Abstract
The inability to achieve optimal diabetes glucose control in people with diabetes is multifactorial, but one contributor may be inadequate control of postprandial glucose. In patients treated with multiple daily injections of insulin, both the dose and timing of meal‐related rapid‐acting insulin are key factors in this. There are conflicting opinions and evidence on the optimal time to administer mealtime insulin. We performed a comprehensive literature search to review the published data, focusing on the use of rapid‐acting insulin analogues in patients with Type 1 diabetes. Pharmacokinetic and pharmacodynamic studies of rapid‐acting insulin analogues, together with postprandial glucose excursion data, suggest that administering these 15–20 min before food would provide optimal postprandial glucose control. Data from clinical studies involving people with Type 1 diabetes receiving structured meals and rapid‐acting insulin analogues support this, showing a reduction in post‐meal glucose levels of ~30% and less hypoglycaemia when meal insulin was taken 15–20 min before a meal compared with immediately before the meal. Importantly, there was also a greater risk of postprandial hypoglycaemia when patients took rapid‐acting analogues after eating compared with before eating.
What's new?
Taking rapid‐acting insulin 15–20 min before a meal provides significant improvements in post‐meal control; we recommend this whenever safely possible.
People with diabetes who routinely bolus pre‐meal have better HbA1c values, according to large registry data.
Post‐meal bolusing may increase the risk of hypoglycaemia.
Advice about timing of bolus needs to be tailored in some special circumstances (e.g. pregnancy, emergency work, gastroparesis).
What's new?
Taking rapid‐acting insulin 15–20 min before a meal provides significant improvements in post‐meal control; we recommend this whenever safely possible.
People with diabetes who routinely bolus pre‐meal have better HbA1c values, according to large registry data.
Post‐meal bolusing may increase the risk of hypoglycaemia.
Advice about timing of bolus needs to be tailored in some special circumstances (e.g. pregnancy, emergency work, gastroparesis).
Introduction
The importance of optimal glycaemic control in preventing the micro‐ and macrovascular complications associated with diabetes has been well documented 1, 2. Despite this, a significant percentage of people with diabetes do not achieve target glycaemic control. The UK National Diabetes Audit 2015–2016 found that HbA1c levels were >58 mmol/mol (7.5%) in 70.8% of people with Type 1 diabetes and 34.3% in those with Type 2 diabetes 3. Data published in the USA in 2013 estimated that 47.8% of people with diabetes had HbA1c levels of >53 mmol/mol (7%) 4. The inability to achieve optimal glycaemic control in diabetes is multifaceted, as highlighted by Khunti et al. 5; however, postprandial hyperglycaemia is one likely key contributing factor 6. High postprandial blood glucose (BG) levels also contribute to greater glycaemic variability, another marker of poor glycaemic control 7. Epidemiological studies show an association between impaired glucose tolerance and cardiovascular risk and outcome 8.
Prandial insulin replacement is important. In individuals without diabetes, prandial insulin makes up ~50% of the total daily pancreatic output. Most of the prandial insulin is secreted within the first hour after the meal 9. The International Diabetes Federation consensus statement recommends that 2‐h post‐meal glucose levels should not exceed 7.8 mmol/l, as this level is seldom seen in those without diabetes 10. The American Diabetes Association specifies a postprandial glucose target of 10 mmol/l at 2 h 11. There is evidence that postprandial glucose excursions beyond these levels increase the risk of retinopathy 12 and greater carotid intima‐media thickness, and lead to oxidative stress, inflammation and endothelial dysfunction 13, 14, 15. Furthermore, there is evidence to suggest that post‐meal hyperglycaemia is also associated with decreased myocardial blood flow and an increased risk of cancer 16, 17. Meanwhile, the pre‐meal target in the intensive arm of the Diabetes Control and Complications Trial was 3.9–6.7 mmol/l and resulted in a significant reduction in vascular complications 18.
In people with either Type 1 or insulin‐requiring Type 2 diabetes treated with multiple daily injections (MDI), short‐ or rapid‐acting insulin is given with meals to cover mealtime glucose excursions. The pharmacology of the insulin compared with the glucose profile from the food ingested govern the extent of post‐meal glucose excursions. In the pre‐rapid‐acting insulin analogue era, regular human insulin (RHI) was the mainstay of bolus insulin therapy; however, recognition of its slow onset of action and delayed peak led to the recommendation to take it ≥30 min pre‐meal. In practice, many people did not do this. To address this, rapid‐acting insulin analogues were introduced in the 1990s.
There are currently three rapid‐acting analogues marketed in the USA and Europe: insulin lispro (Humalog; Eli Lilly, Indianapolis, IN, USA), insulin aspart (Novolog/NovoRapid; Novo Nordisk, Bagsvaerd, Denmark) and insulin glulisine (Apidra; Sanofi, Paris, France). Additionally, a fourth rapid‐acting analogue, fast‐acting insulin aspart (faster aspart; Fiasp; Novo Nordisk, Bagsvaerd, Denmark), has recently been approved for marketing in Europe and other parts of the world. For insulin lispro, proline and lysine at positions 28 and 29 on the B‐chain of human insulin are reversed. With insulin aspart, proline at position 28 on the B‐chain of human insulin is replaced with aspartic acid and for insulin glulisine, arginine at position 3 on the B‐chain is replaced with lysine, and lysine at position 29 on the B‐chain is replaced with glutamic acid. These changes reduce the ability of the insulin molecules to aggregate, and the dimers and monomers are more rapidly absorbed after subcutaneous (s.c.) injection. Next‐generation and faster rapid‐acting insulin analogues have also been developed that boast superior insulin absorption rates and early glucose‐lowering effects when compared with rapid‐acting analogues. Faster aspart is insulin aspart set in a new formulation with vitamin B3 (also known as nicotinamide) and arginine.
Manufacturers of rapid‐acting insulin analogues recommend injecting immediately before food or soon thereafter, and this is common practice for many people who feel more confident of the amount of carbohydrate eaten after they have eaten it 19, 20, 21. Most structured education programmes recommend injections pre‐meal, but often the precise timing is not specified and, in clinical practice, we observe many patients injecting their mealtime insulin post‐meal. For this reason, we conducted a systematic literature review of studies evaluating the timing of rapid‐acting insulin in an attempt to obtain some clarity on this important topic.
