Abstract
Objectives
Tobacco usage is the strongest risk factor in the development of oral squamous cell carcinoma (OSCC), which mandates careful screening for oral cancers in smokers. However, there are indications that oral potentially malignant lesions, such as oral epithelial dysplasia (OED), in non-smokers (NS) have a higher cancer risk than those in smokers. Without tobacco as an etiology, the development of these lesions in NS may suggest genetic susceptibility. The increasing incidence of OSCC in NS calls for a better understanding of the natural history of OED in NS as compared to that of smokers.
Materials and Methods
Patients from a population-based longitudinal study with more than 10 years of follow up were analyzed. Of the 455 patients with primary OED (233 mild and 212 moderate dysplasia), 139 were NS and 306 were smokers. Demographic and habit information, clinical information (lesion site, size and appearance; toluidine blue and fluorescent visualization), microsatellite analysis for loss of heterozygosity (LOH) and outcome (progression) were compared between the two groups.
Results and Conclusions
The majority of patients with OED were smokers. Of these, more were males, non-Caucasians and heavy drinkers. A significantly higher number of OED in NS were in the tongue, whereas a significantly higher number of OED in smokers were in the floor of mouth (FOM). OED in NS showed a greater than 2-fold increase in cancer progression. Strikingly, OED located in the FOM in NS showed a 38-fold increase in cancer progression as compared to those in smokers.
Keywords: Oral cancer, Oral premalignant lesions, Oral leukoplakia, Pathology, oral, Precancerous conditions, Neoplasm, epithelial, Cancer progression, Predictive markers, Biomarkers, Non-smokers
INTRODUCTION
Tobacco usage is the strongest risk factor for the development of oral squamous cell carcinoma (OSCC)(1–4), which mandates careful screening for oral cancers in smokers. However, OSCC does develop in non-smokers (NS), and there are indications that oral potentially malignant lesions (OPML) in NS possess a higher cancer risk than those in smokers.(5–8) Without tobacco as an etiology, the development of these lesions in NS may suggest genetic susceptibility. Tobacco cessation efforts have resulted in a drop in oral cancer rates associated with this habit (9), leading to a growing interest in the increased proportion of cases occurring among NS. (10) The increasing incidence of oral cancer in NS petition a better understanding of the natural history of OPML in NS as compared to that of smokers.
OPML with a histological diagnosis of oral epithelial dysplasia (OED) are at an increased risk of progressing to oral cancer than those without dysplasia. (11–13) Although the presence of dysplasia provides an indication of risk for higher grades of dysplasia (14, 15), it is a relatively poor predictor for OED with low-grade (mild/moderate) dysplasia, which represent the majority. (16) A more precise risk stratification is required for low-grade lesions.
The study of OPMLs has been the focus of our research team for more than 2 decades, mainly with respect to the development of markers that would help in differentiating progressing from non-progressing mild/moderate dysplasia. The markers included clinical visual aids, such as toluidine blue (TB) staining (17), fluorescent visualization (FV) (18, 19) and microsatellite analysis of loss of heterozygosity (LOH).(5)
Microsatellite analysis for loss of heterozygosity (LOH) analysis is used to assess the loss of chromosomal regions that contain known or putative tumour suppressor genes. The Oral Cancer Prediction Longitudinal (OCPL) study being conducted at the BC Cancer Agency in Vancouver (British Columbia, Canada) has reported a risk prediction model which uses LOH at key chromosomal loci to stratify lesions to risk of malignant progression. (5) To date, this PCR-based assay is the only marker that has been shown to predict malignant progression of low-grade OED and has been prospectively validated in an independent cohort of patients from community settings. (5, 20) Furthermore, it has been optimized for work with archival tissue and small DNA quantities. (21–24)
Several studies have examined clinical characteristics and the prognosis of OSCC in NS. However, this question has not been explored thoroughly with respect to OED. (25–30) Not only is the natural history of OED in NS poorly understood, but the path to prevention and intervention of disease is not well defined in this group. There is a gap in the knowledge surrounding the clinicopathological and genetic characterization and the risk of progression in this growing category. This information is critical to the evolution of precision medicine in this subgroup by allowing for medical decisions, practices, and interventions to be tailored to the individual patient based on their predicted risk of disease.
This study reports on findings within the ongoing OCPL study, of which the overall goal is to establish a risk model for the malignant progression of low-grade OED. The purpose of the present study was to characterize the clinicopathological features and the genetic profile of low-grade OED in NS, as well as to compare progression rates and time to progression between NS and smokers with OED. By describing the clinical characteristics of OED in NS, we seek to better define this unique subset of patients and ultimately aid in the prevention, diagnosis, and management of this disease.
MATERIALS AND METHODS
Since January 1, 1997, the OCPL study has prospectively enrolled and followed patients with low-grade OED to a primary endpoint of malignant progression to severe dysplasia, carcinoma in situ (CIS), or SCC. Participants in the study were identified through a centralized population-based biopsy service, the BC Oral Biopsy Service, where community dentists and specialists across British Columbia (population 4.6 million, in 2014) send biopsies for histological diagnosis. Patients with a diagnosis of low-grade OED were referred by these community clinicians, upon recommendation from the OBS, for follow up to Oral Dysplasia Clinics, where they were invited to participate in the OCPL study. Study protocol and ethical approval was obtained from the University of British Columbia/BC Cancer Agency Research Ethics Board, and participants were accrued to the study using written informed consent.
