Abstract
High‐resolution measurement of medication adherence is essential to personalized drug therapy. A US Food and Drug Administration (FDA)‐cleared device, using an edible ingestion sensor (IS), external wearable patch, and paired mobile device can detect and record ingestion events. Oral medications must be combined with an IS to generate precise “digitized‐medication” ingestion records. We developed a Good Manufacturing Practice protocol to repackage oral medications with the IS within certified Capsugel capsules, termed co‐encapsulation (CoE). A randomized bioequivalence study of CoE‐IS‐Rifamate (Isoniazid/Rifampin 150/300 mg) vs. native‐Rifamate was conducted in 12 patients with active Mycobacterium tuberculosis and demonstrated bioequivalence using the population method ratio test (95% confidence interval). Subsequently, CoE‐IS‐medications across all biopharmaceutical classes underwent in vitro dissolution testing utilizing USP and FDA guidelines. CoE‐IS medications tested met USP dissolution specifications and were equivalent to their native formulations. CoE combines oral medications with the IS without altering the quality of the native formulation, generating “digitized” medications for remote capture of dosing histories.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
☑ This is the first description of how to combine a novel sensor‐based technology with oral medications of interest to yield “digitized medications” allowing remote capture of precise detailed dosing histories.
WHAT QUESTION DID THIS STUDY ADDRESS?
☑ The study addresses GMP methods for combining the ingestion sensor (IS) with oral medications of interest.
WHAT THIS STUDY ADDS TO OUR KNOWLEDGE
☑ This study provides a GMP protocol for a simple method of combining the IS with medications of interest via CoE. We demonstrate bioequivalence between a native and CoE‐IS drug formulation for MTB treatment and demonstrate CoE‐IS medications falling in all Biopharmaceutical Classification System classes met USP dissolution specifications and showed equivalence to their native formulations. This study establishes CoE‐IS drug formulations as a method to “digitize” oral medications, allowing remote capture of detailed dosing histories.
HOW THIS MIGHT CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE
☑ Such formulations are ushering in a new era in adherence monitoring and adherence support to enhance personalized drug therapeutics.
Personalized medicine is often described as providing “the right patient with the right drug, at the right dose, at the right time.”1, 2 High‐resolution measurement of medication adherence is critical for improving drug adherence in clinical practice.3 Individual drug‐taking behavior, inevitably associated with variable timing and taking of doses relative to specified dosing regimens, is a major source of variability in drug exposure.4, 5, 6 Such variations in drug‐taking behavior may account for lack of efficacy, leading to unnecessary changes in therapy, such as stopping the drug or switching to another drug, or increased dosages and possible induction of toxicity. Poor adherence may also account for the development of resistance to therapy in an individual.3, 4, 6 Medication nonadherence is highly prevalent, common to all therapeutic fields, and until recently lacked taxonomy for describing and defining adherence to medications.7 Approximately 50% of patients do not adhere to prescribed medications over time8 and the economic burden is estimated to result in a total direct and indirect cost of $290 billion annually in the US.9, 10
A major obstacle in precision medication management has been the difficulty in accurately generating precise, detailed dosing histories based on medication ingestion. The current standard approach in clinical use, primarily for patients with Mycobacterium tuberculosis (MTB), is directly observed therapy (DOT), where a healthcare worker watches a patient swallow each dose of medication. DOT is expensive, difficult to carry out, and thus rarely correctly implemented. Even this method does not guarantee actual ingestion has taken place, as patients may hide pills in their mouth.11, 12, 13 Alternative approaches, including patient questionnaires, pill counts, and prescription refill rates used as methods for assessing adherence have been shown to be biased, and furthermore lack details of the dosing history.8, 14, 15 Electronic monitoring methods, such as the medication event monitoring system (MEMS) that relies on the opening of a cap with a microswitch and memory chip on a medication container as a proxy measure of ingestion, emerged in the 1980s. MEMS has pioneered our current understanding of medication dosing histories and their implications for clinical pharmacology,4 including validated MEMS‐based drug concentration projections.16 However, the limitations of these systems, such as mismatches between electronic cap opening and actual intake, or patients obviating electronic assessment by decanting pills into a different container, have been well documented.17, 18, 19, 20
A novel sensor platform to monitor actual medication ingestion (Proteus Digital Health, Redwood City, CA) presents the opportunity to capture medication ingestion and analyze and support medication adherence in a manner conducive to personalized medicine. We term the system a Digital Health Feedback System or DHFS. The DHFS allows for the date‐ and time‐stamping of actual ingestions of oral medications rather than surrogate measures of ingestion. This system consists of an ingestion sensor, ∼1 mm3 (1 × 1 × 0.45 mm microchip), coated with very thin layers of commonly ingested excipients (i.e., minerals and metals) (Figure 1 a), a small adhesive‐backed detector patch worn on the torso, and a paired mobile device. When ingested with a medication, the sensor readily separates from the carrier, is energized, and communicates with the detector patch.21 The detector patch interprets the information as unique to the ingested sensor. The detector patch worn also records physiological metrics including heart rate, step activity, and sleep/rest. Data from the patch are transmitted wirelessly, via bluetooth technology, to a paired device, such as a mobile phone, tablet, or a personal computer. Subsequently, all of the data on the paired device are uploaded to a secured, centralized data storage location21 (Figure 1 b). These data are available in near‐real‐time to the patient user on their mobile device and, with patient permission, to healthcare personnel and other significant persons, who can access these data from a secure web portal.22, 23, 24, 25
Figure 1.
