Abstract
Background
Electrical excitation initiates myocardial mechanical contraction and coordinates myocardial pumping. We hypothesized that ECG global electrical heterogeneity (GEH) and its longitudinal changes are associated with cardiac structure and function.
Methods and Results
Participants from the Atherosclerosis Risk in Communities (ARIC) study (N=5,114; 58% female; 22% African Americans) with resting 12-lead ECGs (visits 1–5) and echocardiographic assessment of left ventricular ejection fraction (LVEF), LV global longitudinal strain, LV mass index (LVMi), LV end-diastolic (LVEDVi), and end-systolic volume index (LVESVi) at visit 5 were included. Longitudinal analysis included ARIC participants (N=14,609) with measured GEH at visits 1–4. GEH was quantified by spatial ventricular gradient, QRS-T angle, and sum absolute QRST integral (SAI QRST). Cross-sectional and longitudinal regressions were adjusted for manifest and subclinical cardiovascular disease (CVD). Having 4 abnormal GEH parameters was associated with a 6.4%(95% CI 5.5–7.3%) LVEF decline, a 24.2 (95%CI 21.5–26.9) g/m2 increase in LVMi, a 10.3 (95%CI 8.9–11.7) mL/m2 increase in LVEDVi, and a 7.8 (95%CI 6.9–8.6) mL/m2 increase in LVESVi. Altogether, clinical and ECG parameters accounted for approximately 1/3rd of LV volume and 20% of systolic function variability. The associations were significantly stronger in CVD. SAI QRST increased by 20 mV*ms for each 3-year period in participants who demonstrated LV dilatation at visit 5. Sudden cardiac death victims demonstrated rapid GEH worsening, while those with LV dysfunction demonstrated slow GEH worsening. Healthy aging was associated with a distinct pattern of SVG azimuth decrement.
Conclusion
GEH is a marker of subclinical abnormalities in cardiac structure and function.
Journal Subject Terms: Electrocardiology (ECG), Epidemiology, Echocardiography, Electrophysiology, Aging
Keywords: electrocardiography, longitudinal cohort study, left ventricular hypertrophy, left ventricular systolic function, left ventricle geometry, global electrical heterogeneity, vectorcardiography
Introduction
The heart is an electrical generator and electromechanical pump1. Electrical excitation initiates mechanical contraction, orchestrates excitation-contraction coupling1, and coordinates myocardial pumping. Changes in the frequency2, regularity3, and order4 of cardiac electrical excitation can cause electrical heterogeneity, dyssynchrony, ventricular dysfunction, and ultimately clinical pump failure2–4. While the molecular biology of excitation-contraction coupling is well understood1, and mechanisms of dyssynchrony-induced cardiomyopathy are recognized, its prognostic markers are not identified5. Additionally, there is no inexpensive and widely available method for monitoring the degree of or changes in electrical dyssynchrony over time.
Recently we described ECG parameters depicting global electrical heterogeneity (GEH)6. The GEH concept is based on the theory of Wilson’s electrical gradient vector7, which characterizes the degree of heterogeneity of total recovery time8 across the ventricles, and points towards the area where the total recovery time is shortest. Five vectorcardiographic parameters (spatial QRS-T angle, sum absolute QRST integral (SAI QRST), spatial ventricular gradient (SVG) vector magnitude, azimuth, and elevation) characterize GEH6. We recently showed that GEH characterizes an electrophysiological substrate independently associated with the risk of sudden cardiac death (SCD)6. However, the relationships between GEH and cardiac structure and function have not been previously studied and have not been compared with traditional global ECG parameters (QRS and QTc intervals and electrocardiographic left ventricular hypertrophy (LVH) measures). We hypothesized that GEH is associated with echocardiographic measures of cardiac structure and function in the general community population with and without ventricular conduction abnormalities, LVH, and cardiovascular disease (CVD). We also hypothesized that longitudinal patterns of GEH changes over time differ in participants with different clinical outcomes (with vs. without left ventricular (LV) dysfunction, SCD, non-sudden cardiac death, and non-cardiac death).
Methods
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Study sample
The Atherosclerosis Risk in Communities (ARIC) study is an ongoing, prospective cohort study evaluating risk factors, progression, and outcomes of atherosclerosis in 15,792 participants (45% male, 74% white) enrolled in four United States communities in 1987–1989. ARIC study design and protocol have been previously described9. Follow-up of ARIC participants included three triennial visits through 1998; each visit included a 12-lead ECG recording10. A fifth examination was conducted between June 1st, 2011, and August 30th, 2013. Out of 10,740 surviving participants, 6,538 participants (now aged 67–91 years) attended visit 5, and 6,118 underwent echocardiography, whereas 5,981 underwent 12-lead ECG recording at rest. The study protocol was approved by institutional review boards at each field center, and all participants signed informed consent.
Our cross-sectional study sample included ARIC cohort participants who underwent both echocardiography and 12-lead ECG during Visit 5 (on the same day) with echocardiographic images and ECG recordings of acceptable quality for analysis, and the ability to measure GEH. We excluded participants with reported race other than white or black (n=18), without echocardiographic examination (n=598), with atrial fibrillation at the time of echocardiography, with absent or uninterpretable ECGs (n=162), and those with missing covariates (n=272). The final study population included 5,114 participants with analyzable data on cardiac structure and function and ECG GEH. In addition, longitudinal analysis included all ARIC cohort participants (N=14,609) with measured GEH at visits 1–4, as reported previously6.
Definition of clinical covariates
For cross-sectional analysis, demographic and clinical characteristics were assessed at the time of the 5th visit. Coronary heart disease (CHD) was defined as a history of myocardial infarction (MI), a history of angina pectoris, or a history of coronary revascularization (either via coronary artery bypass surgery or percutaneous coronary intervention). At baseline (visit 1) history of MI was defined by participant self-report. At subsequent visits, it was defined as baseline history plus adjudicated events between baseline and the visit of interest. Heart failure (HF)11 was defined as evidence of symptomatic HF as defined by stage 3 of the Gothenburg criteria12 which required the presence of specific cardiac and pulmonary symptoms in addition to medical treatment of HF, self-reported use of HF medications, an adjudicated HF hospitalization (since 2005), or HF hospitalization with International Classification of Diseases-9th Revision, code 428× (before 2005). Hypertension was defined as a systolic blood pressure (BP; average between second and third measures) of ≥140 mmHg, a diastolic BP ≥ 90 mmHg, or physician-reported history of hypertension and use of anti-hypertensive medications. Diabetes mellitus (DM) was defined as a non-fasting glucose level of at least 200 mg/dL, a fasting (≥8 hours) glucose level of ≥126 mg/dL, a self-reported a physician diagnosis of DM, or the use of diabetic medications.
Concentrations of high sensitivity cardiac troponin T (hsTnT) and N-terminal pro–B-type natriuretic peptide (NT-proBNP) were measured as previously described13 at the 4th (1996–1998) and 5th (2011–2013) study visits.
For longitudinal analyses, we used time-updated clinical covariates: incident CHD, HF, diabetes, and hypertension. Follow-up of the study participants included annual telephone calls, local hospital surveillance, three triennial visits through 1998, and the 5th study visit (2011–2013), as previously reported10. Incident HF was diagnosed as first HF hospitalization with ICD-9 code 428.0–428.9 in any position of the hospital discharge list as well as characteristic signs and symptoms of congestion, characteristic chest X-ray findings, and/or assessment of left ventricular (LV) function14. Incident CHD was defined as a definite or probable MI, angina, or a coronary revascularization procedure6. Risk factors (BMI, smoking status, total cholesterol, high density lipoproteins (HDL), triglycerides, systolic and diastolic blood pressure) were assessed at each visit.
Assessment of global electrical heterogeneity and traditional ECG characteristics on ECG
GEH was quantified by five parameters, as previously described6 (Supplemental Figure 1). Spatial mean QRS-T angle was measured as the three-dimensional angle between the mean QRS- and T-vectors15. The magnitude and direction (azimuth and elevation) of the mean SVG vector (vectorial sum of the mean QRS- and the mean T-vectors) were determined6. SAI QRST was computed as the arithmetic sum of areas under the QRST curve on XYZ leads, with baseline defined as the voltage at the end of the T-wave16, 17. As SVG magnitude is largely independent of the specific ventricular activation sequence18, we did not exclude participants with ventricular conduction abnormalities. Sex- and race-specific thresholds were used to define abnormal GEH parameters, as previously described6.
Heart rate, corrected QT interval (QTc), and QRS duration were measured on the median beat by the General Electric (GE) 12SL algorithm (GE Marquette, Milwaukee, WI). Prolonged QTc was defined as exceeding 450 ms in men and 470 ms in women19. Sex-specific Cornell product was calculated for assessment of ECG-LVH ( > 2440 mm*ms)20. Presence of ventricular conduction abnormalities were defined by the Minnesota Code (MC)21 (left bundle branch block MC 7.1, right bundle branch block MC 7.2 and 7.3, fascicular block MC 7.6–7.7, bi-fascicular block MC 7.8), or ventricular-paced rhythm.
We measured GEH and traditional ECG parameters on all ECGs recorded during visits 1–5.
