Abstract
Aims
To assess ertugliflozin in patients with type 2 diabetes who are inadequately controlled by metformin and sitagliptin.
Materials and Methods
In this double‐blind randomized study (Clinicaltrials.gov NCT02036515), patients (glycated haemoglobin [HbA1c] 7.0% to 10.5% [53‐91 mmol/mol] receiving metformin ≥1500 mg/d and sitagliptin 100 mg/d; estimated glomerular filtration rate [eGFR] ≥60 mL/min/1.73 m2) were randomized to ertugliflozin 5 mg once‐daily, 15 mg once‐daily or placebo. The primary efficacy endpoint was change from baseline in HbA1c at Week 26; treatment was continued until Week 52.
Results
A total of 464 patients were randomized (mean baseline HbA1c, 8.0% [64.3 mmol/mol]; eGFR, 87.9 mL/min/1.73 m2). After 26 weeks, placebo‐adjusted least squares (LS) mean changes in HbA1c from baseline were −0.7% (−7.5 mmol/mol) and −0.8% (−8.3 mmol/mol) for ertugliflozin 5 and 15 mg, respectively (both P < .001); 17.0%, 32.1% and 39.9% of patients receiving placebo, ertugliflozin 5 mg or ertugliflozin 15 mg, respectively, had HbA1c <7.0% (53 mmol/mol). Significant reductions in fasting plasma glucose, body weight (BW) and systolic blood pressure (SBP) were observed with ertugliflozin relative to placebo. The positive effects of ertugliflozin on glycaemic control, BW and SBP were maintained through Week 52. A higher incidence of genital mycotic infections was observed in male and female patients receiving ertugliflozin (3.7%‐14.1%) vs placebo (0%‐1.9%) through Week 52. The incidence of urinary tract infections, symptomatic hypoglycaemia and hypovolaemia adverse events were not meaningfully different across groups.
Conclusions
Ertugliflozin added to metformin and sitagliptin was well‐tolerated, and provided clinically meaningful, durable glycaemic control, BW and SBP reductions vs placebo over 52 weeks.
Keywords: DPP‐IV inhibitor, drug development, glycaemic control, SGLT2 inhibitor, sitagliptin, type 2 diabetes
1. INTRODUCTION
Type 2 diabetes mellitus (T2DM) is a progressive disease, for which many patients require combination therapy to maintain glycaemic control over time.1 Metformin is the standard first‐line pharmacotherapy for the treatment of T2DM, unless it is contra‐indicated or not tolerated.1 Dipeptidyl peptidase‐4 (DPP‐4) inhibitors, such as sitagliptin, are commonly used as second‐line therapy and exert an antihyperglycaemic effect by increasing concentrations of incretin hormones, thereby enhancing insulin secretion.2, 3 While the combination of metformin and sitagliptin has been shown to provide good glycaemic efficacy,4, 5 as the disease progresses and glycaemic control declines, some patients may benefit from the addition of a third agent with a complementary mode of action.1
Sodium‐glucose cotransporter 2 (SGLT2) inhibitors represent the latest class of agents approved for the treatment of T2DM.6, 7 They have an insulin‐independent mode of action and inhibit renal glucose reabsorption, resulting in enhanced urinary glucose excretion and thereby reducing plasma glucose and glycated haemoglobin (HbA1c) concentrations. SGLT2 inhibitors have also been shown to reduce body weight and blood pressure (BP)7 and are generally well‐tolerated, with a low risk of hypoglycaemia.
Combination therapy with an SGLT2 inhibitor and a DPP‐4 inhibitor offers 2 antihyperglycaemic agents with different, complementary mechanisms of action.2, 3 Furthermore, it has been hypothesized that DPP‐4 inhibitors may offset the increase in the rate of endogenous glucose production induced by SGLT2 inhibitors, potentially leading to additive effects on HbA1c reduction.8
Ertugliflozin is a highly selective SGLT2 inhibitor currently being evaluated in the VERTIS (eValuation of ERTugliflozin effIcacy and Safety) clinical trial programme. Two doses of ertugliflozin (5 and 15 mg once daily [QD]) were selected for Phase 3 studies, as these doses were predicted to provide >80% and >90% of maximal pharmacology for urinary glucose excretion and glycaemic (HbA1c, fasting plasma glucose [FPG]) and body weight endpoints based on Phase 2 studies.9, 10
The aim of this study (VERTIS SITA2) was to compare the safety and efficacy of the addition of ertugliflozin (5 and 15 mg QD) to that of placebo in patients with T2DM and inadequate glycaemic control while receiving a combination of metformin and sitagliptin.
2. MATERIAL AND METHODS
2.1. Study design
Protocol MK‐8835‐006 was a randomized, double‐blind, placebo‐controlled, parallel‐group Phase 3 study (ClinicalTrials.gov identifier: NCT02036515). It was conducted over 52 weeks in 2 phases; the primary time point was at Week 26 (Phase A) and treatment was continued into a 26‐week extension (Phase B).
The trial was conducted at 104 centres across 12 countries (Appendix S1). The trial started on April 7, 2014; the last patient completed Phase A on November 18, 2015 and Phase A + B on June 6, 2016. The study was conducted in accordance with the ethical principles that have their origin in the Declaration of Helsinki, and that are consistent with Good Clinical Practice and the applicable regulatory requirements. The study was approved by the appropriate institutional review boards and regulatory agencies. All participating patients provided written, informed consent. The protocol and statistical analysis plan were developed by the Sponsors in consultation with an external Scientific Advisory Committee.
2.2. Patient population
Adult patients with T2DM according to American Diabetes Association guidelines,11 who were receiving stable treatment with metformin (≥1500 mg/d, any formulation) and sitagliptin (100 mg/d) for ≥8 weeks, and had an HbA1c level of 7.0% to 10.5% (53‐91 mmol/mol) at the screening visit, entered a 2‐week single‐blind, placebo run‐in period prior to randomization. Patients undergoing this regimen for <8 weeks, receiving metformin ≥1500 mg/d along with a sulphonylurea, or receiving lower doses of metformin and/or another DPP‐4 inhibitor at screening, were eligible if they met the above criteria after the appropriate dose/medication adjustment, stabilization or washout period (Appendix S1).
Key exclusion criteria included: history of type 1 diabetes mellitus or assessment as possibly having type 1 diabetes mellitus, confirmed with a C‐peptide <0.23 nmol/L (0.7 ng/mL); history of ketoacidosis; history of myocardial infarction, unstable angina, arterial revascularization, stroke, transient ischaemic attack or functional class III–IV heart failure according to the New York Heart Association within 3 months of screening; mean value for triplicate sitting systolic BP (SBP) >160 mm Hg and/or diastolic BP (DBP) >90 mm Hg (patients receiving BP medication must have a stable regimen for ≥4 weeks prior to randomization); treatment in the previous 12 weeks with insulin of any type or antihyperglycaemic agents (AHA) other than metformin, DPP‐4 inhibitors or sulphonylureas; active, obstructive uropathy or indwelling urinary catheter; estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2; serum creatinine ≥115 μmol/L (1.3 mg/dL) in men or ≥106 μmol/L (1.2 mg/dL) in women; FPG >14.4 mmol/L (260 mg/dL) prior to the placebo run‐in period and confirmed within 7 days.