The current study is a focused analysis which used a subgroup of the OCPL study population. Eligibility criteria for this analysis required a histologically confirmed primary mild or moderate OED with lesion clinicopathogical and tobacco history available and no prior history of oral cancer. Participants where followed a minimum of 12 months, or to progression, whichever occurred first. No participants were excluded, unless they did not meet the criteria. A total of 445 subjects met the selection criteria and were included in the present analysis, with a median follow-up time of 55.4 months (3.3 – 241.4 months). Of the 445 cases reported, 269 were reported in a previously published study involving patients with primary OPML. (5)
Detailed past and present tobacco and alcohol habits were collected by a standardized questionnaire at study entry. Past and current smoking status, as well as amount and form of tobacco (cigarette, pipe, cigar or smokeless tobacco), were documented. Pipe, cigar and smokeless tobacco were recorded if the subject indicated that they had used this form of tobacco more than once per week for one year or longer. (31) Cigarette equivalents were calculated as one pipe equaled 3 cigarettes, and one cigar equaled 2 cigarettes. Smoker was defined as having consumed more than 100 cigarettes (or the equivalent) in one’s life time. (32) Periods of time where a subject had temporarily or permanently quit smoking were recorded. Lifetime smoking history over the subject’s entire life, including amount smoked per day during specific age categories, was collated as a pack-year calculation. A pack-year was defined as the equivalent of smoking 20 cigarettes (1 pack) per day for 1 year. Average weekly alcohol consumption was recorded. One alcoholic drink was defined as 8 ounces of beer, 4 ounces of wine or 1 ounce of spirits. Heavy drinker was defined as consumption of more than 14 drinks per week for women and 21 drinks per week for men. (33, 34)
Clinicopathological data, including lesion site, size, appearance, lesion margin characteristics, as well as information on FV retention and TB positivity were included in the analysis. Lesion size was measured using a calibrated probe and recorded with a bidirectional measurement in millimeters. Lesion appearance was documented as either homogenous (same colour and texture throughout) or as non-homogenous (colour and texture not uniform). Lesion margins were either ill-defined or well-defined. Index lesions were assessed for FV and TB status as previously described. (17, 19) LOH analysis was performed on index biopsies collected at baseline, and lesions were classified as low, intermediate or high risk of progression, using previously published methods. (5, 35)
Clinical follow-up visits occurred every 6 months. Comparative biopsies of the index site were performed upon significant clinical change or approximately every 24 months if no significant change. Outcome was histologically proven progression to severe dysplasia, CIS, or SCC. Inclusion of severe dysplasia as the progression endpoint was based on our findings that without treatment, progression occurred in 32% of patients in 3 years; 60% in 5 years. (15)
Data analyses were carried out using SPSS® Version 24.0 software (Armonk, NY: IBM Corp). The threshold for significance was set at P < 0.05, and all tests were 2-tailed. The inferential analysis included separate bivariate analyses between each independent and dependent variable. Categorical variables were tested using the Chi-square Test or Fisher’s Exact Test when more than 20% of cells contained expected frequencies of < 5. Quantitative variables were tested using an independent samples T-test; those that were not normally distributed were tested with the Mann-Whitney U test. Interaction effects between tobacco and gender, site and alcohol were evaluated with respect to progression, using a binomial logistic regression model. The main analyses were based on the time-to-event outcome. Time to endpoint was calculated from date of the index biopsy to endpoint date or to last follow-up date (as of Nov 15, 2016), if no progression occurred. Time-to-progression curves and 3-year and 5-year progression rates were estimated using Kaplan–Meier analysis and the Log Rank test. Hazard ratios and the corresponding 95% confidence intervals (95% CI) were determined using the Cox proportional hazards regression model.
RESULTS
Sociodemographic and Lifestyle Characteristics
A total of 445 subjects were included in the analysis. Approximately one third (31%) of the subjects were NS. Sixty-nine percent of subjects were smokers; 3.4% had reported having used chewing tobacco, 6.5% reported using cigars and 4.9% reported smoking a pipe. Table 1 shows the distribution of cases of OED according to sociodemographic and lifestyle variables in NS as compared to smokers. The majority were Caucasian and over the age of 40, and males were more likely to be smokers than females were. Age at diagnosis was not significantly associated with smoking status. Gender and ethnicity were significant for smoking status (P = 0.01 and P < 0.001, respectively). Alcohol consumption was also associated with smoking status. Heavy consumers of alcohol were 6.6 times more likely to have smoked than those who were light drinkers or who abstained (95% CI, 2.58 – 16.76; P < 0.001). Gender, ethnicity and alcohol category were each tested in multivariate analysis to see if interaction with smoking status was predictive of malignant progression. When combined with smoking status, neither gender (P= 0.36), ethnicity (P = 0.86), or alcohol consumption (P = 0.85), was significantly associated with progression.
Table 1.
Distribution of cases according to sociodemographic and lifestyle variables
| ALL | Non-Smokera (%)* | Smokerb (%)* | P value | |
|---|---|---|---|---|
| Total | 445(100) | 139 | 306 | |
| Age at diagnosis (n= 444) | ||||
| Mean (years ± SD) | 58.8 ± 11.86 | 60.1 ± 12.43 | 58.2 ± 11.55 | .10 |
| Age Category (n=444) | ||||
| <40 years | 18 (4) | 6 (4) | 12 (4) | .35 |
| 40 – 60 years | 227 (51) | 64 (46) | 163 (53) | |
| ≥60 years | 199 (45) | 69 (50) | 130 (43) | |
| Gender (n=445) | ||||
| Female | 220 (49) | 81 (58) | 139 (45) | .01 |
| Male | 225 (51) | 58 (42) | 167 (55) | |
| Ethnicity (n=445) | ||||
| Caucasian | 368 (83) | 99 (71) | 269 (88) | <.001 |
| Asian | 37 (8) | 21 (15) | 16 (5) | |
| South Asian | 29 (7) | 14 (10) | 15 (5) | |
| Otherc | 11 (2) | 5 (4) | 6 (2) | |
| Alcohol Categoryd (n=441) | ||||
| None/Light | 376 (85) | 133 (96) | 243 (80) | <.001 |
| Heavy | 65 (15) | 5 (4) | 60 (20) | |
Column percentage reported
Non-smoker was defined as less than 100 cigarettes in life time
Smoker was defined as consumption of more than 100 cigarettes in life time
3 Hispanic, 2 African American, 2 North American Aboriginal/First Nations, 1 Mixed, 1 Unknown
Heavy drinker is defined as consumption of more than 14 drinks per week for women and 21 drinks per week for men. 1 drink = 8oz beer or 4oz wine or 1oz spirits
Clinicopathological Features
The first aim of the study was to characterize the clinicopathological features of OED in NS. Clinical features, including lesion size, texture, colour, appearance, margin characteristics, FV status and TB status, did not differ significantly between smokers and NS (Table 2). Smokers were more likely to have OED at the palate, retromolar trigone or floor of the mouth (FOM) (P < 0.001). Dysplastic lesions on the tongue were 7.3 times more likely to progress than OED elsewhere in the oral cavity (95% CI, 1.71 – 31.11; P < 0.001). Lesion size (P < 0.001), non-homogenous appearance (P = 0.01), loss of FV (P = 0.01), TB positivity (P = 0.001), and grade of dysplasia (P = 0.002) were also significantly associated with progression. Strikingly, when lesion site was analyzed together with smoking status, interaction analysis revealed that NS with a lesion on the FOM possessed a 38-fold increased risk of progression as compared to smokers (95% CI, 3.35 – 440.26; P < 0.003).
