Abstract
Obstructive sleep apnea (OSA) is a global disease with a rising incidence along with its comorbidities, especially with metabolic syndrome. One of the main components contributing to sleep apnea is obesity; as well as diabetes mellitus type 2 (T2DM), hypercholesterolemia, and hypertension. OSA is a condition that requires management and the disease can be treated by using CPAP therapy. The awareness of this global issue is rising, and health care systems are providing preventive measures, diagnosis and the treatment options. The major preventable risk factors to decrease obesity are the awareness of lifestyle modification (eating behaviors, smoking, drinking alcohol, etc.) and understanding the importance of exercise. If these lifestyle modifications are widely applied, then not only will the consequences of obesity and sleep apnea be reduced, but also the incidence of cardiovascular disease will decrease greatly. Public awareness of the importance of weight loss by lifestyle modification or bariatric surgery to improve the quality of life is needed. These preventive actions, screening measures, and treatment strategies for obesity and OSA can significantly reduce the incidence of obesity, as well as OSA and the related comorbidities such as cardiovascular disease, atherosclerosis, and depression. Finally, health care costs will also be reduced.
Keywords: Exercise, Obesity, OSA, Public health, Sleep
Introduction
Obesity incidence is increasing globally; the prevalence of obesity in Asian countries has risen to about 30 [1]. There is a linear correlation between obesity and OSA. In obese people, fat deposits in the upper respiratory tract narrow the airway; there is a decrease in muscle activity in this region, leading to hypoxic and apneic episodes, ultimately resulting in sleep apnea. These hypoxia/apnea episodes lead to a decrease in oxygen that is available in body tissues and blood vessels. The decreased oxygenation causes tissue hypoxia, which is the main contributing factor to atherosclerosis, the main risk factor for Cardiovascular Diseases (CVD) [2].
A four-year longitudinal study [3] of overweight and obese American adults demonstrates that change in weight is directly proportionate to sleep disordered breathing (SDB). Those with the greatest weight gain had a more severe apnea-hypopnea index (AHI). The risk of OSA increases with age and body mass index (BMI); other associated factors seen in a cohort of Australian men includes sedentary lifestyle, tobacco abuse, and heavy alcohol use [4]. OSA is strongly correlated with multiple disease conditions, including type 2 diabetes mellitus (T2DM), hyperlipidemia, hypertension, heart failure, cardiovascular diseases (CVD) and depression [4,5].
Obesity and Sleep
Obesity is defined as a BMI≥ 30, whereas a BMI ≥ 25.0 indicates the person is overweight [6]. Compared to men, women have lower rates of being overweight or obese. The major contributing factors to obesity include environment, eating behavior, and physical inactivity. Psychosocial circumstances and genetics also play important roles in obesity [7].
People who are obese (with a BMI of more than 30) with shorter sleep duration have twice as many subjective sleep problems compared to non-obese people [8]. Being obese or overweight is associated with decreased amount of sleep compared to non-obese patients [9]. Obesity is associated with poor sleep quantity and quality; thus weight reduction can ameliorate sleep problems [10,11]. As a result preventing weight gain had a positive effect on sleep quality and duration in adult Black women [12] (Figure 1).
Figure 1.
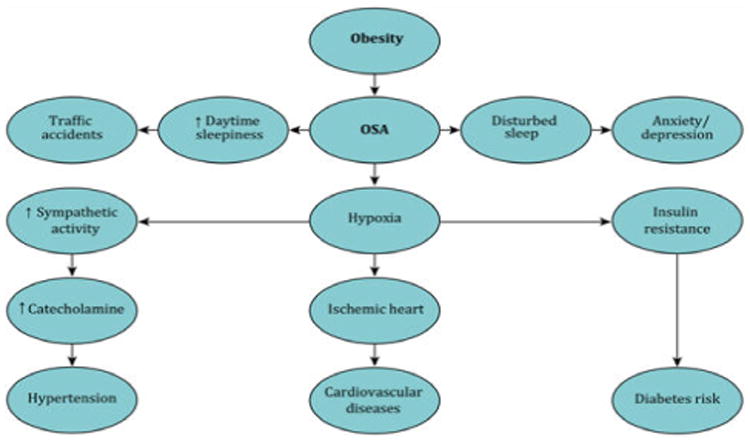
A schematic diagram that shows the obesity and associated comorbidities.
Weight gain is a slow process sustained by lifestyle factors such as lack of sleep, sedentary lifestyle, excessive caloric intake, and genetics. Short sleepers and obese people can easily suffer major depression. In male Chinese subjects, obesity has also been associated with short sleep duration (less than 6 hours) and prolonged working hours (greater than 9 hours) [13]. Short sleep duration and dietary intake can cause hormonal imbalances. One such imbalance is a decrease in melatonin, leading to alterations in the metabolic circadian rhythm predisposing to weight gain and metabolic syndrome [14]. There are also alterations in the actions of the hormones leptin and insulin. Obese individuals develop resistance to both of these hormones. These hormones decrease food need and increase energy metabolism. Ghrelin, which is released by the stomach stimulates appetite and is also affected by sleep disturbances. An increase in the level of ghrelin and a decrease in the level of leptin is also noted in chronic short sleepers [15,16]. These altered levels in short sleepers predispose to obesity because of the associated increased food intake [14,17-19]. Factors which predispose to obesity, such as poor sleep and excessive caloric intake are also the major predisposing factors for diabetes and the other components of the metabolic syndrome [20-24]. OSA has further-reaching consequences than simply being tired, in OSA patients, excessive daytime sleepiness, anxiety, and poor concentration due to lack of sleep can lead to traffic accidents [25] (Figure 2).