Assessment of cardiac structure and function on echocardiogram
Details of the echocardiographic imaging and analysis protocol have been thoroughly described previously22. All echocardiograms were acquired during Visit 5 with a dedicated machine (Phillips iE33 Ultrasound systems with Vision 2011) per a detailed acquisition protocol that included comprehensive two-dimensional, Doppler, tissue Doppler, and speckle-tracking echocardiography22. Heart rate and systolic and diastolic blood pressure were measured at the time of echocardiography. Dedicated and blinded analysts at the Brigham and Women’s Hospital Cardiac Imaging Core Laboratory performed quantitative measures on all echocardiograms according to American Society of Echocardiography (ASE) recommendations and standards23, 24. LV volumes and the LVEF were assessed using the modified Simpson rule.
Cardiac structure measurements included left-ventricular (LV) end-diastolic volume (LVEDV), LV end-systolic volume (LVESV), and LV mass index (LVMi). LV dimensions and mass were indexed to body surface area (BSA) as per ASE guidelines23, 24. As recommended, LVEDV index (LVEDVi) > 74 mL/m2 for men and > 61 mL/m2 for women and LVESV index (LVESVi) > 31 mL/m2 for men and > 24 mL/m2 for women were considered abnormal24. Echo-LVH was defined if LVMi exceeded 115 g/m2 in men and 95 g/m2 in women.24
Systolic LV function measurements included LV ejection fraction (LVEF) and global longitudinal LV strain (GLS) derived from speckle-tracking echocardiography. Speckle-tracking analysis was performed using Tom-Tec Cardiac Performance Analysis package (TomTec, Unterschleissheim, Germany). GLS was obtained from apical 4- and 2-chamber views, as previously described22. LVEF of less than 52% for men and less than 54% for women was defined as abnormal24. Peak GLS > −14% was considered abnormal24, 25.
Statistical analysis
All continuous variables are presented as means ± standard deviation (SD). Square root transformed SVG elevation and square root transformed absolute SVG azimuth were used in all adjusted analyses to normalize distribution of these two circular variables. Due to a U-shaped association between SVG magnitude and echocardiographic parameters, we squared SVG magnitude for its entry as a predictor in linear regression models.
Comparison of Visit 5 responders to alive Visit 5 nonresponders
To determine how ARIC study participants who participated in visit 5 (“Visit 5 responders”) are similar to alive ARIC study participants who did not participate in Visit 5 (“Visit 5 nonresponders”), we compared baseline (Visit 1) clinical characteristics of Visit 5 responders vs. Visit 5 nonresponders, using t-test and chi-squared test, as appropriate.
Cross-sectional analyses
ANOVA was used for unadjusted comparisons of LV structure and function in participants with abnormal GEH parameters. To assess continuous relationships between electrocardiographic and echocardiographic measures, we used multivariable linear regression models. Each ECG predictor variable was entered into a separate regression model. To answer whether cross-sectional associations between ECG and LV structure and function were mostly due to prevalent or subclinical CVD, we constructed multiple models. Model 1 was minimally adjusted for age, gender, and race. Model 2 was additionally adjusted for known confounders of cardiac structure and function26, 27: CHD, HF, hypertension, diabetes, BMI, smoking, presence of ventricular conduction abnormalities, as well as heart rate and systolic and diastolic blood pressure measured at the time of echocardiography. Model 3 was additionally adjusted for QRS duration. Standardized beta coefficients were used to compare the strength of the association across ECG predictors, reflecting the change of outcome variable per 1 SD of each ECG predictor variable increase. Subgroup analysis was performed in Model 2 to determine significant interactions between ECG parameters and clinical characteristics in their effect on LV structure and function. In addition, associations between ECG and echocardiographic parameters were also evaluated using fully adjusted (Model 2) multivariable linear regression models incorporating cubic splines with 4 knots.
Longitudinal analysis I: comparison of longitudinal ECG changes in participants who did vs. did not develop LVH, LV dilatation, and systolic dysfunction
To determine whether longitudinal changes in ECG parameters were associated with LV structure and function, we performed several longitudinal analyses. First, a t-test was used to compare differences in baseline (measured at visit 1) ECG parameters in participants with vs. without LV systolic dysfunction, dilatation, or hypertrophy, as diagnosed by echocardiography at visit 5 (median 24 years later). Next, we compared patterns of visit-to-visit changes in ECG parameters over time (from visit 1 to visit 5) in participants who did vs. did not develop LVH, LV dilatation, and systolic dysfunction. Generalized estimating equations (GEE) were used to fit population-averaged generalized linear models. Within-participant correlation structure was estimated as autoregressive of order 1. The canonical link function for the Gaussian family was used. Robust standard error was reported. Time-updated ECG variables served as continuous time-updated outcomes. Separate models were constructed for each ECG variable.
To answer the question of whether LVH, LV dilatation, and LV systolic dysfunction were associated with different patterns of ECG changes over time, these binary echocardiographic variables were entered in the models one-by-one. The pattern of dynamic ECG changes was described in each subgroup: in participants with and without LVH, LV dilatation, and systolic dysfunction. All linear GEE models were rigorously adjusted for time-updated confounders representing known pathways of myocardial injury. Model 1 was adjusted by gender and race. Model 2 was in addition adjusted for prevalent and incident HF and CHD. Model 3 was further adjusted for all time-updated covariates (diabetes, hypertension, BMI, current smoking status, levels of total cholesterol, HDL, triglycerides, systolic and diastolic blood pressure, heart rate, presence of ventricular conduction abnormalities, hsTnT and NTpro-BNP, age), and exact time interval (in days) between each ECG recording.
Longitudinal analysis II: comparison of ECG change patterns in healthy aging participants vs. those who developed LVH, LV dilatation, LV systolic dysfunction, or died due to SCD, non-sudden cardiac death, or non-cardiac death
We performed a comparison of longitudinal ECG changes in ARIC study participants who died due to SCD, non-sudden cardiac death, and non-cardiac death before the 5th visit, and those who survived and were diagnosed with LV systolic dysfunction, LVH, and LV dilatation on the 5th visit echocardiogram. For appropriate comparison, in survivors we used patterns of changes in ECG parameters from visit 1 to visit 4 (median 12 years). Linear GEE models were constructed in 3 subgroups of competing death outcomes6, and 5 LV dysfunction subgroups. All models were adjusted for sex and race.
Statistical analyses were performed using STATA MP 15.0 (StataCorp LP, College Station, TX). We applied the Bonferroni correction for multiple testing. As we tested 8 predictors and 5 outcomes, a P-value < 0.00125 (0.05/40) was considered statistically significant.
Results
Study population
Clinical characteristics of the study participants (obtained at visit 5) are shown in Table 1. Prevalent CHD and HF were infrequent, whereas CHD risk factors were common. LV systolic function and LV volumes were within the normal range in most participants. Approximately 10% of participants had ECG LVH. Abnormal GEH was observed more frequently than traditional ECG abnormalities: two thirds of participants had at least one abnormal GEH parameter, and 38% of participants had at least two abnormal GEH parameters. No participants had abnormal SVG magnitude.
Table 1.
Clinical characteristics of study participants at visit 5, and comparison of baseline characteristics of visit 5 responders vs. alive visit 5 non-responders
| Visit 5 responders (n=5,114) | Alive Visit 5 nonresponders (n=4,200) | |||
|---|---|---|---|---|
|
| ||||
| Parameter | Visit 5 characteristics | Visit 1 characteristics | Visit 1 characteristics | P-value |
| Age (SD), y | 75.9(5.1) | 51.7(5.0) | 54.1(5.7) | <0.0001 |
| Female gender, n (%) | 2,949(57.7%) | 2,949(57.7) | 2,562(61.0) | 0.001 |
| Black, n (%) | 1,099(21.5%) | 1,099(21.5) | 1,060(25.2) | <0.0001 |
| Body mass index (SD), kg/m2 | 28.5(5.5) | 26.9(4.6) | 27.8(5.4) | <0.0001 |
| Coronary heart disease, n (%) | 729(14.3%) | 87(1.7) | 127(3.1) | <0.0001 |
| Heart failure, n (%) | 741(14.5%) | 105(2.1) | 168(4.1) | <0.0001 |
| Hypertension, n (%) | 3,762(73.6%) | 1,180(23.2) | 1,410(33.7) | <0.0001 |
| Diabetes, n(%) | 1,583(31.0%) | 258(5.1) | 381(9.2) | <0.0001 |
| Current smoking, n (%) | 291(5.7%) | 895(17.5) | 1,021(24.3) | <0.0001 |
|
| ||||
| Systolic blood pressure (SD), mmHg | 130.3(17.8) | 116.0(15.6) | 121.1(17.9) | <0.0001 |
| Diastolic blood pressure (SD), mmHg | 66.5(10.5) | 72.6(10.3) | 73.5(11.0) | 0.0001 |
| Heart Rate (SD), bpm | 62.3(10.3) | 65.0(9.4) | 66.3(9.8) | <0.0001 |
|
| ||||
| Spatial QRS-T angle (SD),º | 71.2(36.5) | 58.5(25.3) | 58.1(26.8) | 0.509 |
| Abnormal6 Spatial QRS-T angle, n (%) | 1,940(37.9) | 1,105(21.6) | 971(23.1) | 0.081 |
| SVG magnitude (SD), mV | 1.44(0.47) | 1,725(458) | 1,693(476) | 0.002 |
| Abnormal6 SVG magnitude, n (%) | 0 | 2,496(48.8) | 1,875(44.6) | <0.0001 |
| SAI QRST (SD), mV*ms | 144.1(66.3) | 141.8(45.5) | 137.8(46.0) | <0.0001 |
| Abnormal6 SAI QRST, n (%) | 970(19.0) | 980(19.2) | 741(17.6) | 0.060 |
| sqrtSVG azimuth (SD) | 5.12(2.40) | 4.83(1.88) | 4.84(1.91) | 0.749 |
| Abnormal6 sqrtSVG azimuth, n (%) | 1,439(28.1) | |||
| sqrtSVG elevation (SD) | 8.64(1.16) | 8.06(0.96) | 8.11(0.99) | 0.016 |
| Abnormal6 sqrtSVG elevation, n (%) | 2,398(46.9) | |||
|
| ||||
| QRS duration (SD), ms | 96.1(21.2) | 91.6(10.9) | 91.2(11.8) | 0.105 |
| Abnormal ventricular conduction, n (%) | 696(13.6) | 146(2.9) | 156(3.7) | 0.020 |
| QTc (SD), ms | 424.4(22.7) | 413.2(16.4) | 416.4(19.9) | <0.0001 |
| Prolonged QTc, n (%) | 348(6.8) | 97(1.9) | 134(3.2) | <0.0001 |
| Sex-specific Cornell product (SD), mm*ms | 1625(895) | 1385(538) | 1443(565) | <0.0001 |
| ECG-Left Ventricular Hypertrophy, n (%) | 523(10.2) | 178(3.5) | 180(4.3) | 0.040 |
|
| ||||
| LVEF (SD), % | 65.3(6.6) | |||
| Abnormal LVEF, n (%) | 188(3.7) | |||
| GLS (SD), % | −17.9(2.6) | |||
| Abnormal GLS, n (%) | 356(7.0) | |||
| Left Ventrocuar Mass Index (SD), g/m2 | 79.8(20.8) | |||
| Echo- Left Ventricular Hypertrophy, n (%) | 561(11.0) | |||
| LVEDV index (SD), mL/m2 | 43.4(11.1) | |||
| Abnormal LVEDV index, n (%) | 117(2.3) | |||
| LVESV index (SD), mL/m2 | 15.4(6.7) | |||
| Abnormal LVESV index, n (%) | 170(3.3) | |||
Comparison of Visit 5 responders to alive Visit 5 nonresponders
Visit 5 nonresponders as compared to responders were older, were more likely to be female and black, and had higher rates of prevalent CVD and CVD risk factors (Table 1). Nevertheless, there were no significant differences in most baseline ECG GEH parameters between these two groups, with a few exceptions. SVG magnitude and SAI QRST were significantly smaller in Visit 5 nonresponders in spite of significantly longer QTc and larger Cornell product (Table 1).