Table 2.
Clinicopathological and histopathological features according to smoking status
| ALL | Non-Smokera (%)* | Smokerb ((%)* | P value | Odds Ratio (95%CI) | No Progression (%) | Progression (%) | P value | Odds Ratio (95%CI) | |
|---|---|---|---|---|---|---|---|---|---|
| Total | 445 | 139 | 306 | 385 | 60 | ||||
| Size at diagnosis (n=402) | |||||||||
| Median (mm2 (IQRc)) | 160 (50 – 378)) | 160 (65 – 414) | 155 (48 – 360) | .54 | 135 (48 – 324) | 297 (108 – 600) | <.001 | ||
| Site (n=445) | |||||||||
| gingiva | 54 (12) | 18 (13) | 36 (12) | <.001 | 1 | 52 (14) | 2 (1) | <.001 | 1 |
| buccal/vestibule mucosa | 63 (14) | 19 (14) | 44 (14) | 1.16 (0.53 – 2.53) | 58 (15) | 5 (8) | 2.24 (0.42 – 12.1) | ||
| palate/retromolar/trigone | 54 (12) | 9 (6) | 45 (15) | 2.50 (1.01 – 6.23) | 49 (13) | 5 (8) | 2.65 (0.49 – 14.32) | ||
| tongue | 201 (45) | 85 (61) | 116 (38) | 0.68 (0.36 – 1.28) | 157 (41) | 44 (73) | 7.29 (1.71 – 31.11) | ||
| FOMd | 73 (16) | 8 (6) | 65 (21) | 4.06 (1.61 – 10.27) | 69 (18) | 4 (1) | 1.51 (0.27 – 8.55) | ||
| Appearance (n=385) | |||||||||
| Homogenous | 221 (57) | 67 (54) | 154 (59) | .36 | 1 | 199 (60) | 22 (40) | .01 | 1 |
| Non-homogenous | 164 (43) | 57 (46) | 107 (41) | 0.82 (0.53 – 1.26) | 131 (40) | 33 (60) | 2.28 (1.27 – 4.08) | ||
| Marginse (n=343) | |||||||||
| Well-defined | 114 (33) | 34 (30) | 80 (35) | .31 | 1 | 101 (34) | 13 (30) | .66 | 1 |
| Ill-defined | 229 (67) | 81 (70) | 148 (65) | 0.77 (0.48 – 12.6) | 199 (66) | 30 (70) | 1.17 (0.59 – 2.34) | ||
| FVf Results (n=272) | |||||||||
| FV retention | 98 (36) | 30 (32) | 68 (38) | .26 | 1 | 92 (39) | 6 (39) | .01 | 1 |
| FV loss or equivocal | 174 (64) | 65 (68) | 109 (62) | 0.74 (0.44 – 1.26) | 145 (61) | 29 (83) | 3.07 (1.23 – 7.67) | ||
| TBg Results (n=387) | |||||||||
| TB negative | 300 (78) | 90 (73) | 210 (80) | .16 | 1 | 266 (80) | 34 (61) | .001 | 1 |
| TB positive or equivocal | 87 (22) | 33 (27) | 54 (20) | 0.70 (0.43 –1.16) | 65 (20) | 22 (39) | 2.65 (1.45 – 4.83) | ||
| Diagnosis (n=445) | |||||||||
| Mild Dysplasia | 233 (52) | 71 (51) | 162 (53) | .72 | 1 | 213 | 20 | .002 | 1 |
| Moderate Dysplasia | 212 (48) | 68 (49) | 144 (47) | 0.93 (0.62 – 1.39) | 172 | 40 | 2.48 (1.40 – 4.39) | ||
| Length of Follow-up§ | |||||||||
| Median months of follow-up (range) | 55.4 (3.3 – 241.4) | 59.9 (3.3 – 222.7) | 55.1 (3.6 – 241.4) | .51 | 59.9 (12.0 – 241.4) | 32.0 (3.3 – 222.7) | <.001 | ||
Column percentage reported
Non-smoker was defined as less than 100 cigarettes in life time
Smoker was defined as consumption of more than 100 cigarettes in life time
IQR = interquartile range
FOM = floor of mouth
discrete = well-defined; diffuse = ill-defined
FV = fluorescence visualization
TB = toluidine blue
Months to last follow-up or progression, whichever occurred first
Outcome
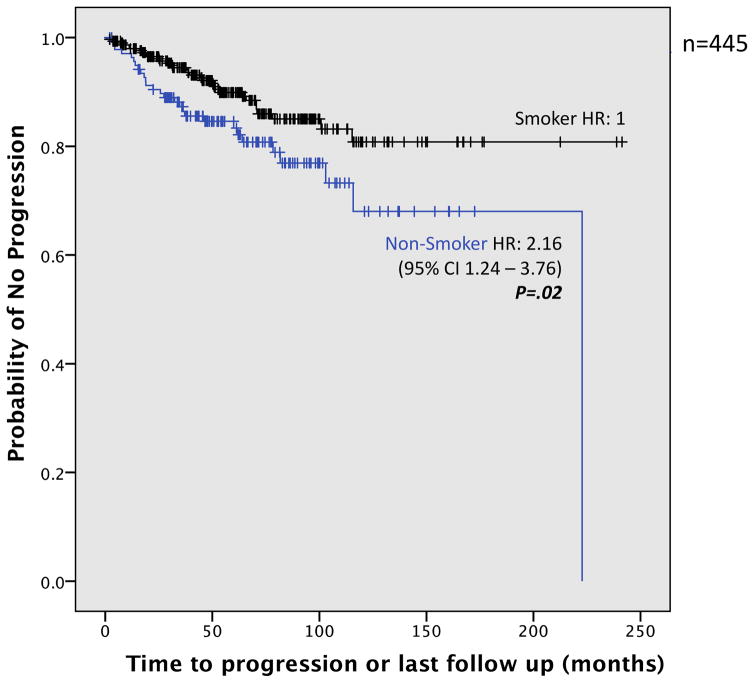
The second aim of the study was to explore whether there were differences in progression between smokers and non-smokers with OED. Out of 445 subjects, 60 (13%) cases progressed (Table 3); 33 to severe dysplasia (7%), 5 to CIS (1%), and 22 to SCC (5%). A significantly higher proportion of progression occurred in NS: NS were more than twice as likely to progress than those who smoked (95% CI, 1.24 – 3.76; P = 0.006). When smokers were further categorized into former smoker (FS) and continuing smoker (CS), NS possessed a 4-fold increased risk of progression as compared to that of CS (P = 0.004). Amount of smoking was also negatively associated with progression: NS possessed more than twice the risk of heavy smokers (HR=2.31; 95% CI, 1.16 – 4.60; P=.02).