Patients with adequate compliance during the placebo run‐in period (≥80% based on pill count) were randomized 1:1:1 to receive ertugliflozin 5 mg QD, ertugliflozin 15 mg QD, or placebo using a computer‐generated randomization schedule. Patients were expected to take study medication in the morning without regard to food. Patients, investigators and the sponsor remained blinded during the 26‐week Phase A. The sponsor became unblinded after the Week 26 database lock to permit writing of the Phase A clinical study report; the investigators and patients remained blinded during the 26‐week Phase B extension.
Glycaemic rescue therapy with open‐label glimepiride (or insulin glargine if glimepiride was not considered appropriate) was prescribed for patients meeting glycaemic rescue criteria, which became more stringent over time (Appendix S1).
Prespecified protocol discontinuation criteria for renal function were either serum creatinine concentrations consistently ≥133 μmol/L (1.5 mg/dL) in men or ≥124 μmol/L (1.4 mg/dL) in women, or eGFR consistently (repeat measurement performed within 7 days) <50 mL/min/1.73 m2.
2.3. Efficacy assessments
The primary efficacy endpoint was change from baseline in HbA1c at Week 26. Prespecified key secondary efficacy endpoints subject to inferential hypothesis testing with strict alpha control were change from baseline at Week 26 in FPG, body weight and SBP, and the proportion of patients with HbA1c <7.0% (53 mmol/mol) at Week 26. Other efficacy endpoints, not included in the alpha‐controlled testing procedure, were change from baseline at Week 26 in DBP and homeostasis model assessment of β‐cell function (HOMA‐β), proportion of patients who received glycaemic rescue therapy through Week 26, and effect of ertugliflozin on quality of life (as assessed by the EuroQol five dimensions three level [EQ‐5D‐3L] questionnaire).12 No formal hypothesis testing was conducted at Week 52.
Efficacy assessments (HbA1c, FPG, body weight, SBP, DBP) were performed at Weeks 0 (baseline), 6, 12, 18, 26, 39 and 52. Body weight was measured in duplicate with a standardized digital scale. Sitting BP was measured in triplicate using an automated oscillometric BP measuring device. HOMA‐β was calculated using FPG and fasting C‐peptide levels, which were measured at baseline, Weeks 26 and 52, using the calculator released by the University of Oxford in 2004.13 The EQ‐5D‐3L assessment was performed at baseline, and at Weeks 26 and 52.
2.4. Safety assessments
Safety analysis was conducted at Week 26 (Phase A) and Week 52 (Phases A + B). Safety assessments included the number of patients with adverse events (AEs), including AEs of special interest prespecified for inferential testing without multiplicity control (symptomatic hypoglycaemia [defined as episodes with clinical symptoms reported by the investigator as hypoglycaemia; biochemical documentation not required], and AEs associated with genital mycotic infection [analysed by gender], urinary tract infection and hypovolaemia). In addition, documented hypoglycaemia (symptomatic and asymptomatic), defined as episodes with a glucose level ≤3.9 mmol/L (70 mg/dL), with or without symptoms, was recorded.
Prespecified laboratory, electrocardiogram (ECG) and postural BP parameters were assessed, as well as changes over time in laboratory parameters (including eGFR and lipid panels [including low‐density lipoprotein cholesterol (LDL‐C) and high‐density lipoprotein cholesterol (HDL‐C)]), ECG measurements and vital signs.
Orthostatic BP (defined in Appendix S1) was assessed at baseline, Weeks 6 and 26.
2.5. Statistical analyses
A planned sample size of approximately 405 patients (135 patients per group) was estimated to provide 97% power to detect a true difference of 0.5% in the mean change from baseline in HbA1c between a given ertugliflozin dose and placebo, based on a 2‐sided test at 5% level of significance, assuming approximately 19% attrition at Week 26, and assuming a standard deviation of 1.0.
Primary and key secondary efficacy endpoints were tested at Week 26 in the following order: HbA1c, FPG, body weight, proportion of patients with HbA1c <7.0% (53 mmol/mol) and SBP. For each endpoint, the 15‐mg dose was tested vs placebo, followed by the 5‐mg dose vs placebo. Each test was performed at the 0.05 level, and testing continued until a P value ≥.05 was obtained.
Efficacy analyses included all randomized patients who received ≥1 dose of study drug and had ≥1 measurement of the respective endpoint. Post‐rescue efficacy data were treated as missing in all efficacy analyses. A longitudinal data analysis (LDA) model14 was used to evaluate continuous endpoints, with fixed effects for treatment, prior antihyperglycaemic agents (metformin + DPP‐4 inhibitor / metformin + sulphonylurea), baseline eGFR (continuous), time (categorical) and interaction of time by treatment with a constraint that the true mean at baseline is common to all treatment groups, which is valid because of randomization. Missing data were handled implicitly by the model. Logistic regression was used to evaluate the proportion of patients with HbA1c <7.0% (53 mmol/mol), fitted with terms for treatment, baseline eGFR (continuous) and baseline HbA1c (continuous), with missing data imputed via multiple imputation using the LDA model described above.
In a prespecified analysis, HbA1c reduction from baseline at Week 26 was assessed in the subgroups shown in Table S1 (Appendix S1) using a repeated measures analysis of covariance model.
Statistical testing was not performed for Week 52 efficacy endpoints; however, 95% confidence intervals (CIs) are provided for between‐group comparisons.
Safety analyses included all randomized, treated patients. Data following initiation of glycaemic rescue were included for analysis of serious AEs (SAEs), deaths and discontinuations because of AEs, and were excluded for the other endpoints at Week 26. For Week 52, all safety analyses included post‐rescue observations, with the exception of those related to hypoglycaemia. P values and 95% CIs for between‐group differences in pre‐specified AEs were computed using the Miettinen and Nurminen method.15 LDL‐C and HDL‐C were assessed by an LDA model similar to that used for the primary endpoint. Changes from baseline in eGFR and other safety endpoints were summarized descriptively.
3. RESULTS
3.1. Patient disposition and baseline characteristics
In total, 464 patients were randomized and 462 were analyzed (two patients in the ertugliflozin 15 mg group did not receive study medication) (Figure S1, Appendix S1). Baseline demographics were generally similar between groups (Table 1), except for a higher proportion of males in the placebo group vs ertugliflozin groups. The mean age was 59.1 years; 72.9% of patients were White and 20.3% were Asian. The overall median metformin dose at baseline was 2000 mg/d.
Table 1.