Cross-sectional association of global ECG measures with LV structure and systolic function at study visit 5
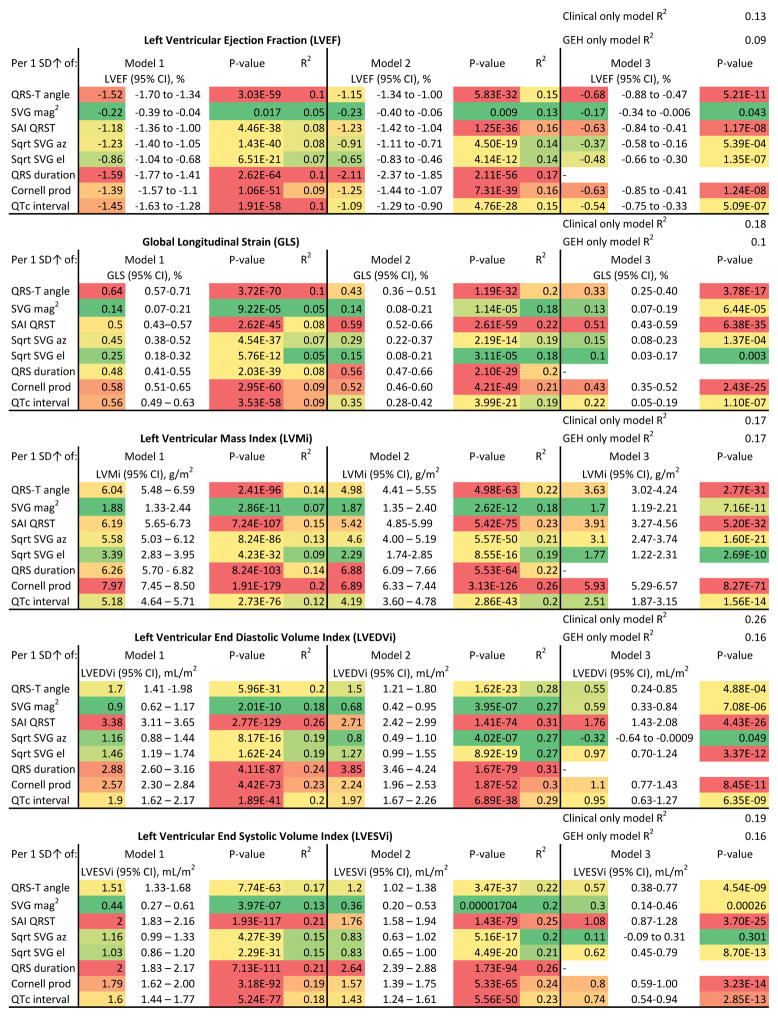
In unadjusted analyses, the number of abnormal GEH parameters was associated with LV systolic function, LV volumes and LV mass indices (Figure 1). In adjusted regression analyses, all ECG metrics at visit 5 were robustly associated with echocardiographic measures of cardiac structure and systolic function at visit 5, except SVG magnitude, which did not associate with LVEF (Figure 2). All GEH ECG variables were monotonically associated with echocardiographic metrics (Figure 3 and Supplemental Figures 2–5). Adjustment for prevalent and subclinical CVD only slightly attenuated the strength of these associations, suggesting an independent cross-sectional association between ECG GEH characteristics and LV structure and function, although the strength of associations ranged from negligible to weak (Figure 2). Further adjustment for QRS duration confirmed an independent association between GEH and LV structure and function, and revealed differences between the 5 GEH parameters. QRS duration fully explained the associations of SVG elevation with GLS and of SVG azimuth with LV volumes (Figure 2).
Figure 1. Cross-sectional analysis.
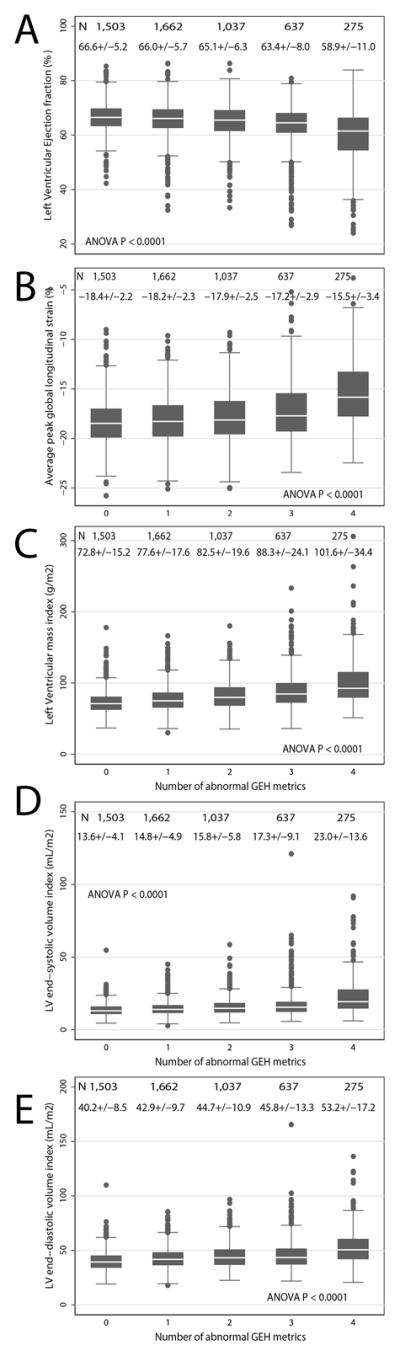
Boxplot of left ventricular ejection fraction (A), global longitudinal strain (B), left ventricular mass index (C), left ventricular end-systolic volume index (D), and left ventricular end-diastolic index (E) in participants with increasing number of abnormal global electrical heterogeneity parameters. Median (white horizontal line crossing the box) and interquartile range (IQR) (box) of each echocardiographic parameter are shown. Whiskers specify the adjacent values, defined as the most extreme values within 1.5 IQR of the nearer quartile.
Figure 2. Cross-sectional difference (95% CI) in left ventricular structure and function indices per 1 SD of ECG metric increase.
Model 1 was adjusted for age, gender, and race. Model 2 was additionally adjusted for CHD, HF, hypertension, diabetes, BMI, smoking, presence of ventricular conduction abnormalities, as well as heart rate and systolic and diastolic blood pressure measured at the time of echocardiography. Model 3 in addition was adjusted for QRS duration. Heat map color coded the most significant findings (largest effect size and R2, and smallest P-value) as red. Color transitioned to the least significant findings (smallest effect size and R2, and the largest / less significant P-value) as green.
