ABSTRACT
Objective
A high ratio of soluble fms‐like tyrosine kinase‐1 (sFlt‐1) to placental growth factor (PlGF) has been linked to pre‐eclampsia (PE). We evaluated the sFlt‐1/PlGF ratio as a predictive marker for early‐onset PE in women at risk of PE.
Methods
This prospective, Spanish, multicenter study included pregnant women with a risk factor for PE, including intrauterine growth restriction, PE, eclampsia or hemolysis, elevated liver enzymes and low platelet count syndrome in previous pregnancy, pregestational diabetes or abnormal uterine artery Doppler. The primary objective was to show that the sFlt‐1/PlGF ratio at 20, 24 and 28 weeks' gestation was predictive of early‐onset PE (< 34 + 0 weeks). Serum sFlt‐1 and PlGF were measured at 20, 24 and 28 weeks. Multivariate logistic regression was used to develop a predictive model.
Results
A total of 819 women were enrolled, of which 729 were suitable for analysis. Of these, 78 (10.7%) women developed PE (24 early onset and 54 late onset). Median sFlt‐1/PlGF ratio at 20, 24 and 28 weeks was 6.3 (interquartile range (IQR), 4.1–9.3), 4.0 (IQR, 2.6–6.3) and 3.3 (IQR, 2.0–5.9), respectively, for women who did not develop PE (controls); 14.5 (IQR, 5.5–43.7), 18.4 (IQR, 8.2–57.9) and 51.9 (IQR, 11.5–145.6) for women with early‐onset PE; and 6.7 (IQR, 4.6–9.9), 4.7 (IQR, 2.8–7.2) and 6.0 (IQR, 3.8–10.5) for women with late‐onset PE. Compared with early‐onset PE, the sFlt‐1/PlGF ratio was significantly lower in controls (P < 0.001 at each timepoint) and in women with chronic hypertension (P < 0.001 at each timepoint), gestational hypertension (P < 0.001 at each timepoint) and late‐onset PE (P < 0.001 at each timepoint). A prediction model for early‐onset PE was developed, which included the sFlt‐1/PlGF ratio plus mean arterial pressure, being parous and previous PE, with areas under the receiver–operating characteristics curves of 0.86 (95% CI, 0.77–0.95), 0.91 (95% CI, 0.85–0.97) and 0.93 (95% CI, 0.86–0.99) at 20, 24 and 28 weeks, respectively, and was superior to models using the sFlt‐1/PlGF ratio alone or uterine artery mean pulsatility index.
Conclusions
The sFlt‐1/PlGF ratio can improve prediction of early‐onset PE for women at risk of this condition. Copyright © 2017 ISUOG. Published by John Wiley & Sons Ltd.
Keywords: biomarker, early‐onset prediction, hypertension, PlGF, pre‐eclampsia, sFlt‐1, sFlt‐1/PlGF ratio
INTRODUCTION
Pre‐eclampsia (PE) affects 2–5% of pregnancies1, 2, 3, 4, 5 and can result in intrauterine growth restriction (IUGR), renal or hepatic impairment, HELLP syndrome (hemolysis, elevated liver enzyme levels and low platelet count), eclampsia, and maternal and fetal mortality5, 6, 7. Early and late manifestations of PE differ in time of onset of symptoms, relative frequency, placental morphology, genetic risk and risk of adverse outcomes8, 9, 10, 11, 12, 13. Early‐onset PE is associated with a higher incidence of adverse perinatal outcomes, including oligohydramnios, Apgar score < 7, stillbirth and early neonatal death, compared with late‐onset PE14, 15. As early intervention is important to improve maternal and fetal outcomes16 and the classical clinical markers of PE (hypertension and proteinuria) are poorly predictive of those who will develop the condition, markers of angiogenesis have been examined as aids to PE prediction.
A key feature of PE is placental insufficiency. Dysregulation of pro‐ and antiangiogenic factors is thought to be causally linked to the condition7, 17, 18; before and during PE, maternal serum concentrations of antiangiogenic soluble fms‐like tyrosine kinase‐1 (sFlt‐1) are increased and levels of proangiogenic placental growth factor (PlGF) are decreased19, 20. A high sFlt‐1/PlGF ratio has been linked with PE and demonstrated before clinical onset of the condition, and differences in sFlt‐1 and PlGF have been observed between early‐ and late‐onset PE21, 22, 23, 24, 25, 26, 27, 28, 29. The Elecsys® immunoassay sFlt‐1/PlGF ratio is CE‐IVD (Conformité Européenne–In Vitro Diagnostics) approved as a diagnostic aid for PE with gestational age‐specific cut‐off values, and as an aid in short‐term prediction of PE in women with suspected PE26, 30, 31. The Prediction of Short‐Term Outcome in Pregnant Women with Suspected PE Study (PROGNOSIS) developed a cut‐off‐based PE prediction model. Optimum sFlt‐1/PlGF ratio cut‐off levels of ≤ 38 and > 38 were identified to rule out and rule in, respectively, PE, in women with singleton pregnancy at 24 + 0 to 36 + 6 weeks' gestation32. However, the predictive value of the sFlt‐1/PlGF ratio has not been examined specifically for early‐onset PE.
This study, the Study of Early Pre‐eclampsia in Spain (STEPS), aimed to evaluate the sFlt‐1/PlGF ratio at 20, 24 and 28 weeks as a predictive marker for early‐onset PE in women at risk of PE.
METHODS
Study design and participants
STEPS was a prospective, double‐blind, multicenter (10 study sites in Spain) study, performed between October 2010 and March 2013, and enrolled pregnant women at risk of PE. To be considered at risk of PE, women had to meet one of the following inclusion criteria: PE, eclampsia, HELLP syndrome or IUGR in a previous pregnancy; pre‐existing chronic hypertension without proteinuria; gestational hypertension (new‐onset hypertension in pregnancy); pre‐existing renal disease (kidney transplantation or creatinine clearance < 60 mL/min); pre‐existing diabetes mellitus Type I (insulin dependent); mean uterine artery Doppler pulsatility index (UtA‐PI) > 1.45 (at 19–20 weeks); thrombophilia (antiphospholipid syndrome, protein C deficiency, protein S deficiency, antithrombin deficiency, factor V Leiden mutation); multiple pregnancy; age ≥ 40 years and conceived with assisted reproductive technologies (ART). Women with two or more of the following risk factors were also included: nulliparity; body mass index ≥ 35 kg/m2; diastolic blood pressure > 80 mmHg at study inclusion; age ≥ 40 years; and family history (mother or sister) of PE, eclampsia or HELLP syndrome. Women were excluded if they were both hypertensive and had proteinuria or if major fetal malformations/chromosome disorders were observed.
Women provided informed, signed consent. The protocol was approved by applicable national/regional independent ethics committees and institutional review boards (Table S1). The study adhered to the Guidelines for Good Clinical Practice.
The primary objective of the study was to demonstrate that the sFlt‐1/PlGF ratio was a predictive marker for early‐onset PE. Secondary objectives included evaluation of sFlt‐1/PlGF ratio as a predictor of late‐onset PE and the use of the sFlt‐1/PlGF ratio for differentiation of hypertension from PE.
Diagnostic criteria
For consistency, investigators used predefined diagnostic criteria (Table 1) based on the Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy33. PE was defined as newly occurring hypertension (systolic blood pressure ≥ 140 mmHg and/or diastolic blood pressure ≥ 90 mmHg) with newly occurring proteinuria after 20 weeks. To be considered early onset, PE had to occur before 34 + 0 weeks.
Table 1.
