumor, antioxidant and hypoglycemic properties [5, 6, 8, 11] In addition, ginseng also has been used for the development of physical strength, especially in patients who suffered from severe fatigue [1, 10]. Wang et al. [18] also showed that ginseng polysaccharides from the P. ginseng have anti-fatigue activity, also reflected in the effects on the physiological markers for fatigue. However, to the best of our knowledge, there is no prior report on the effects of a fixed combination formula of these two plant extracts on the exercise improvement or anti-fatigue. The purpose of this study was to evaluate the potential beneficial effects of the mix formulation of these two herbal medicines (AG formulation) on fatigue and ergogenic functions following physiological challenge.
MATERIALS AND METHODS
Materials
A commercially available supplement, the mix formulation of Antrodia camphorata and Panax ginseng (AG formulation), was provided by Formosa Biomedical Technology Corp. (Taipei City, Taiwan).
Animals and treatment
Male ICR mice were purchased from BioLASCO (A Charles River Licensee Corp., Yilan, Taiwan). All animals were fed a chow diet (no. 5001; PMI Nutrition International, Brentwood, MO, U.S.A.) and distilled water ad libitum, and were maintained on a regular cycle (12-h light/dark) at room temperature (24 ± 2°C) and 60–70% humidity. The bedding was changed and cleaned twice per week. All animal experimental protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of National Taiwan Sport University, and the study conformed to the guidelines of the protocol IACUC-10311 approved by the IACUC ethics committee.
ICR mice at 6 months old were randomly divided into 4 groups (n=10 per group): (1) the vehicle control group (Vehicle group); (2) supplementation with AG-1X group (AG-1X group); (3) supplementation with AG-3X group (AG-3X group); and (4) supplementation with AG-6X group (AG-6X group). The AG-1X, AG-3X and AG-6X would be 0.984, 2.952 and 5.904 g/kg/day, respectively. AG formulation was dissolved in distilled water. The Vehicle, AG-1X, AG-3X and AG-6X groups received the same volume of distilled water equivalent to an individual’s body weight. The water and food intake were monitored daily, and BW was recorded weekly. Oral gavage was used to administer AG formulation or distilled water once a day for 28 consecutive days. Food intake and water consumption were monitored daily, and the body weight was recorded weekly.
Exhaustive swimming test
Measurement of exhaustive swimming test was carried out according to the method of Kan et al. [4]. Mice were individually placed in a columnar swimming pool (65 cm high with a radius of 20 cm) with 40 cm of water depth maintained at 24 ± 1°C. A weight equivalent to 5% of the body weight was attached to the root of the tail, and the swimming time was recorded from the beginning to exhaustion for each mouse in the various groups. Exhaustion was determined by observing failure to swim, and the swimming period was regarded as the time spent by the mouse floating in the water, struggling, and making necessary movements until strength exhaustion and drowning. When a mouse was unable to remain on the water surface, it was assessed. Exhaustive swimming test was measured 30 min after the last treatment was administered. The swimming time from beginning to exhaustion was used to evaluate the endurance performance.
Forelimb grip strength
A low-force testing system (Model-RX-5, Aikoh Engineering, Nagoya, Japan) was used to measure the forelimb absolute grip strength as we previously described [2], and the maximal force (grams) was recorded. A force transducer equipped with a metal bar (2 mm in diameter and 7.5 cm long) was used to measure the amount of tensile force by each mouse. We grasped the mouse at the base of the tail and lowered it vertically toward the bar. The mouse was pulled slightly backwards by the tail while the 2 paws (forelimbs) grasped the bar, which triggered a “counter pull”. This grip strength meter recorded the grasping force in grams. Grip strength was measured 30 min after the last treatment was administered. The maximal force (grams) exerted by the mouse counter pull was used as the forelimb grip strength.
Fatigue-associated biochemical indices
After 4 weeks of the intervention, mice underwent a 15-min swimming test without weight loading to evaluate fatigue-associated biochemical variables as in our previous studies [2, 22]. Blood samples were collected before and after the swimming exercise, as well as after resting 20 min. Serum was collected by centrifugation at 1,500 g and 4°C for 10 min. Lactate, ammonia and glucose levels were determined by use of an autoanalyzer (Hitachi 7060, Hitachi, Tokyo, Japan). After 30 days of the intervention, mice underwent a 90-min swimming test after resting 60 min to evaluate fatigue-associated creatine kinase (CK) and blood urea nitrogen BUN.
Tissue glycogen determination
The muscles and liver were excised and weighed for a subsequent glycogen content analysis. The method of glycogen analysis was described in our previous studies [2].
Histological staining of tissues
Different tissues were collected and fixed in 10% formalin after mice was sacrificed. After the formalin fixed, tissues were then embedded in paraffin and cut into 4-µm thick slices for morphological and pathological evaluations. Tissue sections were stained with hematoxylin and eosin (H & E) and examined by light microscopy with a CCD camera (BX-51, Olympus, Tokyo, Japan) by a clinical pathologist.
Statistical analyses
All results are expressed as mean ± SEM (n=10). The significance of difference was calculated by a one-way ANOVA with Duncan’s test, and values of <0.05 were considered significant. We also used the Cochran-Armitage test of trend to examine the relationship of dosage.
RESULTS
Effect of 4-week AG supplementation on body weight, food intake and water intake
Results of body weight, food intake and water intake are shown in Table 1. One-way ANOVA results indicated that there were no significant differences in the body weight, food intake or water intake among the Vehicle, AG-1X, AG-3X and AG-6X groups.