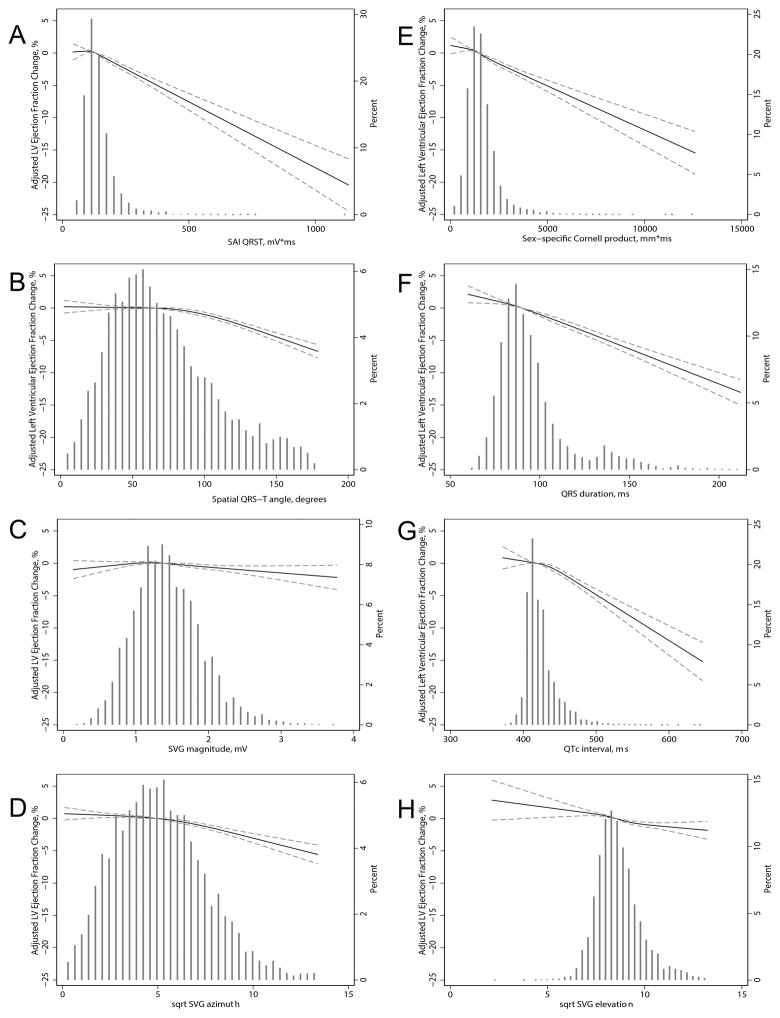
Figure 3. Cross-sectional analysis.
Fully adjusted (by age, sex, race, CHD, HF, hypertension, diabetes, BMI, smoking, presence of ventricular conduction abnormalities, heart rate, systolic and diastolic blood pressure) association between (A) SAI QRST, (B) spatial QRS-T angle, (C) SVG magnitude, (D) sqrt-SVG azimuth, (E) Cornell product, (F) QRS duration, (G) QTc interval, (H) sqrt-SVG elevation and LV ejection fraction. The models adjusted for age, sex, race, BMI, CHD, HF, hypertension, diabetes, smoking, and blood pressure. Restricted cubic spline with 95% confidence interval shows change in LVEF (Y-axis) in response to ECG predictor (X-axis); 50th percentile of predictor variable is selected as the reference.
Higher GEH was significantly associated with worse LV systolic function (Figure 2). For every 10 ms increase in QRS duration, LVEF (Figure 3) decreased by 1% (95%CI 0.9–1.1%). Every 100 mV*ms increase in SAI QRST was associated with a 2% (95%CI 1.6–2.1%) LVEF drop. The presence of ECG-LVH was associated with a 3% (95% CI 2.4–3.6%) LVEF reduction, and having 4 abnormal GEH parameters was associated with a 6.4% (95% CI 5.5–7.3%) LVEF decline. SAI QRST had a strong association with GLS (Supplemental Figure 2). Although each ECG measure separately explained a relatively small proportion (approximately 10%) of population-level variability of LV systolic function parameters, altogether, electrocardiographic and clinical characteristics were responsible for about 20% of systolic function variability (Figure 2).
Cross-sectional associations between GEH and LVMi were stronger than those between GEH and LV systolic function (Figure 2 and Supplemental Figure 3). Having 4 abnormal GEH metrics was associated with 24.2 (95%CI 21.5–26.9) g/m2 increase in LVMi, whereas having ECG-LVH was associated with only a 13.7 (95%CI 11.9–15.5) g/m2 increase in LVMi. For every 10 ms increase in QRS duration, LVMi increased by 3.2 (95%CI 2.9–3.6) g/m2. Altogether, ECG and clinical characteristics explained up to one quarter of LVMi variability.
SAI QRST strongly associated with LVEDVi, explaining 26% of LVEDVi variability (Figure 2). Altogether, clinical and ECG parameters accounted for approximately one third of LV volume variability (Supplemental Figures 4 and 5). Each 10 ms increase in QRS duration resulted in a 1.8 (95%CI 1.6–2.0) mL/m2 increase in the LVEDVi. Having 4 abnormal GEH parameters was associated with a 10.3 (95%CI 8.9–11.7) mL/m2 increase in LVEDVi, and a 7.8 (95%CI 6.9–8.6) mL/m2 increase in LVESVi.
Subgroups analyses
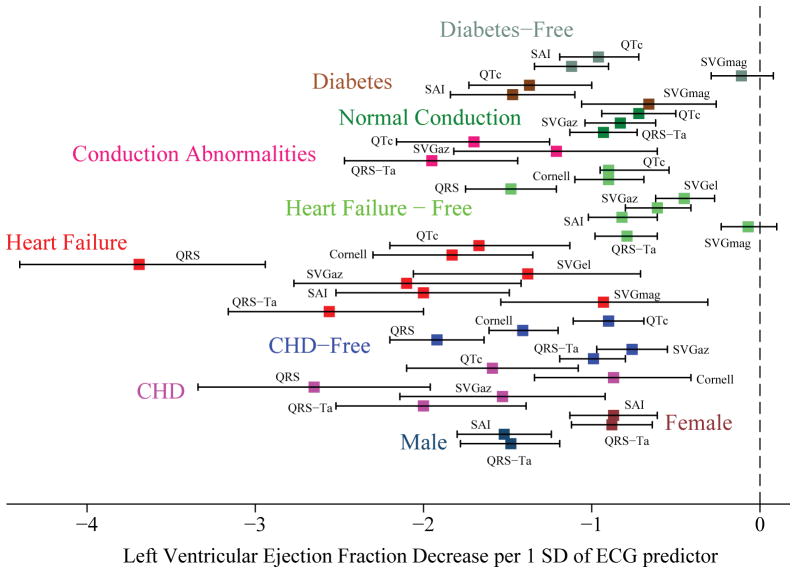
We observed numerous interactions between ECG parameters and clinical characteristics in their effect on LV echocardiographic measures of cardiac structure and function (Supplemental Table 1). Overall, the associations between ECG parameters and LV structure and function were stronger in the subgroups of participants with prevalent and subclinical CVD (Figure 4 and Supplemental Figures 6–8), with few exceptions. SAI QRST was more strongly associated with LVEDVi and LVESVi in hypertension-free participants and individuals with normal conduction, as compared to hypertensive participants and those with conduction abnormalities (Supplemental Table 1). Cornell product was more strongly associated with LVEF in CHD-free participants as compared to those with CHD (Figure 4).
Figure 4. Cross-sectional analysis.
Change (with 95% confidence interval) in LVEF per 1 standard deviation of ECG predictor in the study subgroups.
Association of longitudinal changes in ECG parameters over 24 years with LV structure and function
Measurement of visit 1 baseline ECG parameters was performed 23.6 ±1.0 years prior to the echocardiographic assessment of LV structure and function. Study participants with LV systolic dysfunction and abnormal LVESVi at visit 5 had wider (by 12 degrees) baseline QRS-T angles and larger (by 20 mV*ms) SAI QRST (Supplemental Table 2). SVG elevation (but not azimuth or magnitude) was also different ~24 years prior to the diagnosis of LV systolic dysfunction. Differences in QRS duration (3 ms) were statistically significant, but very small. Of note, baseline GEH parameters (especially SAI QRST) did not differ in individuals with vs. without echo-LVH which was diagnosed 24 years later. In contrast, baseline Cornell product was significantly larger in the subgroup with echo-LVH (Supplemental Table 2).
