Abstract
Skin and oral mucosa substitutes are a therapeutic option for closing hard‐to‐heal skin and oral wounds. Our aim was to develop bi‐layered skin and gingiva substitutes, from 3 mm diameter biopsies, cultured under identical conditions, which are compliant with current European regulations for advanced therapy medicinal products. We present in vitro mode of action methods to (i) determine viability: epithelial expansion, proliferation (Ki‐67), metabolic activity (MTT assay); (ii) characterize skin and gingiva substitutes: histology and immunohistochemistry; and (iii) determine potency: soluble wound healing mediator release (enzyme‐linked immunosorbent assay). Both skin and gingiva substitutes consist of metabolically active autologous reconstructed differentiated epithelium expanding from the original biopsy sheet on a fibroblast populated connective tissue matrix (donor dermis). Gingival epithelium expanded 1.7‐fold more than skin epithelium during the 3 week culture period. The percentage of proliferating Ki‐67‐positive cells located in the basal layer of the gingiva substitute was >1.5‐fold higher than in the skin substitute. Keratins 16 and 17, which are upregulated during normal wound healing, were expressed in both the skin and gingiva substitutes. Notably, the gingiva substitute secreted higher amounts of key cytokines involved in mitogenesis, motogenesis and chemotaxis (interleukin‐6 > 23‐fold, CXCL8 > 2.5‐fold) as well as higher amounts of the anti‐fibrotic growth factor, hepatocyte growth factor (>7‐fold), compared with the skin substitute. In conclusion, while addressing the viability, characterization and potency of the tissue substitutes, important intrinsic differences between skin and gingiva were discovered that may explain in part the superior quality of wound healing observed in the oral mucosa compared with skin.
Keywords: ATMP, cytokine, gingiva substitute, mode of action, skin substitute, wound healing
1. INTRODUCTION
Skin and oral mucosa substitutes are a therapeutic option for closing hard‐to‐heal skin and oral wounds. For skin, several autologous and allogeneic dermal and bi‐layered (epidermis and dermis) constructs have been described over the past years to heal venous and arterial leg ulcers, diabetic foot ulcers and pressure ulcers (Falanga & Sabolinski, 1999; Jones, Nelson, & Al‐Hity, 2013; Kirsner et al., 2012; Marston, Hanft, Norwood, & Pollak, 2003; Veves, Falanga, Armstrong, & Sabolinski, 2001; Wu, Marston, & Armstrong, 2010; Zaulyanov & Kirsner, 2007). Also, application of autologous epidermal cells has been described for treating large burn wounds (Gardien et al., 2016; Gravante et al., 2007; Wood et al., 2012). Difficult‐to‐heal wounds in the oral mucosa arise from tumour excision, cleft palate repair and large trauma. Autografts still remain the golden standard for soft tissue augmentation in the oral cavity. The most commonly used oral soft tissue grafts are free gingival grafts, free buccal mucosa grafts and buccal fat pad grafts (Wolff et al., 2016). However, the amount of donor material available for grafting is often limited. Therefore, solutions are also being sought in the area of tissue engineering.
The major advantage of living skin and oral mucosa substitutes over acellular dressings and biomaterials is their capability to function as a living pump, continuously secreting a cocktail of cytokines, chemokines and growth factors, which promote angiogenesis, granulation tissue formation and re‐epithelialization (Spiekstra, Breetveld, Rustemeyer, Scheper, & Gibbs, 2007). However, no human tissue‐engineered oral substitutes for clinical oral applications are yet commercially available. Furthermore, the few skin substitutes that were starting to be introduced in Europe have been confronted with a new legislational hurdle when the European Union placed advanced therapy medicinal products (ATMPs) under Regulation (EC) no. 1394/2007 in 2008. In addition to strict production quality and safety assessment, and the use of clinical grade culture medium, extensive characterization, viability and potency assessment of ATMPs (mode of action) now needs to be performed.
Previously, we described a method for producing an autologous bi‐layered skin (Gibbs et al., 2006) and oral mucosa (gingiva) (Vriens et al., 2008) substitute from very small 3 mm punch biopsies during just a 3 week culture period. The unique method of constructing skin substitutes (SS) and gingival substitutes (GS) involves isolation of the intact epithelial sheet from the biopsy and fibroblasts from the dermis or lamina propria, respectively, followed by proliferation and migration of keratinocytes and melanocytes out of the epithelial sheet over acellular human dermis, and migration of fibroblasts into the dermis (patent no. WO 2005/068614 A2). Both SS and GS maintained many of the characteristics of the original biopsy. The SS consists of a multilayered orthokeratinized epithelium, whereas the GS consists of a parakeratinized epithelium in which the terminally differentiated cells in the upper layers retained remnants of nuclei. Several keratins, as well as loricrin and involucrin expression, were tissue specific, representative of the original biopsy tissue (Gibbs et al., 2006; Vriens et al., 2008).
Before the implementation of the European ATMP regulations the SS was extensively studied in a phase I clinical trial, with over 100 hard‐to‐heal therapy‐resistant ulcers treated (Blok et al., 2013). The GS was implemented in a proof‐of‐concept pilot study, closing three gingiva lesions (Vriens et al., 2008). Excellent safety data and promising efficacy data were obtained in both studies.
The aim of this study was to update the production procedure of both constructs in order for the SS and GS to be fully compliant with current European regulations. The culture medium was adapted to clinical grade standards and extensive quality controls were incorporated with regards to viability, characterization and potency (mode of action) under cGMP. In this study we identified the mode of action in vitro of the second generation SS and GS and in doing so can describe important intrinsic differences between SS and GS. These tissue‐specific differences may explain in part the superior quality of wound healing generally observed in oral mucosa compared with skin (Engeland, Bosch, Cacioppo, & Marucha, 2006; Kiecolt‐Glaser, Marucha, Malarkey, Mercado, & Glaser, 1995; Larjava et al., 2011; Mak et al., 2009).
2. MATERIALS AND METHODS
2.1. Skin and gingiva tissue
Healthy human skin and gingiva biopsies were obtained after informed consent from patients undergoing corrective abdominal plastic surgery (skin) and dental implant surgery (gingiva). Skin and gingiva tissue was used anonymously and in accordance with the Code for Proper Use of Human Tissue, as formulated by the Dutch Federation of Medical Scientific Societies (www.fmwv.nl) and following procedures approved by the VU University Medical Centre institutional review board. No clinical signs of inflammation or scar were present in the tissues used (determined by the surgeon or the dentist). The gingiva biopsies (epithelium and lamina propria) were obtained from the edentulous area. After tooth extraction, the extraction site was left to heal for at least 3–6 months before an implant was placed. Prior to placing the implant a 6 mm diameter biopsy was removed. The biopsy was sent to the research laboratory within 24 h after harvesting and was further biopsied in the research laboratory into 3 mm diameter biopsies. Abdominal skin tissue was received with epidermis, dermis and subcutaneous fat present. The fat was removed and then 3 mm diameter biopsies were taken. Therefore, both skin and gingiva biopsies used for the experiments were 3 mm diameter and approximately 2–3 mm thick.
