ABSTRACT
Introduction
Earlier small case series and clinical observations reported on chronic pain playing an important role in facioscapulohumeral dystrophy (FSHD). The aim of this study was to determine the characteristics and impact of pain on quality of life (QoL) in patients with FSHD.
Methods
We analyzed patient reported outcome measures collected through the U.K. FSHD Patient Registry.
Results
Of 398 patients, 88.6% reported pain at the time of study. The most frequent locations were shoulders and lower back. A total of 203 participants reported chronic pain, 30.4% of them as severe. The overall disease impact on QoL was significantly higher in patients with early onset and long disease duration. Chronic pain had a negative impact on all Individualised Neuromuscular Quality of Life Questionnaire domains and overall disease score.
Discussion
Our study shows that pain in FSHD type 1 (FSHD1) is frequent and strongly impacts on QoL, similar to other chronic, painful disorders. Management of pain should be considered when treating FSHD1 patients. Muscle Nerve 57: 380–387, 2018
Keywords: facioscapulohumeral dystrophy, INQoL, pain, patient registry, patient reported outcome measures, quality of life
Abbreviations
- FSHD
facioscapulohumeral muscular dystrophy
- FSHD1
facioscapulohumeral muscular dystrophy type 1
- INQoL
Individualised Neuromuscular Quality of Life Questionnaire
- IR
interquartile range
- JWMDRC
John Walton Muscular Dystrophy Research Centre
- NSAIDs
nonsteroidal anti‐inflammatory drugs
- QoL
quality of life
- PROM
patient reported outcome measures
- SF‐MPQ
Short Form of the McGill Pain Questionnaire
- SMCHD1
structural maintenance of chromosomes flexible hinge domain containing 1
Facioscapulohumeral muscular dystrophy (FSHD) is the second most common muscle condition in adults, with an overall incidence of 1:20,000.1, 2 FSHD is an autosomal dominant and genetically heterogeneous disorder; in 95% of patients (FSHD1), it is associated with the loss of part of a D4Z4 repeated sequence in chromosome 4q35. In 5% of patients (FSHD2), mutations in the structural maintenance of chromosomes flexible hinge domain containing 1 (SMCHD1) gene are found. The D4Z4 methylation status changes in FSHD and the D4Z4 hypomethylation leads to chromatin relaxation of D4Z4 and expression of DUX4.3
FSHD symptoms usually start around the second decade and are most commonly characterized by asymmetric weakness affecting the face, shoulder, and arms, followed by the distal lower extremities and pelvic girdle. Not all patients have the complete phenotype of FSHD, and clinical severity varies widely among patients, including great variability of weakness within families. Chronic pain is a significant problem in different neuromuscular conditions,4, 5 such as limb‐girdle muscular dystrophy 1C, myotonic dystrophies, and FSHD6, 7; in some cases, pain may even be the first disease manifestation.8, 9 Reports about pain in FSHD‐phenotype patients predate the development of genetic testing.
Recent studies have suggested that pain may be present in the majority of FSHD patients, ranging from 76% to more than 80% of the FSHD population, with 19% reporting severe pain.5, 10, 11, 12, 13, 14, 15, 16 In some cases, FSHD patients reported severe, difficult to control, multifocal muscle pain as the most disabling aspect of their condition.9 Even so, pain is often undertreated. Some studies have shown that pain in FSHD negatively impacts quality of life (QoL) and increases disease burden.13, 15 However, the data available is scarce, often not FSHD‐specific and usually clinician‐reported. The studies address small heterogeneous cohorts that include other neuromuscular disorders.5, 10 Moreover, epidemiology, etiology, pathophysiology, and management of pain have not yet been addressed, nor has the interaction between pain and its impact on QoL.
The aim of the study was to determine the frequency, localization, and intensity of pain in the FSHD1 population registered in the U.K. FSHD registry; and to evaluate the influence of pain, age, sex, disease duration, and ambulatory status on QoL.
MATERIALS AND METHODS
Patients and Setting
We have analyzed data obtained from the U.K. FSHD Patient Registry that is curated from the John Walton Muscular Dystrophy Research Centre (JWMDRC) at Newcastle University (https://www.fshd-registry.org/uk/participants/questionnaires/index.en.html). The Registry started in May 2013. A cutoff point for data analysis was established in February 2017. This patient driven registry17 is based on the recommendations reported at the 171st European Neuromuscular Centre workshop on the care and management of FSHD.18 In addition to these items, several patient reported outcome measures (PROM) on pain and QoL and were added after consultation with the patient community about their own research priorities. The registry has received full ethical (Newcastle and North Tyneside 113/NE/0048, February 2013), management (Newcastle upon Tyne Hospitals Trust R&D 6573, February 2013), and data protection (Caldicott February 2013) approvals.
