Abstract
Aim
Open myomectomy (OM) was previously frequently performed; however, laparoscopic myomectomy (LM) has recently become more common. Nevertheless, myoma can recur after both LM and OM. In this study, we report our retrospective investigation of myoma recurrence by comparing LM and OM.
Methods
A total of 474 patients underwent LM and 279 patients underwent OM. The patients were followed‐up postoperatively from six months to eight years. Recurrence was confirmed when a myoma with a diameter of ≥ 1 cm was detected. Post‐LM, post‐OM and cumulative recurrence rates were investigated, and a Cox hazard test was performed.
Results
The cumulative recurrence rates between the two groups were 76.2% (LM) vs. 63.4% (OM) at eight years postoperatively. A log‐rank test revealed a significant difference between the two groups. Cox hazard testing revealed that LM, a larger number of enucleated myoma masses and the absence of postoperative gestation significantly contributed to the postoperative recurrence rate.
Conclusions
LM yielded a higher recurrence rate than OM, likely a result of manual myoma removal in OM, which is a more exhaustive extraction of smaller myoma masses than performed in LM. In other words, fewer residual myoma masses after OM contribute to a lower postoperative recurrence rate.
Keywords: laparoscopic myomectomy, laparoscopic surgery, open myomectomy, recurrence, uterine myoma
Introduction
Myoma is a common gynecologic disease that occurs in 20% of women aged > 30 years and in 40% of women aged > 40 years.1, 2 Open myomectomy (OM) was previously frequently performed to treat uterine myoma; however, laparoscopic myomectomy (LM) has recently become more common. Nevertheless, myoma can recur after both LM and OM.
Yoo et al. reported myoma recurrence rates of 11.7%, 36.1%, 52.9% and 84.4% at one, three, five, and eight years after LM, respectively.3 They also reported that recurrence was less frequent in patients with < 2 myoma masses, myoma masses of a size comparable to that of 13 weeks’ gestation, patients who did not deliver postoperatively and patients aged < 35 years.3 They concluded that age, tumor number and size, the presence of pelvic disease and postoperative parity were risk factors for recurrence. Furthermore, Nezhat et al. reported recurrence rates of 31.7% and 51.4% at three and five years, respectively, while Fedele et al. reported a recurrence rate of 51% at five years.4, 5 Our department has reported recurrence rates of 15.3%, 43.8% and 62.1% at one, three and five years after LM, respectively. We further identified age, the largest diameter of a myoma mass and the number of myoma masses as risk factors for recurrence.6 There are several other reports of recurrence after LM.3, 4, 5, 6, 7
To compare outcomes between OM and LM, Rossetti et al. conducted a randomized study on cases of myoma recurrence, excluding patients with < 7 masses, smallest masses sized < 3 cm and submucous myoma. They reported recurrence rates of 23% following treatment with open surgery and 27% following treatment with laparoscopic surgery, with no statistically significant difference between these rates.7
In recent years, LM has become the preferred option over OM because of the less invasive nature and positive surgical outcomes.7 Open surgery is applied only in cases in which LM cannot be indicated, including cases with a myoma diameter > 10 cm and 10–20 myoma masses. The same policy is observed in our facility, in which more LMs than OMs are performed. An OM is not performed if LM is indicated for a case. A comparison of recurrence rates between LM and OM would be meaningful; however, we have found no study that compared these recurrence rates using actual accumulated data.
In a comparative review, Hirschelmann and De Wilde reported a higher recurrence rate after LM than open surgery.8 However, the data used for the comparison were derived from different articles, as no large comparative study has been conducted using data from a single site.4, 8
Therefore, in this study, we report our retrospective investigation of myoma recurrence by comparing LM and open surgery.
Methods
A total of 474 patients underwent LM and 279 patients underwent OM at a single hospital between January 1995 and December 2014. The patients were followed‐up postoperatively from six months to eight years, during which time they were assessed for recurrence semiannually using transvaginal ultrasound and magnetic resonance imaging (MRI). Recurrence was confirmed when a myoma with a diameter of ≥ 1 cm was detected by transvaginal ultrasound or MRI.