Methods
Data for the present review were collected through searches of PubMed: a specific search over the past 30 years and a more general search over the past 10 years. A search of ProQuest was also conducted which captured the Embase and Biosis databases. Search terms included: ‘diabetes’, ‘diabetes mellitus’, ‘Type 2 diabetes’, ‘Type 1 diabetes’, ‘T1D’, ‘T2D’, ‘bolus insulin’, ‘prandial insulin’, ‘insulin analogue’, ‘insulin aspart’, ‘insulin lispro’, ‘insulin glulisine’, ‘postprandial excursions’, ‘postprandial hyperglycaemia’, ‘postprandial administration’, ‘preprandial administration’, ‘post‐meal administration’, ‘pre‐meal administration’, ‘insulin timing’, ‘time of dose’, ‘timing of bolus’, ‘timing of prandial’, ‘dosing’, ‘flexibility’, ‘pharmacokinetics’ and ‘pharmacodynamics’. Studies on faster aspart were identified by the original search. A study on BC lispro was reviewed and included after the published search. The specific search of PubMed over the past 30 years yielded 1432 results, and the more general 10‐year search yielded 1990 results. The ProQuest search yielded 770 results. The authors reviewed the abstracts of all papers produced by the search and evaluated for relevance papers that specifically looked at the glycaemic effect of timing of rapid‐acting insulin in Type 1 or Type 2 diabetes. We identified 19 studies that could potentially be relevant to our review, with 11 being included (Fig. 1). Additional studies included in the review were obtained from references of studies identified by the search.
Figure 1.
Review flow diagram.
Evidence from pharmacokinetic and pharmacodynamic studies
Pharmacokinetic (PK) studies carried out in people with Type 1 diabetes show that all three rapid‐acting insulin analogues have similar PK and pharmacodynamic (PD) profiles (Table 1) 22, 23. They demonstrate peak plasma insulin concentrations approximately double those of RHI, and a time to maximum concentration less than half that of RHI, with concentrations of the analogues falling more rapidly, returning to levels <20% of peak concentrations at about 4 h (Fig. 2) 19, 20, 21.
Table 1.
Pharmacokinetic and pharmacodynamic studies
| Study | Rapid‐acting insulin analogues | PK characteristics INS‐Tmax, min | PD characteristics GIR‐Tmax, min |
|---|---|---|---|
| Plank et al. 22 |
Insulin aspart Insulin lispro |
43.8 ± 3.9 46.7 ± 4.7 |
n/a |
| Heise et al. 23 |
Insulin glulisine Insulin lispro |
100 ± 40 92 ± 38 |
196 ± 73 198 ± 65 |
| Arnolds et al. 24 * |
Insulin glulisine Insulin aspart |
90 (40–120) 90 (50–150) |
186 (155–263) 156 (83–245) |
| Heise et al. 25 † |
Faster aspart Insulin aspart |
62.9 (3.73) 69.7 (3.73) |
124.3 (5.87) 135.2 (5.87) |
| De la Peňa et al. 28 ‡ |
Lispro 100 Lispro 200 |
45 (30–180) 60 (30–180) |
120 (56) 126.6 (49) |
| Homko et al. 29 |
Aspart Insulin lispro |
30 30 |
120 120 |
| Swan et al. 30 | Insulin aspart | 60 | 100 |
| Andersen et al. 26, * |
BC lispro Insulin lispro |
45 (25–120) 60 (25–105) |
109 (65–221) 117 (71–225) |
Values are means (sd) unless stated otherwise. *Values are medians (range). †Values are means (sem). ‡Values are means (CV[%]).
BC lispro, BioChaparone insulin lispro; CV[%], coefficient of variance; GIR‐Tmax, time to maximum glucose infusion rate; INS‐Tmax, time to maximum serum insulin concentration; n/a, not available; PD, pharmacodynamic; PK, pharmacokinetic.
Figure 2.

Pharmacokinetics of bolus insulins. Panels (a), (b) and (c) are reproduced from Home et al. Diabetes Obes Metab 2012; 14: 780–788. Panel (d) is reproduced with permission from Andersen et al. EASD 2016; ePoster #931. Panels (e) and (f) are reproduced from Heise et al. Diabetes Obes Metab 2015; 17: 682–688 25, under a Creative Commons licence. s.c., subcutaneous.
One study showed little difference between the analogues: insulin aspart reached t50% of peak(ins) at 19.6 ± 1.7 min and insulin lispro at 16.7 ± 1.8 min (P = 0.29), and each analogue reached tpeak(ins) at 43.8 ± 3.9 min and 46.7 ± 4.7 min, respectively (P = 0.66) 22. When compared with insulin aspart and insulin lispro, the PK properties of insulin glulisine differ slightly in the majority of published studies, with a faster onset of action observed for glulisine. Heise et al. 23 showed that the time to 10% of total insulin area under the curve (INS‐AUC) was faster with insulin glulisine compared with insulin lispro at either dose (0.2 U/kg: 0.7 ± 0.2 vs 0.8 ± 0.2 h; 0.4 U/kg: 0.8 ± 0.2 vs 0.9 ± 0.2 h; P < 0.001) 23. When compared with insulin aspart, faster absorption rates were noted with insulin glulisine (shorter times to 10% and 20% of INS (max); P = 0.0005 each) 24.
Heise et al. 25 also investigated the PK properties of faster aspart, comparing it with insulin aspart. A faster initial onset of absorption of faster aspart vs insulin aspart was supported by a significantly earlier onset of appearance (4.9 vs 11.2 min) and time to reach half the maximum concentration (t50%Cmax(ins) 20.7 vs 31.6 min). With faster aspart, the time to onset of appearance and t50%Cmax(ins) were reduced by 57% and 35%, respectively, compared with insulin aspart. The tmax(ins) for faster aspart was 62.9 min and, for insulin aspart, it was 69.7 min (Fig. 2) 25.
Andersen et al. 26 looked at the PK properties of ultra‐rapid BC lispro vs insulin lispro (0.4 U/kg vs 0.2 U/kg). Onset of action was significantly earlier with BC lispro when compared with insulin lispro, with a median (min;max) t50%Cmax(ins) of 15 (6;32) vs 27 (12;43) min, respectively. The median (min;max) tmax(ins) for BC lispro was 45 (25;120) min and for insulin lispro it was 60 (25;105) min (Fig. 2) 26.
In clinical practice, PD assessment holds more relevance than PK assessment. The glucose‐clamp technique is the ‘gold standard’ for assessing insulin PD characteristics, providing data on onset, peak and duration of action, which are key in determining optimal bolus timing 27. Euglycaemia is maintained after insulin administration via a concomitant intravenous glucose infusion at a variable rate. This variable glucose infusion rate (GIR) is indicative of whole‐body insulin action. Clamp studies have demonstrated discordance between rapid‐acting insulin analogue PK properties (absorption time) and PD properties (action time), which creates an obstacle in successfully replicating prandial, physiological insulin action (we have not found any clinical data that explain the differences seen between the PK and PD characteristics of these insulin analogues). For example, a study comparing PD profiles of insulin lispro 100 U/ml and insulin lispro 200 U/ml found that, although the times to maximum insulin concentration were 45 and 60 min, respectively, the times to maximum GIR were similar (120 vs 126.6 min for insulin lispro 100 vs 200 U/ml) 28. Another study highlights the potential difference in timing of important outcomes. Directly comparing insulin aspart with insulin lispro, this study demonstrated a maximum insulin concentration of 30 min for both (P = 0.24), but a maximum GIR at 120 min, also for both (P = 0.61; Fig. 3) 29. Swan et al. 30 investigated the effect of puberty on the PD and PK properties of insulin pump therapy in adolescents, and found that the peak action of insulin aspart was not observed until 90 min, 40 min after peak insulin concentration was reached.