Measures
Pain
The Short Form of the McGill Pain Questionnaire (SF‐MPQ) was used to report pain. The SF‐MPQ is widely used, well‐validated, and reliable and has previously been used in FSHD1.19 The SF‐MPQ consists of 15 descriptors (11 sensory and 4 affective) which are rated on an intensity scale as 0 = none, 1 = mild, 2 = moderate or 3 = severe, with higher scores representing more pain. Sensory (ranging from 0 to 33), affective (ranging from 0 to 12), and total pain scores (ranging from 0 to 45) and Present Pain Intensity Index (ranging from 0 to 5) were analyzed. An FSHD specific PROM, named universal pain assessment tool, developed by the multi‐disciplinary team at JWMDRC was also used. The universal pain assessment tool is in the process of validation and it includes sections on pain intensity, current pain and chronic pain, and in addition questions regarding medication and other nonpharmacological therapies (sections of the universal pain assessment tool used in this study are shown in Supplementary Table S1, which is available online). For the purpose of the study, “current pain” was defined as any pain experienced in the last 7 days and “chronic pain” as any persistent pain experienced for at least 12 weeks within a year in the last 5 years. The universal pain assessment tool clearly provided the time definition of current and chronic pain according to study pain definitions. To analyze pain intensity, the pain was considered to be severe when reported as horrible or excruciating.
Individualized Neuromuscular Quality of Life Questionnaire
Patients completed the Individualised Neuromuscular Quality of Life Questionnaire (INQoL), a widely used and well‐validated neuromuscular disease specific measure of QoL.20, 21 The final score for each section and total INQoL score is presented as a percentage of the maximum detrimental impact where 100 is the greatest impact on QoL.
Data Analysis
Location and dispersion indexes of noncontinuous variables were used to describe the sample with median and interquartile range (IR). Categorical variables were described as percentages. All statistics discussed here are presented as percentages of the total patient answers and not as percentages of the whole cohort unless otherwise stated. For comparison between groups, Chi‐square tests, Mann‐Whitney U test, and Kruskal‐Wallis test were used, as appropriate. Correlations were calculated using nonparametric Spearman's coefficient. To evaluate influence of age, age of onset, disease duration, gender, D4Z4 repeat, SF‐MPQ total score, and chronic pain on INQoL we performed a multivariate linear regression. Differences were considered significant at P < 0.05, and all statistical tests were two tailed. All data were analyzed with IBM SPSS Statistics 22.
RESULTS
Clinical Data
Four hundred and two genetically confirmed FSHD1 patients were included in this study. Figure 1 shows the progress of participants included. In total, 398 patients have been included for further analysis. All patients are followed‐up by neurologists with experience in neuromuscular disorders to ensure the FSHD1 diagnosis. Of these 398 UK genetically confirmed FSHD1 patients, 383 (96.2%) answered the SF‐MPQ, 367 (92.2%) and 365 (91.7%) answered current and chronic pain questions in the universal pain assessment tool, respectively, and 340 (85.4%) provided answers to INQoL.
Figure 1.
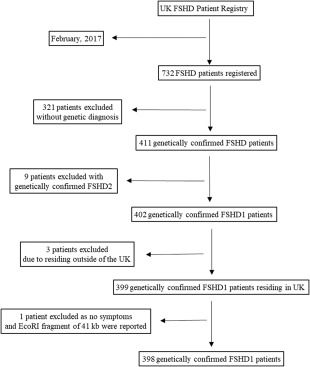
Flow diagram of the progress of participants included in the U.K. FSHD Patient Registry and the number of patients excluded.
The median age of the entire cohort was 47.0 (interquartile range: 60.6) years. Demographic data is presented in Table 1. In 6 patients, the size of the p13E‐11 EcoRI fragments was > 39 kb (3 patients had 39 kb, and 3 patients had 40, 41, and 45 kb, respectively); although, they have neither SMCHD1 test nor methylation studies. These 6 patients were included in the study because all reported symptoms and the treating neuromuscular specialist confirmed clinical features consistent with FSHD. There was no EcoRI fragment information in 45 of the cases.
Table 1.