Baseline demographics and disease characteristics
| Placebo (n = 153) | Ertugliflozin 5 mg (n = 156) | Ertugliflozin 15 mg (n = 153) | Total (n = 462) | |
|---|---|---|---|---|
| Age, years | 58.3 (9.2) | 59.2 (9.3) | 59.7 (8.6) | 59.1 (9.0) |
| Male, n (%) | 100 (65.4) | 81 (51.9) | 82 (53.6) | 263 (56.9) |
| Race / ethnicity, n (%) | ||||
| White | 108 (70.6) | 114 (73.1) | 115 (75.2) | 337 (72.9) |
| Asian | 33 (21.6) | 33 (21.2) | 28 (18.3) | 94 (20.3) |
| Black or African American | 3 (2.0) | 2 (1.3) | 4 (2.6) | 9 (1.9) |
| American Indian or Alaska Native | 5 (3.3) | 1 (0.6) | 5 (3.3) | 11 (2.4) |
| Multiple | 4 (2.6) | 6 (3.8) | 1 (0.7) | 11 (2.4) |
| Hispanic or Latino | 24 (15.7) | 23 (14.7) | 25 (16.3) | 72 (15.6) |
| Region, n (%) | ||||
| North America | 30 (19.6) | 32 (20.5) | 31 (20.3) | 93 (20.1) |
| South America | 14 (9.2) | 10 (6.4) | 9 (5.9) | 33 (7.1) |
| Europe | 65 (42.5) | 70 (44.9) | 74 (48.4) | 209 (45.2) |
| Asia | 44 (28.8) | 44 (28.2) | 39 (25.5) | 127 (27.5) |
| Body weight, kg | 86.4 (20.8) | 87.6 (18.6) | 86.6 (19.5) | 86.9 (19.6) |
| BMI, kg/m2 | 30.3 (6.4) | 31.2 (5.5) | 30.9 (6.1) | 30.8 (6.0) |
| Duration of T2DM, years | 9.4 (5.6) | 9.9 (6.1) | 9.2 (5.3) | 9.5 (5.7) |
| HbA1c, mmol/mol | 64.3 (10.2) | 64.5 (9.4) | 64.0 (9.1) | 64.3 (9.6) |
| HbA1c, % | 8.0 (0.9) | 8.1 (0.9) | 8.0 (0.8) | 8.0 (0.9) |
| FPG, mmol/L | 9.4 (2.1) | 9.3 (2.1) | 9.5 (2.2) | 9.4 (2.1) |
| FPG, mg/dL | 169.6 (37.8) | 167.7 (37.7) | 171.7 (39.1) | 169.7 (38.2) |
| SBP, mm Hg | 130.2 (13.3) | 132.1 (12.5) | 131.6 (13.2) | 131.3 (13.0) |
| DBP, mm Hg | 78.5 (7.6) | 78.4 (7.3) | 78.8 (7.2) | 78.6 (7.4) |
| Background AHA therapy at screening, n (%) | ||||
| Biguanides | 153 (100) | 156 (100) | 153 (100) | 462 (100) |
| DPP‐4 inhibitors | 102 (66.7) | 107 (68.6) | 100 (65.4) | 309 (66.9) |
| Sulphonylureas | 52 (34.0) | 52 (33.3) | 54 (35.3) | 158 (34.2) |
| Two agents | 152 (99.3) | 152 (97.4) | 152 (99.3) | 456 (98.7) |
| Three or more agents | 1 (0.7) | 4 (2.6) | 1 (0.7) | 6 (1.3) |
| One or more blood pressure medications, n (%) | 111 (72.5) | 112 (71.8) | 109 (71.2) | 332 (71.9) |
| RAS agents | 99 (64.7) | 94 (60.3) | 95 (62.1) | 288 (62.3) |
| Beta blockers | 41 (26.8) | 44 (28.2) | 39 (25.5) | 124 (26.8) |
| Calcium channel blockers | 31 (20.3) | 30 (19.2) | 36 (23.5) | 97 (21.0) |
| Diuretics | 36 (23.5) | 29 (18.6) | 31 (20.3) | 96 (20.8) |
| Other anti‐hypertensives | 9 (5.9) | 8 (5.1) | 8 (5.2) | 25 (5.4) |
| eGFR, mL/min/1.73 m2 | 89.9 (17.5) | 87.0 (17.5) | 86.9 (15.6) | 87.9 (16.9) |
| C‐peptide | 2.2 (1.5) | 2.2 (1.0) | 2.3 (1.0) | – |
Abbreviations: AHA, antihyperglycaemic agents; BMI, body mass index, DBP, diastolic blood pressure; DPP‐4, dipeptidyl peptidase 4; eGFR, estimated glomerular filtration rate; FPG, fasting plasma glucose; HbA1c, glycated haemoglobin; SBP, systolic blood pressure; SD, standard deviation; T2DM, type 2 diabetes mellitus.
Data are presented as mean (± SD), unless otherwise stated.
3.2. Efficacy
At Week 26, significantly greater reductions in HbA1c from baseline were seen in the ertugliflozin groups relative to the placebo group (placebo‐adjusted least squares [LS] means [95% CI] HbA1c changes at Week 26: −0.7% [−0.9, −0.5] and −0.8% [−0.9, −0.6], respectively; P <.001 for both comparisons) (Table 2; Figure 1A). The corresponding placebo‐adjusted changes from baseline at Week 26 in HbA1c in mmol/mol were: ertugliflozin 5 mg: −7.5 mmol/mol (−9.5, −5.5); ertugliflozin 15 mg: −8.3 mmol/mol (−10.3, −6.3).
Table 2.
Change in glycated haemoglobin (HbA1c) from baseline at Week 26 and Week 52
| Week 26 | Week 52a | |||||
|---|---|---|---|---|---|---|
| Placebo | Ertugliflozin 5 mg | Ertugliflozin 15 mg | Placebo | Ertugliflozin 5 mg | Ertugliflozin 15 mg | |
| Baseline | n = 152 | n = 155 | n = 152 | |||
| Mean (SD), mmol/mol | 64.3 (10.2) | 64.5 (9.4) | 64.0 (9.1) | |||
| Mean (SD), % | 8.0 (0.9) | 8.1 (0.9) | 8.0 (0.8) | |||
| Week 26 or 52 | n = 119 | n = 138 | n = 138 | n = 73 | n = 120 | n = 115 |
| Mean (SD), mmol/mol | 61.4 (10.7) | 55.7 (8.0) | 54.8 (8.9) | 56.7 (7.2) | 54.0 (8.0) | 53.0 (7.5) |
| Mean (SD), % | 7.7 (1.0) | 7.2 (0.7) | 7.2 (0.8) | 7.3 (0.7) | 7.1 (0.7) | 7.0 (0.7) |
| Change from baseline | ||||||
| n = 153 | n = 156 | n = 153 | n = 153 | n = 156 | n = 153 | |
| Mean (SD), mmol/mol | −1.7 (10.4) | −8.9 (8.8) | −9.4 (9.5) | −3.1 (9.3) | −9.2 (10.0) | −10.7 (8.7) |
| Mean (SD), % | −0.2 (1.0) | −0.8 (0.8) | −0.9 (0.9) | −0.3 (0.9) | −0.8 (0.9) | −1.0 (0.8) |
| LS mean (95% CI), mmol/mol | −1.0 (−2.5, 0.5) | −8.5 (−1.0, −7.1) | −9.4 (−10.8, −7.9) | 0.2 (−1.7, 2.1) | −8.1 (−9.8, −6.5) | −8.9 (−10.6, −7.2) |
| LS mean (95% CI), % | −0.1 (−0.2, 0.0) | −0.8 (−0.9, −0.6) | −0.9 (−1.0, −0.7) | 0.0 (−0.2, 0.2) | −0.7 (−0.9, −0.6) | −0.8 (−1.0, −0.7) |
| Difference in LS means vs placebo | ||||||
| n = 153 | n = 156 | n = 153 | n = 153 | n = 156 | n = 153 | |
| mmol/mol (95% CI) | – | −7.5 (−9.5, −5.5)* | −8.3 (−10.3, −6.3)* | – | −8.3 (−10.8, −5.9) | −9.1 (−11.5, −6.6) |
| % (95% CI) | – | −0.7 (−0.9, −0.5)* | −0.8 (−0.9, −0.6)* | – | −0.8 (−1.0, −0.5) | −0.8 (−1.1, −0.6) |
Abbreviations: CI, confidence interval; LS, least squares; SD, standard deviation; n, number of patients included in the analysis.
