Abstract
Cardiovascular (CV) disease is the leading cause of death and morbidity in patients with type 2 diabetes. Five CV risk factors (blood pressure, resting heart rate, body weight, cholesterol levels and blood glucose) are monitored routinely as safety and efficacy endpoints in randomized clinical trials for diabetes therapies. To determine if different glucagon‐like peptide‐1 receptor agonists (GLP‐1RAs) had varying effects on these CV risk factors, we reviewed 16 head‐to‐head trials directly comparing GLP‐1RAs that included at least one of the five factors. Few trials reported statistical differences between GLP‐1RAs in terms of systolic blood pressure (SBP), body weight and total cholesterol. Liraglutide increased heart rate vs its comparators in three separate trials. All GLP‐1RAs reduced glycated haemoglobin (HbA1c), but exenatide twice daily and lixisenatide had statistically smaller effects compared with other GLP‐1RAs. These descriptive data indicate that individual GLP‐1RAs affect CV risk factors differently, potentially because of their individual pharmacokinetics and/or size. Short‐acting GLP‐1RAs appeared to result in smaller changes in SBP and total cholesterol compared with continuous‐acting treatments, while large GLP‐1RAs had a reduced effect on body weight compared with small GLP‐1RAs. For glycaemic control, short‐acting GLP‐1RAs had a greater impact on postprandial glucose levels vs continuous‐acting GLP‐1RAs, but for fasting plasma glucose levels and HbA1c, continuous‐acting treatments had the greater effect. No differentiating trends were obvious in heart rate data. These diverse actions of GLP‐1RAs on CV risk factors should aid individualized patient treatment.
Keywords: cardiovascular disease, GLP‐1 analogue, incretin therapy, randomized trial
1. INTRODUCTION
In 2015, the International Diabetes Federation estimated there were 415 million people with diabetes across the globe, and in high‐income countries up to 91% of those had type 2 diabetes.1 In patients with type 2 diabetes, cardiovascular (CV) disease is the leading cause of death and morbidity.1 The risk of developing CV disease is linked to several factors, some of which are considered modifiable lifestyle risk factors, including lack of physical exercise, smoking and diet.2, 3 Other risk factors are monitored routinely in clinical practice, and as safety and efficacy endpoints in randomized clinical trials (RCTs), including blood pressure,1 resting heart rate,4 body weight,5 cholesterol levels,1 and blood glucose.1 These monitored CV risk factors form the focus of the present review.
Meta‐analyses and population studies have examined how these five monitored risk factors impact CV disease development, across different populations, which have not always included patients with type 2 diabetes. For example, a 10 mm Hg decrease in systolic blood pressure (SBP) reduces the risk of CV disease events by 11%.6 If resting heart rate increases to ≥83 beats per minutes (bpm), the risk of CV death increases by 31%.7 Similarly, a 5‐unit increase in body mass index (BMI) results in a 16% increased risk of coronary heart disease5 and a 1‐mmol/L decrease in LDL cholesterol reduces the annual rate of CV disease by ~20%.8 Finally, a 0.9% decrease in glycated haemoglobin (HbA1c) results in a significant reduction in non‐fatal myocardial infarction (MI) events by 17%.9 These specific numbers may be relevant to particular population groups only in the cited references, but as changes in each of these monitored factors are known to decrease the risk of CV disease development, it is important to consider how treatment of type 2 diabetes may influence them.
There are several treatment options available for patients with type 2 diabetes, including glucagon‐like peptide‐1 receptor agonists (GLP‐1RAs).10, 11, 12 GLP‐1RAs reduce hyperglycaemia by increasing insulin secretion and decreasing glucagon secretion in a glucose‐dependent manner.11 The glucose‐dependent nature of these actions means that hyperglycaemia is reduced with a low risk of hypoglycaemia.11 Through their impact on the GLP‐1 signalling pathway, this class of agonists also facilitates weight loss and exerts effects on several CV variables.13
Currently, there are six GLP‐1RAs approved for the treatment of patients with type 2 diabetes in the USA and Europe: exenatide twice daily14, 15; exenatide once weekly16, 17; lixisenatide once daily18, 19; liraglutide once daily20, 21; albiglutide once weekly22, 23; and dulaglutide once weekly.24, 25 Data from phase III studies of other (currently unapproved) GLP‐1RAs, semaglutide and ITCA 650 are also available.26, 27, 28 ITCA 650 is an osmotic miniature pump that continuously releases exenatide to the subcutis for up to 12 months.29 Other GLP‐1RAs are in development (e.g. efpeglenatide30); however, there are currently limited clinical data for these treatments.
In 2008, the US Food and Drug Administration issued guidelines whereby the pharmaceutical industry must demonstrate that any new treatment for patients with type 2 diabetes will not result in an unacceptable increase in CV risk.31 Of the resulting CV outcome trials (CVOTs; all completed in patients at high risk of CV disease), three have published results with GLP‐1RAs. The first trial demonstrated that lixisenatide did not significantly affect the rate of major adverse CV events vs standard care.32 The next two of these trials demonstrated that liraglutide33 and semaglutide34 had a beneficial effect on CV outcomes compared with current standard of care. Some other GLP‐1RA CVOTs have finished, but the data are yet to be published, with only headline results from press releases available stating that they are non‐inferior vs placebo (for ITCA 650 in FREEDOM‐CVO, NCT0145589635 and for exenatide once weekly in EXSCEL, NCT0114433836). Other CVOTs are ongoing, including REWIND with dulaglutide (NCT01394952),37 HARMONY with albiglutide (NCT02465515)38 and PIONEER‐6 with oral semaglutide (NCT02692716).39 It is uncertain if GLP‐1RAs prevent the development of CV disease in patients with low CV risk.40
While treatment guidelines consider GLP‐1RAs as one treatment class,10, 11 there are some intrinsic differences between the molecules in their pharmacokinetics, structure and size (Table 1), which may influence their impact on CV disease. To determine if the different GLP‐1RAs had varying effects on CV risk factors, the published literature was searched for head‐to‐head trials directly comparing GLP‐1RAs and detailing at least one of the five CV risk factors of interest: blood pressure; heart rate; body weight; lipid profile; and glycaemic control. We were also interested in inflammation markers, but as measurements of inflammation do not yet form part of routine RCT practice in patients with type 2 diabetes, we used the identified trials to determine if any such head‐to‐head data exist.
Table 1.
Classification of GLP‐1RAs according to their pharmacokinetics, structure and size
| Pharmacokinetics | Structure | Size | |||
|---|---|---|---|---|---|
| Short‐acting | Continuous‐acting | Exendin‐4‐based | GLP‐1‐based | Small (<5 kDa) | Large (~63–73 kDa) |
| Exenatide twice daily14 | Albiglutide22 | Exenatide twice daily14 | Albiglutide22 | Exenatide twice daily14 | Albiglutide22 |
| Lixisenatide18 | Dulaglutide24 | Exenatide once weekly41 | Dulaglutide24 | Exenatide once weekly16 | Dulaglutide24 |
| Exenatide once weekly16 | ITCA 65029 | Liraglutide20 | ITCA 65029 | ||
| ITCA 65029 | Lixisenatide41 | Semaglutide26, 42 | Liraglutide20 | ||
| Liraglutide20 | Lixisenatide18 | ||||
| Semaglutide26, 42 | Semaglutidea 26, 42 | ||||
Precise molecular weight is not included in the cited references, but it is referred to as a small molecule.