Diagnostic criteria in Study of Early Pre‐eclampsia in Spain (STEPS)
| Diagnosis | Criteria |
|---|---|
| Hypertension | Systolic blood pressure ≥ 140 mmHg and/or diastolic blood pressure ≥ 90 mmHg (on two occasions at least 6 h apart) |
| Chronic hypertension | Hypertension (systolic blood pressure ≥ 140 mmHg and/or diastolic blood pressure ≥ 90 mmHg) diagnosed before pregnancy or in first half of pregnancy (< 20 weeks) and continued for > 12 weeks after delivery |
| Proteinuria | For determination of urinary protein using test strips, a value of 1+ was not considered reliable for diagnosis of PE. Data were reconfirmed with protein test on 24‐h urine (≥ 0.3 g protein/24 h); in an emergency, if it was not possible to determine protein in 24‐h urine, protein determination was carried out on isolated urine sample (≥ 30 mg protein/dL or protein/creatinine ratio ≥ 30 mg protein/mmol creatinine) |
| Gestational hypertension | New‐onset hypertension (systolic blood pressure ≥ 140 mmHg and/or diastolic blood pressure ≥ 90 mmHg) after 20 weeks of pregnancy, which resolved by 12 weeks postpartum |
| PE | New‐onset hypertension (systolic blood pressure ≥ 140 mmHg and/or diastolic blood pressure ≥ 90 mmHg) and new‐onset proteinuria after 20 weeks of pregnancy |
| Severe PE | PE plus one or more of the following: systolic blood pressure ≥ 160 mmHg and/or diastolic blood pressure ≥ 110 mmHg (on two occasions at least 6 h apart); proteinuria (>5 g protein/24 h or test strip ≥ 3+ in two urine samples collected at random at least 4 h apart); impairment of renal function (serum creatinine ≥ 1.2 mg/dL unless known to be elevated previously or oliguria < 500 mL/24 h); pulmonary edema; impairment of hepatic function (elevated liver enzymes, epigastric pain or right upper quadrant pain caused by distension of Glisson's capsule); neurological symptoms (cerebral or visual disturbances, severe headache); hematological disturbances (thrombocytopenia, hemolysis); IUGR |
| Eclampsia | New‐onset tonic–clonic convulsions in women with PE, not attributable to any other cause |
| Early‐ and late‐onset PE | Early onset: PE developing < 34 + 0 weeks; late onset: PE developing ≥ 34 + 0 weeks |
| HELLP syndrome | Increased ASAT (> 70 IU/L); decreased platelet count (< 100 000/μL); increased LDH (> 600 IU/L) |
| IUGR | Estimated fetal weight or abdominal circumference < 10th percentile (adjusted for gender/race in accordance with tables normally used by study center). Presence of pathological process that inhibits expression of normal intrinsic growth potential. Pathological process must be demonstrated at least once after 22 weeks, according to either oligohydramnios (amniotic fluid index < 10th percentile) or pathological flow in umbilical artery (pulsatility index > 95th percentile) |
| SGA neonate | Estimated fetal weight or abdominal circumference < 10th percentile (adjusted for gender/race in accordance with tables normally used by study center); no pathological process |
| Preterm birth | Delivery before end of 37 weeks (e.g. gestational age of 36 + 6 weeks would be recorded as 36 completed weeks of pregnancy and baby would be defined as preterm) |
ASAT, aspartate aminotransferase; HELLP, hemolysis, elevated liver enzymes and low platelet count; IUGR, intrauterine growth restriction; LDH, lactate dehydrogenase; PE, pre‐eclampsia; SGA, small‐for‐gestational‐age.
Data collection and visits
At gestational weeks 19–20 (Visit 1), 23–24 (Visit 2) and 27–28 (Visit 3), participants underwent a blood test to determine the sFlt‐1/PlGF ratio, Doppler examination of the uterine arteries and assessment of blood pressure (measured by validated automated devices), proteinuria, PE status, hemoglobin, platelets and uric acid levels. Postpartum, additional data were collected, including blood pressure, type of delivery, Apgar score, weight of placenta, neonatal outcomes (perinatal/fetal death, delivery < 34 weeks, IUGR, placental abruption, respiratory distress syndrome, necrotizing enterocolitis, intraventricular hemorrhage) and maternal outcomes (maternal death, pulmonary edema, acute renal failure, cerebral hemorrhage, cerebral thrombosis, disseminated intravascular coagulation). Unplanned visits could be carried out in the event of complications.
Serum samples (≥ 2 mL) were collected according to a standard operating procedure and were analyzed at the individual study sites. Results were checked for consistency between study sites by central analysis at Hospital Universitario Central de Asturias.
Maternal serum levels of sFlt‐1 and PlGF were determined using the fully automated Elecsys sFlt‐1 and Elecsys PlGF assays on the cobas® e electrochemiluminescence immunoassay platform (Roche Diagnostics GmbH, Mannheim, Germany) and the sFlt‐1/PlGF ratio was calculated27, 30, 31. The sFlt‐1/PlGF ratio results were concealed from both patients and carers to ensure that they did not affect the clinical monitoring of patients.
Adverse events were recorded, although the study was non‐interventional.
Statistical analysis
To obtain 100 cases of PE, it was calculated that 800 pregnant women would need to be included in the study, based on a presumed prevalence of PE of 12% (including both singleton and multiple pregnancies). The sFlt‐1/PlGF ratio was log‐transformed to correct for right skewness prior to any calculation. Differences in means between independent groups were assessed using analysis of variance (ANOVA) or Student's t‐test in the case of homogeneity of variances, and using generalized least squares in the case of heteroscedasticity. Appropriateness of the methods was assessed by evaluation of the plots of residuals.
To develop a predictive model of PE, multivariate logistic regression was used considering maternal characteristics, medical history and biomarkers as potential predictors. The variables that were finally included in the early‐PE prediction model were selected according to the results of a logistic regression model with L1 penalization (‘lasso’ technique)34. The coefficients derived from the multivariate analysis were used as weights in a nomogram to predict early PE. Performances of the models were evaluated by receiver–operating characteristics (ROC) curves and areas under the curve (AUC) with 95% CIs.
All statistical analyses were performed using R (version 3.1.2) and R‐packages rms (version 4.2‐1) and ROCR (version 1.0‐5).
RESULTS
Study participants
Overall, 729 women were eligible for analysis, including 447 with singleton pregnancy and 282 with multiple pregnancy (twin pregnancy, n = 276; triplet pregnancy, n = 6). A total of 78 (10.7%) women developed PE (singleton pregnancy, n = 42; multiple pregnancy, n = 36), of which 24 were early‐onset PE (singleton pregnancy, n = 14; multiple pregnancy, n = 10) and 54 were late‐onset PE (singleton pregnancy, n = 28; multiple pregnancy, n = 26) (Figure 1). The number of participants per study site is reported in Table S2. Women who developed early‐onset PE had higher systolic and diastolic blood pressures, mean arterial blood pressure (MAP) and lower gestational age at delivery compared with the control group (women who did not develop PE/hypertension during the entire pregnancy) (Table 2).
Figure 1.
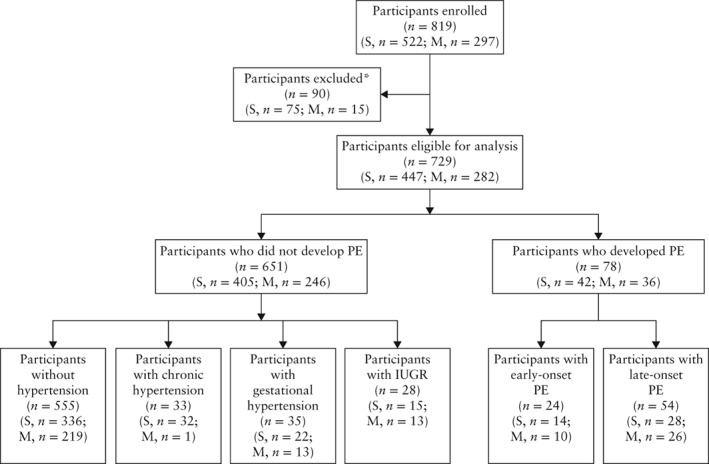
Flowchart of participants in Study of Early Pre‐eclampsia in Spain (STEPS). *Reasons for exclusion: inclusion criteria not met (n = 4); signed consent given but did not start study (n = 28); miscarriage (n = 7); termination of pregnancy due to fetal malformations (n = 8); lost to follow‐up (n = 13); placental abruption at 26 weeks (n = 1); completed follow‐up until 28 weeks but data could not be retrieved because of delivery in another setting (n = 29). IUGR, intrauterine growth restriction; M, multiple pregnancy; PE, pre‐eclampsia; S, singleton pregnancy.
Table 2.