Average age, parity, weight of the uterus, number of myoma masses, largest diameter of myoma, surgical duration, blood loss, postoperative hospital stay, rate of preoperative gonadotropin‐releasing hormone agonist (GnRHa) therapy and post myomectomy pregnancy rate were compared between the groups.
Post‐LM and post‐OM recurrence rates were investigated, as well as the cumulative recurrence rates at one, three, five and eight years postoperatively.
In addition, Cox hazard testing was performed regarding LM or OM, age, parity, use of GnRHa, number of removed myoma masses, largest myoma diameter and presence of postoperative gestation.
Surgical technique
Laparoscopic myomectomy was performed using the following technique. After insertion of a uterine manipulator, a pneumoperitoneum needle was inserted through the umbilicus, and pneumoperitoneum was achieved using the closed method. Subsequently, the first trocar was inserted through the umbilicus and the laparoscope was inserted. The second and third trocars were placed in the left and right lower abdomen, and the fourth trocar was placed on the left side of the umbilicus. The size of the trocars was 5 mm, except for the fourth, which was 12 mm for the retrieval of myoma masses and intracorporeal suturing. To reduce blood loss, vasopressin diluted 100‐fold was locally injected through the surface of the myoma masses.9, 10 The myometrium was incised with an ultrasonic knife and the myoma masses were grasped, pulled and enucleated with a borer. The uterine incision was closed in two to three layers with 1‐vicryl suture on a CT‐1 needle. Enucleated myoma nodules were then retrieved from the body using a morcellator. The surgery was considered complete after hemostasis was confirmed, the intraperitoneal cavity was washed and a Seprafilm hyaluronic acid or Interceed carboxymethylcellulose membrane was applied to the incision sites.11, 12
Open myomectomy was performed as follows. An abdominal median incision was made and the myoma masses were incised with an electrosurgical knife and enucleated while retracted with threads. The uterine wound was divided into two or three layers, each sutured with VICRYL CTB1‐1. Homeostasis was confirmed, the intraperitoneal cavity was washed and Seprafilm was applied to close the wound.
Statistical analysis
The Kaplan–Meier method was used to evaluate the cumulative recurrence rates. A log‐rank test was performed to compare the groups, while a Student's t‐test was used to test the difference between the averages of the groups. A chi‐square test was used to compare the rates. P < 0.05 was considered statistically significant in all tests.
Results
The patients’ clinical background and surgical outcomes following LM and OM procedures are shown in Table 1. There were significant differences in mean age 38 (LM) vs 36 (OM) years; mean parity (0.5 (LM) vs 0.2 (OM)); rate of GnRHa administration (29.7% (LM) vs 16.0% (OM)); mean number of enucleated myoma masses (3.6 (LM) vs 6.1 (OM)); mean largest myoma diameter (7.0 (LM) vs 9.1 (OM) cm); mean surgical duration (148 (LM) vs 126 (OM) min); mean blood loss (249 (LM) vs 156 (OM) mL); and mean postoperative hospital days (3.5 (LM) vs 11.9 (OM) days). There was no significant difference in mean body mass index (21.7 (LM) vs 22.1 (OM)) or post‐myomectomy pregnancy rate (14.6% (LM) vs 15.1% (OM)).
Table 1.
Clinical background of patients and surgical outcomes by technique
| Characteristics and outcomes | Laparoscopic myomectomy (n = 474) | Open myomectomy (n = 279) | P |
|---|---|---|---|
| Age (year) | 37.6 ± 5.2 (24–52) | 36.0 ± 5.8 (23–53) | < 0.001 |
| Parity | 0.5 ± 0.8 (0–4) | 0.2 ± 0.5 (0–3) | < 0.001 |
| Body mass index (kg/m2) | 21.7 ± 3.5 (15.8–36.6) | 22.1 ± 3.3 (15.4–35.0) | 0.061 |
| GnRH agonist (%) | 29.7 (141/474) | 16.1 (45/279) | < 0.001 |
| Number of myomas | 3.7 ± 4.1 (1–32) | 6.5 ± 8.6 (1–65) | < 0.001 |
| Largest diameter (cm) | 7.0 ± 2.6 (1.0–20.0) | 9.0 ± 4.6 (1.0–30.0) | < 0.001 |
| Surgical duration (min) | 148 ± 58 (53–422) | 127 ± 48 (25–315) | < 0.001 |
| Blood loss (mL) | 207 ± 225 (9–1325) | 554 ± 536 (34–2875) | < 0.001 |
| Postoperative hospital stay (days) | 3.5 ± 1.8 (2–17) | 11.7 ± 3.9 (3–36) | < 0.001 |
| Post myomectomy pregnancy rate (%) | 14.6 (69/474) | 15.1 (42/279) | 0.853 |
GnRH, gonadotropin‐releasing hormone agonist.