2.2. Construction of SS and GS (Figure 1)
Figure 1.
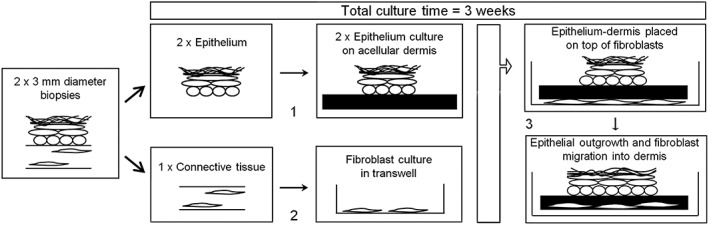
Schematic showing culture method and quality controls. (1) air‐exposed culture = 7–10 days. Viability control: Visual inspection (macroscopically) of attachment, >1 mm outgrowth on to dermis. (2) submerged culture = 7–10 days. Viability control: Microscopic inspection (phase contrast) > 50% confluent, adherent fibroblasts. (3) air‐exposed culture = 11–14 days. After the 3 week culture period, from each batch of skin substitute or gingiva substitute, 2 × 3 mm diameter biopsies were taken from the outgrowth region for (i) metabolic activity (MTT assay) and (ii) for characterization by histology and immunohistochemistry; potency by assessing soluble wound healing mediator release into culture medium (enzyme‐linked immunosorbent assay). For this extensive characterization study, further tissue samples were taken to assess epithelial differentiation and outgrowth
SS and GS were constructed essentially as described previously (Gibbs et al., 2006; Vriens et al., 2008). For this study, tissue from seven gingiva donors and seven skin donors was received in the culture facility. With the exception of one skin and two gingiva donors (infection in the incoming biopsy), tissue from the donors was successfully cultured as determined by >1 mm visible epithelial outgrowth from the original epithelial sheet; a stratified epithelium being present; and >50% confluent fibroblasts in the transwell at the time point in which the epithelium and fibroblasts are combined. The average age of gingiva donors was 69 years (standard deviation ±1.6) and of skin donors was 36 years (standard deviation ±7.4). Gingiva donors were mostly male (4/5), whereas skin donors were mostly female (5/6). The results described here are derived from five GS donors and six SS donors.
In brief, a single batch of SS or GS was constructed from two 3 mm diameter skin or gingiva biopsies and one piece of acellular human donor dermis (1.5 × 2.5 cm2). Acellular dermis was prepared from glycerol‐preserved donor skin (Euro Tissue Bank, Beverwijk, the Netherlands). Glycerol and dead donor cells were removed by repeated washing over a period of approximately 10 days in Dulbecco's phosphate‐buffered saline (PBS; 1×) ATMP‐ready (PAA, Pasching, Austria) with 50 μg/ml gentamicin (Centrafarm, Etten‐Leur, the Netherlands) at 37°C. Remnants of dead epidermis were gently scraped off using a spatula until only the white acellular dermis remained. The acellular dermis, with basement membrane intact (collagen IV, collagen VII, BP180 and HSPG expression), was stored at 4°C in Dulbecco's PBS (1×) ATMP‐ready until used to construct SS and SG (Gibbs et al., 2006). Next, two epithelial sheets were separated from the connective tissue of two biopsies from a single skin or gingiva donor after overnight incubation on dispase II (Roche, Mannheim, Germany) at 4°C. Intact epithelial sheets were placed with the differentiated side upwards on to the basement membrane side of the acellular donor dermis and cultured at the air–liquid interface on keratinocyte medium consisting of Dulbecco's modified Eagle medium (DMEM; SAFC, Zwijndrecht, the Netherlands)/Ham's F‐12 (SAFC) (3:1) containing 5% Fetal‐Clone III (Thermo Scientific, Waltham, MA, USA), 2.5 μg/ml isoprenalin (Monico SPA, Venice, Italy), 0.5 μg/ml Solu‐Cortef (Pfizer, Capelle a/d IJssel, the Netherlands), 0.5 μg/ml Actrapid (NovoNordisk, Bagsvaerd, Denmark), 50 μg/ml gentamicin and 2 ng/ml LONG EGF (Repligen, Lund, Sweden).
Primary fibroblasts were isolated by incubating the connective tissue from one of the biopsies in dispase (BD Biosciences, Breda, the Netherlands)/collagenase (Nordmark, Uetersen, Germany) for 2 h at 37°C. The entire digest from the biopsy containing the skin or gingiva fibroblasts was transferred to a transwell (0.4 μm pore size, cat no. 3450, Costar, Corning Inc., New York, NY, USA) and the adherent fibroblast cells were cultured for 7–10 days in DMEM containing 5% Fetal‐Clone III and 50 μg/ml gentamycin. After 7–10 days of culturing the primary fibroblasts and epithelial sheet apart, the acellular donor dermis containing the epithelial sheet was placed on to the fibroblasts in order to allow fibroblast migration into the reticular side of the dermis (Monsuur et al., 2016). The SS and GS were further cultured air exposed in keratinocyte medium (see above), but with only 1% Fetal‐Clone III and without gentamicin.
Culture medium was renewed twice a week and SS and GS were harvested 3 weeks after initiating culture from the original biopsy. At this point the substitutes would otherwise be applied to an ulcer or oral lesion. Culture supernatant collected over the previous 3–4 days of culturing was stored at −20°C until further analysis by enzyme‐linked immunosorbent assay (ELISA). Harvested cultures were formalin‐fixed and paraffin‐embedded using standard methods. The culture medium and method of culture were accepted by the Dutch authorities as being fully compliant with European ATMP requirements.
2.3. Histology, epithelial outgrowth, immunohistochemistry and MTT assay
Paraffin‐embedded tissue sections (5 μm) were used to assess histology (haematoxylin and eosin staining; H&E) and for immunohistochemical staining of keratins, Ki‐67, vimentin, loricrin and involucrin.
Epithelial outgrowth from the biopsy was analysed on H&E‐stained sections covering the entire width of the dermis with a Zeiss Axioscoop 20 microscope and NIS‐Elements AR 3.2 software (Figure 2A–C). The distance between the two epithelial end sections was measured in mm, the diameter of the initial epithelial sheet subtracted and the remaining value expressed as mm outgrowth from the original epithelial sheet.
Figure 2.