Demographic data and frequency of pain
| Total | Males | Females | |
|---|---|---|---|
| Epidemiological | |||
| Gender | 398 | 197 (49.6%) | 201 (50.4 %) |
| Age (years)* | 47.0 (60.6) | 48.0 (25.0) | 46.0 (25.0) |
| Age onset (years)a | 17.0 (21.2) | 19.0 (22.0)b | 13.5 (22.5)b |
| Disease duration (years)a | 26.0 (27.0) | 24.0 (22.5) | 27.5 (30.7) |
| Genetic test (D4Z4 repeat kb)a | 25.0 (10.0) | 25.0 (10.0) | 24.0 (11.3) |
| Motor function, total (%) | |||
| Ambulatory‐unassisted | 193 (49.4%) | 91 (47.4%) | 102 (51.3%) |
| Ambulatory‐assisted | 145 (37.1%) | 78 (40.6%) | 67 (33.7%) |
| Non‐ambulatory | 53 (13.6%) | 23 (12.0%) | 30 (15.1%) |
| Wheelchair use, total (%) | |||
| No use | 244 (62.2%) | 125 (65.1%) | 119 (59.5%) |
| Part‐time | 94 (24.0%) | 44 (22.9%) | 50 (25.0%) |
| Full‐time | 54 (13.8%) | 23 (12.0%) | 31 (15.5%) |
| SF‐MPQ | |||
| Presence of pain Total (%) | 339 (85.2 %) | 164 (83.2%) | 175 (87.1%) |
| Sensory Scorea | 5.0 (9.0) | 3.0 (9.0)c | 6.0 (9.0)c |
| Affective Scorea | 1.0 (3.0) | 1.0 (3.0) | 2.0 (4.0) |
| Total scorea | 6.0 (12.5) | 5.0 (11.5) | 7.0 (12.5) |
| Present Pain Intensity Indexa | 1.0 (2.0) | 1.0a (2.0) | 1.0 (1.0) |
| Chronic pain | |||
| Chronic pain Total (%) | 203 (55.6%) | 92 (50.3%)c | 111 (61.0%)c |
| Severe chronic pain Total (%) | 69 (30.4%) | 29 (26.6%) | 40 (33.9%) |
Expressed in median and interquartile range.
P < 0.01.
P < 0.05.
Pain
Of those completing the SF‐MPQ, 339 patients (88.5%) reported experiencing pain to some degree. The sensory score in females was significantly higher (P < 0.05) than in males (Table 1). Three hundred and twenty‐five (88.6%) participants reported experiencing current pain. Figure 2 shows the percentage of patients that reported the highest levels of pain and the pain severity in different locations. The severe lower back pain was reported more frequently in females (33 patients; 17.7%) than in males (14 patients; 11.2%) with statistical significance (P < 0.05). No significant differences were observed regarding current pain localization across EcoRI fragments size, age of onset or motor function as showed in Table 2. Of the 365 responders, 203 (55.6%) people reported experiencing chronic pain, 69 patients (30.4%) reported this pain as severe. The chronic pain was more frequently reported in females (111 patients; 61.0%) than in males (92 patients, 50.3%) (P < 0.05). The most common location of chronic pain was the shoulder joint in 165 patients (45%). No association was seen between chronic pain and EcoRI fragments size, age of onset, or motor function.
Figure 2.
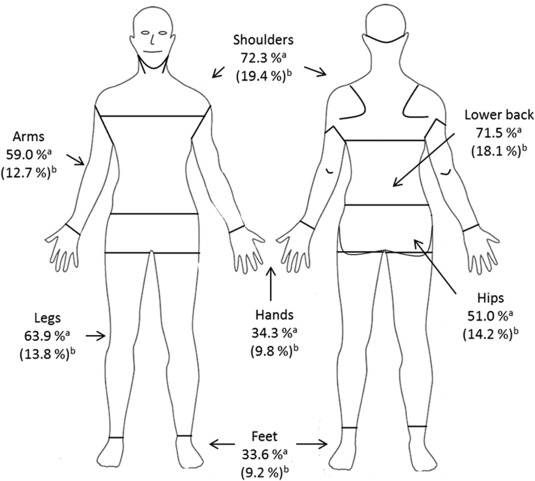
Description of the localization and the severity of the current pain in each localization expressed in percentage. aPercentage of patients suffering current pain in each localization. bPercentage of patients suffering severe current pain (pain rated as horrible or excruciating) in each localization.
Table 2.
Characteristics of pain in different sub‐groups of FSHD1 patients
| Total | SF‐MPQa | Severe current pain localizations Total (%) | Chronic pain Total (%) | |||
|---|---|---|---|---|---|---|
| Total pain score | Shoulder | Lower back | Presence chronic pain | Severe chronic pain | ||
| Age of onset (years) | ||||||
| 0‐9 | 107 | 7.0 (9.0) | 13 (17.8%) | 19 (24.1 %) | 62 (64.6 %) | 23 (34.8%) |
| 10‐19 | 104 | 7.5 (13.0) | 14 (20.9%) | 11 (16.7%) | 53 (54.6%) | 19 (33.3%) |
| 20‐39 | 99 | 6.0 (13.0) | 16 (23.5%) | 11 (16.7%) | 55 (58.5%) | 22 (34.4%) |
| > 40 | 56 | 5.0 (7.7) | 7 (17.1%) | 5 (13.5%) | 27 (50.0%) | 4 (13.3%) |
| Disease duration (years) | ||||||
| 0‐19 | 138 | 6.0 (12.0) | 22 (22.7%) | 13 (15.1%) | 75 (56.4%) | 22 (26.5%) |
| 20‐39 | 130 | 9.0 (13.0) | 19 (20.2%) | 22 (22.9%) | 70 (57.4%) | 30 (38.5%) |
| > 40 | 98 | 12.0 (12.0) | 9 (15.5%) | 11 (16.7%) | 52 (60.5%) | 16 (28.6%) |
| Fragment size, total | ||||||
| ≤ 18 Kb | 82 | 5.0 (14.0) | 6 (13.6 %) | 14 (25.9 %) | 38 (54.3 %) | 14 (33.3%) |
| > 18 Kb | 271 | 6.0 (8.0) | 39 (20.5%) | 28 (15.5%) | 144 (56.9%) | 46 (28.9%) |
| Motor function | ||||||
| Ambulatory‐unassisted | 193 | 6.0 (10.0)b | 17 (15.5%) | 24 (18.9%) | 95 (51.1%) | 29 (27.4% ) |
| Ambulatory‐assisted | 145 | 9.0 (13.0)b | 27 (24.1%) | 24 (22.9%) | 83 (60.6%) | 34 (37.4%) |
| Non‐ambulatory | 53 | 6.0 (11.0)b | 3 (8.1%) | 3 (9.7%) | 28 (58.3%) | 6 (20.0%) |
Expressed in median and interquartile range.