2. SEARCHES PERFORMED AND TRIALS INCLUDED
The 6 GLP‐1RAs approved for the treatment of patients with type 2 diabetes and semaglutide with a published head‐to‐head study formed the focus of the initial literature searches. PubMed was searched using the following search strings, with the filter of “clinical trial” from inception to March 20, 2017 (ie, the cut‐off date for this review):
(lixisenatide AND exenatide) OR (lixisenatide AND liraglutide) OR (lixisenatide AND albiglutide) OR (lixisenatide AND dulaglutide) OR (lixisenatide AND semaglutide): 3 hits and all 3 included
(exenatide [ti] AND liraglutide [ti]) OR (exenatide [ti] AND albiglutide [ti]) OR (exenatide [ti] AND dulaglutide [ti]) OR (exenatide [ti] AND semaglutide [ti]): 7 hits, 3 included (4 excluded: 1 detailed glucose fluctuations only, 1 lipidaemia only, 1 patient‐reported outcomes only, 1 was a switching study and so the data were not directly relevant)
(liraglutide [ti] AND albiglutide [ti]) OR (liraglutide [ti] AND dulaglutide [ti]) OR (liraglutide [ti] AND semaglutide [ti]): 5 hits and all 5 included
(albiglutide [ti] AND dulaglutide [ti]) OR (albiglutide [ti] AND semaglutide [ti]): 0 hits
(dulaglutide [ti] AND semaglutide [ti]): 0 hits
(exenatide once weekly [ti] AND twice daily [ti]): 5 hits, 2 included (3 excluded: 1 excluded as a pooled analysis study; 1 as it was patient‐reported outcomes; and 1 as it was a post hoc analysis)
This provided 12 studies (one with two publications) for inclusion; however, we were aware of other head‐to‐head studies not identified through this search from reading other review articles and attending congresses. These four studies were:
the Lira‐Lixi study,43 which was removed by the “clinical trial” filter;
Rosenstock et al.'s study on albiglutide,44 which did not contain exenatide in its title;
SUSTAIN‐3,45 which has been presented at a congress only;
Nakatani et al.,46 which did not list any of the GLP1‐RAs by name in its title.
In total, 16 trials were identified; 15 of which recorded several of the CV risk factors and one focused solely on heart rate and ECG outputs46 (Table 2). All were multicentre studies with >100 patients enrolled, apart from the Nakatani et al.46 study that recruited 60 patients from a single site in Japan. The results from all 16 studies are compared in the present review, acknowledging that cross‐trial comparisons should always be conducted with caution, and being aware of the limitations such as differences in study populations and trial design, including the use of concomitant medications.
Table 2.
Head‐to‐head GLP‐1RA trials included in this review article
| Trial | GLP1‐RAs investigated | Study duration | Study type | Randomization ratio | Number of patients | Location(s) |
|---|---|---|---|---|---|---|
| DURATION‐147 | Exenatide 2 mg once weekly vs exenatide 10 μg twice daily | 30 wk | Randomized, open‐label, non‐inferiority | 1:1 | 303 | Canada, USA |
| LEAD‐648 | Liraglutide 1.8 mg once daily vs exenatide 10 μg twice daily | 26 wk | Randomized, open‐label | 1:1 | 464 | Multinational |
| DURATION‐549 | Exenatide 2 mg once weekly vs exenatide 10 μg twice daily | 24 wk | Randomized, open‐label | 1:1 | 254 | USA |
| DURATION‐650 | Exenatide 2 mg once weekly vs liraglutide 1.8 mg once daily | 26 wk | Randomized, open‐label | 1:1 | 912 | Multinational |
| GetGoal‐X51 | Lixisenatide 20 μg once daily vs exenatide 10 μg twice daily | 24 wk | Phase III, randomized, open‐label | 1:1 | 639 | Multinational |
| HARMONY‐752 | Albiglutide 50 mg once weekly vs liraglutide 1.8 mg once daily | 32 wk | Phase III, randomized, open‐label, non‐inferiority | 1:1 | 812 | Multinational |
| AWARD‐153 | Dulaglutide 1.5 mg or 0.75 mg once weekly vs exenatide 10 μg twice daily | 52 wk in total (primary endpoint at 26 wk) | Randomized, placebo‐controlled, double blind | 2:2:2:1 | 978 | Mexico, Argentina, USA |
| AWARD‐654 | Dulaglutide 1.5 mg once weekly vs liraglutide 1.8 mg once daily | 26 wk | Phase III, randomized, open‐label | 1:1 | 599 | Multinational |
| Lira‐Lixi43 | Liraglutide 1.8 mg once daily vs lixisenatide 20 μg once daily | 26 wk | Randomized, open‐label | 1:1 | 404 | Multinational |
| Rosenstock et al.44 | Albiglutide 4, 15, 30 mg once weekly vs albiglutide 15, 30, 50 mg every second week vs albiglutide 50 or 100 mg monthly vs exenatide 10 μg twice dailya | 16 wk treatment followed by 11 wk wash‐out | Phase II, randomized, placebo‐controlled, double blind | (10 treatment arms)a | 361 | Chile, Dominican Republic, Mexico, USA |
| Nauck et al.42 | Semaglutide 0.1, 0.2, 0.4, 0.8 mg and 0.8, 1.6 mg with dose escalation once weekly vs liraglutide 1.2, 1.8 mg once dailya | 12 wk | Phase II, randomized, placebo controlled, blind (semaglutide vs placebo), open‐label liraglutide | (9 treatment arms)a | 415 | Multinational |
| Kapitza et al.55 | Lixisenatide 20 μg once daily vs liraglutide 1.8 mg once daily | 28 days | Randomized, open‐label | 1:1 | 148 | Germany |
| Meier et al.56 | Lixisenatide 20 μg once daily vs liraglutide 1.2, 1.8 mg once dailya | 8 wk | Randomized, open‐label | 1:1:1a | 142 | Germany |
| Miyagawa et al.57 (and Odawara et al.58) | Dulaglutide 0.75 mg once weekly vs liraglutide 0.9 mg once daily | 26 wk (52‐wk results reported in58) |
Randomized, blind (dulaglutide vs placebo), open‐label liraglutide | 4:2:1 (dulaglutide: liraglutide: placebo) | 492 | Japan |
| SUSTAIN‐345 | Exenatide ER 2.0 mg once weekly vs semaglutide 1.0 mg once weekly | 56 wk | Phase III, randomized, open‐label | 1:1 | 813 | Europe, USA |
| Nakatani et al.46 | Lixisenatide 20 μg once daily vs liraglutide 0.9 mg once daily | A minimum of 1 wk at the maximum dose | Randomized, open‐label (heart rate/ECG) | 1:1 | 60 | Japan (single centre) |
Abbreviations: ER, extended‐release.
To aid comparisons in this review, only the highest doses of the GLP‐1‐RA were included in subsequent figures.
3. BLOOD PRESSURE
According to a report by Lim et al.,59 hypertension is one of the leading risk factors globally for disease burden.59 To aid accurate measurements of blood pressure, the Joint National Committee on the Prevention, Detection, Evaluation and Treatment of High Blood Pressure has published guidelines.60 Very few of the included studies detailed the specific methods used to measure blood pressure; these were: LEAD‐6 followed national guidelines48, 60; Kapitza et al.55 measured blood pressure when patients were in a supine resting position and before treatment injection; in the Lira‐Lixi trial, measurements were according to standard clinical practice43; Miyagawa et al. (and Odawara et al. in the extension) used seated blood pressure measurements57, 58; and Meier et al.56 measured the mean 24‐hour day and night SBP and diastolic blood pressure (DBP).
Two trials reported a significant treatment difference in SBP between two continuous‐acting GLP‐1RAs (Figure 1).45, 58 Liraglutide 0.9 mg once daily decreased SBP significantly more than dulaglutide 0.75 mg once weekly at 26 weeks and 52 weeks (P = .013 and P = .007, respectively),57, 58 and semaglutide 1.0 mg once weekly also significantly decreased SBP compared with exenatide 2 mg once weekly at 56 weeks (P = .016).45 Both of these studies had the longest duration out of the included trials (52 and 56 weeks, respectively).45, 58 These were the only two trials that used these specific doses of liraglutide and semaglutide. In the other 12 trials, there were no significant differences reported between the GLP‐1RAs investigated (Figure 1). For DBP, no significant between‐treatment differences were reported in any of the included trials.42, 43, 44, 45, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58
Figure 1.