Table 1. Effects of 4-week AG supplementation on the body weight (BW), diet intake and water intake in mice.
| Vehicle | AG-1X | AG-3X | AG-6X | |
|---|---|---|---|---|
| Beginning BW (g) | 39.4 ± 0.6a) | 39.5 ± 0.3a) | 39.6 ± 0.9a) | 39.3 ± 0.5a) |
| Final BW (g) | 41.4 ± 0.6a) | 41.4 ± 0.7a) | 41.4 ± 1.1a) | 41.5 ± 0.6a) |
| Diet intake (g/mouse/day) | 7.8 ± 0.1a) | 7.8 ± 0.1a) | 7.5 ± 0.1a) | 7.4 ± 0.1a) |
| Diet intake (kcal/mouse/day) | 23.5 ± 0.4a) | 23.5 ± 0.4a) | 22.6 ± 0.4a) | 22.4 ± 0.2a) |
| Water (ml/mouse/day) | 11.0 ± 0.1a) | 10.6 ± 0.3a) | 10.8 ± 0.3a) | 10.4 ± 0.2a) |
Data are the mean ± SEM (n=10). Different letters in a given row indicated a significant difference at P<0.05 according to a one-way ANOVA.
Effect of 4-week AG supplementation on an exhaustive swimming test
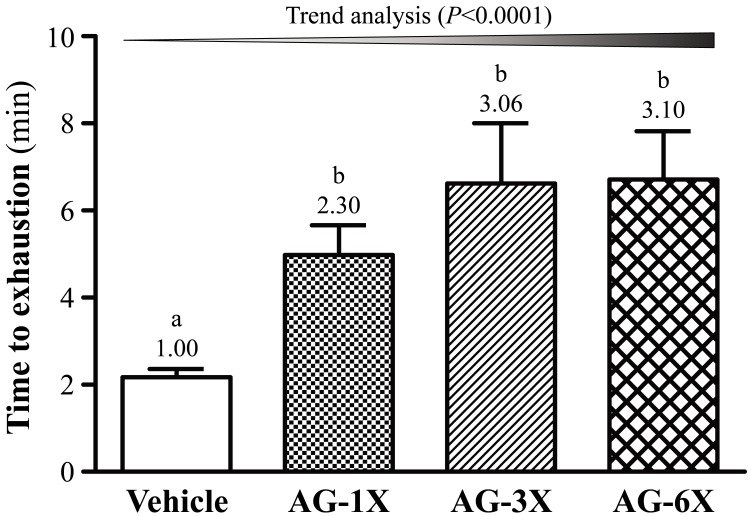
As shown in Fig. 1, the exhaustion time of AG-1X group was 4.98 ± 0.68 min (2.30-fold greater than that of the Vehicle group; P=0.0443); the exhaustion time of the AG-3X group was 6.62 ± 1.38 min (3.00-fold greater than that of the Vehicle group; P=0.0022); the exhaustion time of the AG-6X group was 6.71 ± 1.11 min (3.10-fold greater than that of the Vehicle group; P=0.0018), indicating that AG-1X, AG-3X and AG-6X groups exhibited an anti-fatigue effect. In addition, there was a significant dose-dependent effect on endurance swimming performance (P<0.0001).
Fig. 1.
Effect of a 4-week AG supplementation on endurance swimming performance in mice. Mice were pretreated with the Vehicle, AG-1X, AG-3X and AG-6X for 28 days. Data are the mean ± SEM (n=10). Different letters indicated a significant difference at P<0.05 according to a one-way ANOVA.
Effect of 4-week AG supplementation on forelimb grip strength
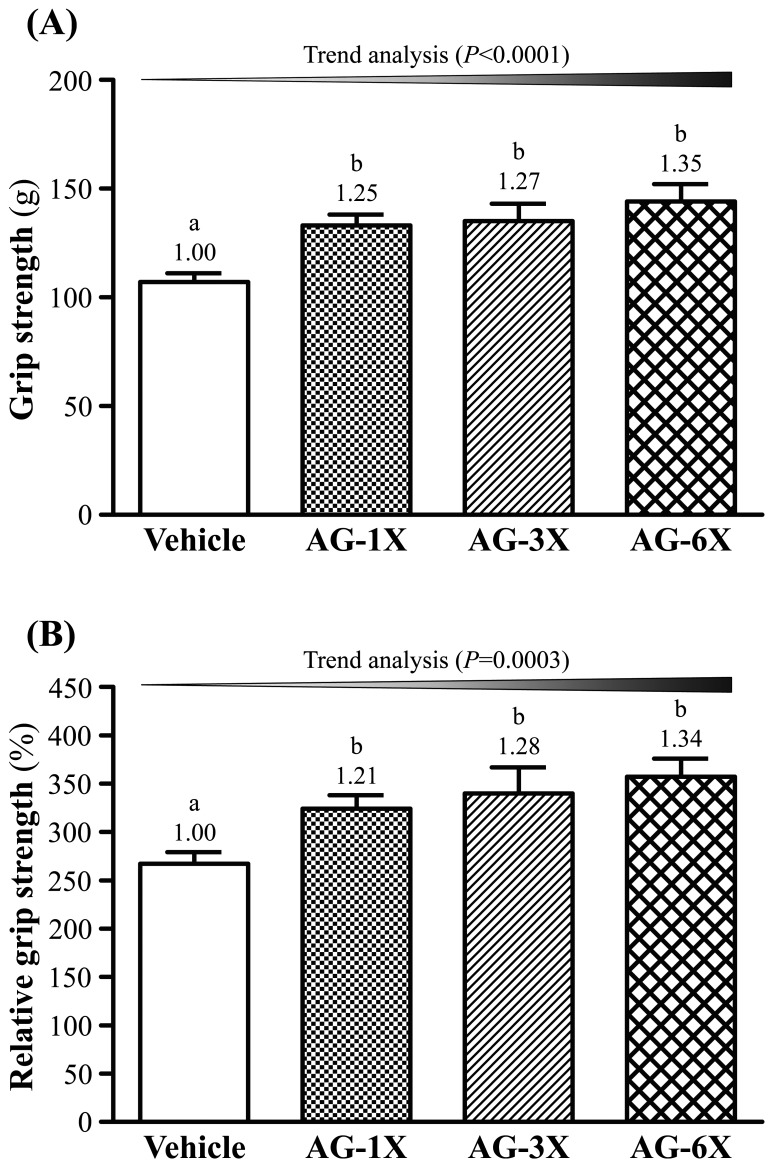
The grip strength was higher in the AG-1X, AG-3X and AG-6X groups than the Vehicle group (133 ± 5, 135 ± 8 and 144 ± 8 g vs. 107 ± 4 g) (P<0.05) (Fig. 2A). Thus, the grip strength in the AG-1X, AG-3X and AG-6X groups significantly increased by 24% (P=0.0048), 26% (P=0.0025) and 35% (P=0.0002), respectively, compared to the Vehicle group. In addition, there was a significant dose-dependent effect on the grip strength (P<0.0001).
Fig. 2.