Baseline characteristics of women who developed early‐ or late‐onset pre‐eclampsia (PE) and those who did not develop PE (controls)
| Characteristic | Controls (n = 651) | Early‐onset PE (n = 24) | Late‐onset PE (n = 54) |
|---|---|---|---|
| Age (years) | 34.6 ± 5.3 | 35.6 ± 3.9 | 34.7 ± 0.7 |
| Body mass index (kg/m2) | 26.7 ± 6.0 | 28.5 ± 6.4 | 27.9 ± 7.3 |
| Systolic blood pressure (mmHg) | 119.1 ± 13.7 | 127.9 ± 13.5* | 125.0 ± 16.0* |
| Diastolic blood pressure (mmHg) | 73.7 ± 11.3 | 77.5 ± 8.5* | 77.7 ± 12.0* |
| Mean arterial pressure (mmHg) | 88.9 ± 11.1 | 94.3 ± 8.1* | 93.4 ± 12.2* |
| Multiple pregnancy | 246 (37.8) | 10 (41.7) | 26 (48.1) |
| Gestational age at delivery (weeks) | 37.5 ± 2.7 | 31.8 ± 3.5* | 36.6 ± 1.4 |
| Birth weight of first infant (g) | 2911 ± 721 (n = 646) | 1745 ± 830 (n = 22)* | 2759 ± 584 (n = 54) |
| Birth weight of second infant (g) | 2303 ± 552 (n = 243) | 1807 ± 378 (n = 9)† | 2285 ± 364 (n = 26) |
| Birth weight of third infant (g) | 1221 ± 689 (n = 4) | 1445 ± 304 (n = 2) | — (n = 0) |
| Nulliparous | 272 (41.8) | 15 (62.5) | 31 (57.4) |
| Previous PE | 101 (15.5) | 9 (37.5)* | 14 (25.9) |
| Family history of PE | 23 (3.5) | 1 (4.2) | 5 (9.3) |
| Previous IUGR | 55 (8.4) | 3 (12.5) | 5 (9.3) |
| Chronic hypertension | 81 (12.4) | 6 (25.0) | 12 (22.2) |
| Gestational hypertension | 4 (0.6) | 0 (0) | 3 (5.6) |
| Nephropathy | 6 (0.9) | 0 (0) | 1 (1.9) |
| Diabetes mellitus Type 1 | 42 (6.5) | 1 (4.2) | 1 (1.9) |
| Thrombophilia | 50 (7.7) | 1 (4.2) | 3 (5.6) |
| Conceived by assisted reproduction | 93 (14.3) | 5 (20.8) | 11 (20.4) |
| Smoker at enrollment | 80 (12.3) | 1 (4.2) | 3 (5.6) |
| Abnormal UtA Doppler | 8 (1.2) | 1 (4.2) | 2 (3.7) |
Data are given as mean ± SD or n (%). PE groups compared with controls using Dunnett's test:
P < 0.001;
P < 0.05, after adjustment by Bonferroni correction.
IUGR, intrauterine growth restriction; UtA, uterine artery.
sFlt‐1, PlGF and sFlt‐1/PlGF ratio measurements
In the control group, median sFlt‐1/PlGF ratio remained low (< 7) between 20 and 28 weeks' gestation (Table 3). In women who developed early‐onset PE, median sFlt‐1/PlGF ratio was already higher (14.5) at 20 weeks' gestation and increased further to 18.4 at 24 weeks and 51.9 at 28 weeks. There was little change in the median sFlt‐1/PlGF ratio between 20 and 28 weeks in women who developed late‐onset PE, remaining low throughout at < 7.
Table 3.
Measurements of soluble fms‐like tyrosine kinase‐1 (sFlt‐1), placental growth factor (PlGF) and sFlt‐1/PlGF ratio in maternal serum at 20, 24 and 28 weeks in women who developed early‐ or late‐onset pre‐eclampsia (PE) and in those who did not develop PE (controls)
| Biomarker | Controls | Early‐onset PE | Late‐onset PE |
|---|---|---|---|
| 20 weeks | |||
| n | 612 | 21 | 52 |
| PlGF (pg/mL) | 264.5 (172.0–403.6) | 193.1 (68.0–262.4) | 267.8 (151.5–414.0) |
| sFlt‐1 (pg/mL) | 1623.0 (1081.0–2531.0) | 1972.0 (1331.0–3473.0) | 1967.0 (1120.5–2903.8) |
| sFlt‐1/PlGF ratio | 6.3 (4.1–9.3) | 14.5 (5.5–43.7) | 6.7 (4.6–9.9) |
| 24 weeks | |||
| n | 580 | 20 | 52 |
| PlGF (pg/mL) | 424.5 (277.0–615.6) | 168.9 (62.1–329.7) | 415.0 (259.7–595.7) |
| sFlt‐1 (pg/mL) | 1725.0 (1123.5–2674.3) | 3127.5 (1961.8–4202.5) | 1882.5 (1134.5–3115.8) |
| sFlt‐1/PlGF ratio | 4.0 (2.6–6.3) | 18.4 (8.2–57.9) | 4.7 (2.8–7.2) |
| 28 weeks | |||
| n | 557 | 16 | 49 |
| PlGF (pg/mL) | 540.0 (339.0–821.5) | 176.5 (67.2–278.6) | 335.0 (263.0–485.9) |
| sFlt‐1 (pg/mL) | 1826.0 (1231.0–2766.0) | 6370.0 (2385.3–8788.3) | 2499.0 (1522.0–3681.0) |
| sFlt‐1/PlGF ratio | 3.3 (2.0–5.9) | 51.9 (11.5–145.6) | 6.0 (3.8–10.5) |
Data are given as median (interquartile range) unless stated otherwise.
Mean sFlt‐1 levels and PlGF levels were significantly different between singleton and multiple pregnancies at 20, 24 and 28 weeks (P = 0.001). However, the mean sFlt‐1/PlGF ratio was only significantly different at 28 weeks' gestation (P = 0.001) (Table S3) and, at all timepoints, the difference between median values in singleton and multiple pregnancies was small.
sFlt‐1/PlGF ratio: prediction of PE
Compared with control participants, the sFlt‐1/PlGF ratio was consistently significantly higher in women with early‐onset PE (P < 0.001 at all timepoints) (Figure 2). Women with early‐onset PE also had significantly higher sFlt‐1/PlGF ratios at 20, 24 and 28 weeks relative to women with chronic or gestational hypertension and women with late‐onset PE (Figure 3). Differences between early‐onset PE and control/hypertension/late‐onset PE became more pronounced as the pregnancy progressed.
Figure 2.
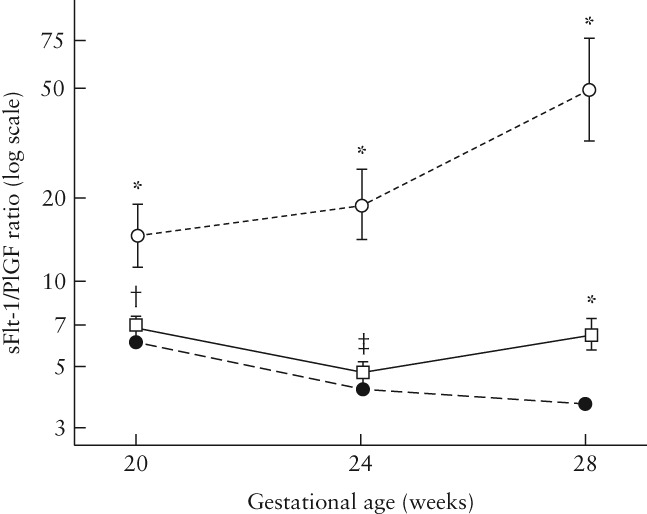
Soluble fms‐like tyrosine kinase‐1 (sFlt‐1)/placental growth factor (PlGF) ratio at 20, 24 and 28 weeks in control group of women who did not develop pre‐eclampsia (PE;  ) and in those who developed early‐onset (
) and in those who developed early‐onset ( ) or late‐onset (
) or late‐onset ( ) PE. Comparison with controls: *P < 0.001; †P = 0.15; ‡P = 0.21.
) PE. Comparison with controls: *P < 0.001; †P = 0.15; ‡P = 0.21.
Figure 3.
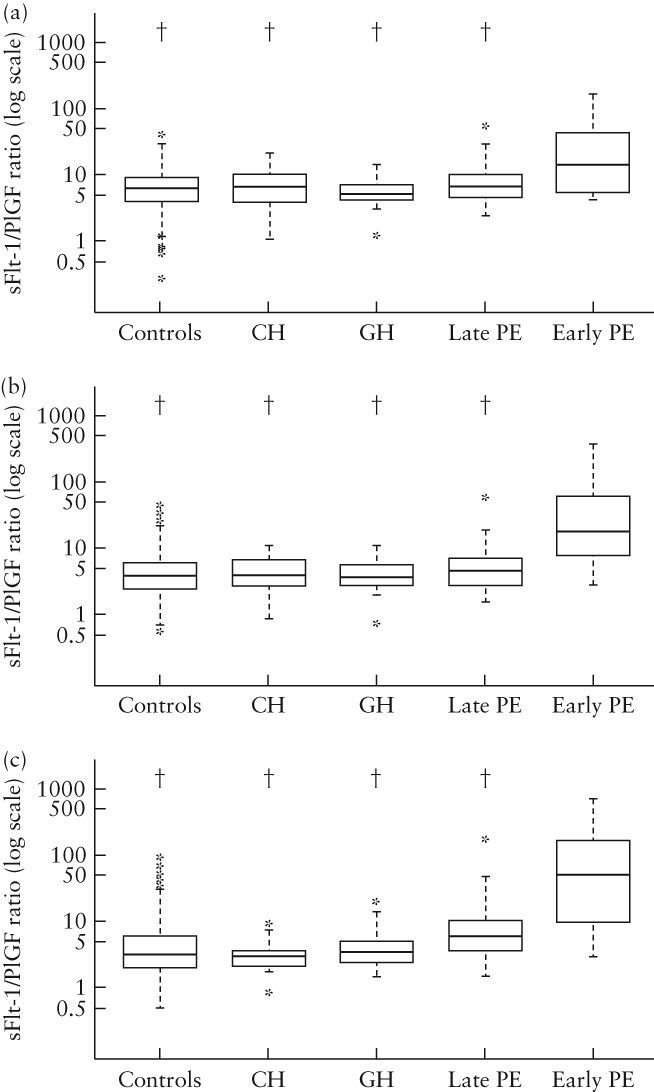
Box‐and‐whisker plots of soluble fms‐like tyrosine kinase‐1 (sFlt‐1)/placental growth factor (PlGF) ratio in control women who did not develop pre‐eclampsia (PE) and in those who developed chronic hypertension (CH), gestational hypertension (GH), late‐onset PE or early‐onset PE at: (a) 20 weeks, (b) 24 weeks and (c) 28 weeks. †P < 0.001 in comparison with early‐onset PE. Boxes with internal lines represent median and interquartile range, whiskers are 1.5 × interquartile range and stars are outliers.
