Abstract
Objectives
Neck failure in patients with oral squamous cell carcinoma (OSCC) carries a poor outcome, yet the management of patients who initially present with clinically node-negative (cN0) neck is not clearly defined.
Patients and Methods
Retrospective review of patients with cN0 OSCC treated at Memorial Sloan Kettering Cancer Center from 1985 to 2012, focusing on rate, pattern and predictors of neck failure, salvage treatment, and survival outcomes.
Results
Of 1,302 patients, 806 (62%) underwent elective neck dissection (END) and 496 (38%) had observation. 190 patients (15%) developed neck recurrence. Median follow-up was 58.5 months (range 1–343); 5-year neck recurrence-free survival (NRFS) was 85% and 80% for the END and observation group respectively (p = 0.06). Patients with neck failure had poorer outcomes than patients without neck failure (5-year overall survival, 37% vs. 74% [p < 0.001]; disease-specific survival [DSS], 41% vs. 91% [p < 0.001]). Independent predictors of neck failure were smoking, primary tumor subsite (hard palate and upper gum), and extranodal extension. 87% of patients underwent salvage treatment (END: 81.1%; observation: 94%). Salvage surgery with adjuvant (chemo) radiation had better DSS than surgery alone or nonsurgical salvage.
Conclusions
In our cohort of patients with initially cN0 OSCC triaged to END vs. observation using clinical parameters, 15% developed neck failure. Salvage treatment was feasible in most cases but survival was poorer compared to patients without neck failure. Surgery followed by adjuvant (chemo) radiation resulted in the best outcome.
Keywords: oral squamous cell carcinoma, elective neck dissection, observation, neck recurrence, salvage surgery, radiotherapy
INTRODUCTION
The incidence of oral squamous cell carcinoma (OSCC) has gradually increased during the last 4 decades [1]. Despite advances in surgical techniques, radiation therapy (RT), and systemic agents, 5-year overall survival (OS) has plateaued at 65% [2]. With the exception of prevention measures and early-detection educational initiatives, there have been no major changes in the management of patients with OSCC, which is primarily surgical [3, 4]. Disease-specific survival (DSS) and OS, are, in large part, dependent on achieving local and regional control [5]. Regional recurrence in patients who present with clinically node-negative (cN0) neck is uncommon but carries a poor prognosis [6]. Several prognostic factors, including primary tumor (T) stage, tumor depth, perineural invasion (PNI), lymphovascular invasion (LVI), and aggressive tumor histological subtype, have been associated with an increased risk of neck recurrence [7, 8]. Other factors, such as the subsite of the primary tumor within the oral cavity, have not been adequately addressed and may be significant [9].
Neck recurrences are typically treated with salvage surgery, with or without adjuvant therapy [10]. This salvage treatment must be individually tailored according to the extent of the neck recurrence, the feasibility of surgical resection, and the functional and aesthetic consequences of resection, in addition to host factors that may be relevant. In some cases, achieving regional control may be challenging, especially in the setting of previous RT or chemoradiotherapy (CRT).
We reviewed our 27-year experience in neck management in patients with OSCC who initially presented with cN0 neck. Specifically, we sought to determine the incidence and pattern of neck recurrence in our cohort, the effect of neck recurrence on survival, the clinical and pathological factors predictive of neck recurrence, the rate and type of salvage treatment used, and lastly, the outcomes following salvage treatment.
PATIENTS AND METHODS
After approval was obtained from the Institutional Review Board at Memorial Sloan Kettering Cancer Center, 1,302 patients with cN0 neck were identified from a cohort of 1,866 patients who underwent surgical resection for OSCC from 1985 to 2012.
Clinical and pathological characteristics were recorded. Clinical factors consisted of sociodemographic characteristics (sex, age, smoking status, and alcohol consumption), tumor subsites, and American Joint Committee on Cancer (AJCC) clinical stage. The oral cavity subsites were categorized as buccal mucosa, floor of mouth, hard palate (HP), lower gum, retromolar trigone, tongue, and upper gum (UG). Pathological factors included histological grade, PNI, (LVI), margins, tumor thickness, AJCC pathological T (pT) stage, pathological N (pN) stage, and extra capsular spread (ECS). Salvage treatment was defined as any potentially curative treatment, surgical or nonsurgical, given to a patient following a recurrence in the neck. The salvage rate refers to the percentage of patients who received the salvage treatment.
This study included three survival endpoints of interest: neck recurrence-free survival (NRFS), DSS, and OS, which were calculated from the date of initial curative surgery. Salvage DSS was calculated from the date of neck recurrence. NRFS, DSS, and salvage DSS were censored at the last recorded date of disease assessment. OS was censored at the last date a patient was known to be alive, regardless of disease status. A neck recurrence event was documented only if proven by biopsy. A death was considered an event for DSS if the patient was known to have active disease at the last disease assessment before death. Death from any cause was considered an event for OS.
Associations were identified by using the chi-square test. Univariate survival analysis was performed using the Kaplan-Meier method, and the log-rank test was used to determine significance. A p-value less than 0.05 were considered statistically significant. Multivariable analysis was performed using Cox proportional hazard regression.
RESULTS
Clinical and pathological characteristics and initial management
Table 1 shows the clinical and pathological characteristics of patients, stratified by modality of neck management. Of the 1,302 patients with cN0 necks, (55%) were males, and the median age was 63 years (range, 15–96 years). The most common tumor subsite was the tongue (53%). Elective neck dissection (END) was carried out in 806 patients (62%). The remaining 496 patients (38%) were selected for observation based on the following criteria: (1) patients with small T1 tumors < 2 mm in thickness, according to preoperative clinical and radiological evaluation; (2) elderly patients with medical comorbidities who have substantially increased risk of severe postoperative complications from more extensive surgery; and (3) patients with tumors of the HP and UG, in whom the risk of occult neck metastases was traditionally thought to be low and who therefore underwent observation of the cN0 neck irrespective of T stage.
Table 1.
