Abstract
Aims
Nasal glucagon (NG) is a nasally‐administered glucagon powder, absorbed through the nasal mucosa, designed for treatment of severe hypoglycaemia. This study evaluated the safety, pharmacokinetics (PK) and pharmacodynamics (PD) of NG in otherwise healthy participants with common colds and after recovery from cold symptoms, with and without concomitant nasal decongestant.
Materials and Methods
This was a single‐centre, open‐label study. Cohort 1 participants (N = 18) received 2 doses of NG: one while experiencing nasal congestion and another after recovery from cold symptoms. Cohort 2 participants (N = 18), who also had colds with nasal congestion, received a single dose of NG 2 hours after treatment with the decongestant oxymetazoline. Total symptoms score and other safety measures were assessed before and after NG administration.
Results
NG was well tolerated, without serious adverse events. Common adverse events (transient lacrimation, nasal discomfort, rhinorrhea and nausea) were more frequent in both Cohorts 1 and 2 during nasal congestion. Glucagon levels peaked 18 minutes post‐dose and glucose levels peaked 30 to 42 minutes post‐dose in all groups. Nasal congestion, with or without concomitant nasal decongestant, did not significantly affect PK of NG. Although glucose AUECs0‐t was different between Cohort 1 with nasal congestion and Cohort 2, glucose concentrations at 30 minutes appeared similar in all groups.
Conclusions
There were no clinically relevant differences in safety or PK/PD of NG associated with nasal congestion or concomitant administration of nasal decongestant, suggesting that NG can be used to treat severe hypoglycaemia in individuals experiencing nasal congestion.
Keywords: glucagon, hypoglycaemia
1. INTRODUCTION
Severe hypoglycaemia is an ever‐present concern among individuals treated with insulin or insulin secretagogues. In 2011, hypoglycaemia was the first‐listed diagnosis noted in more than 280 000 hospital emergency room visits in the USA.1 In a survey of individuals with type 1 diabetes (T1D), 11.8% reported seizure or loss of consciousness because of hypoglycaemia within the previous 12 months.2 Fear of hypoglycaemia may influence patients towards higher blood glucose, increasing risks of hyperglycaemia‐associated complications.3, 4
Glucagon is an effective treatment for severe hypoglycaemia. Aqueous glucagon is unstable; thus, glucagon emergency kits contain powdered glucagon which must be reconstituted before being administered.5, 6 When treating individuals who are disoriented, unconscious, seizing or convulsing in an emergency situation, people without medical training may find the reconstitution process difficult, time‐consuming and prone to error. Delays, errors, or failure to administer glucagon during severe hypoglycaemia may negatively impact patient outcomes.7 Recently, a study has shown that reconstituted recombinant glucagon delivered intranasally in euglycaemic individuals is rapidly absorbed into the systemic circulation and acts to increase blood glucose.8 A form of glucagon that does not require reconstitution could be more beneficial, as it could be administered more quickly.
Nasal glucagon (NG), a novel drug/device combination product, is being developed for treatment of severe hypoglycaemia. The needle‐free, ready‐to‐use device delivers a dry powdered formulation into the patient's nostril. Administration of injectable glucagon requires several steps, including reconstitution, and is prone to error. In a simulation study, NG administration was faster and provided greater probability of success in delivering the full dose by caregivers (94% vs 13%) and others (93% vs 0%), with fewer errors compared to injectable glucagon.9
Nasal administration is effective for delivery of medications and vaccines.10 The human nasal cavity has a volume of 15 to 20 mL and a surface area of approximately 150 cm2. Because of this large surface area and the rich blood supply, medications can be readily absorbed through the nasal mucosa.10 Most commonly, nasally‐administered drugs are for the treatment of acute or chronic nasal symptoms (eg, decongestants). However, drugs that act systemically are also administered nasally,10 including treatments for migraine (eg, zolmitriptan and sumatriptan),10 pain (eg, fentanyl)10, 11 and drug overdose (eg, naloxone).12 Lipophilic drugs are well absorbed from the nasal cavity and often have pharmacokinetic profiles and bioavailability comparable to intravenously‐injected drugs.13
The NG formulation contains the lipophilic excipient dodecylphosphocholine, which helps to enhance absorption. Dodecylphosphocholine contains a choline group, a phosphate group and a saturated aliphatic chain. All 3 moieties are present in phospholipids and lecithins which are ubiquitous in mammalian cell membranes. The synthetic glucagon in the formulation is a single‐chain, 29‐amino acid polypeptide identical to the human recombinant DNA‐derived glucagon used in injectable emergency kits.
NG is delivered with a single‐use device, inserted into the patient's nostril. When the plunger on the device is depressed, glucagon powder is propelled into the anterior region of the nasal cavity (Figure 1).14 The powder then dissolves and is passively absorbed into the blood stream via the moist membrane of the nasal mucosa without requiring the patient to actively inhale or breathe deeply.
Figure 1.