Women with late‐onset PE were not easily differentiated from control participants by the sFlt‐1/PlGF ratio at 20 and 24 weeks (difference was non‐significant at 20 (P = 0.15) and 24 (P = 0.21) weeks, Figure 2). At 28 weeks, there was a statistically significant difference in the sFlt‐1/PlGF ratio between women with late‐onset PE and control participants (P < 0.001), although the numerical difference in the median ratio was small (2.7) (Table 3).
Development of a prediction model for early‐onset PE
Prediction models for early‐onset PE were developed, which included variations of the following factors: sFlt‐1/PlGF ratio, PlGF, UtA‐PI, MAP, being parous, previous PE and use of ART. The AUC was optimal for a model including the sFlt‐1/PlGF ratio, MAP, being parous and previous PE (hereafter referred to as the ‘early‐onset PE prediction model’) (Figure S1) compared with models that used the sFlt‐1/PlGF ratio alone or UtA‐PI alone (Table 4). The accuracy of the prediction model was not substantially improved by including ART or UtA‐PI and ART in the model. At 20 and 24 weeks, including these two parameters in the model increased the AUC from 0.86 (95% CI, 0.77–0.95) to 0.87 (95% CI, 0.79–0.96), and from 0.91 (95% CI, 0.85–0.97) to 0.92 (95% CI, 0.85–0.97), respectively. However, at 28 weeks, including UtA‐PI and ART in the model reduced the AUC from 0.93 (95% CI, 0.86–0.99) to 0.91 (95% CI, 0.82–0.99) (Figure 4). A nomogram for prediction risk is presented in Figures S2 and S3. The detection rate for early‐onset PE using different prediction models is reported in Table 5.
Table 4.
Prediction of early‐onset pre‐eclampsia (PE) at 20, 24 and 28 weeks using different individual parameters and early‐onset PE prediction model
| Prediction parameter | AUC (95% CI) |
|---|---|
| 20 weeks | |
| Early‐onset PE prediction model | 0.86 (0.77–0.95) |
| MAP | 0.67 (0.55–0.79) |
| UtA‐PI | 0.50 (0.35–0.66) |
| PlGF | 0.70 (0.58–0.82) |
| sFlt‐1 | 0.61 (0.49–0.74) |
| sFlt‐1/PlGF ratio | 0.77 (0.65–0.89) |
| 24 weeks | |
| Early‐onset PE prediction model | 0.91 (0.85–0.97) |
| MAP | 0.72 (0.62–0.83) |
| UtA‐PI | 0.55 (0.39–0.72) |
| PlGF | 0.81 (0.72–0.90) |
| sFlt‐1 | 0.71 (0.58–0.84) |
| sFlt‐1/PlGF ratio | 0.86 (0.76–0.96) |
| 28 weeks | |
| Early‐onset PE prediction model | 0.93 (0.86–0.99) |
| MAP | 0.77 (0.66–0.89) |
| UtA‐PI | 0.63 (0.45–0.80) |
| PlGF | 0.86 (0.78–0.94) |
| sFlt‐1 | 0.81 (0.67–0.95) |
| sFlt‐1/PlGF ratio | 0.89 (0.79–0.98) |
Early‐onset PE prediction model includes soluble fms‐like tyrosine kinase 1(sFlt‐1)/placental growth factor (PlGF) ratio, mean arterial pressure (MAP), being parous and previous PE.
AUC, area under the receiver–operating characteristics curve; UtA‐PI, uterine artery pulsatility index.
Figure 4.
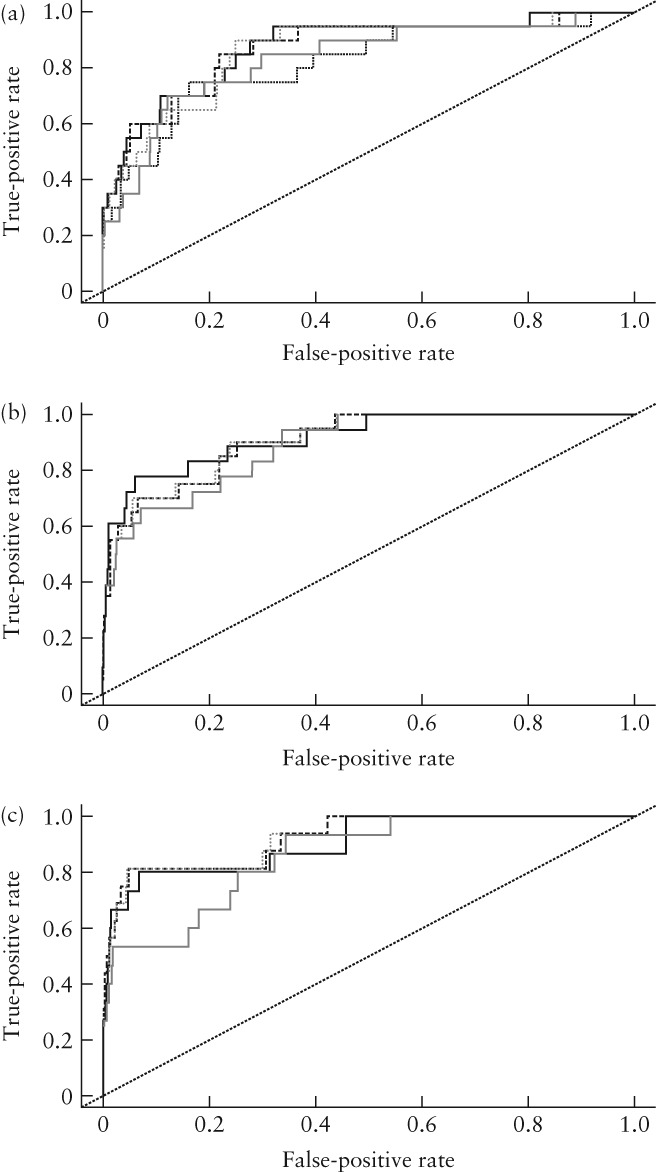
Receiver–operating characteristcs curves for prediction of early‐onset pre‐eclampsia (PE) using different models at 20 weeks (a), 24 weeks (b) and 28 weeks (c). Tables S4 and 5 present numerical values for areas under curves. Early‐onset PE prediction model ( ) includes soluble fms‐like tyrosine kinase‐1 (sFlt‐1)/placental growth factor (PlGF) ratio, mean arterial pressure (MAP), being parous and previous PE. ART, assisted reproductive technologies; UtA‐PI, uterine artery pulsatility index.
) includes soluble fms‐like tyrosine kinase‐1 (sFlt‐1)/placental growth factor (PlGF) ratio, mean arterial pressure (MAP), being parous and previous PE. ART, assisted reproductive technologies; UtA‐PI, uterine artery pulsatility index.
 , sFlt‐1/PlGF ratio, MAP, being parous, previous PE, ART.
, sFlt‐1/PlGF ratio, MAP, being parous, previous PE, ART.
 , sFlt‐1/PlGF ratio, MAP, being parous, previous PE, UtA‐PI, ART.
, sFlt‐1/PlGF ratio, MAP, being parous, previous PE, UtA‐PI, ART.
 , MAP, being parous, previous PE, ART, PlGF.
, MAP, being parous, previous PE, ART, PlGF.
 , MAP, being parous, previous PE, UtA‐PI, ART, PlGF.
, MAP, being parous, previous PE, UtA‐PI, ART, PlGF.
Table 5.