Figure 2.
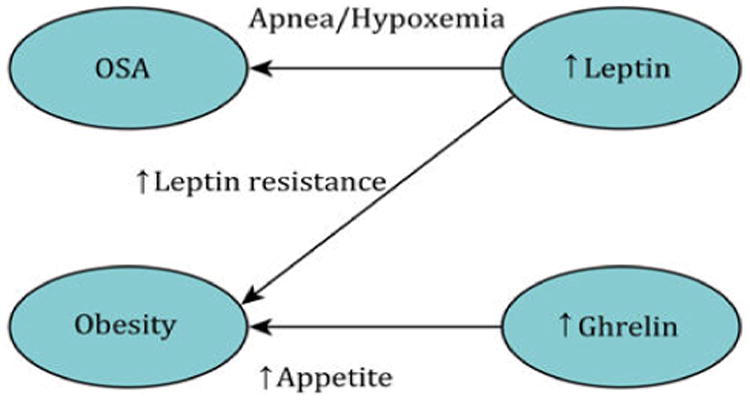
A schematic diagram that shows the correlations between OSA, Obesity, Leptin, and Ghrelin.
Obstructive sleep apnea syndrome
In obstructive sleep apnea syndrome (OSAS), patients experience recurrent apnea and hypopnea episodes due to the complete or partial collapse of the upper airway. In obese people, the narrowing of upper respiratory muscles occurs because of the accumulation of fatty tissues. The obstruction in breathing due to the narrowing of the upper airway causes a marked increase in intrathoracic pressure and triggers apnea and hypoxia [26]. There is an increased sympathetic activation due to apnea/hypoxia episodes in OSAS patients [27]. Episodes of hypoxemia/apnea can drop the oxyhemoglobin saturation from 95% to 80%, depending on the length of the period of apnea. OSA is an independent risk factor for cardiovascular and cerebrovascular diseases. Due to the hypoxia-related to OSAS, the oxidative stress leads to overproduction of reactive oxygen species, which can cause endothelial dysfunction and result in atherosclerosis. The inflammatory marker C-reactive protein (CRP), tumor necrosis factor α (TNF α), and interleukin-6 (IL-6) were increased in patients with OSA and significantly elevated when the AHI was 15 or greater [28].
Leptin is a hormone involved in feeding and metabolizing energy. Increased levels of leptin are found in patients with OSAS. The level of leptin hormone is correlated with the severity of OSAS [29]. Another study shows that increased levels of the leptin hormone are present in both obese and obstructive sleep apnea patients, with the leptin level being directly proportional to the severity of the syndrome [30]. In OSA patients, serum leptin levels are 50% higher compared to controls.
Epidemiology, prevalence and gender differences in OSA
The prevalence of OSA in the general population is 3 to 7% for men and 2 to 5% for women [31]. The rates are especially high for those who are obese. The converse is also true: those with OSA are at risk for obesity. Inadequate sleep during the night and daytime sleepiness predispose patients to weight gain [32]. The prevalence of sleep apnea in obese people who go for bariatric surgery is 77%. Polysomnographic (PSG) testing is recommended for all obese people who are candidates for bariatric surgery [33].
The rates of OSA vary amongst women based on their menopausal status. In premenopausal women, the prevalence of OSA is 0.6%, which is very low. Postmenopausal women who are on hormone replacement therapy (HRT) have a prevalence of 0.5%; however, postmenopausal women who do not use HRT have a higher prevalence: 2.7%. Post-menopausal women not on HRT have rates of OSA almost equal to that of men [34]. The prevalence of OSA is higher in men than women, with the exception of postmenopausal women [35]. Despite the increased prevalence of sleep apnea in men, women present with increased comorbidities, including a morning headache, insomnia, mood problems, and anxiety [36].
Differences in ethnicity
Most studies done in regards to ethnicity differences and OSA have been done between Blacks and Whites. In a cross-sectional study of OSA prevalence in South Asian patients with T2DM compared to that of white Europeans, whites were nearly twice as likely to have OSA (36.2% vs. 51.4%). In addition, the severity of the condition was lower for South Asians than for Europeans [37]. In the Sleep Heart Health Study, a multi-ethnic cohort was studied to explore the symptoms of SDB in relation to race and ethnicity. Frequent snoring was more common in Hispanic men and woman as well as Black women compared to others [38]. In African-Americans, the risk of OSA is seen at a younger age than in whites [39]. In a study comparing Far East Asian men with white men, OSA was more common and more severe in Far East Asian men, although they had a lower BMI [40]. Authors noted that craniofacial differences may play a role in these increased rates of OSA in Far East Asian men. In the Middle East, obesity plays a bigger role in OSA. A review article exploring this topic found that studies were done in countries such as Dubai, United Arab Emirates found up to 22% of participants are at risk for OSA (Table 1) [41,42].
Table 1.
