Abstract
Objectives
The purpose of this study was to assess the safety and performance of Stellarex Drug‐coated balloon (DCB).
Background
DCB coatings differ in excipients, paclitaxel dose, and coating morphologies. Due to these differences, a class effect with DCBs has not been demonstrated. Consequently, each DCB needs to be evaluated independently based on its own clinical study results.
Methods
The ILLUMENATE Global Study is a prospective, multicenter, single‐arm study. Patients with intermittent claudication or ischemic rest pain due to superficial femoral artery (SFA) and/or popliteal peripheral artery disease (PAD) were treated with the Stellarex DCB. The primary efficacy endpoint was primary patency, defined as freedom from restenosis with peak systolic velocity ratio ≤2.5 or clinically‐driven target lesion revascularization (CD‐TLR) at 12 months. The primary safety endpoint was freedom from device and procedure‐related death through 30 days postprocedure and freedom from target limb major amputation and CD‐TLR through 12 months.
Results
In total, 417 lesions were treated in 371 patients. The mean lesion length was 7.5 ± 5.3 cm, 40.8% of lesions were severely calcified per core laboratory fluoroscopy criteria and 31.3% were total occlusions. Primary patency by independent duplex core lab evaluation was 81.4% and the freedom from CD‐TLR was 94.8% day 365 per Kaplan‐Meier estimate. The majority of patients experienced improvements in their Rutherford classification (90.3%) and walking impairment questionnaire score (83.6%) at 12 months compared to baseline.
Conclusions
This study validated previous positive findings and confirms the strong safety profile and effectiveness outcomes.
Keywords: peripheral arterial disease, drug‐coated balloon, superficial femoral artery
1. INTRODUCTION
Endovascular treatment of symptomatic peripheral atherosclerotic disease (PAD) of the lower extremities is the preferred and recommended treatment of choice for many patients today 1, 2. Percutaneous transluminal angioplasty (PTA) is very effective in restoring blood flow but short‐term restenosis rate due to recoil and neointimal hyperplasia is high and documented to be around 50% at 1 year in recently published trials 3, 4. The restenosis rate is higher in occlusions and long lesions as well as in highly calcified lesions 5, 6. Stents have been used to prevent restenosis from elastic recoil, but do not prevent neointimal hyperplasia due to the mechanical interaction of the stent and the vessel wall 6, 7, 8. Multiple improvements in stent design were implemented to improve primary patency such as improved radial force, higher flexibility, and more resistance to stent fractures 9. Results with paclitaxel‐eluting stents in the superficial femoral artery (SFA) showed a significant improvement of primary patency as compared to bare metal stents 10, 11. However there is still significant controversy on their role especially in the popliteal artery 12.
Another approach in treating these lesions is the use of paclitaxel‐coated angioplasty balloons where an active antiproliferative drug is delivered to the vessel wall while performing the angioplasty 13. The absorption of an effective amount of drug into the arterial wall depends on the drug dose, coating morphology, and the excipient 14. Differences in drug‐coated balloon (DCB) construction can lead to differences in efficacy, primary patency rates and clinically driven target lesion revascularization (CD‐TLR) rates 3, 4. Promising patency rates and target lesion revascularization rates have already been documented with this technique without the need for a permanent implant 3, 4, 15. A limiting factor in using DCBs has been the amount of calcium in the vessel wall, due to the intrinsic limitations of an angioplasty balloon in severely calcified vessels and the potential barrier to drug absorption 16.
The Stellarex DCB (Spectranetics Corp., Colorado Springs, CO) has a low dose (2 µg/mm2) paclitaxel concentration in a hybrid‐crystalline formulation coupled with the excipient polyethylene glycol (PEG), designed to limit drug loss and facilitate effective drug tissue transfer. Initial studies on this DCB have revealed primary patency rates at 12 months of 89.5% in the First‐in‐Human trial 17, 89.0% in the European Randomized Trial 18, and 82.3% in the ILLUMENATE Pivotal Trial 19. The freedom from CD‐TLR rate was 90 to 95% in each study 17, 18, 19. The purpose of the ILLUMENATE Global Study was to expand on these findings.
2. METHODS
2.1. Study Design
The ILLUMENATE Global Study is an international, multicenter, single‐arm study designed to continue to assess the safety and performance of the Stellarex™ in patients with de novo or restenotic lesions in the SFA and/or popliteal arteries down to the trifurcation. The study received approval by the independent ethics committees. Patients provided written informed consent prior to enrollment. Eligible patients had symptomatic leg ischemia (Rutherford 2–4) with angiographic evidence of stenosis within the superficial femoral artery and/or popliteal artery down to the trifurcation, total lesion length ≤200 mm with a possibility of 1 or 2 target lesions, and reference vessel diameter 4 to 6 mm. Included patients were required to have at least one run‐off vessel patent to the foot. Key study inclusion and exclusion criteria are provided in Table 1. The trial was prospectively registered at ClinicalTrials.gov (NCT01927068).