Figure 3.
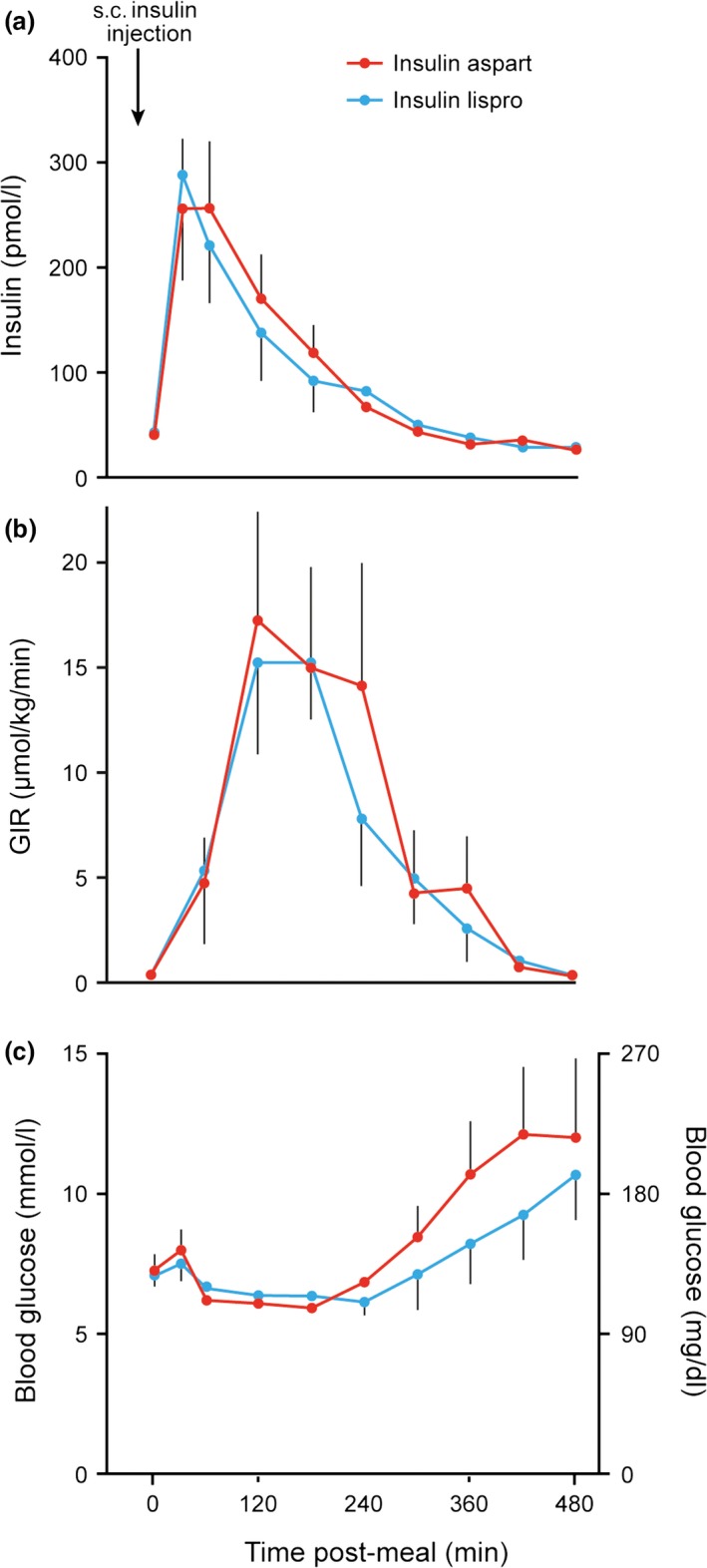
(a): Serum insulin levels before and after subcutaneous (s.c.) injection (at 0 min) of insulin aspart or insulin lispro in seven patients with Type 1 diabetes. (b) Glucose infusion rate (GIR) needed to prevent hypoglycaemia in the same seven patients. (c) Plasma glucose concentrations before and after s.c. injection of insulin aspart or insulin lispro in the same seven patients. Figure reproduced from Homko et al. Diabetes Care 2003; 26: 2027–2031 33.
The PD characteristics of faster aspart and BC lispro may be superior when compared with their respective rapid‐acting analogue counterparts. When comparing faster aspart with insulin aspart, the onset of glucose‐lowering effect was earlier with faster aspart, with a significantly earlier t50%GIRmax (38.3 vs 46.1 min). The time to reach the peak effect, tGIRmax, was also shorter (124.3 vs 135.2 min, ratio 0.83, 95% CI 0.73; 0.94) 25. BC lispro displayed similar differences compared with insulin lispro with a significantly earlier t50%GIRmax (31 vs 42 min, respectively; P < 0.001) and significantly earlier tGIRmax (109 vs 117 min, respectively; P = 0.0005) 26.
Continuous glucose monitoring (CGM) studies have shown that postprandial glucose levels peak at a mean of 70–80 min after eating in people with diabetes 31. CGM measures interstitial glucose, with a lag of 4–10 min in relation to BG levels 32. Although peak insulin levels are seen 40–60 min post‐injection, peak insulin action occurs around 100–120 min after injection. Given this, it is reasonable to expect that the optimal time to administer rapid‐acting insulin analogues is 15–20 min prior to eating, to synchronize insulin action peaks with postprandial glucose excursions, thus minimizing postprandial hyperglycaemia.
Evidence from clinical studies
Conflicting literature exists on optimal prandial bolus timing in clinical practice. Two studies in particular favour injection of prandial insulin 15–20 min before eating. Cobry et al. 33 carried out a crossover study in 23 young people with Type 1 diabetes (mean age 18.3 ± 4.4 years) on insulin pump therapy. The trial had three treatment arms: delivering an insulin glulisine bolus by insulin pump 20 min prior to a meal (−20 min), immediately before the meal (0 min) or 20 min after meal initiation (+20 min). At 60 min, the −20 min arm showed significantly lower glycaemic excursions than both the 0 min arm and the +20 min arm (−20 min = 10.0 ± 3.70 mmol/l vs 12.33 ± 3.27 mmol/l and 13.1 ± 2.59 mmol/l, respectively). At 120 min after meal initiation, the −20 min arm likewise showed significantly lower BG values than both the 0 min and +20 min arms (−20 min = 9.79 ± 3.9 mmol/l vs 11.5 ± 2.7 mmol/l and 11.4 ± 2.8 mmol/l, respectively; Table 2). Peak BG levels were also significantly lower in the −20 min arm compared with the 0 min arm and in the +20 min arm (11.2 ± 0.44 mmol/l) compared with the 0 min arm (13.55 ± 0.40 mmol/l; P = 0.0001) and the +20 min arm (13.7 ± 0.47 mmol/l; P < 0.0001; Fig. 4). No difference in BG readings was observed when insulin was administered immediately prior to the meal compared with 20 min post‐meal. Hypoglycaemic episodes recorded were highest in the +20 min arm compared with the 0 min and −20 min arms (five vs one vs four), respectively 33.