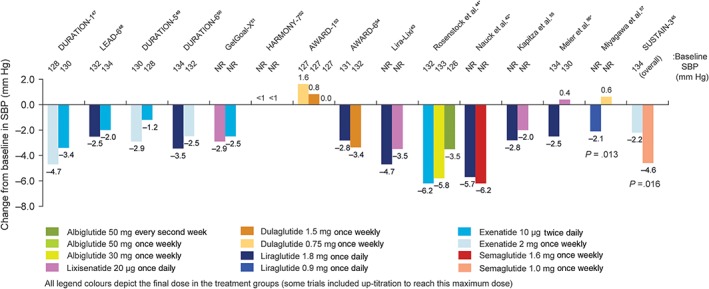
Change in systolic blood pressure in head‐to‐head comparison trials of GLP‐1RAs. Only significant P values are included. *To aid comparisons in this review, only the highest doses of the GLP‐1RA in any given dosing schedule in these trials were included. NR, not reported
4. HEART RATE
Methods used to measure heart rate were detailed in just three publications: Kapitza et al.55used ECG measurements, after 10 minutes’ rest in the supine position, before injection of the study drug; Meier et al.56 monitored heart rate over 24 h (including day and night means); and Nakatani et al.46 used a 24‐hour Holter ECG.
In 8 of the 9 trials with liraglutide, the increases in heart rate it induced were numerically larger than with comparators43, 46, 48, 52, 54, 55, 56, 57 and in three of these the differences were significant (P ≤ .0012 in all three trials)43, 48, 56 (Figure 2). In the remaining trial with liraglutide, semaglutide 1.6 mg once weekly resulted in a larger heart rate increase from baseline than liraglutide 1.8 mg once daily.42 There were no other statistically significant differences in heart rate reported with the other GLP‐1RAs investigated in these head‐to‐head trials at the primary endpoint (Figure 2).
Figure 2.
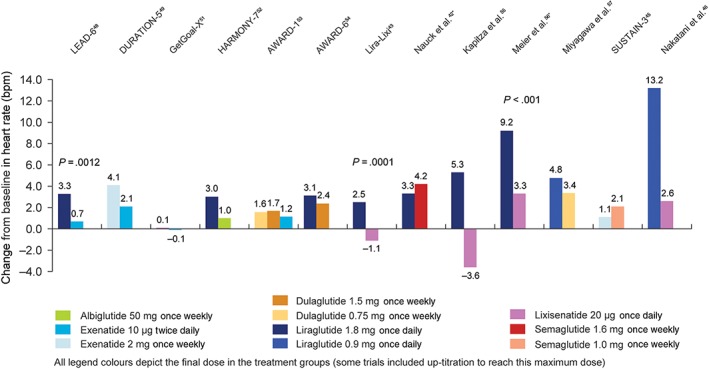
Change in heart rate in head‐to‐head comparison trials of GLP‐1RAs. Only significant P values are included. *To aid comparisons in this review, only the highest doses of the GLP‐1RA in any given dosing schedule in these trials were included
In two trials, differences in heart rate observed between GLP‐1RAs were not sustained over time. In AWARD‐1, dulaglutide once weekly significantly increased heart rate at week 26 (+2.8 bpm, both doses) compared with exenatide 10 μg twice daily (+1.2 bpm; P < .05 for both dulaglutide 0.75 mg and 1.5 mg once weekly), but at week 52 there were no significant differences between these GLP‐1RA treatments or compared with placebo.53 In HARMONY‐7, there was a transient increase in heart rate of 5.7 bpm at week 4 with liraglutide treatment, which was not evident in the albiglutide treatment arm. At week 32, the changes from baseline were smaller (+3 bpm with liraglutide and +1 bpm with albiglutide).52 In light of this observation, it should be noted that the 13.2 bpm increase reported by Nakatani et al.46 with liraglutide 0.9 mg once daily was measured after 1 week of treatment with this dose and so may not be indicative of any long‐term change in this CV risk factor.
5. BODY WEIGHT
Statistical comparisons showed that liraglutide 1.8 mg once daily resulted in a significantly greater decrease in body weight from baseline compared with exenatide 2 mg once weekly,50 albiglutide 50 mg once weekly,52 dulaglutide 1.5 mg once weekly54 and lixisenatide 20 μg once weekly,55 in four separate trials (Figure 3). In an additional three trials, there were no significant between‐treatment differences in body weight between liraglutide 1.8 mg once daily and exenatide 10 μg twice daily48 or lixisenatide 20 μg once daily43, 56 (Figure 3). The decrease in body weight achieved with semaglutide 1.0 mg once weekly was significantly greater than that achieved with exenatide 2 mg once weekly (P < .0001)45 and numerically greater than that achieved with liraglutide 1.8 mg once daily42 (estimated treatment difference = −2.2 kg; 95% confidence interval [CI] –3.2 to −1.3, but not corrected for multiple testing; Figure 3).
Figure 3.
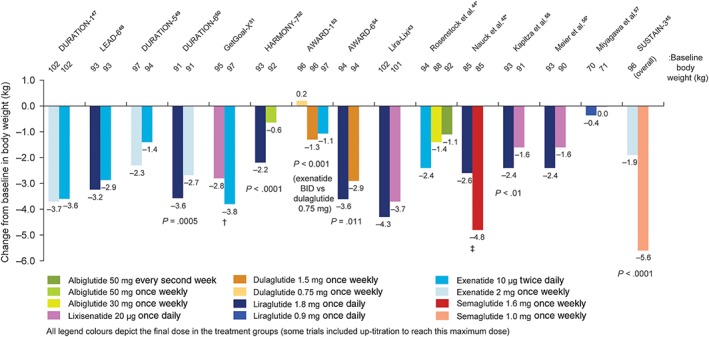
Change in body weight in head‐to‐head comparison trials of GLP‐1RAs. Only significant P values are included. *To aid comparisons in this review, only the highest doses of the GLP‐1RA in any given dosing schedule in these trials were included. †P‐value not reported for weight difference of 1.02 kg (95% CI: 0.456 to 1.581). ‡Mean change greater for semaglutide 1.6 mg vs liraglutide 1.8 mg based on the unadjusted CI (treatment difference: −2.2 kg [95% CI –3.2 to −1.3])
6. LIPID PROFILES
Lipid profiles were reported in 10 of the 16 trials included in this review42, 43, 44, 47, 48, 49, 50, 52, 54, 57, 58 (Figure 4); however, two of the trials reported the lipid profiles in a manner that did not allow them to be plotted on Figure 4 (Nauck et al. only provided lipid data for semaglutide and not liraglutide,42 while the Lira‐Lixi study reported ratios based on decrease from baseline43). Only 4 of the 10 trials specifically stated that fasting lipids were measured.42, 43, 47, 49 As the other trials included the measurement of fasting plasma or serum glucose, we have assumed that the lipid profiles were measured using the same blood samples and represent fasting lipid profiles of the patients.44, 48, 50, 52, 54, 57 Within these 10 trials, total cholesterol and HDL cholesterol were reported in all,42, 43, 44, 47, 48, 49, 50, 52, 54, 57 nine trials reported LDL cholesterol42, 43, 44, 47, 48, 49, 50, 52, 54 and nine reported triglycerides.42, 43, 44, 47, 48, 49, 52, 54, 57, 58 Other lipids examined included VLDL cholesterol,42, 43, 48 free fatty acids,43, 44, 48, 52 non‐HDL cholesterol50, 54 and apolipoprotein B.48
Figure 4.