Table 1.
Key inclusion and exclusion criteria
| Key inclusion criteria |
| Symptomatic leg ischemia requiring treatment of superficial femoral artery and/or popliteal artery up to the trifurcation |
| Age ≥18 yr |
| Life expectancy >1 yr |
| Rutherford‐Becker classification 2–4 |
| De novo or restenotic lesion stenosis within superficial femoral artery and/or popliteal artery |
| Lesion length ≤20 cm |
| Lesion treatable by no more than two devices |
| Successful wire crossing of lesion |
| Target reference vessel diameter 4–6 mm |
| Patent (<50% stenosis) inflow artery |
| At least one patent (<50% stenosis) tibio‐peroneal run‐off vessel to the foot |
| Key exclusion criteria |
| Female who is pregnant, lactating, or intends to become pregnant; male intending to father children during study |
| Contraindication to dual antiplatelet therapy |
| Hemorrhagic stroke within 3 mo |
| Planned vascular interventions within 14 days before or 30 days after the index procedure |
| Previous bypass graft to target limb |
| Chronic renal insufficiency (dialysis dependent, or serum creatinine >2.5 mg/dl) |
| Acute or subacute intraluminal thrombus in target vessel |
| Severe calcification that precludes adequate PTA treatment |
| In‐stent restenosis or restenosis of the target lesion following previous treatment with drug‐coated balloon |
| Use of adjunctive therapies (i.e. laser, atherectomy, cryoplasty, scoring/cutting balloon, brachytherapy) |
| Aneurysm in aorta, target vessel, iliac artery, or popliteal artery |
2.2. Procedures
Pretreatment evaluations included a clinical assessment, medical history, medication history, and assessment for study eligibility. Patients who met eligibility criteria were scheduled to undergo treatment with the DCB. Prior to the study procedure and for the duration of the study, subjects were required to receive acetylsalicylic acid (ASA) of at least 81 mg daily. According to the study protocol, patients were recommended to receive either clopidogrel (preferred medication), prasugrel, or ticlopidine per hospital standard of care preoperatively or should have received a loading dose of this product immediately postprocedure. It was recommended to continue dual antiplatelet therapy for a minimum of 4 weeks; however, the optimal duration for each subject was to be determined by the investigator. Following successful lesion crossing and predilatation with an uncoated balloon (with a diameter 1 mm less than vessel diameter), angiographic inclusion and exclusion criteria were evaluated (Table 1). If these criteria were met, patients were treated with the Stellarex DCB. No more than two DCBs were allowed to treat each lesion. In cases of residual stenosis >50% or flow‐limiting dissection (grade D or greater), postdilatation for at least 2 min using a commercially available uncoated balloon was required. Provisional stent implantation with a bare metal stent was allowed if postdilatation failed to resolve the residual stenosis or dissection. Patients returned for clinical visits at 1, 6, and 12 months and are scheduled to continue returning at 24 and 36 months. Each visit included clinical assessment, functional status, adverse event reporting WIQ score, EQ‐5D, and duplex ultrasound. Patients’ adverse events will be collected through 5 years.
2.3. Outcomes
The primary safety endpoints were freedom from device and procedure‐related death through 30 days and freedom from target limb major amputation and CD‐TLR through 12 months. The primary efficacy endpoint was primary patency through 12 months, defined as absence of target lesion restenosis (peak systolic velocity ratio ≤2.5) and freedom from CD‐TLR. Primary patency was evaluated on a per lesion basis. Technical success was defined as final in‐lesion residual diameter stenosis ≤50% (as determined by the angiographic core laboratory) without a device malfunction of the study device. Procedural success (per patient) was defined as lesion success without the occurrence of major adverse events during the procedure. Lesion success required final in‐lesion residual diameter stenosis ≤50% determined by the angiographic core laboratory. CD‐TLR was defined as any revascularization of a restenotic target lesion (peak systolic velocity ratio ≥2.5 by duplex ultrasound or percent diameter stenosis >50% by angiography) associated with a worsening in Rutherford class (increase ≥1 class) or ankle‐brachial index (ABI) (decrease greater than 0.15 units) that was referable to the target lesion. Additional outcomes included target limb major amputation, cardiovascular death, walking capacity using the Walking Impairment Questionnaire, and quality of life evaluated by the EQ‐5D. Data were regularly monitored and 100% source verified. Independent core laboratories analyzed all images including duplex ultrasonography (VasCore, Massachusetts General Hospital, Boston, MA) and angiography (BIDMC, Angiographic Core Laboratory, Boston, MA). An independent Clinical Events Committee adjudicated adverse events and an independent Data Safety and Monitoring Board monitored the study for safety.
2.4. Data Analysis
Outcomes are reported using the intent‐to‐treat population, which includes all patients who were enrolled, as opposed to the per protocol analysis which excluded patients where treatment deviated from the protocol, such as stented patients or those who were not predilated. Continuous data were reported as mean and standard deviation; categorical data were reported as frequencies and percentages. Longitudinal changes in clinical outcomes were assessed with paired t‐test. The Kaplan‐Meier method was used to evaluate time‐to‐event data through 12 months. Data were analyzed with SAS version 9.4. Statistical significance was set at P < 0.05 for all comparisons unless otherwise stated.