The cumulative recurrence rates between the two groups were 11.0% versus 5.3% at postoperative year 1, 41.6% versus 34.2% at postoperative year 3, 57.3% versus 49.6% at postoperative year 5 and 76.2% versus 63.4% at postoperative year 8 (Fig. 1). There was a significant difference in the cumulative recurrence rates between the groups, as indicated by the log‐rank test.
Figure 1.
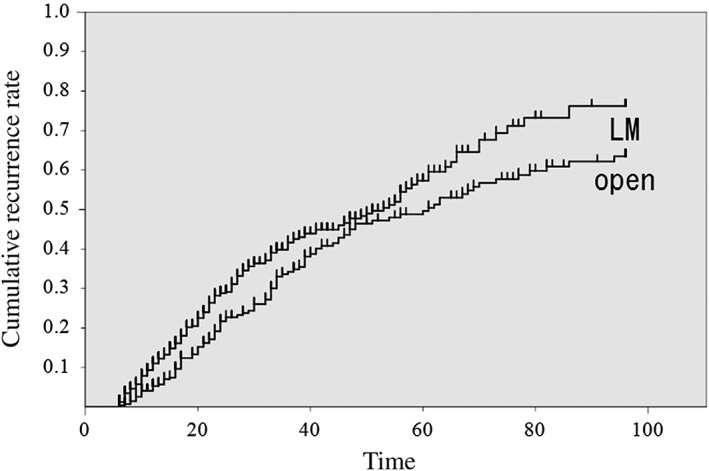
Comparison of the cumulative recurrence rates between the groups. The cumulative recurrence rates after laparoscopic myomectomy were 11.0 ± 1.5%, 41.6 ± 2.8%, 57.3 ± 3.5% and 76.2 ± 4.9% at postoperative years one, three, five and eight, respectively. The cumulative recurrence rates after open myomectomy were 5.3 ± 1.4%, 34.2 ± 3.4%, 46.9 ± 3.9% and 63.4 ± 4.3% at postoperative years one, three, five and eight, respectively.
The Cox hazard test revealed that LM, a larger number of enucleated myoma masses and the absence of postoperative gestation significantly contributed to the postoperative recurrence rate (Table 2).
Table 2.
Cox proportional hazards regression analysis for post‐myomectomy recurrence risk factors
| Risk factors | β | Standard error (β) | Z | P | Relative risk | 95% confidence interval |
|---|---|---|---|---|---|---|
| Laparoscopic myomectomy | 0.515 | 0.142 | 3.636 | < 0.001 | 1.674 | 1.268–2.210 |
| Age | 0.007 | 0.012 | 0.561 | 0.575 | ||
| Parity | −0.065 | 0.089 | 0.723 | 0.469 | ||
| Body mass index | −0.009 | 0.021 | 0.405 | 0.146 | ||
| GnRH agonist | 0.007 | 0.134 | 0.056 | 0.956 | 1.008 | 0.775–1.310 |
| Number of myoma masses | 0.041 | 0.007 | 6.229 | < 0.001 | ||
| Largest diameter | 0.013 | 0.016 | 0.802 | 0.423 | ||
| Post myomectomy pregnancy | −0.542 | 0.201 | 2.692 | 0.007 | 0.582 | 0.392–0.863 |
GnRH, gonadotropin‐releasing hormone agonist.
