Abstract
Background
The X‐ACT study aimed to examine the effect of omalizumab treatment on quality of life (QoL) in chronic spontaneous urticaria (CSU) patients with angioedema refractory to high doses of H1‐antihistamines.
Methods
In X‐ACT, a phase III, double‐blind, placebo‐controlled study, CSU patients (18‐75 years) with ≥4 angioedema episodes during the 6 months before inclusion were randomized (1:1) to receive omalizumab 300 mg or placebo every 4 weeks for 28 weeks. Angioedema‐related QoL, skin‐related QoL impairment, and psychological well‐being were assessed.
Results
Ninety‐one patients were randomized and 68 (omalizumab, n = 35; placebo, n = 33) completed the 28‐week treatment period. At baseline, the mean (SD) total Angioedema QoL (AE‐QoL; 56.2 [18.7] and 59.9 [19.2]) and Dermatology Life Quality Index (DLQI; 14.6 [5.7] and 16.6 [7.3]) score were high in the omalizumab and placebo group, respectively. At Week 4 (after the first treatment), the least squares mean difference in the AE‐QoL and DLQI score between groups was −17.6 (P < .001) and −7.2 (P < .001), respectively. Significant QoL improvements in the omalizumab vs placebo groups continued until Week 28, but returned to placebo levels at the follow‐up visit. The mean (SD) baseline 5‐item World Health Organization Well‐being Index was 10.0 (5.5, omalizumab) and 7.7 (5.3, placebo), which increased above the depression threshold (<13) from Week 4 and throughout with omalizumab but not placebo treatment. Compared to placebo, omalizumab was also associated with decreased fear of suffocation due to angioedema.
Conclusions
Our findings support omalizumab treatment in patients with severe H1‐antihistamine‐refractory CSU with angioedema.
Keywords: angioedema, chronic spontaneous urticaria, H1‐antihistamine‐refractory, omalizumab, quality of life
1. INTRODUCTION
Chronic spontaneous urticaria (CSU) involves the repeated occurrence of wheals (hives), angioedema, or both for more than 6 weeks, without any specific external trigger.1, 2 Angioedema is an important symptom associated with CSU; it is characterized by a sudden swelling of the dermis, subcutaneous tissue, mucosa, and/or submucosa, which usually occurs around the eyes, lips and mouth, arms and legs, genitalia, or hands.3 Approximately 33%‐67% of CSU patients reportedly experience wheals and angioedema, and 1%‐13% reportedly experience only angioedema.4 Furthermore, recent data from the observational AWARE study indicate that 46.1% of German CSU patients, who remained symptomatic despite treatment with at least the approved dose of H1‐antihistamines, had both, wheals and angioedema, while 2.9% exclusively had angioedema.5 Angioedema is a major driver of quality‐of‐life (QoL) impairment in patients with CSU.6 Owing to the unpredictable development of disfigurement and/or functional impairment, angioedema episodes can have a significant impact on daily activities and social interactions in CSU patients.4 Furthermore, patients report missing work or school owing to their angioedema.
Omalizumab is approved as an add‐on therapy in patients with CSU refractory to H1‐antihistamines.7 Several randomized controlled trials (RCTs) have shown that omalizumab effectively controls the symptoms of CSU (itching and wheals)8, 9, 10, 11, 12, 13, 14 and improves the QoL in CSU patients.8, 9, 10, 11, 12, 13, 14, 15, 16 We recently reported that subcutaneous omalizumab (300 mg) also reduces the frequency and severity of angioedema in H1‐antihistamine‐refractory CSU patients.4 Furthermore, omalizumab reduced QoL impairment in the same patient cohort,4 as assessed using the validated CSU‐specific QoL Questionnaire (CU‐Q2oL). However, a number of questions remain unanswered for H1‐antihistamine‐refractory CSU patients with angioedema: (1) Are specific domains of QoL and psychological well‐being more severely affected than other aspects at baseline? (2) Do angioedema‐related QoL scores and psychological well‐being improve over the course of omalizumab treatment? and (3) Are improvements in symptom severity correlated with changes in QoL?
Here, we present results from the X‐ACT (Xolair Effects on Angioedema in Chronic Spontaneous Urticaria Treatment) study, to bridge the gaps in the knowledge on angioedema‐related QoL impairment and improvement with omalizumab treatment.
2. METHODS
2.1. X‐ACT study design
X‐ACT was a randomized, double‐blind, placebo‐controlled, multicenter, phase III study that has previously been described in detail.4 The trial was conducted between January 2013 and May 2014 in 24 centers across Germany. Briefly, the study was divided into a 2‐week screening period, a 28‐week double‐blind treatment phase in which patients were randomized (1:1) to receive subcutaneous injections of omalizumab 300 mg or placebo every 4 weeks from baseline (Week 0) until Week 24, and an 8‐week follow‐up phase (Figure 1). The study protocol was reviewed by an Independent Ethics Committee or Institutional Review Board for all study centers in Germany. All patients provided written informed consent prior to study initiation (ClinicalTrials.gov number: NCT01723072; EudraCT number: 2011‐004254‐25).
Figure 1.

X‐ACT study design. N = 68 patients (omalizumab, n = 35; placebo, n = 33) completed the 28‐week treatment period. Patients received omalizumab or placebo as add‐on therapy to the screening period‐defined up‐dosed H1‐antihistamine treatment
2.2. Study patients
Male and female CSU patients aged 18‐75 years with a history of angioedema (at least 4 episodes within the last 6 months before study enrollment), who remain symptomatic despite the use of two‐to‐four times the recommended dose of second‐generation H1‐antihistamine treatment, with an urticaria activity score (UAS) over 7 days (UAS7) of ≥14 and a CU‐Q2oL score of ≥30 at baseline were included in the study. Patients underwent a clinical examination by an experienced physician, and a thorough history was taken; the presence of any form of chronic inducible urticaria was explicitly checked for, including delayed pressure urticaria which can be mistaken for angioedema. Patients were excluded from the study if they had non‐urticaria‐associated angioedema (eg, low C1‐inhibitor levels), serious psychological disturbance, metabolic and pathological conditions, history of malignancy, hypersensitivity to omalizumab, or had received treatment with omalizumab within the previous 6 months.