3. RESULTS
Between July 2013 and June 2015, 371 patients (417 lesions) were enrolled at 37 sites in Europe, Australia, and New Zealand (listed in the appendix). Patients were predominantly male (73.0%) with mean age 68 years. Most patients presented as Rutherford class 2 (33.4%) or 3 (57.7%) and 33.7% of patients had diabetes (Table 2). Lesion characteristics as assessed by the angiographic core laboratory, are presented in Table 3. The mean lesion length was 7.5 ± 5.3 cm, reference vessel diameter was 4.9 ± 0.8 mm, and 31% had total occlusions. Severe calcification (assessed and defined by the core lab as calcific radiopacities noted on both sides of the arterial wall and extending more than 1 cm of length prior to contrast injection or digital subtraction) was present in 40.8% of patients. A total of 341 patients (384 lesions) completed the 12‐month follow‐up visit. A patient accountability flowchart is provided in Figure 1.
Table 2.
Baseline patient characteristics.
| Variable | Value |
|---|---|
| Demographics | |
| Age (yr) | 68.2 ± 9.3 |
| Male | 73.0% (271/371) |
| Body mass index (kg/m2) | 27.0 ± 4.2 |
| Clinical presentation | |
| Rutherford Class | |
| 1 | 0.3% (1/371) |
| 2 | 33.4% (124/371) |
| 3 | 57.7% (214/371) |
| 4 | 6.2% (23/371) |
| 5 | 2.4% (9/371) |
| Ankle‐brachial index | 0.70 ± 0.20 |
| Medical historya | |
| Smoking history | 81.9% (304/371) |
| Hypertension | 79.5% (295/371) |
| Hyperlipidemia | 74.7% (277/371) |
| Diabetes mellitus | 33.7% (125/371) |
| Cerebrovascular disease | 17.3% (64/371) |
| Myocardial infarction | 17.3% (64/371) |
| Angina | 13.2% (49/371) |
| Chronic obstructive pulmonary disease | 13.2% (49/371) |
| Previous revascularization | |
| Lower limb | 42.3% (157/371) |
| Ipsilateral Limb | 26.4% (98/371) |
| Coronary | 33.4% (124/371) |
Data reported as mean ± standard deviation (n) or % (n/N).
Variables with frequency >10% reported.
Table 3.
Baseline lesion characteristics
| Variable | Value |
|---|---|
| No. treated lesions | |
| One | 87.6% |
| Two | 12.4% |
| Lesion typea | |
| De novo | 94.0% |
| Restenotic | 6.0% |
| Lesion location | |
| Proximal SFA | 11.1% |
| Mid SFA | 41.8% |
| Distal SFA | 33.2% |
| Proximal popliteal | 9.1% |
| Mid popliteal | 3.8% |
| Distal popliteal | 1.0% |
| Lesion length (mm) | 75.0 ± 52.7 |
| Reference vessel diameter (mm) | 4.9 ± 0.8 |
| Diameter stenosis (%) | 80.3 ± 17.4 |
| Total occlusion | 31.3% |
| Severe calcification | 40.8% |
| 0–1 Patent run‐off vessel | 31.1% |
Data reported as mean ± standard deviation (n) or % (n/N).
Per investigator assessment.
Abbreviations: SFA, superficial femoral artery.
Figure 1.
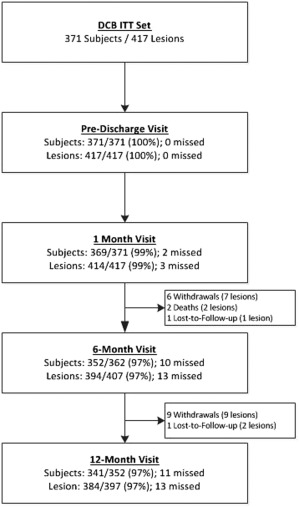
Patient flow diagram
Most (98%) patients underwent predilatation with an uncoated balloon before treatment with DCB. Three patients were screen failures due to the need for adjunctive therapies and 12 were excluded because of severe calcification that precluded adequate PTA treatment. Mean DCB inflation time was 3.4 min per lesion. Final in‐lesion residual diameter stenosis was 25 ± 12%. Acute success rates were 98% for lesion success, 96% for technical success, and 97% for procedural success (Table 4). A bail‐out stent was placed in 17% (72/417) of lesions. Of the stented lesions, 59.2% (42/71; one lesion unknown) were total occlusions and 40.3% (27/67; five lesions unknown) were severely calcified at baseline. A total of 81.9% of subjects were on aspirin every day through the 12‐month follow‐up visit and 76.5% of subjects received clopidogrel (or equivalent) for 28 days after treatment. In total, 65.8% of subjects were on the recommended dual antiplatelet therapy regimen.
