Abstract
The cardiovascular safety of liraglutide, a glucagon‐like peptide‐1 receptor agonist approved for weight management at a dose of 3.0 mg, was evaluated post hoc using data from 5908 participants in 5 randomized, double‐blind, placebo‐controlled clinical trials. Participants were randomized to liraglutide or a comparator group (placebo or orlistat). The objective was to evaluate whether cardiovascular risk was increased with liraglutide treatment. The primary composite outcome of this time‐to‐event analysis was the first occurrence of cardiovascular death, nonfatal myocardial infarction or nonfatal stroke. These cardiovascular events were adjudicated prospectively for three of the trials and retrospectively for two trials by an event adjudication committee. The primary outcome was analyzed using a Cox proportional hazards model, stratified by trial. With liraglutide 3.0 mg, 8 participants had positively adjudicated cardiovascular events (1.54 events/1000 person‐years) compared to 10 participants in the comparators group (3.65 events/1000 person‐years). The hazard ratio for liraglutide 3.0 mg compared to comparators was 0.42 (95% confidence interval, 0.17‐1.08). In this analysis, liraglutide 3.0 mg treatment was not associated with excess cardiovascular risk. However, the wide confidence intervals and retrospective adjudication of events in two of the trials are limitations of the analysis.
Keywords: antiobesity drug, cardiovascular disease, clinical trial, GLP‐1, GLP‐1 analogue, liraglutide
1. INTRODUCTION
Obesity is a chronic disease, and a risk factor for the development of type 2 diabetes mellitus (T2DM) and cardiovascular disease; obesity is also associated with an increased risk of cardiovascular mortality.1 While weight loss can lead to improvements in cardiovascular risk factors (waist circumference, blood pressure, lipid profile, cardiovascular biomarkers and glucose homeostasis), its association with improved cardiovascular outcomes is less clear.2, 3
Several anti‐obesity medications have been linked to increased blood pressure, heart rate and cardiovascular risk, leading to marketing authorization withdrawal.4 Regulatory authorities now require all new obesity and T2DM medications to demonstrate no excess cardiovascular health risks.5, 6, 7, 8 Cardiovascular‐outcomes trials with lorcaserin and phentermine/topiramate for weight management are planned.
Liraglutide, a glucagon‐like peptide‐1 receptor agonist (GLP‐1 RA), is approved for the treatment of T2DM at doses up to 1.8 mg, and has been shown to reduce the risk of cardiovascular complications.9 Liraglutide 3.0 mg, as an adjunct to a reduced‐calorie diet and increased physical activity, is approved in several regions worldwide for weight management. Treatment with liraglutide 3.0 mg during the phase 3a development programme called SCALE (Satiety and Clinical Adiposity – Liraglutide Evidence in individuals with and without diabetes) has been associated with significantly greater and sustained weight loss compared to placebo and with improvements in a variety of cardiometabolic risk factors.10, 11, 12, 13, 14 The cardiovascular outcomes from the SCALE clinical trials have not been reported. Therefore, the primary objective of this report was to evaluate whether liraglutide 3.0 mg treatment was associated with excess cardiovascular risk. The composite outcome was the time to first occurrence of cardiovascular death, nonfatal myocardial infarction or nonfatal stroke.
2. METHODS
2.1. Trials and participants included in the analysis
The weight management clinical development programme for liraglutide included a 2‐year phase 2 dose‐finding trial and 4 randomized, double‐blind, placebo‐controlled phase 3a SCALE trials.10, 11, 12, 13, 14 This post hoc analysis was of pooled data from these 5 trials (of up to 3 years' duration) and it evaluated cardiovascular risk associated with liraglutide at doses up to 3.0 mg.