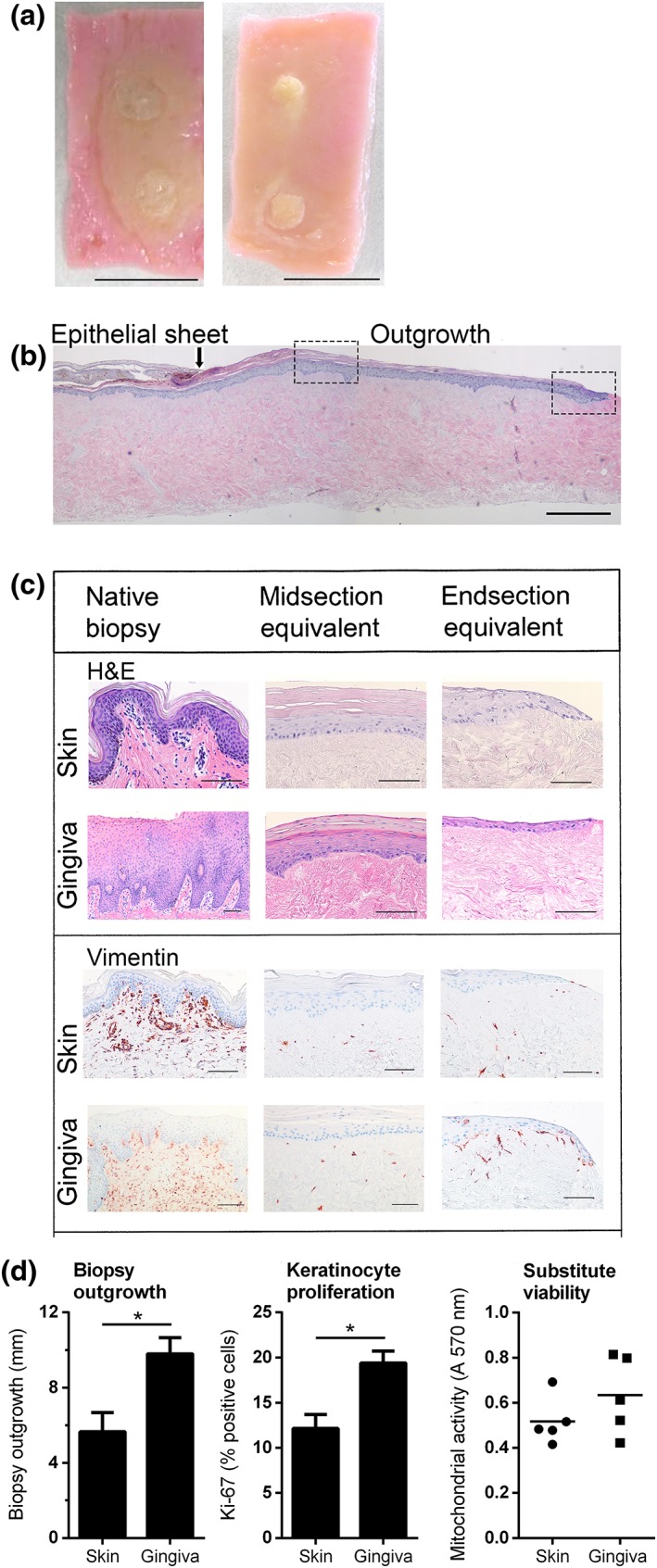
Gingiva substitutes show enhanced epithelial outgrowth and proliferation compared with skin substitutes. (A) representative macroscopic view of a skin substitute (left) and a gingiva substitute (right) at the time of harvesting after 3 weeks of culturing. Scale bar = 1 cm. (B) representative microscopic image. The original epithelial sheet of the biopsy and the outgrowing epithelial tissue of a skin substitute are shown with haematoxylin and eosin (H&E) staining. The arrow indicates the end of the epithelial sheet of the biopsy and the beginning of the epithelial outgrowth). The left box indicates the location of the ‘midsection’ in Figure 2C; the right box indicates the ‘end section'. Scale bar = 0.5 mm. (C) Representative H&E and vimentin staining is shown for native tissue, the midsection of the substitute as well as the migrating front of the substitute (end section). The skin substitute represents orthokeratinized epithelium, whereas the gingiva substitute represents parakeratinized epithelium. Scale bar = 100 μm. (D) extent of outgrowth from the epithelial sheet of the biopsy (left), percentage of Ki‐67‐positive keratinocytes (middle) and cell viability as determined by MTT assay (right) is shown for skin substitutes and gingiva substitutes. An absorbance above 0.05 indicates mitochondrial activity and cell viability. Bars represent means ± standard error of the mean of n = 6 skin donors for skin substitutes and n = 5 gingiva donors for gingival substitutes. *p < 0.05, **p < 0.01
Immunohistochemical staining was performed essentially as described previously (Vriens et al., 2008). For staining of keratin 6, 10, 13, 16, 17, Ki‐67 and vimentin, antigen retrieval was performed by immersion of the slides in 0.01 m citrate buffer (pH 6.0) for 15 min at 100°C, followed by slow cooling to room temperature for 3 h. For loricrin and involucrin, endogenous peroxidase was inhibited by 20 min incubation in 0.3% H2O2 in methanol solution. Subsequently, loricrin was pre‐incubated with goat serum (X0907; Dako, Glostrup, Denmark). All sections were incubated for 1 h with primary antibodies; keratin 6: clone KA12 (cat. no. 61090; Progen Biotechnik, Heidelberg, Germany); keratin 10: clone DE‐K10 (cat. no. 11414; Progen Biotechnik); keratin 13: clone 1C7 (cat. no. MON3017; Monosan, Uden, the Netherlands); keratin 16: clone LL025 (cat. no. MONX10691; Monosan); keratin 17: clone E3 (cat. no. MONX10692; Monosan); Ki‐67: clone Mib1 (cat. no. M7240; Dako); loricrin: clone AF‐62 (cat. no. PRB‐145P‐100; Covance Inc., Princeton, NJ, USA); involucrin: clone SY5 (cat. no. NCI‐INV; Novocastra, Leica Biosystems, Newcastle, UK); vimentin: clone V9 (cat. no. M0725; Dako). For keratin 13 staining, incubation with post‐antibody blocking and incubation with powervision‐HRP horseradish peroxidase solution was performed. Slides for all stainings were incubated with Envision (K4001; Dako), except for loricrin, which was incubated with goat‐anti rabbit‐biotin (E0432; Dako) and streptavidin‐HRP (P0397; Dako) both for 30 min.
The proliferation index is expressed as the number of Ki‐67 positively stained nuclei/total number of basal epidermal cells × 100%. From three different regions of each section, approximately 100 basal cells were counted and then averaged.
Metabolic activity was analysed using the MTT assay with 3 mm biopsies taken from the visible outgrowth of the epithelium. Biopsies were incubated in a 2 mg/ml MTT solution (Sigma‐Aldrich) for 2 h at 37°C and transferred to an isopropanol/HCl solution (3:1). The next day the colour intensity was measured at 570 nm.
2.4. Quantification of cytokine and growth factor secretion
For the quantification of interleukin‐6 (IL‐6), CCL2, CCL5, CCL27, CCL28, vascular endothelial growth factor (VEGF), hepatocyte growth factor (HGF) and basic fibroblast growth factor (bFGF) in culture supernatants, ELISA reagents were used in accordance with the manufacturer's specifications. Commercially available paired ELISA antibodies and recombinant proteins obtained from R&D Systems Inc. (Minneapolis, MN, USA) were used. For CXCL8, a Pelipair reagent set (Sanquin, Amsterdam, the Netherlands) was used. As SS and GS are derived from two 3 mm punch biopsies growing on a single piece of acellular dermis (1.5 × 2.5 cm) with 12.5 ml culture medium, the results are expressed as pg/ml derived from the 12.5 ml culture supernatant and 3.75 cm2 (1.5 × 2.5 cm) tissue substitute. This method of analysis was accepted by the Dutch authorities as a means of expressing standardized potency of an autologous tissue substitute designed for clinical applications. The detection limits of the ELISAs were 30 pg/ml.