2.3. Study objectives and endpoints
The primary objective of the X‐ACT trial was to evaluate the efficacy of once‐monthly add‐on injections of omalizumab (300 mg; subcutaneously for 28 weeks) compared to placebo on the QoL of CSU patients with angioedema refractory to H1‐antihistamines (CU‐Q2oL).4 Key secondary endpoints included changes in angioedema disease activity, as well as angioedema‐specific and overall skin‐related QoL impairment following omalizumab treatment.
2.4. Patient‐reported outcomes: disease activity, QoL, and psychological well‐being
The Angioedema Activity Score (AAS) was completed by patients on a daily basis and recorded in a paper‐based symptom diary. The AAS is a validated questionnaire that consists of an opening question (Have you had a swelling episode in the last 24 h?), followed by the five specific AAS questions to determine the severity and impact of the angioedema episode.12 The daily AAS values are added over 7 days and, in the present study, weekly scores were recorded at every patient visit (baseline/Week 0, and Weeks 4, 8, 12, 16, 20, 24, 28, and 36). Weekly AAS (AAS7) scores range from 0 to 105, with higher scores indicative of a higher disease activity.
The Angioedema Quality of Life (AE‐QoL) is a validated, angioedema‐specific 17‐item questionnaire that includes four subdomains: functioning, fatigue/mood, fears/shame, and food. Each item is answered on a 5‐point Likert scale (0 = not important to 4 = very important). The results of all the answered questions are summed up and transferred to a scale ranging from 0 to 100, with higher scores indicative of a higher QoL impairment.17, 18
Two separate questions related to patients' fear of a life‐threatening angioedema episode were included to explore this potential complication further: (1) Were you afraid of a life‐threatening swelling episode? and (2) Were you afraid of suffocating due to the swelling episode? Questions were answered on a 5‐point Likert scale (0 = never to 4 = very often), with higher scores indicative of an increased fear of a life‐threatening angioedema attack. The results of both questions were analyzed descriptively by visit, and missing scores were not replaced.
The Dermatology Life Quality Index (DLQI) is a validated, dermatology‐specific 10‐item questionnaire with six subheadings: symptoms and feelings, daily activities, leisure, work and school, personal relationships, and treatment. The DLQI score ranges between 0 and 30, with higher scores indicative of a higher QoL impairment.19
The 5‐item World Health Organization Well‐being Index (WHO‐5) is a validated, 5‐item, positively‐worded questionnaire that measures patient well‐being in terms of mood, vitality, and general interests. The maximum score is 25, and values lower than 13 are indicative of signs of depression.20
2.5. Statistical analysis
Subjects were analyzed according to the treatment to which they were randomized. All results presented here are based on the full analysis set (FAS; all randomized subjects who received at least one dose of the study drug). The secondary endpoint analysis was performed by comparing the two treatments with respect to change in AE‐QoL and DLQI scores using an analysis of covariance (ANCOVA) model with treatment and center as factors and baseline score as a covariate. The analysis was conducted in the FAS using the last observation carried forward (LOCF) and for the DLQI and observed values for the AE‐QoL questionnaire. Data for the omalizumab treatment group were compared to those of the placebo group. The AAS7 was analyzed analogously to the AE‐QoL and DLQI as a mean difference from baseline (Visit 2) to Week 28 (Visit 9) in an ANCOVA analysis conducted on the FAS using observed cases. The WHO‐5 and the questions on fear of a life‐threatening angioedema episode were regarded as exploratory questionnaires. Pearson correlation coefficients were computed to explore the correlations between different endpoints.
3. RESULTS
A total of 91 CSU patients were randomized to one of two groups: omalizumab (n = 44) or placebo (n = 47; Figure 1). On average, patients experienced 2.7 and 3.4 angioedema‐burdened days during the screening phase (Week −2 to Week −1) in the omalizumab and placebo group, respectively. The mean (SD) AAS7 scores at baseline were also high in both the omalizumab (22.5 [20.6]) and placebo (28.1 [24.1]) groups indicating that angioedema activity was high (Table 1). Baseline demographics and disease characteristics have been reported in detail previously.4
Table 1.
Baseline values of health‐related QoL impairment, angioedema disease activity, and patient well‐being
| Baseline | ||
|---|---|---|
| Omalizumab n = 44 | Placebo n = 47 | |
| Weekly AAS (AAS7) | ||
| Total score | 22.5 (20.6) | 28.1 (24.1) |
| Angioedema‐burdened days | 2.7 (2.34) | 3.5 (2.35) |
| AE‐QoL | ||
| Total score | 56.2 (18.7) | 59.9 (19.2) |
| DLQI | ||
| Total score | 14.6 (5.7) | 16.6 (7.3) |
| WHO‐5 index | ||
| Total score | 10.0 (5.5) | 7.7 (5.3) |
Data are presented as mean (SD). AAS, Angioedema Activity Score; AE‐QoL, Angioedema Quality of Life Questionnaire; DLQI, Dermatology Life Quality Index; QoL, quality of life; SD, standard deviation; WHO‐5, 5‐item World Health Organization Well‐being Index.