Effect of a 4-week AG supplementation on (A) forelimb grip strength and (B) relative forelimb grip strength in mice. Mice were pretreated with the Vehicle, AG-1X, AG-3X and AG-6X for 28 days. Data are the mean ± SEM (n=10). Different letters indicated a significant difference at P<0.05 according to a one-way ANOVA.
As shown in Fig. 2B, the relative absolute grip strength of the AG-1X (324 ± 14%), AG-3X (340 ± 27%) and AG-6X (357 ± 19%) groups significantly increased by 21, 27 and 34%, respectively, compared to the Vehicle group (267 ± 12%). Additionally, there was a significant dose-dependent effect on the relative grip strength (P=0.0003).
Effect of 4-week AG supplementation on lactate after a 15-min swimming test
After 4-week of the intervention, mice underwent a 15-min swimming test to evaluate the levels of lactate before and after the swimming exercise, as well as after resting 20 min, as shown in Table 2. Before swimming, there were no significant differences in the levels of blood lactate among the Vehicle, AG-1X, AG-3X and AG-6X groups. After swimming, the levels of blood lactate of the AG-1X (4.9 ± 0.1 mmol/l), AG-3X (4.8 ± 0.1 mmol/l) and AG-6X (4.8 ± 0.2 mmol/l) groups were significantly lower by 20% (P=0.0006), 21% (P=0.0002) and 21% (P=0.0003), respectively, than that of the Vehicle group (6.1 ± 0.4 mmol/l). At rest for 20 min, there were no significant differences in the levels of blood lactate among the Vehicle, AG-1X and AG-3X groups. But, the blood lactate of the AG-6X (2.2 ± 0.2 mmol/l) groups significantly decreased by 21%, respectively, compared to the Vehicle group (2.8 ± 0.1 mmol/l).
Table 2. Effect of a 4-week AG supplementation on blood lactate before and after the swimming exercise, as well as after resting 20 min.
| Time point | Vehicle | AG-1X | AG-3X | AG-6X |
|---|---|---|---|---|
| Lactate (mmol/l) | ||||
| Before swimming [A] | 2.5 ± 0.1a) | 2.5 ± 0.2a) | 2.5 ± 0.1a) | 2.5 ± 0.1a) |
| After swimming [B] | 6.1 ± 0.4b) | 4.9 ± 0.1a) | 4.8 ± 0.1a) | 4.8 ± 0.2a) |
| At rest for 20 min [C] | 2.8 ± 0.1b) | 2.6 ± 0.1ab) | 2.5 ± 0.1ab) | 2.2 ± 0.2a) |
| Increase ratio [B/A] | 2.51 ± 0.17b) | 2.03 ± 0.13a) | 1.95 ± 0.12a) | 1.95 ± 0.08a) |
| Clearance (%) [(B–C)/B] | 0.53 ± 0.03a) | 0.47 ± 0.02a) | 0.48 ± 0.03a) | 0.54 ± 0.04a) |
Mice were pretreated with the vehicle, AG-1X, AG-3X and AG-6X for 28 days. Data are the mean ± SEM (n=10). Different letters indicate a significant difference at P<0.05 according to a one-way ANOVA.