P <.001 vs placebo. Italic rows present the data in a different set of units.
Statistical testing was not performed at Week 52.
Figure 1.
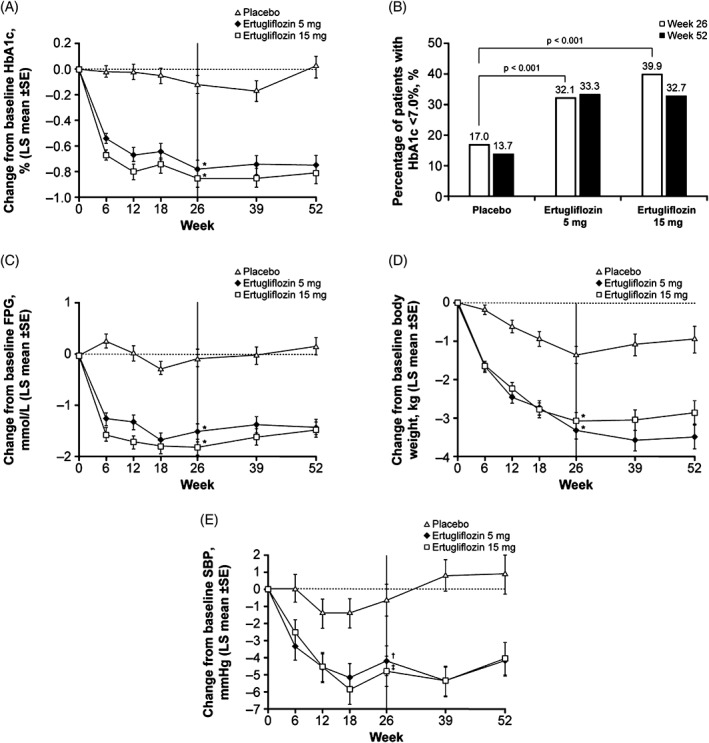
A, Change over time in glycated haemoglobin (HbA1c); B, percentage of patients with HbA1c <7.0% at Week 26 and Week 52; C, change over time in fasting plasma glucose (FPG); D, change over time in body weight; E, change over time in systolic blood pressure (SBP). LS, least squares; SE, standard error. *P < .001 vs placebo; † P = .019 vs placebo; ‡ P = .002 vs placebo
At Week 26, HbA1c reductions were greater in the ertugliflozin groups relative to the placebo group across all HbA1c subgroup categories (Table S1, Appendix S1). Larger placebo‐adjusted reductions in HbA1c were observed in those with higher than median baseline HbA1c (patients with baseline HbA1c ≤ median 7.9%: −0.6% [95% CI: −0.9, −0.4] and −0.6% [95% CI: −0.8, −0.3], for ertugliflozin 5 mg and ertugliflozin 15 mg, respectively; baseline HbA1c > median 7.9%: −0.7% [95% CI: −1.0, −0.5] and −1.0% [95% CI: −1.3, −0.7], respectively).
A higher proportion of ertugliflozin‐treated patients had HbA1c <7.0% (53 mmol/mol) at Week 26 compared to the placebo group (Table 3). The odds of having HbA1c <7.0% (53 mmol/mol) at Week 26 were significantly greater in the ertugliflozin groups vs the placebo group (both P < .001) (Table 3; Figure 1B).
Table 3.
Summary of key secondary efficacy endpoints at Week 26 and Week 52
| Week 26 | Week 52a | |||||
|---|---|---|---|---|---|---|
| Placebo(n = 153) | Ertugliflozin 5 mg (n = 156) | Ertugliflozin 15 mg (n = 153) | Placebo (n = 153) | Ertugliflozin 5 mg (n = 156) | Ertugliflozin 15 mg (n = 153) | |
| Patients with HbA1c <7.0% (<53 mmol/mol) | ||||||
| Number of patients, n (%) | 26 (17.0) | 50 (32.1) | 61 (39.9) | 21 (13.7) | 52 (33.3) | 50 (32.7) |
| Adjusted odds ratio relative to placebo (95% CI) | – | 3.2 (1.7, 5.7)* | 4.4 (2.4, 8.0)* | – | 3.6 (2.0, 6.6) | 4.0 (2.2, 7.3) |
| Fasting plasma glucose | ||||||
| LS mean change from baseline (95% CI), mmol/L | −0.1 (−0.4, 0.2) | −1.5 (−1.8, −1.2) | −1.8 (−2.1, −1.5) | 0.2 (−0.2, 0.5) | −1.4 (−1.7, −1.1) | −1.5 (−1.8, −1.2) |
| Pairwise comparison vs placebo, difference in LS means (95% CI), mmol/L | – | −1.4 (−1.8, −1.0)* | −1.7 (−2.2, −1.3)* | – | −1.6 (−2.0, −1.2) | −1.6 (−2.1, −1.2) |
| LS mean change from baseline (95% CI), mg/dL | −1.8 (−7.7, 4.2) | −26.9 (−32.6, −21.2) | −33.0 (−38.7, −27.4) | 3.2 (−3.1, 9.5) | −25.6 (−30.9, −20.2) | −26.4 (−31.8, −21.0) |
| Pairwise comparison vs placebo, difference in LS means (95% CI), mg/dL | – | −25.2 (−32.8, −17.5)* | −31.3 (−38.9, −23.7)* | – | −28.8 (−36.4, −21.1) | −29.6 (−37.3, −21.9) |
| Body weight, kg | ||||||
| LS mean change from baseline (95% CI) | −1.3 (−1.8, −0.9) | −3.4 (−3.8, −2.9) | −3.0 (−3.5, −2.6) | −1.0 (−1.6, −0.3) | −3.5 (−4.1, −2.9) | −2.8 (−3.4, −2.2) |
| Pairwise comparison vs placebo, difference in LS means (95% CI) | – | −2.0 (−2.7, −1.4)* | −1.7 (−2.4, −1.1)* | – | −2.5 (−3.4, −1.6) | −1.9 (−2.8, −1.0) |
| Systolic blood pressure, mm Hg | ||||||
| LS mean change from baseline (95% CI) | −0.9 (−2.7, 0.9) | −3.8 (−5.5, −2.1) | −4.8 (−6.6, −3.1) | 0.8 (−1.4, 3.1) | −4.2 (−6.0, −2.3) | −4.1 (−6.0, −2.2) |
| Pairwise comparison vs placebo, difference in LS means (95% CI) | – | −2.9 (−5.4, −0.5)# | −3.9 (−6.4, −1.5)† | – | −5.0 (−7.8, −2.2) | −4.9 (−7.8, −2.1) |
Abbreviations: CI, confidence interval; HbA1c, glycated haemoglobin; LS, least squares.