Table 2.
Clinical studies
| Study | Rapid‐acting insulin analogue | CSII or MDI | Time of insulin administration in relation to mealtime, min | Most effective time at lowering postprandial hyperglycaemia, min | Postprandial glucose levels, mmol/l |
|---|---|---|---|---|---|
| Cobry et al. 33 | Insulin glulisine | CSII | −20, 0, +20* | −20 | 11.0 ± 3.8 vs 13.7 ± 3.0 vs 13.8 ± 2.3 (max) |
| Luijf et al. 34 | Insulin aspart | CSII | −30, −15, 0 | −15 | 11.74 ± 0.8 vs 9.26 ± 0.72 vs 12.29 ± 0.93 (max) |
| Brunner et al. 35 | Insulin aspart | MDI | 0, +15 | 0 | 11.2 (10.4–12.0) vs 13.2 (12.3–14.2) (max) |
| Schernthaner et al. 36 | Insulin lispro | MDI | −20, 0, +15* | −20 | n/a |
| Jovanovic et al. 38 | Insulin aspart | MDI | −5 to 0, +30* | −5 to 0 | 5.7 ± 0.5 vs 8.3 ± 0.55 (max) |
| Schernthaner et al. 37 | Insulin lispro | MDI | 0, +30 | 0 | 7.71 ± 1.83 vs 8.66 ± 2.13 (mean) |
*Up to 15, 20 and 30 min after commencement of eating.
CSII, continuous subcutaneous insulin infusion; max, maximum postprandial glucose level; mean, mean postprandial glucose level; MDI, multiple daily injections; n/a, not available.
Figure 4.

Mean blood glucose levels after meal initiation in three treatment arms: Pre: delivering an insulin glulisine bolus by insulin pump 20 min prior to a meal (−20 min); Start: immediately before the meal (0 min); and Post: 20 min after meal initiation (+20 min). Figure reproduced from Cobry et al. Diabetes Technol Ther 2010; 12: 173–137 33.
Luijf et al. 34 studied 10 people with Type 1 diabetes on insulin pump therapy with a mean age of 45.5 ± 12.1 years, in a three‐way, randomized, crossover trial. Insulin aspart was administered at 30, 15 or 0 min before mealtime. Each participant was provided with a breakfast similar to their usual breakfast. Area under the glucose curve was lower in the −15 min arm (0.41 ± 0.51 mmol/l/min) than in the −30 min arm (1.89 ± 0.72 mmol/l/min; P = 0.029) and 0 min arm (2.11 ± 0.66 mmol/l/min; P = 0.030). Maximum glucose excursion was almost 30% lower in the −15 min arm (4.77 ± 0.52 mmol/l) than in the −30 min arm (6.48 ± 0.76 mmol/l; P = 0.025) and 0 min arm (6.93 ± 0.76 mmol/l; P = 0.022). Time spent in the +3.5 to +10 mmol/l range was higher in the −15 min arm (224.5 ± 25.0 min) than in the 0 min arm (90.5 ± 23.2 min; P = 0.001). There was no significant difference in occurrence of hypoglycaemia between arms (P = 0.901) 34.
While both these studies were performed exclusively in people using insulin pumps, the results should be applicable to people using MDI regimens, as the bolus aspect of these therapies is very similar. Importantly, participants in both of these studies had quite tight glucose control immediately prior to commencement of the study: 5.5–10 mmol/l in the Cobry et al. 33 study and 3.5–7.8 mmol/l in the Luijf et al. study 34. In the former, test meals consisted of a known, fixed amount of carbohydrate that was not specified and <20 g of fat. The protein content was not revealed. In the latter study, the nutritional content of the meal was not mentioned, which is an important missing variable. The infusion sites of the pumps were also not mentioned.
Several studies have compared preprandial (immediately before eating) and postprandial administration of rapid‐acting insulin analogues (Table 2). Brunner et al. 35 compared insulin aspart administered immediately before (0 min) and 15 min after the start of the meal, along with RHI 15 min before and immediately before the meal 35. This was a well‐designed study in which participants’ glucose levels were kept within a range of 100.8–140.4 mg/dl (5.6–7.8 mmol/l) prior to commencement, with a variable insulin infusion. A standardized breakfast was used (543 kcal, 55% carbohydrate, 17% protein and 28% fat). That study showed that insulin aspart at 0 min was superior to insulin aspart at +15 min and was similar to RHI at −15 min. The lowest postprandial glucose level achieved was in insulin aspart at 0 min but was higher than most target values at 11.2 mmol/l, compared with 13.2 mmol/l with insulin aspart at +15 min. Insulin aspart injected 15 min before mealtime was not investigated in that study. Importantly, late hypoglycaemia occurred in 21% of the experiments (0 min, n = 6; +15 min, n = 6) 35.
One of the most comprehensive studies was performed by Schernthaner et al. 36 comparing RHI at −40, −20 and 0 min and insulin lispro at −20, 0 and +15 min on postprandial glucose levels. Participants in that study had a standardized meal consisting of 584.5 kcal, 45.5 g of carbohydrate, 35 g of protein and 28 g of fat. BG excursions at 60 min after injection were significantly lower with insulin lispro at −20 min when compared with all other treatments, particularly insulin lispro 0 min and +15 min (−1.12 ± 2.13 vs 0.19 ± 1.72 vs 2.20 ± 1.49 mmol/l, respectively). At 90 and 120 min, insulin lispro −20 min and 0 min were superior to all other treatments, with insulin lispro 0 min performing much better at 90 and 120 min than at 60 min when compared with insulin lispro −20 min (−1.44 ± 1.60 vs −1.27 ± 1.89 [90 min]; −1.79 ± 1.66 vs −0.99 ± 1.89 mmol/l [120 min], respectively). AUCs of all six treatments showed insulin lispro −20 min (−2.19 mmol/h/1) and insulin 0 min (−2.15 mmol/h/1) to be significantly (P < 0.001) lower than all other treatments. The AUC of insulin lispro +15 min was +1.98 mmol/h/1. In total, 13 hypoglycaemic events were experienced: for early hypoglycaemia, there were three in the RHI −40 min group, one in the RHI −20 min group, two in the insulin lispro −20 min group and one in the insulin lispro +15 min group. There was one late hypoglycaemic episode in each of the RHI −40 min, −20 min and 0 min groups and three in the insulin lispro +15 min group. No hypoglycaemic events were seen with insulin lispro 0 min. It should be noted that the pre‐meal glucose was not as well controlled as other studies, with large variation (3.3–11.1 mmol/l) 36.