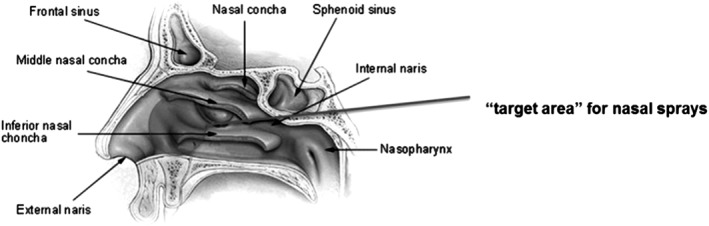
Diagram of the nasal passage and sinuses. [For illustrative purposes only.] From Intranasal Drug Administration — An Attractive Delivery Route for Some Drugs by Degenhard Marx, Gerallt Williams and Matthias Birkhoff (http://dx.doi.org/10.5772/59468). Use of the figure does not assert or imply any connection with, sponsorship or endorsement by the Original Authors and is subject to Creative Commons License 3.0 (https://creativecommons.org/licenses/by/3.0/legalcode)
NG was well‐tolerated in preclinical studies15 and in clinical trials in adult and paediatric patients with T1D.16, 17, 18 In a crossover study of 75 adults with T1D, a 3‐mg NG dose was noninferior to 1 mg injectable glucagon in treating insulin‐induced hypoglycaemia.17 NG was also effective in treating naturally‐occurring hypoglycaemia in home and school settings in children and adolescents, as well as in home and work settings in adults with T1D.18, 19
To date, all studies of NG have been conducted in individuals without notable pre‐existing nasal congestion. However, common cold and associated nasal congestion occur frequently. In one survey, 85% of respondents reported experiencing at least 1 cold within the past year; nasal congestion was among the most common cold symptoms reported.20 Moreover, some congestion in the nasal passageways is common even in healthy individuals.21 Therefore, it is important to understand whether the pharmacokinetics (PK) and pharmacodynamics (PD) of NG are affected when nasal congestion is present. Nasal decongestants used for the treatment of cold symptoms may also alter blood glucose levels,22 as well as affect absorption of nasally‐administered medications.11 This study, therefore, enrolled participants with common cold, and compared the effects of NG in participants with nasal congestion and after recovery from cold symptoms, as well as in participants with nasal congestion treated with a decongestant.
2. MATERIAL AND METHODS
This single‐centre, open‐label, repeated‐measures, parallel‐design, phase I study examined the safety and PK/PD of a single 3‐mg dose of NG in otherwise healthy participants with nasal congestion resulting from common cold and after recovering from cold symptoms, as well as in participants with nasal congestion resulting from common cold who had been treated with a nasal decongestant (oxymetazoline, Dristan Long Lasting Nasal Mist 0.05%, Pfizer Consumer Healthcare). The study (Clinicaltrials.gov identifier NCT02778100) was conducted from March to April of 2013 in Montréal, Québec according to the Declaration of Helsinki and was approved by the local institutional review board. Informed consent was obtained from all participants.
All participants had common cold symptoms at study enrollment, as evidenced by a score of 2 or 3 for nasal congestion and/or nasal discharge on the Jackson cold scale,23 which assesses 8 symptoms of common cold (runny nose, nasal congestion, sneezing, cough, malaise, sore throat, fever/chills and headache) on a scale from 0 (absent) to 3 (severe). Peak nasal inspiratory flow was measured to confirm nasal congestion approximately 1 hour before each treatment in Cohort 1 and approximately 30 minutes prior to administration of nasal decongestant in Cohort 2. Eligible participants were adult, light‐, non‐ or ex‐smokers, 18 to 50 years old inclusively, with body mass index ≥18.5 and <30.0 kg/m2. Aside from cold symptoms, participants were healthy as determined by medical history, physical examination (including vital signs), nasal examination, bilateral anterior rhinoscopy, electrocardiogram (ECG) and clinical laboratory tests, as well as screening for human immunodeficiency virus, hepatitis B, and hepatitis C, and abuse of alcohol and drugs.
2.1. Eligible participants were enrolled into two cohorts.
2.1.1. Cohort 1
The first 18 participants enrolled were assigned to Cohort 1. These participants received NG treatment twice: first, while suffering from untreated cold symptoms, and again 7 to 28 days later (≥2 days after recovery from cold symptoms). Participants were required to have a Jackson cold scale score of 0 before the second NG treatment.
2.1.2. Cohort 2
The subsequent 18 participants enrolled, also experiencing common cold with nasal congestion, were assigned to Cohort 2. These participants received NG only once. Two hours before NG administration, they were treated with nasal decongestant (oxymetazoline), sprayed twice into each nostril.
2.2. NG administration and study procedures
All participants fasted for 10 hours (overnight) before a single 3‐mg dose of NG was administered in 1 nostril. Participants were closely monitored for safety. Blood samples were collected for measurement of glucagon and glucose concentrations 30 and 15 minutes before, just before (time 0) and at 5, 10, 15, 20, 30, 40, 60, 90, 120, 150, and 180 minutes after NG administration.