Effect of AG supplementation on ammonia and glucose after a 15-min swimming test
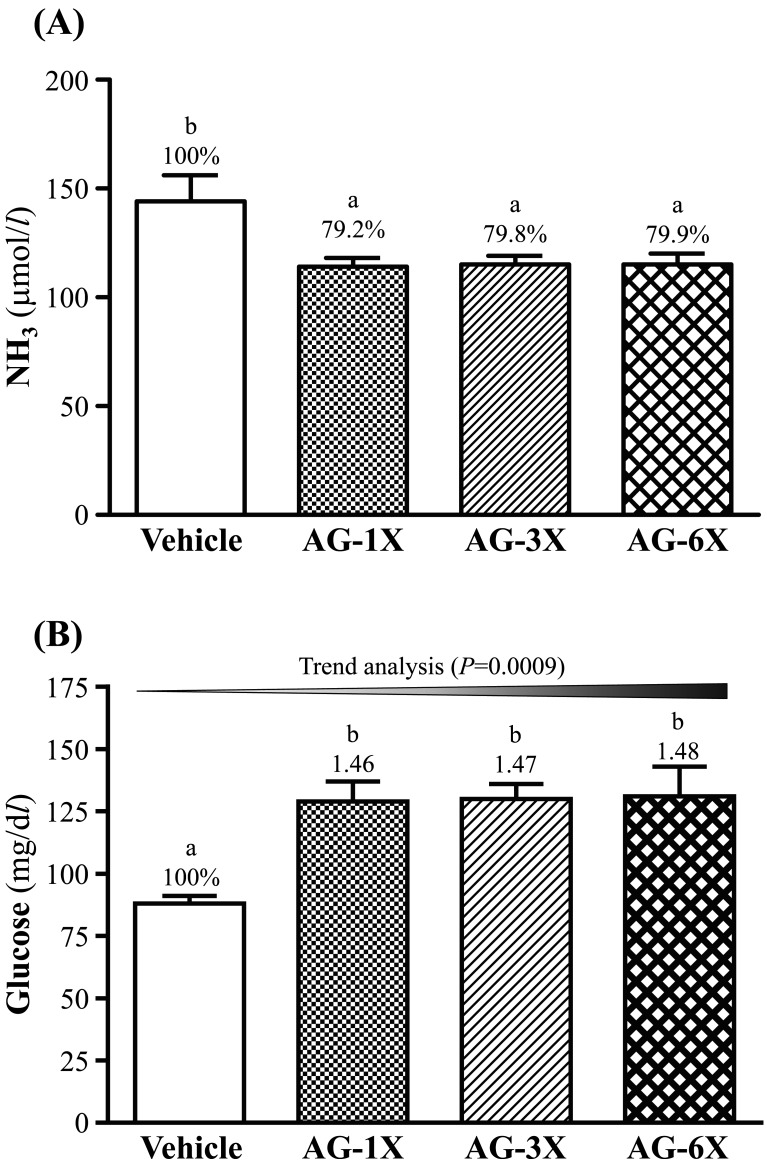
After AG supplementation for 28 days, serum ammonia levels were lower in the AG-1X (114 ± 4 µmol/l; 21% lower than that of the Vehicle group, P=0.0058), AG-3X (115 ± 4 µmol/l; 20% lower than that of the Vehicle group, P=0.0071) and AG-6X (115 ± 5 µmol/l; 20% lower than that of the Vehicle group, P=0.0074) than the Vehicle group (144 ± 12 µmol/l) after the swimming test (Fig. 3A).
Fig. 3.
Effect of 4-week AG supplementation on serum levels of (A) ammonia (NH3) and (B) glucose after a 15-min swim test. Mice were pretreated with the vehicle, AG-1X, AG-3X and AG-6X for 28 days. Data are the mean ± SEM (n=10). Different letters indicate a significant difference at P<0.05 according to a one-way ANOVA.
As shown in Fig. 3B, the blood glucose levels of the AG-1X, AG-3X and AG-6X groups were significantly 1.46-fold (P=0.0010), 1.47-fold (P=0.0008) and 1.48-fold (P=0.0007) higher than that of the Vehicle group.
Effect of 4-week AG supplementation on BUN and CK after a 90-min swimming test
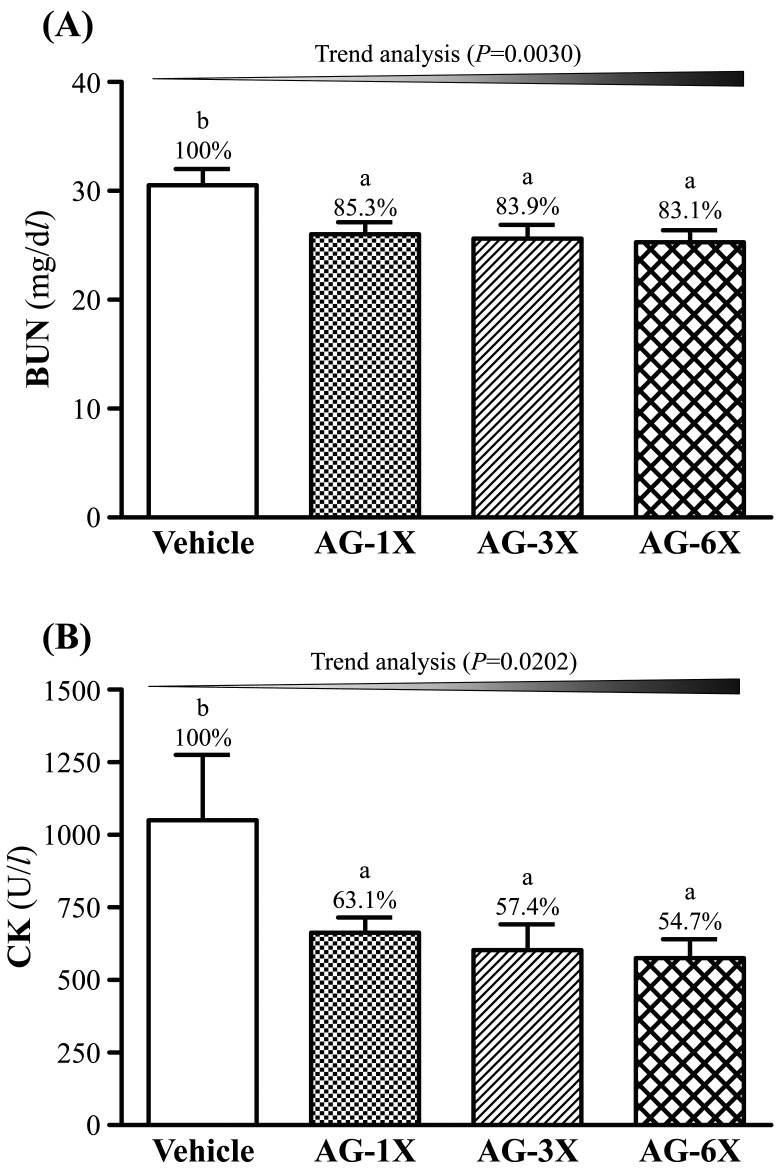