Table 3.
Distribution of risk factor variables according to outcome
| ALL | No Progression (%)* | Progressed (%)* | P value | Odds Ratio (95%CI) | |
|---|---|---|---|---|---|
| Total | 445 (100) | 385 | 60 | ||
| Tobacco History (n=445) | |||||
| NSa | 139 (31) | 111 (29) | 28 (47) | .006 | 2.16 (1.24 – 3.76) |
| Smokerb | 306 (69) | 274 (71) | 32 (53) | 1 | |
| Tobacco History (n=445) | |||||
| NSa | 139 (31) | 111 (29) | 28 (47) | .004 | 3.93 (1.65 – 9.37) |
| FSc | 190 (43) | 165 (43) | 25 (42) | 2.34 (0.99 – 5.65) | |
| CSd | 116 (26) | 109 (28) | 7 (12) | 1 | |
| Total Pack-yeare (n=445) | |||||
| Median pack-year (IQRf) | 10.5 (0.0 – 30.0) | 12.8 (0.0 – 30.9) | 0.0 (0.0 – 17.1) | .05§ | |
| Tobacco Amount Category (n=445) | |||||
| NSa | 139 (31) | 111 (29) | 28 (47) | .02 | 2.31 (1.16 – 4.60) |
| Lightg | 159 (36) | 141 (37) | 18 (30) | 1.17 (0.56 – 2.44) | |
| Heavyh | 142 (32) | 128 (34) | 14 (23) | 1 | |
| Alcohol Categoryh (n=441) | |||||
| None/Light | 376 (85) | 324 (85) | 52 (87) | .74 | 1 |
| Heavy | 65 (15) | 57 (15) | 8 (13) | 0.87 (0.40 – 1.94) | |
Column percentage reported
exponential distribution of data, logarithmic transformation applied
NS = non-smoker; defined as less than 100 cigarettes in life time
Smoker was defined as consumption of more than 100 cigarettes in lifetime
FS = former smoker; defined as smoker who quit smoking at or before diagnosis
CS = current smoker; defined as smoker who continued to smoke after diagnosis
A pack-year is defined as the equivalent of smoking 20 cigarettes per day for 1 year.
IQR = interquartile range
Light smoker was defined as smoker and pack-year total less than median (23.8)
Heavy smoker was defined as smoker and pack-year total greater than median (23.8)
Heavy drinker is defined as consumption of more than 14 drinks per week for women and 21 drinks per week for men. 1 drink = 8oz beer or 4oz wine or 1oz spirits
Time to progression occurred faster in NS as well (Figure 1). Table 4 compares the probability of progression in NS and in smokers, showing 3- and 5-year rates. Both 3-year and 5-year progression rates were higher in NS than those in smokers (3-year: 12.7% vs. 5.5%; 5-year: 16.6% vs. 10.1%, respectively) (P = 0.002). Length of follow up did not differ significantly between the groups (median time of 66.2 months for NS, 60.4 months for smokers; P = 0.07).
Figure 1.
Kaplan–Meier plot of time to progression in smokers vs. non-smokers. Smoker was defined as > 100 cigarettes in lifetime; Non-smoker was defined as <100 cigarettes in lifetime.
Table 4.
Probability of progression in smokers versus non-smokers
| ALL | Non-Smokera | Smokerb | P value | |
|---|---|---|---|---|
| Total | 445 | 139 | 306 | |
| Probability of Progression† | ||||
| 3-year (95% CI) | 12.7 (9.8 – 15.6) | 5.5 (4.1 – 6.9) | .02 | |
| 5-year (95% Ci) | 16.6 (13.2 – 20) | 10.1 (8.1 – 12.8) | ||
Non-smoker was defined as less than 100 cigarettes in life time
Smoker was defined as consumption of more than 100 cigarettes in life time
Progression defined as progression to severe dysplasia, carcinoma in-situ, squamous cell carcinoma
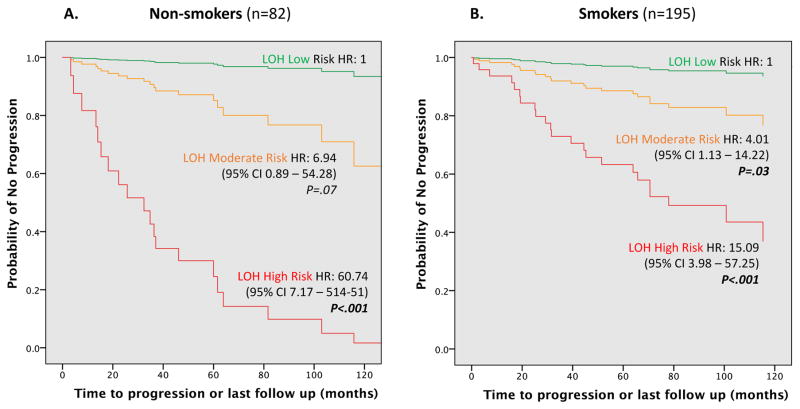
When the LOH risk model was used to examine outcome in NS compared to that in smokers, Cox regression analysis showed that LOH risk patterns were strongly associated with progression and was sensitive in both groups. Overall, lesions in the high-risk category had a 25-fold increased risk of progression (95% CI 8.50 – 76.69; P < 0.001) as compared to those in a low-risk category. However, NS in a high-risk category possessed much higher risk (HR = 60.74; 95% CI, 7.17 – 514.51; P < 0.001) than smokers (HR=15.09; 95% CI, 3.98 – 57.25; P < 0.001) (Figure 2).