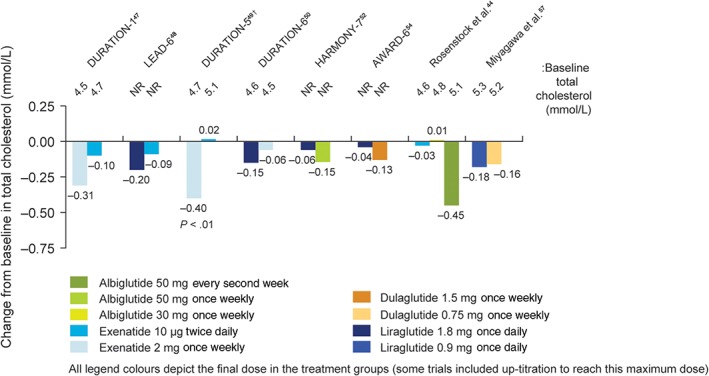
Change in total cholesterol levels in head‐to‐head comparison trials of GLP‐1RAs. Only significant P values are included. *To aid comparisons in this review, only the highest doses of the GLP‐1RA in any given dosing schedule in this trial were included. †Cholesterol was reported in mg/dL in the publication and so was converted to mmol/L for this figure. NR, not reported
Just one trial reported a statistically significant difference between two GLP‐1RAs and placebo (Figure 4).49 In DURATION‐5, exenatide 2 mg once weekly statistically reduced total and LDL cholesterol levels from baseline (−0.40 and −0.17 mmol/L, respectively), but exenatide 10 μg twice daily did not (+0.02 and +0.07 mmol/L, respectively; P < 0.01, for comparison between the two treatments).49 In LEAD‐6, liraglutide was associated with significantly greater reductions in triglycerides and free fatty acid values compared with exenatide 10 μg twice daily (P < .05, P < .01, respectively); however, VLDL cholesterol increased by 0.20 mmol/L in the liraglutide group and by 0.27 mmol/L in the exenatide group (treatment difference = −0.07 mmol/L; P = .028).48
7. GLYCAEMIC CONTROL
Statistically significant differences in HbA1c between the GLP‐1RAs were reported in several of the trials,43, 45, 47, 48, 49, 50, 53, 55, 56 three trials were of non‐inferiority design,51, 52, 54 two did not complete formal statistical comparisons between the GLP‐1RAs tested42, 44 and one trial did not measure HbA1c (as it focused on heart rate)46 (Figure 5). In five of the six trials that included exenatide 10 μg twice daily, the HbA1c response was smaller with it than with comparators (P < .01 vs exenatide 2 mg once weekly47, 49; P < .01 vs liraglutide 1.8 mg once daily48; P < .01 vs dulaglutide 1.5 mg once weekly and 0.75 mg once weekly53; no between‐treatment statistics were reported vs albiglutide 30 mg once weekly and 50 mg every second week44). The remaining trial with exenatide twice daily demonstrated the non‐inferiority of lixisenatide 20 μg once daily to exenatide 10 μg twice daily.51
Figure 5.
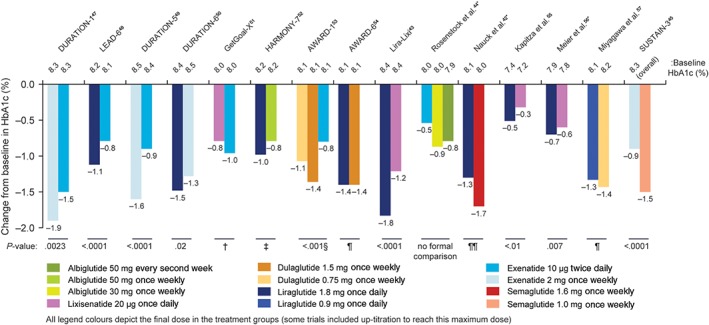
Change in HbA1c in head‐to‐head comparison trials of GLP‐1RAs. *To aid comparisons in this review, only the highest doses of the GLP‐1RA in any given dosing schedule in these trials were included. †Non‐inferiority P value not reported (95% CI 0.033 to 0.297, meeting predefined non‐inferiority margin). ‡Non‐inferiority P value = .0846 (not meeting predefined non‐inferiority margin). §For both doses of dulaglutide vs exenatide twice daily. ¶Non‐inferiority P value <.001 (meeting predefined non‐inferiority margin). ¶¶Mean change greater for semaglutide vs liraglutide based on the unadjusted CI
In three separate trials, the HbA1c improvements with lixisenatide 20 μg once daily were significantly smaller compared with liraglutide 1.8 mg once daily (P < .01 for all).43, 55, 56 Liraglutide 1.8 mg also had a greater impact on HbA1c vs exenatide 10 μg twice daily48 and exenatide 2 mg once weekly,50 but not compared with semaglutide 1.6 mg once weekly42 or dulaglutide 1.5 mg once weekly54 (Figure 5).
At least one other measure of glycaemic control (fasting plasma glucose [FPG], fasting serum glucose [FSG], or postprandial plasma glucose [PPG] levels) was reported in all but one of the included trials.42, 43, 44, 45, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57 The 15 trials that reported HbA1c also included FPG or FSG levels.42, 43, 44, 45, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57 In the majority of these, if a significant treatment difference between two GLP‐1RAs was reported in HbA1c, a similar difference was found in FPG or FSG levels42, 43, 45, 47, 48, 49, 50, 51, 52; however, this was not the case in two trials. In the study by Meier et al.,56 there was a significant difference between liraglutide 1.8 mg once daily and lixisenatide 20 μg once daily for HbA1c, but no difference in FPG levels. Similarly in the GetGOAL‐X trial, the difference in HbA1c between lixisenatide 20 μg once daily and exenatide 10 μg twice daily appeared significant as the 95% CI did not cross zero (treatment difference: 0.17%, 95% CI 0.033 to 0.297) and the two GLP‐1RAs were similar in FPG levels (treatment difference: 0.23 mmol/L, 95% CI –0.052 to 0.522).51
Ten of the trials reported PPG levels,42, 43, 45, 47, 48, 53, 54, 55, 56, 58 five of which used the 7‐point self‐monitored plasma glucose (SMPG),45, 47, 48, 54, 58 three used the area under the curve after the start of a standardized breakfast test meal,42, 55, 56 one used the 8‐point SMPG53 and one the 9‐point SMPG.43 In six of these, the trends were in the opposite direction to HbA1c, where treatments with larger reductions in HbA1c had smaller reductions in PPG compared with the comparators within any particular trial.42, 43, 47, 48, 55, 56 The majority of these trials also showed that there was a tendency for short‐acting treatments to reduce PPG more than continuous‐acting treatments, while continuous‐acting treatments reduced FPG/FSG more than their short‐acting counterparts.43, 47, 48, 55, 56
8. INFLAMMATION
While the focus of the present review was the CV risk factors routinely monitored in RCTs, other CV risk factors exist, including the presence of inflammation markers within patient samples.3 A meta‐analysis of data from >200 000 patients demonstrated a strong correlation between C‐reactive protein (CRP) levels and the development of CV disease,61 but many studies of other markers were limited by possible reporting bias.62 It is, therefore, considered at present that CRP is the only inflammatory marker with a proven link to CV risk.3 Of the 16 trials included in this review, DURATION‐6 was the only trial to publish CRP levels (with a high sensitivity assay).50 CRP levels improved in both treatment arms (exenatide 2 mg once weekly and liraglutide 1.8 mg once daily) by similar amounts (treatment difference: −2.48 nmol/L; 95% CI –14.83 to 9.43).50
9. DISCUSSION
It is well established that all GLP‐1RAs improve glycaemic control42, 43, 44, 45, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57 and the present comparison of head‐to‐head trials shows that they also improve three of the other four CV risk factors examined (decreased blood pressure, total cholesterol and body weight). However, all GLP‐1RAs increased heart rate from baseline, but liraglutide 1.8 mg once daily was the only GLP‐1RA to do so statistically significantly vs its comparators in three trials. Through the descriptive comparisons made here, it would appear that the individual GLP‐1RAs affect CV risk factors differently, potentially because of individual pharmacokinetics, molecular structure or size, thereby possibly complicating any treatment class effects. The diverse actions of GLP‐1RAs on CV risk factors mean that they can be used to tailor treatments for patients based on their individual needs and may also partly explain the different results from the published GLP‐1RA CVOTs.