The levels of BUN of the AG-1X (26.0 ± 1.1 mg/dl), AG-3X (25.6 ± 1.3 mg/dl) and AG-6X (25.6 ± 1.3 mg/dl) were significantly lower 15% (P=0.0199), 16% (P=0.0114) and 16% (P=0.0122), respectively, compared to that of the Vehicle group (30.5 ± 1.5 mg/dl) (Fig. 4A). Additionally, there was a significant dose-dependent effect on the BUN (P=0.0030).
Fig. 4.
Effect of AG supplementation on serum levels of (A) BUN and (B) CK after a 90-min swim test. Mice were pretreated with the vehicle, AG-1X, AG-3X and AG-6X for 30 days. Data are the mean ± SEM (n=10). Different letters indicated a significant difference at P<0.05 according to a one-way ANOVA.
As shown in Fig. 4B, the serum CK levels of the AG-1X (663 ± 53 U/l), AG-3X (603 ± 89 U/l) and AG-6X (575 ± 65 U/l) groups were lower 37% (P=0.0380), 43% (P=0.0177) and 45% (P=0.0121), respectively, compared to the vehicle control (1051 ± 224 U/l). There was a significant dose-dependent effect on the CK (P=0.0202).
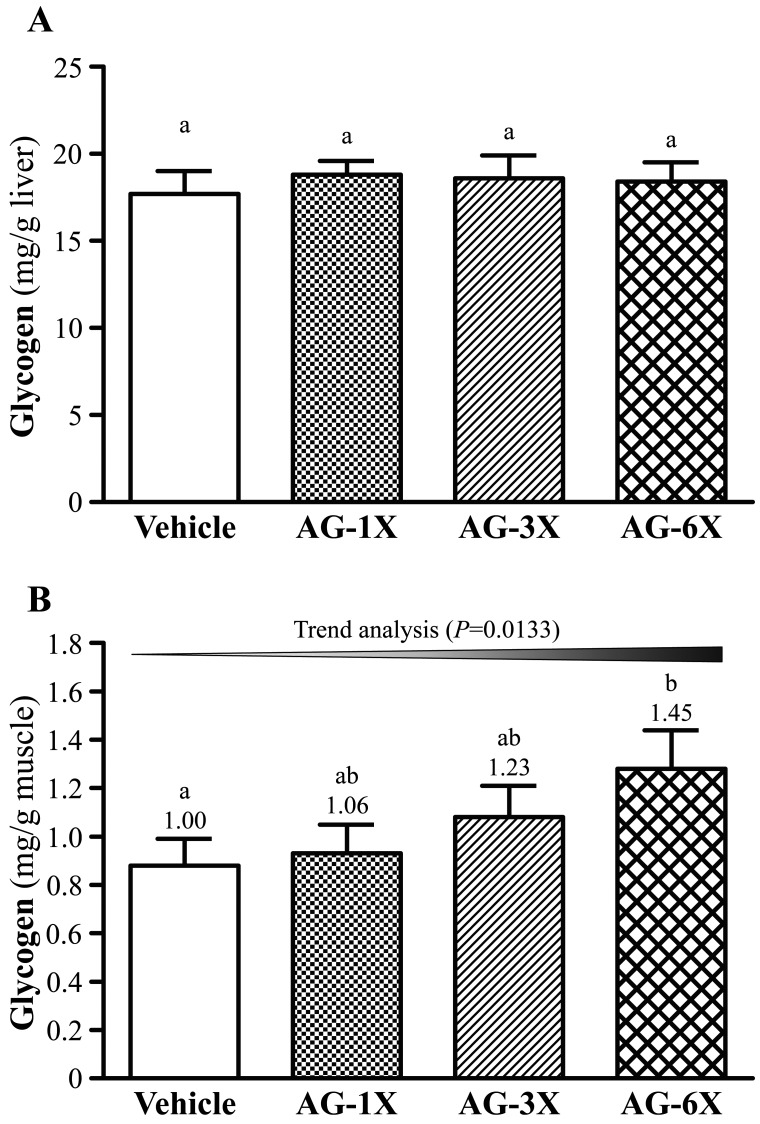
Effect of AG supplementation on liver and muscular glycogen
As shown in Fig. 5A, we found that glycogen contents of liver tissues did not show significant differences among the Vehicle, AG-1X, AG-3X and AG-6X groups. However, muscular glycogen levels of the AG-1X (0.93 ± 0.1 mg/g), AG-3X (1.08 ± 0.13 mg/dl) and AG-6X groups (1.28 ± 0.16 mg/g; 1.45-fold higher than that of the OC group, P=0.0432) were higher than that of the Vehicle group (0.88 ± 0.11 mg/dl) (Fig. 5B).