The mean (SD) AE‐QoL total scores (omalizumab 300 mg: 56.2 [18.7]; placebo: 59.9 [19.2]) were also high at baseline, reflecting poor QoL in these patients (Table 1). The most severely affected subdomains of the AE‐QoL were fears/shame, fatigue/mood, and functioning (Table 2). The subdomain of food was less severely affected, but still indicated impairment in both groups. The mean DLQI total scores (omalizumab: 14.6 [5.7]; placebo: 16.6 [7.3]) also reflected poor QoL. The most severely affected subdomains of the DLQI score were symptoms and feelings, daily activities, and leisure. Total and subdomain scores were comparable for the omalizumab and placebo groups at baseline (Tables 1 and 2).
Table 2.
Subdomain scores (AE‐QoL and DLQI; observed values for the FAS) and fear of life‐threatening swelling episodes and suffocation due to swelling from baseline to follow‐up at Week 36
| Baseline | Week 4 | Week 12 | Week 28 | Follow‐up | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Omalizumab | Placebo | Omalizumab | Placebo | Omalizumab | Placebo | Omalizumab | Placebo | Omalizumab | Placebo | |
| AE‐QoL | n = 44 | n = 47 | n = 38 | n = 36 | n = 35 | n = 29 | n = 34 | n = 25 | n = 33 | n = 23 |
| Functioning | 52.3 (26.07) | 57.4 (28.75) | 17.4 (19.83) | 46.8 (29.63) | 10.2 (15.47) | 37.1 (31.69) | 6.6 (11.91) | 30.5 (32.19) | 23.7 (27.05) | 29.6 (29.57) |
| Fatigue/mood | 56.0 (22.82) | 58.0 (22.06) | 32.1 (24.73) | 43.2 (25.64) | 21.7 (20.93) | 40.0 (26.76) | 18.4 (19.06) | 42.4 (23.68) | 28.6 (24.85) | 40.3 (28.12) |
| Fear/shame | 66.9 (23.08) | 72.4 (19.99) | 36.6 (26.72)a | 58.7 (18.86) | 23.3 (24.24) | 46.1 (28.45) | 16.3 (19.34) | 46.8 (27.25) | 34.2 (27.34) | 44.5 (28.87) |
| Food | 32.7 (37.28) | 32.7 (31.87) | 21.4 (30.33) | 21.2 (24.61) | 14.3 (24.28) | 17.7 (26.41) | 11.8 (20.40) | 16.5 (25.70) | 13.6 (20.34) | 14.7 (23.13) |
| n = 44 | n = 46 | n = 43 | n = 45 | n = 37 | n = 34 | n = 22 | n = 24 | n = 32 | n = 28 | |
| Were you afraid of a life‐threatening swelling episode? | ||||||||||
| Never | 7 (15.9) | 6 (13.0) | 19 (44.2) | 15 (33.3) | 20 (54.1) | 11 (32.4) | 14 (63.6) | 7 (29.2) | 12 (37.5) | 10 (35.7) |
| Rarely | 11 (25.0) | 6 (13.0) | 11 (25.3) | 7 (15.6) | 6 (16.2) | 9 (26.5) | 5 (22.7) | 7 (29.2) | 10 (31.3) | 4 (14.3) |
| Occasionally | 11 (25.0) | 16 (34.8) | 3 (7.0) | 16 (35.6) | 9 (24.3) | 6 (17.6) | 3 (13.6) | 7 (29.2) | 6 (18.8) | 10 (35.7) |
| Often | 8 (18.2) | 11 (23.9) | 7 (16.3) | 6 (13.3) | 1 (2.7) | 3 (8.8) | 0 (0.0) | 1 (4.2) | 3 (9.4) | 2 (7.1) |
| Very often | 7 (15.9) | 7 (15.2) | 3 (7.0) | 1 (2.2) | 1 (2.7) | 5 (14.7) | 0 (0.0) | 2 (8.3) | 1 (3.1) | 2 (7.1) |
| Were you afraid of suffocating due to the swelling episode? | ||||||||||
| Never | 17 (38.6) | 14 (30.4) | 24 (55.8) | 19 (42.2) | 24 (64.9) | 16 (47.1) | 16 (72.7) | 11 (45.8) | 18 (56.3) | 12 (42.9) |
| Rarely | 7 (15.9) | 8 (17.4) | 10 (23.3) | 13 (28.9) | 9 (24.3) | 7 (20.6) | 5 (22.7) | 7 (29.2) | 8 (25.0) | 7 (25.0) |
| Occasionally | 10 (22.7) | 10 (21.7) | 3 (7.0) | 6 (13.3) | 2 (5.4) | 5 (14.7) | 1 (4.5) | 4 (16.7) | 3 (9.4) | 7 (25.0) |
| Often | 5 (11.4) | 9 (19.6) | 4 (9.3) | 7 (15.6) | 1 (2.7) | 4 (11.8) | 0 (0.0) | 1 (4.2) | 2 (6.3) | 0 (0.0) |
| Very often | 5 (11.4) | 5 (10.9) | 2 (4.7) | 0 (0.0) | 1 (2.7) | 2 (5.9) | 0 (0.0) | 1 (4.2) | 1 (3.1) | 2 (7.1) |
| DLQIb | n = 44 | n = 47 | n = 39 | n = 37 | n = 36 | n = 30 | n = 34 | n = 28 | n = 32 | n = 25 |
| Symptoms and feelings | 4.1 (1.26) | 4.3 (1.35) | 1.6 (1.37) | 3.5 (1.66) | 1.3 (1.41) | 2.8 (2.04) | 1.0 (1.39) | 2.5 (1.71) | 2.3 (2.01) | 2.7 (1.95) |
| Daily activities | 3.1 (1.78) | 3.0 (1.47) | 1.0 (1.14) | 2.6 (1.67) | 0.9 (1.20) | 2.0 (1.65) | 0.6 (1.01) | 1.4 (1.67) | 1.7 (1.75) | 1.7 (1.75) |
| Leisure | 3.0 (1.63) | 3.6 (1.93) | 1.1 (1.30) | 2.5 (2.09) | 0.7 (0.98) | 2.1 (2.08) | 0.7 (1.07) | 1.4 (1.75) | 1.8 (1.88) | 1.6 (1.87) |
| Work and school | 1.3 (1.05) | 1.6 (1.08) | 0.4 (0.64) | 1.0 (0.90) | 0.3 (0.52) | 1.0 (1.05) | 0.3 (0.62) | 0.8 (1.03) | 0.7 (0.87) | 0.8 (1.00) |
| Personal relationships | 2.4 (1.43) | 2.9 (2.18) | 0.6 (0.91) | 2.1 (1.84) | 0.5 (0.81) | 1.7 (1.95) | 0.4 (0.89) | 1.8 (1.99) | 1.3 (1.72) | 1.6 (2.00) |
| Treatment | 0.7 (0.76) | 1.2 (0.97) | 0.4 (0.49) | 0.8 (0.76) | 0.1 (0.32) | 0.8 (0.91) | 0.1 (0.29) | 0.6 (0.84) | 0.5 (0.80) | 0.6 (0.82) |
Data are from N = 91 and presented as mean (SD).