9.1. Pharmacokinetics: duration of biological activity
As there were no statistically significant differences in blood pressure in estimated treatment differences between short‐ and continuous‐acting GLP‐1RAs, we examined the changes from baseline to determine if perhaps any underlying trend existed in this classification of GLP‐1RAs. Short‐acting GLP‐1RAs (exenatide twice daily and lixisenatide once daily) appeared to result in smaller changes from baseline in SBP compared with continuous‐acting treatments (exenatide once weekly, liraglutide once daily, albiglutide once weekly and every second week, dulaglutide once weekly, semaglutide once weekly). For total cholesterol, the only reported significant difference between two molecules was between exenatide twice daily and exenatide once weekly, with the continuous‐acting GLP‐1RA inducing the larger decrease.49 A possible underlying reason to explain this could be that short‐acting treatments are associated with shorter exposure and, thus, intermittent increases in GLP‐1 receptor activity, whereas continuous‐acting agonists ensure 24‐hour exposure and, thus, continuously increased receptor activity.13
There were no obvious trends in heart rate when results from treatment with continuous‐acting vs short‐acting molecules were compared, apart from the continuous‐acting liraglutide 1.8 mg once daily resulting in greater statistically significant increases vs short‐acting exenatide twice daily48 and lixisenatide once daily.43, 56 Changes in heart rate may be sensitive to the precise timing of the measurement, in that if the heart rate of a patient on a short‐acting treatment is measured just prior to a dose, no obvious effect may be detected. This was evident from the hourly heart rate measurements reported by Meier et al.56 and Nakatani et al.46; however, over the course of several weeks, it would appear that the initial increases in heart rate reported with continuous‐acting treatments decline,52, 53 which is suggestive of some treatment tolerability or tachyphylaxis for this particular CV risk factor.
For glycaemic control measurements, it appeared that continuous‐acting treatments had a greater effect on HbA1c and FPG vs short‐acting treatments, but for PPG, short‐acting GLP‐1RAs had the greater effect, as described previously.13 The impact of these two specific glycaemic factors on CV disease risk is unknown.
Improvements in blood pressure and glycaemic control are known to improve diabetes‐related death, microvascular disease, stroke, MI and nephropathy63, 64, 65; however, the included studies were generally 26 weeks in duration, so it remains difficult to know how between‐group differences in the effects of GLP‐1RAs on CV risk factors may impact patient CV outcomes. These outcomes may not be apparent until several years after initiating treatment with these agonists.
A comparison between CVOTs (typically 2–5 years in duration) and these head‐to‐head trials may indicate the impact that changes in the five risk factors may have on CV outcomes. Again, such cross‐trial comparisons need to be made with care because of differences in study design, particularly inclusion criteria. Three CVOTs have examined the impact of GLP‐1RAs on major adverse CV events, including death from CV causes, non‐fatal MI or non‐fatal stroke with a median 2–3 years of follow‐up.32, 33, 34 These trials showed that the continuous‐acting GLP‐1RAs liraglutide and semaglutide reduce the risk of major adverse CV events compared with standard care,33, 34 while the short‐acting GLP‐1RA lixisenatide does not have a significant impact on these events compared with standard care.32 Within these longer‐term CVOTs, SBP, glycaemic control, body weight and heart rate showed similar changes as in the head‐to‐head comparisons. However, it is interesting to note that the reported time points for these parameters in these three publications varied as follows: lixisenatide 20 μg once daily at week 12,32 liraglutide 1.8 mg once daily (significant differences compared with placebo for the four parameters noted) at month 3633 and semaglutide (significant differences in all four) at week 104.34 Each of these comparisons was vs placebo with standard of care, which included other glucose‐lowering treatment, dependent on the trial protocol. Cholesterol levels were only reported in SUSTAIN‐6, and the changes were significant with semaglutide 0.5 mg once weekly and not the 1.0 mg once‐weekly dose vs placebo.34 In addition to the different study populations and intrinsic study design differences, the question arises as to whether the results of the CVOTs are attributable to differences in the effects of liraglutide, semaglutide and lixisenatide on these CV risk factors alone or to other factors, for example, other pharmacological or cardioprotective mechanisms (e.g. reductions in atherosclerosis).
9.2. Molecular structure (exendin‐4‐based vs human GLP‐1‐based)
The molecular structure of the treatments did not appear to have an impact on any of the five CV risk factors examined, with no apparent trends between exendin‐4‐based (exenatide twice daily, exenatide once weekly and lixisenatide14, 16, 18) or human GLP‐1‐based (liraglutide, albiglutide, dulaglutide and semaglutide20, 22, 24) treatments. As the precise signalling pathways between GLP‐1 and CV risk factors have yet to be determined,66 it is unknown how these two different molecular structures may affect the pathways.
The immunogenicity of these treatments appears to be related to their molecular structure, with some GLP‐1RA treatments more immunogenic than others.13 The proportion of patients with antibodies after treatment with exendin‐4‐based GLP‐1RAs ranges from 44% to 70%.15, 17, 19 This proportion is lower after treatment with GLP‐1 analogues (range from 1.6% to 8.6%).21, 23, 25 As there did not appear to be a link between structure and CV risk factors, it would also appear that the presence of antibodies to the treatments does not play a role here.
Results from ongoing CVOTs of GLP‐1RAs will also be informative in this research area. To date, three CVOTs have shown that three exendin‐based therapies (lixisenatide,32 ITCA 65035 and exenatide once weekly36) are non‐inferior compared with placebo for CV outcomes. Two other CVOTs showed that two GLP‐1‐based therapies (liraglutide33 and semaglutide34) may have cardioprotective effects. It will be interesting to see the full results of the EXSCEL (exenatide once weekly) study, as it is a continuous‐acting GLP‐1RA, but only its non‐inferiority to placebo has been reported.36 Other ongoing CVOTs may help determine if molecular structure or activity duration of GLP‐1RAs is correlated with CV benefit.
9.3. Size
The molecular weight of GLP‐1RAs appeared to influence their impact on body weight and heart rate, which was reflected in the significant treatment differences reported in some of the head‐to‐head trials. In the included studies, large GLP‐1RAs (>60 kDa; albiglutide and dulaglutide) had a reduced effect on body weight compared with smaller GLP‐1RAs (<5 kDa; exenatide twice daily, exenatide once weekly, liraglutide, lixisenatide and semaglutide). Their impact on heart rate followed a similar trend, but it remains unknown if there is any link between the two parameters. Although it is commonly believed that only molecules <500 Da are able to cross the blood–brain barrier,67 there is evidence that lixisenatide, liraglutide and exenatide can effect neuronal function, either by crossing the blood–brain barrier or through interacting with GLP‐1 receptors present in circumventricular organs.68, 69, 70 It is possible that the larger GLP‐1RAs may not be able to penetrate into the brain and/or affect central nervous system signalling to the same extent as the smaller agonists,13, 41, 70 thereby reducing their known effects on satiety and cardiomechanisms.41
It is interesting to note that, although the size of the molecule tended to correlate with biological activity, i.e. the largest GLP‐1RAs having the longest half‐lives,23, 25 this was not the case for all treatments because of different formulations. In particular, exenatide once weekly has a molecular weight of just 4 kDa, but through the adherence of exenatide to microspheres, it is released within the body for up to 10 weeks.16 This disconnect between size and half‐life may explain some of the inconsistencies observed in some of the CV risk factor trends.