Prediction rates of early‐onset pre‐eclampsia (PE) using different models at 20, 24 and 28 weeks
| Detection rate (%) | ||
|---|---|---|
| Prediction model | FPR = 5% | FPR = 10% |
| 20 weeks | ||
| Early‐onset PE prediction model | 45 | 60 |
| sFlt‐1/PlGF ratio, MAP, being parous, previous PE, UtA‐PI and ART | 50 | 60 |
| sFlt‐1/PlGF ratio, MAP, being parous, previous PE and ART | 55 | 60 |
| MAP, being parous, previous PE, UtA‐PI, ART and PlGF | 35 | 55 |
| MAP, being parous, previous PE, ART and PlGF | 45 | 45 |
| 24 weeks | ||
| Early‐onset PE prediction model | 60 | 70 |
| sFlt‐1/PlGF ratio, MAP, being parous, previous PE, UtA‐PI and ART | 72 | 78 |
| sFlt‐1/PlGF ratio, MAP, being parous, previous PE and ART | 60 | 70 |
| MAP, being parous, previous PE, UtA‐PI, ART and PlGF | 56 | 67 |
| MAP, being parous, previous PE, ART and PlGF | 56 | 67 |
| 28 weeks | ||
| Early‐onset PE prediction model | 81 | 81 |
| sFlt‐1/PlGF ratio, MAP, being parous, previous PE, UtA‐PI and ART | 73 | 80 |
| sFlt‐1/PlGF ratio, MAP, being parous, previous PE and ART | 81 | 81 |
| MAP, being parous, previous PE, UtA‐PI, ART and PlGF | 53 | 53 |
| MAP, being parous, previous PE, ART and PlGF | 53 | 53 |
Early‐onset PE prediction model includes soluble fms‐like tyrosine kinase 1(sFlt‐1)/placental growth factor (PlGF) ratio, mean arterial pressure (MAP), being parous and previous PE.
Areas under receiver–operating chracteristics curves for each model are provided in Table S4. ART, assisted reproductive technologies; FPR, false‐positive rate; UtA‐PI, uterine artery pulsatility index.
We also compared the performance of a standard prediction model (maternal history, MAP and UtA‐PI) with the same model but including the sFlt‐1/PlGF immunoassay ratio to estimate early‐onset PE risk at 20, 24 and 28 weeks' gestation. The addition of the sFlt‐1/PlGF immunoassay ratio substantially increased the detection rate at all gestational ages studied (assuming a false‐positive rate of both 5% and 10%) (Table 6).
Table 6.
Performance of standard prediction model and same model plus soluble fms‐like tyrosine kinase‐1 (sFlt‐1)/placental growth factor (PlGF) ratio to estimate risk of early‐onset pre‐eclampsia at 20, 24 and 28 weeks
| Detection rate (%) | |||
|---|---|---|---|
| Prediction model | AUC (95% CI) | FPR = 5% | FPR = 10% |
| 20 weeks | |||
| Standard prediction model (maternal history, MAP, UtA‐PI) | 0.81 (0.71–0.89) | 17 | 48 |
| Standard prediction model plus sFlt‐1/PlGF ratio | 0.91 (0.85–0.97) | 60 | 70 |
| 24 weeks | |||
| Standard prediction model (maternal history, MAP, UtA‐PI) | 0.87 (0.79–0.94) | 40 | 60 |
| Standard prediction model plus sFlt‐1/PlGF ratio | 0.95 (0.90–0.99) | 72 | 83 |
| 28 weeks | |||
| Standard prediction model (maternal history, MAP, UtA‐PI) | 0.89 (0.83–0.95) | 42 | 58 |
| Standard prediction model plus sFlt‐1/PlGF ratio | 0.95 (0.90–1.00) | 80 | 80 |
AUC, area under receiver–operating characteristics curve; FPR, false‐positive rate; MAP, mean arterial pressure; UtA‐PI, uterine artery pulsatility index.
sFlt‐1 and PlGF as single biomarkers: development of PE
Women with early‐onset PE had lower PlGF (P < 0.001 at 20, 24 and 28 weeks) and higher sFlt‐1 (P = 0.018, P < 0.001 and P < 0.001 at 20, 24 and 28 weeks, respectively) compared with those who did not develop early‐onset PE (women who developed late‐onset PE and those who did not develop any PE combined) (Figure S4).
A comparison of prediction models that included the sFlt‐1/PlGF ratio with models that used sFlt‐1 or PlGF alone was performed by evaluating their respective AUCs and Akaike information criterion (AIC), which measures goodness of fit35. The AUC for prediction models that included the sFlt‐1/PlGF ratio (0.86–0.87, 0.91–0.92 and 0.91–0.93 at 20, 24 and 28 weeks' gestation, respectively) was greater than that of models that used single biomarkers (0.81–0.83, 0.88–0.90 and 0.88–0.91 for sFlt‐1 and 0.79–0.83, 0.85–0.89 and 0.86–0.89 for PlGF at 20, 24 and 28 weeks' gestation, respectively) (Table S4).
Data consistency
No inconsistencies were found between site and central testing (data not shown).
DISCUSSION
Substantial evidence supports the use of the sFlt‐1/PlGF ratio in PE diagnosis and prediction20, 21, 23, 27, 36, 37, 38, 39, 40. However, differences between early‐ and late‐onset PE suggest different etiologies; thus, different ‘rules’ for the sFlt‐1/PlGF ratio could be applied. In a study of 257 women with suspected PE, the optimal sFlt‐1/PlGF ratio cut‐off to diagnose PE < 34 weeks' and ≥ 34 weeks' gestation was 23 (92.0% sensitivity, 81.1% specificity) and 45 (83.7% sensitivity, 72.6% specificity), respectively41. In PROGNOSIS, a sFlt‐1/PlGF ratio cut‐off ≤ 38 ruled out PE within 1 week in women with suspected PE and singleton pregnancy at 24 + 0 to 36 + 6 weeks' gestation32. PROGNOSIS had a higher prevalence of PE compared with our study (19% vs 11%, respectively), possibly due to the fact that PROGNOSIS enrolled women with suspicion of PE, while we enrolled women with a moderate or high risk of developing PE. The prevalence of PE in our study falls between the estimated ranges for women with moderate or high risk for PE (5.29–6.19% and 16.09–19.49%, respectively)42.
In STEPS, the sFlt‐1/PlGF ratio was significantly different between women who did not develop PE and those who did. The combination of the sFlt‐1/PlGF ratio with other clinical measures produced a predictive model with considerably increased specificity and sensitivity compared with using UtA‐PI or sFlt‐1/PlGF ratio alone. We also evaluated how the models used to estimate early‐onset PE risk would perform when using the single biomarkers, instead of the sFlt‐1/PlGF ratio. Based on both AUC and AIC, models with the sFlt‐1/PlGF ratio demonstrated consistently the highest predictive performance. Using our early‐onset PE prediction model (sFlt‐1/PlGF ratio, MAP, being parous, previous PE), early‐onset PE could be predicted from 20 weeks onward, with an AUC of 0.86 and 60% sensitivity for a false‐positive rate of 10%. A previous model developed without serum biomarkers, which used a history of diabetes, hypertension and MAP, reported an AUC of 0.83 with 55% sensitivity for a false‐positive rate of 10% (these were not high‐risk women)43.
Other studies have included sFlt‐1 and PlGF in their models. An observational study of women at high risk of PE developed a prediction model for early‐onset PE using sFlt‐1 at 28 + 0 to 31 + 6 weeks' gestation, which had an AUC of 0.85 (67% sensitivity, 96% specificity)44. A model including gestational age, UtA‐PI and sFlt‐1/PlGF ratio showed an association with perinatal complications with an AUC of 0.89 (64% sensitivity, 95% specificity)45. Another model combined PlGF with maternal characteristics, obstetric history and UtA‐PI to predict early‐onset PE in the first trimester with an AUC of 0.9446. Although an abnormal UtA‐PI was associated with the development of PE in our study, it was not included in our model since it did not substantially improve PE prediction. From a practical perspective, the UtA can be difficult to locate in the first trimester and the International Society of Ultrasound in Obstetrics and Gynecology does not include UtA Doppler as part of the routine first‐trimester fetal ultrasound examination47. Other studies have also not included UtA‐PI in their models48, 49.