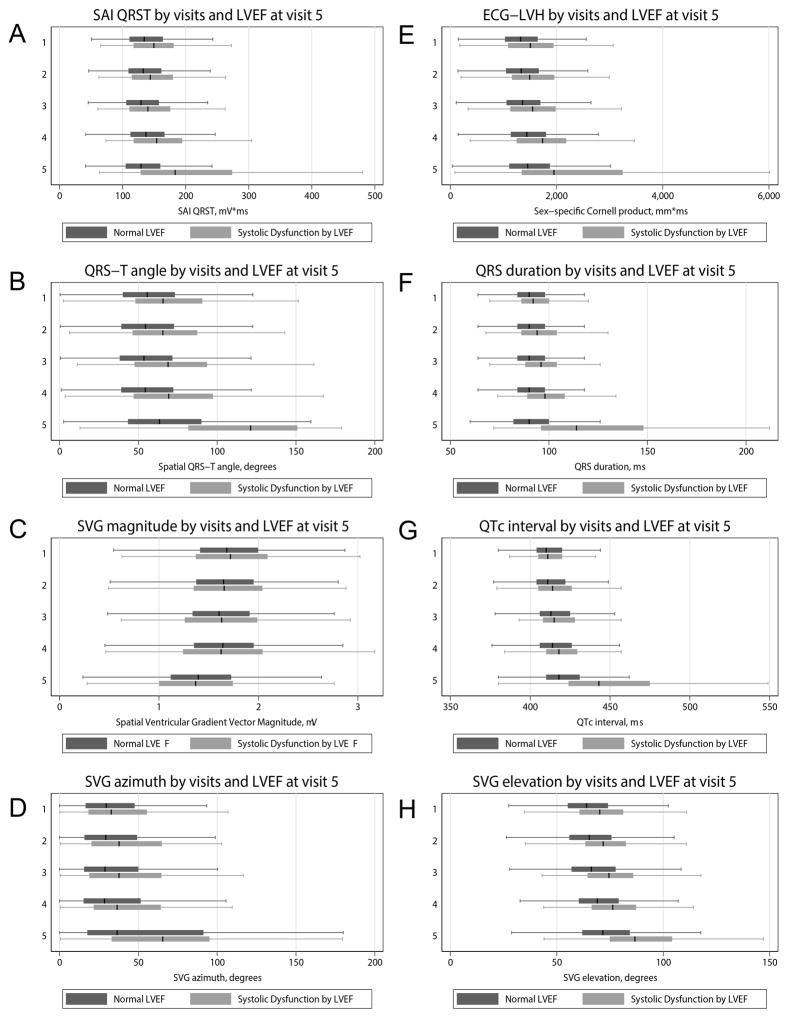
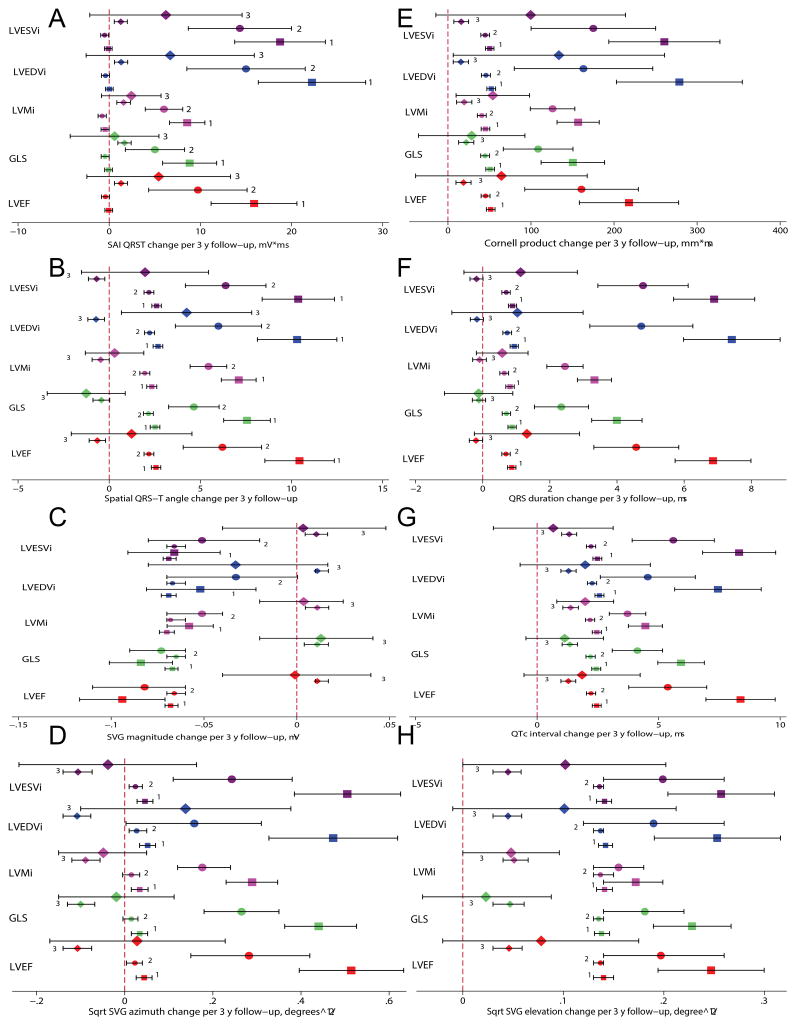
Unadjusted (Figure 5 and Supplemental Figures 9–12) and adjusted longitudinal analyses (Figure 6 and Supplemental Table 3) showed that patterns of longitudinal changes in all but one ECG parameter were significantly and dramatically different in participants with normal vs. abnormal LV structure and function. The pattern of temporal SVG magnitude changes, however, was an exception: it very slightly and monotonically decreased in all individuals. SAI QRST did not change in healthy aging participants. In contrast, SAI QRST increased on average by 20 mV*ms over each 3-year period in participants who demonstrated LV dilatation at visit 5. Similar patterns were confirmed for longitudinal changes of QRS-T angle (about 10 degrees/3-years), SVG azimuth and elevation, Cornell product (about 200 mm*ms/3-years), and QTc intervals (5–8 mm/3-years). Systolic dysfunction and LV dilatation were associated with larger longitudinal changes in GEH ECG parameters, whereas echo-LVH was associated with smaller longitudinal ECG parameter changes.
Figure 5. Longitudinal analysis.
Boxplot of (A) SAI QRST, (B) spatial QRS-T angle, (C) SVG magnitude, (D) sqrt-SVG azimuth, (E) Cornell product, (F) QRS duration, (G) QTc interval, (H) sqrt-SVG elevation at each study visit (1–5) in participants with and without abnormal LVEF, measured at visit 5.
Figure 6. Longitudinal analysis I (24 years follow-up).
Change (with 95% confidence interval) in (A) SAI QRST, (B) spatial QRS-T angle, (C) SVG magnitude, (D) sqrt-SVG azimuth, (E) Cornell product, (F) QRS duration, (G) QTc interval, (H) sqrt-SVG elevation per median 3 years, over 24 years of follow-up (visits 1–5) in participants with (large size mark) vs. without (medium size mark) abnormal LVEF (red), abnormal GLS (mint), echo-LVH (magenta), abnormal LVEDVi (blue), and abnormal LVESVi (purple). Model 1 (square) was adjusted by gender and race. Model 2 (circle) was in addition adjusted for prevalent and incident HF and CHD. Model 3 (diamond) was further adjusted for all time-updated covariates (diabetes, hypertension, BMI, current smoking status, total cholesterol, HDL, triglycerides, systolic and diastolic blood pressure, heart rate, presence of ventricular conduction abnormalities, hsTnT and NTpro-BNP, age), and exact time interval (in days) between each ECG recording.
Longitudinal ECG changes in participants who developed LV systolic dysfunction, LVH, and LV dilatation were fully explained by clinically manifest and subclinical CVD and subclinical myocardial injury (Figure 6 and Supplemental Table 3). In contrast, after rigorous adjustment, uniform (across participants) and statistically significant longitudinal changes in all but one ECG parameter were observed in survivors with normal LV function. Healthy aging was characterized by a slight decrease in QRS-T angle (1º/3-years) and SVG azimuth, and a slight increase in SAI QRST (1–2 mV*ms/3-years), Cornell product (20 mm*ms/3-years), QTc (1.3 ms/3-years), and SVG elevation and magnitude. The degree of heterogeneity in ECG changes across healthy aging participants was very small. QRS duration was an exception, however; after full adjustment, there were no statistically significant longitudinal trends in QRS duration in healthy aging participants (Figure 6). Longitudinal changes in QRS duration were fully explained by subclinical myocardial injury not only in LV dysfunction, but also in a healthy aging group.
Comparison of longitudinal ECG trends in participants with different modes of death and LV dysfunction over 12-years of follow-up
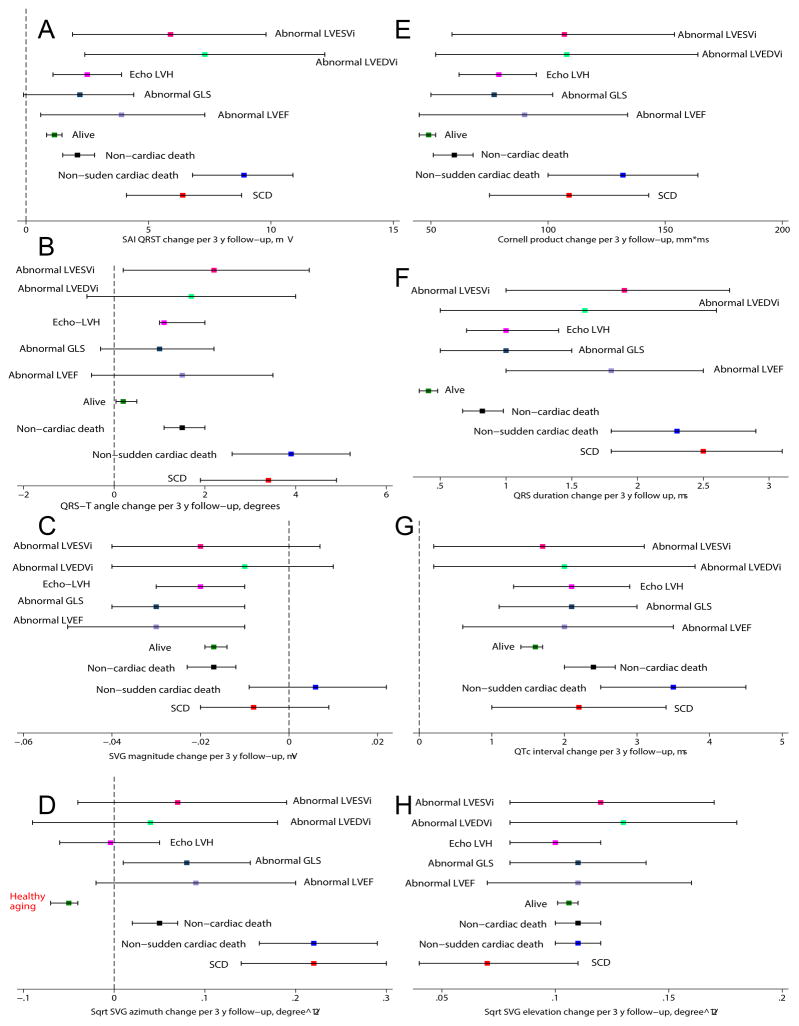
Longitudinal changes in ECG parameters in ARIC cohort participants with different causes of death, in comparison to surviving participants, (Figure 7 and Supplemental Figure 13) showed that cardiac death victims experienced the fastest GEH worsening (Supplemental Table 4). The pattern of ECG changes in SCD victims and victims of non-sudden cardiac death was similar to that as described above for individuals with LV dysfunction (Figure 6). The major difference between these subgroups was in the speed of GEH worsening; while SCD victims were characterized by rapid worsening of GEH over time, those who developed LV dysfunction demonstrated slow progression of GEH worsening. One additional distinct feature of healthy aging was the opposite direction of SVG azimuth changes over time (decreasing sqrt-SVG azimuth; Figure 7D). Due to shorter follow-up (median 12 years vs. 24 years), longitudinal analysis II had less statistical power compared to longitudinal analysis I, which explains the wider confidence intervals in Figure 7. However, point estimates reported by both longitudinal analyses were consistent.