9.4. Clinically important differences
In the present narrative review, we focused on the statistical differences reported in the included publications; however, the size of these differences compared with published minimal clinically important differences are also worthwhile of consideration. While detailed studies on blood pressure and lipid profiles have been published,6, 8 the minimal clinically important differences in these CV risk factors in patients with type 2 diabetes receiving treatments for blood glucose are unknown.71 From the meta‐analysis by Emdin et al.6 it would appear that a 10‐mm Hg decrease in SBP is beneficial for patients with diabetes, but the largest statistical differences here were 2.7 mm Hg57 and 2.4 mm Hg.45 Similarly, for lipids, the largest treatment differences in total cholesterol were 0.42 mmol/L,44 which is unlikely to be clinically significant. The minimal clinically important difference in heart rate is also unknown and there remains debate in the field as to whether it is an independent CV risk factor or not.72 For body weight, patients with type 2 diabetes who are overweight are recommended to lose 5% to 10% body weight as an initial goal, but smaller losses in weight may also be beneficial71 and as such the clinically important difference remains undetermined. The largest difference in body weight between two GLP‐1RAs in one trial was 3.7 kg, from a baseline of 96 kg, representing a 3.9% loss in weight,45 which may have a clinical impact. Finally, for HbA1c, a clinically minimal important difference of 0.5% is recognized.71 This magnitude difference was reported in four of the trials (DURATION‐5,49 AWARD‐1,53 Lira‐Lixi43 and SUSTAIN‐345), in which exenatide once weekly,49 dulaglutide,53 liraglutide43 and semaglutide45 were both statistically and clinically significantly different from their comparators.
9.5. Limitations
Narrative reviews, such as this, are inherently limited by lack of a fully systematic approach and any additional analyses. While cross‐trial comparisons should always be made with caution because of differences in study design and baseline population demographics, investigating the trends across these 16 trials has highlighted some areas that could be examined in detail in future mechanistic studies, head‐to‐head trials, network meta‐analyses and outcomes models. The lack of detail within the publications relating to methods used to measure blood pressure, heart rate and lipids in particular also contributed to making the comparisons difficult. For example, measurements performed in the morning under fasting conditions would be unlikely to capture any direct effect if a short‐acting GLP‐1RA was administered 12–24 hours before this. In contrast, 24‐hour blood pressure measurements would be expected to capture any effects of such treatments.
Concomitant medication and prior CV events used as inclusion and exclusion criteria in the trials may also have affected the results presented here. Some publications specifically mentioned that lipid‐lowering medications had to be maintained at pre‐enrolment doses unless otherwise advised by the trial investigator (e.g. LEAD‐649), but others do not mention their use at all within the published eligibility criteria (e.g. AWARD‐153). Seven of the trials listed a CV‐related exclusion criterion,42, 44, 48, 50, 51, 52, 54 but there was no mention that CV events were considered as an eligibility criterion in other trials.43, 45, 47, 49, 53, 55, 56, 57 The lack of details provided within the publications for the inclusion/exclusion criteria used for most of these trials makes it difficult to suggest any hypotheses as to how these may have impacted the results.
In terms of the CV risk factors examined, we were interested in inflammation, but CRP levels were only published in one of the trials.50 In the future, if further evidence emerges in favour of inflammatory markers as CV risk factors, it may be that they will be monitored routinely in RCTs, as for the main risk factors included in this review, but we were limited on this point by the available data. Lack of data also limited analysis of BMI, which is closely related to CV disease.5 Within the context of head‐to‐head trials in type 2 diabetes, it is body weight, and not BMI, that is commonly used as a study endpoint, which complicates this area.
A fundamental limitation of this narrative review is that just two of the included 16 trials were double‐blind in design44, 53 and two partially blinded.42, 57, 58 The other trials were open‐label and so at risk of bias, which has been demonstrated with other treatments.73, 74, 75 It is unknown how this affected the reported results.
10. CONCLUSION
The data reviewed here indicate that there may be differences between short‐acting and continuous‐acting GLP‐1RAs, and between small and large GLP‐1RA molecules in terms of their effects on CV risk factors. Within the five CV risk factors investigated, the molecular structure of the GLP‐1RAs did not seem to have an effect; for now, only GLP‐1‐based GLP‐1RAs with continuous activity have shown beneficial effects on CV outcomes. Improvements in CV risk factors with these GLP‐1RAs may contribute to these beneficial CV effects; however, improvements in CV risk factors do not always translate into improvements in CV outcomes, as has been shown with the short‐acting GLP‐1RA lixisenatide. The mechanisms underlying reductions in CV risk with certain GLP‐1RAs merit further investigation.
ACKNOWLEDGMENTS
Medical writing and editorial support was provided by Gillian Groeger, PhD, and Izabel James, MBBS, both from Watermeadow Medical, an Ashfield company, part of UDG Healthcare plc, funded by Novo Nordisk. The content of the manuscript was reviewed by Novo Nordisk for medical accuracy only.
Author contributions
F. K. K. devised the concept of the review; N. B. D., T. V. and F. K. K. contributed to the content of the manuscript; all authors reviewed drafts of the content, approved the submission of the manuscript and accept responsibility for all aspects of the work.
Conflict of interest
N. B. D. reports no conflicts of interest. Within the last 36 months, T. V. has served on scientific advisory panels and/or speaker's bureaus for, served as a consultant to and/or received research support from Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, MSD/Merck, Novo Nordisk, Sanofi and Takeda. Within the last 36 months, F. K. K. has served on scientific advisory panels and/or speaker's bureaus for, served as a consultant to and/or received research support from Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Gubra, MSD/Merck, Novo Nordisk, Sanofi and Zealand Pharma.
Dalsgaard NB, Vilsbøll T, Knop FK. Effects of glucagon‐like peptide‐1 receptor agonists on cardiovascular risk factors: A narrative review of head‐to‐head comparisons. Diabetes Obes Metab. 2018;20:508–519. https://doi.org/10.1111/dom.13128
Funding information Novo Nordisk Foundation Center for Basic Metabolic Research, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark.
References
- 1. International Diabetes Foundation . IDF Diabetes Atlas. 7th ed. Brussels, Belgium: IDF; 2015. www.diabetesatlas.org. Accessed February 15, 2017. [Google Scholar]
- 2. Goff DC Jr, Lloyd‐Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25) (suppl 2)):S49–S73. [DOI] [PubMed] [Google Scholar]
- 3. Piepoli MF, Hoes AW, Agewall S, et al. European guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts): developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur J Prev Cardiol. 2016, 2016;23(11):Np1‐np96. [DOI] [PubMed] [Google Scholar]
- 4. Fox K, Borer JS, Camm AJ, et al. Resting heart rate in cardiovascular disease. J Am Coll Cardiol. 2007;50(9):823–830. [DOI] [PubMed] [Google Scholar]
- 5. Bogers RP, Bemelmans WJ, Hoogenveen RT, et al. Association of overweight with increased risk of coronary heart disease partly independent of blood pressure and cholesterol levels: a meta‐analysis of 21 cohort studies including more than 300 000 persons. Arch Intern Med. 2007;167(16):1720–1728. [DOI] [PubMed] [Google Scholar]
- 6. Emdin CA, Rahimi K, Neal B, Callender T, Perkovic V, Patel A. Blood pressure lowering in type 2 diabetes: a systematic review and meta‐analysis. JAMA. 2015;313(6):603–615. [DOI] [PubMed] [Google Scholar]
- 7. Diaz A, Bourassa MG, Guertin MC, Tardif JC. Long‐term prognostic value of resting heart rate in patients with suspected or proven coronary artery disease. Eur Heart J. 2005;26(10):967–974. [DOI] [PubMed] [Google Scholar]
- 8. Baigent C, Blackwell L, Emberson J, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta‐analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ray KK, Seshasai SR, Wijesuriya S, et al. Effect of intensive control of glucose on cardiovascular outcomes and death in patients with diabetes mellitus: a meta‐analysis of randomised controlled trials. Lancet. 2009;373(9677):1765–1772. [DOI] [PubMed] [Google Scholar]
- 10. International Diabetes Foundation . IDF global guideline for type 2 diabetes, 2012. http://www.idf.org/sites/default/files/IDF-Guideline-for-Type-2-Diabetes.pdf. Accessed February 16, 2017.