Fig. 5.
Effect of AG supplementation on levels of (A) hepatic and (B) muscular glycogen. Mice were pretreated with the vehicle, AG-1X, AG-3X and AG-6X for 30 days. Data are the mean ± SEM (n=10). Different letters indicated a significant difference at P<0.05 according to a one-way ANOVA.
Effect of AG supplementation on histopathological evaluation of tissues
The pathological histology of the major organs, including the liver, muscle, heat, kidney and lung tissues were shown in Fig. 6. The groups did not differ in histological observations of liver, muscle, heat, kidney and lung tissues of the mice in AG-1X, AG-3X and AG-6X groups, in comparison with the Vehicle group. There were no clinical signs of organ-specific toxicity on AG treatments.
Fig. 6.
Effect of AG supplementation on histopathological evaluation of tissues, including (A) liver, (B) muscle, (C) heart, (D) kidney and (E) lung. Mice were pretreated with the vehicle, AG-1X, AG-3X and AG-6X for 30 days. Specimens were photographed with a light microscope (Olympus BX51). (Magnification: × 200, Scale bar, 40 µm).
DISCUSSION
In an animal model, swimming to exhaustion could directly measure anti-fatigue effects. The model is a high reproducibility to evaluate the endurance capacity [26]. Reduced susceptibility to fatigue was interpreted from a longer swimming time. Huang et al. [2] showed that mice treated with 50 mg/kg and 200 mg/kg ethanol extract of A. camphorata fruiting body significantly increased by 1.60- (P=0.016) and 2.15-fold (P<0.0001), respectively, compared with the vehicle treatment. Wang et al. [18] showed that ginseng polysaccharides (WGP), neutral ginseng polysaccharides (WGPN) and acidic ginseng polysaccharides (WGPA) at 200, 200 and 40 mg/kg, respectively, significantly attenuated the immobility times following exposure to the forced swim test. Saito et al. [10] showed that 0.1 and 0.5 mg/kg of 20 (R)-ginsenoside Rg3 (20 (R)-Rg3) from P. ginseng could significantly prolong the weight loaded swimming time of mice. Thus, in this study the mix formulation of these two herbal medicines (AG formulation) at 0.984, 2.952 and 5.904 g/kg/day significantly attenuated the exhaustion time.
The grip strength currently is used widely to evaluate anti-fatigue effects. Huang et al. [2] showed that mice treated with 50 mg/kg and 200 mg/kg ethanol extract of A. camphorata fruiting body significantly increased the grip strength by 1.13- (P=0.005) and 1.13-fold (P=0.005), respectively, compared to the vehicle treatment. Our data is consistent with their result. Therefore, AG supplementation significantly increased the exercise performance.
To explore the mechanism, some biochemical parameters including lactase, ammonia, glucose, BUN and CK were determined in mice after they had swum. Gycolysis is the main energy source for short-term high-intensity exercise, and blood lactate is the glycolysis product of carbohydrates through anaerobic glycolysis [24]. The increased blood lactate may decrease the pH value of muscle tissue or blood, thus the phenomenon can induce various side effects of several biochemical and physiological response [2]. Therefore, blood lactate for biochemical parameter in blood is response to the fatigue [7]. In this study, AG supplementation reduced blood lactate further decreasing the fatigue. Huang et al. [2] showed that mice treated with 50 mg/kg and 200 mg/kg ethanol extract of A. camphorata fruiting body significantly lowered blood lactate by 21% (P=0.0046) and 31% (P<0.0001), respectively, compared to the vehicle treatment.
Proteins and amino acids metabolize to produce ammonia which is related to fatigue [14]. The increase in ammonia in response to exercise can be managed by the use of amino acids or carbohydrates that interfere with ammonia metabolism [9]. The increase in the ammonia level is connected with both peripheral and central fatigue during exercise [2]. Therefore, the blood ammonia level is an important blood biochemical parameter related to fatigue. In our study, it is suggested that when AG supplementation for 4 weeks, the ammonia level after the swimming test might be significantly improved compared to the vehicle control. Huang et al. [2] showed that mice treated with 50 and 200 mg/kg ethanol extract of A. camphorata fruiting body significantly lowered ammonia levels by 35 and 41%, respectively, compared to the vehicle treatment.
The homeostatic regulation of blood glucose plays an important role during prolonged exercise [17]. Hypoglycemia deprives the active functioning of the brain during exercise that often leads to the inability to continue exercise [19]. Thus, blood glucose homeostasis is an important blood biochemical parameter related to fatigue. Our result indicated that AG formulation can regulate blood-glucose levels to reduce fatigue. Huang et al. [2] showed that mice treated with 200 mg/kg ethanol extract of A. camphorata fruiting body significantly increased the glucose content by 1.3-fold compared to the vehicle treatment. In the present study, we also observed beneficial effects of AG supplementation on the exhaustive exercise challenge and measured other physiological effects after 30 days of AG supplementation.