Figure 2.
Cox proportional hazards regression model analysis for loss of heterozygosity (LOH) risk patterns in non-smokers (A) compared to smokers (B) with risk stratification by the previously reported LOH model. (5) Non-smoker (NS) was defined as less than 100 cigarettes in life time; Smoker was defined as consumption of more than 100 cigarettes in lifetime. Low Risk was defined as 9p Retained; Moderate Risk was defined as 9p LOH (Loss of Heterozygosity), or 9p LOH + 17p LOH, or 9p LOH + 4q LOH; High Risk was defined as 9p LOH + 17p LOH + 4q LOH.
DISCUSSION
This study characterizes both the clinicopathological features and the genetic profile of OED in NS and associates these findings with outcome in a large number of patients in longitudinal follow up. Although several studies have explored the association between clinical or genomic characteristics and outcome of OSCC in NS (25–28, 30, 36, 37), few studies have explored these considerations with respect to OED. Although previous studies have reported a higher transformation rate in NS,(5–8, 37, 38) this study is more comprehensive in that the primary focus is to compare multiple parameters (histological, clinicopathological and genetic) between smokers and NS, as well as to evaluate the interaction of smoking status with these parameters in association with progression. In 2012, Ho et al. (38) found that non-smoking status and tongue subsite had the highest risk of transformation. Our study has supported the findings of previous studies in OPML, by confirming that NS with OED possess a significantly elevated risk for progression, and has presented new findings in interaction analysis with clinical features, the genetic risk models, as well as the proportion and time to progression among smokers and NS. This study was conducted within the framework of a prospective clinical trial, the OCPL Study, the largest longitudinal study attempted to date, and is unique in that it draws from a community-based rather than a high-risk population. The study design demonstrates clear temporal sequence between exposure and outcome. The limitations are the same inherent limitations as those of any prospective cohort study: it requires a large sample size and long follow up. Long latency periods increase the study time, complexity, and cost, as well as increase the potential of loss to follow-up. Another potential limitation comes from the self-reported smoking data which requires the participant to recall and report this information accurately.
Tobacco use is considered one of the most significant risk factors for OSCC. (1–4). However, this environmental exposure is not the only pathway to oral cancer. Alcohol consumption is also recognized as an independent risk factor for OSCC. (3, 4, 39–42) There is also evidence that suggests tobacco and alcohol act synergistically to contribute to OSCC risk. (3, 4, 43–45) Although alcohol was strongly associated with smoking status in this data set, alcohol alone had no association with progression (P = 0.65). Like other studies that have examined alcohol and tobacco interaction in the etiology of OSCC, results of this study are hampered by the low numbers of heavy drinkers who do not use tobacco (n = 5). The interaction between alcohol category (none/light vs. heavy) and smoking (NS vs. smoker) was not predictive of progression (P = 0.85). Similarly, interaction between number of tobacco pack-years and weekly alcohol consumption was also not predictive of progression (P = 0.19).
With increasing evidence of the etiological role of human papilloma virus (HPV) in the development of cancers of many human organs and tissues, one could hypothesize that HPV may play an important etiological role for oral SCC in NS. However unpublished data from our lab has shown a higher percentage of oral SCC with HPV DNA in smokers (9%, 13/135) than that of NS (3%, 2/76) although the difference was not significant (P = 0.09).
The data presented in this analysis confirm that although smokers are more likely to develop OED, when OED does occur in NS, they are at higher risk for cancer progression. Our findings not only clearly demonstrate a significantly elevated risk for malignant progression in NS with OED, but also reveal that OED in NS progress more quickly than smoking associated OED. LOH markers can delineate high risk lesions, regardless of risk habits, and should be an important consideration in the management of OED.
Cancer development is believed to be underlined by accumulation of mutations of driver genes through exposure to environmental carcinogens or hereditary predisposition. Recently Tomasetti and Vogelstein (46) have proposed a third theory for the mutation - mutations resulting from the random mistakes made during normal DNA replication, or replicative errors. It has been proposed that up to two-thirds of human cancers are a result of such errors. (47) It is possible that OED in NS is driven either by inherited predisposition and/or by replicative errors, versus smokers whose OED is more likely attributable to mutations that are environmental in etiology. To test the hypothesis that the progression risk model would differ in the OED of NS and those of smokers, we examined the chromosomal changes in regions of hypothesized tumour suppressor genes at 3p, 4q, 8p, 9p, 11q, 13q and 17p. The previously published LOH risk model, which uses LOH at 9p, 17p and 4q to predict the cancer risk of OED, was still the best risk model and equally predictive of progression in both smokers and NS. (5) The similarities in the prediction models could be interpreted as showing that the genetic alterations are similar between smokers and NS, regardless of how these changes are acquired, i.e., through environmental carcinogens, genetic predisposition or replicative errors. On the other hand, OED in NS may involve unique genetic mutations, which are driving progression, which have not yet been identified. Further genomic characterization, using methods such as next genome sequencing (NGS), would be needed to provide valuable insight into the differences in the molecular pathogenesis of OSCC associated with cigarette smoking and that of NS.