Values were not used if they were assessed after intake of corticosteroid rescue medication during the previous 6 wk (excluding baseline values). AE‐QoL, Angioedema Quality of Life Questionnaire; DLQI, Dermatology Life Quality Index; FAS, full analysis set; SD, standard deviation.
n = 37.
Maximum range for: Symptoms and feelings, 0‐6; Daily activities, 0‐6; Leisure, 0‐6; Work and school, 0‐3; Personal relationship, 0‐6; Treatment, 0‐3.
3.1. AE‐QoL and DLQI scores improve rapidly with omalizumab treatment
The AE‐QoL and DLQI scores decreased rapidly following initiation of omalizumab treatment, and the improvements increased further throughout the treatment period (Figure 2A and B). The least squares (LS) mean difference in the AE‐QoL total scores between groups was −17.6 (P < .001) at Week 4, −26.0 (P < .001) at Week 12, −16.3 (P = .006) at Week 20, and −22.7 (P < .001) at Week 28. The LS mean difference in DLQI scores between groups was −7.2 (P < .001) at Week 4, −8.0 (P < .001) at Week 12, −5.8 (P < .001) at Week 20, and −6.1 (P < .001) at Week 28. The mean (SD) total AE‐QoL (omalizumab: 27.7 [22.9]; placebo: 36.2 [24.4]) and DLQI (omalizumab, 7.8 [7.8]; placebo, 11.2 [8.6]) scores approached placebo levels at the follow‐up visit 12 weeks after the last omalizumab injection.
Figure 2.

LS mean changes in (A) AE‐QoL total scores (observed values), (B) DLQI scores (LOCF), and (C) weekly AAS scores (zero was imputed for missing AAS day sum scores) from baseline. The values represented are the LS mean change from baseline for the FAS, using analysis of covariance. *indicates P < .05, **indicates P < .01, and ***indicates P < .001 (LS mean for difference between treatment groups; ANCOVA model). The black triangles indicate omalizumab/placebo administration (Weeks 4, 8, 12, 16, 20, and 24). AAS, Angioedema Activity Score; AE‐QoL, Angioedema Quality of Life Questionnaire; ANCOVA, analysis of covariance; DLQI, Dermatology Life Quality Index; FAS, full analysis set; LOCF, last observation carried forward; LS, least square
The mean AAS7 scores decreased rapidly following omalizumab treatment initiation, with significant differences observed as early as Week 4 (Visit 3).4 AAS7 scores decreased further throughout the omalizumab treatment period; the LS mean difference between groups was −15.6 (P < .001) at Week 4, −14.1 (P = .002) at Week 12, −7.0 (P = .056) at Week 20, and −9.8 (P = .036) at Week 28. Following discontinuation of omalizumab (Week 36), the mean (SD) AAS7 scores increased to placebo levels (omalizumab, 10.2 [19.6]; placebo, 9.8 [12.0]).
Analyses of the AE‐QoL subdomain scores showed that, after 4 weeks of treatment, patients in the omalizumab group had significantly greater mean improvements from baseline in three subdomains of the AE‐QoL compared to the placebo group as follows:functioning, −33.1 vs −6.3 (P < .001); fatigue/mood, −24.3 vs −12.6 (P = .030); fear/shame, −29.3 vs −12.3 (P = .007). The AE‐QoL food subdomain mean scores improved in both the omalizumab and the placebo arms of the study (−10.2 vs −6.3 [P = .444]). At Week 28, the mean change from baseline for the DLQI subdomains in the omalizumab treatment and placebo groups were as follows: symptoms and feelings, −3.2 vs. −1.9 (P = .005); daily activities, −2.5 vs. −1.9 (P = .208); leisure, −2.3 vs. −2.4 (P = .833); work and school, −1.0 vs. −0.8 (P = .509); personal relationship, −2.1 vs. −1.8 (P = .500); and treatment, −0.6 vs. −0.8 (P = .412) (Table 2).
3.2. Fear of life‐threatening angioedema is reduced during omalizumab treatment
At baseline, when we asked patients whether they were afraid of a life‐threatening swelling episode, 66.7% responded “occasionally,” “often,” or “very often.” Similarly, 48.9% of patients were “occasionally” to “very often” afraid that “they could suffocate due to a swelling episode,” emphasizing the gravity of the burden of disease these patients experience (Table 2).