Table 4.
Procedural data
| Variable | Value |
|---|---|
| Procedure time (min) | 61.0 ± 28.5 |
| Fluoroscopy time (min) | 10.4 ± 8.3 |
| Predilatation performed | 98.1% |
| Total inflation time (min) | 3.4 ± 1.8 |
| Flow‐limiting dissection | 0.3% |
| Postdilatation | 28.3% |
| Bail‐out stent placement | 17.3% |
| Diameter stenosis postprocedure | 24.7 ± 11.8 |
| Lesion success | 97.6% |
| Technical success | 95.8% |
| Procedural success | 97.3% |
Data reported as mean ± standard deviation or %.
3.1. Follow‐Up Data
The freedom from a primary safety endpoint event was 94.8% through 365 days (Figure 2A). There were 20 primary safety events in 19 patients including 19 CD‐TLRs, 1 major amputation, and no 30‐day device‐ or procedure‐related death. The major amputation occurred in a patient who was classified with Rutherford class 5 at baseline (a protocol deviation) and underwent multiple interventions of the popliteal target lesion before amputation. The primary effectiveness endpoint, primary patency, was met in 77.2% (285 of 369) of lesions through the 12‐month follow‐up interval (day 395). Patency failures included 22 CD‐TLRs, 55 lesions that were restenosed per Duplex ultrasound assessment but did not result in a CD‐TLR), and 7 missing 12‐month data where restenosis was identified at an earlier follow‐up interval. The Kaplan‐Meier estimate of primary patency was 81.4% at 365 days (Figure 2B). The Kaplan‐Meier estimate for freedom from CD‐TLR through day 365 is 94.8% as shown in Figure 2C. In patients with an ABI <0.9 at baseline and with data at 12‐month follow‐up, ABI increased from 0.64 ± 0.15 to 0.90 ± 0.20 (P ≤ 0.001). Rutherford classification at 12 months decreased by at least one category compared to baseline in 90.3% of patients (Table 5). Scores on the Walking Impairment Questionnaire increased from 32 ± 23 at baseline to 61 ± 30 (P < 0.001) and health utility scores on the EQ‐5D questionnaire significantly improved from 0.67 ± 0.19 to 0.80 ± 0.21 (P < 0.001), amongst those with data at 12 months.
Figure 2.
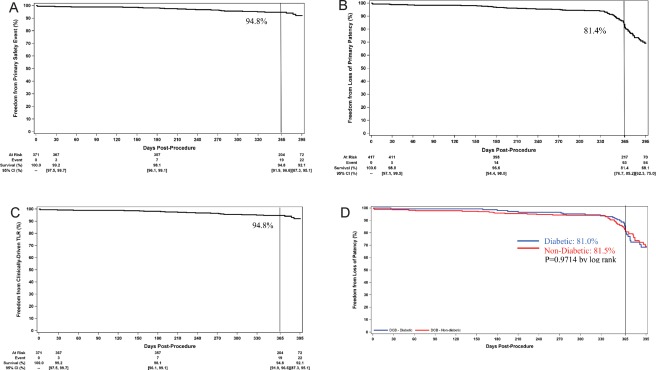
(A) Freedom from a primary safety event through 1 year: the freedom from a primary safety event at day 365 was 94.8% and 92.1% at the end of the follow‐up window (day 395). (B) Freedom from loss of primary patency through 1 year: the primary patency rate at day 365 was 81.4% and 69.1% at the end of the follow‐up window (day 395). (C) Freedom from CD‐TLR through 1 year: freedom from CD‐TLR at day 365 was 94.8% and 92.1% the end of the follow‐up window (day 395). (D) Freedom from loss of primary patency rates of patients with diabetes and patients without diabetes: primary patency rates of patients with diabetes (blue line) was not different than patients without diabetes (red line) (day 365 estimates: 81.0% vs. 81.5%, P = 0.9717 by log rank). Rates at the end of the follow‐up window should be interpreted with caution as the number of patients at risk is low due to subjects being censored prior to day 395, at the time of the 12‐month visit [Color figure can be viewed at wileyonlinelibrary.com]
Table 5.
Key 12‐month outcomes
| Assessment | 12‐Mo result |
|---|---|
| Primary patencya | 81.4% |
| Freedom from primary safety eventa | 94.8% |
| All‐cause mortalityb | 0.6% (2/355) |
| CD‐TLRb | 6.2% (22/354) |
| Improvement in Rutherford classificationc | 90.3% (306/339) |
| Change from baseline | −2.1 ± 1.1 (339) |
| Improvement in composite WIQ scorec | 83.6% (265/317) |
| Change from baseline | 28.7 ± 29.3 (317) |
| Improvement in EQ 5‐D indexc | 66.8% (219/328) |
| Change from baseline | 0.14 ± 0.22 (328) |
KM estimate.
Through day 395, the denominator includes patients with an event or those without an event having follow‐up on or past the opening of the follow‐up window.