2.3. Analytical methods
Glucagon levels in plasma samples were analysed using a radioimmunoassay (20 pg/mL quantification limit; Millipore Human Glucagon Assay, performed by Northwest Lipid Metabolism and Diabetes Research Laboratories, University of Washington, Seattle, Washington). Plasma glucose was measured using a Synchron® System, which determines GLUCm concentration by an oxygen rate method using a Beckman Coulter Oxygen electrode (Gamma‐Dynacare Medical Laboratories, St‐Laurent, Québec, Canada).
2.4. Safety and tolerability
Safety and tolerability measures included adverse events (AEs) up to 5 hours after NG administration, physical examination, nasal examination, bilateral anterior rhinoscopy, standard laboratory evaluations, vital signs and electrocardiogram (ECG). AEs were classified by System Organ Class and Preferred Term using the Medical Dictionary for Regulatory Activities Version 13.1.
Participants rated their nasal symptoms (runny nose, nasal congestion, nasal itching and sneezing) and non‐nasal symptoms (watery eyes, itchy eyes, redness of eyes, itching of ears and itching of throat) individually from 0 (absent) to 3 (severe) on the Total Symptom Score questionnaire.24, 25 These ratings were collected at approximately 30 minutes prior to and at 15, 30, 60 and 180 minutes after each glucagon administration.
2.5. Statistical methods
Three analysis groups were defined: (1) Cohort 1 participants with cold symptoms; (2) Cohort 1 participants without cold symptoms; (3) Cohort 2 participants.
PK and PD parameters of glucose were derived using non‐compartmental analysis based on raw concentrations (Area Under the Curve [AUC]0‐t [for PK], Area Under the Effect Curve [AUEC]0‐t [for PD], maximum concentration [Cmax] and time to maximum concentration [Tmax]). Individual concentrations and PK/PD parameters were summarized by analysis group, with descriptive statistics including median for Tmax, and mean and standard deviation (or coefficient of variation) for other parameters.
Comparison of natural logarithm (ln)‐transformed PK/PD parameters of glucagon and glucose between analysis group 1 and 2, as well as between analysis group 1 and 3 was conducted using a mixed analysis of variance (ANOVA) model including participant as random effect and presence of cold symptoms or use of decongestant as fixed effects. Predicted geometric means along with 90% confidence intervals were derived. Analyses for PK, PD and safety included all participants who received ≥1 dose of study drug.
Total nasal and non‐nasal symptom scores at pre‐glucagon and various post‐glucagon administration time‐points were summarized by analysis group with descriptive statistics including mean and standard error. As a post‐hoc analysis, percentages of participants with a total nasal and non‐nasal symptom score ≥2 at pre‐glucagon and various post‐glucagon administration time‐points were also summarized by analysis group.
Change in vital signs from 1 h pre‐ to 45 minutes post‐glucagon administration was summarized by analysis group. Within‐group difference was assessed using Wilcoxon Signed Rank test. SAS version 9.1 or higher (SAS Institute Inc., Cary, North Carolina) was used. Unless otherwise specified, all tests of statistical significance were evaluated at a nominal level of 0.05 using 2‐tailed test procedures.
2.6. Power calculations
To achieve statistical power of ≥80% to demonstrate that nasal decongestant use had no significant impact on the glucose AUEC0‐t (assuming true mean difference is zero) and allowing one standard deviation (1.35 hour*mmol/L) as acceptable difference for equivalence, 18 participants per cohort were required; this also provided ≥90% power to demonstrate that nasal congestion had no significant impact on the glucose AUEC0‐t under the same assumption.
3. RESULTS
3.1. Study participants
A total of 36 participants were enrolled in this study, 18 in each cohort. Baseline demographics were similar for both cohorts (Table S1). Peak nasal inspiratory air flow measurements confirmed nasal congestion in all participants with colds. Jackson cold scores at baseline ranged from 4 to 15 (Cohort 1 with nasal congestion) and 4 to 13 (Cohort 2). Participants in Cohort 1 were required by protocol to have Jackson cold scores of 0 after recovery from cold symptoms. One participant in Cohort 1 withdrew from the study before receiving a second NG dose (Figure S1). All other participants completed the trial.
3.2. Glucagon pharmacokinetics
3.2.1. Cohort 1
Glucagon profiles were similar for Cohort 1 participants while experiencing nasal congestion and after recovery from cold symptoms (Figure 2A), with glucagon concentrations rising slightly faster when nasal congestion was present. Glucagon concentrations increased substantially above baseline with mean peak concentrations (Cmax) of 1198.4 pg/mL (with nasal congestion) and 801.5 pg/mL (after recovery from cold symptoms) (Table 1). Tmax was 18 minutes, both with nasal congestion and after recovery from cold symptoms. The glucagon PK observed during nasal congestion did not show any statistically significant differences compared to that after recovery from cold symptoms in this cohort (Table 2).
Figure 2.