- 11. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient‐centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38(1):140–149. [DOI] [PubMed] [Google Scholar]
- 12. Garber AJ, Abrahamson MJ, Barzilay JI, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm–2016 executive summary. Endocr Pract. 2016;22(1):84–113. [DOI] [PubMed] [Google Scholar]
- 13. Lund A, Knop FK, Vilsboll T. Glucagon‐like peptide‐1 receptor agonists for the treatment of type 2 diabetes: differences and similarities. Eur J Intern Med. 2014;25(5):407–414. [DOI] [PubMed] [Google Scholar]
- 14. Amylin Pharmaceuticals . Byetta® (exenatide) injection, prescribing information, 2009. http://www.accessdata.fda.gov/drugsatfda_docs/label/2009/021773s9s11s18s22s25lbl.pdf. Accessed February 16, 2017.
- 15. AstraZeneca AB . Byetta summary of product characteristics, 2011. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000698/WC500051845.pdf. Accessed February 22, 2017.
- 16. Amylin Pharmaceuticals . Bydureon™ (exenatide extended‐release for injectable suspension), prescribing information, 2012. http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/022200s000lbl.pdf. Accessed February 16, 2017.
- 17. AstraZeneca AB . Bydureon summary of product characteristics, 2016. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002020/WC500108241.pdf. Accessed February 22, 2017.
- 18. Sanofi‐Aventis US . Adlyxin (lixisenatide), prescribing information, 2016. http://www.accessdata.fda.gov/drugsatfda_docs/label/2016/208471Orig1s000lbl.pdf. Accessed February 16, 2017.
- 19. Sanofi‐Aventis . Lyxumia summary of product characteristics, 2016. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002445/WC500140401.pdf. Accessed February 22, 2017.
- 20. Novo Nordisk A/S . Victoza® (liraglutide injection), prescribing information, 2010. https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/022341lbl.pdf. Accessed February 16, 2017.
- 21. Novo Nordisk A/S . Victoza summary of product characteristics, 2016. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/001026/WC500050017.pdf. Accessed February 22, 2017.
- 22. GlaxoSmithKline . Tanzeum (albiglutide), prescribing information, 2014. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/125431s000lbl.pdf. Accessed February 16, 2017.
- 23. GlaxoSmithKline . Eperzan summary of product characteristics, 2017. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002735/WC500165117.pdf. Accessed February 22, 2017.
- 24. Eli Lilly and Company . Trulicity (dulaglutide), prescribing information, 2017. http://pi.lilly.com/us/trulicity-uspi.pdf. Accessed February 16, 2017.
- 25. Eli Lilly . Trulicity summary of product characteristics, 2016. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002825/WC500179470.pdf. Accessed Febuary 22, 2017.
- 26. Sorli C, Harashima SI, Tsoukas GM, et al. Efficacy and safety of once‐weekly semaglutide monotherapy versus placebo in patients with type 2 diabetes (SUSTAIN 1): a double‐blind, randomised, placebo‐controlled, parallel‐group, multinational, multicentre phase 3a trial. Lancet Diabetes Endocrinol. 2017;5(4):251–260. [DOI] [PubMed] [Google Scholar]
- 27. Henry RR, Schwartz B, Kjems L, Huang H, Baron M. ITCA‐650 improves glycemic control and reduces the need to advance antidiabetes therapy. Presented at the American Diabetes Association 2017; June 9–13, 2017; San Diego, CA; Poster 1078. [Google Scholar]
- 28. Rosenstock J, Buse JB, Azeem R, Kjems L, Baron MA. Clinical impact of ITCA 650 in type 2 diabetes: a randomized, double‐blind, placebo‐controlled, 39‐week trial. Diabetes. 2015;64(suppl 1):A73. [Google Scholar]
- 29. Henry RR, Rosenstock J, Logan D, Alessi T, Luskey K, Baron MA. Continuous subcutaneous delivery of exenatide via ITCA 650 leads to sustained glycemic control and weight loss for 48 weeks in metformin‐treated subjects with type 2 diabetes. J Diabetes Complications. 2014;28(3):393–398. [DOI] [PubMed] [Google Scholar]
- 30. Davies M, Thiman M, Kugler A. Efpeglenatide: a once monthly GLP‐1 RA in the pipeline. Austin J Endocrinol Diabetes. 2016;3(4):1053. [Google Scholar]
- 31. US Food and Drug Administration . Guidance for industry. Diabetes mellitus — evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes, 2008. www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm071627.pdf. Accessed March 16, 2017.
- 32. Pfeffer MA, Claggett B, Diaz R, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373(23):2247–2257. [DOI] [PubMed] [Google Scholar]
- 33. Marso SP, Daniels GH, Brown‐Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834–1844. [DOI] [PubMed] [Google Scholar]
- 35. Intarcia Therapeutics . Press Release: Intarcia announces successful cardiovascular safety results in phase 3 FREEDOM‐CVO Trial for ITCA 650, 2016. https://www.intarcia.com/media/press-releases/2016-may-6-cardiovascular-safety.html. Accessed April 25, 2017.
- 36. AstraZeneca AB . Bydureon EXSCEL trial meets primary safety objective in type‐2 diabetes patients at wide range of cardiovascular risk, 2017. https://www.astrazeneca.com/media-centre/press-releases/2017/bydureon-exscel-trial-meets-primary-safety-objective-in-type-2-diabetes-patients-at-wide-range-of-cardiovascular-risk-23052017.html. Accessed June 5, 2017.
- 37. Eli Lilly and Company . Researching cardiovascular events with a weekly incretin in diabetes (REWIND), 2017. https://clinicaltrials.gov/ct2/show/NCT01394952?term=NCT01394952&rank=1. Accessed June 22, 2017.
- 38. GlaxoSmithKline . Effect of albiglutide, when added to standard blood glucose lowering therapies, on major cardiovascular events in subjects with type 2 diabetes mellitus, 2017. https://clinicaltrials.gov/ct2/show/NCT02465515?term=NCT02465515&rank=1. Accessed June 22, 2017.
- 39. Novo Nordisk A/S . A trial investigating the cardiovascular safety of oral semaglutide in subjects with type 2 diabetes (PIONEER 6), 2017. https://clinicaltrials.gov/ct2/show/NCT02692716. Accessed March 28, 2017.
