Abstract
Aims
The comparative efficacy of Roux‐en‐Y gastric bypass (RYGB) and sleeve gastrectomy on Type 2 diabetes remission and the role of weight loss are unclear. The DiaRem diabetes remission prediction score uses HbA1c, age and diabetes medications but not diabetes duration. The aim of this study was to compare the DiaRem with the DiaBetter score that includes diabetes duration, upon combined (complete plus partial) 2‐year post‐surgery diabetes remission in people following RYGB and sleeve gastrectomy, and to investigate the relationship between weight loss and diabetes remission.
Methods
A retrospective single‐centre cohort study of obese people with diabetes who underwent RYGB (107) or sleeve gastrectomy (103) and a validation cohort study (173) were undertaken. Diabetes remission, % weight loss, DiaRem, DiaBetter scores and areas under receiving operator characteristic (ROC) curves were calculated. The relationship between % weight loss and diabetes remission was investigated using logistic regression.
Results
The proportion of people achieving diabetes remission was highest for those with the lowest DiaBetter and DiaRem scores. Areas under the ROC curves were comparable [DiaBetter: 0.867 (95%CI: 0.817–0.916); DiaRem: 0.865 (95%CI: 0.814–0.915), P=0.856]. Two‐year % weight loss was higher post RYGB [26.6 (95%CI: 24.8–28.4)] vs post‐sleeve gastrectomy [20.6 (95%CI: 18.3–22.8), P<0.001]. RYGB had 151% higher odds of diabetes remission [OR 2.51 (95%CI: 1.12–5.60), P=0.025]. This association became non‐significant when adjusted for % weight loss.
Conclusion
DiaBetter and DiaRem scores predict diabetes remission following both procedures. Two‐year % weight loss plays a key role in determining diabetes remission.
What's new?
Roux‐en‐Y gastric bypass (RYGB) and sleeve gastrectomy can lead to Type 2 diabetes remission in people with obesity and Type 2 diabetes. The impact of post‐surgery weight loss and surgical procedure type upon Type 2 diabetes remission rates are unclear.
The DiaRem score predicts post‐RYGB Type 2 diabetes remission but does not include Type 2 diabetes duration, which negatively associates with Type 2 diabetes remission rates.
We show that the novel DiaBetter Score, which includes Type 2 diabetes duration, predicts Type 2 diabetes remission at 2 years following RYBG and sleeve gastrectomy with comparable predictive power to DiaRem.
Importantly, we show that percentage weight loss rather than procedure type determines Type 2 diabetes remission 2 years postoperatively.
What's new?
Roux‐en‐Y gastric bypass (RYGB) and sleeve gastrectomy can lead to Type 2 diabetes remission in people with obesity and Type 2 diabetes. The impact of post‐surgery weight loss and surgical procedure type upon Type 2 diabetes remission rates are unclear.
The DiaRem score predicts post‐RYGB Type 2 diabetes remission but does not include Type 2 diabetes duration, which negatively associates with Type 2 diabetes remission rates.
We show that the novel DiaBetter Score, which includes Type 2 diabetes duration, predicts Type 2 diabetes remission at 2 years following RYBG and sleeve gastrectomy with comparable predictive power to DiaRem.
Importantly, we show that percentage weight loss rather than procedure type determines Type 2 diabetes remission 2 years postoperatively.
Introduction
Bariatric surgery is the most effective weight loss treatment for people with a BMI of 40 kg/m2 and of 35 kg/m2 or more with an obesity‐associated co‐morbid condition 1. Bariatric surgery leads to marked, sustained weight loss with amelioration of obesity‐related diseases, prevention or delay of incident Type 2 diabetes and cardiovascular disease, and reduced mortality 2. Bariatric surgery is particularly beneficial for people with Type 2 diabetes; up to 60% are able to maintain normoglycaemia without hypoglycaemic therapy (remission) at 2 years post surgery 2. Importantly, longitudinal case‐matched studies show that bariatric surgery reduces the incidence of microvascular complications even in people who subsequently experience Type 2 diabetes relapse 3.