Clinical and pathological characteristics of patients with clinically negative neck nodes, stratified by neck management
| VARIABLE | OBSERVED (N=496) N (%) |
ELECTIVE NECK DISSECTION (N=806) N (%) |
P |
|---|---|---|---|
| Sex | 0.037 | ||
| Female | 243 (49) | 347 (43) | |
| Male | 253 (51) | 459 (57) | |
| Age, years | < 0.001 | ||
| <60 | 169 (34) | 382 (47) | |
| ≥60 | 327 (66) | 424 (53) | |
| Alcohol | 0.277 | ||
| Never | 154 (31) | 227 (28) | |
| Ever | 340 (69) | 574 (71) | |
| Not Knowna | 2 (0.4) | 5 (0.6) | |
| Tobacco | 0.872 | ||
| Never | 175 (35) | 280 (35) | |
| Ever | 320 (65) | 522 (65) | |
| Not Knowna | 1(0.2) | 4 (0.4) | |
| cT stage | < 0.001 | ||
| T1 | 300 (61) | 264 (33) | |
| T2 | 129 (26) | 380 (47) | |
| T3 | 18 (4) | 70 (9) | |
| T4 | 30 (6) | 85 (11) | |
| Not Knowna | 19 (4) | 7 (1) | |
| Site | < 0.001 | ||
| Buccal Mucosa | 29 (6) | 51 (6) | |
| Floor of Mouth | 65 (13) | 108 (13) | |
| Hard Palate | 34 (7) | 2 (0.2) | |
| Lower Gum | 39 (8) | 127 (16) | |
| Retromolar Trigone | 14 (3) | 48 (6) | |
| Tongue | 224 (45) | 466 (21) | |
| Upper Gum | 91 (18) | 4 (0.4) | |
| LVI | < 0.001 | ||
| No | 294 (59) | 517 (64) | |
| Yes | 19 (4) | 87 (11) | |
| Not Knowna | 183 (37) | 202 (25) | |
| PNI | < 0.001 | ||
| No | 283 (57) | 406 (50) | |
| Yes | 30 (6) | 198 (25) | |
| Not Knowna | 183 (37) | 202 (25) | |
| Grade | < 0.001 | ||
| Well | 157 (32) | 146 (18) | |
| Moderate | 221 (45) | 529 (66) | |
| Poor | 26 (5) | 102 (13) | |
| Not Knowna | 92 (19) | 29 (4) | |
| pT Stage | < 0.001 | ||
| T1 | 310 (63) | 435 (54) | |
| T2 | 62 (13) | 204 (25) | |
| T3 | 9 (2) | 33 (4) | |
| T4 | 39 (8) | 99 (12) | |
| Not Knowna | 76 (15) | 35 (4) | |
| Adjuvant RT | 7% | 35% |
cT: clinical T stage; LVI: lymphovascular invasion; PNI: perineural invasion; pT: pathological T stage; RT: radiation therapy
not included in chi square analysis.
Clinical and pathological characteristics of patients, stratified by adjuvant treatment are shown in Supplementary Table 1. As expected, patients who underwent END were more likely to have adverse pathological factors, such as LVI, PNI, higher histological grade, and higher pT stage. In the END group, 282 patients (35.0%) received adjuvant RT or CRT after primary surgery. Thirty-five patients (7.1%) in the observation group received adjuvant RT or CRT after surgery of the primary site. In accordance with National Comprehensive Cancer Network guidelines, the RT field in patients with adverse factors included both the primary site and the neck [11].
Incidence of neck recurrence
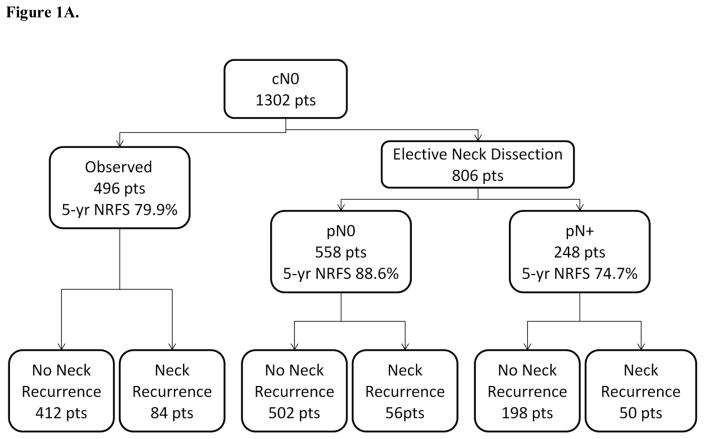
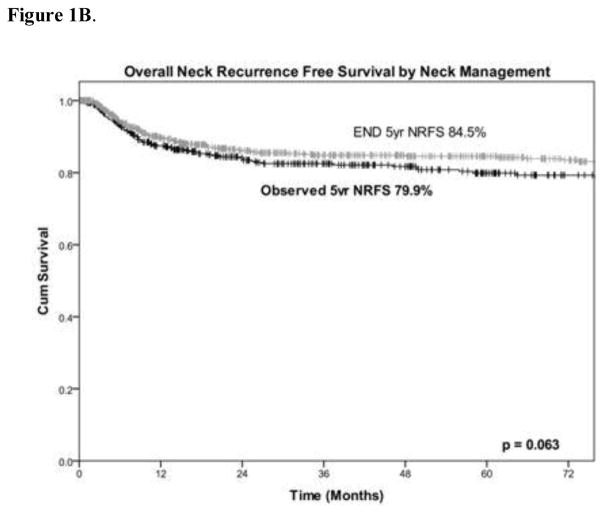
Figure 1A shows the incidence of neck recurrence, stratified by management of the cN0 neck. 190 patients (15%) had neck recurrence. The overall 5-year NRFS was 83%. NRFS trended towards being better in the ED group compared to the observation group, but this was not statistically significant (85% vs. 80% [p = 0.063]; Figure 1B). The median time to neck recurrence was 8.3 months. Among the patients in the END group, those who were pN+ had the highest rate of neck recurrence (50 of 248 [20%]), with a 5-year NRFS of 75%, compared with those who were pN− (56 of 558 [10%]), with a 5-year NRFS of 89%. Among the patients in the observation group, the rate of neck recurrence was 17% (84 of 496), with a 5-year NRFS of 80%.
Figure 1.
(A) Initial management and outcomes of all patients, described by a flow diagram and (B) a Kaplan-Meier curve of neck recurrence–free survival.