Schernthaner et al. 37 also performed a 6‐month crossover study on 31 people with Type 1 diabetes receiving insulin lispro either preprandially or postprandially for a 3‐month period followed by the alternate regimen for a further 3 months. This study was unique, as it examined clinical outcomes by measuring HbA1c, fructosamine and eight‐point BG levels. HbA1c decreased in the preprandial group and increased in the postprandial group (−0.15 ± 0.41% vs +0.11 ± 0.48%; P = 0.008). Fructosamine levels also reduced in the preprandial group (− 15 ± 31 μmol/l vs 1 ± 39 μmol/l), although eight‐point BG levels were not statistically significantly different between the groups. This study highlights the negative impact that postprandial insulin administration can have on glycaemic control with long‐term use. There was no difference in hypoglycaemic events between the two groups 37.
Jovanovic et al. 38 compared the injection of insulin aspart immediately before mealtime to immediately afterwards in 19 people with Type 1 diabetes on MDI regimens and found that total glucose AUC during the meal test was 22% smaller when insulin aspart was injected immediately before the study meal (mean [se] 23 014 [1832] mg/dl/min) than when injected immediately after the meal (mean [se], 29 535 [2243] mg/dl/min; P < 0.001). There were some weaknesses in that study, however, because preprandial glucose levels were higher on the postprandial injection study day and the fat and protein content of the meals was not fixed, with lower intake on days with pre‐meal injections 38.
Bode et al. 39 conducted a double‐blind, randomized, crossover, active‐controlled trial comparing 2‐h postprandial BG level response after 2 weeks of continuous s.c. insulin infusion with faster aspart or insulin aspart. Insulin was administered immediately before consumption of a standardized meal and all participants wore blinded CGM devices. Faster aspart provided a statistically significantly greater glucose‐lowering effect after the meal than did insulin aspart: ΔBG of 3.03 vs 4.02 mmol/l. At 1 h post‐meal, BG levels were −1.64 mmol/l lower with faster aspart than with insulin aspart (P = 0.006). This study suggests potential benefit with the newer, faster, rapid‐acting analogue insulins on postprandial hyperglycaemia when given pre‐meal 39. The impacts of using either insulin 15 min pre‐meal, or a comparison of aspart given 15 min pre‐meal and the faster‐acting analogue just before eating, remain to be determined.
Data from the Type 1 exchange registry support our findings, with the majority of people in the excellent control group administering pre‐meal boluses as recommended 40.
It is notable that most structured education programmes and algorithms for dose adjustment are based on pre‐meal glucose (Dose Adjustment for Normal Eating [DAFNE], Diabetes Teaching and Treatment Programme [DTTP]). As CGM technologies become more widely used, patients will be exposed to more post‐meal glucose data. Indeed, in a study using intermittently monitored continuous s.c. monitoring, in which the glucose sensor records a continuous trace of plasma glucose‐equivalent measures in the hours prior to screening, there was a significant shift towards delivering meal boluses 15–20 min pre‐meal from immediate or post‐meal administration, as users identified timing of bolus as a key factor in post‐meal glucose control 41. The American Diabetes Association recommends checking post‐meal glucose at 2 h, aiming for a target of <10 mmol/l 11. At 2 h, glucose is likely to be falling, and there is a need for recommendations that take into account the insulin on board, which again may be influenced by the timing of the bolus.
Dose timing may be less critical in people with Type 2 diabetes, at least while they retain useful amounts of endogenous insulin. A study by Gredal et al. 42 assessed the optimal dose and timing of aspart in people with Type 2 diabetes. No difference in postprandial glucose profile was demonstrated whether insulin aspart 0.04 IU/kg was administrated 15 or 30 min before mealtime. Doubling the dose increased the risk of hypoglycaemia 42.
Ratner et al. 43 investigated the effect of insulin glulisine injected either preprandially (0–15 min) or postprandially (+20 min) on glycaemic control and weight gain in people with Type 2 diabetes. Participants were also taking insulin glargine once daily ± metformin. This study lasted for 52 weeks, with 322 people completing the study. At study end, insulin glulisine achieved similar glycaemic control whether it was administered before or after meals (HbA1c: 7.04% pre‐meal vs 7.16% post‐meal; P = non‐significant). Overall hypoglycaemia incidence and severe hypoglycaemia rates were not significantly different between pre‐meal and post‐meal groups; however, symptomatic and nocturnal hypoglycaemia rates were higher in the postprandial group. There was no significant difference in weight gain 43.
Many people with Type 2 diabetes requiring insulin therapy use biphasic or mixed insulin. Warren et al. 44 compared biphasic aspart insulin (BIAsp 30, a biphasic formulation of insulin aspart, 30% soluble and 70% protamine‐crystallized) injected 5 min before or 15–20 min after eating in an elderly population (aged >65 years) with Type 2 diabetes. Mean plasma glucose values during a 4‐h meal test at the end of each treatment were similar for pre‐ and postprandial BIAsp 30 (8.5 ± 3.2 mmol/l and 8.9 ± 3.3 mmol/l, respectively; difference not significant). The mean BG increment from self‐measured BG values, however, was slightly but significantly greater after postprandial injection than after preprandial injection (treatment difference: 0.9 mmol/l, 95% CI 0.03;1.63). No increased risk of hypoglycaemia was seen with postprandial injection 44.
Effect of other factors on postprandial glucose control
When interpreting data from the described studies, it is important to consider other factors that can adversely affect postprandial glycaemia and potentially skew results.
The nutritional content of food, particularly the protein and fat content, has been shown to affect postprandial glycaemia. Some studies have shown that meals containing carbohydrates that are high in dietary fat cause sustained late postprandial hyperglycaemia. One study showed that the addition of 35 g dietary fat increased postprandial glucose concentrations by 2.3 mmol/l at 5 h and another demonstrated that the addition of 50 g fat caused significant hyperglycaemia over 5 h 45, 46. Protein has also been shown to increase postprandial glucose levels, with one study reporting that the addition of 35 g of protein to a 30‐g carbohydrate meal resulted in a 2.6‐mmol/l increase in BG at 5 h 47. There are also data to suggest that food order has a significant role to play. A study by Shukla et al. 48 showed that when protein and fat were consumed 15 min before carbohydrate, the mean post‐meal glucose levels were lower by 28.6% (P = 0.001), 36.7% (P = 0.001) and 16.8% (P = 0.03) at 30, 60 and 120 min, respectively, and the incremental AUC was 73% lower. The glycaemic index of food may also affect postprandial BG levels, as foods with a high glycaemic index cause a large and rapid rise in BG, whereas those with a low glycaemic index produce small fluctuations in BG 49. It has also been shown that large carbohydrate meals may contribute to late postprandial hyperglycaemia 50. These studies highlight the importance of knowing and understanding the nutritional content of meals, as this can have a bearing on prandial glucose levels and insulin requirements. We may speculate that bolusing 15–20 min before eating is of most importance with high‐glycaemic index foods, and that dividing administration of doses may allow optimum postprandial glucose control for meals of high fat and/or protein content. One study found that an 8‐h dual‐wave bolus given pre‐meal using an insulin pump provided the best postprandial glucose control after a high‐fat meal 51.