It is generally accepted that OED is at risk of progression to SCC (11–13), although no universally accepted guidelines for the management of low-grade OED exist. Therefore, it is suggested that the secondary prevention of SCC, from OED, should utilize, not only the histological diagnosis of dysplasia, but also more objective biomarkers of the risk of transformation. The need to find molecular markers for the risk of OSCC, and the importance of the implications for the prevention and early detection has been highlighted by others.(23, 48–50) The term precision medicine, or personalized medicine, refers to the ability to make medical decisions and offer treatment or interventions tailored to the individual patient based on their predicted risk of disease. (51) The ability to identify low-grade lesions that are at risk for progression paves the way for interception, or the idea that premalignant lesions (OPML) can actively be treated to reduce the risk of the lesion becoming a full blown cancer. (52). These high-risk individuals could be offered more aggressive treatment options and more intensive follow-up; they are also prime candidates to target for chemoprevention trials.
Conclusion
Clinicians should be diligent in screening for cancer in both smokers and NS. Tobacco remains one of the strongest risk factors for the development of OSCC, yet for patients with a histologically confirmed OED, NS have increased cancer risk. With smoking eliminated as an etiology, their development in these patients suggest either genetic susceptibility or replicative errors. These findings substantiate the risk of progression in NS and emphasize the need for clinicians to consider smoking history and the molecular profiles in the triage and management of OED. The consideration of smoking history and LOH risk category marks the evolution of a systematic decision-making process for this very heterogeneous group of lesions and an important move towards clinical application of these markers in a way that minimizes patient morbidity while maximizing health system and cost efficiency. This information is critical to the evolution of precision medicine in this subgroup by allowing for medical decisions, practices, and interventions to be tailored to the individual patient based on their predicted risk of disease.
HIGHLIGHTS.
The majority of patients with OED were smokers; yet NS with OED were at a higher risk of progression
OED in smokers and NS were similar in size and appearance, but differed with respect to site
NS with an OED at the FOM possessed a 38-fold increased risk of progression compared to smokers
LOH markers can identify high-risk lesions, and are sensitive regardless of smoking risk habits
Acknowledgments
This work was supported by grants from the British Columbia Cancer Foundation, the National Institutes of Health (R01DE13124), and the National Institute of Dental and Craniofacial Research (R01DE17013).
Footnotes
CONFLICT OF INTEREST STATEMENT
None declared.
ROLE OF THE FUNDING SOURCE
The funding source(s) had no involvement in the study design; data collection; analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Warnakulasuriya S. Living with oral cancer: Epidemiology with particular reference to prevalence and life-style changes that influence survival. Oral Oncology. 2010;46(6):407–10. doi: 10.1016/j.oraloncology.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 2.International Agency for Research on Cancer, World Health Organization, Iarc Working Group on the Evaluation of Carcinogenic Risks to Humans Lyon F. Tobacco smoke and involuntary smoking. Lyon, France; Geneva: IARC Press; 2004. [Google Scholar]
- 3.Hashibe M, Brennan P, Benhamou S, Castellsague X, Chu C, Curado MP, et al. Alcohol drinking in never users of tobacco, cigarette smoking in never drinkers, and the risk of head and neck cancer: Pooled analysis in the international head and neck cancer epidemiology consortium. Journal of the National Cancer Institute. 2007;99(10):777–89. doi: 10.1093/jnci/djk179. [DOI] [PubMed] [Google Scholar]
- 4.Hashibe M, Hunt J, Wei M, Buys S, Gren L, Lee YCA. Tobacco, alcohol, body mass index, physical activity, and the risk of head and neck cancer in the prostate, lung, colorectal, and ovarian (PLCO) cohort. Head and Neck-Journal for the Sciences and Specialties of the Head and Neck. 2013;35(7):914–22. doi: 10.1002/hed.23052. [DOI] [PubMed] [Google Scholar]
- 5.Zhang L, Poh CF, Williams M, Laronde DM, Berean K, Gardner PJ, et al. Loss of heterozygosity (LOH) profiles--validated risk predictors for progression to oral cancer. Cancer Prevention Research (Phila) 2012;5(9):1081–9. doi: 10.1158/1940-6207.CAPR-12-0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Einhorn J, Wersall J. Incidence of oral carcinoma in patients with leukoplakia of the oral mucosa. Cancer. 1967;20(12):2189–93. doi: 10.1002/1097-0142(196712)20:12<2189::aid-cncr2820201218>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 7.Schepman KP, van der Meij EH, Smeele LE, van der Waal I. Malignant transformation of oral leukoplakia: a follow-up study of a hospital-based population of 166 patients with oral leukoplakia from The Netherlands. Oral Oncol. 1998;34(4):270–5. [PubMed] [Google Scholar]
- 8.Holmstrup P, Vedtofte P, Reibel J, Stoltze K. Long-term treatment outcome of oral premalignant lesions. Oral oncology. 2006;42(5):461–74. doi: 10.1016/j.oraloncology.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. Data and Statistics. Atlanta (GA): Office of Smoking and Health, National Center for Chronic Disease Prevention and Health Promotion; 2017. [accessed 2017 Jul 27]. Available from: https://www.cdc.gov/tobacco/data_statistics/index.htm. [Google Scholar]