Location and pattern of neck recurrence
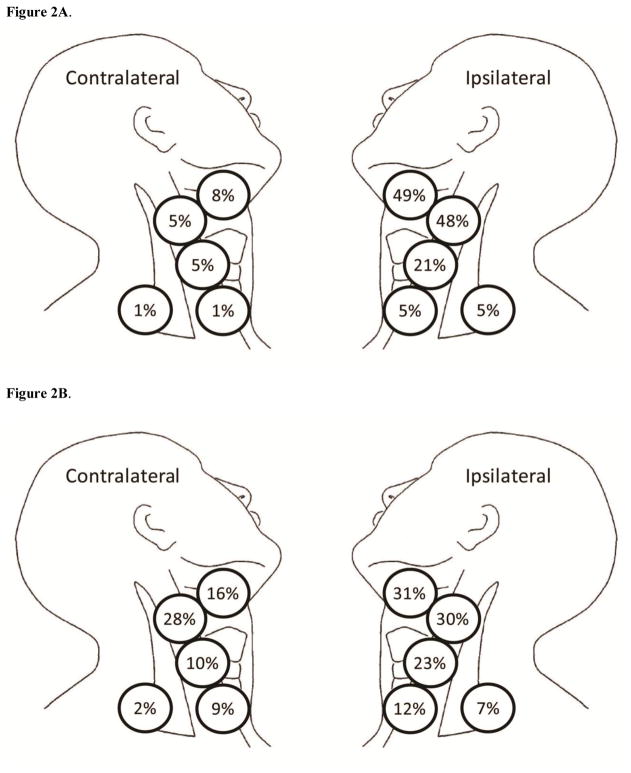
Figure 2 shows the pattern of neck recurrence in the observation group (Figure 2A) and the END group (Figure 2B), with each percentage representing the proportion of patients with a recurrence in a specific level of the neck. In the observation group, 76 of 84 patients (90%) had an ipsilateral neck recurrence, and 12 patients (14%) had a contralateral neck recurrence; neck levels I and II were most commonly affected (49% and 48%, respectively, in the ipsilateral neck). In the END group, 73 of 106 patients (69%) had an ipsilateral neck recurrence, representing in-field recurrences. However, there were more recurrences in the contralateral neck (which represents out-of-field recurrences) among patients in the END group (41/106; 39%) than in the observation group. Again, neck levels I and II were the most commonly affected in both the ipsilateral and contralateral neck recurrence groups. Among patients with midline primary tumors (n = 66), 9 developed neck recurrence, 5 of which were in the END group.
Figure 2.
(A) Anatomical illustration showing location and pattern of neck recurrence in patients in the observation and (B) elective neck dissection groups. Each percentage represents the percentage of patients with recurrence who recurred in that specific level of the neck.
Effect of neck recurrence on survival
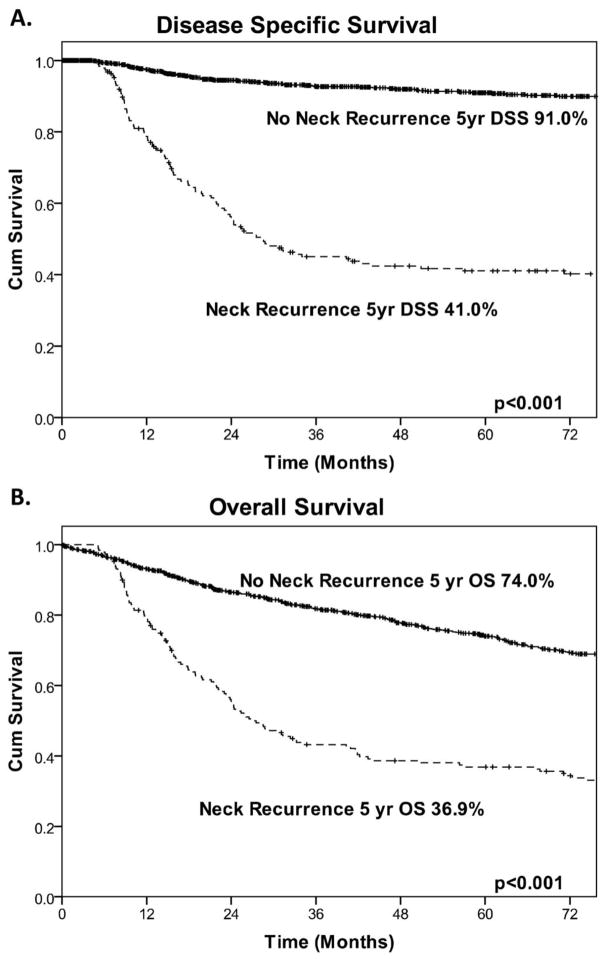
The median follow-up time was 58.5 months. The 5-year OS and DSS for all patients was 68% and 82%, respectively. Patients who developed neck recurrence had poorer outcomes than patients who did not (Figure 3; - 5-year OS, 37% vs. 74% [p < 0.001]; DSS, 41% vs. 91% [p < 0.001]). When stratified by neck management, patients in the observation group who developed neck recurrence had the best 5-year DSS (56%, Supplementary Figure 1A). Patients in the END group with pN− who developed neck recurrence had a DSS of 45% (Supplementary Figure 1B). In contrast, patients in the END group with pN+ who developed neck recurrence had the worst 5-year DSS (11%, Supplementary Figure 1C).
Figure 3.
Kaplan-Meier curves demonstrating the effect of neck recurrence on (A) disease-specific survival (DSS) and (B) overall survival (OS)
Factors predictive of neck recurrence on univariate and multivariable analyses
Table 2 shows the clinical factors predictive of overall neck NRFS. Only tumor subsite and tobacco use were predictive of neck recurrence on multivariable analysis (p = 0.029 and p = 0.014, respectively). Patients with HP and UG tumors had the highest risk of neck recurrence (hazard ratio, 2.63 and 2.33, respectively) and the worst outcomes (5-year overall NRFS, 70% and 71%, respectively), compared with patients with other tumor subsites. One likely explanation for this finding is that these two groups mostly included patients who underwent observation (94% and 96%, respectively) and/or had high pT stage tumors (3 patients were pT3, and 39 patients were pT4) (Supplementary Figure 2). 21 patients with HP and UG tumors were upstaged following surgery: 18 patients from cT1/T2 to pT4, and 3 patients from cT3 to pT4.
Table 2A.