2.5. Statistical analysis
All data are presented as mean ± standard error mean. The results represent SS and GS data obtained from six skin donors (n = 6) and five gingiva donors (n = 5), respectively. Therefore, each n represents a separate donor and also an independent experiment. SS were cultured in duplicate from each donor and the two values were averaged to a single value per donor, but due to the limited amount of tissue available, GS were cultured in single fold from each donor. Differences between SS and GS were calculated with two‐tailed, unpaired t‐tests. Statistics were calculated in GraphPad Prism (San Diego, CA, USA). Differences were considered significant when *p < 0.05, **p < 0.01, ***p < 0.005.
3. RESULTS
As the SS and GS were produced under identical culture procedures they provide an excellent tool to investigate similarities and differences between the mode of action of the two tissue substitutes. Below we show in vitro mode of action data concerning (i) viability: epithelial expansion, proliferation (Ki‐67), metabolic activity (MTT assay); (ii) characterization: histology and immunohistochemistry; and (iii) potency: soluble wound healing mediator release (ELISA).
3.1. Viability: GS show enhanced epithelial outgrowth and proliferation compared with SS
Metabolic activity, which correlates to cell viability, was determined with an MTT assay. No difference was observed between the metabolic activities of 3 mm diameter punch biopsies removed from SS and GS, and all values obtained were above those of dead acellular donor dermis, clearly indicating that the tissue substitutes were viable (Figure 2D, right panel).
Both SS and GS consisted of a viable differentiated epithelium growing out from the original 3 mm diameter epithelial sheet and a fibroblast populated human connective tissue matrix. Similar to the native biopsies, the SS had a multilayered orthokeratinized epithelium, whereas the GS had a parakeratinized epithelium in which the terminally differentiated cells in the upper layers retained remnants of nuclei. Rete ridge formation in the SS and GS was not as pronounced as in the native biopsies (Figure 2A–C). This is in line with the previously reported first generation SS and GS (Vriens et al., 2008). As epithelialization is achieved by keratinocyte migration and proliferation, first the histology and extent of outgrowth from the skin and gingival epithelial sheets over the connective tissue matrix after the 3 week culture period was compared. The histology of the mid‐section adjacent to the original epithelial sheet was analysed separately from the outermost migrating epithelial front and both were compared with the intact healthy native biopsy (Figure 2C). Histology of the mid‐section of the SS closely resembled that of the native biopsy, with a stratum basale, stratum spinosum, stratum granulosum and stratum corneum. The mid‐section of the GS also resembled the native biopsy with the cells within the upper most differentiated layers retaining remnants of nuclei. Whereas SS epithelium had approximately the same number of living cell layers as the native tissue biopsy, GS had clearly less cell layers in the midsection compared with the native tissue biopsy. In contrast to the mid‐sections, the migrating fronts of both SS and GS contained no well‐defined differentiated cell layers. Also, the GS migrating front was thinner and more extended than the SS migrating front, which is indicative of a faster epithelial outgrowth (Figure 2C). Indeed, the gingival epithelium expanded 1.7‐fold more than skin epithelium during the 3 week culture period (Figure 2D). Furthermore, the percentage of proliferating Ki‐67‐positive cells was >1.5‐fold higher in GS compared with SS (Figures 2D, 3). Taken together, our findings on epithelial migration and proliferation correlate to the higher turnover and wound closure (epithelialization) capacity of gingiva compared with skin.
Figure 3.
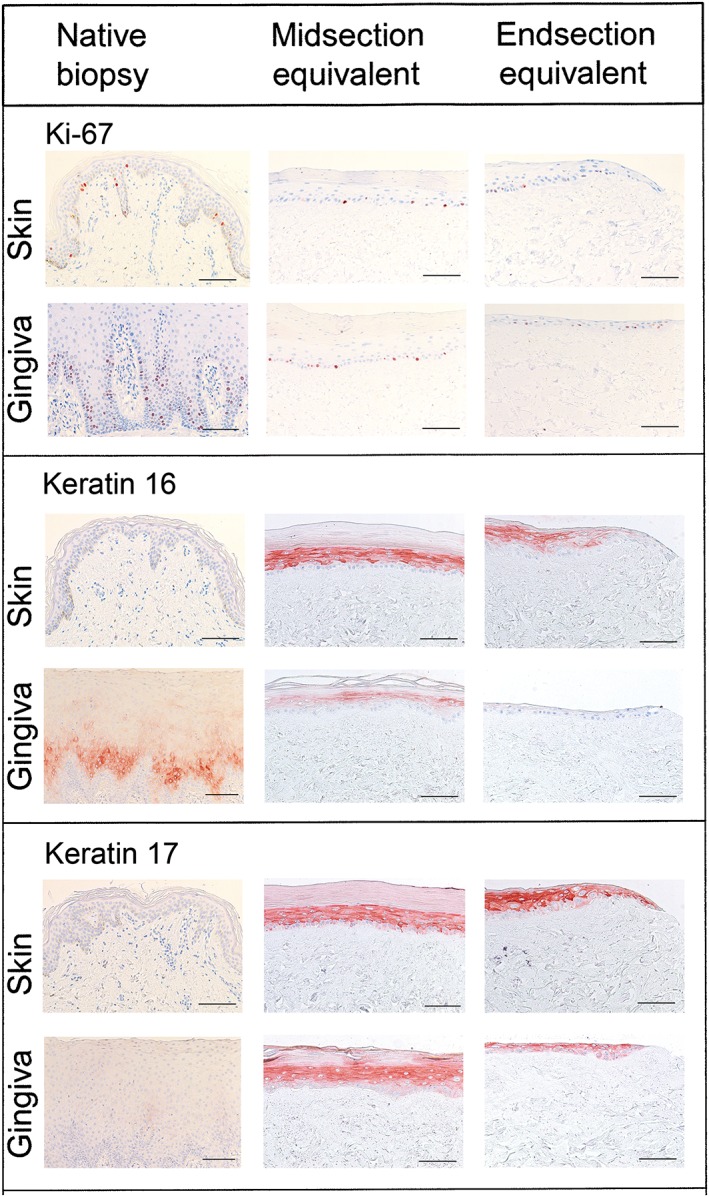
Histology and immunohistochemistry of native skin and gingiva biopsies and skin and gingiva substitutes. Representative Ki‐67 and keratin 16 and 17 staining is shown for native biopsy tissue, the midsection and the migrating front (end section) of skin and gingiva substitutes. Scale bar = 100 μm
3.2. Characterization: Keratins 16 and 17 are strongly expressed in expanding skin and gingiva epithelium
Differential keratin expression is a characteristic of epithelial tissues derived from different body locations. For example, keratin 16 is expressed in native gingiva, but is absent in native skin (Figure 3). Furthermore, keratins 16 and 17 are described to be upregulated during wound healing as they disrupt the rigid structure of the epithelium, thus allowing keratinocytes to migrate over each other and over the connective tissue matrix (Paladini, Takahashi, Bravo, & Coulombe, 1996). Notably, both keratins were expressed in the midsections of the SS as well as the GS. Whereas keratin 17 was also expressed in the migrating fronts of both SS and GS, keratin 16 was clearly absent in the most undifferentiated frontal cells in both the SS and the GS (Figure 3). Keratins 6, 10, 13, loricrin and involucrin showed differential expression in the SS and GS, which was representative of the original biopsy tissue and in line with our previous findings with first generation SS and GS (Gibbs et al., 2006; Vriens et al., 2008) (Table 1).