A high-intensity exercise challenge can cause physical and chemical damage to the tissue, and it can also cause sarcomeric damage and muscular cell necrosis [20]. When muscle damage has occurred or is occurring, Muscle cells would release CK into the blood. Thus, CK is known to be an accurate indicator of muscle damage in clinical. In this study, AG treatment can reduce the serum CK level, indicating that AG treatment may ameliorate high-intensity exercise challenge-induced sarcomeric damage or muscular cell necrosis. Huang et al. [2] showed that mice treated with 50 and 200 mg/kg ethanol extract of A. camphorata fruiting body significantly decreased the serum CK level by 41% and 54%, respectively, than the vehicle treatment.
Liver glycogen is also an index of fatigue. The role of hepatic glycogen is to complement the consumption of blood glucose to maintain blood glucose in the physiologic range. Fatigue occurs when liver glycogen is mostly consumed [3]. In addition, reduced muscular glycogen severely limits exercise performance [21]. After we administered AG to mice for 30 days, the levels of muscular glycogen were notably lower with AG than vehicle treatment after the swim test. Therefore, AG should enhance muscular glycogen. Additionally, muscular glycogen levels were increased in response to AG treatments. These results illuminate the release of glucose from muscular glycogen for energy recovery with AG and the statistical significance on trend analysis after exercise (P=0.0133). Huang et al. [2] showed that mice treated with 50 and 200 mg/kg ethanol extract of A. camphorata fruiting body significantly decreased the muscular glycogen level by 1.20- and 1.28-fold, respectively, than the vehicle treatment.
In summary, we provide evidence that when combining A. camphorata and ginseng supplementation for 4 weeks, the muscle strength and endurance performance of mice significantly improved compared to the vehicle treatment. Huang et al. [2] also found ergostane and lanostane skeleton triterpenoids may be important antifatigue active components in A. camphorata. In addition, ginseng polysaccharides from the P. ginseng have anti-fatigue activity, also reflected in the effects on the physiological markers for fatigue [18]. Therefore, the anti-fatigue activity of AG formulation maybe due to the bioactive components of triterpenoids from A. camphorate and ginseng polysaccharides from the P. ginseng. This study provides science-based evidence to support traditional claims of antifatigue results with AG formulation and suggests a use for AG formulation as an ergogenic and antifatigue agent.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Acknowledgments
This research was supported by the Ministry of Science and Technology of Taiwan, the successor to the National Science Council (grant no. NSC97-2410-H134-023 to City C. Hsieh) and Chang Gung Memorial Hospital Grants (CMRPF1G0041, CMRPF1G0171 to Chien-Yu Hsiao). The authors are grateful to Dr. Chien-Chao Chiu for their technical assistance in animal experiments.
REFERENCES
- 1.Banerjee U., Izquierdo J. A.1982. Antistress and antifatigue properties of Panax ginseng: comparison with piracetam. Acta Physiol. Lat. Am. 32: 277–285. [PubMed] [Google Scholar]
- 2.Huang C. C., Hsu M. C., Huang W. C., Yang H. R., Hou C. C.2012. Triterpenoid-rich extract from Antrodia camphorata improves physical fatigue and exercise performance in mice. Evid. Based Complement. Alternat. Med. 2012: 364741. doi: 10.1155/2012/364741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jia J. M., Wu C. F.2008. Antifatigue activity of tissue culture extracts of Saussurea involucrate. Pharm. Biol. 46: 433–436. doi: 10.1080/13880200802055909 [DOI] [Google Scholar]
- 4.Kan N. W., Ho C. S., Chiu Y. S., Huang W. C., Chen P. Y., Tung Y. T., Huang C. C.2016. Effects of resveratrol supplementation and exercise training on the exercise performance in middle-aged mice. Molecules 21: E661. doi: 10.3390/molecules21050661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Konno C., Murakami M., Oshima Y., Hikino H.1985. Isolation and hypoglycemic activity of panaxans Q, R, S, T and U, glycans of Panax ginseng roots. J. Ethnopharmacol. 14: 69–74. doi: 10.1016/0378-8741(85)90030-3 [DOI] [PubMed] [Google Scholar]
- 6.Lewis R., Wake G., Court G., Court J. A., Pickering A. T., Kim Y. C., Perry E. K.1999. Non-ginsenoside nicotinic activity in ginseng species. Phytother. Res. 13: 59–64. doi: [DOI] [PubMed] [Google Scholar]
- 7.Li M., Donglian C., Huaixing L., Bende T., Lihua S., Ying W.2008. Anti-fatigue effects of salidroside in mice. J. Med. Coll. PLA. 23: 88–93. doi: 10.1016/S1000-1948(08)60028-3 [DOI] [Google Scholar]