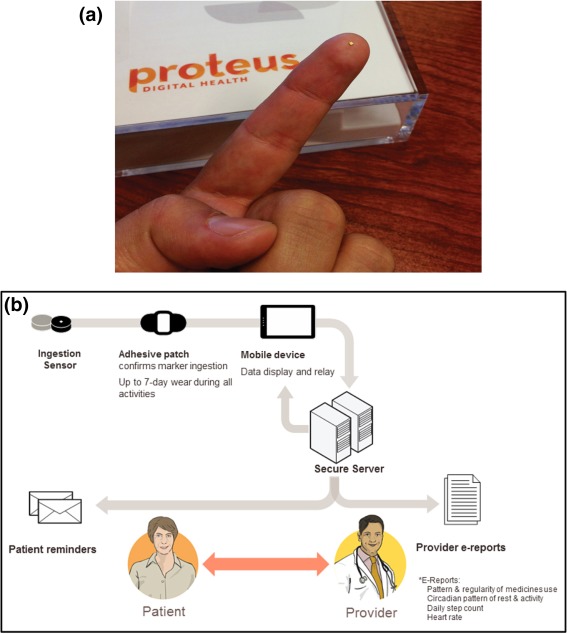
(a) The edible ingestion sensor (IS) is a microchip 1 × 1 × 0.45 mm coated with very thin layers of commonly ingested excipients (minerals and metals); the active layers are thin films of magnesium and cuprous chloride with a gold underlayer that acts as a current collector.20 (Photo courtesy of Tall Grass Pictures, San Diego, CA.) (b) Overview of the DHFS (figure courtesy of Proteus Digital Health).
The DHFS appears reliable, and highly accurate, with a positive detection accuracy of sensor‐detected ingestions of ∼99% (99.1%; 321/324 ingested under direct observation, 95% confidence interval (CI) 97.3–99.7).21 From a total of almost 30,000 sensor ingestions, no serious adverse events were observed in clinical studies.21 Early versions of the patch were associated with self‐limited, localized skin rashes in ∼10% of users.21, 22 The Proteus Digital Health Feedback Device (DHFD) was issued CE Mark approval in 2010 to market its ingestible sensor and personal physiologic monitor system in the European Union and was approved for marketing as a medical device by the US Food and Drug Administration (FDA) Center for Devices and Radiological Health (CDRH) in 2012.
Early studies using the DHFS were performed using the ingestion sensor (IS) embedded in a small white excipient tablet (Figures 1 a, 2) swallowed in close succession by, but separately from, the medication of interest.23, 24, 25, 26 This method relegates the IS to a proximate marker of an ingestion event, not the actual ingestion event. To allow the DHFS to be utilized to its full potential in clinical research and practice, a simple, reliable method of combining the IS with oral medications that does not alter the product quality of the oral medication is needed. Over‐encapsulation (in which an active drug is placed inside an opaque capsule shell, often involving the addition of a backfilled excipient) has been a widely used, highly effective technique for blinding solid oral dosage forms in comparative clinical trials since the 1960s.27, 28 Good Manufacturing Practice (GMP) for over‐encapsulation requires provision of data to show that product quality has not been altered, stability data to justify an expiration date for use, along with careful consideration of the nature of the capsule shell and the presence or absence of backfill.27, 28
Figure 2.
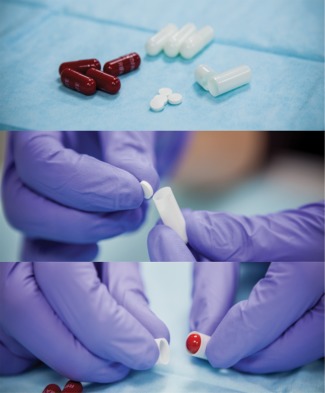
The co‐encapsulation process showing Rifamate medication (red), gelatin capsule (white), and the IS tablet (white) containing the edible ingestion sensor shown in Figure 1. (Photo courtesy of Tall Grass Pictures, San Diego, CA.)
An adaptation of over‐encapsulation is co‐encapsulation (CoE). CoE, as presented here, describes a technique in which an FDA‐approved medication is combined with the FDA‐approved IS tablet within a certified locked gelatin, or HPMC capsule, with or without micro‐cellulose backfill (Figure 2). This process is identical to over‐encapsulation, with the exception of the presence of the IS tablet. By means of this process a target medication becomes “digitized” for use with the DHFS so that its ingestion can be detected by the patch and then uploaded to a paired device for remote detection.