A highlights on the worldwide studies concerning OSA, obesity, and related comorbidities.
| Study Title | Study Design | Total Number (N) | Major Associated Comorbidities | Obesity | Sleep Disturbances (OSA) | P-Values | Reference |
|---|---|---|---|---|---|---|---|
| Quality of sleep and risk for obstructive sleep apnea in ambulant individuals with T2DM at a tertiary referral hospital in Kenya: A cross-sectional, comparative study. | Cross-sectional, comparative study | 223 males + females | T2 dm, hypertension, depression, COPDS | Positive | Positive | 0.001 | [5] |
| Sleep apnea in Australian men: Disease burden, co-morbidities, and correlates from the Australian longitudinal study on male health. | Longitudinal study | 13,423 adult males | T2 dm, hypertension, depression | Positive | Positive | <0.001 | [4] |
| OSA and multiple anthropometric indices of general obesity and abdominal obesity among young adults | Cross-sectional study | 2911 males + females | Anxiety/depression | Positive | Positive | <0.001 | [2] |
| Longitudinal study of moderate weight change and SDB. | Prospective cohort study | 690 males + females | Anxiety/depression | Positive | Positive | 0.01 | [3] |
| Short sleep duration combined with OSA is associated with visceral obesity in Korean adults | Cross-sectional study. | 838 males + females | Anxiety/depression | Positive | Positive | <0.001 | [42] |
| Association of short sleep duration with weight gain and obesity at 1-year follow-up: A large-scale prospective study. (Japan) | Prospective | 35,247 males + females | Anxiety/depression | Positive | Positive | <0.01 | [1] |
Treatment implications
Weight loss, physical activity, and diet control
Obesity and low levels of physical activity are associated with moderate to severe OSA. Exercise helps in decreasing weight, blood pressure, depression, anxiety, and fatigue [28]. Eating disorders such as bulimia can also be the causative factor of obesity; these mental and psychological issues should be addressed to overcome obesity and its comorbidities in early childhood or at the earliest age of diagnosis [43]. To prevent adult obesity, early childhood prevention by families is key in controlling this global issue. Unfortunately, high-quality studies in this area are greatly lacking [44].
CPAP
The conventional treatment for OSA is continuous positive airway pressure (CPAP). This therapy uses a machine to deliver a constant airflow to a patient's airway via a nasal, facial, or oral device that maintains airway patency during sleep. CPAP treatment significantly relieves OSAS symptoms and improves functional status in both males and females. Women's physiological changes during pregnancy and menopause lead to different sleep patterns and clinical presentations of OSAS when compared to men [28].
Avoidance of lying in a supine position or, using topical nasal corticosteroids are other therapeutic approaches. The psychoactive drug modafinil has also prescribed to patients who do not respond to CPAP effectively [45].
Weight loss by surgical procedures
In the primary care setting, physicians performing BMI screenings markedly increased from 2008 to 2013 from 54% to 73%. However, the management and implications of obesity at primary healthcare facilities still need to be improved, and behavioral-based treatment should be given priority [46].
Weight loss is the key factor in the treatment of OSA. It can be achieved with exercise, diet changes, and/or medications. One study showed that short sleepers (less than 7 hours) had improvement in metabolic indices and greater weight loss when they increased their sleep more than 7 hours [47]. Short sleepers (6 hours or less) who go to sleep late tend to eat later at night, increasing their daily intake of calories and becoming more prone to gain weight [48].
Surgical procedures are more beneficial than medical procedures for losing weight and addressing the comorbidities associated with obesity. If dieting and increased sleep measures fail, then bariatric surgery could be an alternative treatment option to lose weight. After losing weight through bariatric surgery, OSA, and metabolic derangements greatly improved [49,50]. While the treatment combination CPAP and surgery to lose weight has beneficial effects in the treatment of OSA, patients need close monitoring to prevent surgical complications [51].
There was a great improvement noticed in OSA on polysomnographic studies before and after the surgical procedure; improvements were also noticed in metabolic syndrome. Bariatric surgery is becoming the major treatment option for patients in which weight loss through diet, exercise, and CPAP therapy has failed [52-54]. In obese renal transplant patients, LSG (laparoscopic sleeve gastrectomy) surgical procedures greatly reduce the post-transplant complications [55]. Another study shows bariatric surgery reduces microvascular complications, especially in those with prediabetic state [56].
The concentration of the enzyme heme oxygenase is increased in patients with severe OSA and morbid obesity; bariatric surgery decreases this enzyme concentration. As result, inflammatory process and insulin resistance decrease. Obesity-related OSA is best treated by a combination of surgical procedures and CPAP therapy [51,57]. OSAS and obesity are correlated problems; while treating OSAS, obesity should also be addressed in obese patients, even if that means using surgical procedures [58].
Complications of surgical procedures
There are very few microvascular complications noted with bariatric surgery. In patients with pre-diabetes, it is actually more beneficial in preventing microvascular complications such as diabetic nephropathy, neuropathy, and retinopathy [56]. Obese patients who have previously undergone bariatric surgery who subsequently cardiac surgery, have a higher risk of coronary artery complications compared to patients with no previous bariatric surgery [59].
People who have bariatric surgery are also at increased risk for vitamin deficiencies, depending on the type of procedure. Vitamin B deficiencies are often reported after bariatric surgical procedures, and the patients can develop neurological complications, such as muscle weakness, polyneuropathy, and abnormal gait. Wernicke-Korsakoff neuropathy is one side effect that is not reversible; other complications can be treated by taking vitamins [60]. After bariatric surgery, bleeding, ulceration, fistula formation, and stenosis of the anastomosis can occur; all these complications can be repaired endoscopically or in an open procedure [61].