Eligible individuals had a body mass index (BMI) ≥27 kg/m2 with at least 1 weight‐related comorbidity, or a BMI ≥30 kg/m2. Those with uncontrolled hypertension (systolic blood pressure ≥160 mm Hg and/or diastolic blood pressure ≥ 100 mm Hg) were excluded. Participants received once‐daily subcutaneous treatment with liraglutide or placebo, starting at a dose of 0.6 mg with weekly 0.6‐mg increments to the maintenance dose of 3.0 mg. Additional liraglutide doses were included in the SCALE Diabetes trial (1.8 mg) and the phase 2 trial (1.2, 1.8 and 2.4 mg); the latter also included orlistat as an active comparator. Diet and exercise advice, given at approximately monthly intervals, was similar for all trials. Table S1 provides details of the trials and their primary outcomes; full methodology details have been reported previously.10, 11, 12, 13, 14
2.2. Cardiovascular event adjudication
Cardiovascular events were adjudicated prospectively by a blinded event adjudication committee for 3 of the SCALE trials (Table S1). For the phase 2 trial and the SCALE Maintenance trial, events were identified by searching the trial database for specific Medical Dictionary for Regulatory Activities (MedDRA) terms and serious adverse events recorded by trial investigators, as described in Appendix S1. Events found in each search were then adjudicated retrospectively and independently by blinded medical experts.
2.3. Statistical analysis
The primary analysis compared the incidence of cardiovascular events for the approved weight management dose of liraglutide 3.0 mg compared to a pooled comparator group (placebo or orlistat) in the weight management trials. Additional supportive analyses included all liraglutide doses from the trials compared to all comparators, liraglutide 3.0 mg compared to placebo, and analyses excluding participants with T2DM, that is, those from the SCALE Diabetes trial. For both the primary and supportive analyses, the time from first treatment dose to occurrence of the first event was analysed using a Cox proportional hazards model stratified by trial, with treatment as the explanatory variable. All analyses included events observed between the date of first treatment administration and the last date of treatment plus 30 days. Participants who discontinued prematurely were censored at the date of last treatment plus 30 days. Analyses were performed using SAS version 9.4 (SAS Institute, Cary, North Carolina).
3. RESULTS
Data from 5908 participants in the weight management trials were included in this analysis: liraglutide 3.0 mg, n = 3384; all liraglutide doses, n = 3872; comparators, n = 2036 (1941 receiving placebo, 95 receiving orlistat). Baseline characteristics of the pooled population are shown in Table S2. Mean and categorical weight loss achieved in each of the trials is shown in Table S3.
The incidence of cardiovascular events in each trial and analysis is shown in Table 1. Figure 1A shows the Kaplan–Meier curve of time to first occurrence of an event for the primary analysis. The primary composite analysis plus analyses of each of the 3 individual components are depicted in Figure 1B. With liraglutide 3.0 mg, 8 participants underwent positively adjudicated events (1.54 events/1000 person‐years) compared to 10 participants in the comparator group (3.65 events/1000 person‐years). The hazard ratio for the primary analysis was 0.42 (95% confidence interval (CI), 0.17‐1.08). Across analyses, hazard ratios ranged from 0.31 to 0.94, favouring liraglutide, with upper limits of the 95% CI ranging from 1.08 to 4.67 (Table 1). For all liraglutide doses vs comparators, the hazard ratio was 0.50 (95% CI, 0.21‐1.18). In the SCALE Obesity and Prediabetes trial, which represented approximately 80% of total person‐years, the hazard ratio was 0.70 (95% CI, 0.20‐2.50). All participants with positively adjudicated cardiovascular events are shown in Table S4.
Table 1.