Similar to GMP for over‐encapsulation, CoE formulations require provision of data to show that product quality has not been altered, with careful consideration of the nature of the capsule shell and the presence or absence of backfill. In terms of the product quality, bioequivalence is a highly rigorous test to evaluate whether CoE of the drug with the IS within a certified capsule significantly alters the pharmacokinetics (PK) of the drug. Alternatively, dissolution testing can be used as a proxy to assess the effect of the capsule and the IS on the availability of the active drug for absorption in the GI tract. Dissolution testing is frequently used by the pharmaceutical industry to confirm the adequacy of an over‐encapsulated formulation prior to double‐blinded clinical trials.27 Dissolution is the process whereby a solid enters into solution. In pharmaceutics it is defined as the amount of drug substance that enters solution per unit time under standardized conditions.29 Since drug absorption and pharmacological activity depend on the availability of drug substance in a dissolved state, suitable dissolution characteristics are important for a satisfactory medicine formulation, and dissolution kinetics are important in determining the overall bioavailability of the drug.29 In vitro dissolution kinetics have been incorporated into the USP since 1970 and British Pharmacopeia since 1973. The availability of drug substance in the dissolved state provides a quality control test for CoE digitized medications that, although not identical in rigor to bioequivalence, is less costly and time‐consuming compared to a full bioequivalence study.
Here we present a protocol following GMP for repackaging oral medications of interest in certified gelatin or HPMC capsules in combination with the IS. We then present data evaluating the bioequivalence of CoE formulations of antituberculous medications with native drug formulations in active TB patients. This is followed by dissolution analyses performed following FDA guidelines evaluating the quality of the CoE formulations of the IS with multiple oral medications used in clinical areas in which medication adherence is of considerable importance, including tuberculosis, diabetes, hypertension, and hyperlipidemia.
RESULTS
Repackaging procedure
Table 1 lists procedures for repacking Rifamate (Sanofi Aventis, Bridgewater, NJ) to yield digitized CoE IS‐Rifamate. Procedures adapted to capsule fillers are shown in Supplementary Table 1. The CoE repackaging procedure described was followed for all other CoE‐IS oral medications used in the dissolution studies. Details of the medication strength, capsule size, and specifications for the CoE‐IS formulations are listed in Supplementary Table 2.
Table 1.
Standard operating procedure for UCSD research pharmacy repackaging procedures for co‐encapsulated IS‐Rifamate dispensed to patients
| Upon arrival of the patient, collect the prescription and follow the procedures below: |
| 1. Make sure the working counter is clutter free. |
|
2. Assemble all the materials needed for repackaging: • Empty opaque white, size 00EL Capsugel capsules • Rifamate 300/150 mg capsules • Proteus Ingestible Sensors (ISs) ‐ use ISs from a sealed bottle or from a partial bottle opened within the same calendar day. Destroy partial bottles the next day. • HDPE bottle • Repackaging worksheet and log • Prescription labels • Polylined Sterile Field • Sterile gloves / hair cover / lab coat (disposal gown and mask‐optional) • Isopropyl alcohol and gauzes • Pill counter tray and spatula |
| 3. Place on hair cover and mask (optional). |
| 4. Wash your hands. |
| 5. Place on lab coat (or disposable gown). |
| 6. Wipe down the repackaging area as well as the pill counter tray and spatula with gauzes saturating with isopropyl alcohol. |
| 7. Remove a disposable Polylined Sterile Field and place it on the dry, clean surface of the counter. |
| 8. Using the pill tray and spatula, count out the appropriate number of empty gelatin capsules, ISs, and Rifamate, and place them on the sterile field in separate piles. |
| 9. Wash your hands again. |
| 10. Place the sterile gloves on and begin to assemble the capsules. |
| 11. Open the empty gelatin capsule. |
| 12. Place the IS in the body of the capsule. |
| 13. Place a Rifamate on top of the IS in the body of the capsule. |
| 14. Close the gelatin capsule with the top until it snaps firmly in place. |
| 15. Once all capsules are completed, visually inspect each capsule to make sure all the components are in place. The IS and Rifamate can be readily identified through the white opaque capsule. |
| 16. There should be no left over components (i.e. Rifamate, ISs, or empty gelatin capsules) once the repackaging is complete. If there are, all capsules will need to be reassembled, inventory evaluated, and procedure repeated. |
| 17. Place the Co‐encapsulated IS‐Rifamate capsules in the HDPE container and close the HDPE container. |
| 18. Dispose of the sterile field and gloves in the trash. |
| 19. Fill out the repackaging worksheet and logs. |
|
20. Place the following labels on the HDPE container: • Prescription label • AVRC lot and exp: date label (35 days from when the IS bottle is open) • Label explaining the storage conditions of the product • Label stating the drug has been repackaged by the UCSD Research Pharmacy. |
| 21. Fill out the drug accountably log. |
| 22. File a copy of the repackaging worksheet in the study log book. File the original in the UCSD Research Pharmacy Repackaging Log Book. |
| 23. Dispense the finished prescription to the patient or study coordinator. |
Bioequivalence study
Demographics and baseline characteristics of enrolled subjects were as follows: Mean age 41.1 years, range 18–65, 71% male, 43% single. The majority of participants were Hispanic (64%), followed by Asian (14%), White not Hispanic (14%), and Black (7%).