Score at 12 months compared to baseline.
Abbreviations: CD‐TLR, clinically‐driven target lesion revascularization; WIQ, walking impairment questionnaire.
The data were stratified by the diabetic status. Patients with diabetes were more likely to be obese (35.5% vs. 16.0%, P < 0.001), have had a previous coronary revascularization (47.2% vs. 26.4%, P < 0.001) and had more severely calcified lesions (50.3% vs. 35.5%, P = 0.004). Other key lesion characteristics in diabetics versus nondiabetics were: mean lesion length: 7.1 cm vs. 7.7 cm (P = 0.230), CTO: 22.4% vs. 36.2% (P = 0.004), and restenotic (10.2% vs. 3.7%, P = 0.008). The primary patency rate per Kaplan‐Meier estimate at day 365 in patients with diabetes was 81.0% vs. 81.5% in patients without diabetes (log‐rank P value = 0.9714) (Figure 2D). The rates of freedom from CD‐TLR at day 365 were 95.1% and 90.3%, respectively (P value by log‐rank = 0.4738).
The primary patency rate at day 365 per Kaplan‐Meier estimate was higher in males than females (84.5% vs.72.8%, log‐rank P value = 0.015). The rate of freedom from CD‐TLR was 96.2% in males and 90.7% in females at day 365 (P value by log‐rank = 0. 0370). Baseline characteristics were similar between genders including a mean lesion length was 7.5 and 7.4 cm and total occlusion was present in 29.8% and 35.4%, in males and females, respectively. As expected, the reference vessel diameter, was larger in males (5.01 mm vs. 4.52 mm, P < 0.001). Procedural characteristics were similar, with the exception of a higher stenting rate in males (21.0% vs. 15.0%, P = 0.192) while the occurrence of geographic miss was lower in males (11.6% vs. 18.4%, P = 0.067). Geographic miss was defined by the angiographic core lab as segments proximal or distal to the treated lesion that were subjected to injury during pre and/or postdilatation but were not covered by study device.
In total, 72 lesions in 72 patients were stented. The majority were in subjects with a single treated lesion (n = 60) while twelve subjects had an additional treated lesion that was not stented. The baseline demographics of the subjects with a stented lesion were similar to the entire cohort. Of the stented lesions, the mean lesion length was 8.5 cm, 59.2% were CTOs, and 40.3% were severely calcified. Reasons for bail‐out stenting included 36 residual stenoses >50% only, 12 dissections only, and 24 both residual stenosis and dissection. However, not all of these lesions met the protocol‐required degree of residual stenosis or grade of dissection (grade D or greater) for stenting based on the site‐reported data. The angiographic core laboratory data were reviewed for the subjects with stented lesions. According to the core laboratory data 39 (9.4%) lesions met the protocol requirements for stenting. Per Kaplan‐Meier (KM) estimate at day 365, the freedom from CD‐TLR rate was 88.6% and the primary patency rate was 78.3% for stented patients.
4. DISCUSSION
The ILLUMENATE Global Study builds on the growing body of evidence with the Stellarex DCB. The primary patency rate observed in this study was 81.4%, consistent with the 1 year primary patency rates in the ILLUMENATE FIH Study (89.5%), ILLUMENATE Pivotal Study (82.3%), and the ILLUMENATE EU RCT (89.0%) 17, 18, 19. The primary patency rate in the DCB arms of IN.PACT SFA at 12 months was 86.6% 20 and 73.5% in LEVANT 2 [3]. The CD‐TLR rate at 1 year in the ILLUMENATE Global Study was 6.2%, also consistent with results observed in ILLUMENATE Pivotal (7.9%) 19 and ILLUMENATE EU RCT (5.9%) 21. This outcome is also comparable to 4.6% in IN.PACT SFA 22 and half the rate observed in the LEVANT 2 Study (12.3%) 3.
This study enrolled a particularly high number of CTOs (31.3%) and the high proportion of lesions that were located within the distal femoropopliteal region; 47.1% of lesions were located in the distal SFA and popliteal artery, including 28 (13.9%) of which were located entirely within the popliteal artery. Popliteal involvement has been shown to have a significantly lower patency rate than lesions located solely in the SFA 23.
The periprocedural stent rate was 17.3%, higher than in IN.PACT SFA (7.3%) and LEVANT 2 (2.5%). ILLUMENATE Pivotal reported a stent rate of 6.0% and in ILLUMENATE EU RCT it was 15.4%. Periprocedural stent rates are inconsistent over different studies without a definitive explanation other than investigator dependency. Assessment of angiographic core lab data within this study indicted 9.4% of lesions treated met the predefined criteria for bail‐out stenting. Therefore, the stenting rate does not appear to be related to the performance of the study device, but rather due to investigator treatment practices.