Overall NRFS by clinical factors
| Variable | Patients (N = 1302) | Univariate Analysisb | Multivariate Analysisc | ||
|---|---|---|---|---|---|
|
| |||||
| 5-year NRFS, % | P | Hazard Ratio (CI) | P | ||
| Sex | 0.564 | ||||
| Female | 590 | 82.7 | |||
| Male | 712 | 82.6 | |||
| Age | 0.169 | ||||
| <60 | 551 | 85.2 | |||
| ≥60 | 751 | 80.8 | |||
| Alcohol | 0.148 | ||||
| Never | 381 | 80.3 | |||
| Ever | 914 | 83.7 | |||
| Not Knowna | 7 | ||||
| Tobacco | 0.011 | 0.029 | |||
| Never | 455 | 79.0 | Reference | ||
| Ever | 842 | 84.8 | 0.72 (0.53–0.97) | ||
| Not Knowna | 5 | ||||
| Site | 0.006 | 0.014 | |||
| Retromolar Trigone | 62 | 93.8 | Reference | ||
| Lower Gum | 166 | 89.2 | 1.54 (0.51–4.62) | ||
| Buccal Mucosa | 80 | 91.2 | 1.64 (0.49–5.45) | ||
| Tongue | 690 | 81.6 | 2.28 (0.84–6.21) | ||
| Floor of Mouth | 173 | 83.5 | 2.53 (0.88–7.34) | ||
| Upper Gum | 95 | 70.9 | 3.82 (1.32–11.06) | ||
| Hard Palate | 36 | 69.7 | 4.30 (1.34–13.80) | ||
| Nodal Dissection | 0.063 | ||||
| None | 496 | 79.9 | |||
| Elective | 806 | 84.5 | |||
NRFS: neck recurrence-free survival; CI: confidence interval.
not included in analyses
Kaplan Meier
Cox proportional hazard
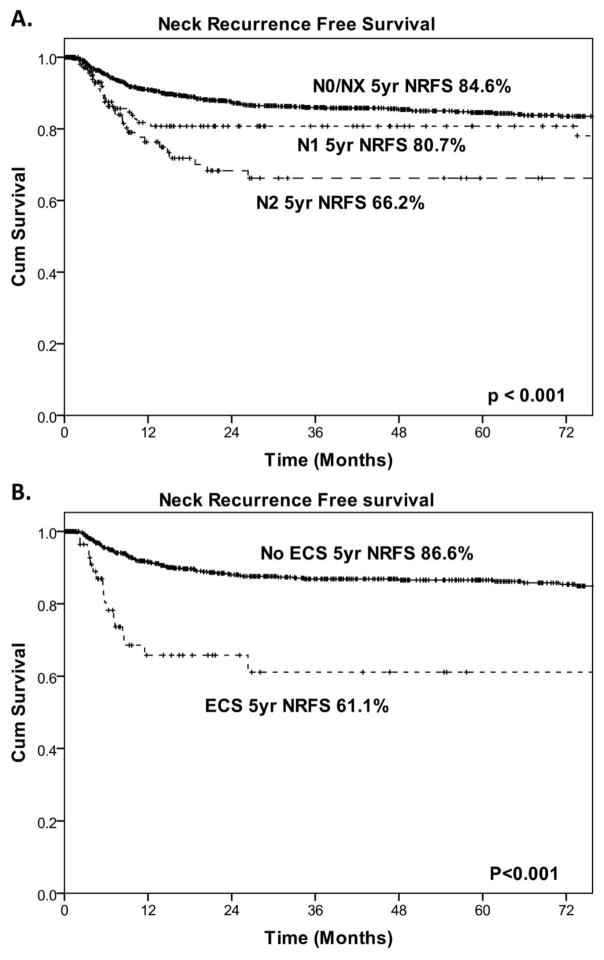
Table 3 shows the pathological factors that were predictive of overall NRFS. On univariate analysis of the entire cohort, histological grade, PNI, LVI, tumor thickness ≥ 4 mm, and pN status were significant predictors of NRFS (Figure 5). However, when all variables were included in a multivariable model, none of the variables were independently predictive of NRFS. On further evaluation of patients in the END group only, all pathological variables, as well as ECS, were significantly predictive of NRFS on univariate analysis. On multivariable analysis, the only significant independent predictor of NRFS was ECS (Table 4 and Figure 4).
Figure 5.
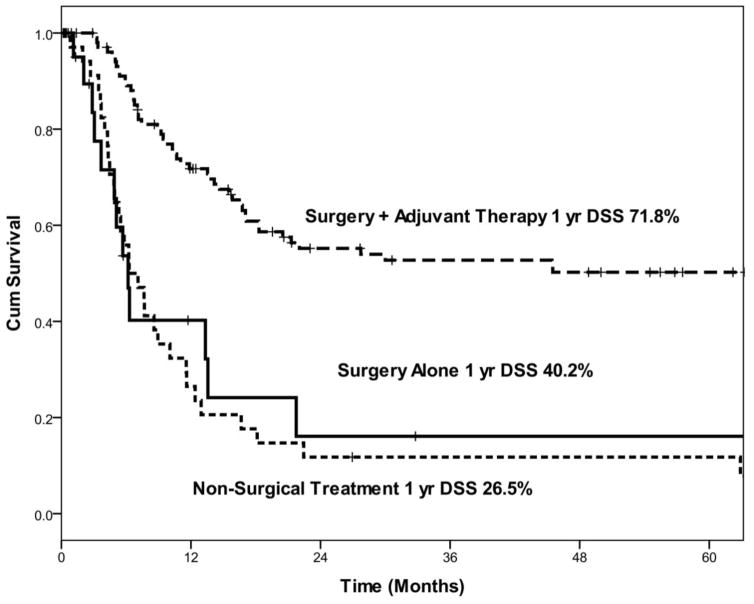
Kaplan-Meier curves demonstrating disease-specific survival (DSS) by salvage treatment modality.
Figure 4.
(A) Kaplan-Meier curves demonstrating neck recurrence–free survival (NRFS) stratified by pathological N (pN) stage and (B) the presence of extra capsular spread (ECS).
Rate and type of salvage treatment
Overall, 87% of patients with neck recurrence received some type of salvage treatment. Of 190 patients, 106 (56%) underwent salvage surgery with adjuvant RT or CRT, 25 (13%) had salvage surgery alone, and 34 (18%) received nonsurgical treatment with CRT. Thirteen percent of patients did not receive any salvage treatment for several reasons: they had comorbidities, they refused such treatment, or they chose palliative measures. Patients in the observation group were more likely to have salvage treatment (94%) than patients in the END group (81%). Among patients in the END group, those with pN− were more likely to have salvage treatment (86%) than patients with pN+ (76%).