The Cmax and AUC values for Isoniazid (INH) and Rifampin (RIF) in the CoE IS‐Rifamate vs. native Rifamate periods using noncompartmental methods were as follows. The median INH Cmax was 3.85 and 4.27 mcg/ml for native and CoE IS‐Rifamate, respectively; median INH AUC0‐12h were 13.34 and 12.50 mcg/ml for native and CoE IS‐Rifamate, respectively. The median rifampin Cmax was 12.12 and 11.79 mcg/ml for native Rifamate and CoE IS‐Rifamate, respectively; median rifampin AUC0‐12h were 45.19 and 43.76 mcg/ml for native Rifamate and CoE IS‐Rifamate, respectively. The mean value, with standard deviation, of the concentration of INH and RIF vs. time for CoE IS‐Rifamate vs. Native Rifamate (NR) formulations across all subjects is shown in Figure 3 a,b.
Figure 3.
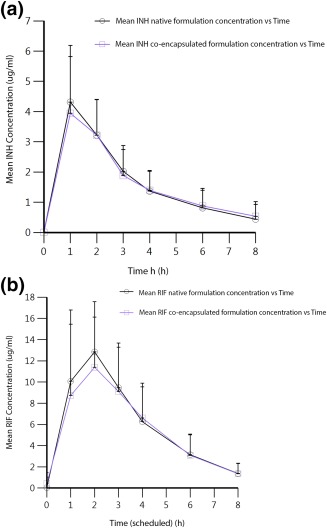
(a) Mean value with standard deviation concentration vs. time curves for INH co‐encapsulated (CoE) IS vs. Native (NR) Rifamate formulations. (b) Mean value with standard deviation concentration vs. time curves for RIF co‐encapsulated (CoE) IS vs. Native (NR) Rifamate formulations.
The bioequivalence (BE) analysis was done with standard BE tools in the FDA‐recognized Phoenix WinNonlin software (Certara, Princeton, NJ). A standard BE method, the population ratio method within the BE tool, was chosen and summary data presented in Table 2. Phoenix WinNonlin linear mixed effects modeling showed that INH AUC and Cmax were BE using the population ratio method test at the 95% CI. Similarly, Rifampin AUC and Cmax were BE at the 95% CI.
Table 2.
Summary data of bioequivalence (BE) analysis, via standard BE population ratio method, following noncompartmental pharmacokinetic analysis of Isoniazid and Rifampin in Native (N) and Co‐encapsulated (CoE) formulations of Rifamate using tools within FDA‐recognized Phoenix WinNonlin software (Certera)
| Isoniazid | Rifampin | |||
|---|---|---|---|---|
| Cmax | AUC | Cmax | AUC | |
| Bioequivalence confidence level | 95.00 | 95.00 | 95.00 | 95.00 |
| Ratio N/CoE | 94.547746 | 97.194316 | 82.072826 | 90.668841 |
| Ratio test | Bioequivalence shown | Bioequivalence shown | Bioequivalence shown | Bioequivalence shown |
No adverse events were observed during the PK testing associated with either CoE IS‐Rifamate or native Rifamate.
Dissolution studies
Antituberculous medications
Rifinah (Sanofi‐Aventis) is a solid tablet formulation of INH 150 mg and rifampin 300 mg that is available worldwide outside the USA. Figure 4 shows the dissolution characteristics of CoE IS‐Rifinah. Both the INH and rifampin components in CoE IS‐Rifinah show that the mean percentage dissolved reaches 100% in less than 40 min. This exceeds the USP Dissolution Acceptance Criteria for INH and Rifampin (i.e., not less than 75% of rifampin and not less than 80% of INH present in the dosage form released in 45 min).
Figure 4.
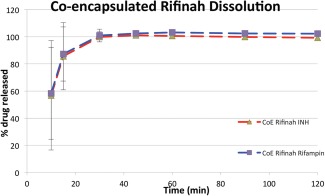
Dissolution of CoE Rifinah 150/300 mg; the percentage of drug released vs. time is shown for INH and Rifampin. USP specifications for Rifampin and INH drug release by 45 min are 80% and 85%, respectively.
Hypoglycemic medications
USP specification for glipizide 5 mg tablets is NLT 80% (Q) in 45 min. USP Stage 2 testing requirement was met, since no unit is less than the (Q (80%)) –15% for both the commercial tablet and CoE configuration arms (Supplementary Table 3). The similarity factor, f2, was calculated to be 70. The glipizide 5 mg tablets and the digitized CoE glipizide 5 mg formulation are considered equivalent.
All metformin commercial dosages and CoE metformin commercial doses met the USP acceptance criteria for metformin HCl dissolution (NLT 80% (Q) in 30 min). For metformin, the similarity factor, f2, was calculated to be 38. The profile of the commercial doses shows very rapid dissolution (i.e., at least 80% dissolved in 10 min). The discrepancy in dissolution profile agreement is in the early stage of the profile, as the capsule shell may add a lag of between 5 and 15 min to the dissolution process. There is good agreement between the two dosage forms beyond the 20‐min timepoint. The dosage forms are considered equivalent. Figure 5 shows the comparative dissolution studies with individual drugs evaluated indicated on each graph.