Gastric emptying rate is also an important variable that can influence postprandial glycaemia in both people with and without diabetes, with significant inter‐individual variability. Pre‐meal glucose affects gastric emptying, with hyperglycaemia causing a ‘physiological’ slowing, as may meal composition and other concomitant medication such as glucagon‐like peptide‐1 receptor agonists 52, 53, 54. People with gastroparesis may also need a different bolus profile, such as a dual‐wave or a square‐wave, to mimic the delayed gastric absorption of carbohydrate 55. One study examined the intra‐individual variability in postprandial glucose excursions in a small cohort of people with Type 1 diabetes on MDI, using standardized test meals with either insulin lispro (15 min pre‐meal) or regular human insulin (30 min pre‐meal). The intra‐individual coefficients of variance of the mean glucose excursions after the meals were significant, and also lower with insulin lispro, at most time points: 1 h, 66% vs 71%; 2 h, 49% vs 69%; 4 h, 66% vs 75% and 5 h, 49% vs 72% 56.
There are, of course, some circumstances in which safety or practicality governs the timing of insulin. Examples may be people working in critical environments or where they cannot guarantee eating of food 15–20 min after a bolus, when eating out at social events or when predicting the exact carbohydrate content of the meal ahead is not possible. In these situations, pre‐ rather than post‐meal administration remains optimal but, if safety or convenience leads to occasional post‐meal administration of prandial insulin, rapid‐acting analogues are the safer option.
Administration of fast‐acting analogues by a parent uncertain of a child's appetite within 15 min after the child starts to eat may be an acceptable compromise between parental anxiety and normoglycaemia, but it should not be allowed to become a habit as the child's behaviour becomes more predictable, and adults with diabetes should be discouraged from postprandial injections.
Another special circumstance, in the opposite direction, is pregnancy. A study by Murphy et al. 57 showed that postprandial BG levels are impaired by significantly slower glucose disposal in late gestation, with the authors suggesting that optimal bolus timing in late pregnancy may be 30–40 min pre‐meal compared to 15–20 min in early pregnancy.
The site at which s.c. insulin is injected can also affect the PK characteristics of insulin. Abdominal injecting of rapid‐acting insulin analogues results in the highest concentration of insulin at the earliest time when compared with insulin administration in the arm, thigh or buttocks 58.
Conclusions
The data from the present review of the literature provide clear clinical evidence for the superiority and safety of injecting 15–20 min pre‐food, with almost 30% lower post‐meal glucose levels, a lower AUC for hyperglycaemia and less post‐meal hypoglycaemia when the pre‐meal glucose levels are in range. We therefore recommend that people with diabetes should aim to do this whenever possible, accepting that there may be individual circumstances when this is not practical. Postprandial administration of rapid‐acting insulin analogues is a less effective method of controlling BG levels in the postprandial phase, and carries a significant risk of hypoglycaemia.
The PK/PD studies of rapid‐acting insulin analogues show that the time to maximum insulin levels is between 40 and 60 min, but time to peak insulin effect is up to 120 min after injection. Given that BG levels peak before the maximum peak effect of insulin has been reached, it makes sense to administer rapid‐acting insulin analogues 15–20 min before mealtimes to try to synchronize BG and insulin peaks in an attempt to avoid postprandial hyperglycaemia. Fear of hypoglycaemia by adopting this approach may prevent patients from following this advice but may be allayed by discussion of the lack of insulin action during that time. Indeed, the data show that risk of post‐meal hypoglycaemia is highest with analogue insulins when administered 20 min after the start of eating. The delay in injecting after a meal in adults with Type 1 diabetes using post‐meal insulin administration may often be greater than that, which would be expected to exacerbate the problems further.
As our understanding of post‐meal glucose control increases, we may need to develop better strategies to cope with complex meals that may require different time–action profiles. With the advent of newer faster‐acting analogues, we will need further clinical studies to understand the optimum timing of these insulins; however, given the profile of post‐meal glucose excursions, the time to peak insulin action would need to be <45 min if these insulins were to be effectively used after eating.
Funding sources
The authors thank Liam Gillies, PhD and Helen Marshall of Watermeadow Medical – an Ashfield company, part of UDG Healthcare PLC, for providing editorial and submission support in accordance with Good Publication Practice (GPP3) guidelines (www.ismpp.org/gpp3), funded by Novo Nordisk.
Competing interests
D.S. has no conflicts of interest to declare. P.C. has received travel support, speaker fees and honoraria for Novo Nordisk, Lilly, Sanofi, Astra Zeneca, Medtronic, Roche, Diasend, Ascenecia, Abbott and Dexcom. S.A. has attended advisory boards for Medtronic, NovoNordisk and Roche.
Diabet. Med. 35, 306–316 (2018)
References
- 1. Diabetes Control and Complications Trial Research Group , Nathan DM, Genuth S, Lachin J, Cleary P, Crofford O et al The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus. N Engl J Med 1993; 329: 977–986. [DOI] [PubMed] [Google Scholar]
- 2. Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ et al Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group . Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005; 353: 2643–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Health and Social Care Information Centre . National Diabetes Audit 2015‐2016. Report 1: Care Processes and Treatment Targets. 2013. Available at http://www.content.digital.nhs.uk/catalogue/PUB23241. Last accessed 18 June 2017.
- 4. Ali MK, Bullard KM, Saaddine JB, Cowie CC, Imperatore G, Gregg EW. Achievement of goals in U.S. diabetes care, 1999–2010. N Engl J Med 2013; 368: 1613–1624. [DOI] [PubMed] [Google Scholar]
- 5. Khunti K, Wolden ML, Thorsted BL, Andersen M, Davies MJ. Clinical inertia in people with type 2 diabetes: a retrospective cohort study of more than 80,000 people. Diabetes Care 2013; 36: 3411–3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Scheen AJ, Schmitt H, Jiang HH, Ivanyi T. Factors associated with reaching or not reaching target HbA1c after initiation of basal or premixed insulin in patients with type 2 diabetes. Diabetes Metab 2017; 43: 69–78. [DOI] [PubMed] [Google Scholar]
- 7. Hirsch IB, Brownlee M. Should minimal blood glucose variability become the gold standard of glycemic control? J Diabetes Complications 2005; 19: 178–181. [DOI] [PubMed] [Google Scholar]
- 8. DECODE Study Group . Glucose tolerance and cardiovascular mortality: comparison of fasting and 2‐hour diagnostic criteria. Arch Intern Med 2001; 161: 397–405. [DOI] [PubMed] [Google Scholar]
- 9. Polonsky KS, Given BD, Van Cauter E. Twenty‐four‐hour profiles and pulsatile patterns of insulin secretion in normal and obese subjects. J Clin Invest 1988; 81: 442–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. International Diabetes Federation . Guideline for Management of Post‐meal Glucose. 2007. Available at www.idf.org/webdata/docs/Guideline_PMG_final.pdf. Last accessed 25 January 2017.