Outcomes of salvage treatment
When we evaluated only patients who received salvage treatment after neck recurrence, patients treated with surgery with adjuvant RT +/− chemotherapy had significantly better 1-year DSS (72%) than patients treated with surgery alone (40%) or patients treated with nonsurgical salvage treatment (27%) (Figure 5).
DISCUSSION
In this study, we describe our experience over a 27-year period in the management of the cN0 neck in patients with oral cavity cancer. We sought to determine the incidence and pattern of neck recurrence in our group of patients, the effect of neck recurrence on survival, identify the clinical and pathological factors predictive of neck recurrence, and determine the outcome of salvage treatment.
In our experience, approximately 15% of patients with OSCC who initially presented with cN0 necks nodes developed neck recurrence. Our neck recurrence rate is similar to those reported in other studies, ranging from 14%–22% [12, 13]. NRFS trended towards being better in the ED group compared to the observation group, though this was not statistically significant (85% vs. 80%). This observation is in keeping with the results of the randomized controlled trial reported by D’Cruz which reported improved survival in patients treated with elective neck dissection [14]. In our study, neck recurrence was most common in patients with pN+ neck disease who underwent END. In contrast, patients with pN− neck who underwent END had the lowest rate of neck recurrence. In our series, patients with cN0 neck who underwent observation had a higher than expected recurrence rate (17%). The observation group included patients with UG and HP cancers, some with high T stage. These patients were traditionally thought to have a low risk of neck recurrence. However, recent publications [15,16,17] have shown that even these subsites carry a significant risk of occult metastases for tumors staged T2 and higher. If we remove UG and HP patients from the observation group, the NRFS at 5 years increases to 83.3% from 79.9% for the entire observation group. Our findings highlight the importance of carefully selecting patients for observation. Since 2012, patients at our institution with UG and HP tumors staged T2 or higher undergo END.
The pattern of neck recurrence provides an interesting perspective on the anatomical complexity of cervical lymphatic drainage. Whereas most recurrences occurred in the ipsilateral neck, the END group experienced more contralateral neck recurrences than the observation group. This is most likely the result of diverted lymphatic drainage of in-transit metastases in the dissected neck, a concern that has been previously raised by others [18,19]. Nonetheless, we identified ipsilateral neck recurrences among patients in the END group. These may represent in-transit metastases that were not removed during the initial neck dissection, occurred after the initial neck dissection, or were not included in the radiation field [20]. Alternatively, one could speculate that patients who have in-field failures after RT have inherently more aggressive tumors.
Patients who developed neck failure had poorer survival outcomes than those who did not. Patients in the END group with pN+ neck who developed neck recurrence had the poorest survival. In contrast, patients in the observation group who developed neck recurrence had significantly better survival than the other groups, as the majority of these patients can have a salvage neck dissection in a previously undissected neck.
We found that smoking and primary tumor subsite (HP and UG) were the only independent clinical predictors of neck recurrence. Smoking is an obvious risk factor for the development of OSCC, and it is also a substantial negative prognosticator of survival [21]. This may be explained by smoking-related cancers having a different mutation profile, such as p53 [22, 23], or by the long-standing theory of “field cancerization” [24]. These patients are also more likely to develop local recurrence and/or second primary tumors, both adversely affecting survival. Many patients with primary HP and UG cancers at our institution underwent observation, irrespective of T stage. This reflected the management policy of our institution at the time, which was based on the belief that these patients had negligible rates of occult cervical nodal metastases. Our data show that approximately 10% of patients in the observation group (48 of 496) had tumors that were pT3/T4, and almost half of these (21 patients) were upstaged following surgery to pT4 stage tumors. All the patients who were upstaged to pT4 had HP or UG tumors. As these patients also had higher rates of neck recurrence, our data clearly indicate that our previous policy selecting patients with HP and UG cancers for observation was inappropriate. In 2011, we reported our 21-year experience with these cancers [15], which in 2012 led to a change in our management policy for HP and UG cancers in which patients with UG and HP tumors staged T2 or higher undergo END. Furthermore, a recent multi-institutional collaborative report concluded that in patients with primary HP and UG tumors, END was associated with lower rates of recurrence and improved survival [16].
Pathological factors predictive of neck recurrence were histological grade, PNI, LVI, tumor thickness ≥ 4 mm, pN stage, and ECS. However, on multivariable analysis, only ECS was an independent predictor of neck recurrence in the END group. Most studies investigating predictors of neck recurrence in early-stage OSCC treat locoregional recurrence as a single entity, rather than looking at local and regional recurrences separately [25]. Spiro et al. found tumor thickness ≥ 4 mm to be a significant factor for locoregional recurrence and survival in patients with OSCC [26]. Another study investigating patients with OSCC of the oral tongue treated with surgery of the primary site and neck dissection found tumor thickness to be of significant prognostic importance [27]. Using a retrospective cohort of 148 patients, Huang et al. concluded that the only independent predictors of locoregional recurrence were LVI and tumor thickness (which they referred to as non-T4 muscular invasion) [28]. Puri and colleagues concluded that ECS is one of the most important predictors of survival, locoregional recurrence, and distant metastases in patients with head and neck squamous cell carcinoma; thus, these patients require an aggressive therapeutic approach [29]. Greenberg et al. suggested that patients with ECS or multiple positive lymph nodes (with or without ECS) on pathological review might benefit from intensified regional and systemic adjuvant therapy [30].