Figure 5.

The comparative dissolution profiles of CoE IS vs. native formulations of the antihypertensive, hypoglycemic, and lipid‐lowering drugs used in the comparative dissolution studies with individual drugs evaluated indicated on each graph.
Antihypertensive medications: hydrochlorothiazide, amlodipine besylate, lisinopril, and losartan
All CoE IS‐hydrochlorothiazide and native commercial dosages met the acceptance criteria (NLT 60% (Q) in 60 min). The similarity factor, f2, was calculated to be 87. The native hydrochlorothiazide 25 mg tablets and CoE IS‐hydrochlorothiazide 25 mg formulation are considered equivalent.
In the case of lisinopril, the CoE configuration met the USP acceptance criteria (NLT 80% (Q) in 30 min). In addition, the similarity factor, f2, was calculated to be 55. Both the commercial tablet and the native lisinopril 40 mg commercial tablets and CoE IS‐lisinopril 40 mg are considered equivalent.
The amlodipine besylate 5 mg commercial tablets and CoE IS‐amlodipine besylate 5 mg commercial tablets all met the USP acceptance criteria of NLT 75% (Q) in 30 min. For amlodipine besylate the similarity factor, f2, was calculated to be 60. The native amlodipine besylate 5 mg commercial tablets and CoE IS‐ amlodipine besylate 5 mg are considered equivalent.
During the evaluation of losartan 100 mg tablets, one CoE sample did not meet the tablet acceptance criteria in the USP (NLT 75% (Q) in 30 min). The profile of this specific sample showed low dissolution values throughout the run, an observation that may be caused by a content uniformity issue (a drug unit outside the typical range of 85% to 115% of label claim). However, the total batch results passed the USP requirements for dissolution for Stage 1 and Stage 2 when n = 12 units are evaluated (Supplementary Table 3). The losartan f2 was 38%, the difference between the curves occurred prior to 30 min reflecting an observed lag of 5 to 15 min due to gelatin capsule dissolution; with excellent curve agreement beyond that timepoint. The CoE IS‐losartan 100 mg and the native formulation are considered equivalent (Figure 5).
Lipid‐lowering medications
All native and CoE IS‐atorvastatin commercial dosages met the recommended acceptance criteria of NLT 80% (Q) in 30 min. The similarity factor, f2, was calculated to be 36, again the discrepancy affecting f2 is in the early part of the profile, where the reduction in release rate is likely due to the capsule shell. The dissolution behavior of CoE IS‐atorvastatin meets the recommended dissolution criteria, and is considered equivalent to the native dosage form (Figure 5).
DISCUSSION
The DHFS is a medical device initially approved by the FDA CDRH in 2012, with a subsequent 2015 clearance for “tracking and trending of (medication) intake times.” For precise, unambiguous data on ingestion and the opportunity to specifically identify the ingestion of a complex regimen the IS must be combined with the drug(s) of interest. Ideally, the IS would be directly incorporated into solid dosage forms, including immediate‐release or extended‐release formulations, as is currently under study with certain dosage forms (NCT02219009). In the meantime, there are simple and reliable methods to combine the IS with oral medications of interest. This article describes an established GMP protocol for repackaging oral solid dose formulations in combination with the IS into certified gelatin or HPMC capsules in a process termed CoE (Table 1, Figure 2). CoE is identical to over‐encapsulation strategies used in placebo‐controlled double‐blinded studies, except for the addition of the IS tablet.
The initial PK studies performed evaluated the BE of INH and Rifampin in CoE IS‐Rifamate compared to native Rifamate in a rigorously designed randomized, crossover study. The CoE IS and native formulations demonstrated FDA‐acceptable evidence of BE. We performed a BE study, as this was the first use of the CoE IS‐INH/Rifampin formulations in active TB patients with a serious infectious disease and provided lead‐in data for an ongoing clinical trial. In the context of the treatment of TB, the DHFS is referred to as Wirelessly Observed Therapy (WOT) and digitized Rifamate is currently being used in a randomized controlled clinical trial comparing WOT to DOT during the continuation phase of TB treatment (NCT01960257).30 Given the conclusive result of the BE study on Rifamate, only dissolution studies were performed on an equivalent combination formulation of INH and Rifampin used widely outside the US, Rifinah 150/300 mg (Sanofi‐Aventis), and these exceeded USP specifications. From these data we conclude that the CoE IS‐Rifamate and IS‐Rifinah are equivalent to their native formulations and can be used clinically in patients with active tuberculosis.