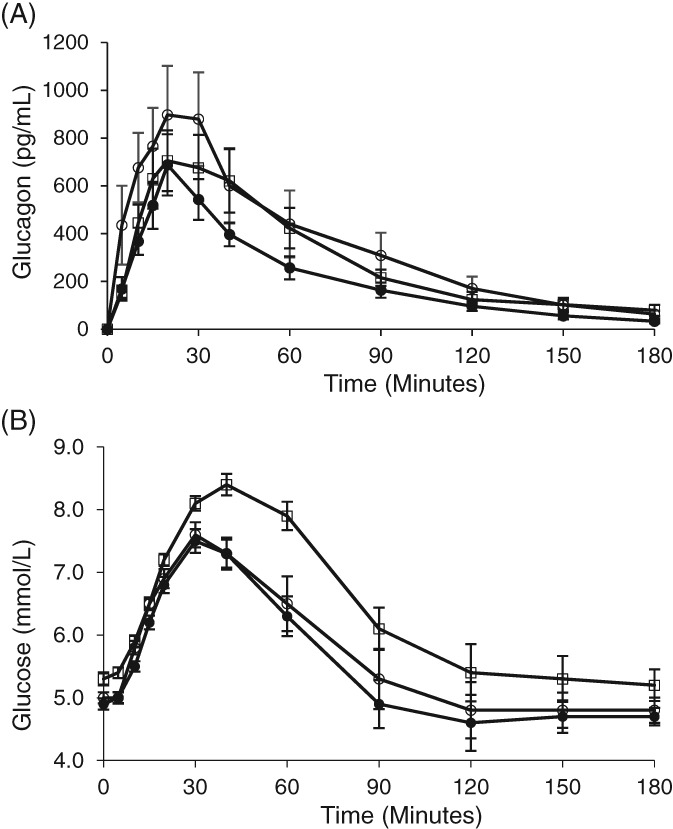
Glucagon and glucose concentrations over time following nasal glucagon administration. A single 3‐mg dose of nasal glucagon was administered at time 0. Glucagon concentrations are shown in Panel A. Glucose concentrations are shown in Panel B. White circles = Cohort 1 NG with nasal congestion. Black circles = Cohort 1 NG without cold symptoms. White squares = Cohort 2 (NG with nasal congestion, treated with decongestant). Values are given as means (adjusted for baseline concentration) ± standard error; NG = nasal glucagon; 1 mmol/L = 18 mg/dL
Table 1.
Summary of PK parameters of plasma glucagon and PD parameters of plasma glucose
| PK parameters (glucagon) | Cohort 1 | Cohort 2 | |
|---|---|---|---|
| NG + Common cold (N = 18) | NG + No cold symptoms (N = 17) | NG + Common cold + Decongestant (N = 18) | |
| AUC0‐t, h·pg/mLa | 1198.4 (84.6) | 797.5 (50.1) | 1038.0 (60.7) |
| Cmax, pg/mLa | 1198.4 (83.0) | 801.5 (68.2) | 868.0 (68.8) |
| Tmax, minutesb | 18 (5, 90) | 18 (15, 40) | 18 (10, 60) |
| PD parameters (glucose) | |||
| AUEC0‐t, h·mmol/La | 17.0 (16.9) | 16.4 (13.4) | 19.0 (14.3) |
| Cmax, mmol/La | 8.0 (22.9) | 7.7 (19.4) | 8.8 (17.7) |
| Tmax, minutesb | 30 (15, 60) | 36 (20, 60) | 42 (20, 60) |
Abbreviations: AUC0‐t, area under the curve from time zero; AUEC0‐t, area under the effect curve from time zero; Cmax, maximum concentration; CV, coefficient of variation; NG, nasal glucagon; PD, pharmacodynamics; PK, pharmacokinetics; Tmax, time to maximum concentration; for glucose, 1 mmol/L = 18 mg/dL.
Arithmetic mean (CV%).
Median (minimum, maximum).
Table 2.
Comparison of plasma glucagon PK parameters and plasma glucose PD parameters
| Comparison | Cohort 1 ‐ Common cold vs Cohort 2 ‐ Common cold + Decongestant | Cohort 1 ‐ Common cold vs Cohort 1 ‐ No cold symptoms | ||||
|---|---|---|---|---|---|---|
| PK parameters (glucagon) | Estimated difference | P value | 90% CI for difference of geometric means of transformed values | Estimated difference | P value | 90% CI for difference of geometric means of transformed values |
| LnAUC0‐t (h·pg/mL) | 0.010 | 0.967 | −0.39, 0.415 | 0.230 | 0.331 | −0.165, 0.625 |
| LnCmax (pg/mL) | 0.161 | 0.552 | −0.294, 0.617 | 0.250 | 0.357 | −0.210, 0.710 |
| PD parameters (glucose) | ||||||
| LnAUEC0‐t, h·mmol/L | −0.116 | 0.023 | −0.198, −0.034 | 0.038 | 0.132 | −0.004, 0.079 |
| LnCmax, mmol/L | 0.114 | 0.109 | −0.231, 0.003 | −0.026 | 0.605 | −0.060, 0.112 |
Abbreviations: AUC0‐t, area under the curve from time zero; AUEC0‐t, area under the effect curve from time zero; Cmax, maximum concentration; CI, confidence interval; Ln, natural logarithm.