Public health implications
To prevent obesity and its serious complications, patients as well as policymakers, health care workers, marketing and food industries all play a role [62]. To prevent obesity and its complications, early childhood development interventions mediated by parents and family education is also very important. Preparing healthy meals, increasing physical activities, and encouraging lifestyle modifications at an early age. Early childhood education about what constitutes a healthy lifestyle and its adoption are very important. Parents can make children more active by involving them in chores or encouraging play beyond video games. Also adopting healthier cooking methods such as baking or grilling instead of frying food should be emphasized. Avoidance of foods with high fructose corn syrup such as juice or soda is also important. Ironically in Australia, obesity persists despite a national dietary campaign which has led to a reduction in sugary beverage consumption [63]. Local community campaigns can gain attention and motivate people to avoid sugary drinks and other sugar-filled beverages and food. This avoidance and awareness might be detrimental to soda industries and other sugar manufacturing products globally, but this will allow us to appropriately address this rising global problem and its comorbidities worldwide [64]. Engaging the entire family in making healthy choices leads to a healthy society in the future [65].
To improve public health, healthcare workers must address the important lifestyle modifications of diet and exercise, as well as the proper duration and quality of sleep [66]. Public messages should be given that emphasize the harmful effects of excessive food intake and lack of physical exertion [67]. Primary care physicians can play a vital role in preventing obesity and related comorbidities by educating patients, recommending different strategies, and involving a multi-disciplinary team of healthcare workers to provide effective obesity therapy and prevention to their patients. A dietician can be an integral part of this team as dieticians can aid patients in developing adoption of a balanced diet by providing healthy food education [68]. Dieticians can aid patients in developing adoption of a balanced diet by providing healthy food education. Through a proper diet, greater weight loss can be achieved [69].
To achieve a better quality of life, specific and concrete measures should be taken to address the underlying factors related to obesity. Surgical procedures are vital treatment options, which can give a better choice for overcoming obesity and its associated comorbidities. These bariatric surgeries are safe and effective. Guidance and awareness of the costs and side effects of these procedures can impact better public health by primary care physicians and healthcare workers [70].
Discussion
The prevalence of OSA is increasing globally due to the growing occurrence of obesity in society. In obese people, the fat deposits in the upper respiratory tract make breathing difficulties during sleep thus causing OSA, [71]. OSA is strongly associated with obesity [72]. Obese people must be screened for OSA and disorders that are associated with it. Both obesity and OSA patients have a greater risk of metabolic syndrome [73]. Diet control in obese people can improve sleep problems and associated depression, anxiety, and insomnia. In young adults, disturbed sleep management should be addressed along with stress and depression management. Keeping in mind that the attention should also be placed on emotional issues. Involving a person in healthy group discussions and therapies, encouraging them to actively participate in these groups, can strengthen psychological, mental and social health. Health care workers, especially primary care physicians, can play a vital role in solving this growing public health problem [74,75].
OSA is a rising prevalent problem, associated mainly with obesity. The gold standard of treatment is CPAP therapy and ensuring that patients adhere to the therapy. If the condition does not improve, then surgical procedures are recommended to overcome this problem [76]. Surgical weight loss procedures for obese people are highly recommended, even by non-surgical teams and societies [77]. Bariatric surgical procedures are safe, even in the elderly population, so this should not be a deterrent to undergo the operations [78-80].
Conclusion
This review highlights the impact of obesity on OSA is evident. Indeed, in several populations across different cultures, the positive relationship between obesity and OSA was pronounced. This represents an important public health crisis that demands multi-layered interventions. To cope with this increasing and seriously preventable health issue, we emphasize the need to minimize the consumption of junk and fast food, increase the consumption of fresh fruits and vegetables. Other important habits are drinking water instead of juice, soda or alcohol; as well as smoking cessation. In addition to making dietary changes engaging in physical exercise is necessary. If medical and/or lifestyle medication therapies fail, then surgical interventions are another option to treat obesity and its associated comorbidities. Surgical procedures which can drastically reduce obesity and its related comorbidities ultimately improving mortality rates. To improve public health, obesity and its associated comorbidities should be seriously considered. Solving this issue is not only the responsibility of an individual and a healthcare worker, but it should be properly addressed by government officials to improve the health of individuals and make an overall healthier society.
Acknowledgments
Funding: This work is supported by the following funding agencies: R25-HL105444 and R25-HL116378 (NHLBI); R01-MD007716 (NIMHD) to GJL. However, the funders had no role in study design, data collection or analysis.
Abbrevations
- OSA
Obstructive Sleep Apnea
- T2DM
Type 2 Diabetes Mellitus
- MetS
Metabolic Syndrome
- CVD
Cardiovascular Diseases
- AHI
Apnea-Hypopnea Index
- SDB
Sleep Disordered Breathing
- BMI
Body Mass Index
- OSAS
Obstructive Sleep Apnea Syndrome
- CRP
C-Reactive Protein
- TNF-α
Tumor Necrosis Factor α
- HRT
Hormone Replacement Therapy
- PSG
Polysomnographic
- CPAP
Continuous Positive Airway Pressure
Footnotes
Conflicts of Interest: The authors declared that there are no conflicts of interest.