Rigorous BE studies are expensive, time‐consuming, and we currently consider such studies unnecessary if comparative dissolution data are equivalent between the CoE IS‐enabled and native formulations of a drug. In vitro dissolution testing nevertheless requires expert protocol planning and evaluation for drugs of different biopharmaceutical classes, hence we have included descriptions of the design specifications of the CoE formulations and their testing conditions (Supplementary Tables). Dissolution data assesses the effect of the capsule on the availability of the drug for absorption in the GI tract and, if adequate, attest that the quality of CoE drug product has not been altered. In this research, dissolution studies were performed with drugs falling in all Biopharmaceutical Classification System classes and all used certified, hard gelatin capsules, with or without microcrystalline cellulose backfill. As CoE of drug tablets with the IS and excipient backfill does not represent a significant manufacturing or process change to the commercially approved drug, the similarity value, f2, was calculated from dissolution profiles for information only. The dissolution profile is dominated in the early stage by disintegration; subsequently, dissolution of the active component is the primary driver. The presence of a capsule shell can create a lag in initiation of the disintegration process, accounting for early differences observed in comparability curves between some native and CoE formulations. All of the CoE drug dissolution profiles studied met either USP specifications, or met both USP specifications and f2 comparability testing equivalence to the native commercial drug tablet, following unit sample specifications in USP monograph <711> (Supplementary Table 3).31
Over‐encapsulation of drugs in placebo/double blind studies is routine. Our work is consistent with the existing literature and provides strong evidence that the process of CoE and the presence of the IS within this formulation is of little or no consequence to plasma drug concentrations or to the dissolution of native drug. The digitized CoE formulations developed and evaluated as described in this article are currently being used in clinical research and practice.30, 32, 33
The DHFS can accurately monitor the pattern of individual patients dosing histories, including precise ingestion timing, and can, by the design of the IS, detect multiple digitized medications simultaneously. As this technology comes into greater use it is likely the DHFS will have the most impact in areas where precise data on oral medication dose taking and timing is critical for determining the true dose–response relationship. In clinical trials lack of knowledge about dose‐taking and timing may lead to an incorrect conclusion on lack of effectiveness or estimates of toxicity for a candidate drug or the development of inappropriate dosing regimens. In clinical practice, lack of information or biased estimates of dose‐taking and timing can lead to inappropriate drug dose increases, which may lead to increased risk of toxicity or the abandonment of that drug or even its entire class of drugs. Precision data on dose‐taking and timing is particularly critical to the effective use of drugs with a narrow therapeutic window such as hypoglycemic and antiepileptic medications5, 34 and is essential to understanding the PK and dynamics that lead to the development of resistance against antibacterial and antiviral agents.3
In addition to allowing precision remote capture of oral medication adherence, the DHFS has the capacity to help support individual patient medication adherence by providing near‐real‐time reminders for missed doses, as well as allowing individualized caregiver contact to maintain adherence.30, 32, 33 The data generated by the device may also deepen our understanding of factors influencing medication‐taking and timing in individual patients. Early exploration of multimodal data visualizations combining detailed medication ingestion and dense physiological data, such as sleep/rest and activity cycles, indicate that the DHFS can provide insights on daily behavioral patterns in relation to medication‐taking patterns.23
This research provides a practical model for digitization of oral medications using CoE that can be used with the DHFS to generate complete and accurate dosing histories. Evaluation of antiretroviral CoE formulations is currently under way, in addition to other oral drug classes. Desirable developments of this system include incorporating the IS directly into a target medication during the manufacturing process itself. Formulations of medications, either CoE with the IS or having the IS incorporated inside the medication, are ushering in a new era in precision medication dosing, adherence monitoring, and adherence support.
METHODS
Bioequivalence study
Twelve patients with active TB on rifampin and isoniazid during the continuation phase of TB therapy were enrolled in a crossover, randomized intensive PK study comparing CoE IS‐Rifamate to native Rifamate. All subjects participating in the PK study signed a consent form. Full Institutional Review Board (IRB) approval was obtained from UCSD Human Subjects Protection Program (#130841).
Experimental design
Upon entry, subjects were randomized to one of two initial sequences: CoE IS‐Rifamate or native Rifamate. Fourteen days after starting treatment with the study medication (CoE IS‐Rifamate or native Rifamate), patients underwent PK sampling, then began 14 days of the alternate formulation, followed by a second round of PK sampling (Supplementary Table 5). Randomization was performed centrally via the study clinical database. At each sampling visit, the time of administration of the last three doses of Rifamate was collected.
Pharmacokinetic sampling
A predose Ctrough blood sample was obtained and the daily dose of CoE IS‐Rifamate, or native Rifamate administered. The subject remained fasting for 2 h. PK samples were collected at eight timepoints: 0 (prior to dosing), 1, 2, 3, 4, 6, 8, and 24 h. Plasma samples were frozen at –70°C within 40 min of collection. Plasma was stored at –70°C (or colder) until shipped on dry ice to the specialized testing laboratory (Peloquin Laboratory).