P < 0.05.
Therapies
Medication to manage pain was taken by 367 (92.2%) patients. When specified, nonsteroidal anti‐inflammatory drugs (NSAIDs) were the most frequent drugs used followed by opioids as described in Figure 3. Less than half of patients (162 patients, 46.2%) have used physiotherapy to help with pain management; a dramatic reduction in pain was reported by 4.4% (7 patients). Sixty‐two patients (38.8%) reported some reduction in pain, and 80 (50.0%) reported no reduction. Eleven patients (6.9%) experienced an increase in their pain after physiotherapy. We did not find any statistical difference in response to physiotherapy between any groups studied. In this survey, 370 patients (93%) reported using nonpharmacologic therapies to help manage pain (Fig. 4).
Figure 3.
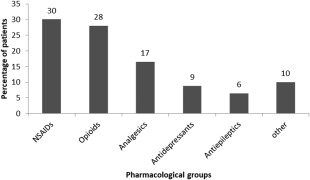
Most frequently pharmacological groups used to manage pain.
Figure 4.
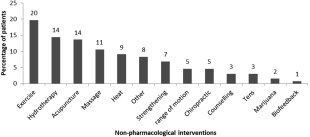
Most frequently nonpharmacologic interventions used to manage pain.
Quality of Life
The median overall INQoL score was 53.1 (IR: 34.3) suggesting relatively moderate sickness‐related dysfunction in this group of FSHD1 patients. The sub‐domains with the highest median scores, indicating the greatest impact on QoL were muscle weakness, body image, and activities. The least impact on QoL is in the areas of muscle locking and relationships. The weakness score in males was significantly higher than in females (P < 0.01). No other domain was affected by gender (Table 3). A higher total INQol score is seen in patients with a shorter EcoRI fragment (P < 0.05). The overall INQol increases with statistical significance with younger age of onset (rho = ‐0.22) and longer disease duration (rho = +0.23) (P < 0.05).
Table 3.
INQoL subscale scores and overall score comparisons between genders, and absence or presence of chronic pain
| INQoL | Gender | Chronic pain | |||
|---|---|---|---|---|---|
| Total | Males | Females | No | Yes | |
| Weakness score | 63.2 (26.4) | 73.7 (36.8)a | 68.4 (47.4)a | 57.9 (47.4)a | 79.0 (31.6)a |
| Locking score | 10.5 (22.4) | 10.5 (10.5) | 10.5 (13.2) | 10.5 (0.0)a | 10.5 (26.3)a |
| Pain score | 44.7 (31.6) | 31.6 (52.6) | 36.8 (47.4) | 10.5 (15.8)a | 63.2 (42.2)a |
| Fatigue score | 50.0 (34.2) | 47.4 (52.6) | 47.4 (52.6) | 26.3 (42.1)a | 68.4 (43.4)a |
| Activities score | 57.4 (58.6) | 52.8 (43.1) | 53.2 (54.9) | 37.0 (38.5)a | 67.6 (37.3)a |
| Independence score | 50.0 (64.6) | 34.7 (53.5) | 33.3 (55.6) | 25.0 (35.4)a | 55.6 (50.2)a |
| Relationship score | 20.4 (16.7) | 20.4 (28.7) | 18.5 (35.6) | 13.0 (21.3)a | 30.6 (37.1)a |
| Feelings score | 54.2 (56.2) | 38.9 (42.4) | 41.7 (39.6) | 27.8 (33.3)a | 50.0 (40.3)a |
| Body image score | 59.7 (51.4) | 55.6 (52.8) | 58.3 (55.6) | 41.7 (50.0)a | 61.1 (50.0)a |
| QoL Score | 53.1 (34.3) | 52.2 (30.0) | 51.1 (36.3) | 41.1 (30.4)a | 60.6 (29.5)a |
Values are expressed in median and interquartile range.
P < 0.01.