Roux‐en‐Y gastric bypass (RYGB), which involves creating a small stomach pouch through which ingested nutrients empty rapidly into the mid‐jejunum, bypassing the duodenum and proximal jejunum, and sleeve gastrectomy, which involves removing ~ 70% of the stomach fundus, are the two most commonly performed bariatric procedures 1. Postoperative weight loss is highly variable 4. RYGB and sleeve gastrectomy differentially impact upon nutrient‐stimulated circulating levels of several gut hormones known to influence insulin secretion and glucose control 5. However, it remains unclear whether RYGB or sleeve gastrectomy benefit people with Type 2 diabetes equally 6, 7.
Randomized clinical trials have shown that bariatric surgery leads to a superior glycaemic control compared with medical therapy, with a greater proportion of people experiencing Type 2 diabetes remission, lower HbA1c levels and a decrease in hypoglycaemic medications 2, 7, 8. Consequently, bariatric surgery is now recognized as a treatment option for people with Type 2 diabetes with a BMI of 35 kg/m2 and above 9. Furthermore, the new guidelines from the Second Diabetes Surgery Summit (DSS‐II), endorsed by 45 international professional organizations, urge that bariatric surgery is considered in subjects with a BMI of 30–34.9 kg/m2 who fail to respond to conventional treatment 9. However, access to bariatric surgery is limited and postoperative weight loss and glycaemic improvement are variable 4. Longer Type 2 diabetes duration, low preoperative C‐peptide levels, insulin usage and poor postoperative weight loss are associated with reduced likelihood of Type 2 diabetes remission 3. Predictive outcome scores for Type 2 diabetes remission encapsulating these factors have been developed and may be useful in prioritizing those most likely to benefit. However, C‐peptide, and fasted insulin and glucose are not routinely measured in the real‐world setting 10. The DiaRem score, developed in people who had undergone RYGB, is based upon age, HbA1c and hypoglycaemic medications and associates with Type 2 diabetes remission 11. However, a recent study reported that the DiaRem score, which does not include Type 2 diabetes duration, has limited negative predictive power (for a low chance of remission) in an ethnically diverse British cohort at 1 year after RYGB 12. In addition, there are no studies examining the performance of the DiaRem score at predicting Type 2 diabetes remission following sleeve gastrectomy.
We undertook a retrospective cohort study and a separate validation study in people with 2‐year outcome data following RYGB or sleeve gastrectomy to compare the predictive value of the DiaRem score with a novel score based upon Type 2 diabetes duration, HbA1c and hypoglycaemic medication usage, the DiaBetter score upon Type 2 diabetes remission. Next, we examined the effect of procedure type, RYGB or sleeve gastrectomy, upon 2‐year post‐surgery weight loss and glycaemic outcomes. Finally, we examined the relationship between Type 2 diabetes remission and weight loss.
Methods
A retrospective cohort study was undertaken at University College London Hospitals (UCLH) National Health Service (NHS) Foundation Trust, London, UK. Demographic, anthropometric and clinical data were collected by review of prospectively maintained clinical records within a single bariatric surgery unit at UCLH Bariatric Centre for Weight Management and Metabolic Surgery. People aged 18 years or over, with a BMI ≥ 35.0 kg/m2 and Type 2 diabetes, who underwent either primary RYGB or sleeve gastrectomy procedures with complete 2‐year follow‐up data were included. People undergoing bariatric surgery were evaluated pre‐ and postoperatively by a multidisciplinary team consisting of physicians, surgeons, clinical nurse specialists, psychologists, dieticians and anaesthetists. Procedure selection was based on informed patient preference after standardized counselling including the provision of written and verbal information detailing the risks and benefits of each procedure and the multidisciplinary team decision. Informed written consent was obtained prior to surgery. Post surgery, people were advised to follow a liquid diet for 2 weeks, followed by softer foods for a further 2 weeks, before resuming a solid diet thereafter. The postoperative follow‐up schedule comprised of appointments at 4–6 weeks, then 3‐monthly during the first year and 6–12‐monthly thereafter. RYGB and sleeve gastrectomy were undertaken as described previously 13. Age was calculated as the difference between the date of birth and date of surgery. Baseline BMI was calculated from the weight measured in kg, divided by the square of height measured in metres on the day of surgery. Postoperative weight loss was determined relative to the weight on the day of surgery. Percentage weight loss was chosen as the outcome measure for weight change, as it is less influenced by baseline BMI than per cent excess weight loss.