After 28 weeks of omalizumab treatment, only a minority of patients still experienced these fears: 13.6% were “occasionally” to “very often” afraid of life‐threatening swelling compared to 41.7% in the placebo group, and 4.5% were “occasionally” to “very often” afraid of suffocating owing to a swelling episode compared to 25.1% in the placebo group. Reduced fear of a life‐threatening angioedema episode was evident as early as 4 weeks after starting omalizumab treatment but increased upon discontinuation of treatment, that is, at Week 28, 63.6% of patients were “never” afraid of life‐threatening angioedema episodes compared to 29.2% in the placebo group, and during the follow‐up period, these proportions were 37.5% and 35.7% in the omalizumab and placebo groups, respectively (Table 2).
3.3. General psychological well‐being improves with omalizumab treatment
At baseline, the WHO‐5 score indicated a signal for depression in both groups (mean score below the depression threshold of 13; Table 1); however, only 4.5% of patients in the omalizumab group and 8.5% of patients in the placebo group had a previous or current diagnosis of depression. In the omalizumab group, the mean (SD) WHO‐5 score was 10.0 (5.5) at baseline and increased above the depression threshold to 18.6 (5.1) by the end of the treatment period. The corresponding results in the placebo group were 7.7 (5.3) at baseline and 11.5 (5.8) at Week 28 (LS mean difference between groups at Week 28, P < .001; Figure 3).
Figure 3.
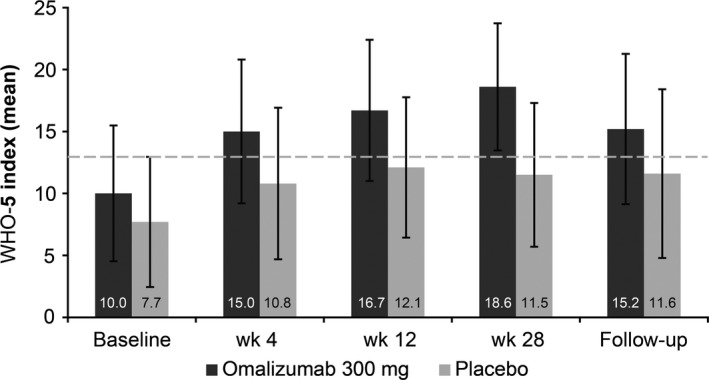
WHO‐5 scores at baseline, Week 4, Week 12, Week 28, and the follow‐up visit at Week 36. A score <13 indicates that the patient is showing signs of depression. Data are presented as mean (SD) WHO‐5 scores (observed cases). SD, standard deviation; WHO‐5, 5‐item World Health Organization Well‐being Index
At Week 28, 82.4% of the omalizumab‐treated patients showed no signal for depression, that is, a WHO‐5 score of ≥13 points, compared to only 41.9% of patients in the placebo group. The probability of reaching the critical threshold (score 13) at the end of the treatment period was 5.6 times higher (odds ratio [OR]: 5.55, 95% confidence interval [CI]: 1.7‐18.2) in the omalizumab group compared to the placebo group (P = .005, Wald test). Furthermore, impaired psychological well‐being, assessed using the WHO‐5, correlated well (Pearson correlation coefficient) with the markedly impaired QoL scores at baseline (AE‐QoL, −0.618 [P < .001]; DLQI, −0.614 [P < .001]) and at Week 28 (AE‐QoL, −0.666 [P < .001]; DLQI, −0.591 [P < .001]).
3.4. Improvement in angioedema symptoms is correlated with health‐related QoL
Angioedema‐related QoL improved with improved AAS scores. Changes in total AE‐QoL and AAS7 scores from baseline were significantly correlated at Week 12 (0.526, P < .001) and at Week 28 (0.501, P < .001). Furthermore, changes in weekly AAS scores correlated with changes in DLQI and WHO‐5 scores at Week 12 (0.518, P < .001 and −0.319, P = .007, respectively) and Week 28 (0.546, P < .001 and −.321, P = .009, respectively).
Subgroup analyses revealed that female patients had higher AE‐QoL scores at baseline compared to male patients (P = .001; Table 3). Although baseline AE‐QoL impairment was significantly higher in females compared to males, there was no significant influence of any factors investigated (age [P = .694], sex [P = .757], disease duration [P = .815], or angioedema activity [baseline AAS7; P = .516]) on the response to treatment (Table 3).
Table 3.
AE‐QoL total scores by subgroups at baseline and at Week 28 (observed values for the FAS)
| Baseline | Week 28 | |||
|---|---|---|---|---|
| Omalizumab | Placebo | Omalizumab | Placebo | |
| Overall | 56.2 (18.69; n = 44) | 59.9 (19.24; n = 47) | 14.1 (14.82; n = 34) | 38.1 (23.56; n = 25) |
| Sex | ||||
| Male | 43.6 (19.49; n = 14) | 54.0 (12.56; n = 14) | 12.1 (14.74; n = 10) | 31.3 (22.84; n = 7) |
| Femalea | 62.0 (15.35; n = 30) | 62.5 (21.12; n = 33) | 15.0 (15.08; n = 24) | 40.8 (23.93; n = 18) |
| Age class | ||||
| <40 y | 59.1 (15.99; n = 14) | 57.1 (15.97; n = 22) | 13.4 (13.31; n = 10) | 41.4 (29.11; n = 7) |
| ≥40 y | 54.8 (19.93; n = 30) | 62.5 (21.73; n = 25) | 14.4 (15.67; n = 24) | 36.8 (21.87; n = 18) |
| Disease duration | ||||
| <2 y | 51.6 (19.19; n = 13) | 52.9 (19.19; n = 13) | 12.9 (9.53; n = 10) | 33.8 (14.9; n = 7) |
| ≥2 y | 58.1 (18.44; n = 31) | 62.6 (18.85; n = 34) | 14.6 (16.69; n = 24) | 39.8 (26.35; n = 18) |
| Disease severity (AAS) | ||||
| <20 | 49.3 (20.1; n = 25) | 56.4 (17.66; n = 22) | 15.8 (17.1; n = 19) | 30.9 (20.65; n = 12) |
| ≥20 | 65.2 (11.97; n = 19) | 63.1 (20.36; n = 25) | 12.0 (11.52; n = 15) | 44.8 (24.86; n = 13) |
AAS, Angioedema Activity Score; AE‐QoL, Angioedema Quality of Life Questionnaire; FAS, full analysis set; SD, standard deviation.