The pain INQoL score is higher in ambulant populations (P < 0.01).SF‐MPQ sensory, affective, and total pain scores correlated with INQoL pain score (rho = +0.79, rho = + 0.68, rho = + 0.80, respectively, P < 0.01), and INQoL total score (rho = +0.48, rho = + 0.50, rho = + 0.51, respectively, P < 0.01). On the other hand, the presence of severe pain in the shoulders, severe lower back pain, chronic pain, and severe chronic pain correlated with the INQoL pain score (rho = +0.54, rho = + 0.46, rho = + 0.67, rho = +0.44, respectively, P < 0.01), and the INQoL total score (rho = +0.32, rho = + 0.29, rho = + 0.38, rho = +0.29, respectively, P < 0.01). Multiple regression analysis showed that longer disease duration was related to greater deterioration on INQol; interestingly, the total SF‐MPQ score and presence of chronic pain was also directly related to an increase in total INQoL scores as showed in Table 4.
Table 4.
Association of INQol index with demographic, genetic, and pain presence
| INQoL index | ||
|---|---|---|
| Univariate analysis (P) | Multivariate analysis (beta, P) | |
| Age | 0.338 | NI |
| Age of onset | 0.001 | ‐0.057, 0.323 |
| Disease duration | 0.001 | + 0.173, 0.003 |
| Gender | 0.529 | NI |
| D4Z4 repeat | 0.062 | NI |
| SF‐MPQ total score | 0.001 | + 0.400, 0.001 |
| Chronic pain | 0.001 | +0.128, 0.029 |
| Adjusted R2: 0.284 | ||
NI, not included in the multivariate analysis since it was non‐significant in univariate analysis.
DISCUSSION
This study demonstrates that pain is highly prevalent in FSHD1. Our data are consistent with previous studies carried out in smaller mixed disease cohorts, and supports the conclusion that pain is a common complaint in patients with FSHD1, similar to or possibly worse than in other neuromuscular conditions, even though a direct comparative study was not conducted.5, 14 Furthermore, pain is reported in a higher percentage in our cohort that would be expected in the general population, where is ranges from 10% to 55%.22, 23 The frequency and intensity of pain reported in our population are similar to what has been reported previously in osteoarthritis or rheumatoid arthritis.24, 25 Chronic pain is a substantial problem in FSHD1 and needs to be tackled in a holistic way with exercise/physiotherapy, psychosocial intervention and tailored pharmacological treatment.
Gender influences FSHD1 clinical expression. Males are characterized by a lower age at onset of motor impairment and by a more severe disability.26 However, our data shows that female FSHD1 patients experience pain more frequently than males, suggesting that clinical impairment is not the only underlying factor leading to pain in FSHD1 patients. Although commonly reported, the mechanisms behind gender differences in pain perception are unknown; biopsychosocial mechanisms, influence of sex hormones or endogenous opioid function have all been proposed.27, 28, 29 Further research is required to explore if there is any specific mechanism in FSHD1 contributing to gender difference in pain.30
Pain was reported most frequently in the shoulders and lower back. These localizations are consistent with the findings of previous studies.10 We hypothesize that the degree of pain in these two locations may be exacerbated by the fact that the muscles in this region are amongst the weakest muscle groups in FSHD1. The abnormal posture, with forward shoulders and exaggerated lumbar lordosis as a consequence of the weakness, may be a cause of pain. Lumbosacral spine movements and kinetics are essential to normal movement; therefore, lower back pain might negatively impact on the patients' ability to stand or walk. This type of pain could be partially due to the asymmetrical, multifocal pattern of muscle involvement that in itself affects movement, generating a circle of pain, muscle atrophy, and functional impairment. Muscular involvement of the hands is less common in FSHD131; therefore, it is unsurprising that this was an area where pain was less frequently reported. These findings and the possible correlation of pain with the areas of greater weakness suggest an interesting area for future study. The development of specific strategies to improve strength, flexibility, and endurance in the areas most affected by pain, could lead to an improvement in the patient's mobility as well as QoL.
FSHD1 is an inheritable muscular condition with a progressive clinical course; therefore, we would expect to see an association between chronic pain and disease duration. No correlation was seen between chronic pain and current age, age of onset or disease duration. We may speculate that the causes of pain in different age groups and in patients with different disease duration times may be attributed to different causes with inflammatory mechanisms and muscle pain probably being more important in the early stages of disease and mechanical problems associated with asymmetrical muscle atrophy and weakness being the cause for pain later in the natural history of the disease. These aspects need to be considered in future studies to achieve an optimal management of pain.
Chronic pain and the severity of it were not significantly correlated with the D4Z4 fragment size or motor function; therefore, our data did not confirm the relationship between genetic pattern, patient ambulatory state, and chronic pain. Conversely, when considering “current pain” both with the SF‐MPQ and the universal pain assessment tool, patients who had no mobility limitations had a tendency to report less pain, as previously reported10; but in terms of chronic pain, the frequency of pain is similar in ambulatory and nonambulatory patients. This controversial data warrant further studies to carefully assess the presence of different type of pain in FSHD1 patients.