- 10.Llewellyn CD, Johnson NW, Warnakulasuriya K. Risk factors for squamous cell carcinoma of the oral cavity in young people - a comprehensive literature review. Oral Oncology. 2001;37(5):401–18. doi: 10.1016/s1368-8375(00)00135-4. [DOI] [PubMed] [Google Scholar]
- 11.Mehanna HM, Rattay T, Smith J, McConkey CC. Treatment and follow-up of oral dysplasia - a systematic review and meta-analysis. Head & neck. 2009;31(12):1600. doi: 10.1002/hed.21131. [DOI] [PubMed] [Google Scholar]
- 12.Lumerman H, Freedman P, Kerpel S. Oral epithelial dysplasia and the development of invasive squamous cell carcinoma. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology and Endodontology. 1995;79(3):321–9. doi: 10.1016/s1079-2104(05)80226-4. [DOI] [PubMed] [Google Scholar]
- 13.Warnakulasuriya S, Kovacevic T, Madden P, Coupland VH, Sperandio M, Odell E, et al. Factors predicting malignant transformation in oral potentially malignant disorders among patients accrued over a 10-year period in South East England. Journal of Oral Pathology & Medicine. 2011;40(9):677–83. doi: 10.1111/j.1600-0714.2011.01054.x. [DOI] [PubMed] [Google Scholar]
- 14.Silverman S, Gorsky M, Kaugars GE. Leukoplakia, dysplasia, and malignant transformation. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology and Endodontology. 1996;82(2):117. doi: 10.1016/s1079-2104(96)80209-5. [DOI] [PubMed] [Google Scholar]
- 15.Zhang L, Lubpairee T, Laronde DM, Rosin MP. Should severe epithelial dysplasia be treated? Oral Oncology. 2016;60:125–9. doi: 10.1016/j.oraloncology.2016.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amagasa T, Yamashiro M, Uzawa N. Oral premalignant lesions: from a clinical perspective. International Journal of Clinical Oncology. 2011;16(1):5–14. doi: 10.1007/s10147-010-0157-3. [DOI] [PubMed] [Google Scholar]
- 17.Zhang L, Williams M, Poh CF, Laronde D, Epstein JB, Durham S, et al. Toluidine blue staining identifies high-risk primary oral premalignant lesions with poor outcome. Cancer research. 2005;65(17):8017–21. doi: 10.1158/0008-5472.CAN-04-3153. [DOI] [PubMed] [Google Scholar]
- 18.Poh CF, Ng SP, Williams PM, Zhang L, Laronde DM, Lane P, et al. Direct fluorescence visualization of clinically occult high-risk oral premalignant disease using a simple hand-held device. Head and Neck-Journal for the Sciences and Specialties of the Head and Neck. 2007;29(1):71–6. doi: 10.1002/hed.20468. [DOI] [PubMed] [Google Scholar]
- 19.Lane PM, Gilhuly T, Whitehead P, Zeng H, Poh CF, Ng S, et al. Simple device for the direct visualization of oral-cavity tissue fluorescence. Journal of Biomedical Optics. 2006;11(2):024006. doi: 10.1117/1.2193157. [DOI] [PubMed] [Google Scholar]
- 20.Lingen MW, Szabo E. Validation of LOH Profiles for Assessing Oral Cancer Risk. Cancer Prevention Research. 2012;5(9):1075–7. doi: 10.1158/1940-6207.CAPR-12-0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Califano J, van der Riet P, Westra W, Nawroz H, Clayman G, Piantadosi S, et al. Genetic progression model for head and neck cancer: implications for field cancerization. Cancer research. 1996;56(11):2488. [PubMed] [Google Scholar]
- 22.Lippman S, Ro JY, Batsakis JG, Lee JS, Fan YH, Hong WK, et al. Frequent microsatellite alterations at chromosomes 9p21 and 3p14 in oral premalignant lesions and their value in cancer risk assessment. Nature medicine. 1996;2(6):682–5. doi: 10.1038/nm0696-682. [DOI] [PubMed] [Google Scholar]
- 23.Lippman SM, Hong WK. Molecular markers of the risk of oral cancer. New England Journal of Medicine. 2001;344(17):1323–6. doi: 10.1056/NEJM200104263441710. [DOI] [PubMed] [Google Scholar]
- 24.Sidransky D. Molecular genetics of head and neck cancer. Curr Opin Oncol. 1995;7(3):229–33. doi: 10.1097/00001622-199505000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Barrowman R, Koo K, Wiesenfeld D, Nastri A, McCullough M. Oral cancer in non-smokers, non-drinkers: a systematic review. International Journal of Oral and Maxillofacial Surgery. 2015;44:e34–e5. [Google Scholar]
- 26.Durr ML, Li D, Wang SJ. Oral cavity squamous cell carcinoma in never smokers: analysis of clinicopathologic characteristics and survival. American journal of otolaryngology. 2013;34(5):388. doi: 10.1016/j.amjoto.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 27.Koch WM, Lango M, Sewell D, Zahurak M, Sidransky D. Head and neck cancer in nonsmokers: a distinct clinical and molecular entity. The Laryngoscope. 1999;109(10):1544–51. doi: 10.1097/00005537-199910000-00002. [DOI] [PubMed] [Google Scholar]
- 28.Li R, Faden DL, Fakhry C, Langelier C, Jiao Y, Wang Y, et al. Clinical, genomic, and metagenomic characterization of oral tongue squamous cell carcinoma in patients who do not smoke: Clinical and genomic study of nonsmokers with oral tongue cancer. Head & Neck. 2015;37(11):1642–9. doi: 10.1002/hed.23807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morse DE, Psoter WJ, Baek LS, Eisenberg E, Cohen D, Cleveland D, et al. Smoking and drinking in relation to depressive symptoms among persons with oral cancer or oral epithelial dysplasia. Head & Neck. 2010;32(5):578. doi: 10.1002/hed.21227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pickering CR, Zhang J, Neskey DM, Zhao M, Jasser SA, Wang J, et al. Squamous cell carcinoma of the oral tongue in young non-smokers is genomically similar to tumors in older smokers. Clinical cancer research: an official journal of the American Association for Cancer Research. 2014;20(14):3842. doi: 10.1158/1078-0432.CCR-14-0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Centers for Disease Control and Prevention. Data and Statistics. Atlanta (GA): Office of Smoking and Health, National Center for Chronic Disease Prevention and Health Promotion; 2017. [accessed 2017 Jul 27]. Available from: https://www.cdc.gov/tobacco/data_statistics/index.htm. [Google Scholar]