- 11. American Diabetes Association . Standards of medical care. Diabetes Care 2004; 27(Suppl. 1): S15–S35. [DOI] [PubMed] [Google Scholar]
- 12. Shiraiwa T, Kaneto H, Miyatsuka T, Kato K, Yamamoto K, Kawashima A et al Post‐prandial hyperglycemia is an important predictor of the incidence of diabetic microangiopathy in Japanese type 2 diabetic patients. Biochem Biophys Res Commun 2005; 336: 339–345. [DOI] [PubMed] [Google Scholar]
- 13. Hanefeld M, Koehler C, Schaper F, Fuecker K, Henkel E, Temelkova‐Kurktschiev T. Postprandial plasma glucose is an independent risk factor for increased carotid intima‐media thickness in non‐diabetic individuals. Atherosclerosis 1999; 144: 229–235. [DOI] [PubMed] [Google Scholar]
- 14. Ceriello A, Quagliaro L, Piconi L, Assaloni R, Da Ros R, Maier A et al Effect of postprandial hypertriglyceridemia and hyperglycemia on circulating adhesion molecules and oxidative stress generation and the possible role of simvastatin treatment. Diabetes 2004; 53: 701–710. [DOI] [PubMed] [Google Scholar]
- 15. Williams SB, Goldfine AB, Timimi FK, Ting HH, Roddy MA, Simonson DC et al Acute hyperglycemia attenuates endothelium‐dependent vasodilation in humans in vivo. Circulation 1998; 97: 1695–1701. [DOI] [PubMed] [Google Scholar]
- 16. Cavalot F, Petrelli A, Traversa M, Bonomo K, Fiora E, Conti M et al Postprandial blood glucose is a stronger predictor of cardiovascular events than fasting blood glucose in type 2 diabetes mellitus, particularly in women: lessons from the San Luigi Gonzaga Diabetes Study. J Clin Endocrinol Metab 2006; 91: 813–819. [DOI] [PubMed] [Google Scholar]
- 17. Stattin P, Björ O, Ferrari P, Lukanova A, Lenner P, Lindahl B et al Prospective study of hyperglycemia and cancer risk. Diabetes Care 2007; 30: 561–567. [DOI] [PubMed] [Google Scholar]
- 18. Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, et al Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005; 353: 2643–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Eli Lilly . Humalog Summary of Product Characteristics. 2011. Available at www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000088/WC500050332.pdf. Last accessed 25 January 2017.
- 20. Novo Nordisk A/S . NovoRapid Summary of Product Characteristics. 2011. Available at www.ema.europa.eu/docs/ en_GB/document_library/EPAR_‐_Product_Information/human/000258/WC500030372.pdf. Last accessed 23 March 2011.
- 21. Sanofi‐Aventis . Apidra Summary of Product Characteristics. 2011. Available at www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000557/WC500025250.pdf. Last accessed 25 January 2017.
- 22. Plank J, Wutte A, Brunner G, Siebenhofer A, Semlitsch B, Sommer R et al A direct comparison of insulin aspart and insulin lispro in patients with type 1 diabetes. Diabetes Care 2002; 25: 2053–2057. [DOI] [PubMed] [Google Scholar]
- 23. Heise T, Nosek L, Spitzer H, Heinemann L, Niemoller E, Frick AD et al Insulin glulisine: a faster onset of action compared with insulin lispro. Diabetes Obes Metab 2007; 9: 746–753. [DOI] [PubMed] [Google Scholar]
- 24. Arnolds S, Rave K, Hovelmann U. Insulin glulisine has a faster onset of action compared with insulin aspart in healthy volunteers. Exp Clin Endocrinol Diabetes 2010; 118: 662–664. [DOI] [PubMed] [Google Scholar]
- 25. Heise T, Hövelmann U, Brøndsted L, Adrian CL, Nosek L, Haahr H. Faster‐acting insulin aspart: earlier onset of appearance and greater early pharmacokinetic and pharmacodynamic effects than insulin aspart. Diabetes Obes Metab 2015; 17: 682–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Andersen G, Alluis B, Meiffren G, Ranson A, Seroussi C, Gaudier M et al Ultra‐rapid BioChaperone insulin lispro (BC‐LIS): linear dose‐response and faster absorption than insulin lispro (LIS). Poster presented at the 52nd European Association for the Study of Diabetes annual meeting, 12–16 September 2016, Munich, Germany. ePoster #931. Available at www.easdvirtualmeeting.org/resources/ultra-rapid-biochaperone-insulin-lispro-bc-lis-linear-dose-response-and-faster-absorption-than-insulin-lispro-lis-3. Last accessed 25 January 2017.
- 27. Committee for Proprietary Medicinal Products (CPMP) The European Agency for the Evaluation of Medicinal Products (EMEA) London. Available at http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2015/03/WC500184161.pdf. Last accessed 18 June 2017.