More than 90% of the patients reported taking medication, the most common treatments were NSAIDs and opioids, used more frequently than in previous studies. Physiotherapy was used to control pain in half of the patients together with other interventions. Although physiotherapy was reported as beneficial by some patients, a small percentage reported an exacerbation of the symptoms after the treatment; whether this worsening is due to lack of access to specialist physiotherapy for neuromuscular diseases should be assessed. It is necessary to identify whether there is a subgroup of FSHD1 patients who may get worse with physiotherapy to improve the therapeutic approach to these patients. On the other hand, worsening of the pain due to disease progression may be incorrectly attributed to therapy by some patients. Our data confirm that there is no consistent management of pain in FSHD1 and that current methods are not adequately relieving pain. Evaluating the patient's response to individual treatment is warranted to develop optimal pain therapy regimens in FSHD1 patients.
We have estimated QoL in this cohort using INQoL. There are difficulties for assessing QoL in neuromuscular diseases; therefore, there are few comprehensive studies available. In general, it is reported that QoL in patients with neuromuscular diseases is low.7, 14, 32, 33 INQoL has been highly rated in terms of its conceptual and measurement model, reliability, validity, and administrative burden in neuromuscular diseases.20 INQoL provides an overall score along with results in different domains. It should be noted that INQoL was not designed specifically for FSHD patients and, therefore, is limited as a true measure of QoL for FSHD. Specifically, INQoL includes questions regarding muscle locking, which has no relevance to this population. In our population, the INQoL domains having the biggest impact were muscle weakness, activities, and body image. The domain least affected, apart from muscle locking, was relationships. Our study has shown that patients perceive a deterioration in QoL with the progression of the disease; the younger the age of onset and the longer the disease duration, the higher the patients' perception of disability. Moreover, the perception of lower QoL was also related to lower EcoRI fragment sizes. These results suggest a relationship between genetic pattern, clinical severity, and perception of QoL.
The analysis of our data shows that chronic pain has a major negative impact on the QoL in FSHD1 consistent with the literature.13, 15 Future research should focus on the identification of the features of pain with the greatest impact on QoL. This may support the development of effective treatments.
In this study, all the questionnaires were patient reported17 without input from clinicians. This gives information about how patients perceive pain and QoL. PROM are an extraordinarily useful tool, favored by regulatory agencies, for monitoring the impact of care on patient well‐being and Qol.34
There are limitations to patient‐reported data and the element of self‐selection to the registry should not be ignored. An important limitation of the current study is the lack of information on physical examination/strength measures; more severely clinically affected individuals may be more likely to experience shoulder and back pain. The patient population may represent the most engaged and active patients who may not be representative of the entire FSHD1 population; however, our data are in line with previously published studies. Furthermore, our study could be improved through the inclusion of longitudinal data, assessing changes in pain and QoL over time. Finally, one of the pain questionnaires (universal pain assessment tool) has not yet been validated; however, there was a good correlation with the well‐validated SF‐MPQ. In answering the universal pain assessment tool, it is important to clarify that patients have to recognize the pain as a result of their FSHD, and that it may be difficult to differentiate this pain from other types of pain.
In conclusion, pain is a frequent symptom in FSHD1 that negatively impacts QoL. Studies have demonstrated that pain in FSHD1 is an inherent feature of the condition that requires further investigations to understand the pathophysiology, to study the relation with disease severity and functional disability.
Supporting information
Additional supporting information may be found in the online version of this article.
Supporting Information Table S1
ACKNOWLEDGMENTS
The authors acknowledge the work of past and present members of the steering committee Mark Busby, Andrew Graham, David Hilton‐Hones, Hanns Lochmüller, Cheryl Longman, Peter Lunt, Fiona Norwood, Richard Orrell, Marita Pohlschmidt, Mark Roberts, Stuart Watt, Suzanne Watt, and Tracey Willis. We also acknowledge the following doctors and healthcare professionals for referring patients to the registry and providing genetic data; Anne‐Marie Childs, Charlotte Brierley, Cheryl Longman, Chiara Marini‐Bettolo, Chris Turner, Christopher McDermott, David Dick, David Hilton‐Jones, Elizabeth Househam, Fiona Norwood, Hanns Lochmüller, John Winer, Margaret Phillips,Maria Farrugia, Mark Busby, Mark Roberts, Mark Rogers, Matt Parton, Meriel McEntagart, Paul Maddison, Peter Lunt, Richa Kulshrestha, Tracey Willis, Richard Orrell, Richard Petty, Ros Quinlivan, Sandya Tirupathi, Simon Hammans, and Volker Straub. Furthermore, we acknowledge Marcel Kiel for developing the registry software and for its ongoing maintenance. None of the authors have received any financial assistance/income during the period of the research activity and generation of the current report.