Sample analysis
INH: All high‐performance liquid chromatography (HPLC) procedures for analysis of samples for the INH concentration were performed with a Thermo Scientific (Pittsburgh, PA) Surveyor HPLC system with UV detector. The standard curves for the INH concentration in plasma covered a range of from 20–0.40 mcg/ml. The overall validation precision was 0.77–2.33% coefficient of variation (% CV) across all standard concentrations, while the within sample precision was 0.68% CV. The absolute recovery of INH from plasma was 94%. Rifampin: All HPLC procedures for analysis of the samples for the RIF concentration were performed with a Thermo Scientific Specrta HPLC system with photodiode array UV detector. The standard curves for the RIF concentration in plasma covered a range of from 50–0.05 mcg/ml. The overall validation precision was 1.27–9.41% CV across all standard concentrations, while the within sample precision was 3.22% CV. The absolute recovery of RIF from plasma was 94%.
Statistical analysis
The analysis was done with standard BE tools in the FDA‐recognized Phoenix WinNonlin software. A standard BE method, the population ratio method within the BE tool, was chosen following procedures described below.
The PK data for each subject was compiled and analyzed using noncompartmental PK analyses (Phoenix/WinNonlin software v. 6.4, Certara). Individual estimates of primary and secondary parameters (t1/2, CL/F, Cmax, Ctrough, and AUC) were determined. Individual PK parameters were compared within subjects comparing native to CoE INH and Rifampin by calculating geometric mean ratios of Isoniazid and Rifampin PK parameters, and 95% CIs were calculated around these ratios. Bioequivalence testing of the native and CoE formulations was performed using the population ratio method
Dissolution studies
Dissolution studies were performed following FDA and USP guidelines and compared native commercial grade medications to CoE formulations. Supplementary Table 4 lists the dissolution buffers and volume required, USP apparatus (baskets or paddles), rotational speed, analysis method for dissolution samples for each FDA‐approved oral drug tested. Result acceptance criteria were defined as follows: The quantity, Q, is the amount of dissolved active ingredient. Q specifications are presented in each individual drug tablet USP monograph. Unit sample acceptance criteria per USP Monograph <711> and drug tablet USP monographs29 were used to assess the feasibility of respective CoE configurations and is presented in Supplementary Table 3. The similarity value, f2, is calculated for information only. Generally, an f2 of 50–100 ensures sameness or equivalence for the two curves.31
AUTHOR CONTRIBUTIONS
S.H.B., C.P., and T.B. wrote the article; S.H.B., C.P., R.H., L.M., C.A.B., and T.F.B. designed the research; S.H.B., C.P., F.S., L.M., and K.M. performed the research; S.H.B., C.P., F.S.S., and T.F.B. analyzed the data; G.S. contributed new reagents/analytical tools.
Supporting information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
ACKNOWLEDGMENTS
Research conducted in this study was supported by an Innovation Award to S.H.B. from the Alliance Healthcare Foundation (AHF), San Diego, CA; Specialists in Global Health (SIGH), Encinitas, CA; NIH R43 AI100479‐01A1, NIH R01 MH110057 and Proteus Digital Health Inc., Redwood City, CA.
References
- 1. FDA . Paving the Way for Personalized Medicine: FDA's Role in a New Era of Medical Product Development. March 16, 2014.
- 2. Sadee, W. & Dai, Z. Pharmacogenetics/genomics and personalized medicine. Hum. Mol. Genet. 14(Spec No 2), R207–214 (2005). [DOI] [PubMed] [Google Scholar]
- 3. Vrijens, B. High‐fidelity measurement of patients' medication adherence: A missing link in precision medicine [Internet]. National Institutes of Health Office of Behavioral and Social Sciences Rese 2016. (arch). Podcast.
- 4. Blaschke, T.F. , Osterberg, L. , Vrijens, B. & Urquhart, J. Adherence to medications: insights arising from studies on the unreliable link between prescribed and actual drug dosing histories. Annu. Rev. Pharmacol. Toxicol. 52, 275–301 (2012). [DOI] [PubMed] [Google Scholar]
- 5. Assawasuwannakit, P. , Braund, R. & Duffull, S.B. A framework for quantifying the influence of adherence and dose individualization. Clin. Pharmacol. Ther. 99, 452–459 (2016). [DOI] [PubMed] [Google Scholar]
- 6. Urquhart, J. & Vrijens, B. Commentary on “A Framework for Quantifying the Influence of Adherence and Dose Individualization.” Clin. Pharmacol. Ther. 99, 354–356 (2016). [DOI] [PubMed] [Google Scholar]
- 7. Vrijens, B. et al A new taxonomy for describing and defining adherence to medications. Br. J. Clin. Pharmacol. 73, 691–705 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Osterberg, L. & Blaschke, T. Adherence to medication. N. Engl. J. Med. 353, 487–497 (2005). [DOI] [PubMed] [Google Scholar]
- 9. Greener, M. Wasted medicines and avoidable adverse events: a multibillion pound problem. J. Med. Econ. 9, 27–44 (2006). [Google Scholar]
- 10. Ernst, F.R. & Grizzle, A.J. Drug‐related morbidity and mortality: updating the cost‐of‐illness model. J. Am. Pharm. Assoc. (Wash). 41, 192–199 (2001). [DOI] [PubMed] [Google Scholar]
- 11. Falvo, D. Effective Patient Education: A Guide to Increased Adherence, 4th ed (Jones & Bartlett Learning; Burlington, MA, 2011). [Google Scholar]
- 12. An Unusual Case of Cheeking. <http://www.jailmedicine.com/an‐unusual‐case‐of‐cheeking/>.