References
- 1.Watanabe M, Kikuchi H, Tanaka K, Takahashi M. Association of short sleep duration with weight gain and obesity at 1-year follow-up: a large-scale prospective study. Sleep. 2010;33(2):161–167. doi: 10.1093/sleep/33.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen X, Pensuksan WC, Lohsoonthorn V, Lertmaharit S, Gelaye B, et al. Obstructive sleep apnea and multiple anthropometric indices of general obesity and abdominal obesity among young adults. Int J Soc Sci Stud. 2014;2(3):89–99. doi: 10.11114/ijsss.v2i3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peppard PE, Young T, Palta M, Dempsey J, Skatrud J. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA. 2000;284(23):3015–3021. doi: 10.1001/jama.284.23.3015. [DOI] [PubMed] [Google Scholar]
- 4.Senaratna CV, English DR, Currier D, Perret JL, Lowe A, et al. Sleep apnoea in Australian men: disease burden, co-morbidities, and correlates from the Australian longitudinal study on male health. BMC Public Health. 2016;16(Suppl 3):1029. doi: 10.1186/s12889-016-3703-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sokwalla SM, Joshi MD, Amayo EO, Acharya K, Mecha JO, et al. Quality of sleep and risk for obstructive sleep apnoea in ambulant individuals with type 2 diabetes mellitus at a tertiary referral hospital in Kenya: a cross-sectional, comparative study. BMC Endocr Disord. 2017;17(1):7. doi: 10.1186/s12902-017-0158-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuczmarski RJ, Flegal KM. Criteria for definition of overweight in transition: background and recommendations for the United States. Am J Clin Nutr. 2000;72(5):1074–1081. doi: 10.1093/ajcn/72.5.1074. [DOI] [PubMed] [Google Scholar]
- 7.Guilcher SJT, Kaufman-Shriqui V, Hwang J, O'Campo P, Matheson FI, et al. The association between social cohesion in the neighborhood and body mass index (BMI): An examination of gendered differences among urban-dwelling Canadians. Prev Med. 2017;99:293–298. doi: 10.1016/j.ypmed.2017.02.022. [DOI] [PubMed] [Google Scholar]
- 8.Vgontzas AN, Lin HM, Papaliaga M, Calhoun S, Vela-Bueno A, et al. Short sleep duration and obesity: the role of emotional stress and sleep disturbances. Int J Obes (Lond) 2008;32(5):801–809. doi: 10.1038/ijo.2008.4. [DOI] [PubMed] [Google Scholar]
- 9.Vorona RD, Winn MP, Babineau TW, Eng BP, Feldman HR, et al. Overweight and obese patients in a primary care population report less sleep than patients with a normal body mass index. Arch Intern Med. 2005;165(1):25–30. doi: 10.1001/archinte.165.1.25. [DOI] [PubMed] [Google Scholar]
- 10.Gangwisch JE, Malaspina D, Boden-Albala B, Heymsfield SB. Inadequate sleep as a risk factor for obesity: analyses of the NHANES I. Sleep. 2005;28(10):1289. doi: 10.1093/sleep/28.10.1289. [DOI] [PubMed] [Google Scholar]
- 11.Kohatsu ND, Tsai R, Young T, Vangilder R, Burmeister LF. Sleep duration and body mass index in a rural population. Arch Intern Med. 2006;166(16):1701–1705. doi: 10.1001/archinte.166.16.1701. [DOI] [PubMed] [Google Scholar]
- 12.Steinberg DM, Christy J, Batch BC, Askew S, Moore RH. Preventing Weight Gain Improves Sleep Quality Among Black Women: Results from a RCT. Ann Behav Med. 2017;51(4):555–566. doi: 10.1007/s12160-017-9879-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ko GT, Chan JC, Chan AW, Wong PT, Hui SS, et al. Association between sleeping hours, working hours and obesity in Hong Kong Chinese: the ‘better health for better Hong Kong’ health promotion campaign. Int J Obes (Lond) 2007;31(2):254–260. doi: 10.1038/sj.ijo.0803389. [DOI] [PubMed] [Google Scholar]
- 14.Baron KG, Reid KJ, Kim T, Van Horn L, Attarian H, et al. Circadian timing and alignment in healthy adults: associations with BMI, body fat, caloric intake and physical activity. Int J Obes (Lond) 2016;41(2):203–209. doi: 10.1038/ijo.2016.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chaput JP, Després JP, Bouchard C, Tremblay A. Short sleep duration is associated with reduced leptin levels and increased adiposity: results from the Quebec family study. Obesity (Silver Spring) 2007;15(1):253–261. doi: 10.1038/oby.2007.512. [DOI] [PubMed] [Google Scholar]
- 16.Cummings DE, Foster KE. Ghrelin-leptin tango in body-weight regulation. Gastroenterology. 2003;124(5):1532–1535. doi: 10.1016/s0016-5085(03)00350-0. [DOI] [PubMed] [Google Scholar]
- 17.Schwartz MW, Morton GJ. Obesity: keeping hunger at bay. Nature. 2002;418(6898):595–597. doi: 10.1038/418595a. [DOI] [PubMed] [Google Scholar]
- 18.Spiegel K, Leproult R, L'hermite-Balériaux M, Copinschi G, Penev PD, et al. Leptin levels are dependent on sleep duration: relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. J Clin Endocrinol Metab. 2004;89(11):5762–5771. doi: 10.1210/jc.2004-1003. [DOI] [PubMed] [Google Scholar]