3.2.2. Cohort 2
The glucagon profile for Cohort 2 was similar to the 2 profiles for Cohort 1 (Table 1), with Cmax and AUC0‐t values for Cohort 2 falling between those for Cohort 1. Tmax was the same as that for both Cohort 1 profiles. There were no statistically significant differences between the profiles for Cohort 1 with nasal congestion and Cohort 1 without nasal congestion or the profile for Cohort 2 (Table 2).
3.3. Glucose pharmacodynamics
3.3.1. Cohort 1
Blood glucose increased within 5 minutes after NG administration in participants with nasal congestion and after recovery from cold symptoms (Figure 2B) with similar profiles. Plasma glucose increased substantially within 30 minutes and reached Cmax values of 8.0 mmol/L (144 mg/dL) during nasal congestion and 7.7 mmol/L (139 mg/dL) after recovery from cold symptoms, with median Tmax of 30 minutes and 36 minutes, respectively (Table 1).
3.3.2. Cohort 2
Blood glucose also rose within 5 minutes after NG administration in participants with nasal congestion treated with decongestant. Cmax and Tmax for Cohort 2 were 8.8 mmol/L (158 mg/dL) and 42 minutes, respectively. After 30 minutes, the glucose concentration for Cohort 2 was 8.1 mmol/L (146 mg/dL). The glucose profile in Cohort 2 was similar to both of the profiles in Cohort 1 participants during the first 30 minutes (Figure 2B). From 30 to 90 minutes after NG administration, glucose concentrations were numerically higher in Cohort 2, resulting in a statistically significantly larger AUEC0‐t (P = .023) for Cohort 2 (Table 1, Table 2, Figure 2B). Difference in LnAUEC0‐t values was not statistically significant between Cohort 1 with nasal congestion and Cohort 2 after adjusting for baseline glucose concentration (P = .503). No other statistically significant differences in PD parameters were observed (Table 2).
3.4. Total nasal and non‐nasal symptom score
3.4.1. Cohort 1
Before NG administration, total symptom scores were substantially higher for participants experiencing nasal congestion compared to the same participants after recovery from cold symptoms (Figure S2A). Regardless of whether or not participants were experiencing nasal congestion, mean scores rose 1 to 2 points within 15 minutes after glucagon administration, plateaued or declined by 30 minutes, and were at or below baseline scores within 3 hours.
While experiencing nasal congestion, all (100%) Cohort 1 participants had total symptom scores ≥2 from 30 minutes before glucagon administration until 1 hour afterwards (Figure S2B). A score of ≥2 indicates the presence of ≥2 mild symptoms or ≥1 moderate or severe symptom. After recovery from cold symptoms, no Cohort 1 participants (0%) had scores ≥2 before glucagon administration; 15 minutes after administration, 35% had scores ≥2 and the percentage dropped thereafter, reaching 0 within 3 hours (Figure S2B).
3.4.2. Cohort 2
Scores for Cohort 2 were between the 2 sets of scores for Cohort 1 and followed a similar pattern. The mean score rose within 15 minutes after NG administration and fell below baseline scores by 3 hours.
Among Cohort 2 participants, 78% had total symptom scores ≥2 prior to glucagon administration; this percentage was higher at 15 and 30 minutes after glucagon administration and returned to baseline 1 hour post dose (Figure S2B).
3.5. Adverse events
No serious AEs were reported during this study (Table 3). All 36 study participants (100%) experienced ≥1 AE, mostly mild or moderate in severity. Four severe AEs were observed in Cohort 1 participants experiencing nasal congestion: nasal congestion (1 participant) and rhinorrhea (3 participants). After recovery from cold symptoms, participants experienced no severe AEs following NG administration. Three severe AEs were observed in Cohort 2 (congestion treated with decongestant): increased lacrimation (1 participant) and nausea (2 participants). The most consistently observed AE was increased lacrimation, a transient increase in tearing most likely explained by the nasal route of administration. AEs, especially those associated with common cold, were considerably more frequent in Cohort 1 participants experiencing nasal congestion compared with participants after recovery from cold symptoms, or with participants in Cohort 2. Other adverse events which are well known glucagon side effects, such as nausea and vomiting, were also reported.
Table 3.