Salvage was feasible in many of the patients in our series who developed neck recurrence. Most underwent surgery with or without adjuvant treatment; the rest were treated nonsurgical. Previous studies have reported that patients with stage I/II disease who were treated initially with surgery alone were the best candidates for salvage in the case of recurrence [31]. The reported salvage rate, which represents the percentage of patients who are eligible for salvage treatment, varies across the literature. Most studies report salvage rates of 29%–76% [32, 33, 34, 35]. In a study by Schwartz and colleagues that investigated patients with recurrent OSCC, the authors found that among patients with early-stage primary tumors, patients who had recurrence > 6 months after initial treatment and patients who were amenable to salvage surgery benefitted the most from salvage therapy [32]. This finding provides a 3-dimensional insight into the management of neck recurrence in patients with OSCC, which depends not only on tumor factors but also on host factors and treatment modality. In our study, salvage treatment was more likely among patients in the observation group than patients in the END group. Patients in the observation group were more likely to present with nodal disease confined to the neck at levels I, II, and III. The rate of salvage surgery among patients in the END group was lower, and it was particularly apparent in patients who had previously received adjuvant RT for pN+. Such patients experience recurrence in a less predictable fashion, often in the contralateral neck. Salvage treatment is also more difficult in these patients, owing to scarring and fibrosis from previous surgery and radiation-induced fibrosis. In our experience, salvage neck dissection with adjuvant RT or CRT achieved superior outcomes than surgery alone or nonsurgical treatment. Previous studies have highlighted the importance of selecting patients who are most likely to benefit from surgical salvage. Most studies on salvage treatment for neck recurrence in patients with OSCC have found that surgery is the primary modality used in these patients, and the addition of a second modality may enhance regional control and OS [33]. Sklenicka et al. found that salvage surgery significantly increased OS after regional recurrence [34]. Similarly, Ord and colleagues found that the best salvage results were achieved with surgery followed by adjuvant therapy [35].
By using a large single-institution database, we have attempted to answer several important questions related to the management of the cN0 neck in OSCC. However, our study does have several limitations that require discussion. This study, by its retrospective nature, has limitations associated with retrospective data collection. The first is selection bias, by physician, patient, or institution, with regard to initial treatment, follow-up, and salvage treatment. The importance of proper selection is mentioned in the discussion section.. We have also highlighted the erroneous selection of patients with HP and UG primary tumors for observation and have shown that such patients have a high incidence of occult metastases and should be managed with END if the primary tumor is T2 or higher. The decision of when to perform END is strongly influenced by patient and physician bias, as is the decision regarding postoperative adjuvant radiation. Unfortunately, such selection bias cannot be corrected for in retrospective studies and can only be addressed in properly conducted randomized controlled trials.
Another important limitation is the presence of misclassification bias. Before 1990, we often did not perform CT or ultrasound imaging of the neck. The status of the neck was determined by clinical examination alone. It is possible that our cohort of patients with cN0 necks included patients who had clinically unrecognized lymph node metastases all along. This would overestimate the recurrence rate in the observation group and is another explanation for why the neck failure rate in our observation group was higher than expected.
CONCLUSION
In our cohort of patients with initially cN0 OSCC triaged to END vs observation from 1985 to 2012, approximately 15% experienced neck failure. Although many of these patients were eligible for salvage treatment, patients with neck recurrence had significantly poorer survival—particularly those in the END group with pN+ necks—than patients without neck recurrence. Outcomes following salvage treatment were best in the observation group when surgery was supplemented with adjuvant RT plus or minus chemotherapy. Careful patient selection should stratify patients with a higher risk of harboring occult lymph metastases to undergo END. Conversely, patients with lower risk can be observed with close follow-up. In particular, patients selected for observation should undergo careful preoperative imaging to determine the true extent of the primary tumor and neck nodes, and the selection algorithm should consider the significant risk of occult neck metastases associated with less common subsites in the oral cavity, such as the UG and HP.
Supplementary Material
Table 2B.
Overall NRFS by pathological factors
| Variable | Patients | Univariate Analysisb | Multivariate Analysisc | ||
|---|---|---|---|---|---|
|
| |||||
| 5 year NRFS, % | P | Hazard Ratio (CI) | P | ||
| LVI | 0.147 | ||||
| No | 811 | 84.2 | 0.002 | Reference | |
| Yes | 106 | 73.0 | 1.46 (0.88–2.42) | ||
| Not Knowna | 385 | ||||
| PNI | 0.496 | ||||
| No | 689 | 84.2 | 0.005 | Reference | |
| Yes | 228 | 79.4 | 1.61 (0.76–1.78) | ||
| Not Knowna | 385 | ||||
| Grade | 0.299 | ||||
| Well | 303 | 87.2 | 0.046 | Reference | |
| Intermediate | 750 | 80.3 | 1.34 (0.78–2.31) | ||
| Poor | 128 | 77.7 | 1.72 (0.87–3.41) | ||
| Not Knowna | 121 | ||||
| pN Stage | 0.056 | ||||
| N0/NX | 1054 | 84.6 | < 0.001 | Reference | |
| N1 | 129 | 80.7 | 1.21(0.70–2.07) | ||
| N2 | 119 | 66.2 | 1.95 (1.13–3.37) | ||
| Thickness | 0.089 | ||||
| < 4 mm | 357 | 88.5 | < 0.001 | Reference | |
| ≥ 4 mm | 781 | 79.8 | 1.58 (0.93–2.66) | ||
| Not Knowna | 164 | ||||
| pT Stage | |||||
| T1 | 745 | 84.1 | 0.074 | ||
| T2 | 266 | 79.5 | |||
| T3 | 42 | 83.3 | |||
| T4 | 138 | 74.4 | |||
NRFS: neck recurrence-free survival; LVI: lymphovascular invasion; PNI: perineural invasion; pN: pathological N stage; pT: pathological T stage; CI: confidence interval.
not included in analyses
Kaplan-Meier
Cox proportional hazard
Table 2C.
Overall NRFS for only patients with elective neck dissection
| Variable | Patients | Univariate Analysisb | Multivariate Analysisc | ||
|---|---|---|---|---|---|
|
| |||||
| 5 year NRFS, % | P | Hazard Ratio (CI) | P | ||
| LVI | 0.222 | ||||
| No | 517 | 86.3 | 0.007 | Reference | |
| Yes | 87 | 74.7 | 1.43 (0.80–2.56) | ||
| Not Knowna | 202 | ||||
| PNI | 0.224 | ||||
| No | 406 | 87.4 | 0.001 | Reference | |
| Yes | 198 | 79.1 | 1.37 (0.82–2.29) | ||
| Not Knowna | 202 | ||||
| Grade | 0.572 | ||||
| Well | 146 | 90.7 | 0.077 | Reference | |
| Intermediate | 529 | 83.6 | 1.51 (0.64–3.57) | ||
| Poor | 102 | 79.0 | 1.69 (0.64–4.49) | ||
| Not Knowna | 29 | ||||
| Thickness | 0.119 | ||||
| < 4 mm | 128 | 89.9 | 0.016 | Reference | |
| ≥ 4 mm | 607 | 82.6 | 2.10 (0.83–5.36) | ||
| Not Knowna | 71 | ||||
| ECS | < 0.001 | ||||
| pN0 | 558 | 88.6 | < 0.001 | Reference | |
| pN+ECS− | 148 | 79.2 | 1.62 (0.91–2.87) | ||
| pN+ECS+ | 72 | 61.1 | 3.99 (2.09–7.61) | ||
| Not Knowna | 28 | ||||
| pT Stage | 0.129 | ||||
| T1 | 435 | 87.3% | |||
| T2 | 204 | 79.3% | |||
| T3 | 33 | 83.2% | |||
| T4 | 99 | 78.2% | |||
NRFS: neck recurrence-free survival; LVI: lymphovascular invasion; PNI: perineural invasion; ECS: extracapsular spread; pN: pathological N stage; pT: pathological T stage; CI: confidence interval.
not included in analyses
Kaplan-Meier
Cox proportional hazard
Highlights.