A recent study demonstrated that a prospective screening model at 19–24 weeks' gestation, involving maternal factors, UtA‐PI, MAP and PlGF, was superior to screening by maternal factors alone. The performance of the model was inversely related to the gestational age at which delivery became necessary; detection rates (false‐positive rate of 10%) for PE < 32 weeks, between 32 + 0 and 36 + 6 weeks, and ≥ 37 weeks were 99%, 85% and 46%, respectively. However, this study evaluated PlGF and sFlt‐1 separately; it did not assess the sFlt‐1/PlGF ratio50. Of note, the study defined early‐onset PE as requiring delivery before 32 weeks' gestation, rather than before 34 weeks. A related study showed that a two‐stage screening model, in which UtA‐PI and PlGF measurements were reserved for at‐risk individuals, achieved similar detection rates for preterm PE (< 37 weeks' gestation), compared with screening the whole population by maternal factors, MAP, UtA‐PI and PlGF51.
Various guidelines recommend PE screening based on maternal history52, 53, 54. However, the addition of MAP, UtA‐PI and angiogenic serum markers to the assessment of maternal history has been shown to increase the PE detection rate between 12 and 36 weeks' gestation55, 56, 57, 58. In STEPS, the addition of the sFlt‐1/PlGF ratio increased the detection rate at all gestational ages studied, supporting the inclusion of the sFlt‐1/PlGF ratio in the risk estimation of early‐onset PE.
The prospective, longitudinal design and large cohort in our study provided a robust dataset and the angiogenic marker results were hidden from the investigators to avoid bias in the diagnosis of outcomes. However, despite the large sample size, there were relatively small numbers of women in the early‐onset PE group and the results of this study, which included women at risk of developing PE, cannot be applied to a low‐risk population, i.e. in screening for PE. The data were validated using the Elecsys immunoassay sFlt‐1/PlGF ratio and the predictive value may differ when other assays are used. The developed prediction model for early‐onset PE has to be validated in an independent prospective study cohort in a comparable target population, and interventional studies are required to confirm the clinical utility of the results.
STEPS provides further evidence that the addition of the sFlt‐1/PlGF ratio to clinical protocols for women at risk of PE improves prediction of early‐onset PE in the second trimester. This complements the findings of PROGNOSIS, which showed that, in women with signs and/or symptoms of PE and a sFlt‐1/PlGF ratio above 38, the positive predictive value for PE within the following 4 weeks was 36.7%32. In STEPS, women who developed early‐onset PE had a median sFlt‐1/PlGF ratio at 28 weeks' gestation of 51.9, indicating that these women had an increased risk of developing PE in the following 4 weeks before 34 + 0 weeks' gestation. Better prediction of PE could facilitate targeting of monitoring and therapeutic procedures towards at‐risk women and allow better utilization of healthcare resources.
DISCLOSURES
The STEPS study was sponsored by Roche Diagnostics Spain who were involved in study design, interpretation of the data and writing of the manuscript. ELECSYS and cobas are trademarks of Roche. A.P. is a consultant for Roche, GE Healthcare, Ferring, Italfarmaco, EFFIK, Merck and Gynea. J.L.D. is a consultant for Roche and Italfarmaco. M.H. is employed by Roche Diagnostics and has shares in F. Hoffmann‐La Roche.
STEPS INVESTIGATORS
Azahar Romero and Francisco Cabrera, Hospital Materno Infantil de Canarias, Gran Canaria, Spain; María Vázquez, Francisco Moreno and Óscar Vaquerizo, Hospital Universitario Central de Asturias, Oviedo, Spain; Myriam Miguel, Catalina de Paco, Miriam Pertegal and Alicia Arteaga, Hospital Virgen de la Arrixaca, Murcia, Spain; M. Jesús Franco and Blanca Envid, Hospital Miguel Servet, Zaragoza, Spain; Elena Martin, José Luis Bartha and Antonio Buño, Hospital de la Paz, Madrid, Spain; Gema Pérez, Silvia Roig, Rosa Gómez and David Hervás, Hospital Universitario y Politécnico La Fe, Valencia, Spain; Ángel Aguaron de la Cruz and Nieves López, Hospital Gregorio Marañón, Madrid, Spain; Victoria Melero and Mercedes Calero, Hospital Puerta del Mar, Cadiz, Spain; Marta de Ramón and Antonio Paya, Hospital del Mar, Barcelona, Spain.
Supporting information
Table S1 Ethics Committee approval details
Table S2 Participant recruitment by study site
Table S3 Comparison of angiogenic factors at 20, 24 and 28 weeks in women with singleton vs multiple pregnancy
Table S4 Performance of different prediction models using soluble fms‐like tyrosine kinase‐1 (sFlt‐1)/placental growth factor (PlGF) ratio, sFlt‐1 or PlGF to estimate risk of early‐onset pre‐eclampsia (PE) at 20, 24 and 28 weeks
Figure S1 Formulae used in prediction model for early‐onset pre‐eclampsia (PE). MAP, mean arterial pressure.
Figure S2 Nomogram for estimation of risk of early‐onset pre‐eclampsia (PE) at 24 weeks. To calculate probability of early‐onset PE for a given patient, the value for each predictor is obtained by drawing a vertical line straight upward from that factor to the ‘points’ axis. The points are then summed and the sum located on the total points nomogram, and the probability of early‐onset PE is located by drawing a vertical line downward to the ‘risk of PE’ line. MAP, mean arterial pressure; PlGF, placental growth factor; sFlt‐1, soluble fms‐like tyrosine kinase‐1.
Figure S3 Worked example of the use of the nomogram for estimation of risk of early‐onset pre‐eclampsia (PE) at 24 weeks. To calculate visually risk of early‐onset PE in a parous patient with previous PE at 24 weeks, with mean arterial pressure (MAP) of 100 mmHg and soluble fms‐like tyrosine kinas‐1 (sFlt‐1)/placental growth factor (PlGF) ratio of 50, points are assigned for each item by plotting a line from the item to the points line. Being parous equates to 0 points; 100 mmHg MAP corresponds to 9 points; previous PE equates to 38 points; and sFlt‐1/PlGF ratio of 50 (In = 3.91) gives 62 points. Total number of points is 109; drawing a vertical line downward from total points axis to ‘risk of PE’ line, the risk is estimated at approximately 80%.
Figure S4 Placental growth factor (PlGF) (a) and soluble fms‐like tyrosine kinase‐1 (sFlt‐1) (b) at 20, 24 and 28 weeks in women who developed early‐onset pre‐eclampsia (PE) and in control participants who did not develop PE.
ACKNOWLEDGMENTS
We thank all women who participated in the STEPS study and the recruitment officers, nurses, midwives and midwifery staff who supported the study. We thank the STEPS investigators and Mª José Ramirez and Nuria Piella (Roche Diagnostics, Spain). Support for third‐party writing assistance for this manuscript was provided by Emma McConnell, PhD (Gardiner‐Caldwell Communications), and was funded by Roche Diagnostics.
The copyright line for this article was changed on 14 February 2018 after original online publication.
Contributor Information
A. Perales, Email: perales_alf@gva.es
STEPS investigators:
Azahar Romero, Francisco Cabrera, María Vázquez, Francisco Moreno, Óscar Vaquerizo, Myriam Miguel, Catalina de Paco, Miriam Pertegal, Alicia Arteaga, M. Jesús Franco, Blanca Envid, Elena Martin, José Luis Bartha, Antonio Buño, Gema Pérez, Silvia Roig, Rosa Gómez, David Hervás, Ángel Aguaron de la Cruz, Nieves López, Victoria Melero, Mercedes Calero, Marta de Ramón, and Antonio Paya
REFERENCES
- 1. WHO. The World Health Report 2005: Make every mother and child count. http://www.who.int/whr/2005/en/ [Accessed 10 November 2015].