Complete or partial Type 2 diabetes remission were defined using the DSS‐II Consensus group criteria [HbA1c < 42 mmol/mol (6.0%) or < 48 mmol/mol (6.5%) respectively for 12 months or longer without any Type 2 diabetes medication] 9. Type 2 diabetes duration was defined as the difference between the date of Type 2 diabetes diagnosis and date of surgery. At 2 years post surgery, the number of people with combined Type 2 diabetes remission (total and partial remission) and no remission were calculated. The DiaBetter score was designed according to the TRIPOD guidelines 14, and based upon HbA1c, Type 2 diabetes duration and anti‐diabetic medications (Table 1) with a score ranging between 0 and 3 for each variable. The scoring categories for HbA1c and Type 2 diabetes duration were obtained by dividing our population in quartiles.
Table 1.
DiaBetter Score. A score ranging between 0 and 3 was allocated for HbA1c, Type 2 diabetes duration and antidiabetic medications. Different scores for HbA1c and Type 2 diabetes duration were obtained dividing our population into quartiles. The medication score was obtained according to the usual medical history of people with Type 2 diabetes and of their pancreatic function (diet, metformin, other medications and finally insulin). By combining these three scores an estimate of Type 2 diabetes severity was obtained with a baseline total score ranging from 0 to 9 points. People were stratified in five groups based on score
| Score 0 | Score 1 | Score 2 | Score 3 | ||
|---|---|---|---|---|---|
| HbA1c mmol/mol (%) | ≤ 48 (≤ 6.5) | 49 (6.6) ‐ 55 (7.2) | 56 (7.3) ‐ 68 (8.4) | ≥ 69 (8.5) | |
| Duration of Type 2 Diabetes (years) | ≤ 2.0 | 2.1 – 5.0 | 5.1 ‐10.0 | ≥ 10.1 | |
| Anti‐Diabetes Medication | None | Metformin | Other non‐insulin drug | Insulin alone or in combination | |
| Total Score (0‐9) | 0 or 1 | 2 or 3 | 4 or 5 | 6 or 7 | 8 or 9 |
| Group | 1 | 2 | 3 | 4 | 5 |
The DiaBetter score (range 0–9; Table 1) and DiaRem score (range 0–22; Table 2) were calculated for each person. People were then stratified into one of five score groups for both DiaBetter and DiaRem. The distribution of the HbA1c level, Type 2 diabetes duration and anti‐diabetic medications in each DiaBetter score group and the odds of Type 2 diabetes remission by these individual predictor variables are presented in Tables S1 and S2 The percentage of people with Type 2 diabetes remission within each group was calculated. Baseline population characteristics and pre‐ and post‐surgery determinants of Type 2 diabetes remission by procedure type were examined using chi‐square tests and t‐tests. Nonparametric receiver operating characteristic (ROC) curves, a graph of the sensitivity vs 1‐specificity of the diagnostic test 15 were used to compare the diagnostic value of the DiaBetter and DiaRem using the continuous scores. Logistic regression was utilized to examine odds of Type 2 diabetes remission by procedure type adjusted for baseline Type 2 diabetes score (basic model). These analyses were further adjusted for age, gender and BMI and per cent weight loss at 2 years post surgery, measured on a continuous scale. Finally, odds of remission were tested using per cent weight loss measured on a continuous scale, categorized into 5% weight loss groups and in quintiles as exposure. Using the likelihood ratio test, we evaluated the assumption of linearity and found that the odds of Type 2 diabetes remission increased linearly with each 5% weight loss category and thus a pooled odds ratio is presented. The basic model controlling for the DiaBetter and DiaRem was further adjusted for age (in the DiaBetter model only), sex and baseline BMI to rule out potential confounders, as these variables have been associated with both Type 2 diabetes remission and per cent weight loss at 2 years post surgery. All analyses were performed in Stata 13 (Stata Corp., College Station, TX, USA). A separate validation analysis was undertaken in an independent cohort of 173 people, 114 RYGB and 59 sleeve gastrectomy, from two centres: UCLH (new cohort, 113) and Centro Hospitalar de Entre o Douro e Vouga, Santa Maria da Feira, Portugal (60) to validate the DiaBetter score.