Table 1.
Summary of (immuno)histological comparison between skin and gingiva substitute
| Characteristic | Skin substitute | Gingiva substitute |
|---|---|---|
| Keratin 6 | SS, SG | SB‐U |
| Keratin 10 | SS, SG | SB‐Ia |
| Keratin 13 | Absent | ± SBb |
| Keratin 16 | SB | SB |
| Keratin 17 | SB | SB |
| Loricrin | SG | SB‐U |
| Involucrin | SG | SB‐U |
| Ki‐67 | BL | BL |
BL, basal layer; SG, stratum granulosum; SS, stratum spinosum; SB, suprabasal; SB‐U, upper suprabasal layers; SB‐I, intermittent expression in suprabasal layers.
Present in two gingival substitutes only
Present in three gingival substitutes only
3.3. Potency: GS secrete higher amounts of wound healing mediators than SS
The potency of both SS and GS can be ascribed to the type and amount of secreted mediators that stimulate wound healing and vascularization. Therefore, cytokines (Figure 4A) and growth factors (Figure 4B) secreted by SS and GS that have been described to play a role in wound healing (Table 2) were compared.
Figure 4.
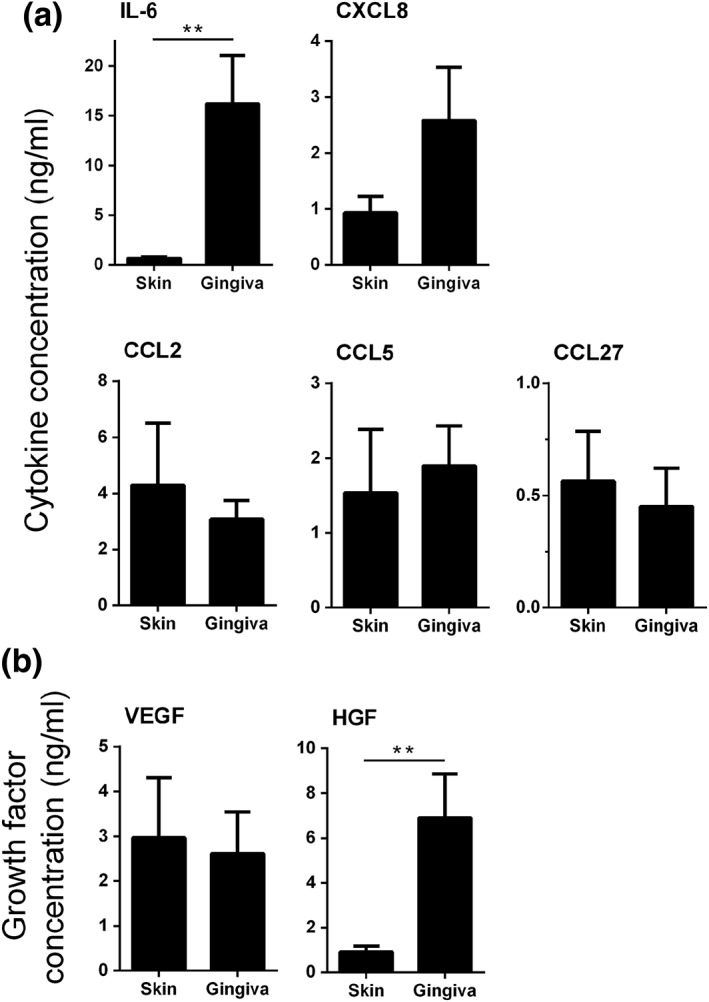
Wound healing mediators secreted by skin and gingiva substitutes. (A) cytokine secretion [interleukin‐6 (IL‐6), CXCL8, CCL2, CCL5 and CCL27] and (B) growth factor secretion [vascular endothelial growth factor (VEGF), hepatocyte growth factor (HGF)] by skin and gingiva substitutes are shown. Protein levels in the culture supernatants were determined by means of enzyme‐linked immunosorbent assay (ELISA). Background amounts of cytokines and growth factors within the fibroblast culture medium were below the detection limits of the ELISA (data not shown). Bars represent means ± standard error of the mean of n = 6 skin donors for skin substitutes and n = 5 gingiva donors for gingival substitutes. *p < 0.05, **p < 0.01
Table 2.
Cytokines and growth factors involved in wound healing
| Name | Function in wound healing | Reference |
|---|---|---|
| Interleukin‐6 |
Immune response during infection and after trauma Neutrophil chemoattractant Fibroblast proliferation, keratinocyte migration and proliferation |
Efron & Moldawer, 2004; Werner & Grose, 2003 |
| CXCL8 |
Mediator in innate immune response Neutrophil and macrophage activation and chemotaxis Keratinocyte proliferation |
Efron & Moldawer, 2004; Kroeze et al., 2012a |
| CCL2/MCP‐1 | Macrophages, T‐cell and mast cell chemoattractant | Werner & Grose, 2003 |
| CCL5/RANTES | Fibroblast migration | Kroeze et al., 2009 |
| CCL27/CTACK |
Effector cell recruitment to sites of epithelial injury Keratinocyte chemoattractant |
Hieshima et al., 2003
Kroeze et al., 2009 |
| CCL28/MEC | Effector cell recruitment to sites of epithelial injury | Hieshima et al., 2003 |
| Hepatocyte growth factor |
Keratinocyte migration and proliferation Angiogenesis Anti‐fibrosis |
Werner & Grose, 2003; Crestani et al., 2012 |
| Vascular endothelial growth factor | Stimulation of vasculogenesis and angiogenesis | Efron & Moldawer, 2004 |
| Basic fibroblast growth factor |
Fibroblast proliferation, keratinocyte migration and proliferation Endothelial growth and migration Collagen remodelling |
Efron & Moldawer, 2004 |
GS secreted higher amounts than SS of key cytokines involved in mitogenesis, motogenesis and chemotaxis (IL‐6 > 23‐fold, CXCL8 > 2.5‐fold), as well as higher amounts of the anti‐fibrotic growth factor HGF (>7‐fold) (Figure 4). No differences were found between GS and SS with regards to chemokines CCL2, CCL5, CCL27 and growth factor VEGF secretion. CCL28 and bFGF were undetectable in both SS and GS culture supernatants.