Ethical Publication Statement: We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Funding: This work has been funded by Muscular Dystrophy UK formerly the Muscular Dystrophy Campaign, (MC1/1064/1 and MC3/1064/3 and MC4/1064/4) from 2012 to 2017. The registry is part of the TREAT‐NMD Alliance (http://www.treat-nmd.eu). H.L. receives funding from the Medical Research Council as part of the MRC Centre for Neuromuscular Diseases (reference G1002274, Grant ID 98482), and by the European Union Seventh Framework Programme (FP7/2007‐2013) under Grant Agreement No. 305444 (RD‐Connect) and 305121 (Neuromics).
Conflicts of Interest: None of the authors has any conflict of interest to disclose.
REFERENCES
- 1. Mostacciuolo ML, Pastorello E, Vazza G, Miorin M, Angelini C, Tomelleri G, et al. Facioscapulohumeral muscular dystrophy: epidemiological and molecular study in a north‐east Italian population sample. Clin Genet 2009;75:550–555. [DOI] [PubMed] [Google Scholar]
- 2. Flanigan KM, Coffeen CM, Sexton L, Stauffer D, Brunner S, Leppert MF. Genetic characterization of a large, historically significant Utah kindred with facioscapulohumeral dystrophy. Neuromuscul Disord 2001;11:525–529. [DOI] [PubMed] [Google Scholar]
- 3. Gatica LV, Rosa AL. A complex interplay of genetic and epigenetic events leads to abnormal expression of the DUX4 gene in facioscapulohumeral muscular dystrophy. Neuromuscul Disord 2016;26:844–852. [DOI] [PubMed] [Google Scholar]
- 4. Chio A, Mora G, Lauria G. Pain in amyotrophic lateral sclerosis. Lancet Neurol 2017;16:144–157. [DOI] [PubMed] [Google Scholar]
- 5. Guy‐Coichard C, Nguyen DT, Delorme T, Boureau F. Pain in hereditary neuromuscular disorders and myasthenia gravis: a national survey of frequency, characteristics, and impact. J Pain Symptom Manage 2008;35:40–50. [DOI] [PubMed] [Google Scholar]
- 6. Scalco RS, Gardiner AR, Pitceathly RD, Hilton‐Jones D, Schapira AH, Turner C, et al. CAV3 mutations causing exercise intolerance, myalgia and rhabdomyolysis: expanding the phenotypic spectrum of caveolinopathies. Neuromuscul Disord 2016;26:504–510. [DOI] [PubMed] [Google Scholar]
- 7. Peric M, Peric S, Rapajic N, Dobricic V, Savic‐Pavicevic D, Nesic I, et al. Multidimensional aspects of pain in myotonic dystrophies. Acta Myol 2015;34:126–132. [PMC free article] [PubMed] [Google Scholar]
- 8. Hilbert JE, Ashizawa T, Day JW, Luebbe EA, Martens WB, McDermott MP, et al. Diagnostic odyssey of patients with myotonic dystrophy. J Neurol 2013;260:2497–2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bushby KM, Pollitt C, Johnson MA, Rogers MT, Chinnery PF. Muscle pain as a prominent feature of facioscapulohumeral muscular dystrophy (FSHD): four illustrative case reports. Neuromuscul Disord 1998;8:574–579. [DOI] [PubMed] [Google Scholar]
- 10. Jensen MP, Hoffman AJ, Stoelb BL, Abresch RT, Carter GT, McDonald CM. Chronic pain in persons with myotonic dystrophy and facioscapulohumeral dystrophy. Arch Phys Med Rehabil 2008;89:320–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jensen MP, Abresch RT, Carter GT. The reliability and validity of a self‐report version of the FIM instrument in persons with neuromuscular disease and chronic pain. Arch Phys Med Rehabil 2005;86:116–122. [DOI] [PubMed] [Google Scholar]
- 12. Della Marca G, Frusciante R, Vollono C, Iannaccone E, Dittoni S, Losurdo A, et al. Pain and the alpha‐sleep anomaly: a mechanism of sleep disruption in facioscapulohumeral muscular dystrophy. Pain Med 2013;14:487–497. [DOI] [PubMed] [Google Scholar]
- 13. Padua L, Aprile I, Frusciante R, Iannaccone E, Rossi M, Renna R, et al. Quality of life and pain in patients with facioscapulohumeral muscular dystrophy. Muscle Nerve 2009;40:200–2005. [DOI] [PubMed] [Google Scholar]
- 14. Abresch RT, Carter GT, Jensen MP, Kilmer DD. Assessment of pain and health‐related quality of life in slowly progressive neuromuscular disease. Am J Hosp Palliat Care 2002;19:39–48. [DOI] [PubMed] [Google Scholar]