P < .001 vs placebo;
P = .019 vs placebo;
P = .002 vs placebo. Italic rows present the data in a different set of units.
Statistical testing was not performed at Week 52.
Significantly greater reductions from baseline were observed at Week 26 for ertugliflozin 5 mg and 15 mg compared to placebo in the key secondary endpoints of FPG, body weight and SBP (Table 3; Figure 1C–E). DBP was not prespecified as a key secondary endpoint; placebo‐adjusted reductions in DBP of 1.2 and 1.4 mm Hg were observed in the ertugliflozin 5 mg and 15 mg groups, respectively (Table S2, Appendix S1). Across treatment groups, the proportion of patients receiving BP‐lowering medication did not change in a meaningful manner during the study (Table S3, Appendix S1).
The effects of ertugliflozin on HbA1c, FPG, body weight and SBP at Week 26 were sustained through Week 52 (Figure 1). Fewer ertugliflozin‐treated patients received glycaemic rescue medication at or before Week 26 (1.3% and 2.0% in the ertugliflozin 5 mg and ertugliflozin 15 mg groups, respectively) compared with the placebo group (16.3%; nominal P < .001 for both comparisons) (Table S2, Appendix S1). A similar trend was observed at Week 52 (Table S2, Appendix S1).
LS mean increases from baseline in HOMA‐β (%) at Week 26 were greater in the ertugliflozin 5 and 15 mg groups than in the placebo group, with the improvement in HOMA‐β lasting to Week 52 (nominal P < .001 for both comparisons) (Table S2, Appendix S1). The mean change from baseline in EQ‐5D‐3L score was negligible in all groups (data not shown).
3.3. Safety
The proportion of patients with 1 or more AEs was similar across groups (Table 4). The incidence of serious AEs and AEs leading to discontinuation were low and similar across treatment groups. No deaths were reported during the treatment period. The observed incidence of drug‐related AEs was higher in the ertugliflozin groups compared with the placebo group, largely the result of drug‐related AEs associated with genital mycotic infections.
Table 4.
Summary of overall safety and prespecified adverse events (AEs)
| Number of patients, n (%) | Week 26 | Week 52 | ||||
|---|---|---|---|---|---|---|
| Placebo (n = 153) | Ertugliflozin 5 mg (n = 156) | Ertugliflozin 15 mg (n = 153) | Placebo (n = 153) | Ertugliflozin 5 mg (n = 156) | Ertugliflozin 15 mg (n = 153) | |
| Overall safety (ER and IR) a | Overall safety (all IR) | |||||
| One or more AEs | 74 (48.4) | 65 (41.7) | 67 (43.8) | 97 (63.4) | 90 (57.7) | 92 (60.1) |
| AEs related to study drugb | 13 (8.5) | 17 (10.9) | 22 (14.4) | 18 (11.8) | 19 (12.2) | 32 (20.9) |
| One or more serious AEs | 5 (3.3) | 7 (4.5) | 3 (2.0) | 8 (5.2) | 13 (8.3) | 3 (2.0) |
| Serious AEs related to study drugb | 0 (0) | 0 (0) | 1 (0.7) | 1 (0.7) | 0 (0) | 0 (0) |
| Deaths | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| AEs leading to discontinuation | 1 (0.7) | 5 (3.2) | 1 (0.7) | 6 (3.9) | 7 (4.5) | 6 (3.9) |
| Prespecified AEs (ER) | Pre‐specified AEs (IR, except hypoglycaemia) | |||||
| Genital mycotic infection (women) | 1/53 (1.9) | 6/75 (8.0) | 9/71 (12.7)* | 1 (1.9) | 9 (12.0)* | 10 (14.1)* |
| Genital mycotic infection (men) | 0/100 (0) | 4/81 (4.9)* | 3/82 (3.7) | 0 (0) | 4 (4.9)* | 3 (3.7) |
| Urinary tract infection | 3 (2.0) | 4 (2.6) | 7 (4.6) | 10 (6.5) | 5 (3.2) | 11 (7.2) |
| Symptomatic hypoglycaemiac | 4 (2.6) | 6 (3.8) | 1 (0.7) | 6 (3.9) | 7 (4.5) | 3 (2.0) |
| Hypovolaemia | 1 (0.7) | 1 (0.6) | 0 (0) | 2 (1.3) | 1 (0.6) | 0 (0) |
Abbreviations: AE, adverse event; ER, analysis excluding events occurring after initiation of rescue medication; IR, analysis including events occurring after initiation of rescue medication.
P < .05 vs placebo.
For Week 26 safety analyses, data following initiation of glycaemic rescue were excluded from incidence of “one or more AEs” and from “AEs related to study drug.”
As reported by the investigator.
Event with clinical symptoms reported by the investigator as hypoglycaemia (biochemical documentation not required).
Genital mycotic infections were more common among male and female patients who received ertugliflozin than among those who received placebo (Table 4). The incidence of urinary tract infections was low and not meaningfully different between treatment groups (Table 4). The incidence of symptomatic hypoglycaemia (Table 4) and documented hypoglycaemia was low and similar between the ertugliflozin and placebo groups over 26 weeks (documented hypoglycaemia: placebo: 3.3%, ertugliflozin 5 mg: 4.5%, ertugliflozin 15 mg: 2.0%) and 52 weeks (placebo: 7.2%, ertugliflozin 5 mg: 5.1%, ertugliflozin 15 mg: 5.2%). No cases of severe hypoglycaemia were reported in the ertugliflozin groups and 1 case was reported in the placebo group. The incidence of polyuria, pollakiuria and nocturia AE was low and similar across treatment groups.
Hypovolaemia AEs were low overall, and not meaningfully different between treatment groups (Table 4). Although infrequent in all groups, small increases in the proportion of patients with orthostasic changes in SBP were observed in the ertugliflozin groups relative to the placebo group (Table S4, Appendix S1). The proportion of patients experiencing diastolic orthostatic hypotension increased from baseline to Week 26 in the placebo group (10.7%‐15.1%) and a trend similar in magnitude was observed for the ertugliflozin 15 mg group (12.0%‐18.6%), but not for the ertugliflozin 5 mg group in which there was essentially no difference between baseline and Week 26 (15.4% and 16.3%).
After 52 weeks of treatment, the most common AEs (incidence >5% in any treatment group) were nasopharyngitis (placebo: 3.3%; ertugliflozin 5 mg: 5.1%; ertugliflozin 15 mg: 3.9%), urinary tract infection (placebo: 5.2%; ertugliflozin 5 mg: 1.3%; ertugliflozin 15 mg: 3.3%) and hypoglycaemia (placebo: 7.2%; ertugliflozin 5 mg: 4.5%; ertugliflozin 15 mg: 3.3%).