4. DISCUSSION
In general, oral wounds heal more rapidly and with better final scar quality than skin wounds (Engeland et al., 2006; Kiecolt‐Glaser et al., 1995; Larjava et al., 2011; Mak et al., 2009). In this study we characterized and compared the mode of action of SS and GS that were cultured under identical procedures and are fully compliant with the current European regulations for ATMPs. Taken together, our results show that gingiva tissue is intrinsically more primed towards enhanced wound healing compared with skin, given the higher keratinocyte migration and proliferation, as well as enhanced secretion of wound healing and anti‐fibrotic mediators (IL‐6, CXCL8 and HGF) in GS when compared with SS.
The autologous SS and GS have been designed for the clinical setting in which biopsies are taken from the patient to be treated. Each SS and GS is 1.5 × 2.5 cm2 and therefore can be used to cover 3.75 cm2 wound surface. For larger wounds, more biopsies are required. We have previously shown that large chronic ulcers (>150 cm2) can successfully be treated with the first generation SS. Oral wounds are generally smaller than skin wounds and therefore will require less biopsies to construct sufficient GS pieces to close the wound (Blok et al., 2013; Gibbs et al., 2006; Vriens et al., 2008).
It was not possible to determine directly the efficacy of fibroblast migration into the reticular side of the dermis as the individual SS and GS were too small to take samples to isolate and quantify cells migrating into the dermis. It was considered unethical in the autologous patient setting to take extra biopsies to construct an extra SS and GS in order to introduce a quality control on the exact number of fibroblasts within the construct. Therefore quality controls during culture that confirmed fibroblast viability (>50% confluent after approximately 1 week of culture) at the time point when fibroblasts are combined with the epithelial sheet growing on the dermis were accepted. During the following 2 weeks (3 weeks of culture total), fibroblasts migrated into the dermis (Monsuur et al., 2016). Many fibroblasts remain in the lower regions of the dermis, with few cells migrating into the upper regions. However, as we have previously shown that soluble mediators HGF, IL‐6, CCL2, CCL5 and CXCL8 are secreted by SS only when fibroblasts are present (Spiekstra et al., 2007) and as we show that vimentin is detectable in both SS and GS, we can conclude that viable fibroblasts as well as epithelial cells are present in SS and GS.
Clinical studies show that gingiva has a higher number of epithelial cell layers and a higher turnover than skin and also has a faster rate of wound closure. Our results clearly show that the increased proliferation and migration capacity of gingiva compared with skin is an intrinsic property of the epithelium, and that our GS and SS closely mimic corresponding native healing tissues. However, as we did not observe an increased number of epithelial cell layers in GS compared with SS we can conclude that the transition of proliferating keratinocytes into terminally differentiating keratinocytes and the following stratification into a multilayered epithelium is regulated in part by extrinsic environmental factors, possibly for example by soluble mediators present in saliva (Brand, Ligtenberg, & Veerman, 2014).
Upon injury, keratin 16 and 17 are strongly induced in post‐mitotic cells at the wound edge. It is thought that these keratins may promote reorganization of the cytoplasmic array of keratin filaments (Paladini et al., 1996). This is an event that precedes the onset of keratinocyte migration into the wound site. In the mid‐section of SS, which mimics actively proliferating and migrating epidermis, keratin 16 and 17 were highly expressed in the suprabasal cell layers, similar to the corresponding region in GS. However, we found that in the very outermost migrating front of both SS and GS, where only one or two undifferentiated keratinocyte cell layers are present, keratin 16 was absent. In line with these findings, an elevated level of keratin 16 in mice has been shown to partially impair keratinocyte migration, although the mechanism is unknown (Wawersik, Mazzalupo, Nguyen, & Coulombe, 2001). Taken together, these findings suggest that keratin 16 and 17 enable keratinocytes to migrate over each other, but keratin 16, in contrast to keratin 17, may not be involved in keratinocyte migration over the connective tissue matrix. Minor discrepancies (regarding GS proliferation and keratin 17 expression) were observed when comparing our results with our previous study (Vriens et al., 2008). These can most probably be attributed to the fact that in our previous study gingiva was obtained from molar tooth extractions, whereas in this study gingival biopsies were obtained from the edentulous area prior to placing dental implants. The SS and GS were further extensively characterized with the aid of immunohistochemistry. Close correlations were found between the tissue substitutes and their native tissues with regards to epithelial keratin, loricrin and involucrin expression, which again emphasized the specific intrinsic differences between skin and gingiva.
In order to determine the potency of the tissue substitutes, the secretion of cytokines, chemokines and growth factors described to play a role in wound healing was determined (Table 2). Secretion of wound healing mediators IL‐6 and CXCL8 by GS was much higher than by SS, again supporting the greater intrinsic healing capacity of gingiva compared with skin. For CXCL8 and IL‐6, literature is conflicting. There is evidence that elevated levels of CXCL8 stimulate keratinocyte proliferation in vitro (Kroeze et al., 2012a; Rennekampff et al., 2000), but the opposite, inhibition of keratinocyte proliferation, has also been described (Iocono et al., 2000). Furthermore, elevated levels of CXCL8 have been described to contribute to delayed wound healing, as CXCL8 was increased in non‐healing human thermal wounds compared with healing wounds. High amounts of CXCL8 and IL‐6 were detected in wound extracts of non‐healing ulcers (Kroeze et al., 2012b). However, it has also been described that tumour necrosis factor‐α stimulates oral keratinocytes to produce more CXCL8 and IL‐6 than skin keratinocytes (Li, Farthing, Ireland, & Thornhill, 1996a; Li, Ireland, Farthing, & Thornhill, 1996b). In general, IL‐6 and CXCL8 are pleiotropic in nature, exhibiting increases and decreases according to the homeostatic environment.
Interestingly, gingiva not only heals faster than skin, but also heals with negligible final scarring (Larjava et al., 2011; Mak et al., 2009; Szpaderska, Zuckerman, & DiPietro, 2003), although it is currently unknown why. HGF has been shown to have an anti‐fibrotic effect and it also acts as a mitogen and motogen (Crestani et al., 2012). HGF has been described to be anti‐apoptotic on endothelial cells, while at the same time it promotes myofibroblast apoptosis. These properties would clearly be expected to increase the quality of a scar and suggests that the increased amount of HGF secreted by GS compared with SS may contribute to the intrinsic property of gingiva to heal with superior scar quality. Indeed, in the pilot study in which three tooth extraction sites were treated with the first generation GS, the oral lesions healed with negligible scarring (Vriens et al., 2008).