- 2. Ananth CV, Keyes KM, Wapner RJ. Pre‐eclampsia rates in the United States, 1980–2010: age‐period‐cohort analysis. BMJ 2013; 347: f6564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hernandez‐Diaz S, Toh S, Cnattingius S. Risk of pre‐eclampsia in first and subsequent pregnancies: prospective cohort study. BMJ 2009; 338: b2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Skjaerven R, Wilcox AJ, Lie RT. The interval between pregnancies and the risk of preeclampsia. N Engl J Med 2002; 346: 33–38. [DOI] [PubMed] [Google Scholar]
- 5. Duley L. The global impact of pre‐eclampsia and eclampsia. Semin Perinatol 2009; 33: 130–137. [DOI] [PubMed] [Google Scholar]
- 6. Duley L, Meher S, Abalos E. Management of pre‐eclampsia. BMJ 2006; 332: 463–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chaiworapongsa T, Chaemsaithong P, Yeo L, Romero R. Pre‐eclampsia part 1: current understanding of its pathophysiology. Nat Rev Nephrol 2014; 10: 466–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Duckitt K, Harrington D. Risk factors for pre‐eclampsia at antenatal booking: systematic review of controlled studies. BMJ 2005; 330: 565–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Oudejans CBM, van Dijk M, Oosterkamp M, Lachmeijer A, Blankenstein MA. Genetics of preeclampsia: paradigm shifts. Human Genetics 2007; 120: 607–612. [DOI] [PubMed] [Google Scholar]
- 10. Valensise H, Vasapollo B, Gagliardi G, Novelli GP. Early and late preeclampsia: two different maternal hemodynamic states in the latent phase of the disease. Hypertension 2008; 52: 873–880. [DOI] [PubMed] [Google Scholar]
- 11. Borges VT, Zanati S, Peracoli MT, Weel IC, Poiati JR, Matias ML, Peracoli JC. [14‐OR]: Different profile of cardiac morphofunctional changes and brain natriuretic peptide (BNP) between early onset and late onset preeclampsia. Pregnancy Hypertens 2015; 5: 7–8. [Google Scholar]
- 12. Borges VT, Peracoli JC, Romao M, Zanati S, Poiati JR, Weel IC, Peracoli JC. [303‐POS]: Differentiation between early and late pre‐eclampsia by inflammatory cytokines and the association between IL‐1beta and left cardiac hypertrophy. Pregnancy Hypertens 2015; 5: 150. [Google Scholar]
- 13. Stergiotou I, Crispi F, Valenzuela‐Alcaraz B, Bijnens B, Gratacos E. Patterns of maternal vascular remodeling and responsiveness in early‐ versus late‐onset preeclampsia. Am J Obstet Gynecol 2013; 209: 558.e1–558.e14. [DOI] [PubMed] [Google Scholar]
- 14. Madazli R, Yuksel MA, Imamoglu M, Tuten A, Oncul M, Aydin B, Demirayak G. Comparison of clinical and perinatal outcomes in early‐ and late‐onset preeclampsia. Arch Gynecol Obstet 2014; 290: 53–57. [DOI] [PubMed] [Google Scholar]
- 15. Lisonkova S, Joseph KS. Incidence of preeclampsia: risk factors and outcomes associated with early‐ versus late‐onset disease. Am J Obstet Gynecol 2013; 209: 544e1–544.e12. [DOI] [PubMed] [Google Scholar]
- 16. Hall DR, Odendaal HJ, Kirsten GF, Smith J, Grove D. Expectant management of early onset, severe pre‐eclampsia: perinatal outcome. BJOG 2000; 107: 1258–1264. [DOI] [PubMed] [Google Scholar]
- 17. Llurba E, Crispi F, Verlohren S. Update on the pathophysiological implications and clinical role of angiogenic factors in pregnancy. Fetal Diagn Ther 2015; 37: 81–92. [DOI] [PubMed] [Google Scholar]
- 18. Brosens I, Pijnenborg R, Vercruysse L, Romero R. The “Great Obstetrical Syndromes” are associated with disorders of deep placentation. Am J Obstet Gynecol 2011; 204: 193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, Sukhatme VP, Karumanchi SA. Excess placental soluble fms‐like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest 2003; 111: 649–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Levine RJ, Lam C, Qian C, Yu KF, Maynard SE, Sachs BP, Sibai BM, Epstein FH, Romero R, Thadhani R, Karumanchi SA; CPEP Study Group . Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N Engl J Med 2006; 355: 992–1005. [DOI] [PubMed] [Google Scholar]
- 21. Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, Sibai BM, Sukhatme VP, Karumanchi SA. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med 2004; 350: 672–683. [DOI] [PubMed] [Google Scholar]
- 22. Wikstrom AK, Larsson A, Eriksson UJ, Nash P, Norden‐Lindeberg S, Olovsson M. Placental growth factor and soluble FMS‐like tyrosine kinase‐1 in early‐onset and late‐onset preeclampsia. Obstet Gynecol 2007; 109: 1368–1374. [DOI] [PubMed] [Google Scholar]
- 23. Villa PM, Hamalainen E, Maki A, Raikkonen K, Pesonen AK, Taipale P, Kajantie E, Laivuori H. Vasoactive agents for the prediction of early‐ and late‐onset preeclampsia in a high‐risk cohort. BMC Pregnancy Childbirth 2013; 13: 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tripathi R, Rath G, Jain A, Salhan S. Soluble and membranous vascular endothelial growth factor receptor‐1 in pregnancies complicated by pre‐eclampsia. Ann Anat 2008; 190: 477–489. [DOI] [PubMed] [Google Scholar]
- 25. Chaiworapongsa T, Romero R, Espinoza J, Bujoid E, Kim YM, Gocalves LF, Gomez R, Edwin S. Evidence supporting a role for blockade of the vascular endothelial growth factor system in the pathophysiology of preeclampsia: Young Investigator Award. Am J Obstet Gynecol 2004; 190: 1541–1547. [DOI] [PubMed] [Google Scholar]
- 26. Verlohren S, Herraiz I, Lapaire O, Schlembach D, Zeisler H, Calda P, Sabria J, Markfeld‐Erol F, Galindo A, Schoofs K, Denk B, Stepan H. New gestational phase‐specific cutoff values for the use of the soluble fms‐like tyrosine kinase‐1/placental growth factor ratio as a diagnostic test for preeclampsia. Hypertension 2014; 63: 346–352. [DOI] [PubMed] [Google Scholar]
- 27. Verlohren S, Galindo A, Schlembach D, Zeisler H, Herraiz I, Moertl MG, Pape J, Dudenhausen JW, Denk B, Stepan H. An automated method for the determination of the sFlt‐1/PlGF ratio in the assessment of preeclampsia. Am J Obstet Gynecol 2010; 202: 161.e1–161.e11. [DOI] [PubMed] [Google Scholar]
- 28. Verlohren S, Stepan H, Dechend R. Angiogenic growth factors in the diagnosis and prediction of pre‐eclampsia. Clin Sci (Lond) 2012; 122: 43–52. [DOI] [PubMed] [Google Scholar]
- 29. Verlohren S, Herraiz I, Lapaire O, Schlembach D, Moertl M, Zeisler H, Calda P, Holzgreve W, Galindo A, Engels T, Denk B, Stepan H. The sFlt‐1/PlGF ratio in different types of hypertensive pregnancy disorders and its prognostic potential in preeclamptic patients. Am J Obstet Gynecol 2012; 206: 58.e1–58.e8. [DOI] [PubMed] [Google Scholar]
- 30.Roche Diagnostics GmbH. Elecsys sFlt‐1 immunoassay. Method sheet. 2015–10, V 7.
- 31.Roche Diagnostics GmbH. Elecsys PlGF immunoassay. Method sheet. 2015–11, V 8.