Table 2.
DiaRem Score. This score was obtained using four preoperative parameters: (1) HbA1c, (2) age, (3) use of oral hypoglycaemic agents and (4) use of insulin. The total score ranges between 0 and 22 and is obtained adding the score for every single parameter. People were stratified into five score groups as proposed by Still et al. 11
| HbA1c mmol/mol (%) | < 48 (< 6.5) | ≥ 48 (6.5) and ≤ 52 (6.9) | ≥ 53 (7.0) and ≤ 74 (8.9) | ≥ 75 (9.0) | |
| Score 0‐6 | Score 0 | Score 2 | Score 4 | Score 6 | |
| Age (years) | < 40 | 40‐49 | 50‐59 | ≥ 60 | |
| Score 0‐3 | Score 0 | Score 1 | Score 2 | Score 3 | |
| Anti‐Diabetes Medication | None | Metformin | Other non‐insulin drug | Insulin | |
| Score 0‐13 | Score 0 | Score 0 | Score 3 | Score 10 | |
| Total Score (0‐22) | 0‐2 | 3‐7 | 8‐12 | 13‐17 | 18‐22 |
| Group | 1 | 2 | 3 | 4 | 5 |
Results
Some 346 adults with Type 2 diabetes who underwent either primary RYGB or primary sleeve gastrectomy between January 2008 and December 2015 were identified. One hundred and thirty‐six were excluded due to incomplete 2‐year follow‐up data (Fig. S1). Comparison of people with complete vs incomplete data revealed no statistical differences in post‐surgery weight loss or pre‐surgery data except that people with incomplete data were younger (Table S3). In total, 107 people who underwent RYGB and 103 who underwent sleeve gastrectomy were included in the retrospective primary cohort study. There were no differences between the RYGB and sleeve gastrectomy groups in terms of gender, age, ethnicity, mean HbA1c values, Type 2 diabetes duration and hypoglycaemic medications at baseline. There were significant group differences in baseline weight and BMI (higher in the sleeve gastrectomy group), and in insulin usage (higher proportion in the RYGB group). People who underwent RYGB showed higher DiaRem and DiaBetter scores at baseline (Table 3).
Table 3.