Cardiovascular events in the weight management trials, and pooled
| Population | Liraglutide 3.0 mg | Comparator | P value | |||||
|---|---|---|---|---|---|---|---|---|
| Number | Person‐years | Incidence rate Events/1000 person‐years | Number | Person‐years | Incidence rate Events/1000 person‐years | HR (95% CI) | ||
| All trials pooled (N = 5908) | ||||||||
| Liraglutide 3.0 mg vs all comparators a | 8 | 5194 | 1.54 | 10 | 2742 | 3.65 | 0.42 (0.17‐1.08) | .07 |
| Liraglutide 3.0 mg vs placebo | 8 | 5194 | 1.54 | 10 | 2612 | 3.83 | 0.42 (0.17‐1.08) | .07 |
| All liraglutide doses vs all comparatorsb | 11 | 5786 | 1.90 | 10 | 2742 | 3.65 | 0.50 (0.21‐1.18) | .11 |
| Participants without T2DM (N = 5064) | ||||||||
| Liraglutide 3.0 mg vs all comparators | 6 | 4781 | 1.25 | 7 | 2546 | 2.75 | 0.48 (0.16‐1.44) | .19 |
| Liraglutide 3.0 mg vs placebo | 6 | 4781 | 1.25 | 7 | 2416 | 2.90 | 0.48 (0.16‐1.44) | .19 |
| All liraglutide doses vs all comparators | 6 | 5168 | 1.16 | 7 | 2546 | 2.75 | 0.48 (0.16‐1.44) | .19 |
| SCALE trials | ||||||||
| Obesity and Prediabetes 160‐week trial (N = 3723) c | ||||||||
| Liraglutide 3.0 mg vs placebo | 6 | 4325 | 1.39 | 4 | 2023 | 1.98 | 0.70 (0.20‐2.50) | .59 |
| Diabetes 56‐week trial (N = 844); participants with T2DM | ||||||||
| Liraglutide 3.0 mg vs placebo | 2 | 413 | 4.84 | 3 | 196 | 15.3 | 0.31 (0.05‐1.88) | .20 |
| Liraglutide 1.8 mg vs placebo | 3 | 206 | 14.6 | 3 | 196 | 15.3 | 0.94 (0.19‐4.67) | .94 |
| All liraglutide doses vs placebo | 5 | 619 | 8.08 | 3 | 196 | 15.3 | 0.53 (0.13‐2.21) | .38 |
| Maintenance 56‐week trial (N = 422) | ||||||||
| Liraglutide 3.0 mg vs placebo | 0 | 212 | 0 | 1 | 202 | 4.96 | – | – |
| Sleep apnea 32‐week trial (N = 355) | ||||||||
| Liraglutide 3.0 mg vs placebo | 0 | 104 | 0 | 2 | 110 | 18.2 | – | – |
| Phase 2 dose‐finding 2‐year trial (N = 564) d | ||||||||
| Liraglutide 3.0 mg vs all comparators | 0 | 140 | 0 | 0 | 211 | 0 | – | – |
| Liraglutide 3.0 mg vs placebo | 0 | 140 | 0 | 0 | 81 | 0 | – | – |
| All liraglutide doses vs all comparators | 0 | 526 | 0 | 0 | 211 | 0 | – | – |
Abbreviations: CI, confidence interval; HR, hazard ratio; N, total number of participants in each analysis; T2DM, type 2 diabetes mellitus.
The number of participants with adjudicated cardiovascular events and the incidence rate are shown by treatment group. Person‐years were counted until the time of first event or censoring. Cardiovascular events were defined as cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke. Exposure time included 30 days after the last treatment date or last visit, as applicable. The endpoint in all analyses was time to first event; 2 participants had an additional event each, see Table S4.
Primary analysis.
All comparators group included treatment with placebo (n = 1941) and orlistat (n = 95). No events were reported in the orlistat treatment group, from the phase 2 trial.
Excluding participants in the liraglutide group who were re‐randomized to placebo in the re‐randomized period of the 56‐week trial.
For participants randomized to placebo, data are included only up to 1 year, after which participants switched to treatment with liraglutide.
Figure 1.