Up to 15% of patients with oral cancer and a clinically negative neck may recur in the neck.
Neck recurrence free survival is superior in patients who undergo elective neck dissection compared to those who are selected for observation.
Patients who have neck recurrence can be salvaged in 80% of cases but survival is poorer compared to patients with no neck recurrence
Surgery with adjuvant radiation or chemoradiation gives best outcomes.
Acknowledgments
Sources of funding: This work was supported, in part, by NIH/NCI Cancer Center Support Grant P30 CA008748.
Abbreviations
- OSCC
oral squamous cell carcinoma
- cN0
clinically node-negative
- END
elective neck dissection
- PORT
postoperative radiation therapy
- NRFS
neck recurrence-free survival
- RT
radiation therapy
- DSS
disease-specific survival
- OS
overall survival
- CRT
chemoradiotherapy
- AJCC
American Joint Committee on Cancer
- HP
hard palate
- UG
upper gum
- PNI
perineural invasion
- LVI
lymphovascular invasion
- pT
pathological T stage
- pN
pathological N stage
- ECS
extracapsular spread
Footnotes
Conflict of Interest Statement: None declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chi AC, Day TA, Neville BW. Oral cavity and oropharyngeal squamous cell carcinoma--an update. CA Cancer J Clin. 2015:65401–21. doi: 10.3322/caac.21293. [DOI] [PubMed] [Google Scholar]
- 2.Surveillance, Epidemiology, and End Results Program. SEER Cancer Statistics Review (CSR) 1975–2013. National Cancer Institute; Bethesda, MD: Mar, 2016. [Accessed [Apr 29, 2016]]. http://seer.cancer.gov/csr/1975_2013/, based on November 2015 SEER data submission, posted to the SEER website. [Google Scholar]
- 3.Shah JP, Gil Z. Current concepts in management of oral cancer—surgery. Oral Oncol. 2009;45:394–401. doi: 10.1016/j.oraloncology.2008.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Montero PH, Patel SG. Cancer of the oral cavity. Surg Oncol Clin N Am. 2015;24:491–508. doi: 10.1016/j.soc.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Montero PH, Yu C, Palmer FL, Patel PD, Ganly I, Shah JP, et al. Nomograms for preoperative prediction of prognosis in patients with oral cavity squamous cell carcinoma. Cancer. 2014;120:214–221. doi: 10.1002/cncr.28407. [DOI] [PubMed] [Google Scholar]
- 6.Sano D, Myers JN. Metastasis of squamous cell carcinoma of the oral tongue. Cancer Metastasis Rev. 2007;26:645–662. doi: 10.1007/s10555-007-9082-y. [DOI] [PubMed] [Google Scholar]
- 7.Yii NW, Patel SG, Rhys-Evans PH, Breach NM. Management of the N0 neck in early cancer of the oral tongue. Clin Otolaryngol Allied Sci. 1999;24:75–9. doi: 10.1046/j.1365-2273.1999.00224.x. [DOI] [PubMed] [Google Scholar]
- 8.Ganly I, Patel SG, Shah J. Early stage squamous cell cancer of the oral tongue--clinicopathologic features affecting outcome. Cancer. 2012;118:101–11. doi: 10.1002/cncr.26229. [DOI] [PubMed] [Google Scholar]
- 9.Yuen AP, Wei WI, Wong YM, Tang KC. Elective neck dissection versus observation in the surgical treatment of early oral tongue carcinoma. Head Neck. 1997;19:583–8. doi: 10.1002/(sici)1097-0347(199710)19:7<583::aid-hed4>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 10.Yuen AP, Lam KY, Chan AC, Wei WI, Lam K, Ho WK, et al. Clinicopathologic analysis of elective neck dissection for N0 neck of early oral tongue carcinoma. Am J Surg. 1999;177:90–2. doi: 10.1016/s0002-9610(98)00294-3. [DOI] [PubMed] [Google Scholar]
- 11.National Comprehensive Cancer Network. [Accessed Apr 29, 2016];National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN guidelines): Cancer of the Oral Cavity. Version 1. 2015 http://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf.