Data are presented as mean (SD; n).
indicates P < .001 (t test; Satterthwaite method) comparing mean (SD) AE‐QoL total score at baseline between male (48.8 [16.94]) and female (62.3 [18.45]) patients.
4. DISCUSSION
To our knowledge, X‐ACT is the first study designed to investigate the effects of omalizumab 300 mg treatment on the impact of angioedema on QoL in patients with moderate‐to‐severe CSU. Our findings show that antihistamine‐refractory CSU patients with angioedema experience significant QoL impairment, general psychological impairment, and fear of life‐threatening angioedema episodes at baseline, all of which rapidly reduced following the initiation of omalizumab treatment.
We have previously reported that omalizumab treatment significantly reduced the disease activity (AAS7), improved CSU‐related QoL, and reduced number of angioedema‐burdened days per week and size of individual swelling episodes.4 We also observed a rapid improvement in angioedema‐related QoL and psychological well‐being as well as reduced angioedema‐related fears, which continued to improve over the course of omalizumab treatment. The rapid improvements in symptoms and QoL observed in these CSU patients are consistent with previous CSU trials, where omalizumab led to a rapid decrease in CSU symptoms and QoL impairment.12, 13, 21 In addition, a recent analysis of three phase 3 studies showed that omalizumab 300 mg significantly improved total DLQI scores vs. placebo, with a mean decrease from baseline to Week 12 of −10.3 vs. −6.1 (P < .0001) in ASTERIA I, −10.2 vs. −6.1 (P = .0004) in ASTERIA II and −9.7 vs. −5.1 (P < .0001) in GLACIAL.22 The proportion of patients in whom changes in mean total DLQI score from baseline to Week 12 reached a minimal clinically important difference (MCID) of ≥4 was 74.1%, 76.0%, and 77.2% in ASTERIA I, II, and GLACIAL, respectively (P < .01; all studies).22 Clinical observations indicate that the onset of omalizumab effect may be even earlier than 4 weeks, but data from controlled clinical trials are missing.
Improvements in the AE‐QoL total scores in this study indicate a clinically meaningful benefit with omalizumab treatment; mean improvements were approximately 6.5 times higher than the previously reported MCID of the total AE‐QoL score (6 points)18 and correlated well with improved AAS7 scores. Moreover, the change in DLQI score was also above the previously defined MCID threshold of 2.2 to 3.1 points.23 Interestingly, these rapid QoL improvements were not only visible in the total score but also in all but one of the AE‐QoL subdomain scores. The rapid decreases in the AE‐QoL subdomain scores indicate that patient's QoL is restored, their functioning in daily life and work life returns to a normal level, they overcome their sleeping problems and depressive mood, as well as their fears and embarrassment associated with the swellings. This observation surpassed our expectations, as it was thought that some subdomains, such as fear/shame, could have been less responsive to omalizumab treatment, or could have responded more slowly. The rapid reduction in QoL appears to be directly associated with the rapid reduction in the frequency and severity of angioedema. In line with this and as reported previously, symptoms began to reappear, and QoL scores worsened again to placebo levels following omalizumab discontinuation.4, 11, 12, 13
Angioedema can be mediated by histamine or bradykinin. Hereditary angioedema (HAE)12 is bradykinin‐mediated and usually develops over the course of several hours, whereas in CSU, angioedema is histamine‐mediated and develops rapidly.24 For HAE patients, angioedema episodes can range from a minor inconvenience to life‐threatening episodes, with a mortality rate of approximately 30% in the case of laryngeal edema.25 In contrast, angioedema is not considered life‐threatening for CSU patients. Despite this, the majority of patients in the X‐ACT trial reported angioedema‐related fears at baseline, and although no patient experienced a truly life‐threatening swelling episode, they still reported fears of suffocation and of life‐threatening symptoms. During omalizumab treatment, symptom‐related fears decreased compared to the placebo group, but these fears reappeared following omalizumab discontinuation; this further supports the benefit of omalizumab in CSU patients with antihistamine‐refractory angioedema. Patient education and appropriate treatment are required to reduce the fears associated with angioedema in CSU patients. Furthermore, the rapid symptom improvement and reduced angioedema‐associated fears observed with omalizumab treatment could reduce health‐resource utilization, such as the number of patient visits to the emergency department, as well as reducing work productivity loss and/or sick leave duration owing to angioedema.26
Chronic spontaneous urticaria patients are reported to have an increased risk of psychiatric comorbidities, including depression.27, 28, 29 In patients with other chronic diseases, depression has been associated with decreased treatment adherence, sleep disturbance, and negative perception of the illness.30 Here, the mean WHO‐5 score was <13 for both treatment groups at baseline, indicating that depression may be present in these patients and that CSU with angioedema must not be classified as a trivial disease or just a cosmetic problem. Following appropriate treatment of CSU‐related symptoms with omalizumab, approximately 80% of patients showed no sign of depression (≥13 points in the WHO‐5) compared with approximately 40% in the placebo group. Thus, omalizumab treatment is not only associated with improvement in the disease condition but also the psychological symptoms associated with CSU. These results suggest that the psychological symptoms observed in CSU patients may be a reaction to the dermatological symptoms experienced; however, further research is required to confirm this.