Most commonly reported adverse events by system organ class
| Cohort 1 | Cohort 2 | ||
|---|---|---|---|
| Common cold (n = 18) | No cold symptoms (n = 17) | Common cold + Decongestant (n = 18) | |
| Total AEs reported, n | 112 | 64 | 113 |
| Patients with ≥1 AE, n (%) | 18 (100) | 17 (100) | 18 (100) |
| Patients with ≥1 treatment‐related AE, n (%) | 18 (100) | 17 (100) | 18 (100) |
| Serious AEs reported, n | 0 | 0 | 0 |
|
AEs by system organ class
Preferred term, n (%) |
n (%) | n (%) | n (%) |
| Ocular | |||
| Increased lacrimation | 13 (72.2) | 12 (70.6) | 12 (66.7) |
| Ocular hyperaemia | 7 (38.9) | 7 (41.2) | 8 (44.4) |
| Eye pruritus | 5 (27.8) | 0 | 5 (27.8) |
| Respiratory | |||
| Nasal discomfort | 12 (66.7) | 7 (41.2) | 15 (83.3) |
| Rhinorrhea | 10 (55.6) | 5 (29.4) | 14 (77.8) |
| Nasal congestion | 4 (22.2) | 3 (17.6) | 4 (22.2) |
| Sneezing | 5 (27.8) | 3 (17.6) | 3 (16.7) |
| Nervous system | |||
| Dizziness | 4 (22.2) | 5 (29.4) | 5 (27.8) |
| Headache | 4 (22.2) | 3 (17.6) | 5 (27.8) |
| Somnolence | 6 (33.3) | 3 (17.6) | 1 (5.6) |
| Gastrointestinal | |||
| Nausea | 8 (44.4) | 2 (11.8) | 7 (38.9) |
| Vomiting | 1 (5.6) | 0 | 5 (27.8) |
Abbreviation: AE, adverse event.
3.6. Other safety measures
Blood pressure (BP) and pulse were measured 1 hour before NG administration and 45 minutes afterwards. Statistically‐significant increases in BP were observed in each treatment group except for diastolic BP in Cohort 1 participants with cold symptoms, and were more pronounced with the use of nasal decongestant. There were no significant changes in pulse (Table S2). Results of physical examination, nasal examination, bilateral anterior rhinoscopy, clinical pathology and ECG did not suggest treatment‐related safety concerns.
4. DISCUSSION
NG was effective in rapidly raising blood glucose. Comparable PD was observed when participants had nasal congestion and after recovery from cold symptoms (Cohort 1). Participants with nasal congestion treated with decongestant (Cohort 2) had statistically significantly higher glucose AUEC0‐t than participants in Cohort 1 with untreated nasal congestion. This could be an effect of the decongestant used, as oxymetazoline does alter blood glucose.26 Other PD parameters, including Cmax, were not statistically significantly different, regardless of the presence or absence of nasal congestion or administration of nasal decongestant. As seen in Figure 2B, glucose responses at 30 minutes were similar among the Cohorts. The comparable glucose responses within the first 30 minutes are the most important to consider because a rapid and reliable rise in plasma glucose is the most clinically‐meaningful attribute for rescue treatment during severe hypoglycaemia.
No significant differences in PK parameters were observed in this study. Prior data with injected glucagon showed that small variations in glucagon pharmacokinetics would not be expected to significantly impact either the glucose response or the safety profile as intravenous administration of a 2‐mg dose of glucagon did not increase blood glucose concentrations more than a 0.25‐mg dose of glucagon, indicating clear saturation of the PD response (maximal effective concentration of glucagon exceeded even at the lower dose).7 Although lower doses of glucagon are being investigated to maintain euglycaemia and avoid hypoglycaemia when used in the artificial pancreas,27 a supratherapeutic dose that maximizes the glucose response is desirable in the case of severe hypoglycaemia rescue. For NG, 3 mg is the dose identified as non‐inferior to injectable glucagon in glucose response.17
Comparable results were seen with NG in patients with type 1 or type 2 diabetes who were treated with single or repeated 3‐mg doses of NG in a randomized sequence. Repeated doses of NG (doubling the NG dose) resulted in higher glucagon concentrations, but glucose responses resembled those seen with a single dose.28 Although repeat dosing resulted in greater systemic glucagon exposure, it did not result in a clinically‐meaningful increase in glucose response. All NG treatments were generally well‐tolerated. Many observed AEs were related to local tolerability, probably explained by the route of administration. Other AEs are well‐known effects of glucagon administration. These events include nausea, vomiting and increased blood pressure.6 Increased pulse rate is also observed generally with glucagon administration; however, we did not see a significant change in pulse rate in this study. Administration of oxymetazoline, which acts as a vasoconstrictor,22 may also have contributed to the rise in blood pressure, which was more pronounced in participants treated with a decongestant.
Despite the presence of cold symptoms in this study, these PK, PD and safety results are similar to those observed in a study of glucagon in individuals with T1D who were not experiencing nasal congestion,17 indicating that NG PK/PD were not significantly altered by nasal congestion, with or without use of a nasal decongestant. This is in contrast to a study of nasally‐administered fentanyl, where absorption was somewhat compromised and Tmax was delayed by oxymetazoline administration.11
Strengths of the study include having participants in Cohort 1 serve as their own controls, and the use of a single study centre, which ensured consistency. One potential limitation was the use of a single nasal decongestant, although we selected a decongestant that is among the most commonly used.