Compared with non‐pregnant women, pregnant women showed a low relative risk of recurrence (0.582) after surgery. Thus, the relative risk of recurrence (1.674) with LM was higher in non‐pregnant than in pregnant women.
Discussion
Myomectomy is a fertility‐preserving operation that allows for postoperative pregnancy and safe delivery. However, the myoma recurrence rate increases with increasing postoperative years. In Japan, where there is an increasing tendency to delay marriage, many patients do not wish to become pregnant immediately after surgery and thus face an increasing risk of myoma recurrence. By the way, the recurrence rate of those who were not pregnant was 40.5% (260/642).
In terms of procedure, less invasive LM is preferred over OM as it has been reported to be as safe as an OM and as such is increasingly performed at our facility.7, 13, 14, 15, 16, 17 We only perform OM in cases with an extremely large myoma (such as those located at the supraumbilical) or with a large number of myoma masses (such as in diffuse leiomyomatosis). We have now accumulated LM case records and upon analysis found a considerable number of cases of recurrence, which led us to conduct a study of recurrence. Our results show that open surgery produced a lower recurrence rate, although it was used to treat relatively challenging cases. Cox proportional hazard regression analysis indicated that the recurrence rate was 167% higher after LM than OM. This is likely the result of manual myoma removal in OM, which is a more exhaustive extraction of smaller myoma masses than performed during LM. In other words, fewer residual myoma masses left after open surgery contribute to a lower postoperative recurrence rate. Furthermore, GnRHa therapy, which is used more frequently for LM than for OM, may complicate the identification of smaller myoma masses.
This study included subjects treated via LM or OM under different conditions, such as the number of myoma masses, size of myoma or use of GnRHa. Ideally, a study should randomize subjects with identical conditions; however, LM was selected in this study primarily because of the non‐invasive nature of the procedure. Although this preference makes setting an identical condition difficult, the results of our study indicate that OM is a better option than LM when considering only the rate of recurrence.
Laparoscopic myomectomy is the preferable option from the perspective of postoperative adhesion, invasiveness and other criteria.7, 13, 14, 15, 16, 17 We conventionally apply an MRI scan in all LM cases, and have reported that the recurrence rate can be lowered by the preoperative myoma count using MRI findings.18 However, this study showed that LM yielded a higher recurrence rate than OM, despite our beliefs and practices. We must renew our emphasis on reducing the high rate of recurrence after LM and treat cases accordingly. As medical technology advances, so too does imaging quality. The use of hepatic laparoscopy, a new system that integrates computed tomography images into the laparoscopic monitor display, helps to safely and securely remove tumors.19 A similar system or other techniques that will contribute to lowering the recurrence rate are expected for LM.
Disclosure
The authors declare no conflict of interest.
Author contributions
All authors have read and approved the final version of the manuscript.
References
- 1. Hendrickson ME, Kempson RL. Surgical Pathology of the Uterine Corpus. Philadelphia, PA: WB Saunders, 1980. [PubMed] [Google Scholar]
- 2. Parsons L, Sommers SC. Gynecology, 2nd edn. Philadelphia, PA: WB Saunders, 1978. [Google Scholar]
- 3. Yoo EH, Lee PI, Huh CY et al Predictors of leiomyoma recurrence after laparoscopic myomectomy. J Minim Invasive Gynecol 2007; 14: 690–697. [DOI] [PubMed] [Google Scholar]