Modest, transient decreases from baseline in mean eGFR were observed in the ertugliflozin groups at Week 6; values returned to or near to baseline during the study (Figure 2). Through Week 52, 6.0%, 5.2% and 5.3% of patients in the placebo, ertugliflozin 5 mg and ertugliflozin 15 mg groups experienced a decrease from baseline >30% in eGFR. Two patients (1.3%) in the placebo group and none in the ertugliflozin groups experienced a decrease >50% in eGFR from baseline. During the study, 1 patient in the ertugliflozin 15 mg group (baseline eGFR 54 mL/min/1.73 m2) met protocol‐specified discontinuation criteria because of renal problems (eGFR consistently <50 mL/min/1.73 m2). eGFR increased following treatment cessation. Two patients in the ertugliflozin 15 mg group with baseline eGFR values of 61 and 59 mL/min/1.73 m2 discontinued treatment because of AEs of blood creatinine increased and eGFR decreased. eGFR increased to or near to baseline in both patients after treatment discontinuation.
Figure 2.
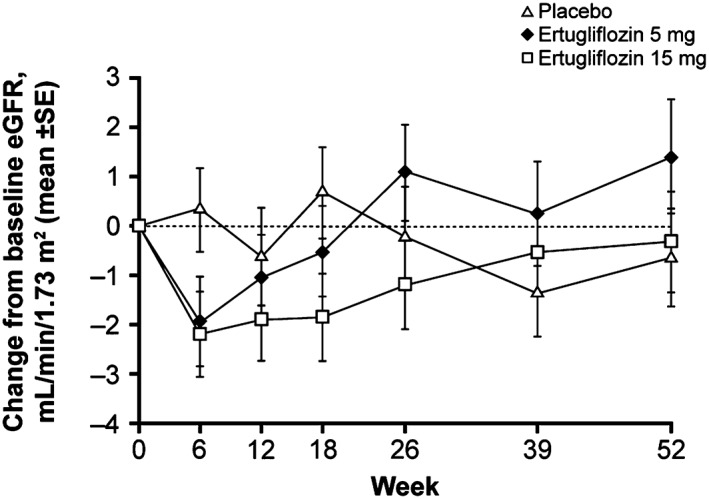
Mean change from baseline in estimated glomerular filtration rate (eGFR) (mL/min/1.73 m2) through Week 52. SE, standard error
Negligible placebo‐corrected LS mean percent changes in LDL‐C were observed at Week 26 (Table S5, Appendix S1) (−0.6% [95% CI: −7.2, 6.0] and 1.3% [95% CI: −5.4, 8.0] for ertugliflozin 5 mg and ertugliflozin 15 mg, respectively). At Week 52, the placebo‐adjusted increases were 4.5% (95% CI: −2.4, 11.4) and 2.9% (95% CI: −4.1, 9.9) for ertugliflozin 5 mg and ertugliflozin 15 mg, respectively. Small increases from baseline in HDL‐C were observed across all groups; these were higher in the ertugliflozin groups vs the placebo group (Table S5, Appendix S1) (placebo‐adjusted difference for ertugliflozin 5 mg and 15 mg: 4.2% and 4.4%, respectively, at Week 26 and 5.0% and 5.7%, respectively, at Week 52).
No patient experienced an event that reflected ketoacidosis. No AEs of pancreatitis were reported. Adjudication‐confirmed fractures occurred in 1 patient in each treatment group (placebo: tibia fracture associated with a fall; ertugliflozin 5 mg: femur fracture and a spinal compression fracture following a hang‐gliding accident; ertugliflozin 15 mg: forearm fracture and radius fracture associated with a fall, with no concurrent report of orthostatic hypotension). None of these patients reported concurrent AEs of hypovolaemia or hypoglycaemia.
4. DISCUSSION
In this Phase 3 placebo‐controlled study (VERTIS SITA2), significant improvements in glycaemic control were obtained with ertugliflozin added to background therapy of metformin and sitagliptin and were sustained over 52 weeks of treatment. Ertugliflozin led to greater reductions in HbA1c compared with placebo across all baseline HbA1c subgroups. Significantly more patients in the ertugliflozin groups met the American Diabetes Association‐recommended HbA1c target of <7.0% (53 mmol/mol)1 compared with the placebo group at Week 26.
At Week 26, statistically significant, clinically meaningful placebo‐adjusted reductions in FPG, body weight and SBP were seen, with these effects being sustained through 52 weeks. The observed SBP reductions were notable, given that over 70% of patients in this study were receiving BP medication at baseline, and SBP was generally well‐controlled (mean SBP approximately 130 mm Hg). No meaningful differences in the proportions of patients receiving anti‐hypertensive medication at Week 26 or Week 52 relative to baseline were observed in the ertugliflozin or placebo groups. The reductions in BP may be explained by the mild diuretic characteristics of SGLT2 inhibitors,16 although other mechanisms may contribute to BP lowering.
These results are consistent with previously reported findings from the VERTIS Phase 3 clinical trial programme.17, 18 The reductions in HbA1c from baseline at Week 26 reported here are reflective of those reported in other studies involving SGLT2 inhibitor addition to metformin and DPP‐4 inhibitor background therapy with similar baseline HbA1c values.19, 20, 21, 22 Because of the progressive nature of T2DM, over time, patients who are receiving dual therapy with metformin and a DPP‐4 inhibitor may require intensification with a third oral agent to maintain glycaemic control, or, for those with HbA1c ≥10% (86 mmol/mol), initiation of injectable insulin therapy.1 The present results support the addition of ertugliflozin as a third‐line agent.
Ertugliflozin monotherapy improves HOMA‐β, a marker of β‐cell function.17 In this study, improvements were also observed despite the fact that patients were already receiving sitagliptin, which is known to improve β‐cell function.23 This is likely to be an indirect effect of reduced glucotoxicity resulting from enhanced urinary elimination of glucose.
Patients with moderate renal impairment were not eligible for enrolment in this study. The glycaemic efficacy of SGLT2 inhibitors is dependent on renal function and the amount of filtered glucose; therefore, in patients with impaired renal function, the glycaemic efficacy of SGLT2 inhibitors is likely to be attenuated, although beneficial effects on body weight and BP have been observed.24, 25, 26, 27
Addition of ertugliflozin 5 mg and 15 mg to metformin and sitagliptin was generally well‐tolerated over 52 weeks of treatment; no clinically important differences were observed between the two ertugliflozin doses. A higher incidence of genital mycotic infections, a known class effect of SGLT2 inhibitors,28 was observed in male and female patients receiving ertugliflozin vs placebo. The low incidence of hypoglycaemia in this study is consistent with the mechanism of action of SGLT2 inhibitors.6, 28 This, together with their mechanism of action which is complementary to that of DPP‐4 inhibitors, makes SGLT2 inhibitors an attractive alternative to sulphonylureas for combination therapy.