Surprisingly, we show that GS are able to secrete the skin‐specific chemokine CCL27, whereas the mucosa homologue CCL28 was undetectable. Both chemokines are lymphocyte chemo‐attractants (Morales et al., 1999) and CCL28 has high homology with CCL27 (Wang et al., 2000). Recently we have shown in another GS model (reconstructed epithelium on a fibroblast populated collagen hydrogel) that gingiva can indeed secrete CCL27 and that it is also inducible with tumour necrosis factor‐α, albeit to a much lower extent than in skin equivalents (Kosten, Buskermolen, Spiekstra, de Gruijl, & Gibbs, 2015). The reason why CCL28 was undetectable is currently unknown, but it is possible that it is directly internalized by other cells in the vicinity, such as fibroblasts.
In this study we describe for the first time SS and GS cultured under identical conditions, which are fully compliant with the current European regulations for ATMPs. In addition to strict culture methods and quality controls, ATMP regulations now require information on the mode of action of the final product. In this study we have presented in vitro methods that were accepted by the Dutch authorities. Taken together, our information on mode of action has highlighted intrinsic differences between the two tissues that could be related to superior oral mucosa healing.
CONFLICT OF INTEREST
S. Gibbs and R. Scheper are co‐founders and shareholders of A‐Skin BV.
ACKNOWLEDGEMENTS
The authors thank Professor E.C.I. Veerman and Dr J.G.M Bolscher for motivating discussions. This study was financed by the Dutch Technology Foundation (Stichting Technische Wetenschappen), grant 10695, the VU University Medical Center and A‐Skin BV, which is a VU University spin‐off SME.
Boink MA, Roffel S, Breetveld M, et al. Comparison of advanced therapy medicinal product gingiva and skin substitutes and their in vitro wound healing potentials. J Tissue Eng Regen Med. 2018;12:e1088–e1097. https://doi.org/10.1002/term.2438
REFERENCES
- Blok, C. S. , Vink, L. , de Boer, E. M. , van Montfrans, C. , van den Hoogenband, H. M. , Mooij, M. C. , … Gibbs, S. (2013). Autologous skin substitute for hard‐to‐heal ulcers: Retrospective analysis on safety, applicability, and efficacy in an outpatient and hospitalized setting. Wound Repair and Regeneration, 21, 667–676. [DOI] [PubMed] [Google Scholar]
- Brand, H. S. , Ligtenberg, A. J. , & Veerman, E. C. (2014). Saliva and wound healing. Monographs in Oral Science, 24, 52–60. [DOI] [PubMed] [Google Scholar]
- Crestani, B. , Marchand‐Adam, S. , Quesnel, C. , Plantier, L. , Borensztajn, K. , Marchal, J. , … Dehoux, M. (2012). Hepatocyte growth factor and lung fibrosis. Proceedings of the American Thoracic Society, 9, 158–163. [DOI] [PubMed] [Google Scholar]
- Efron, P. A. , & Moldawer, L. L. (2004). Cytokines and wound healing: The role of cytokine and anticytokine therapy in the repair response. Journal of Burn Care & Rehabilitation, 25, 149–160. [DOI] [PubMed] [Google Scholar]
- Engeland, C. G. , Bosch, J. A. , Cacioppo, J. T. , & Marucha, P. T. (2006). Mucosal wound healing: The roles of age and sex. Archives of Surgery, 141, 1193–1197. [DOI] [PubMed] [Google Scholar]
- Falanga, V. , & Sabolinski, M. (1999). A bilayered living skin construct (APLIGRAF) accelerates complete closure of hard‐to‐heal venous ulcers. Wound Repair and Regeneration, 7, 201–207. [DOI] [PubMed] [Google Scholar]
- Gardien, K. L. , Marck, R. E. , Bloemen, M. C. , Waaijman, T. , Gibbs, S. , Ulrich, M. M. , & Middelkoop, E. (2016). Outcome of burns treated with autologous cultured proliferating epidermal cells: A prospective randomized multicenter intrapatient comparative trial. Cell Transplantation, 25, 437–448. [DOI] [PubMed] [Google Scholar]
- Gibbs, S. , van den Hoogenband, H. M. , Kirtschig, G. , Richters, C. D. , Spiekstra, S. W. , Breetveld, M. , … de Boer, E. M. (2006). Autologous full‐thickness skin substitute for healing chronic wounds. British Journal Dermatology, 155, 267–274. [DOI] [PubMed] [Google Scholar]
- Gravante, G. , Di Fede, M. C. , Araco, A. , Grimaldi, M. , De, A. B. , Arpino, A. , … Montone, A. (2007). A randomized trial comparing ReCell system of epidermal cells delivery versus classic skin grafts for the treatment of deep partial thickness burns. Burns, 33, 966–972. [DOI] [PubMed] [Google Scholar]
- Hieshima, K. , Ohtani, H. , Shibano, M. , Izawa, D. , Nakayama, T. , Kawasaki, Y. , … Yoshie, O. (2003). CCL28 has dual roles in mucosal immunity as a chemokine with broad‐spectrum antimicrobial activity. Journal of Immunology, 170, 1452–1461. [DOI] [PubMed] [Google Scholar]
- Iocono, J. A. , Colleran, K. R. , Remick, D. G. , Gillespie, B. W. , Ehrlich, H. P. , & Garner, W. L. (2000). Interleukin‐8 levels and activity in delayed‐healing human thermal wounds. Wound Repair and Regeneration, 8, 216–225. [DOI] [PubMed] [Google Scholar]
- Jones, J. E. , Nelson, E. A. , & Al‐Hity, A. (2013). Skin grafting for venous leg ulcers. Cochrane Database of Systematic Reviews, 1, CD001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt‐Glaser, J. K. , Marucha, P. T. , Malarkey, W. B. , Mercado, A. M. , & Glaser, R. (1995). Slowing of wound healing by psychological stress. The Lancet, 346, 1194–1196. [DOI] [PubMed] [Google Scholar]
- Kirsner, R. S. , Marston, W. A. , Snyder, R. J. , Lee, T. D. , Cargill, D. I. , & Slade, H. B. (2012). Spray‐applied cell therapy with human allogeneic fibroblasts and keratinocytes for the treatment of chronic venous leg ulcers: A phase 2, multicentre, double‐blind, randomised, placebo‐controlled trial. The Lancet, 380, 977–985. [DOI] [PubMed] [Google Scholar]
- Kosten, I. J. , Buskermolen, J. K. , Spiekstra, S. W. , de Gruijl, T. D. , & Gibbs, S. (2015). Gingiva equivalents secrete negligible amounts of key chemokines involved in Langerhans cell migration compared to skin equivalents. Journal of Immunology Research, 2015, 627125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroeze, K. L. , Boink, M. A. , Sampat‐Sardjoepersad, S. C. , Waaijman, T. , Scheper, R. J. , & Gibbs, S. (2012a). Autocrine regulation of re‐epithelialization after wounding by chemokine receptors CCR1, CCR10, CXCR1, CXCR2, and CXCR3. Journal of Investigative Dermatology, 132, 216–225. [DOI] [PubMed] [Google Scholar]