Notably, the primary patency in patients with diabetes was similar to those without diabetes. The patency rates were 81.0% and 81.5%, respectively (P = 0.9714 by log rank). These outcomes are consistent with the data observed in the ILLUMENATE EU RCT Trial 21. Previous reports have shown diabetes to increase the risk of restenosis in patients undergoing endovascular treatment with PTA and/stenting or DCB 23, 24.
The primary patency rate in males was significantly higher than females (84.5% vs. 72.8%, log‐rank P value is 0.015). This difference between groups is inconsistent with findings from the two previous trials on the same DCB. In the ILLUMENATE Pivotal Study, a significant treatment effect was observed in both females and males after use of the Stellarex DCB as compared to PTA with no significant difference in primary patency between the genders (80.9% in males vs. 84.1% in females, log rank P value = 0.4851). Likewise, there was no statistical difference in the primary patency rates observed in the ILLUMENATE EU RCT Study when stratified by gender (90.4% in males and 85.3% in females, P = 0.3064) 21. The only differences in vascular characteristics we could identify was a larger reference vessel diameter in males (5.01± 0.86 vs. 4.52 ± 0.69, P < 0.001). Lesion length and number of CTOs were similar in males and females. We know vessel diameter and lesion length are directly related to vessel patency after treatment 25. The angiographic core lab reported geographic miss occurring in females in 18.4% of cases versus 11.6% in males P = 0.067, also a predictor of primary patency failure 26. While inconsistent with previous trials on this DCB, these findings are not unusual. Female gender was an independent predictor of restenosis in a retrospective analysis of 260 patients treated with the IN.PACT Admiral or Pacific DCBs 23. Within the LEVANT 2 study the female cohort had a higher patency rate when treated with an uncoated PTA catheter than with the Lutonix DCB. More investigational work needs to be done to understand the impact of gender on outcomes with DCB treatment in these arteries.
DCB performance and pharmacokinetic profiles differ because of differences in the balloon platform, excipients, drug concentrations, coating morphology, drug solubility, and coating methods 14, 27, 28. A class effect for DCBs has not been shown, therefore each DCB has to be evaluated and outcomes from one cannot be generalized to another. The Stellarex DCB utilizes a low dose of paclitaxel (2 µg/mm2) in a hybrid formulation made of both amorphous and crystalline constituents combined with a polyethylene glycol (PEG) excipient. The hybrid formulation helps maintain coating integrity compared to pure crystalline while allowing for sustained drug tissue release 14.
Limitations of this study include the trial design being nonblinded and single‐arm. Additionally, adjunctive devices such as atherectomy were not assessed and outcomes cannot be generalized beyond lesions that can be successfully predilated with PTA. Longer term follow‐up data will be critical to understand the durability of this treatment.
5. CONCLUSIONS
The ILLUMENATE Global Study is a large prospective, multicenter, single‐arm study using the Stellarex DCB in SFA and/or popliteal arteries down to the trifurcation. The results reported for this study are consistent with the previously reported data with this DCB. The data confirm the high primary patency rate and strong safety profile of the Stellarex DCB.
CONFLICT OF INTEREST
Herman Schroe has received research grants, consulting fees or speaking honoraria from Abbott, Bard, Boston Scientific, Biotronik, Medtronic, Spectranetics, WL Gore. He has no financial association or stock ownership with these companies. Frank Vermassen has received research grants from Medtronic, Spectranetics, Bard and Boston Scientific. He has received modest speaking honoraria from Medtronic, Spectranetics, Bard and Boston Scientific. He has also acted as an “expert witness” for Medtronic and Bard. Antonio Micari is a consultant for Medtronic, Bard, Boston Scientific and Terumo. Wulf Ito and Erwin Blessing have received speaking honoraria from Spectranetics. Thomas Zeller has received honoraria from: Abbott Vascular, Bard Peripheral Vascular, Veryan, Biotronik, Boston Scientific Corp., Cook Medical, Cordis Corp., Gore & Associates, Medtronic, Spectranetics, Straub Medical, TriReme, VIVA Physicians, GLG, and Philips. He has consulted for: Abbott Vascular, Bard Peripheral Vascular, Boston Scientific Corp., Cook Medical, Gore & Associates, Medtronic, and Spectranetics. He has recived research, clinical trial, or drug study funds from: 480 biomedical, Bard Peripheral Vascular, Veryan, Biotronik, Cook Medical, Gore & Associates, Abbott Vascular, Medtronic, Spectranetics, Terumo, TriReme, Philips, Intact Vascular, Caveo Med, Innora, CSI, Bayer Pharma, Mercator, B. Braun, Contego Medical, Pluristem, Shockwave. He is a stock holder of Veryan and QT Medical. Andrew Holden is a Clinical Investigator for Spectranetics, Endologix, Boston Scientific, Medtronic, Cook Medical, Arsenal Medical, Lombard Medical, Bard, Cagent Vacular, Elixir Medical, Gore Medical, Shockwave Medical and Siemens Medical. He has no financial association or stock ownership with these companies. Michael Jaff is a non‐compensated advisor to Abbott Vascular, Boston Scientific, Cordis and Medtronic. He has equity investments in Access Closure, Emblitech, Janacare, MC10, Northwind Medical, PQ Bypass, Primacia, Sano V and Vascular Therapies. He is also a Board Member of VIVA Physicians, a 501 c 3 not‐for‐profit education and research organization. He is a consultant for AOPA, Micell, Primacea, Valian and Volcano/Philips. Yann Goueffic has recived research grants, consulting fees or speaking honoraria from: Abbott, Bard, Boston Scientific, Cook, Hexacath, Medino, Medtronic, Perouse, Spectranetics, Terumo and WL Gore. Shirley Jansen, Koen Keirse and Patrick Peeters have no disclosures.