Figure 7. Longitudinal analysis II (12 years follow-up).
Change (with 95% confidence interval) in (A) SAI QRST, (B) spatial QRS-T angle, (C) SVG magnitude, (D) sqrt-SVG azimuth, (E) Cornell product, (F) QRS duration, (G) QTc interval, (H) sqrt-SVG elevation per median 3 year, over 12 years of follow-up (visits 1–4) in participants with different outcomes: sudden cardiac death (red), non-sudden cardiac death ( blue), non-cardiac death (black), all alive (green), alive with abnormal LVEF (lavender), abnormal GLS (navy), echo-LVH (magenta ), abnormal LVEDVi (mint), abnormal LVESVi (pink).
Discussion
In this large bi-racial community-based cohort of elderly individuals, we demonstrated, in both cross-sectional and longitudinal analyses, that GEH was associated with echocardiographic measures of cardiac structure and function. These findings confirm the concept that electrophysiological properties in general, and the GEH phenotype specifically, reflect subclinical abnormalities in cardiac structure and function, and represents one mechanism by which abnormal electrophysiological substrate may lead to an increased risk of LV dysfunction and/or SCD. Together with clinical characteristics, GEH ECG parameters explained up to one third of variability in LV structure and function, confirming the utility of assessing cardiac electrophysiological properties to better understand cardiac electromechanical pump physiology. Clear differences in GEH observed nearly 25 years prior to diagnosed LV dysfunction, and distinct patterns of gradually increasing GEH were independently associated with LV systolic dysfunction, dilatation, and hypertrophy. Slowly worsening GEH parameters were associated with subsequent LV dysfunction, whereas SCD victims were characterized by rapidly worsening GEH parameters. Observing longitudinal changes in GEH over time offers opportunities for preventive interventions, and this should be further explored in future prospective clinical trials. Longitudinal GEH changes in participants with LV dysfunction are likely explained by underlying CVD and subclinical myocardial injury. Importantly, our study described a distinct pattern of longitudinal changes in SVG azimuth in healthy aging participants. Altogether, our study provided evidence of a potential vicious cycle of abnormal GEH leading to LV dysfunction, which in turn further exacerbates GEH abnormalities.
Vicious cycle of GEH and LV dysfunction
We observed a distinct longitudinal association of SVG direction (SVG azimuth) with normal LV function over a median of 25 years of follow-up in spite of the presence of traditional risk factors28. This observation supports the hypothesis of a primary effect (either favorable or unfavorable) of SVG direction on electromechanical pump function. If favorable, it diminishes the influence of traditional risk factors and potentially counteracts subclinical myocardial injury, preserving LV function. In the case of abnormal GEH it may facilitate development of LV dysfunction, promote deleterious effects of traditional cardiovascular risk factors and subclinical injury, and facilitate progression of CVD substrate. In turn, this progressive CVD substrate may worsen GEH. As we observed, the degree of GEH abnormalities were significantly larger in participants with CVD, and the strength of correlation between LV function measures and GEH measures was stronger in participants with LV dysfunction. Further study of the mechanism of SVG direction effect on cardiac electrophysiology is warranted.
GEH is a marker of early subclinical myocardial injury
We observed that adjustment for CVD, its risk factors, and subclinical myocardial injury (hs troponin T and proBNP) fully explained longitudinal trends in participants with developing LV dysfunction. Therefore, GEH can serve as a marker of subclinical myocardial injury. Previous studies have demonstrated a longitudinal association between hsTnI and SAI QRST29 in HF patients, which is consistent with our findings.
Differences between SCD and LV dysfunction in longitudinal trend of GEH progression
Our findings help to explain differences in the risk of SCD and the risk of progressive LV dysfunction. Rapidly worsening GEH is associated with increased SCD risk, whereas slowly worsening GEH is associated with increased risk of developing LV dysfunction. We previously reported an increased risk of SCD associated with large GEH changes over time6. This finding underscored importance of serial GEH monitoring, as one single measure of GEH might not be sufficient for discrimination of the risk of SCD from non-SCD.
GEH and LV systolic function
In our study, QRS duration had the strongest cross-sectional association with LVEF, whereas SAI QRST had the strongest independent association with GLS. Altogether, GEH explained approximately 10% of systolic function variability in cross-sectional analysis. An inverse relationship between QRS duration and systolic function has been reported in the Framingham Heart Study30. GLS is more sensitive than LVEF for detection of myocardial dysfunction and prediction of adverse cardiovascular outcomes.31, 32 Our findings confirm an association between global ECG measures (both traditional and novel) and LV systolic function. However, the weak-to-negligible strength of the association suggests that electrical and mechanical domains cannot be considered interchangeable, and should be viewed as complementary characteristics of cardiac electromechanical function. Longitudinally increasing QRS-T angle, SAI QRST, and changing SVG direction were strongly associated with LV systolic dysfunction. Early detection of subclinical cardiovascular disease is crucially important for successful prevention of overt CVD and adverse CV outcomes. Further studies are needed to assess how GEH changes in response to cardiovascular medications and life-style modifications. Additionally, in the future, automated GEH measurement can be easily implemented into routine clinical 12-lead ECG analysis, and this could enable widespread GEH monitoring for the early detection of abnormal electrophysiological substrate which might then trigger further testing or treatment.
GEH and cardiac structure
Several studies have shown a positive association between LV size (diameter, volumes, and length) and QRS duration30, 33, 34. In this study, SAI QRST, sex-specific Cornell product, and QRS duration had the strongest association with LV size and mass. Consistent with previous findings of QRS duration studies30, 33, 34, the strength of association between LV structure and GEH was moderate. Agreement between electrocardiographic left ventricular hypertrophy (ECG-LVH) and LV mass has been extensively studied35. ECG-LVH predicts cardiovascular outcomes independent of LV mass36–38. Our observation of a moderate association between GEH and LV mass and size supports the concept of independent electrophysiological substrate6, which only moderately correlates with LV size and mass.
Results of this study potentially explain previously reported discrepancies between ECG-LVH and LV mass35. We observed that the association between Cornell product and LVMi was significantly stronger in African Americans, as compared to whites, in younger participants, and in those with smaller BMI. These observed interactions should be considered in the interpretation of ECG-LVH. Of note, we observed that Cornell product was the strongest longitudinal predictor of LVMi, even if measured 25 years prior to observation of echo-LVH. Thus, a “false-positive” ECG-LVH could be considered as an early pre-clinical stage of LVMi increase. This hypothesis should be tested in a prospective study with longitudinal echocardiographic assessment.
Comparison of ECG parameters
Few prior studies have evaluated associations between QRS-T angle and cardiac structure and function.39, 40 This study is the first comprehensive cross-sectional and longitudinal evaluation of traditional and novel ECG parameters, uncovering differences between traditional ECG parameters and various GEH metrics.
Our study clarifies differences between SAI QRST and Cornell product. Both parameters incorporate QRS complex duration and voltage, and therefore, correlate with each other.6 However, this study demonstrated that in longitudinal analysis SAI QRST did not predict echo-LVH, whereas Cornell product did. Consistently, SAI QRST was more strongly associated with LV volumes in hypertension-free participants compared to hypertensive participants, suggesting that LVH confounds SAI QRST interpretation. In our study, SAI QRST was the strongest longitudinal predictor of LV dilatation. Prospective longitudinal study of LV volumes is needed to study longitudinal relationships between SAI QRST and LV geometry. The strong association of SAI QRST with LV volumes (and geometry of the heart) could be explained by the solid angle theorem41.