Comparison between baseline characteristics for people with Type 2 diabetes (137 females; 73 males) undergoing Roux‐en‐Y gastric bypass or sleeve gastrectomy in the primary cohort
| RYGB (n = 107) | Sleeve gastrectomy (n = 103) | P‐value | |
|---|---|---|---|
| Age (years); mean (sd) | 51.6 (8.0) | 49.7 (8.8) | NS |
| Ethnicity (Caucasian); n (%) | 83 (78) | 76 (74) | NS |
| BMI (kg/m2); mean (sd) | 43.1 (6.3) | 48.2 (7.8) | <0.001 |
| Type 2 diabetes duration (years); mean (sd) | 5.6 (5.1) | 4.7 (5.4) | NS |
| HbA1c; % (sd) | 62, 7.8 (1.5) | 57, 7.3 (1.4) | NS |
| Prescribed Type 2 diabetes medication; n (%) | 85 (52) | 77 (48) | NS |
| Prescribed insulin; n (%) | 26 (70) | 11 (30) | < 0.05 |
| Prescribed metformin; n (%) | 80 (75) | 70 (68) | NS |
| Prescribed sulphonylurea; n (%) | 29 (27) | 24 (23) | NS |
| Prescribed thiazolidinedione; n (%) | 13 (12) | 14 (14) | NS |
| Prescribed DPP‐4 inhibitor; n (%) | 4 (4) | 1 (1) | NS |
| Prescribed SGLT2 inhibitor; n (%) | 0 (0) | 0 (0) | NS |
| Prescribed GLP‐1 receptor agonist; n (%) | 14 (13) | 11 (11) | NS |
| DiaRem total score (sd) | 8.3 (5.2) | 6.0 (4.4) | < 0.001 |
| DiaBetter total score (sd) | 4.4 (2.7) | 3.4 (2.4) | < 0.05 |
RYGB, Roux‐en‐Y gastric bypass; DPP‐4, dipeptidyl peptidase‐4; SGLT2, sodium–glucose co‐transporter‐2; GLP‐1, glucagon‐like peptide‐1; NS, non‐significant
At 2 years post‐surgery, combined Type 2 diabetes remission was achieved in 144 (68.6%) of 210 people. The proportion of people achieving Type 2 diabetes remission was highest for those with the lowest DiaBetter and DiaRem scores and lowest in those with the highest scores, with a significant difference across the groups for both scores (Table 4). The diagnostic value of DiaRem and DiaBetter for Type 2 diabetes combined remission using the values of specificity and sensitivity were similar (Table S4). The area under the ROC curve for the DiaBetter and DiaRem scores were similar [DiaBetter: 0.867 (95% CI: 0.817–0.916) and DiaRem: 0.865 (95% CI: 0.814–0.915), P = 0.856, Fig. 1). The validation cohort baseline characteristics, DiaRem and DiaBetter scores and 2‐year post‐surgery per cent weight loss and percentage of combined remission are presented in Table S5 The proportion of people achieving Type 2 diabetes remission was significantly higher for those with the lowest DiaBetter and DiaRem scores compared with those with the highest scores (Table S5). Area under the ROC curve analyses were similar to the primary cohort (Fig. 2).
Table 4.
Pre‐surgical determinants of Type 2 diabetes combined remission for the primary cohort and for both procedures
| Remission | Total (n = 210) | RYGB (n= 107) | Sleeve gastrectomy (n = 103) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Yes | No | P‐value | Yes | No | P‐value | Yes | No | P‐value | |
| Age (years); mean (sd) | 49.8 ± 8.9 | 52.4 ± 7.1 | < 0.05 | 48.5 ± 9.2 | 52.1 ± 7.4 | NS | 51.0 ± 8.5 | 52.7 ± 6.8 | NS |
| Gender (female vs male); % | 76 vs 55 | <0.05 | 75 vs 55 | <0.05 | 76 vs 54 | <0.05 | |||
| Ethnicity (Caucasian); % | 66 vs 74 | NS | 67 vs 69 | NS | 66 vs 79 | NS | |||
| BMI (kg/m2); mean (sd) | 46.5 ± 7.2 | 43.6 ± 7.8 | < 0.05 | 49.0 ± 7.5 | 46.4 ± 8.5 | NS | 44.2 ± 6.2 | 40.8 ± 5.9 | <0.05 |
| HbA1c (mmol/mol) | 56 | 67 | 53 | 65 | 58 | 69 | |||
| HbA1c (%); mean (sd) | 7.3 ± 1.3 | 8.3 ± 1.5 | < 0.001 | 7.0 ± 1.1 | 8.1 ± 1.6 | < 0.001 | 7.5 ± 1.4 | 8.5 ± 1.5 | < 0.05 |
| DiaRem 0–2 (%) | 100 | 0 | < 0.001 | 100 | 0 | < 0.001 | 100 | 0 | < 0.001 |
| DiaRem 3–7 (%) | 86 | 14 | 89 | 11 | 78 | 22 | |||
| DiaRem 8–12 (%) | 45 | 55 | 60 | 40 | 31 | 69 | |||
| DiaRem 13–17 (%) | 31 | 69 | 40 | 60 | 12 | 88 | |||
| DiaRem 18–22 (%) | 14 | 86 | 18 | 72 | 0 | 100 | |||
| DiaBetter 0–1 (%) | 98 | 2 | <0.001 | 100 | 0 | <0.001 | 97 | 4 | <0.001 |
| DiaBetter 2–3 (%) | 88 | 12 | 86 | 14 | 90 | 10 | |||
| DiaBetter 4–5 (%) | 63 | 37 | 80 | 20 | 48 | 52 | |||
| DiaBetter 6–7 (%) | 39 | 61 | 50 | 50 | 25 | 75 | |||
| DiaBetter 8–9 (%) | 19 | 81 | 21 | 79 | 14 | 86 | |||
NS, non‐significant
Figure 1.