Supporting information
Additional Supporting Information may be found online in the supporting information tab for this article.
Supporting Information
ACKNOWLEDGMENTS
The authors thank Meghan Schadow and Larry Miller for medical writing assistance and Teresa Yurik and Sarah Verdoliva for statistical support. Thank you to Sejal Raval for study management and all of the investigators and clinical staff for their hard work and dedication to this project.
Schroë, H , Holden AH, Goueffic Y, et al. Stellarex drug‐coated balloon for treatment of femoropopliteal arterial disease—The ILLUMENATE Global Study: 12‐Month results from a prospective, multicenter, single‐arm study. Catheter Cardiovasc Interv. 2018;91:497–504. https://doi.org/10.1002/ccd.27348
Funding information This study was sponsored by the Spectranetics Corp., Colorado Springs, CO.
Clinical Trial Registrations: This study was prospectively registered at ClinicalTrials.gov; NCT01927068.
Review all the Editor's Choice articles online at: http://onlinelibrary.wiley.com/journal/10.1002/(ISSN)1522-726X/homepage/cci_editor_s_choice_papers_and_videos.htm
REFERENCES
- 1. Gerhard‐Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: Executive summary. A report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. J Am Coll Cardiol 2017;69:1465–1508. [DOI] [PubMed] [Google Scholar]
- 2. European Stroke O, Tendera M, Aboyans V, et al. ESC Guidelines on the diagnosis and treatment of peripheral artery diseases: Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries: The Task Force on the Diagnosis and Treatment of Peripheral Artery Diseases of the European Society of Cardiology (ESC). Eur Heart J 2011;32:2851–2906. [DOI] [PubMed] [Google Scholar]
- 3. Rosenfield K, Jaff MR, White CJ, et al. Trial of a paclitaxel‐coated balloon for femoropopliteal artery disease. N Engl J Med 2015;373:145–153. [DOI] [PubMed] [Google Scholar]
- 4. Tepe G, Laird J, Schneider P, et al. Drug‐coated balloon versus standard percutaneous transluminal angioplasty for the treatment of superficial femoral and popliteal peripheral artery disease: 12‐Month results from the IN.PACT SFA randomized trial. Circulation 2015;131:495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Armstrong EJ, Saeed H, Alvandi B, et al. Nitinol self‐expanding stents vs. balloon angioplasty for very long femoropopliteal lesions. J Endovasc Ther 2014;21:34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schillinger M, Sabeti S, Loewe C, et al. Balloon angioplasty versus implantation of nitinol stents in the superficial femoral artery. N Engl J Med 2006;354:1879–1888. [DOI] [PubMed] [Google Scholar]
- 7. Laird JR, Katzen BT, Scheinert D, et al. Nitinol stent implantation vs. balloon angioplasty for lesions in the superficial femoral and proximal popliteal arteries of patients with claudication: Three‐year follow‐up from the RESILIENT randomized trial. J Endovasc Ther 2012;19:1–9. [DOI] [PubMed] [Google Scholar]
- 8. Matsumura JS, Yamanouchi D, Goldstein JA, et al. The United States StuDy for EvalUating EndovasculaR TreAtments of Lesions in the Superficial Femoral Artery and Proximal Popliteal By usIng the Protege EverfLex NitInol STent SYstem II (DURABILITY II). J Vasc Surg 2013;58:73–83, e71. [DOI] [PubMed] [Google Scholar]
- 9. Garcia L, Jaff MR, Metzger C, et al. Wire‐interwoven nitinol stent outcome in the superficial femoral and proximal popliteal arteries: Twelve‐month results of the SUPERB trial. Circ Cardiovasc Interv 2015;8:e000937. [DOI] [PubMed] [Google Scholar]
- 10. Dake MD, Ansel GM, Jaff MR, et al. Sustained safety and effectiveness of paclitaxel‐eluting stents for femoropopliteal lesions: 2‐Year follow‐up from the Zilver PTX randomized and single‐arm clinical studies. J Am Coll Cardiol 2013;61:2417–2427. [DOI] [PubMed] [Google Scholar]
- 11. Dake MD, Ansel GM, Jaff MR, et al. Paclitaxel‐eluting stents show superiority to balloon angioplasty and bare metal stents in femoropopliteal disease: Twelve‐month Zilver PTX randomized study results. Circ Cardiovasc Interv 2011;4:495–504. [DOI] [PubMed] [Google Scholar]
- 12. Kroger K, Santosa F, Goyen M. Biomechanical incompatibility of popliteal stent placement. J Endovasc Ther 2004;11:686–694. [DOI] [PubMed] [Google Scholar]