- 15. Miro J, Gertz KJ, Carter GT, Jensen MP. Pain location and intensity impacts function in persons with myotonic dystrophy type 1 and facioscapulohumeral dystrophy with chronic pain. Muscle Nerve 2014;49:900–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nieto R, Raichle KA, Jensen MP, Miro J. Changes in pain‐related beliefs, coping, and catastrophizing predict changes in pain intensity, pain interference, and psychological functioning in individuals with myotonic muscular dystrophy and facioscapulohumeral dystrophy. Clin J Pain 2012;28:47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Evangelista T, Wood L, Fernandez‐Torron R, Williams M, Smith D, Lunt P, et al. Design, set‐up and utility of the UK facioscapulohumeral muscular dystrophy patient registry. J Neurol 2016;263:1401–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tawil R, van der Maarel S, Padberg GW, van Engelen BG. 171st ENMC international workshop: standards of care and management of facioscapulohumeral muscular dystrophy. Neuromuscul Disord 2010;20:471–475. [DOI] [PubMed] [Google Scholar]
- 19. van der Kooi EL, Kalkman JS, Lindeman E, Hendriks JC, van Engelen BG, Bleijenberg G, et al. Effects of training and albuterol on pain and fatigue in facioscapulohumeral muscular dystrophy. J Neurol 2007;254:931–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vincent KA, Carr AJ, Walburn J, Scott DL, Rose MR. Construction and validation of a quality of life questionnaire for neuromuscular disease (INQoL). Neurology 2007;68:1051–1057. [DOI] [PubMed] [Google Scholar]
- 21. Sadjadi R, Vincent KA, Carr AJ, Walburn J, Brooks VL, Pandya S, et al. Validation of the individualised neuromuscular quality of life for the USA with comparison of the impact of muscle disease on those living in USA versus UK. Health Qual Life Outcomes 2011;9:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gureje O, Simon GE, Von Korff M. A cross‐national study of the course of persistent pain in primary care. Pain 2001;92:195–200. [DOI] [PubMed] [Google Scholar]
- 23. Schneiderhan J, Clauw D, Schwenk TL. Primary care of patients with chronic pain. JAMA 2017;317:2367–2368. [DOI] [PubMed] [Google Scholar]
- 24. Jobski K, Luque Ramos A, Albrecht K, Hoffmann F. Pain, depressive symptoms and medication in German patients with rheumatoid arthritis‐results from the linking patient‐reported outcomes with claims data for health services research in rheumatology (PROCLAIR) study. Pharmacoepidemiol Drug Saf 2017;26:766–774. [DOI] [PubMed] [Google Scholar]
- 25. Peat G, McCarney R, Croft P. Knee pain and osteoarthritis in older adults: a review of community burden and current use of primary health care. Ann Rheum Dis 2001;60:91–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ricci G, Scionti I, Sera F, Govi M, D'Amico R, Frambolli I, et al. Large scale genotype‐phenotype analyses indicate that novel prognostic tools are required for families with facioscapulohumeral muscular dystrophy. Brain 2013;136:3408–3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bingefors K, Isacson D. Epidemiology, co‐morbidity, and impact on health‐related quality of life of self‐reported headache and musculoskeletal pain‐‐a gender perspective. Eur J Pain 2004;8:435–450. [DOI] [PubMed] [Google Scholar]
- 28. Bartley EJ, Fillingim RB. Sex differences in pain: a brief review of clinical and experimental findings. Br J Anaesth 2013;111:52–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fillingim RB, King CD, Ribeiro‐Dasilva MC, Rahim‐Williams B, Riley JL III. Sex, gender, and pain: a review of recent clinical and experimental findings. J Pain 2009;10:447–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tonini MM, Passos‐Bueno MR, Cerqueira A, Matioli SR, Pavanello R, Zatz M. Asymptomatic carriers and gender differences in facioscapulohumeral muscular dystrophy (FSHD). Neuromuscul Disord 2004;14:33–38. [DOI] [PubMed] [Google Scholar]
- 31. Wang LH, Tawil R. Facioscapulohumeral dystrophy. Curr Neurol Neurosci Rep 2016;16:66. [DOI] [PubMed] [Google Scholar]
- 32. Sansone VA, Ricci C, Montanari M, Apolone G, Rose M, Meola G. Measuring quality of life impairment in skeletal muscle channelopathies. Eur J Neurol 2012;19:1470–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rakocevic Stojanovic V, Peric S, Paunic T, Pesovic J, Vujnic M, Peric M, et al. Quality of life in patients with myotonic dystrophy type 2. J Neurol Sci 2016;365:158–161. [DOI] [PubMed] [Google Scholar]
- 34. Bartlett SJ, Witter J, Cella D, Ahmed S. Montreal Accord on Patient‐Reported Outcomes Use Series ‐ Paper 6: creating national initiatives to support Patient Reported Outcomes (PRO) development and use‐the PROMIS example. J Clin Epidemiol 2017. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional supporting information may be found in the online version of this article.
Supporting Information Table S1