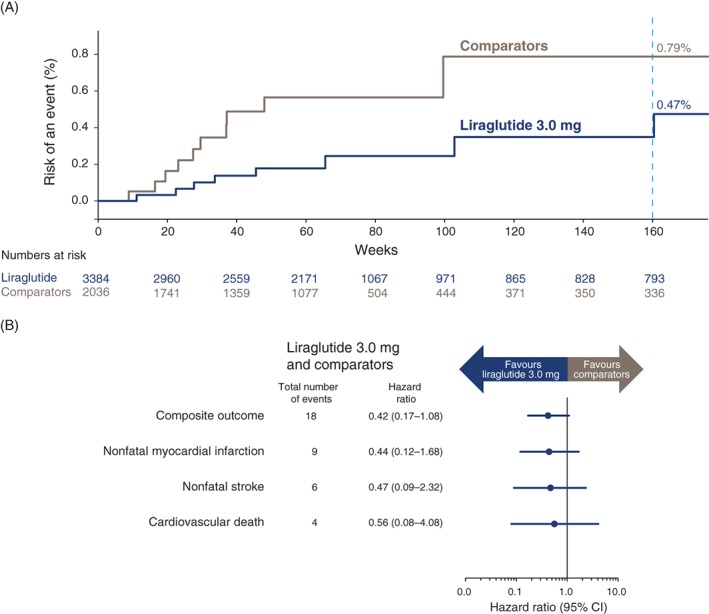
Cardiovascular events in the pooled weight management trials. Time to first event is shown for liraglutide 3.0 mg vs all comparators (A). Primary composite analysis and analyses of the 3 individual components (B). One participant included in the analysis had an additional cardiovascular event (Table S4); however, the composite analysis includes only the first event for each participant
Mean pooled changes in blood pressure and pulse are shown in Table S5 for trials of up to 1‐year duration. Liraglutide 3.0 mg was associated with significantly reduced mean systolic and diastolic blood pressure compared to placebo in each of the trials, with estimated treatment differences of −2.9 (95% CI, −3.5 to −2.3) and −0.8 (−1.3 to −0.4), respectively. Mean resting pulse increased significantly after treatment initiation with liraglutide (estimated treatment difference, 2.5 [95% CI, 2.0‐3.0]), but returned to baseline values upon treatment discontinuation. The estimated treatment difference between liraglutide 3.0 mg and placebo in mean pulse at end‐of‐treatment was 2.5 beats/min (Table S5), with consistent effects seen across the individual trials.
4. DISCUSSION
In this analysis of participants with obesity, or overweight with comorbidities, liraglutide 3.0 mg was associated with a mean increase in pulse and decreased systolic blood pressure. The primary aim of the SCALE programme was to assess the overall efficacy and safety of liraglutide for weight management; this was a post hoc analysis of cardiovascular safety. While the overall number of cardiovascular events was low, liraglutide 3.0 mg was not associated with an increased rate of cardiovascular events as compared with a pooled comparators group.
Trials with sibutramine and the combination of bupropion and naltrexone are the only cardiovascular‐outcomes trials for anti‐obesity medications that have been completed to date.2, 15 No excess cardiovascular risk was observed with bupropion and naltrexone as compared to placebo; however, because of the premature release of interim study results, the cardiovascular safety of this anti‐obesity medication remains unproven.15 In the Sibutramine Cardiovascular OUTcomes (SCOUT) trial, while more events of nonfatal myocardial infarction occurred in a high‐risk population in the sibutramine group compared with the placebo group, greater weight loss was associated with reduced cardiovascular mortality in both treatment groups.2 Sibutramine has since been withdrawn from the market because of concerns about heart attack in those with pre‐existing cardiovascular disease.
The LEADER trial (Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results) was a prospective randomized clinical trial designed to evaluate the cardiovascular safety of liraglutide at doses up to 1.8 mg in 9340 participants with T2DM.9 During a median follow‐up period of 3.8 years, the rate of death from cardiovascular causes, nonfatal myocardial infarction or nonfatal stroke (primary composite outcome) was significantly lower with liraglutide than with placebo, with a hazard ratio of 0.87 (95% CI, 0.78‐0.97); P < .001. Results from another GLP‐1 RA trial, the SUSTAIN‐6 trial (Semaglutide Unabated Sustainability in Treatment of Type 2 Diabetes), indicated that therapy with once‐weekly semaglutide (0.5 or 1.0 mg) for 2 years was also associated with a significant reduction in cardiovascular events, with a hazard ratio of 0.74 (95% CI, 0.58‐0.95).16 Liraglutide 3.0 mg, compared to placebo, has demonstrated positive effects on body weight and a reduced risk of T2DM among individuals with prediabetes while on treatment, as well as a mean decrease in blood pressure.11 Results from the present pooled analysis of the SCALE trials suggest no increased risk and a possible benefit of liraglutide 3.0 mg on cardiovascular safety in an overweight and obese population. The population of the SCALE trials was younger on average, with a higher mean BMI, and was at lower risk of cardiovascular disease compared to the LEADER trial. Moreover, individuals with uncontrolled hypertension were excluded. Whether there is a dose/response relationship for the beneficial effect of liraglutide on cardiovascular risk is currently unknown, but weight loss with liraglutide 3.0 mg was significantly greater than with the 1.8 mg dose,12 and was greater with higher doses compared with lower doses of semaglutide.16
The underlying mechanism of the increase in resting pulse with liraglutide remains to be determined. Preliminary data indicate the presence of GLP‐1 receptors on the sino‐atrial node in nonhuman primates and humans, suggesting a direct chronotropic effect of liraglutide.17
Limitations of this analysis include the wide confidence intervals and the retrospective adjudication of events in 2 of the trials. Moreover, there was no consistent follow‐up for participants who discontinued prematurely (approximately 50% of randomized participants after 3 years) and were censored in the analysis; associations between the risks of censoring and cardiovascular events cannot be ruled out. Strengths of this analysis include the large number of participants and the fact that events were prospectively adjudicated in most of the trials.