- 8.Luo D. H., Fang B. S.2008. Structural identification of ginseng polysaccharides and testing of their antioxidant activities. Carbohydr. Polym. 72: 376–381. doi: 10.1016/j.carbpol.2007.09.006 [DOI] [Google Scholar]
- 9.Prado E. S., de Rezende Neto J. M., de Almeida R. D., Dória de Melo M. G., Cameron L. C.2011. Keto analogue and amino acid supplementation affects the ammonaemia response during exercise under ketogenic conditions. Br. J. Nutr. 105: 1729–1733. doi: 10.1017/S000711451000557X [DOI] [PubMed] [Google Scholar]
- 10.Saito H., Yoshida Y., Takagi K.1974. Effect of Panax Ginseng root on exhaustive exercise in mice. Jpn. J. Pharmacol. 24: 119–127. doi: 10.1254/jjp.24.119 [DOI] [PubMed] [Google Scholar]
- 11.Shin H. J., Kim Y. S., Kwak Y. S., Song Y. B., Kim Y. S., Park J. D.2004. Enhancement of antitumor effects of paclitaxel (taxol) in combination with red ginseng acidic polysaccharide (RGAP). Planta Med. 70: 1033–1038. doi: 10.1055/s-2004-832643 [DOI] [PubMed] [Google Scholar]
- 12.Su W. L., Huang W. C., Chen I. N., Chen W. C., Huang C. C., Huang C. H.2015. Investigation of whole-body vibration training on physiological and biochemical characteristics in mice. Phy. Educ. J. 48: 33–44. [Google Scholar]
- 13.Tang W., Zhang Y., Gao J., Ding X., Gao S.2008. The anti-fatigue effect of 20(R)-ginsenoside Rg3 in mice by intranasally administration. Biol. Pharm. Bull. 31: 2024–2027. doi: 10.1248/bpb.31.2024 [DOI] [PubMed] [Google Scholar]
- 14.Tashiro S.1922. Studies on alkali genesis in tissues: I. Ammonia production in the nerve fiber during excitation. Am. J. Physiol. 60: 519–543. [Google Scholar]
- 15.Tharakan B., Dhanasekaran M., Brown-Borg H. M., Manyam B. V.2006. Trichopus zeylanicus combats fatigue without amphetamine-mimetic activity. Phytother. Res. 20: 165–168. doi: 10.1002/ptr.1773 [DOI] [PubMed] [Google Scholar]
- 16.Tsai T. C., Tung Y. T., Kuo Y. H., Liao J. W., Tsai H. C., Chong K. Y., Chen H. L., Chen C. M.2015. Anti-inflammatory effects of Antrodia camphorata, a herbal medicine, in a mouse skin ischemia model. J. Ethnopharmacol. 159: 113–121. doi: 10.1016/j.jep.2014.11.015 [DOI] [PubMed] [Google Scholar]
- 17.Wagenmakers A. J., Beckers E. J., Brouns F., Kuipers H., Soeters P. B., van der Vusse G. J., Saris W. H.1991. Carbohydrate supplementation, glycogen depletion, and amino acid metabolism during exercise. Am. J. Physiol. 260: E883–E890. [DOI] [PubMed] [Google Scholar]
- 18.Wang J., Li S., Fan Y., Chen Y., Liu D., Cheng H., Gao X., Zhou Y.2010. Anti-fatigue activity of the water-soluble polysaccharides isolated from Panax ginseng C. A. Meyer. J. Ethnopharmacol. 130: 421–423. doi: 10.1016/j.jep.2010.05.027 [DOI] [PubMed] [Google Scholar]
- 19.Wang J. J., Shieh M. J., Kuo S. L., Lee C. L., Pan T. M.2006. Effect of red mold rice on antifatigue and exercise-related changes in lipid peroxidation in endurance exercise. Appl. Microbiol. Biotechnol. 70: 247–253. doi: 10.1007/s00253-005-0051-5 [DOI] [PubMed] [Google Scholar]
- 20.Warren G. L., Ingalls C. P., Lowe D. A., Armstrong R. B.2001. Excitation-contraction uncoupling: major role in contraction-induced muscle injury. Exerc. Sport Sci. Rev. 29: 82–87. doi: 10.1097/00003677-200104000-00008 [DOI] [PubMed] [Google Scholar]
- 21.Williams J. H., Batts T. W., Lees S.2013. Reduced muscle glycogen differentially affects exercise performance and muscle fatigue. International Scholarly Research Notices 2013: 371235. [Google Scholar]
- 22.Wu R. E., Huang W. C., Liao C. C., Chang Y. K., Kan N. W., Huang C. C.2013. Resveratrol protects against physical fatigue and improves exercise performance in mice. Molecules 18: 4689–4702. doi: 10.3390/molecules18044689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.You L., Zhao M., Regenstein J. M., Ren J.2011. In vitro antioxidant activity and in vivo anti-fatigue effect of loach (Misgurnus anguillicaudatus) peptides prepared by papain digestion. Food Chem. 124: 188–194. doi: 10.1016/j.foodchem.2010.06.007 [DOI] [PubMed] [Google Scholar]
- 24.Yu B., Lu Z. X., Bie X. M., Lu F. X., Huang X. Q.2008. Scavenging and anti-fatigue activity of fermented defatted soybean peptides. Eur. Food Res. Technol. 226: 415–421. doi: 10.1007/s00217-006-0552-1 [DOI] [Google Scholar]
- 25.Yu F. R., Liu Y., Cui Y. Z., Chan E. Q., Xie M. R., McGuire P. P., Yu F. H.2010. Effects of a flavonoid extract from Cynomorium songaricum on the swimming endurance of rats. Am. J. Chin. Med. 38: 65–73. doi: 10.1142/S0192415X10007774 [DOI] [PubMed] [Google Scholar]
- 26.Zhang Y., Yao X., Bao B., Zhang Y.2006. Anti-fatigue activity of a triterpenoid-rich extract from Chinese bamboo shavings (Caulis bamfusae in taeniam). Phytother. Res. 20: 872–876. doi: 10.1002/ptr.1965 [DOI] [PubMed] [Google Scholar]