- 19.Taheri S, Lin L, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004;1(3):e62. doi: 10.1371/journal.pmed.0010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ayas NT, White DP, Al-Delaimy WK, Manson JE, Stampfer MJ, et al. A prospective study of self-reported sleep duration and incident diabetes in women. Diabetes Care. 2003;26(2):380–384. doi: 10.2337/diacare.26.2.380. [DOI] [PubMed] [Google Scholar]
- 21.Horne J. Short sleep is a questionable risk factor for obesity and related disorders: statistical versus clinical significance. Biol Psychol. 2008;77(3):266–276. doi: 10.1016/j.biopsycho.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 22.Knutson KL, Spiegel K, Penev P, Van Cauter E. The metabolic consequences of sleep deprivation. Sleep Med Rev. 2007;11(3):163–178. doi: 10.1016/j.smrv.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Cauter E, Spiegel K, Tasali E, Leproult R. Metabolic consequences of sleep and sleep loss. Sleep Med. 2008;9(Suppl 1):S23–S28. doi: 10.1016/S1389-9457(08)70013-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yaggi HK, Araujo AB, McKinlay JB. Sleep duration as a risk factor for the development of type 2 diabetes. Diabetes Care. 2006;29(3):657–661. doi: 10.2337/diacare.29.03.06.dc05-0879. [DOI] [PubMed] [Google Scholar]
- 25.Harrison Y, Horne JA. Should we be taking more sleep? Sleep. 1995;18(10):901–907. [PubMed] [Google Scholar]
- 26.Destors M, Tamisier R, Galerneau LM, Lévy P, Pepin JL. Pathophysiology of obstructive sleep apnea syndrome and its cardiometabolic consequences. Presse Med. 2017;46(4):395–403. doi: 10.1016/j.lpm.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 27.Phillips BG, Kato M, Narkiewicz K, Choe I, Somers VK. Increases in leptin levels, sympathetic drive, and weight gain in obstructive sleep apnea. Am J Physiol Heart Circ Physiol. 2000;279(1):H234–H237. doi: 10.1152/ajpheart.2000.279.1.H234. [DOI] [PubMed] [Google Scholar]
- 28.Jehan S, Auguste E, Zizi F, Pandi-Perumal SR, Gupta R, et al. Obstructive Sleep Apnea: Women's Perspective. J Sleep Med Disord. 2016;3(6) [PMC free article] [PubMed] [Google Scholar]
- 29.Ulukavak Ciftci T, Kokturk O, Bukan N, Bilgihan A. Leptin and ghrelin levels in patients with obstructive sleep apnea syndrome. Respiration. 2005;72(4):395–401. doi: 10.1159/000086254. [DOI] [PubMed] [Google Scholar]
- 30.Ozturk L, Unal M, Tamer L, Celikoglu F. The association of the severity of obstructive sleep apnea with plasma leptin levels. Arch Otolaryngol Head Neck Surg. 2003;129(5):538–540. doi: 10.1001/archotol.129.5.538. [DOI] [PubMed] [Google Scholar]
- 31.Lurie A. Obstructive sleep apnea in adults: epidemiology, clinical presentation, and treatment options. Adv Cardiol. 2011;46:1–42. doi: 10.1159/000327660. [DOI] [PubMed] [Google Scholar]
- 32.Gami AS, Caples SM, Somers VK. Obesity and obstructive sleep apnea. Endocrinol Metab Clin North Am. 2003;32(4):869–894. doi: 10.1016/s0889-8529(03)00069-0. [DOI] [PubMed] [Google Scholar]
- 33.Sareli AE, Cantor CR, Williams NN, Korus G, Raper SE, et al. Obstructive sleep apnea in patients undergoing bariatric surgery-a tertiary center experience. Obes Surg. 2011;21(3):316–327. doi: 10.1007/s11695-009-9928-1. [DOI] [PubMed] [Google Scholar]
- 34.Bixler EO, Vgontzas AN, Lin HM, Ten Have T, Rein J, et al. Prevalence of sleep-disordered breathing in women: effects of gender. Am J Respir Crit Care Med. 2001;163(3 Pt 1):608–613. doi: 10.1164/ajrccm.163.3.9911064. [DOI] [PubMed] [Google Scholar]
- 35.Young T, Palta M, Dempsey J, Skatrud J, Weber S, et al. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328(17):1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 36.Basoglu OK, Tasbakan MS. Gender differences in clinical and polysomnographic features of obstructive sleep apnea: a clinical study of 2827 patients. Sleep Breath. 2017:1–9. doi: 10.1007/s11325-017-1482-9. [DOI] [PubMed] [Google Scholar]
- 37.Amin A, Ali A, Altaf QA, Piya MK, Barnett AH, et al. Prevalence and Associations of Obstructive Sleep Apnea in South Asians and White Europeans with Type 2 Diabetes: A Cross-Sectional Study. J Clin Sleep Med. 2017;13(4):583–589. doi: 10.5664/jcsm.6548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O'Connor GT1, Lind BK, Lee ET, Nieto FJ, Redline S, et al. Variation in symptoms of sleep-disordered breathing with race and ethnicity: the Sleep Heart Health Study. Sleep. 2003;26(1):74–79. [PubMed] [Google Scholar]
- 39.Redline S, Tishler PV, Hans MG, Tosteson TD, Strohl KP, et al. Racial differences in sleep-disordered breathing in African-Americans and Caucasians. Am J Respir Crit Care Med. 1997;155(1):186–192. doi: 10.1164/ajrccm.155.1.9001310. [DOI] [PubMed] [Google Scholar]
- 40.Li KK, Kushida C, Powell NB, Riley RW, Guilleminault C. Obstructive Sleep Apnea Syndrome: A Comparison Between Far-East Asian and White Men. Laryngoscope. 2000;110(10):1689–1693. doi: 10.1097/00005537-200010000-00022. [DOI] [PubMed] [Google Scholar]