- 13. Welfare IDoHa . Case Management. In: Welfare IDoHa, editor. 2007.
- 14. Aronson, J.K. Compliance, concordance, adherence. Br. J. Clin. Pharmacol. 63, 383–384 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Laine, C. et al Adherence to antiretroviral therapy by pregnant women infected with human immunodeficiency virus: a pharmacy claims‐based analysis. Obstet. Gynecol. 95, 167–173 (2000). [DOI] [PubMed] [Google Scholar]
- 16. Vrijens, B. et al Successful projection of the time course of drug concentration in plasma during a 1‐year period from electronically compiled dosing‐time data used as input to individually parameterized pharmacokinetic models. J. Clin. Pharmacol. 45, 461–467 (2005). [DOI] [PubMed] [Google Scholar]
- 17. Bova, C.A. et al Use of electronic monitoring devices to measure antiretroviral adherence:practical considerations. AIDS Behav. 9, 103–110 (2005). [DOI] [PubMed] [Google Scholar]
- 18. Wendel, C.S. et al Barriers to use of electronic adherence monitoring in an HIV clinic. Ann. Pharmacother. 35, 1010–1015 (2001). [DOI] [PubMed] [Google Scholar]
- 19. Olivieri, N.F. , Matsui, D. , Hermann, C. & Koren, G. Compliance assessed by the Medication Event Monitoring System. Arch. Dis. Child. 66, 1399–1402 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Denhaerynck, K. et al Examining assumptions regarding valid electronic monitoring of medication therapy: development of a validation framework and its application on a European sample of kidney transplant patients. BMC Med. Res. Methodol. 8, 5 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hafezi, H. et al An ingestible sensor for measuring medication adherence. IEEE Trans. Biomed. Eng. 62, 99–109 (2015). [DOI] [PubMed] [Google Scholar]
- 22. Eisenberger, U. et al Medication adherence assessment: high accuracy of the new Ingestible Sensor System in kidney transplants. Transplantation. 96, 245–250 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Browne, S.H. , Behzadi, Y. & Littlewort, G. Let visuals tell the story: medication adherence in patients with type II diabetes captured by a novel ingestion sensor platform. JMIR Mhealth Uhealth. 3, e108 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Au‐Yeung, K.Y. et al Early clinical experience with networked system for promoting patient self‐management. Am. J. Manag. Care. 17, e277–287 (2011). [PubMed] [Google Scholar]
- 25. DiCarlo, L. et al A digital health solution for using and managing medications: wirelessly observed therapy. IEEE Pulse. 3, 23–26 (2012). [DOI] [PubMed] [Google Scholar]
- 26. Kane, J.M. et al First experience with a wireless system incorporating physiologic assessments and direct confirmation of digital tablet ingestions in ambulatory patients with schizophrenia or bipolar disorder. J. Clin. Psychiatry 74, e533–540 (2013). [DOI] [PubMed] [Google Scholar]
- 27. Richardson, M. Over‐encapsulation: Techniques and Challenges of Blinding Clinical Trial Materials. Tablets and Capsules; 2010. Accessed on June 26, 2017. <https://tabletscapsules.com/article/overencapsulation-techniques-and-challenges-of-blinding-clinical-trial-materials/?searchquery=authors&alpha=R&pid=11679&ctracker=article&ctracker_url=https://tabletscapsules.com/wp-content/uploads/pdf/tc_201003001_0014.pdf&ctracker=article_download_pdf>.
- 28. Wan, M. , Orlu‐Gul, M. , Legay, H. & Tuleu, C. Blinding in pharmacological trials: the devil is in the details. Arch. Dis. Child. 98, 656–659 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Suresh, L. Dissolution test is widely used in the pharmaceutical industry for optimization of formulation and quality control of different dosage forms. <http://www.pharmainfo.net/dissolution‐test>.
- 30. Browne, S.H. et al Wirelessly observed therapy (WOT): a new paradigm in TB therapy monitoring. Conference on Retroviruses and Opportunistic Infections; February 23‐26, 2015; Seattle, WA.
- 31. FDA . Guidance for Industry; Dissolution Testing of Immediate Release Solid Oral Dosage Forms. In: Administration DHHSFD, 2007.
- 32. Guzman, Z. First, there were wearables. Now, there are swallowables. Suddenly, a computer on your wrist doesn't seem so amazing. CNBC LLC. 2016.
- 33. Castro, B. ‘Digital Pills’ Help Patients, Doctors Keep Track of Medications. NBC Universal Media, LLC. 2016.
- 34. Assawasuwannakit, P. , Braund, R. & Duffull, S.B. Quantification of the forgiveness of drugs to imperfect adherence. CPT Pharmacometrics Syst. Pharmacol. 4, e00004 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Supporting Information