Treatment with SGLT2 inhibitors causes osmotic diuresis, which may lead to AEs related to volume depletion;6, 29 however, the incidence of hypovolaemia and orthostatic hypotension was low in this study. The risk of volume depletion with an SGLT2 inhibitor is increased in those with moderate renal impairment, advanced age and use of diuretics. In this study, 21.4% of patients reported concomitant diuretic use, and 29.9% were 65 years of age or older. Because of the small sample size of patients using diuretics, as well as the low incidence of hypovolaemia/orthostatic hypotension, the interaction between the two could not be investigated in the present study.
The transient decreases in eGFR observed in this study have been reported with other SGLT2 inhibitors and are probably haemodynamically mediated.6 Recent evidence suggests that SGLT2 inhibitors may have long‐term beneficial effects on renal outcomes,30 which is probably the result of several direct and indirect effects on the kidney through tubuloglomerular feedback and improvements in hyperglycaemia, hypertension, obesity and hyperuricaemia.31, 32
In summary, in patients with T2DM who had inadequate glycaemic control with metformin and sitagliptin, the addition of ertugliflozin provided clinically meaningful and durable glycaemic control and reductions in body weight and SBP. Ertugliflozin was generally well‐tolerated without a meaningful difference in symptomatic hypoglycaemia, urinary tract infection or hypovolaemia AEs, but resulted in a higher incidence of genital mycotic infections in men and women compared to placebo.
Supporting information
Appendix S1. Supplementary methods.
Table S1. Glycated haemoglobin (HbA1c) distribution at baseline and subgroup efficacy analysis (change in HbA1c from baseline at Week 26 per baseline HbA1c).
Table S2. Additional secondary efficacy endpoints at Week 26 and Week 52.
Table S3. Proportion of patients receiving blood pressure medication at baseline, Week 26 and Week 52.
Table S4. Categorical summary of patients meeting pre‐specified criteria for orthostatic change in systolic blood pressure (SBP) and diastolic blood pressure (DBP) over time
Table S5. Percent change in low‐density lipoprotein cholesterol (LDL‐C) and high‐density lipoprotein cholesterol (HDL‐C) from baseline at Week 26 and Week 52.
Figure S1. Patient disposition.
ACKNOWLEDGMENTS
The authors wish to thank the investigators, staff and participants in the VERTIS SITA2 trial (protocol MK‐8835‐006). Data from this study were previously presented at the Annual Meeting of the European Association for the Study of Diabetes (52nd Annual Meeting 2016, Munich, Germany) and the American Diabetes Association's 77th Scientific Sessions (San Diego, California). Medical writing support, including assisting authors with development of the outline, initial draft and incorporation of comments, fact checking, referencing and proofreading, was provided by Camille Bonomelli, PhD and Faye Gould, PhD, ISMPP CMPP, and editorial support, including figure preparation, formatting and submission, was provided by Nicola Jenkins, MA (all of Scion, London, UK). This assistance was funded by Merck & Co., Inc., Kenilworth, New Jersey. The sponsor was involved in the study design, collection, analysis and interpretation of data, as well as data checking of information provided in the manuscript. However, ultimate responsibility for opinions, conclusions and data interpretation lies with the authors.
Conflict of interest
S. D.‐J. is the principal investigator/coinvestigator for clinical trials contracts with the University of Tennessee, funded by AstraZeneca, Novo Nordisk, Inc., Boehringer Ingelheim Pharmaceuticals, Inc.; is a consultant/advisory board member for Amgen, Merck & Co., Inc., Sanofi, AstraZeneca, Novo Nordisk Inc., Boehringer Ingelheim Pharmaceuticals, Inc. and Janssen Pharmaceuticals, Inc.
R. E. was an employee of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, New Jersey, at the time the study was conducted. B. L., J. L., G. A., J. J., D. H., S. H., G. G. and S. S. E. are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, New Jersey and may own stock and/or hold stock options in the company. Y. L. is an employee of MSD R&D (China) Co., Ltd., a subsidiary of Merck & Co., Inc., Kenilworth, New Jersey, and may own stock and/or hold stock options in the Company. S. G. T. and J. P. M. are employees and shareholders in Pfizer Inc.
Author contributions
The authors are responsible for the work described in this paper. S. D.‐J., R. E., J. J., D. H., G. G., S. G. T., J. P. M. and S. S. E. contributed to the design of the study. J. L., R. E., G. A. and B. L. contributed to conduct of the study and data collection. S. D.‐J., J. L., R. E., G. A., J. J., D. H., Y. L., S. H., G. G., J. P. M., S. S. E. and B. L. contributed to the analysis. All authors reviewed the manuscript for important intellectual content and provided final approval of the version to be published. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Dagogo‐Jack S, Liu J, Eldor R, et al. Efficacy and safety of the addition of ertugliflozin in patients with type 2 diabetes mellitus inadequately controlled with metformin and sitagliptin: The VERTIS SITA2 placebo‐controlled randomized study. Diabetes Obes Metab. 2018;20:530–540. https://doi.org/10.1111/dom.13116
Funding information Funding for this research was provided by Merck & Co., Inc., Kenilworth, New Jersey, in collaboration with Pfizer Inc.
REFERENCES
- 1. American Diabetes Association . Standards of medical care in diabetes – 2016. Diabetes Care. 2016;39(suppl 1):S1–S112. [DOI] [PubMed] [Google Scholar]
- 2. Drucker DJ, Nauck MA. The incretin system: glucagon‐like peptide‐1 receptor agonists and dipeptidyl peptidase‐4 inhibitors in type 2 diabetes. Lancet. 2006;368:1696–1705. [DOI] [PubMed] [Google Scholar]
- 3. Karasik A, Aschner P, Katzeff H, Davies MJ, Stein PP. Sitagliptin, a DPP‐4 inhibitor for the treatment of patients with type 2 diabetes: a review of recent clinical trials. Curr Med Res Opin. 2008;24:489–496. [DOI] [PubMed] [Google Scholar]
- 4. Deacon CF. Dipeptidyl peptidase 4 inhibition with sitagliptin: a new therapy for type 2 diabetes. Expert Opin Investig Drugs. 2007;16:533–545. [DOI] [PubMed] [Google Scholar]
- 5. Scott R, Loeys T, Davies MJ, Engel SS. Efficacy and safety of sitagliptin when added to ongoing metformin therapy in patients with type 2 diabetes. Diabetes Obes Metab. 2008;10:959–969. [DOI] [PubMed] [Google Scholar]
- 6. Kalra S, Singh V, Nagrale D. Sodium‐glucose cotransporter‐2 inhibition and the glomerulus: a review. Adv Ther. 2016;33:1502–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nauck MA. Update on developments with SGLT2 inhibitors in the management of type 2 diabetes. Drug Des Devel Ther. 2014;8:1335–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Abdul‐Ghani M. Where does combination therapy with an SGLT2 inhibitor plus a DPP‐4 inhibitor fit in the management of type 2 diabetes? Diabetes Care. 2015;38:373–375. [DOI] [PubMed] [Google Scholar]
- 9. Amin NB, Wang X, Jain SM, Lee DS, Nucci G, Rusnak JM. Dose‐ranging efficacy and safety study of ertugliflozin, a sodium‐glucose co‐transporter 2 inhibitor, in patients with type 2 diabetes on a background of metformin. Diabetes Obes Metab. 2015;17:591–598. [DOI] [PubMed] [Google Scholar]
- 10. Amin NB, Wang X, Mitchell JR, Lee DS, Nucci G, Rusnak JM. Blood pressure‐lowering effect of the sodium glucose co‐transporter‐2 inhibitor ertugliflozin, assessed via ambulatory blood pressure monitoring in patients with type 2 diabetes and hypertension. Diabetes Obes Metab. 2015;17:805–808. [DOI] [PubMed] [Google Scholar]
- 11. American Diabetes Association . Executive summary: standards of medical care in diabetes‐‐2012. Diabetes Care. 2012;35(suppl 1):S4–S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. EuroQol . EQ‐5D‐3L: available modes of administration. 2017. http://www.euroqol.org/eq‐5d‐products/eq‐5d‐3l.html. Accessed May 22, 2017.