- 32. Zeisler H, Llurba E, Chantraine F, Vatish M, Staff AC, Sennstrom M, Olovsson M, Brennecke SP, Stepan H, Allegranza D, Dilba P, Schoedl M, Hund M, Verlohren S. Predictive value of the sFlt‐1:PlGF ratio in women with suspected preeclampsia. N Engl J Med 2016; 374: 13–22. [DOI] [PubMed] [Google Scholar]
- 33. Gifford RW, August PA, Cunningham G, Green LA, Lindheimer MD, McNellis D, Roberts JM, Sibai BM, Taler SJ. Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol 2000; 183: S1–S22. [PubMed] [Google Scholar]
- 34. Tibshirani R. Regression shrinkage and selection via the lasso. J R Stat Soc Series B Stat Methodol 1996; 58: 267–288. [Google Scholar]
- 35. Sakamoto Y, Ishiguro M, Kitagawa G. Akaike Information Criterion Statistics. D. Reidel: Dordrecht, 1986. [Google Scholar]
- 36. Vatten LJ, Eskild A, Nilsen TI, Jeansson S, Jenum PA, Staff AC. Changes in circulating level of angiogenic factors from the first to second trimester as predictors of preeclampsia. Am J Obstet Gynecol 2007; 196: 239e1–239.e6. [DOI] [PubMed] [Google Scholar]
- 37. Rana S, Powe CE, Salahuddin S, Verlohren S, Perschel FH, Levine RJ, Lim KH, Wenger JB, Thadhani R, Karumanchi SA. Angiogenic factors and the risk of adverse outcomes in women with suspected preeclampsia. Circulation 2012; 125: 911–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chaiworapongsa T, Romero R, Korzeniewski SJ, Cortez JM, Pappas A, Tarca AL, Chaemsaithong P, Dong Z, Yeo L, Hassan SS. Plasma concentrations of angiogenic/anti‐angiogenic factors have prognostic value in women presenting with suspected preeclampsia to the obstetrical triage area: a prospective study. J Matern Fetal Neonatal Med 2014; 27: 132–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chaiworapongsa T, Romero R, Korzeniewski SJ, Kusanovic JP, Soto E, Lam J, Dong Z, Than NG, Yeo L, Hernandez‐Andrade E, Conde‐Agudelo A, Hassan SS. Maternal plasma concentrations of angiogenic/antiangiogenic factors in the third trimester of pregnancy to identify the patient at risk for stillbirth at or near term and severe late preeclampsia. Am J Obstet Gynecol 2013; 208: 287.e1–287.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Stepan H, Herraiz I, Schlembach D, Verlohren S, Brennecke S, Chantraine F, Klein E, Lapaire O, Llurba E, Ramoni A, Vatish M, Wertaschnigg D, Galindo A. Implementation of the sFlt‐1/PlGF ratio for prediction and diagnosis of pre‐eclampsia in singleton pregnancy: implications for clinical practice. Ultrasound Obstet Gynecol 2015; 45: 241–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Alvarez‐Fernandez I, Prieto B, Rodriguez V, Ruano Y, Escudero AI, Alvarez FV. New biomarkers in diagnosis of early onset preeclampsia and imminent delivery prognosis. Clin Chem Lab Med 2014; 52: 1159–1168. [DOI] [PubMed] [Google Scholar]
- 42. Duley L, Henderson‐Smart D, Knight M, King J. Antiplatelet drugs for prevention of pre‐eclampsia and its consequences: systematic review. BMJ 2001; 322: 329–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Baschat AA, Magder LS, Doyle LE, Atlas RO, Jenkins CB, Blitzer MG. Prediction of preeclampsia utilizing the first trimester screening examination. Am J Obstet Gynecol 2014; 211: 514.e1–514.e7. [DOI] [PubMed] [Google Scholar]
- 44. Moore Simas TA, Crawford SL, Bathgate S, Yan J, Robidoux L, Moore M, Maynard SE. Angiogenic biomarkers for prediction of early preeclampsia onset in high‐risk women. J Matern Fetal Neonatal Med 2014; 27: 1038–1048. [DOI] [PubMed] [Google Scholar]
- 45. Gomez‐Arriaga PI, Herraiz I, Lopez‐Jimenez EA, Escribano D, Denk B, Galindo A. Uterine artery Doppler and sFlt‐1/PlGF ratio: prognostic value in early‐onset pre‐eclampsia. Ultrasound Obstet Gynecol 2014; 43: 525–532. [DOI] [PubMed] [Google Scholar]
- 46. Akolekar R, Zaragoza E, Poon LCY, Pepes S, Nicolaides KH. Maternal serum placental growth factor at 11 + 0 to 13 + 6 weeks of gestation in the prediction of pre‐eclampsia. Ultrasound Obstet Gynecol 2008; 32: 732–739. [DOI] [PubMed] [Google Scholar]
- 47. Salomon LJ, Alfirevic Z, Bilardo CM, Chalouhi GE, Ghi T, Kagan KO, Lau TK, Papageorghiou AT, Raine‐Fenning NJ, Stirnemann J, Suresh S, Tabor A, Timor‐Tritsch IE, Toi A, Yeo G. ISUOG practice guidelines: performance of first‐trimester fetal ultrasound scan. Ultrasound Obstet Gynecol 2013; 41: 102–113. [DOI] [PubMed] [Google Scholar]
- 48. Myers JE, Kenny LC, McCowan LM, Chan EH, Dekker GA, Poston L, Simpson NA, North RA; SCOPE consortium . Angiogenic factors combined with clinical risk factors to predict preterm pre‐eclampsia in nulliparous women: a predictive test accuracy study. BJOG 2013; 120: 1215–1223. [DOI] [PubMed] [Google Scholar]
- 49. Myatt L, Clifton RG, Roberts JM, Spong CY, Hauth JC, Varner MW, Wapner RJ, Thorp JM Jr, Mercer BM, Grobman WA, Ramin SM, Carpenter MW, Samuels P, Sciscione A, Harper M, Tolosa JE, Saade G, Sorokin Y, Anderson GD; Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Maternal‐Fetal Medicine Units Network (MFMU) . The utility of uterine artery Doppler velocimetry in prediction of preeclampsia in a low‐risk population. Obstet Gynecol 2012; 120: 815–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gallo DM, Wright D, Casanova C, Campanero M, Nicolaides KH. Competing risks model in screening for preeclampsia by maternal factors and biomarkers at 19–24 weeks' gestation. Am J Obstet Gynecol 2016; 214: 619.e1–619.e17. [DOI] [PubMed] [Google Scholar]
- 51. Wright D, Gallo DM, Pugliese SG, Casanova C, Nicolaides KH. Contingent screening for preterm preeclampsia. Ultrasound Obstet Gynecol 2016; 47: 554–559. [DOI] [PubMed] [Google Scholar]
- 52. American Congress of Obstetricians and Gynecologists . Committee Opinion No. 638: First‐Trimester Risk Assessment for Early‐Onset Preeclampsia. Obstet Gynecol 2015; 126: e25–e27. [DOI] [PubMed] [Google Scholar]
- 53. Magee LA, Pels A, Helewa M, Rey E, von Dadelszen P; Committee Hypertension Guideline. Diagnosis, evaluation, and management of the hypertensive disorders of pregnancy: executive summary. J Obstet Gynaecol Can 2014; 36: 416–438. [DOI] [PubMed] [Google Scholar]
- 54. The National Institute for Health and Care Excellence (NICE) . Hypertension in pregnancy: the management of hypertensive disorders during pregnancy. NICE, London, 2015. [Google Scholar]
- 55. Tayyar A, Krithinakis K, Wright A, Wright D, Nicolaides KH. Mean arterial pressure at 12, 22, 32 and 36 weeks' gestation in screening for pre‐eclampsia. Ultrasound Obstet Gynecol 2016; 47: 573–579. [DOI] [PubMed] [Google Scholar]
- 56. O'Gorman N, Tampakoudis G, Wright A, Wright D, Nicolaides KH. Uterine artery pulsatility index at 12, 22, 32 and 36 weeks' gestation in screening for pre‐eclampsia. Ultrasound Obstet Gynecol 2016; 47: 565–572. [DOI] [PubMed] [Google Scholar]
- 57. Tsiakkas A, Cazacu R, Wright A, Wright D, Nicolaides KH. Maternal serum placental growth factor at 12, 22, 32 and 36 weeks' gestation in screening for pre‐eclampsia. Ultrasound Obstet Gynecol 2016; 47: 472–477. [DOI] [PubMed] [Google Scholar]
- 58. Tsiakkas A, Mendez O, Wright A, Wright D, Nicolaides KH. Maternal serum soluble fms‐like tyrosine kinase‐1 at 12, 22, 32 and 36 weeks' gestation in screening for pre‐eclampsia. Ultrasound Obstet Gynecol 2016; 47: 478–483. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Ethics Committee approval details
Table S2 Participant recruitment by study site
Table S3 Comparison of angiogenic factors at 20, 24 and 28 weeks in women with singleton vs multiple pregnancy
Table S4 Performance of different prediction models using soluble fms‐like tyrosine kinase‐1 (sFlt‐1)/placental growth factor (PlGF) ratio, sFlt‐1 or PlGF to estimate risk of early‐onset pre‐eclampsia (PE) at 20, 24 and 28 weeks
Figure S1 Formulae used in prediction model for early‐onset pre‐eclampsia (PE). MAP, mean arterial pressure.
Figure S2 Nomogram for estimation of risk of early‐onset pre‐eclampsia (PE) at 24 weeks. To calculate probability of early‐onset PE for a given patient, the value for each predictor is obtained by drawing a vertical line straight upward from that factor to the ‘points’ axis. The points are then summed and the sum located on the total points nomogram, and the probability of early‐onset PE is located by drawing a vertical line downward to the ‘risk of PE’ line. MAP, mean arterial pressure; PlGF, placental growth factor; sFlt‐1, soluble fms‐like tyrosine kinase‐1.
Figure S3 Worked example of the use of the nomogram for estimation of risk of early‐onset pre‐eclampsia (PE) at 24 weeks. To calculate visually risk of early‐onset PE in a parous patient with previous PE at 24 weeks, with mean arterial pressure (MAP) of 100 mmHg and soluble fms‐like tyrosine kinas‐1 (sFlt‐1)/placental growth factor (PlGF) ratio of 50, points are assigned for each item by plotting a line from the item to the points line. Being parous equates to 0 points; 100 mmHg MAP corresponds to 9 points; previous PE equates to 38 points; and sFlt‐1/PlGF ratio of 50 (In = 3.91) gives 62 points. Total number of points is 109; drawing a vertical line downward from total points axis to ‘risk of PE’ line, the risk is estimated at approximately 80%.
Figure S4 Placental growth factor (PlGF) (a) and soluble fms‐like tyrosine kinase‐1 (sFlt‐1) (b) at 20, 24 and 28 weeks in women who developed early‐onset pre‐eclampsia (PE) and in control participants who did not develop PE.