- 32.Bondy SJ, Victor JC, Diemert LM. Origin and use of the 100 cigarette criterion in tobacco surveys. Tobacco Control. 2009;18(4):317–23. doi: 10.1136/tc.2008.027276. [DOI] [PubMed] [Google Scholar]
- 33.Lim K, Moles DR, Downer MC, Speight PM. Opportunistic screening for oral cancer and precancer in general dental practice: results of a demonstration study. British Dental Journal. 2003;194(9):497–502. doi: 10.1038/sj.bdj.4810069. [DOI] [PubMed] [Google Scholar]
- 34.Allen NE, Beral V, Casabonne D, Kan SW, Reeves GK, Brown A, et al. Moderate Alcohol Intake and Cancer Incidence in Women. Journal of the National Cancer Institute. 2009;101(5):296–305. doi: 10.1093/jnci/djn514. [DOI] [PubMed] [Google Scholar]
- 35.Zhang L, Michelsen C, Cheng X, Zeng T, Priddy R, Rosin MP. Molecular analysis of oral lichen planus. A premalignant lesion? American Journal of Pathology. 1997;151(2):323. [PMC free article] [PubMed] [Google Scholar]
- 36.Jaber MA. Oral epithelial dysplasia in non-users of tobacco and alcohol: an analysis of clinicopathologic characteristics and treatment outcome. Journal of Oral Science. 2010;52(1):13–21. doi: 10.2334/josnusd.52.13. [DOI] [PubMed] [Google Scholar]
- 37.Perry BJ, Zammit AP, Lewandowski AW, Bashford JJ, Dragovic AS, Perry EJ, et al. Sites of origin of oral cavity cancer in nonsmokers vs smokers: possible evidence of dental trauma carcinogenesis and its importance compared with human papillomavirus. JAMA Otolaryngol Head Neck Surg. 2015;141(1):5–11. doi: 10.1001/jamaoto.2014.2620. [DOI] [PubMed] [Google Scholar]
- 38.Ho MW, Risk JM, Woolgar JA, Field EA, Field JK, Steele JC, et al. The clinical determinants of malignant transformation in oral epithelial dysplasia. Oral oncology. 2012;48(10):969–76. doi: 10.1016/j.oraloncology.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 39.Lubin JH, Purdue M, Kelsey K, Zhang Z-F, Winn D, Wei Q, et al. Total Exposure and Exposure Rate Effects for Alcohol and Smoking and Risk of Head and Neck Cancer: A Pooled Analysis of Case-Control Studies. American Journal of Epidemiology. 2009;170(8):937–47. doi: 10.1093/aje/kwp222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Turati F, Garavello W, Tramacere I, Pelucchi C, Galeone C, Bagnardi V, et al. A meta-analysis of alcohol drinking and oral and pharyngeal cancers: results from subgroup analyses. Alcohol and alcoholism (Oxford, Oxfordshire) 2013;48(1):107–18. doi: 10.1093/alcalc/ags100. [DOI] [PubMed] [Google Scholar]
- 41.Bagnardi V, Rota M, Botteri E, Tramacere I, Islami F, Fedirko V, et al. Alcohol consumption and site-specific cancer risk: a comprehensive dose-response meta-analysis. British journal of cancer. 2015;112(3):580. doi: 10.1038/bjc.2014.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maserejian NN, Joshipura KJ, Rosner BA, Giovannucci E, Zavras AI. Prospective Study of Alcohol Consumption and Risk of Oral Premalignant Lesions in Men. Cancer Epidemiology Biomarkers & Prevention. 2006;15(4):774–81. doi: 10.1158/1055-9965.EPI-05-0842. [DOI] [PubMed] [Google Scholar]
- 43.Tanaka T, Ishigamori R. Understanding carcinogenesis for fighting oral cancer. J Oncol. 2011;2011:603740. doi: 10.1155/2011/603740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leemans CR, Braakhuis BJM, Brakenhoff RH. The molecular biology of head and neck cancer. Nature Reviews Cancer. 2011;11(1):9–22. doi: 10.1038/nrc2982. [DOI] [PubMed] [Google Scholar]
- 45.Mashberg A, Boffetta P, Winkelman R, Garfinkel L. Tobacco smoking, alcohol drinking, and cancer of the oral cavity and oropharynx among U.S. veterans. Cancer. 1993;72(4):1369–75. doi: 10.1002/1097-0142(19930815)72:4<1369::aid-cncr2820720436>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 46.Tomasetti C, Vogelstein B. Cancer etiology. Variation in cancer risk among tissues can be explained by the number of stem cell divisions. Science. 2015 Jan 2;347(6217):78–81. doi: 10.1126/science.1260825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tomasetti C, Li L, Vogelstein B. Stem cell divisions, somatic mutations, cancer etiology, and cancer prevention. Science. 2017;355(6331):1330–4. doi: 10.1126/science.aaf9011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lippman SM, Sudbo J, Hong WK. Oral cancer prevention and the evolution of molecular-targeted drug development. J Clin Oncol. 2005;23(2):346–56. doi: 10.1200/JCO.2005.09.128. [DOI] [PubMed] [Google Scholar]
- 49.Pitiyage G, Tilakaratne WM, Tavassoli M, Warnakulasuriya S. Molecular markers in oral epithelial dysplasia: review. J Oral Pathol Med. 2009;38(10):737–52. doi: 10.1111/j.1600-0714.2009.00804.x. [DOI] [PubMed] [Google Scholar]
- 50.Chimenos-Kustner E, Font-Costa I, Lopez-Lopez J. Oral cancer risk and molecular markers. Med Oral Patol Oral Cir Bucal. 2004;9(5):381–4. 77–80. [PubMed] [Google Scholar]
- 51.Burke W, Psaty BM. Personalized medicine in the era of genomics. Jama. 2007;298(14):1682–4. doi: 10.1001/jama.298.14.1682. [DOI] [PubMed] [Google Scholar]
- 52.Blackburn EH. Cancer Interception. Cancer Prevention Research. 2011;4(6):787–92. doi: 10.1158/1940-6207.CAPR-11-0195. [DOI] [PubMed] [Google Scholar]