In summary, there were no clinically relevant differences in safety or PK/PD of NG associated with nasal congestion due to common cold or concomitant administration of nasal decongestant. Although there were modest differences in total glucose excursions, the onset of glucose response and the glucose excursion observed in the first 30 minutes were similar across the 3 groups. Glucagon and glucose levels increased rapidly after treatment, peaking at 18 minutes post dose (glucagon) and 30 to 42 minutes post dose (glucose) in all groups. These results indicate that a 3‐mg dose of NG can be used to treat episodes of severe hypoglycaemia in individuals experiencing nasal congestion.
Supporting information
Table S1. Demographics and baseline characteristics of participants
Table S2. Blood Pressure and Pulse Rate
Figure S1. Participant flow diagram.
Figure S2. Total symptoms scores: scores over time and percentage of participants with scores ≥2 by time.
ACKNOWLEDGMENTS
The participation of the people in the study is gratefully acknowledged.
Assistance with early drafts of this manuscript was provided by David Segarnick, PhD, Chief Medical Officer, MedEvoke (a division of Medisys Health Communication), High Bridge, New Jersey, and was funded by Locemia Solutions, Montréal, Québec, Canada. Medical writing support for later drafts of this manuscript was provided by Jennie G. Jacobson, PhD, of Eli Lilly and Company, Indianapolis, Indiana.
Parts of this study have been presented at the 76th Annual Scientific Sessions of the American Diabetes Association, June 10, 2016, and at the 10th International Conference on Advanced Technologies and Treatments for Diabetes, February 15, 2017.
Conflict of interest
E. S. is a consultant for Algorithme Pharma Inc. M. R. and A. A. S. are employees of Algorithme Pharma Inc. H. D., M. Tr., C. P. and D. C. are employees and stockholders of Locemia Solutions, which funded the study. M. Ta. and E. R. are employees of JSS Medical Research. M. Z. is an employee of and stockholder in Eli Lilly Canada. C. B. G. and S. Z. are employees of and stockholders in Eli Lilly and Company. Algorithme Pharma Inc. and JSS Medical Research was under contract with Locemia Solutions to perform different portions of this study. The authors report no other conflicts of interest.
Author contributions
C. P., E. S., M. R., M. Tr., E. R. and D. C. designed the study. E. S., M. R., M. Tr., H. D. and A. A. S. conducted the study. All authors were involved in analysing and interpreting the data, as well as drafting and reviewing the manuscript. All authors approved the final draft of the manuscript for submission.
Guzman CB, Dulude H, Piché C, et al. Effects of common cold and concomitant administration of nasal decongestant on the pharmacokinetics and pharmacodynamics of nasal glucagon in otherwise healthy participants: A randomized clinical trial. Diabetes Obes Metab. 2018;20:646–653. https://doi.org/10.1111/dom.13134
Funding information Funding was provided by Locemia Solutions, Montréal, Québec, Canada and by Eli Lilly and Company, Indianapolis, Indiana.
REFERENCES
- 1. Wang J, Geiss LS, Williams DE, Gregg EW. Trends in emergency department visit rates for hypoglycaemia and hyperglycaemic crisis among adults with diabetes, United States, 2006–2011. PLoS One. 2015;10:e0134917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Weinstock RS, Xing D, Maahs DM, et al. Severe hypoglycemia and diabetic ketoacidosis in adults with type 1 diabetes: results from the T1D exchange clinic registry. J Clin Endocrinol Metab. 2013;98:3411–3419. [DOI] [PubMed] [Google Scholar]
- 3. Wild D, von Maltzahn R, Brohan E, Christensen T, Clauson P, Gonder‐Frederick L. A critical review of the literature on fear of hypoglycemia in diabetes: implications for diabetes management and patient education. Patient Educ Couns. 2007;68:10–15. [DOI] [PubMed] [Google Scholar]
- 4. Cryer P. Hypoglycemia in Diabetes: Pathophysiology, Prevalence and Prevention. Alexandria, VA: American Diabetes Association; 2012. [Google Scholar]
- 5.Eli Lilly and Company. Information for the user: glucagon for injection (rDNA origin). 2012. http://pi.lilly.com/us/rglucagon-ppi.pdf. Accessed November 3, 2016.
- 6. GlucaGen(r) Hypokit(r) . Instructions for use. 2016. http://www.glucagenhypokit.com/instructions.html. Accessed November 3, 2016.