- 13. University of Oxford . HOMA calculator. 2013. http://www.dtu.ox.ac.uk/homacalculator/.Accessed May 22, 2017.
- 14. Liang K‐Y, Zeger SL. Longitudinal data analysis of continuous and discrete responses for pre‐post designs. Sankhya Ser B. 2000;62:134–148. [Google Scholar]
- 15. Miettinen O, Nurminen M. Comparative analysis of two rates. Stat Med. 1985;4:213–226. [DOI] [PubMed] [Google Scholar]
- 16. Basile JN. The potential of sodium glucose cotransporter 2 (SGLT2) inhibitors to reduce cardiovascular risk in patients with type 2 diabetes (T2DM). J Diabetes Complications. 2013;27:280–286. [DOI] [PubMed] [Google Scholar]
- 17. Terra SG, Focht K, Davies M, et al. Phase III, efficacy and safety study of ertugliflozin monotherapy in people with type 2 diabetes mellitus inadequately controlled with diet and exercise alone. Diabetes Obes Metab. 2017;19:721–728. [DOI] [PubMed] [Google Scholar]
- 18. Eldor R, Pratley R, Golm G, et al. Effect of ertugliflozin plus sitagliptin on glycemic control versus either treatment alone in subjects with T2DM inadequately controlled with metformin. Diabetes 2016;65(Suppl 1):LB34 Abstract 125‐LB.
- 19. Rodbard HW, Seufert J, Aggarwal N, et al. Efficacy and safety of titrated canagliflozin in patients with type 2 diabetes mellitus inadequately controlled on metformin and sitagliptin. Diabetes Obes Metab. 2016;18:812–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Softeland E, Meier JJ, Vangen B, Toorawa R, Maldonado‐Lutomirsky M, Broedl UC. Empagliflozin as add‐on therapy in patients with type 2 diabetes inadequately controlled with linagliptin and metformin: a 24‐week randomized, double‐blind, parallel‐group trial. Diabetes Care. 2017;40:201–209. [DOI] [PubMed] [Google Scholar]
- 21. Mathieu C, Ranetti AE, Li D, et al. Randomized, double‐blind, phase 3 trial of triple therapy with dapagliflozin add‐on to saxagliptin plus metformin in type 2 diabetes. Diabetes Care. 2015;38:2009–2017. [DOI] [PubMed] [Google Scholar]
- 22. Jabbour SA, Hardy E, Sugg J, Parikh S. Dapagliflozin is effective as add‐on therapy to sitagliptin with or without metformin: a 24‐week, multicenter, randomized, double‐blind, placebo‐controlled study. Diabetes Care. 2014;37:740–750. [DOI] [PubMed] [Google Scholar]
- 23. Aschner P, Kipnes MS, Lunceford JK, Sanchez M, Mickel C, Williams‐Herman DE. Effect of the dipeptidyl peptidase‐4 inhibitor sitagliptin as monotherapy on glycemic control in patients with type 2 diabetes. Diabetes Care. 2006;29:2632–2637. [DOI] [PubMed] [Google Scholar]
- 24. Kohan DE, Fioretto P, Tang W, List JF. Long‐term study of patients with type 2 diabetes and moderate renal impairment shows that dapagliflozin reduces weight and blood pressure but does not improve glycemic control. Kidney Int. 2014;85:962–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yale JF, Bakris G, Cariou B, et al. Efficacy and safety of canagliflozin over 52 weeks in patients with type 2 diabetes mellitus and chronic kidney disease. Diabetes Obes Metab. 2014;16:1016–1027. [DOI] [PubMed] [Google Scholar]
- 26. Barnett AH, Mithal A, Manassie J, et al. Efficacy and safety of empagliflozin added to existing antidiabetes treatment in patients with type 2 diabetes and chronic kidney disease: a randomised, double‐blind, placebo‐controlled trial. Lancet Diabetes Endocrinol. 2014;2:369–384. [DOI] [PubMed] [Google Scholar]
- 27. Fioretto P, Zambon A, Rossato M, Busetto L, Vettor R. SGLT2 Inhibitors and the Diabetic Kidney. Diabetes Care. 2016;39(suppl 2):S165–S171. [DOI] [PubMed] [Google Scholar]
- 28. Wu JH, Foote C, Blomster J, et al. Effects of sodium‐glucose cotransporter‐2 inhibitors on cardiovascular events, death, and major safety outcomes in adults with type 2 diabetes: a systematic review and meta‐analysis. Lancet Diabetes Endocrinol. 2016;4:411–419. [DOI] [PubMed] [Google Scholar]
- 29. Monica Reddy RP, Inzucchi SE. SGLT2 inhibitors in the management of type 2 diabetes. Endocrine. 2016;53:364–372. [DOI] [PubMed] [Google Scholar]
- 30. Wanner C, Inzucchi SE, Lachin JM, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375:323–334. [DOI] [PubMed] [Google Scholar]
- 31. Vallon V, Thomson SC. Targeting renal glucose reabsorption to treat hyperglycaemia: the pleiotropic effects of SGLT2 inhibition. Diabetologia. 2017;60:215–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cherney DZ, Perkins BA, Soleymanlou N, et al. Renal hemodynamic effect of sodium‐glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation. 2014;129:587–597. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supplementary methods.
Table S1. Glycated haemoglobin (HbA1c) distribution at baseline and subgroup efficacy analysis (change in HbA1c from baseline at Week 26 per baseline HbA1c).
Table S2. Additional secondary efficacy endpoints at Week 26 and Week 52.
Table S3. Proportion of patients receiving blood pressure medication at baseline, Week 26 and Week 52.
Table S4. Categorical summary of patients meeting pre‐specified criteria for orthostatic change in systolic blood pressure (SBP) and diastolic blood pressure (DBP) over time
Table S5. Percent change in low‐density lipoprotein cholesterol (LDL‐C) and high‐density lipoprotein cholesterol (HDL‐C) from baseline at Week 26 and Week 52.
Figure S1. Patient disposition.