- 40. Dalsgaard NB, Bronden A, Vilsboll T, Knop FK. Cardiovascular safety and benefits of GLP‐1 receptor agonists. Expert Opin Drug Saf. 2017;16(3):351–363. [DOI] [PubMed] [Google Scholar]
- 41. Meier JJ. GLP‐1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat Rev Endocrinol. 2012;8(12):728–742. [DOI] [PubMed] [Google Scholar]
- 42. Nauck MA, Petrie JR, Sesti G, et al. A phase 2, randomized, dose‐finding study of the novel once‐weekly human GLP‐1 analog, Semaglutide, compared with placebo and open‐label Liraglutide in patients with type 2 diabetes. Diabetes Care. 2016;39(2):231–241. [DOI] [PubMed] [Google Scholar]
- 43. Nauck M, Rizzo M, Johnson A, Bosch‐Traberg H, Madsen J, Cariou B. Once‐daily Liraglutide versus Lixisenatide as add‐on to metformin in type 2 diabetes: a 26‐week randomized controlled clinical trial. Diabetes Care. 2016;39(9):1501–1509. [DOI] [PubMed] [Google Scholar]
- 44. Rosenstock J, Reusch J, Bush M, Yang F, Stewart M. Potential of albiglutide, a long‐acting GLP‐1 receptor agonist, in type 2 diabetes: a randomized controlled trial exploring weekly, biweekly, and monthly dosing. Diabetes Care. 2009;32(10):1880–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ahmann A, Capehorn M, Charpentier G, et al. Efficacy and safety of once‐weekly semaglutide vs exenatide ER after 56 weeks in subjects with type 2 diabetes (SUSTAIN 3). Diabetologia. 2016;59(suppl 1):S76. Abstract 147. [Google Scholar]
- 46. Nakatani Y, Kawabe A, Matsumura M, et al. Effects of GLP‐1 receptor agonists on heart rate and the autonomic nervous system using Holter electrocardiography and power Spectrum analysis of heart rate variability. Diabetes Care. 2016;39(2):e22–e23. [DOI] [PubMed] [Google Scholar]
- 47. Drucker DJ, Buse JB, Taylor K, et al. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open‐label, non‐inferiority study. Lancet. 2008;372(9645):1240–1250. [DOI] [PubMed] [Google Scholar]
- 48. Buse JB, Rosenstock J, Sesti G, et al. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26‐week randomised, parallel‐group, multinational, open‐label trial (LEAD‐6). Lancet. 2009;374(9683):39–47. [DOI] [PubMed] [Google Scholar]
- 49. Blevins T, Pullman J, Malloy J, et al. DURATION‐5: exenatide once weekly resulted in greater improvements in glycemic control compared with exenatide twice daily in patients with type 2 diabetes. J Clin Endocrinol Metab. 2011;96(5):1301–1310. [DOI] [PubMed] [Google Scholar]
- 50. Buse JB, Nauck M, Forst T, et al. Exenatide once weekly versus liraglutide once daily in patients with type 2 diabetes (DURATION‐6): a randomised, open‐label study. Lancet. 2013;381(9861):117–124. [DOI] [PubMed] [Google Scholar]
- 51. Rosenstock J, Raccah D, Koranyi L, et al. Efficacy and safety of lixisenatide once daily versus exenatide twice daily in type 2 diabetes inadequately controlled on metformin: a 24‐week, randomized, open‐label, active‐controlled study (GetGoal‐X). Diabetes Care. 2013;36(10):2945–2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Pratley RE, Nauck MA, Barnett AH, et al. Once‐weekly albiglutide versus once‐daily liraglutide in patients with type 2 diabetes inadequately controlled on oral drugs (HARMONY 7): a randomised, open‐label, multicentre, non‐inferiority phase 3 study. Lancet Diabetes Endocrinol. 2014;2(4):289–297. [DOI] [PubMed] [Google Scholar]
- 53. Wysham C, Blevins T, Arakaki R, et al. Efficacy and safety of dulaglutide added onto pioglitazone and metformin versus exenatide in type 2 diabetes in a randomized controlled trial (AWARD‐1). Diabetes Care. 2014;37(8):2159–2167. [DOI] [PubMed] [Google Scholar]
- 54. Dungan KM, Povedano ST, Forst T, et al. Once‐weekly dulaglutide versus once‐daily liraglutide in metformin‐treated patients with type 2 diabetes (AWARD‐6): a randomised, open‐label, phase 3, non‐inferiority trial. Lancet. 2014;384(9951):1349–1357. [DOI] [PubMed] [Google Scholar]
- 55. Kapitza C, Forst T, Coester HV, Poitiers F, Ruus P, Hincelin‐Mery A. Pharmacodynamic characteristics of lixisenatide once daily versus liraglutide once daily in patients with type 2 diabetes insufficiently controlled on metformin. Diabetes Obes Metab. 2013;15(7):642–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Meier JJ, Rosenstock J, Hincelin‐Mery A, et al. Contrasting effects of Lixisenatide and Liraglutide on postprandial glycemic control, gastric emptying, and safety parameters in patients with type 2 diabetes on optimized insulin Glargine with or without metformin: a randomized, open‐label trial. Diabetes Care. 2015;38(7):1263–1273. [DOI] [PubMed] [Google Scholar]
- 57. Miyagawa J, Odawara M, Takamura T, Iwamoto N, Takita Y, Imaoka T. Once‐weekly glucagon‐like peptide‐1 receptor agonist dulaglutide is non‐inferior to once‐daily liraglutide and superior to placebo in Japanese patients with type 2 diabetes: a 26‐week randomized phase III study. Diabetes Obes Metab. 2015;17(10):974–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Odawara M, Miyagawa J, Iwamoto N, Takita Y, Imaoka T, Takamura T. Once‐weekly glucagon‐like peptide‐1 receptor agonist dulaglutide significantly decreases glycated haemoglobin compared with once‐daily liraglutide in Japanese patients with type 2 diabetes: 52 weeks of treatment in a randomized phase III study. Diabetes Obes Metab. 2016;18(3):249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lim SS, Vos T, Flaxman AD, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990‐2010: a systematic analysis for the global burden of disease study 2010. Lancet. 2012;380(9859):2224–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42(6):1206–1252. [DOI] [PubMed] [Google Scholar]
- 61. Kaptoge S, Di Angelantonio E, Pennells L, et al. C‐reactive protein, fibrinogen, and cardiovascular disease prediction. N Engl J Med. 2012;367(14):1310–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tzoulaki I, Siontis KC, Evangelou E, Ioannidis JP. Bias in associations of emerging biomarkers with cardiovascular disease. JAMA Intern Med. 2013;173(8):664–671. [DOI] [PubMed] [Google Scholar]
- 63. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10‐year follow‐up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577–1589. [DOI] [PubMed] [Google Scholar]
- 64. Holman RR, Paul SK, Bethel MA, Neil HA, Matthews DR. Long‐term follow‐up after tight control of blood pressure in type 2 diabetes. N Engl J Med. 2008;359(15):1565–1576. [DOI] [PubMed] [Google Scholar]
- 65. Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560–2572. [DOI] [PubMed] [Google Scholar]
- 66. Ussher JR, Baggio LL, Campbell JE, et al. Inactivation of the cardiomyocyte glucagon‐like peptide‐1 receptor (GLP‐1R) unmasks cardiomyocyte‐independent GLP‐1R‐mediated cardioprotection. Mol Metab. 2014;3(5):507–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Banks WA. Characteristics of compounds that cross the blood‐brain barrier. BMC Neurol. 2009;9(Suppl 1):S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hunter K, Holscher C. Drugs developed to treat diabetes, liraglutide and lixisenatide, cross the blood brain barrier and enhance neurogenesis. BMC Neurosci. 2012;13:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Punjabi M, Arnold M, Ruttimann E, et al. Circulating glucagon‐like peptide‐1 (GLP‐1) inhibits eating in male rats by acting in the hindbrain and without inducing avoidance. Endocrinology. 2014;155(5):1690–1699. [DOI] [PubMed] [Google Scholar]
- 70. Secher A, Jelsing J, Baquero AF, et al. The arcuate nucleus mediates GLP‐1 receptor agonist liraglutide‐dependent weight loss. J Clin Invest. 2014;124(10):4473–4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. National Institute for Health and Care Excellence, Internal Clinical Guidelines Team . Type 2 diabetes in adults: management, 2015. https://www.nice.org.uk/guidance/ng28. Accessed September 6, 2017.
- 72. Wang A, Chen S, Wang C, et al. Resting heart rate and risk of cardiovascular diseases and all‐cause death: the Kailuan study. PLoS One. 2014;9(10):e110985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Bhatt DL, Kandzari DE, O'Neill WW, et al. A controlled trial of renal denervation for resistant hypertension. N Engl J Med. 2014;370(15):1393–1401. [DOI] [PubMed] [Google Scholar]
- 74. Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, Bohm M. Renal sympathetic denervation in patients with treatment‐resistant hypertension (the Symplicity HTN‐2 trial): a randomised controlled trial. Lancet. 2010;376(9756):1903–1909. [DOI] [PubMed] [Google Scholar]
- 75. Persu A, Jin Y, Fadl Elmula FE, Jacobs L, Renkin J, Kjeldsen S. Renal denervation after Symplicity HTN‐3: an update. Curr Hypertens Rep. 2014;16(8):460. [DOI] [PMC free article] [PubMed] [Google Scholar]