- 28. de la Pena A, Seger M, Soon D. Bioequivalence and comparative pharmacodynamics of insulin lispro 200 U/mL relative to insulin lispro (Humalog®) 100 U/mL. Clin Pharmacol Drug Dev 2016; 5: 69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Homko C, Deluzio A, Jimenez C, Kolaczynski JW, Boden G. Comparison of insulin aspart and lispro: pharmacokinetic and metabolic effects. Diabetes Care 2003; 26: 2027–2031. [DOI] [PubMed] [Google Scholar]
- 30. Swan KL, Weinzimer SA, Dziura JD, Steil GM, Voskanyan GR, Steffen AT et al Effect of puberty on the pharmacodynamic and pharmacokinetic properties of insulin pump therapy in youth with type 1 diabetes. Diabetes Care 2008; 31: 44–46. [DOI] [PubMed] [Google Scholar]
- 31. Daenen S, Sola‐Gazagnes A, M'Bemba J. Peak‐time determination of post‐meal glucose excursions in insulin‐treated diabetic patients. Diabetes Metab 2010; 36: 165–169. [DOI] [PubMed] [Google Scholar]
- 32. Boyne MS, Silver DM, Kaplan J, Saudek CD. Timing of changes in interstitial and venous blood glucose measured with a continuous subcutaneous glucose sensor. Diabetes 2003; 52: 2790–2794. [DOI] [PubMed] [Google Scholar]
- 33. Cobry E, McFann K, Messer L, Gage V, VanderWel B, Horton L et al Timing of meal insulin boluses to achieve optimal postprandial glycemic control in patients with type 1 diabetes. Diabetes Technol Ther 2010; 12: 173–177. [DOI] [PubMed] [Google Scholar]
- 34. Luijf YM, van Bon AC, Hoekstra JB, Devries JH. Premeal injection of rapid‐acting insulin reduces postprandial glycemic excursions in type 1 diabetes. Diabetes Care 2010; 33: 2152–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Brunner GA, Hirschberger S, Sendlhofer G, Wutte A, Ellmerer M, Balent B, et al Post‐prandial administration of the insulin analogue insulin aspart in patients with type 1 diabetes mellitus. Diabet Med 2000; 17: 371–375. [DOI] [PubMed] [Google Scholar]
- 36. Schernthaner G, Wein W, Sandholzer K, Equiluz‐Bruck S, Bates PC, Birkett MA. Postprandial insulin lispro. A new therapeutic option for type 1 diabetic patients. Diabetes Care 1998; 21: 570–573. [DOI] [PubMed] [Google Scholar]
- 37. Schernthaner G, Wein W, Shnawa N, Bates PC, Birkett MA. Preprandial vs. postprandial insulin lispro‐a comparative crossover trial in patients with type 1 diabetes. Diabet Med 2004; 21: 279–284. [DOI] [PubMed] [Google Scholar]
- 38. Jovanovic L, Giammattei J, Acquistapace M, Bornstein K, Sommermann E, Pettitt DJ. Efficacy comparison between preprandial and postprandial insulin aspart administration with dose adjustment for unpredictable meal size. Clin Ther 2004; 26: 1492–1497. [DOI] [PubMed] [Google Scholar]
- 39. Bode BW, Johnson JA, Hyveled L, Tamer SC, Demissie M. Improved postprandial glycemic control with faster‐acting insulin aspart in patients with type 1 diabetes using continuous subcutaneous insulin infusion. Diabetes Technol Ther 2017; 19: 25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Simmons JH, Chen V, Miller KM, McGill JB, Bergenstal RM, Goland RS et al Differences in the management of type 1 diabetes among adults under excellent control compared with those under poor control in the T1D Exchange Clinic Registry. Diabetes Care 2013; 36: 3573–3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dover AR, Stimson RH, Zammitt NN. Flash glucose monitoring improves outcomes in a type 1 diabetes clinic. J Diabetes Sci Technol 2017; 11: 442–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gredal C, Rosenfalck A, Dejgaard A, Hilsted J. Optimal dose and timing of insulin aspart to mimic first phase insulin response in patients with recently onset type 2 diabetes. Diabetes Res Clin Pract 2008; 80: 293–298. [DOI] [PubMed] [Google Scholar]
- 43. Ratner R, Wynne A, Nakhle S, Brusco O, Vlajnic A, Rendell M. Influence of preprandial vs. postprandial insulin glulisine on weight and glycaemic control in patients initiating basal‐bolus regimen for type 2 diabetes: a multicenter, randomized, parallel, open‐label study. Diabetes Obes Metab 2011; 13: 1142–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Warren ML, Conway MJ, Klaff LJ, Rosenstock J, Allen E. Postprandial versus preprandial dosing of biphasic insulin aspart in elderly type 2 diabetes patients. Diabetes Res Clin Pract 2004; 66: 23–29. [DOI] [PubMed] [Google Scholar]
- 45. Wolpert HA, Atakov‐Castillo A, Smith SA, Steil GM. Dietary fat acutely increases glucose concentrations and insulin requirements in patients with type 1 diabetes: implications for carbohydrate‐based bolus dose calculation and intensive diabetes management. Diabetes Care 2013; 36: 810–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Smart CE, Evans M, O'Connell SM, McElduff P, Lopez PE, Jones TW et al Both dietary protein and fat increase postprandial glucose excursions in children with type 1 diabetes, and the effect is additive. Diabetes Care 2013; 36: 3897–3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Paterson MA, Smart CE, Lopez PE, McElduff P, Attia J, Morbey C et al Influence of pure protein on postprandial blood glucose levels in individuals with type 1 diabetes mellitus. Diabetes 2014; 63: A15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Shukla AP, Iliescu RG, Thomas CE, Aronne LJ. Food order has a significant impact on postprandial glucose and insulin levels. Diabetes Care 2015; 38: e98–e99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lafrance L, Rabasa‐Lhoret R, Poisson D, Ducros F, Chiasson JL. Effects of different glycaemic index foods and dietary fibre intake on glycaemic control in type 1 diabetic patients on intensive insulin therapy. Diabet Med 1998; 15: 972–978. [DOI] [PubMed] [Google Scholar]
- 50. James ML, Green L, Amiel SA, Choudhary P. Evaluation of the effect of carbohydrate intake on postprandial glucose in patients with type 1 diabetes treated with insulin pumps. J Diabetes Sci Technol 2016; 10: 1287–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Jones SM, Quarry JL, Caldwell‐McMillan M, Mauger DT, Gabbay RA. Optimal insulin pump dosing and postprandial glycemia following a pizza meal using the continuous glucose monitoring system. Diabetes Technol Ther 2005; 7: 233–240. [DOI] [PubMed] [Google Scholar]
- 52. Fraser R, Horowitz M, Maddox A. Hyperglycaemia slows gastric emptying in type I diabetes mellitus. Diabetologia 1990; 33: 675–680. [DOI] [PubMed] [Google Scholar]
- 53. Velehik MG, Reynolds JC. The effect of meal energy content on gastric emptying. J Nucl Med 1998; 30: 1106–1110. [PubMed] [Google Scholar]
- 54. Flint A, Raben A, Astrup A, Holst JJ. Glucagon‐like peptide 1 promotes satiety and suppresses energy intake in humans. J Clin Invest 1998; 101: 515–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sharma D, Morrison G, Joseph F. The role of continuous subcutaneous insulin infusion therapy in patients with diabetic gastroparesis. Diabetologia 2011; 54: 2768–2770. [DOI] [PubMed] [Google Scholar]
- 56. Antsiferov M, Woodworth JR, Mayyorov A. Lower within patient variability in postprandial glucose excursion with regular insulin. Diabetologia 1995; 38(Supp 1): 773. [Google Scholar]
- 57. Murphy HR, Elleri D, Allen JM, Harris J, Simmons D, Rayman G et al Pathophysiology of postprandial hyperglycaemia in women with type 1 diabetes during pregnancy. Diabetologia 2012; 55: 282–293. [DOI] [PubMed] [Google Scholar]
- 58. ter Braak EW, Woodworth JR, Bianchi R, Cerimele B, Erkelens DW, Thijssen JH et al Injection site effects on the pharmacokinetics and glucodynamics of insulin lispro and regular insulin. Diabetes Care 1996; 19: 1437–1440. [DOI] [PubMed] [Google Scholar]