- Kroeze, K. L. , Jurgens, W. J. , Doulabi, B. Z. , van Milligen, F. J. , Scheper, R. J. , & Gibbs, S. (2009). Chemokine‐mediated migration of skin‐derived stem cells: Predominant role for CCL5/RANTES. Journal of Investigative Dermatology, 129, 1569–1581. [DOI] [PubMed] [Google Scholar]
- Kroeze, K. L. , Vink, L. , de Boer, E. M. , Scheper, R. J. , van Montfrans, M. C. , & Gibbs, S. (2012b). Simple wound exudate collection method identifies bioactive cytokines and chemokines in (arterio) venous ulcers. Wound Repair and Regeneration, 20, 294–303. [DOI] [PubMed] [Google Scholar]
- Larjava, H. , Wiebe, C. , Gallant‐Behm, C. , Hart, D. A. , Heino, J. , & Hakkinen, L. (2011). Exploring scarless healing of oral soft tissues. Journal of the Canadian Dental Association, 77, b18. [PubMed] [Google Scholar]
- Li, J. , Farthing, P. M. , Ireland, G. W. , & Thornhill, M. H. (1996a). IL‐1+ and IL‐6 production by oral and skin keratinocytes: Similarities and differences in response to cytokine treatment in vitro. Journal of Oral Pathology & Medicine, 25, 157–162. [DOI] [PubMed] [Google Scholar]
- Li, J. , Ireland, G. W. , Farthing, P. M. , & Thornhill, M. H. (1996b). Epidermal and oral keratinocytes are induced to produce RANTES and IL‐8 by cytokine stimulation. Journal of Investigative Dermatology, 106, 661–666. [DOI] [PubMed] [Google Scholar]
- Mak, K. , Manji, A. , Gallant‐Behm, C. , Wiebe, C. , Hart, D. A. , Larjava, H. , & Hakkinen, L. (2009). Scarless healing of oral mucosa is characterized by faster resolution of inflammation and control of myofibroblast action compared to skin wounds in the red Duroc pig model. Journal of Dermatological Science, 56, 168–180. [DOI] [PubMed] [Google Scholar]
- Marston, W. A. , Hanft, J. , Norwood, P. , & Pollak, R. (2003). The efficacy and safety of Dermagraft in improving the healing of chronic diabetic foot ulcers: Results of a prospective randomized trial. Diabetes Care, 26, 1701–1705. [DOI] [PubMed] [Google Scholar]
- Monsuur, H. N. , Boink, M. A. , Weijers, E. M. , Roffel, S. , Breetveld, M. , Gefen, A. , … Gibbs, S. (2016). Methods to study differences in cell mobility during skin wound healing in vitro. Journal of Biomechanics, 49(8), 1381–1387. [DOI] [PubMed] [Google Scholar]
- Morales, J. , Homey, B. , Vicari, A. P. , Hudak, S. , Oldham, E. , Hedrick, J. , … Zlotnik, A. (1999). CTACK, a skin‐associated chemokine that preferentially attracts skin‐homing memory T cells. Proceedings of the National Academy of Sciences of the United States of America, 96, 14470–14475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paladini, R. D. , Takahashi, K. , Bravo, N. S. , & Coulombe, P. A. (1996). Onset of re‐epithelialization after skin injury correlates with a reorganization of keratin filaments in wound edge keratinocytes: Defining a potential role for keratin 16. Journal of Cell Biology, 132, 381–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennekampff, H. O. , Hansbrough, J. F. , Kiessig, V. , Dore, C. , Sticherling, M. , & Schroder, J. M. (2000). Bioactive interleukin‐8 is expressed in wounds and enhances wound healing. Journal of Surgical Research, 93, 41–54. [DOI] [PubMed] [Google Scholar]
- Spiekstra, S. W. , Breetveld, M. , Rustemeyer, T. , Scheper, R. J. , & Gibbs, S. (2007). Wound‐healing factors secreted by epidermal keratinocytes and dermal fibroblasts in skin substitutes. Wound Repair and Regeneration, 15, 708–717. [DOI] [PubMed] [Google Scholar]
- Szpaderska, A. M. , Zuckerman, J. D. , & DiPietro, L. A. (2003). Differential injury responses in oral mucosal and cutaneous wounds. Journal of Dental Research, 82, 621–626. [DOI] [PubMed] [Google Scholar]
- Veves, A. , Falanga, V. , Armstrong, D. G. , & Sabolinski, M. L. (2001). Graftskin, a human skin equivalent, is effective in the management of noninfected neuropathic diabetic foot ulcers: A prospective randomized multicenter clinical trial. Diabetes Care, 24, 290–295. [DOI] [PubMed] [Google Scholar]
- Vriens, A. P. , Waaijman, T. , van den Hoogenband, H. M. , de Boer, E. M. , Scheper, R. J. , & Gibbs, S. (2008). Comparison of autologous full‐thickness gingiva and skin substitutes for wound healing. Cell Transplantation, 17, 1199–1209. [DOI] [PubMed] [Google Scholar]
- Wang, W. , Soto, H. , Oldham, E. R. , Buchanan, M. E. , Homey, B. , Catron, D. , … Zlotnik, A. (2000). Identification of a novel chemokine (CCL28), which binds CCR10 (GPR2). Journal of Biological Chemistry, 275, 22313–22323. [DOI] [PubMed] [Google Scholar]
- Wawersik, M. J. , Mazzalupo, S. , Nguyen, D. , & Coulombe, P. A. (2001). Increased levels of keratin 16 alter epithelialization potential of mouse skin keratinocytes in vivo and ex vivo. Molecular Biology of the Cell, 12, 3439–3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner, S. , & Grose, R. (2003). Regulation of wound healing by growth factors and cytokines. Physiological Reviews, 83, 835–870. [DOI] [PubMed] [Google Scholar]
- Wolff, J. , Farre‐Guasch, E. , Sandor, G. K. , Gibbs, S. , Jager, D. J. , & Forouzanfar, T. (2016). Soft tissue augmentation techniques and materials used in the oral cavity: An overview. Implant Dentistry, 25(3), 427–434. [DOI] [PubMed] [Google Scholar]
- Wood, F. , Martin, L. , Lewis, D. , Rawlins, J. , McWilliams, T. , Burrows, S. , & Rea, S. (2012). A prospective randomised clinical pilot study to compare the effectiveness of Biobrane(R) synthetic wound dressing, with or without autologous cell suspension, to the local standard treatment regimen in paediatric scald injuries. Burns, 38, 830–839. [DOI] [PubMed] [Google Scholar]
- Wu, S. C. , Marston, W. , & Armstrong, D. G. (2010). Wound care: The role of advanced wound‐healing technologies. Journal of the American Podiatric Medical Association, 100, 385–394. [DOI] [PubMed] [Google Scholar]
- Zaulyanov, L. , & Kirsner, R. S. (2007). A review of a bi‐layered living cell treatment (Apligraf) in the treatment of venous leg ulcers and diabetic foot ulcers. Clinical Interventions in Aging, 2, 93–98. [DOI] [PMC free article] [PubMed] [Google Scholar]