In summary, liraglutide 3.0 mg treatment was not associated with excess cardiovascular risk in this analysis of data from the phase 2 and 3a SCALE clinical trials.
Supporting information
Table S1. Summary of randomized, placebo‐controlled trials included in the pooled analysis.
Table S2. Demographics and baseline characteristics.
Table S3. Mean and categorical changes in body weight at end of treatment in the SCALE trials
Table S4. All participants with positively adjudicated cardiovascular events, by trial.
Table S5. Changes in vital signs between baseline and end of treatment for pooled trials up to 56 weeks of duration.
ACKNOWLEDGMENTS
We gratefully acknowledge the contribution of all trial participants and the clinical trial site personnel who assisted with the trials. We also thank Angela Stocks, PhD (Larix A/S, Copenhagen, Denmark) for editorial and medical writing services funded by Novo Nordisk.
Conflict of interest
M. D. has acted as consultant, advisory board member and speaker for Novo Nordisk, Sanofi‐Aventis, Lilly, Merck Sharp & Dohme, Boehringer Ingelheim, AstraZeneca and Janssen, as an advisory board member for Servier, and as a speaker for Mitsubishi Tanabe Pharma Corporation and Takeda Pharmaceuticals International Inc. and has received grants in support of investigator and investigator‐initiated trials from Novo Nordisk, Sanofi‐Aventis, Lilly, Boehringer Ingelheim and Janssen. L. A. is an advisor or consultant for Novo Nordisk, Eisai, GI Dynamics, Pfizer, Zafgen, ERx and Gelesis; he is an investigator for Aspire and AstraZeneca and he is a shareholder in BMIQ, Myos Corp, Jamieson Labs, Zafgen, Gelesis and ERx. I. C. has performed and still performs clinical trials of obesity treatment and prevention, some of which have been funded by government, but others by the pharmaceutical industry (current trials are funded by the NHMRC [3 trials], Novo Nordisk, Pfizer, BMS and SFI); he has given talks for Novo Nordisk, Servier Laboratories, Ache and Pfizer in the last 3 years and he is president of the World Obesity Federation. A. T. and P. J. are employees of Novo Nordisk and own stock in the company. S. M. reports receiving personal fees from Abbott Vascular and Boston Scientific related to physician education and fees from Novo Nordisk, Sanofi Aventis and Abiomed related to consulting or research activities. Novo Nordisk was responsible for the overall trial designs, and provided a formal review of the manuscript, but the authors had final authority, including choice of journal and the decision to submit the work for publication. All authors had full access to the data included in this analysis, analysed and interpreted the data, critically reviewed the manuscript and approved the final version for submission. All authors also agreed to be accountable for all aspects of the work.
Author contributions
All authors had full access to the data included in this analysis, analysed and interpreted the data, critically reviewed the manuscript and approved the final version for submission. All authors also agreed to be accountable for all aspects of the work. Novo Nordisk was responsible for the overall trial designs, and provided a formal review of the manuscript, but the authors had final authority, including choice of journal and the decision to submit the work for publication.
Davies MJ, Aronne LJ, Caterson ID, et al. Liraglutide and cardiovascular outcomes in adults with overweight or obesity: A post hoc analysis from SCALE randomized controlled trials. Diabetes Obes Metab. 2018;20:734–739. https://doi.org/10.1111/dom.13125
Funding information This study was funded by Novo Nordisk A/S.