Across all studied ECG metrics, QRS duration stood out as the strongest cross-sectional marker of LV structure and systolic function (especially LVEF) in all participants, but especially in the subgroup with HF. At the same time, however, QRS duration, in contrast to all other studied ECG parameters, did not maintain an independent longitudinal trend over time. The patterns of longitudinal changes in QRS duration in both healthy aging and LV dysfunction subgroups was fully explained by CVD risk factors and subclinical myocardial injury. Therefore, QRS duration likely reflects the “end result” of the effect of myocardial injury on electrophysiological substrate, whereas, in contrast, GEH likely reflects the “cause” of myocardial injury. This hypothesis should be further tested in a longitudinal study of LV mechanical function. QRS duration and LVEF are excellent predictors of global and cardiac mortality, but the degree of increased risk for SCD in subjects with increased QRS duration and LVEF is similar to the magnitude of increased total cardiac mortality risk. If there were a mechanistic link between QRS duration/LVEF and SCD, patients with increased QRS duration/decreased LVEF should have risk for SCD increased out of proportion to their total cardiac mortality risk, which have not been shown42–44.
Strengths and Limitations
Several limitations of the study should be noted. Due to the nature of observational analyses, we cannot rule out the possibility of residual confounding. Although we conducted longitudinal ECG analyses, we were not able to conduct longitudinal analysis of the cardiac structure and function, and therefore we cannot determine the exact timing of LV dysfunction onset. Nevertheless, robust longitudinal analyses of ECG changes over time allowed us to characterize differences in longitudinal trends in individuals who experienced different outcomes, and for the first time to describe SVG direction changes over time, likely contributing to the maintenance of cardiovascular health in spite of exposure to CVD risk factors. Baseline clinical characteristics of ARIC Visit 5 responders and nonresponders differed significantly, which could also reduce the generalizability of the study findings. However, in spite of greater exposure to CVD risk factors at baseline, visit 5 responders and nonresponders had similar GEH parameters except for SVG magnitude and SAI QRST. Racial and gender differences in visit 5 responders vs. nonresponders reinforce the importance of enriching future studies of GEH with women and African Americans.
In summary, in a large cohort of middle-aged and elderly men and women we observed that although most echocardiographic measures of cardiac structure and function are in the range of normal values, increasing GEH over time is associated with higher LV mass and volume, and worsening LV systolic function. These associations are independent of demographic characteristics and prevalent and subclinical CVD. GEH is a strong and more sensitive longitudinal predictor of cardiac structure and function as compared to traditional global ECG parameters. Increasing GEH represents a potential mechanism of LV dysfunction. Continuous, long-term monitoring of GEH using ECG patches45 in the future may allow early identification of subclinical myocardial injury which potentially could assist in prompting timely interventions to improve patient outcomes. QRS duration stands out as the ECG marker having the strongest cross-sectional association with “today’s” LV structure and function, but it does not change over time in a clinically relevant way.
Supplementary Material
What is Known?
Myocardial global electrical heterogeneity (GEH) can be measured on a standard 12-lead ECG through vectorcardiographic parameters (spatial QRS-T angle, spatial ventricular gradient [SVG], and Sum-absolute QRST integral)
Abnormal increases in GEH parameters are independently associated with sudden cardiac death (SCD) in an adult community-dwelling population
What the Study Adds?
GEH reflects subclinical abnormalities in cardiac structure and function, and represents one mechanism by which abnormal electrophysiological substrate may lead to an increased risk of left ventricular (LV) dysfunction and SCD.
Healthy aging in spite of a life-long cardiovascular disease risk factor exposure is characterized by longitudinal changes in SVG azimuth, manifest as posterior rotation of the SVG vector.
Slow worsening of GEH over time is associated with subsequent LV dysfunction, whereas rapid worsening of GEH is associated with an increased risk of SCD.
Acknowledgments
The authors thank the staff and participants of the ARIC study for their important contributions.
Sources of Funding: The Atherosclerosis Risk in Communities study has been funded in whole or in part with Federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under Contract nos. (HHSN268201700001I, HHSN268201700003I, HHSN268201700005I, HHSN268201700004I, HHSN2682017000021). This work was supported by 1R01HL118277 (LGT).
Footnotes
Disclosures: None.
References
- 1.Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 2.Spinale FG, Fulbright BM, Mukherjee R, Tanaka R, Hu J, Crawford FA, Zile MR. Relation between ventricular and myocyte function with tachycardia-induced cardiomyopathy. Circ Res. 1992;71:174–87. doi: 10.1161/01.res.71.1.174. [DOI] [PubMed] [Google Scholar]
- 3.Huizar JF, Kaszala K, Potfay J, Minisi AJ, Lesnefsky EJ, Abbate A, Mezzaroma E, Chen Q, Kukreja RC, Hoke NN, Thacker LR, 2nd, Ellenbogen KA, Wood MA. Left ventricular systolic dysfunction induced by ventricular ectopy: a novel model for premature ventricular contraction-induced cardiomyopathy. Circ Arrhythm Electrophysiol. 2011;4:543–9. doi: 10.1161/CIRCEP.111.962381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sweeney MO, Prinzen FW. Ventricular Pump Function and Pacing: Physiological and Clinical Integration. Circulation: Arrhythmia and Electrophysiology. 2008;1:127–139. doi: 10.1161/CIRCEP.108.777904. [DOI] [PubMed] [Google Scholar]
- 5.Gopinathannair R, Etheridge SP, Marchlinski FE, Spinale FG, Lakkireddy D, Olshansky B. Arrhythmia-Induced Cardiomyopathies: Mechanisms, Recognition, and Management. J Am Coll Cardiol. 2015;66:1714–28. doi: 10.1016/j.jacc.2015.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Waks JW, Sitlani CM, Soliman EZ, Kabir M, Ghafoori E, Biggs ML, Henrikson CA, Sotoodehnia N, Biering-Sorensen T, Agarwal SK, Siscovick DS, Post WS, Solomon SD, Buxton AE, Josephson ME, Tereshchenko LG. Global Electric Heterogeneity Risk Score for Prediction of Sudden Cardiac Death in the General Population: The Atherosclerosis Risk in Communities (ARIC) and Cardiovascular Health (CHS) Studies. Circulation. 2016;133:2222–34. doi: 10.1161/CIRCULATIONAHA.116.021306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Waks JW, Tereshchenko LG. Global electrical heterogeneity: A review of the spatial ventricular gradient. J Electrocardiol. 2016;49:824–830. doi: 10.1016/j.jelectrocard.2016.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vassallo JA, Cassidy DM, Kindwall KE, Marchlinski FE, Josephson ME. Nonuniform recovery of excitability in the left ventricle. Circulation. 1988;78:1365–1372. doi: 10.1161/01.cir.78.6.1365. [DOI] [PubMed] [Google Scholar]
- 9.The ARIC Investigators. The Atherosclerosis Risk in Community (ARIC) Study: Design and Objectives. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 10.White AD, Folsom AR, Chambless LE, Sharret AR, Yang K, Conwill D, Higgins M, Williams OD, Tyroler HA. Community surveillance of coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) Study: methods and initial two years’ experience. J Clin Epidemiol. 1996;49:223–233. doi: 10.1016/0895-4356(95)00041-0. [DOI] [PubMed] [Google Scholar]
- 11.Rosamond WD, Chang PP, Baggett C, Johnson A, Bertoni AG, Shahar E, Deswal A, Heiss G, Chambless LE. Classification of heart failure in the atherosclerosis risk in communities (ARIC) study: a comparison of diagnostic criteria. Circulation Heart failure. 2012;5:152–9. doi: 10.1161/CIRCHEARTFAILURE.111.963199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eriksson H, Caidaul K, Larsson B, Ohlson L, Welin L, Wilhelmsen L, SVRDSUDDK Cardiac and pulmonary causes of dyspnoea? validation of a scoring test for clinical-epidemiological use: The Study of Men Born in 1913. European heart journal. 1987;8:1007–1014. doi: 10.1093/oxfordjournals.eurheartj.a062365. [DOI] [PubMed] [Google Scholar]
- 13.Nambi V, Liu X, Chambless LE, de Lemos JA, Virani SS, Agarwal S, Boerwinkle E, Hoogeveen RC, Aguilar D, Astor BC, Srinivas PR, Deswal A, Mosley TH, Coresh J, Folsom AR, Heiss G, Ballantyne CM. Troponin T and N-Terminal ProΓÇôB-Type Natriuretic Peptide: A Biomarker Approach to Predict Heart Failure RiskΓÇöThe Atherosclerosis Risk in Communities Study. Clinical Chemistry. 2013;59:1802–1810. doi: 10.1373/clinchem.2013.203638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsushita K, Blecker S, Pazin-Filho A, Bertoni A, Chang PP, Coresh J, Selvin E. The association of hemoglobin a1c with incident heart failure among people without diabetes: the atherosclerosis risk in communities study. Diabetes. 2010;59:2020–2026. doi: 10.2337/db10-0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oehler A, Feldman T, Henrikson CA, Tereshchenko LG. QRS-T Angle: A Review. Annals Noninvasive Electrocardiol. 2014;19:534–42. doi: 10.1111/anec.12206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sur S, Han L, Tereshchenko LG. Comparison of sum absolute QRST integral, and temporal variability in depolarization and repolarization, measured by dynamic vectorcardiography approach, in healthy men and women. PLoS One. 2013;8:e57175. doi: 10.1371/journal.pone.0057175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tereshchenko LG, Cheng A, Fetics BJ, Butcher B, Marine JE, Spragg DD, Sinha S, Dalal D, Calkins H, Tomaselli GF, Berger RD. A new electrocardiogram marker to identify patients at low risk for ventricular tachyarrhythmias: sum magnitude of the absolute QRST integral. J Electrocardiol. 2011;44:208–216. doi: 10.1016/j.jelectrocard.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.WILSON FN, Macleod AG, Barker PS, JOHNSTON FD. The determination and the significance of the areas of the ventricular deflections of the electrocardiogram. Am Heart J. 1934:46–61. [Google Scholar]