- 12.Schwam ZG, Judson BL. Improved prognosis for patients with oral cavity squamous cell carcinoma: analysis of the National Cancer Database 1998–2006. Oral Oncol. 2016;52:45–51. doi: 10.1016/j.oraloncology.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 13.Kurokawa H, Yamashita Y, Takeda S, Zhang M, Fukuyama H, Takahashi T. Risk factors for late cervical lymph node metastases in patients with stage I or II carcinoma of the tongue. Head Neck. 2002;24:731–6. doi: 10.1002/hed.10130. [DOI] [PubMed] [Google Scholar]
- 14.D’Cruz AK, Vaish R, Kapre N, Dandekar M, Gupta S, Hawaldar R, et al. Elective versus Therapeutic Neck Dissection in Node-Negative Oral Cancer. New Eng Journal Med. 2015;373:521–9. doi: 10.1056/NEJMoa1506007. [DOI] [PubMed] [Google Scholar]
- 15.Morris LG, Patel SG, Shah JP, Ganly I. High rates of regional failure in squamous cell carcinoma of the hard palate and maxillary alveolus. Head Neck. 2011;33:824–30. doi: 10.1002/hed.21547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Givi B, Eskander A, Awad MI, Kong Q, Montero PH, Palmer FL, et al. Impact of elective neck dissection on the outcome of oral squamous cell carcinomas arising in the maxillary alveolus and hard palate. Head Neck. 2016;38(Suppl 1):E1688–94. doi: 10.1002/hed.24302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luryi AL, Chen MM, Mehra S, Roman SA, Sosa JA, Judson BL. Treatment factors associated with survival in early-stage oral cavity cancer: analysis of 6830 cases from the National Cancer Data Base. JAMA Otolaryngol Head Neck Surg. 2015;141:593–8. doi: 10.1001/jamaoto.2015.0719. [DOI] [PubMed] [Google Scholar]
- 18.Lim YC, Lee JS, Koo BS, Kim SH, Kim YH, Choi EC. Treatment of contralateral N0 neck in early squamous cell carcinoma of the oral tongue: elective neck dissection versus observation. Laryngoscope. 2006;116:461–5. doi: 10.1097/01.mlg.0000195366.91395.9b. [DOI] [PubMed] [Google Scholar]
- 19.Habib M, Murgasen J, Gao K, Ashford B, Shannon K, Ebrahimi, et al. Contralateral neck failure in lateralized oral squamous cell carcinoma. ANZ J Surg. 2016;86:188–92. doi: 10.1111/ans.13206. [DOI] [PubMed] [Google Scholar]
- 20.Preis M, Hadar T, Soudry E, Shpitzer T, Stenov Y, Hod R, et al. Early tongue carcinoma: analysis of failure. Head Neck. 2012;34:418–21. doi: 10.1002/hed.21754. [DOI] [PubMed] [Google Scholar]
- 21.Stevens MH, Gardner JW, Parkin JL, Johnson LP. Head and neck cancer survival and life-style change. Arch Otolaryngol. 1983;109:746–9. doi: 10.1001/archotol.1983.00800250040009. [DOI] [PubMed] [Google Scholar]
- 22.Brennan JA, Boyle JO, Koch WM, Goodman SN, Hruban RH, Eby YJ, et al. Association between cigarette smoking and mutation of the p53 gene in squamous-cell carcinoma of the head and neck. N Engl J Med. 1995;332:712–7. doi: 10.1056/NEJM199503163321104. [DOI] [PubMed] [Google Scholar]
- 23.Hsieh LL, Wang PF, Chen IH, Liao CT, Wang HM, Chen MC, et al. Characteristics of mutations in the p53 gene in oral squamous cell carcinoma associated with betel quid chewing and cigarette smoking in Taiwanese. Carcinogenesis. 2001;22:1497–503. doi: 10.1093/carcin/22.9.1497. [DOI] [PubMed] [Google Scholar]
- 24.Braakhuis BJ, Tabor MP, Kummer JA, Leemans CR, Brakenhoff RH. A genetic explanation of Slaughter’s concept of field cancerization: evidence and clinical implications. Cancer Res. 2003;63:1727–30. [PubMed] [Google Scholar]
- 25.Bessell A, Glenny AM, Furness S, Clarkson JE, Oliver R, Conway DI, et al. Interventions for the treatment of oral and oropharyngeal cancers: surgical treatment. Cochrane Database Syst Rev. 2011:CD006205. doi: 10.1002/14651858.CD006205.pub3. [DOI] [PubMed] [Google Scholar]
- 26.Spiro R, Huvos A, Wong G, Spiro JD, Gnecco CA, Strong EW. Predictive value of tumor thickness in squamous carcinoma confined to the tongue and floor of the mouth. Am J Surg. 1986;152:345–50. doi: 10.1016/0002-9610(86)90302-8. [DOI] [PubMed] [Google Scholar]
- 27.Ganly I, Goldstein D, Carlson DL, Patel SG, O’Sullivan B, Lee N, et al. Long-term regional control and survival in patients with “low-risk,” early stage oral tongue cancer managed by partial glossectomy and neck dissection without postoperative radiation: the importance of tumor thickness. Cancer. 2013;119:1168–76. doi: 10.1002/cncr.27872. [DOI] [PubMed] [Google Scholar]
- 28.Huang TY, Hsu LP, Wen YH, Huang TT, Chou YF, Lee CF, et al. Predictors of locoregional recurrence in early stage oral cavity cancer with free surgical margins. Oral Oncol. 2010;46:49–55. doi: 10.1016/j.oraloncology.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 29.Puri SK, Fan CY, Hanna E. Significance of extracapsular lymph node metastases in patients with head and neck squamous cell carcinoma. Curr Opin Otolaryngol Head Neck Surg. 2003;11:119–23. doi: 10.1097/00020840-200304000-00010. [DOI] [PubMed] [Google Scholar]
- 30.Greenberg JS, Fowler R, Gomez J, Mo V, Roberts D, El Naggar AK, et al. Extent of extracapsular spread: a critical prognosticator in oral tongue cancer. Cancer. 2003;97:1464–70. doi: 10.1002/cncr.11202. [DOI] [PubMed] [Google Scholar]
- 31.Tsang RK, Chung JC, To VS, Chan JY, Ho WK, Wei WI. Efficacy of salvage neck dissection for isolated nodal recurrences in early carcinoma of oral tongue with watchful waiting management of initial N0 neck. Head Neck. 2011;33:1482–5. doi: 10.1002/hed.21643. [DOI] [PubMed] [Google Scholar]
- 32.Schwartz GJ, Mehta RH, Wenig BL, Shaligram C, Portugal LG. Salvage treatment for recurrent squamous cell carcinoma of the oral cavity. Head Neck. 2000;22:34–41. doi: 10.1002/(sici)1097-0347(200001)22:1<34::aid-hed6>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 33.Kim J, Kim S, Albergotti WG, Choi PA, Kaplan DJ, Abberbock S, et al. Selection of ideal candidates for surgical salvage of head and neck squamous cell carcinoma: effect of the Charlson-Age Comorbidity Index and oncologic characteristics on 1-year survival and hospital course. JAMA Otolaryngol Head Neck Surg. 2015;141:1059–65. doi: 10.1001/jamaoto.2015.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sklenicka S, Gardiner S, Dierks EJ, Potter BE, Bell RB. Survival analysis and risk factors for recurrence in oral squamous cell carcinoma: does surgical salvage affect outcome? J Oral Maxillofac Surg. 2010;68:1270–5. doi: 10.1016/j.joms.2009.11.016. [DOI] [PubMed] [Google Scholar]
- 35.Ord RA, Kolokythas A, Reynolds MA. Surgical salvage for local and regional recurrence in oral cancer. J Oral Maxillofac Surg. 2006;64:1409–14. doi: 10.1016/j.joms.2006.05.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.