4.1. Limitations
This study only considered CSU patients with wheals and angioedema, although there are CSU patients with angioedema without/with almost no wheals, who require adequate therapy. Based on the rapid improvement in angioedema activity and angioedema‐related QoL observed in the X‐ACT patient population, CSU patients without wheals would also likely benefit from omalizumab treatment; however, further studies are warranted in this specific patient population. Furthermore, only adult patients were enrolled in the X‐ACT study; therefore, it is unclear whether these results can be extrapolated to children and adolescents with CSU. Finally, the use of concomitant psychological medications was permitted in the X‐ACT study and their use may be regarded as a surrogate for the presence of mild psychiatric/psychological comorbidities that may negatively impact QoL and psychological well‐being. However, their use was low and was comparable between the omalizumab (n = 4) and placebo (n = 5) groups. Thus, the expected impact on the outcome parameters is low.
5. CONCLUSIONS
Patients with moderate‐to‐severe CSU with angioedema that is refractory to high doses of H1‐antihistamines have impaired QoL, fear of suffocation due to swelling episodes, and impaired psychological well‐being, including an increased risk for signs of depression. This study revealed that omalizumab is a highly effective treatment for this patient cohort and is associated with a marked and rapid improvement in angioedema symptoms, angioedema‐related QoL, symptom‐related fears, and psychological well‐being. Based on these findings, we recommend that omalizumab is prescribed for the treatment of patients with severe H1‐antihistamine‐refractory CSU with angioedema.
AUTHOR CONTRIBUTIONS
All authors participated in the design of this study and/or data generation. All authors also participated in the interpretation of results, and drafted and revised the manuscript. All authors approved the final version of the manuscript.
CONFLICTS OF INTEREST
Matthias Bräutigam and Christian Sieder are employees of the study sponsor, Novartis Pharma GmbH, Germany. Nadine Chapman‐Rothe is an employee of Novartis Pharma AG, Basel, Switzerland. Martin Metz has received honoraria as a speaker for Bayer Pharma, Dr. R. Pfleger, Essex Pharma, Recordati Pharma, Merck, Moxie, Novartis, LEO, Sanofi, Shire, UCB, and Uriach. Marcus Maurer has received research funding and/or fees for consulting and/or lectures from Genentech, GSK, Faes, Moxie, Novartis, MSD, UCB, und Uriach. Karsten Weller has received honoraria for educational lectures from Dr. R. Pfleger, Essex Pharma (now MSD), Uriach, UCB, Novartis, and Moxie. He has also received honoraria for consulting from Novartis and was involved in clinical research projects of Dr. R. Pfleger, Essex Pharma (now MSD), Faes, Uriach, and Novartis. Petra Staubach has received research funding and/or fees for consulting and/or lectures from Genentech, Novartis, MSD, UCB, Dr. R. Pfleger, Karrer, LEO, Shire, Abbvie, Sobi, CSL Behring, Leti, Pfizer, Janssen, Astellas, Celgene, and Lilly.
ACKNOWLEDGMENTS
The authors thank Áine Abautret‐Daly, PhD, of Novartis Ireland Ltd., and Rukaiyya Khan, PhD, of Novartis Healthcare Pvt. Ltd., for providing medical writing support/editorial support, which was funded by Novartis Pharma AG, Basel, Switzerland, in accordance with the Good Publication Practice (GPP3) guidelines ( http://www.ismpp.org/gpp3).
Staubach P, Metz M, Chapman‐Rothe N, et al. Omalizumab rapidly improves angioedema‐related quality of life in adult patients with chronic spontaneous urticaria: X‐ACT study data. Allergy. 2018;73:576–584. https://doi.org/10.1111/all.13339
Funding information
The study was funded by Novartis Pharma GmbH, Germany.
Petra Staubach and Martin Metz are joint first authors.
Edited by: Werner Aberer
REFERENCES
- 1. Zuberbier T, Aberer W, Asero R, et al. The EAACI/GA(2) LEN/EDF/WAO Guideline for the definition, classification, diagnosis, and management of urticaria: the 2013 revision and update. Allergy. 2014;69:868‐887. [DOI] [PubMed] [Google Scholar]
- 2. Grattan C. The urticarias: pathophysiology and management. Clin Med. 2012;12:164‐167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bork K. An evidence based therapeutic approach to hereditary and acquired angioedema. Curr Opin Allergy Clin Immunol. 2014;14:354‐362. [DOI] [PubMed] [Google Scholar]
- 4. Staubach P, Metz M, Chapman‐Rothe N, et al. Effect of omalizumab on angioedema in H‐antihistamine resistant chronic spontaneous urticaria patients: results from X‐ACT, a randomised controlled trial. Allergy. 2016;71:1135‐1144. [DOI] [PubMed] [Google Scholar]
- 5. Maurer M, Staubach P, Raap U, et al. H1‐antihistamine‐refractory chronic spontaneous urticaria: it's worse than we thought – first results of the multicenter real‐life AWARE study. Clin Exp Allergy. 2017;47:684‐692. [DOI] [PubMed] [Google Scholar]
- 6. Choi WS, Lim ES, Ban GY, et al. Disease‐specific impairment of the quality of life in adult patients with chronic spontaneous urticaria. Korean J Intern Med. 2018;33:185‐192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McCormack PL. Omalizumab: a review of its use in patients with chronic spontaneous urticaria. Drugs. 2014;74:1693‐1699. [DOI] [PubMed] [Google Scholar]