REFERENCES
- 1. The Global BMI Mortality Collaboration . Body‐mass index and all‐cause mortality: individual‐participant‐data meta‐analysis of 239 prospective studies in four continents. Lancet. 2016;388:776–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Caterson ID, Finer N, Coutinho W, et al. Maintained intentional weight loss reduces cardiovascular outcomes: results from the Sibutramine Cardiovascular OUTcomes (SCOUT) trial. Diabetes Obes Metab. 2012;14:523–530. [DOI] [PubMed] [Google Scholar]
- 3. The Look AHEAD Research Group . Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369:145154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. James WPT, Caterson ID, Coutinho W, et al. Effect of sibutramine on cardiovascular outcomes in overweight and obese subjects. N Engl J Med. 2010;363:905–917. [DOI] [PubMed] [Google Scholar]
- 5. Jordan J, Astrup A, Engeli S, Narkiewicz K, Day WW. Cardiovascular effects of phentermine and topiramate: a new drug combination for the treatment of obesity. J Hypertens. 2014;32:1178–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. U.S. Department of Health and Human Services.Public Health Service.Food and Drug Administration . Background memorandum: the role of cardiovascular assessment in the pre and postapproval settings for drugs developed for the treatment of obesity, 2012. https://www.fda.gov/downloads/advisorycommittees/committeesmeetingmaterials/drugs/endocrinologicandmetabolicdrugsadvisorycommittee/ucm297240.pdf. Accessed May 24, 2017.
- 7. Food and Drug Administration . Guidance for industry diabetes mellitus – evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes, 2008. http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm071627.pdf. Accessed May 24, 2017.
- 8. European Medicines Agency‐Commitee for Medicinal Products for Human Use . Reflection paper on assessment of cardiovascular risk of medicinal products for the treatment of cardiovascular and metabolic diseases, 2015. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2015/06/WC500187801.pdf. Accessed May 24, 2107.
- 9. Marso SP, Daniels GH, Brown‐Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Astrup A, Carraro R, Finer N, et al. Safety, tolerability and sustained weight loss over 2 years with the once‐daily human GLP‐1 analog, liraglutide. Int J Obes (Lond). 2012;36:843–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pi‐Sunyer X, Astrup A, Fujioka K, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015;373:11–22. [DOI] [PubMed] [Google Scholar]
- 12. Davies MJ, Bergenstal R, Bode B, et al. Efficacy of liraglutide for weight loss among patients with type 2 diabetes: the SCALE diabetes randomized clinical trial. JAMA. 2015;314:687–699. [DOI] [PubMed] [Google Scholar]
- 13. Wadden TA, Hollander P, Klein S, et al. Weight maintenance and additional weight loss with liraglutide after low‐calorie‐diet‐induced weight loss: the SCALE Maintenance randomized study. Int J Obes (Lond). 2013;37:1443–1451. [DOI] [PubMed] [Google Scholar]
- 14. Blackman A, Foster G, Zammit G, et al. Effect of liraglutide 3.0 mg in individuals with obesity and moderate or severe obstructive sleep apnea: the SCALE Sleep Apnea randomized clinical trial. Int J Obes (Lond). 2016;40:1310–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nissen SE, Wolski KE, Prcela RN, et al. Effect of naltrexone‐bupropion on major adverse cardiovascular events in overweight and obese patients with cardiovascular risk factors. JAMA. 2016;315:990–1004. [DOI] [PubMed] [Google Scholar]
- 16. Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834–1844. [DOI] [PubMed] [Google Scholar]
- 17. Pyke C, Heller RS, Kirk RK, et al. GLP‐1 receptor localization in monkey and human tissue; novel distribution revealed with extensively validated monoclonal antibody. Endocrinology. 2014;155:1280–1290. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Summary of randomized, placebo‐controlled trials included in the pooled analysis.
Table S2. Demographics and baseline characteristics.
Table S3. Mean and categorical changes in body weight at end of treatment in the SCALE trials
Table S4. All participants with positively adjudicated cardiovascular events, by trial.
Table S5. Changes in vital signs between baseline and end of treatment for pooled trials up to 56 weeks of duration.