- 41.Vats MG, Mahboub BH, Al Hariri H, Al Zaabi, Vats D. Obesity and Sleep-Related Breathing Disorders in Middle East and UAE. Canadian Respiratory Journal 2016. 2016:ID 9673054. doi: 10.1155/2016/9673054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim NH, Lee SK, Eun CR, Seo JA, Kim SG, et al. Short sleep duration combined with obstructive sleep apnea is associated with visceral obesity in Korean adults. Sleep. 2013;36(5):723–729. doi: 10.5665/sleep.2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Le LK, Barendregt JJ, Hay P, Mihalopoulos C. Prevention of eating disorders: A systematic review and meta-analysis. Clin Psychol Rev. 2017;53:46–58. doi: 10.1016/j.cpr.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 44.Kornet-van der Aa DA, Altenburg TM, van Randeraad-van der Zee CH, Chinapaw MJ. The effectiveness and promising strategies of obesity prevention and treatment programmes among adolescents from disadvantaged backgrounds: a systematic review. Obes Rev. 2017;18(5):581–593. doi: 10.1111/obr.12519. [DOI] [PubMed] [Google Scholar]
- 45.Morgenthaler TI, Kapen S, Lee-Chiong T, Alessi C, Boehlecke B, et al. Practice parameters for the medical therapy of obstructive sleep apnea. Sleep. 2006;29(8):1031–1035. [PubMed] [Google Scholar]
- 46.Fitzpatrick SL, Stevens VJ. Adult obesity management in primary care, 2008-2013. Prev Med. 2017;99:128–133. doi: 10.1016/j.ypmed.2017.02.020. [DOI] [PubMed] [Google Scholar]
- 47.Verhoef SP, Camps SG, Gonnissen HK, Westerterp KR, Westerterp-Plantenga MS. Concomitant changes in sleep duration and body weight and body composition during weight loss and 3-mo weight maintenance. Am J Clin Nutr. 2013;98(1):25–31. doi: 10.3945/ajcn.112.054650. [DOI] [PubMed] [Google Scholar]
- 48.Spaeth AM, Dinges DF, Goel N. Effects of experimental sleep restriction on weight gain, caloric intake, and meal timing in healthy adults. Sleep. 2013;36(7):981–990. doi: 10.5665/sleep.2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fritscher LG, Canani S, Mottin CC, Fritscher CC, Berleze D, et al. Bariatric surgery in the treatment of obstructive sleep apnea in morbidly obese patients. Respiration. 2007;74(6):647–652. doi: 10.1159/000107736. [DOI] [PubMed] [Google Scholar]
- 50.Romero-Corral A, Caples SM, Lopez-Jimenez F, Somers VK. Interactions between obesity and obstructive sleep apnea: implications for treatment. Chest. 2010;137(3):711–719. doi: 10.1378/chest.09-0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Joosten SA, Hamilton GS, Naughton MT. Impact of Weight Loss Management in OSA. Chest. 2017;152(1):194–203. doi: 10.1016/j.chest.2017.01.027. [DOI] [PubMed] [Google Scholar]
- 52.Axer S, Szabo E, Näslund I. Weight loss and alterations in co-morbidities after revisional gastric bypass: A case-matched study from the Scandinavian Obesity Surgery Registry. Surg Obes Relat Dis. 2017;13(5):796–800. doi: 10.1016/j.soard.2017.01.030. [DOI] [PubMed] [Google Scholar]
- 53.Colquitt JL, Pickett K, Loveman E, Frampton GK. Surgery for weight loss in adults. Cochrane Database Syst Rev. 2014;8:CD003641. doi: 10.1002/14651858.CD003641.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kothari SN, Borgert AJ, Kallies KJ, Baker MT, Grover BT. Long-term (>10-year) outcomes after laparoscopic Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2016;13(6):972–978. doi: 10.1016/j.soard.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 55.Kienzl-Wagner K, Weissenbacher A, Gehwolf P, Wykypiel H, Öfner D, et al. Laparoscopic sleeve gastrectomy: gateway to kidney transplantation. Surg Obes Relat Dis. 2017;13(6):909–915. doi: 10.1016/j.soard.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 56.Carlsson LMS, Sjöholm K, Karlsson C, Jacobson P, Andersson-Assarsson JC, et al. Long-term incidence of microvascular disease after bariatric surgery or usual care in patients with obesity, stratified by baseline glycaemic status: a post-hoc analysis of participants from the Swedish Obese Subjects study. Lancet Diabetes Endocrinol. 2017;5(4):271–279. doi: 10.1016/S2213-8587(17)30061-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tirado R, Masdeu MJ, Vigil L, Rigla M, Luna A, et al. Impact of Bariatric Surgery on Heme Oxygenase-1, Inflammation, and Insulin Resistance in Morbid Obesity with Obstructive Sleep Apnea. Obes Surg. 2017;27(9):2338–2346. doi: 10.1007/s11695-017-2635-4. [DOI] [PubMed] [Google Scholar]
- 58.Hudgel DW. Critical Review: CPAP and Weight Management of Obstructive Sleep Apnea Cardiovascular Co-morbidities. Sleep Med Rev. 2016 doi: 10.1016/j.smrv.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 59.Baimas-George M, Hennings DL, Al-Qurayshi Z, Emad Kandil, DuCoin C. No more broken hearts: weight loss after bariatric surgery returns patients' postoperative risk to baseline following coronary surgery. Surg Obes Relat Dis. 2016;13(6):1010–1015. doi: 10.1016/j.soard.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 60.Suriya Punchai, Zubaidah Nor Hanipah, Gautam Sharma, Emre bucak, Dvir Froylich, et al. Neurologic Manifestations of Vitamin B Deficiency after Bariatric Surgery. Surgery for Obesity and Related Diseases. 2016;12(7):S107. [Google Scholar]