- 8. Zhao ZT, Ji CM, Yu WJ, et al. Omalizumab for the treatment of chronic spontaneous urticaria: a meta‐analysis of randomized clinical trials. J Allergy Clin Immunol. 2016;137:1742‐1750. [DOI] [PubMed] [Google Scholar]
- 9. Metz M, Staubach P, Bauer A, et al. Clinical efficacy of omalizumab in chronic spontaneous urticaria is associated with a reduction of FcεRI‐positive cells in the skin. Theranostics. 2017;7:1266‐1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Maurer M, Altrichter S, Bieber T, et al. Efficacy and safety of omalizumab in patients with chronic urticaria who exhibit IgE against thyroperoxidase. J Allergy Clin Immunol. 2011;128:202‐209. [DOI] [PubMed] [Google Scholar]
- 11. Saini SS, Bindslev‐Jensen C, Maurer M, et al. Efficacy and safety of omalizumab in patients with chronic idiopathic/spontaneous urticaria who remain symptomatic on H1 antihistamines: a randomized, placebo‐controlled study. J Invest Dermatol. 2015;135:67‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Weller K, Groffik A, Magerl M, et al. Development, validation, and initial results of the Angioedema Activity Score. Allergy. 2013;68:1185‐1192. [DOI] [PubMed] [Google Scholar]
- 13. Kaplan A, Ledford D, Ashby M, et al. Omalizumab in patients with symptomatic chronic idiopathic/spontaneous urticaria despite standard combination therapy. J Allergy Clin Immunol. 2013;132:101‐109. [DOI] [PubMed] [Google Scholar]
- 14. Saini S, Rosen KE, Hsieh HJ, et al. A randomized, placebo‐controlled, dose‐ranging study of single‐dose omalizumab in patients with H1‐antihistamine‐refractory chronic idiopathic urticaria. J Allergy Clin Immunol. 2011;128:567‐573. [DOI] [PubMed] [Google Scholar]
- 15. Maurer M, Sofen H, Ortiz B, Kianifard F, Gabriel S, Bernstein JA. Positive impact of omalizumab on angioedema and quality of life in patients with refractory chronic idiopathic/spontaneous urticaria: analyses according to the presence or absence of angioedema. J Eur Acad Dermatol Venereol. 2016;31:1056‐1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zazzali JL, Kaplan A, Maurer M, et al. Angioedema in the omalizumab chronic idiopathic/spontaneous urticaria pivotal studies. Ann Allergy Asthma Immunol. 2016;117:370‐377. [DOI] [PubMed] [Google Scholar]
- 17. Weller K, Groffik A, Magerl M, et al. Development and construct validation of the angioedema quality of life questionnaire. Allergy. 2012;67:1289‐1298. [DOI] [PubMed] [Google Scholar]
- 18. Weller K, Magerl M, Peveling‐Oberhag A, Martus P, Staubach P, Maurer M. The Angioedema Quality of Life Questionnaire (AE‐QoL) – assessment of sensitivity to change and minimal clinically important difference. Allergy. 2016;71:1203‐1209. [DOI] [PubMed] [Google Scholar]
- 19. Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI) – a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19:210‐216. [DOI] [PubMed] [Google Scholar]
- 20. Krieger T, Zimmermann J, Huffziger S, et al. Measuring depression with a well‐being index: further evidence for the validity of the WHO Well‐Being Index (WHO‐5) as a measure of the severity of depression. J Affect Disord. 2014;156:240‐244. [DOI] [PubMed] [Google Scholar]
- 21. Metz M, Ohanyan T, Church MK, Maurer M. Omalizumab is an effective and rapidly acting therapy in difficult‐to‐treat chronic urticaria: a retrospective clinical analysis. J Dermatol Sci. 2014;73:57‐62. [DOI] [PubMed] [Google Scholar]
- 22. Finlay AY, Kaplan AP, Beck LA, et al. Omalizumab substantially improves dermatology‐related quality of life in patients with chronic spontaneous urticaria. J Eur Acad Dermatol Venereol. 2017;31:1715‐1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shikiar R, Harding G, Leahy M, Lennox RD. Minimal important difference (MID) of the Dermatology Life Quality Index (DLQI): results from patients with chronic idiopathic urticaria. Health Qual Life Outcomes. 2005;3:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. James C, Bernstein JA. Current and future therapies for the treatment of histamine‐induced angioedema. Expert Opin Pharmacother. 2017;18:253‐262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hallak B, Konu P, Lang F, Simon C, Monnier P. Acquired form of angioedema of the head and neck related to a deficiency in c1‐inhibitor: a case report with a review of the literature. Case Rep Otolaryngol. 2012;2012:405824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bernstein JA, Moellman J. Emerging concepts in the diagnosis and treatment of patients with undifferentiated angioedema. Int J Emerg Med. 2012;5:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Staubach P, Dechene M, Metz M, et al. High prevalence of mental disorders and emotional distress in patients with chronic spontaneous urticaria. Acta Derm Venereol. 2011;91:557‐561. [DOI] [PubMed] [Google Scholar]
- 28. Barbosa F, Freitas J, Barbosa A. Chronic idiopathic urticaria and anxiety symptoms. J Health Psychol. 2011;16:1038‐1047. [DOI] [PubMed] [Google Scholar]
- 29. Herguner S, Kilic G, Karakoc S, Tamay Z, Tuzun U, Guler N. Levels of depression, anxiety and behavioural problems and frequency of psychiatric disorders in children with chronic idiopathic urticaria. Br J Dermatol. 2011;164:1342‐1347. [DOI] [PubMed] [Google Scholar]
- 30. Brooks AJ, Rowse G, Ryder A, Peach EJ, Corfe BM, Lobo AJ. Systematic review: psychological morbidity in young people with inflammatory bowel disease – risk factors and impacts. Aliment Pharmacol Ther. 2016;44:3‐15. [DOI] [PubMed] [Google Scholar]