- 4. Nezhat FR, Roemisch M, Nezhat CH, Seidman DS, Nezhat CR. Recurrence rate after laparoscopic myomectomy. J Am Assoc Gynecol Laparosc 1998; 5: 237–240. [DOI] [PubMed] [Google Scholar]
- 5. Fedele L, Parazzini F, Luchini L, Mezzopane R, Tozzi L, Villa L. Recurrence of fibroids after myomectomy: A transvaginal ultrasonographic study. Hum Reprod 1995; 10: 1795–1796. [DOI] [PubMed] [Google Scholar]
- 6. Shiota M, Kotani Y, Umemoto M, Tobiume T, Hoshiai H. Recurrence of uterine myoma after laparoscopic myomectomy: What are the risk factors? Gynecol Minim Invasive Ther. 2012; 1: 34–36. [Google Scholar]
- 7. Rossetti A, Sizzi O, Soranna L, Cucinelli F, Mancuso S, Lanzone A. Long‐term results of laparoscopic myomectomy: Recurrence rate in comparison with abdominal myomectomy. Hum Reprod 2001; 16: 770–774. [DOI] [PubMed] [Google Scholar]
- 8. Hirschelmann A, De Wilde RL. Plastic and reconstructive uterus operations by minimally invasive surgery? A review on myomectomy. GMS Interdiscip Plast Reconstr Surg DGPW 2012; 1: 1–13. https://doi.org/10.3205/iprs000009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fletcher H, Frederick J, Hardie M, Simeon D. A randomized comparison of vasopressin and tourniquet as hemostatic agents during myomectomy. Obstet Gynecol 1996; 87: 1014–1018. [DOI] [PubMed] [Google Scholar]
- 10. Frederick J, Fletcher H, Simeon D, Mullings A, Hardie M. Intramyometrial vasopressin as a haemostatic agent during myomectomy. Br J Obstet Gynaecol 1994; 101: 435–437. [DOI] [PubMed] [Google Scholar]
- 11. Diamond MP. Reduction of adhesions after uterine myomectomy by Seprafilm membrane (HAL‐F): A blinded, prospective, randomized, multicenter clinical study. Seprafilm Adhesion Study Group. Fertil Steril 1996; 66: 904–910. [PubMed] [Google Scholar]
- 12. Mais V, Ajossa S, Piras B, Guerriero S, Marongiu D, Melis GB. Prevention of de‐novo adhesion formation after laparoscopic myomectomy: A randomized trial to evaluate the effectiveness of an oxidized regenerated cellulose absorbable barrier. Hum Reprod 1995; 10: 3133–3135. [DOI] [PubMed] [Google Scholar]
- 13. Mais V, Ajossa S, Guerriero S, Mascia M, Solla E, Melis GB. Laparoscopic versus abdominal myomectomy: A prospective, randomized trial to evaluate benefits in early outcome. Am J Obstet Gynecol 1996; 174: 654–658. [DOI] [PubMed] [Google Scholar]
- 14. Seracchioli R, Rossi S, Govoni F et al Fertility and obstetric outcome after laparoscopic myomectomy of large myomata: A randomized comparison with abdominal myomectomy. Hum Reprod 2000; 15: 2663–2668. [DOI] [PubMed] [Google Scholar]
- 15. Cagnacci A, Pirillo D, Malmusi S, Arangino S, Alessandrini C, Volpe A. Early outcome of myomectomy by laparotomy, minilaparotomy and laparoscopically assisted minilaparotomy. A randomized prospective study. Hum Reprod 2003; 18: 2590–2594. [DOI] [PubMed] [Google Scholar]
- 16. Benassi L, Marconi L, Benassi G, Accorsi F, Angeloni M, Besagni F. Minilaparotomy vs laparotomy for uterine myomectomies: A randomized controlled trial. Minerva Ginecol 2005; 57: 159–163. [PubMed] [Google Scholar]
- 17. Alessandri F, Lijoi D, Mistrangelo E, Ferrero S, Ragni N. Randomized study of laparoscopic versus minilaparotomic myomectomy for uterine myomas. J Minim Invasive Gynecol 2006; 13: 92–97. [DOI] [PubMed] [Google Scholar]
- 18. Kotani Y, Shiota M, Umemoto M, Tobiume T, Shimaoka M, Hoshiai H. Efficacy of preoperative gonadotropin‐releasing hormone agonist therapy for laparoscopic myomectomy. Asian J Endosc Surg 2009; 2: 24–28. [Google Scholar]
- 19. Yamanaka J, Okada T, Saito S et al Minimally invasive laparoscopic liver resection: 3D MDCT simulation for preoperative planning. J Hepatobiliary Pancreat Surg 2009; 16: 808–815. [DOI] [PubMed] [Google Scholar]