- 7. Pontiroli AE. Intranasal glucagon: a promising approach for treatment of severe hypoglycemia. J Diabetes Sci Technol. 2015;9:38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dash S, Xiao C, Stahel P, Koulajian K, Giacca A, Lewis GF. Evaluation of the specific effects of intranasal glucagon on circulating glucose and lipid concentrations in healthy males during a pancreatic clamp. Diabetes Obes Metab. 2018;20:328–334. [DOI] [PubMed] [Google Scholar]
- 9. Yale J, Dulude H, Egeth M, et al. Faster use and fewer failures with needle‐free nasal glucagon vs injectable glucagon in severe hypoglycemia rescue: a simulation study. Diabetes Tech Ther. 2017;19:423–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pires A, Fortuna A, Alves G, Falcão A. Intranasal drug delivery: how, why and what for? J Pharm Pharm Sci. 2009;12:288–311. [DOI] [PubMed] [Google Scholar]
- 11. Perelman M, Fisher AN, Smith A, Knight A. Impact of allergic rhinitis and its treatment on the pharmacokinetics of nasally administered fentanyl. Int J Clin Pharmacol Ther. 2013;51:349–356. [DOI] [PubMed] [Google Scholar]
- 12. Krieter P, Chiang N, Gyaw S, et al. Pharmacokinetic properties and human use characteristics of an FDA‐approved intranasal naloxone product for the treatment of opiod overdose. J Clin Pharmacol. 2016;56:1243–1253. [DOI] [PubMed] [Google Scholar]
- 13. Illum L. Nasal drug delivery ‐ possibilities, problems and solutions. J Control Release. 2003;87:187–198. [DOI] [PubMed] [Google Scholar]
- 14. Marx D, Williams G, Birkhoff M. In: Vallisuta O, ed. Intranasal Drug Administration — An Attractive Delivery Route for some Drugs, Drug Discovery and Development ‐ from Molecules to Medicine. InTech; 2015. https://doi.org/10.5772/59468. https://www.intechopen.com/books/drug‐discovery‐and‐development‐from‐molecules‐to‐medicine/intranasal‐drug‐administration‐an‐attractive‐delivery‐route‐for‐some‐drugs. Accessed November 3, 2016. [Google Scholar]
- 15. Reno FE, Normand P, McInally K, et al. A novel nasal powder formulation of glucagon: toxicology studies in animal models. BMC Pharmacol Toxicol. 2015;16:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sherr JL, Ruedy KJ, Foster NC, et al. Glucagon nasal powder: a promising alternative to intramuscular glucagon in youth with type 1 diabetes. Diabetes Care. 2016;39:555–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rickels MR, Ruedy KJ, Foster NC, et al. Intranasal glucagon for treatment of insulin‐induced hypoglycemia in adults with type 1 diabetes: a randomized crossover noninferiority study. Diabetes Care. 2016;39:264–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Deeb L, Dulude H, Zhang M, et al. Nasal glucagon (NG) for the treatment of moderate to severe hypoglycemia (hypo) episodes in children and adolescents with type 1 diabetes (T1D) in home or school settings. Pediatr Diabetes. 2016;17:85. [DOI] [PubMed] [Google Scholar]
- 19. Seaquist E, Dulude H, Zhang M, et al. Nasal glucagon for the treatment of moderate‐to‐severe hypoglycemic episodes in real‐world settings in adults with type 1 diabetes. Diabetes. 2017;66:A94. [Google Scholar]
- 20. Blaiss MS, Dicpinigaitis PV, Eccles R, Wingertzahn MA. Consumer attitudes on cough and cold: US (ACHOO) survey results. Curr Med Res Opin. 2015;31:1527–1538. [DOI] [PubMed] [Google Scholar]
- 21. Djupesland PG. Nasal drug delivery devices: characteristics and performance in a clinical perspective—a review. Drug Deliv Transl Res. 2013;3:42–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cerner Multim, Inc. Oxymetazoline nasal disease interactions. https://www.drugs.com/disease‐interactions/oxymetazoline‐nasal.html. 2017. Accessed June 27, 2017.
- 23. Jackson GG, Dowling HF, Anderson TO , Riff L, Saporta J, Turck M. Susceptibility and immunity to common upper respiratory viral infections‐‐the common cold. Ann Intern Med. 1960;53:719–738. [DOI] [PubMed] [Google Scholar]
- 24. Kim K, Sussman G, Hébert J, Lumry W, Lutsky B, Gates D. Desloratadine therapy for symptoms associated with perennial allergic rhinitis. Ann Allergy Asthma Immunol. 2006;96:460–465. [DOI] [PubMed] [Google Scholar]
- 25. Simons FE, Prenner BM, Finn A Jr; Desloratadine Study GroupEfficacy and safety of desloratidine in the treatment of perennial allergic rhinitis. J Allergy Clin Immunol. 2003;111:617–622. [DOI] [PubMed] [Google Scholar]
- 26.Cerner Multum, Inc. Oxymetazoline solution. https://www.drugs.com/cdi/oxymetazoline-solution.html. 2017. Accessed June 27, 2017.
- 27. Blauw H, Wendl I, DeVries JH, Heise T, Jax T. Pharmacokinetics and pharmacodynamics of various glucagon dosages at different blood glucose levels. Diabetes Obes Metab. 2016;18:34–39. [DOI] [PubMed] [Google Scholar]
- 28. Dulude H, Sicard É, Rufiange M, et al. Pharmacokinetics (PK), Pharmacodynamics (PD), and safety following single or repeated 3 mg doses of nasal glucagon (NG) in adults with type 1 or type 2 diabetes (T1D or T2D). Pediatr Diabetes. 2016;17:85. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Demographics and baseline characteristics of participants
Table S2. Blood Pressure and Pulse Rate
Figure S1. Participant flow diagram.
Figure S2. Total symptoms scores: scores over time and percentage of participants with scores ≥2 by time.