- 13. Speck U, Stolzenburg N, Peters D, Scheller B. How does a drug‐coated balloon work? Overview of coating techniques and their impact. J Cardiovasc Surg 2016;57:3–11. [PubMed] [Google Scholar]
- 14. Granada JF, Stenoien M, Buszman PP, et al. Mechanisms of tissue uptake and retention of paclitaxel‐coated balloons: impact on neointimal proliferation and healing. Open Heart 2014;1:e000117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Scheinert D, Schulte KL, Zeller T, Lammer J, Tepe G. Paclitaxel‐releasing balloon in femoropopliteal lesions using a BTHC excipient: Twelve‐month results from the BIOLUX P‐I randomized trial. J Endovasc Ther 2015;22:14–21. [DOI] [PubMed] [Google Scholar]
- 16. Fanelli F, Cannavale A, Gazzetti M, et al. Calcium burden assessment and impact on drug‐eluting balloons in peripheral arterial disease. Cardiovasc Interv Radiol 2014;37:898–907. [DOI] [PubMed] [Google Scholar]
- 17. Schroeder H, Meyer DR, Lux B, Ruecker F, Martorana M, Duda S. Two‐year results of a low‐dose drug‐coated balloon for revascularization of the femoropopliteal artery: Outcomes from the ILLUMENATE first‐in‐human study. Catheter Cardiovasc Interv 2015;86:278–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schroeder H, Werner M, Meyer DR, et al. Low‐dose paclitaxel‐coated versus uncoated percutaneous transluminal balloon angioplasty for femoropopliteal peripheral artery disease: One‐year results of the ILLUMENATE European randomized clinical trial (randomized trial of a novel paclitaxel‐coated percutaneous angioplasty balloon). Circulation 2017;135:2227–2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Krishnan P, Faries P, Niazi K, et al. Stellarex drug‐coated balloon for treatment of femoropopliteal disease: 12‐Month outcomes from the randomized ILLUMENATE pivotal and pharmacokinetic studies. Circulation 2017. https://doi.org/10.1161/CIRCULATIONAHA.117.028893. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jaff MR. Drug‐Coated Balloon Treatment for Patients with Intermittent Claudication: INsights from the IN.PACT Global Full Clinical Cohort. VIVA: Las Vegas, NV; 2016.
- 21. Schroeder H, Werner M, Meyer DR, et al. Low‐dose paclitaxel‐coated versus uncoated percutaneous transluminal balloon angioplasty for femoropopliteal peripheral artery disease: 1‐Year results of the ILLUMENATE European randomized clinical trial. Circulation 2017;135:2227–2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.French National Commission of Medical Device Evaluation on IN.PACT SFA. Available at: http://www.has-sante.fr/portail/jcms/c_2635037/fr/in-pact-admiral. 2016.
- 23. Schmidt A, Piorkowski M, Gorner H, et al. Drug‐coated balloons for complex femoropopliteal lesions: 2‐Year results of a real‐world registry. JACC Cardiovasc Interv 2016;9:715–724. [DOI] [PubMed] [Google Scholar]
- 24. Paraskevas KI, Baker DM, Pompella A, Mikhailidis DP. Does diabetes mellitus play a role in restenosis and patency rates following lower extremity peripheral arterial revascularization? A critical overview. Ann Vasc Surg 2008;22:481–491. [DOI] [PubMed] [Google Scholar]
- 25. Laird JR, Schneider PA, Tepe G, et al. Durability of Treatment Effect Using a Drug‐Coated Balloon for Femoropopliteal Lesions: 24‐Month Results of IN.PACT SFA. J Am Coll Cardiol 2015;66:2329–2338. [DOI] [PubMed] [Google Scholar]
- 26. Scheinert D, Duda S, Zeller T, et al. The LEVANT I (Lutonix paclitaxel‐coated balloon for the prevention of femoropopliteal restenosis) trial for femoropopliteal revascularization: First‐in‐human randomized trial of low‐dose drug‐coated balloon versus uncoated balloon angioplasty. JACC Cardiovasc Interv 2014;7:10–19. [DOI] [PubMed] [Google Scholar]
- 27. Cortese B, Granada JF, Scheller B, et al. Drug‐coated balloon treatment for lower extremity vascular disease intervention: An international positioning document. Eur Heart J 2016;37:1096–1103. [DOI] [PubMed] [Google Scholar]
- 28. Radke PW, Joner M, Joost A, et al. Vascular effects of paclitaxel following drug‐eluting balloon angioplasty in a porcine coronary model: The importance of excipients. EuroIntervention: 2011;7:730–737. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found online in the supporting information tab for this article.
Supporting Information