- 61.Valli PV, Gubler C. Review article including treatment algorithm: endoscopic treatment of luminal complications after bariatric surgery. Clin Obes. 2017;7(2):115–122. doi: 10.1111/cob.12182. [DOI] [PubMed] [Google Scholar]
- 62.Visscher TL, Lakerveld J, Olsen N, Küpers L, Ramalho S, et al. Perceived Health Status: Is Obesity Perceived as a Risk Factor and Disease? Obes Facts. 2017;10(1):52–60. doi: 10.1159/000457958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brand-Miller JC, Barclay AW. Declining consumption of added sugars and sugar-sweetened beverages in Australia: a challenge for obesity prevention. Am J Clin Nutr. 2017;105(4):854–863. doi: 10.3945/ajcn.116.145318. [DOI] [PubMed] [Google Scholar]
- 64.Schwartz MB, Schneider GE, Choi YY, Li X, Harris J, et al. Association of a community campaign for better beverage choices with beverage purchases from supermarkets. JAMA Intern Med. 2017;177(5):666–674. doi: 10.1001/jamainternmed.2016.9650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lora KR, Cheney M, Branscum P. Hispanic Mothers' Views of the Fathers' Role in Promoting Healthy Behaviors at Home: Focus Group Findings. J Acad Nutr Diet. 2017;117(6):914–922. doi: 10.1016/j.jand.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Horne J. Obesity and short sleep: unlikely bedfellows? Obes Rev. 2011;12(5):84–94. doi: 10.1111/j.1467-789X.2010.00847.x. [DOI] [PubMed] [Google Scholar]
- 67.Zhang Q, Chen X, Liu Z, Varma DS, Wan R, et al. Diet diversity and nutritional status among adults in southwest China. PLoS One. 2017;12(2):e0172406. doi: 10.1371/journal.pone.0172406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bąk-Sosnowska M, Skrzypulec-Plinta V. Health behaviors, health definitions, sense of coherence, and general practitioners' attitudes towards obesity and diagnosing obesity in patients. Arch Med Sci. 2017;13(2):433–440. doi: 10.5114/aoms.2016.58145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sun Y, You W, Almeida F, Estabrooks P, Davy B. The effectiveness and cost of lifestyle interventions including nutrition education for diabetes prevention: a systematic review and meta-analysis. J Acad Nutr Diet. 2017;117(3):404–421. doi: 10.1016/j.jand.2016.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brousseau H, Pohl D. Quality Improvement Processes in Obesity Surgery Lead to Higher Quality and Value, Lower Costs. R I Med J (2013) 2017;100(2):28–30. [PubMed] [Google Scholar]
- 71.Lim DC, Pack AI. Obstructive Sleep Apnea: Update and Future. Annu Rev Med. 2016;68:99–112. doi: 10.1146/annurev-med-042915-102623. [DOI] [PubMed] [Google Scholar]
- 72.Glicksman A, Hadjiyannakis S, Barrowman N, Walker S, Hoey L, et al. Body Fat Distribution Ratios and Obstructive Sleep Apnea Severity in Youth with Obesity. J Clin Sleep Med. 2017;13(4):545–550.s. doi: 10.5664/jcsm.6538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Patinkin ZW, Feinn R, Santos M. Metabolic Consequences of Obstructive Sleep Apnea in Adolescents with Obesity: A Systematic Literature Review and Meta-Analysis. Child Obes. 2017;13(2):102–110. doi: 10.1089/chi.2016.0248. [DOI] [PubMed] [Google Scholar]
- 74.Tan X, Alén M, Wang K, Tenhunen J, Wiklund P, et al. Effect of Six-Month Diet Intervention on Sleep among Overweight and Obese Men with Chronic Insomnia Symptoms: A Randomized Controlled Trial. Nutrients. 2016;8(11) doi: 10.3390/nu8110751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wallace DD, Boynton MH, Lytle LA. Multilevel analysis exploring the links between stress, depression, and sleep problems among two-year college students. J Am Coll Health. 2017;65(3):187–196. doi: 10.1080/07448481.2016.1269111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cao MT, Sternbach JM, Guilleminault C. Continuous positive airway pressure therapy in obstuctive sleep apnea: benefits and alternatives. Expert Rev Respir Med. 2017;11(4):259–272. doi: 10.1080/17476348.2017.1305893. [DOI] [PubMed] [Google Scholar]
- 77.Pohl D, Bloomenthal A. Diabetes, Obesity, and Other Medical Diseases-Is Surgery the Answer. R I Med J (2013) 2017;100(2):15–17. [PubMed] [Google Scholar]
- 78.Quirante FP, Montorfano L, Rammohan R, Dhanabalsamy N, Lee A. Is bariatric surgery safe in the elderly population? Surg Endosc. 2016;31(4):1538–1543. doi: 10.1007/s00464-016-5050-3. [DOI] [PubMed] [Google Scholar]
- 79.Raveendran R, Wong J, Singh M, Wong DT, Chung F. Obesity hypoventilation syndrome, sleep apnea, overlap syndrome: perioperative management to prevent complications. Curr Opin Anaesthesiol. 2017;30(1):146–155. doi: 10.1097/ACO.0000000000000421. [DOI] [PubMed] [Google Scholar]
- 80.Hou C, Zheng B, Yang Y, Wang XG, Zhang B, et al. Weight reduction via life-style modifications results in reverse remodelling and cardiac functional improvement in a patient with obesity. Obes Res Clin Pract. 2017;11(3):364–369. doi: 10.1016/j.orcp.2017.02.003. [DOI] [PubMed] [Google Scholar]