- 19.Moss AJ. Measurement of the QT interval and the risk associated with QTc interval prolongation: a review. Am J Cardiol. 1993;72:23b–25b. doi: 10.1016/0002-9149(93)90036-c. [DOI] [PubMed] [Google Scholar]
- 20.Molloy TJ, Okin PM, Devereux RB, Kligfield P. Electrocardiographic detection of left ventricular hypertrophy by the simple QRS voltage-duration product. Journal of the American College of Cardiology. 1992;20:1180–6. doi: 10.1016/0735-1097(92)90376-x. [DOI] [PubMed] [Google Scholar]
- 21.Prineas RJ, Crow RS, Blackburn HW. The Minnesota code manual of electrocardiographic findings : standards and procedures for measurement and classification. 2. London: Springer; 2010. [Google Scholar]
- 22.Shah AM, Cheng S, Skali H, Wu J, Mangion JR, Kitzman D, Matsushita K, Konety S, Butler KR, Fox ER, Cook N, Ni H, Coresh J, Mosley TH, Heiss G, Folsom AR, Solomon SD. Rationale and design of a multicenter echocardiographic study to assess the relationship between cardiac structure and function and heart failure risk in a biracial cohort of community-dwelling elderly persons: the Atherosclerosis Risk in Communities study. Circ Cardiovasc Imaging. 2014;7:173–81. doi: 10.1161/CIRCIMAGING.113.000736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, 3rd, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh JK, Popescu BA, Waggoner AD. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29:277–314. doi: 10.1016/j.echo.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 24.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39. e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 25.Takigiku K, Takeuchi M, Izumi C, Yuda S, Sakata K, Ohte N, Tanabe K, Nakatani S investigators J. Normal range of left ventricular 2-dimensional strain: Japanese Ultrasound Speckle Tracking of the Left Ventricle (JUSTICE) study. Circ J. 2012;76:2623–32. doi: 10.1253/circj.cj-12-0264. [DOI] [PubMed] [Google Scholar]
- 26.Nadruz W, Jr, Claggett B, Goncalves A, Querejeta-Roca G, Fernandes-Silva MM, Shah AM, Cheng S, Tanaka H, Heiss G, Kitzman DW, Solomon SD. Smoking and Cardiac Structure and Function in the Elderly: The ARIC Study (Atherosclerosis Risk in Communities) Circ Cardiovasc Imaging. 2016;9:e004950. doi: 10.1161/CIRCIMAGING.116.004950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goncalves A, Jhund PS, Claggett B, Shah AM, Konety S, Butler K, Kitzman DW, Rosamond W, Fuchs FD, Solomon SD. Relationship between alcohol consumption and cardiac structure and function in the elderly: the Atherosclerosis Risk In Communities Study. Circ Cardiovasc Imaging. 2015:8. doi: 10.1161/CIRCIMAGING.114.002846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soliman EZ, Zhang ZM, Chen LY, Tereshchenko LG, Arking D, Alonso A. Usefulness of Maintaining a Normal Electrocardiogram Over Time for Predicting Cardiovascular Health. Am J Cardiol. 2017;119:249–255. doi: 10.1016/j.amjcard.2016.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tereshchenko LG, Feeny A, Shelton E, Metkus T, Stolbach A, Mavunga E, Putman S, Korley FK. Dynamic Changes in High-Sensitivity Cardiac Troponin I Are Associated with Dynamic Changes in Sum Absolute QRST Integral on Surface Electrocardiogram in Acute Decompensated Heart Failure. Ann Noninvasive Electrocardiol. 2017:22. doi: 10.1111/anec.12379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dhingra R, Ho Nam B, Benjamin EJ, Wang TJ, Larson MG, D’Agostino RB, Sr, Levy D, Vasan RS. Cross-sectional relations of electrocardiographic QRS duration to left ventricular dimensions: The Framingham Heart Study. Journal of the American College of Cardiology. 2005;45:685–689. doi: 10.1016/j.jacc.2004.11.046. [DOI] [PubMed] [Google Scholar]
- 31.Kalam K, Otahal P, Marwick TH. Prognostic implications of global LV dysfunction: a systematic review and meta-analysis of global longitudinal strain and ejection fraction. Heart. 2014;100:1673–80. doi: 10.1136/heartjnl-2014-305538. [DOI] [PubMed] [Google Scholar]
- 32.Smiseth OA, Torp H, Opdahl A, Haugaa KH, Urheim S. Myocardial strain imaging: how useful is it in clinical decision making? Eur Heart J. 2016;37:1196–207. doi: 10.1093/eurheartj/ehv529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chan DD, Wu KC, Loring Z, Galeotti L, Gerstenblith G, Tomaselli G, Weiss RG, Wagner GS, Strauss DG. Comparison of the relation between left ventricular anatomy and QRS duration in patients with cardiomyopathy with versus without left bundle branch block. Am J Cardiol. 2014;113:1717–22. doi: 10.1016/j.amjcard.2014.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stewart RA, Young AA, Anderson C, Teo KK, Jennings G, Cowan BR. Relationship between QRS duration and left ventricular mass and volume in patients at high cardiovascular risk. Heart. 2011;97:1766–70. doi: 10.1136/heartjnl-2011-300297. [DOI] [PubMed] [Google Scholar]
- 35.Jain A, Tandri H, Dalal D, Chahal H, Soliman EZ, Prineas RJ, Folsom AR, Lima JA, Bluemke DA. Diagnostic and prognostic utility of electrocardiography for left ventricular hypertrophy defined by magnetic resonance imaging in relationship to ethnicity: the Multi-Ethnic Study of Atherosclerosis (MESA) Am Heart J. 2010;159:652–658. doi: 10.1016/j.ahj.2009.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patel N, O’Neal WT, Whalen SP, Soliman EZ. Electrocardiographic left ventricular hypertrophy predicts atrial fibrillation independent of left ventricular mass. Ann Noninvasive Electrocardiol. 2017;22:1–5. doi: 10.1111/anec.12419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Almahmoud MF, O’Neal WT, Qureshi W, Soliman EZ. Electrocardiographic Versus Echocardiographic Left Ventricular Hypertrophy in Prediction of Congestive Heart Failure in the Elderly. Clin Cardiol. 2015;38:365–70. doi: 10.1002/clc.22402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bacharova L, Chen H, Estes EH, Mateasik A, Bluemke DA, Lima JA, Burke GL, Soliman EZ. Determinants of discrepancies in detection and comparison of the prognostic significance of left ventricular hypertrophy by electrocardiogram and cardiac magnetic resonance imaging. Am J Cardiol. 2015;115:515–22. doi: 10.1016/j.amjcard.2014.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Selvaraj S, Ilkhanoff L, Burke MA, Freed BH, Lang RM, Martinez EE, Shah SJ. Association of the frontal QRS-T angle with adverse cardiac remodeling, impaired left and right ventricular function, and worse outcomes in heart failure with preserved ejection fraction. J Am Soc Echocardiogr. 2014;27:74–82. e2. doi: 10.1016/j.echo.2013.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mewton N, Strauss DG, Rizzi P, Verrier RL, Liu CY, Tereshchenko LG, Nearing B, Volpe GJ, Marchlinski FE, Moxley J, Killian T, Wu KC, Spooner P, Lima JA. Screening for Cardiac Magnetic Resonance Scar Features by 12-Lead ECG, in Patients with Preserved Ejection Fraction. Ann Noninvasive Electrocardiol. 2016;21:49–59. doi: 10.1111/anec.12264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Holland RP, Arnsdorf MF. Solid angle theory and the electrocardiogram: physiologic and quantitative interpretations. Prog Cardiovasc Dis. 1977;19:431–57. doi: 10.1016/0033-0620(77)90009-3. [DOI] [PubMed] [Google Scholar]
- 42.Tereshchenko LG, Cheng A, Fetics BJ, Marine JE, Spragg DD, Sinha S, Calkins H, Tomaselli GF, Berger RD. Ventricular arrhythmia is predicted by sum absolute QRST integralbut not by QRS width. J Electrocardiol. 2010;43:548–52. doi: 10.1016/j.jelectrocard.2010.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buxton AE, Sweeney MO, Wathen MS, Josephson ME, Otterness MF, Hogan-Miller E, Stark AJ, Degroot PJ. QRS duration does not predict occurrence of ventricular tachyarrhythmias in patients with implanted cardioverter-defibrillators. J Am Coll Cardiol. 2005;46:310–316. doi: 10.1016/j.jacc.2005.03.060. [DOI] [PubMed] [Google Scholar]
- 44.Dhar R, Alsheikh-Ali AA, Estes NA, III, Moss AJ, Zareba W, Daubert JP, Greenberg H, Case RB, Kent DM. Association of prolonged QRS duration with ventricular tachyarrhythmias and sudden cardiac death in the Multicenter Automatic Defibrillator Implantation Trial II (MADIT-II) Heart Rhythm. 2008;5:807–813. doi: 10.1016/j.hrthm.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kabir MM, Perez-Alday EA, Thomas J, Sedaghat G, Tereshchenko LG. Optimal configuration of adhesive ECG patches suitable for long-term monitoring of a vectorcardiogram. J Electrocardiol. 2017;50:342–348. doi: 10.1016/j.jelectrocard.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.