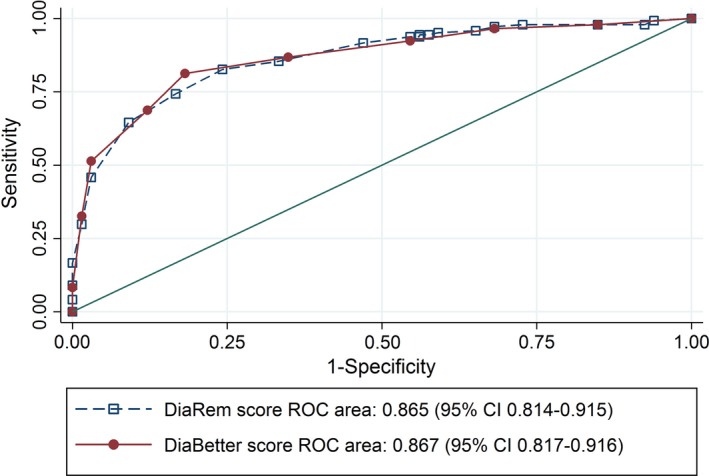
Comparison of the diagnostic value of DiaBetter and DiaRem (continuous scores) in the study population (n = 210) using the nonparametric receiver operating characteristic (ROC) plot. This test is used to classify the accuracy of a prediction model and the closer the area under the curve to a value of 1, the more accurate the model. Sensitivity is shown on the y‐axis and specificity on the x‐axis. DiaBetter prediction score is shown by the filled circles and solid line, and DiaRem by the open squares and a dotted line. Area under the DiaBetter ROC curve and DiaRem ROC curve with 95% confidence intervals and P‐value for the test of equality of ROC areas is shown. The diagonal line is the line of no discrimination, it divides the ROC space into two, the points above the diagonal represent good classification results (better than random), points below the line represent poor results (worse than random).
Figure 2.
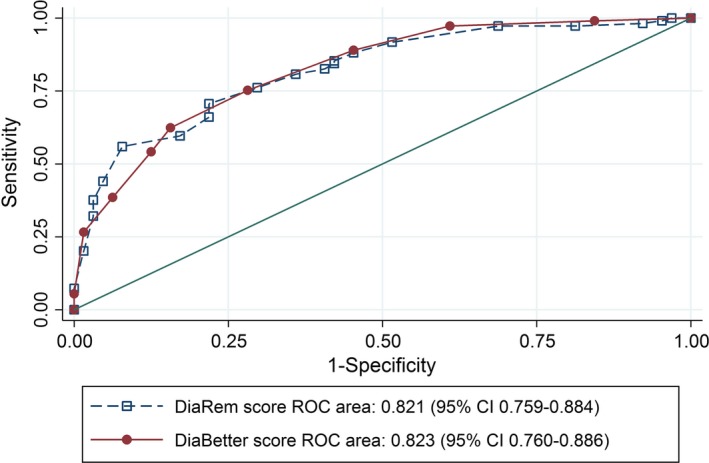
Comparison of the diagnostic value of DiaBetter and DiaRem (continuous scores) in the validation population (n = 173) using the nonparametric receiver operating characteristic (ROC) plot. DiaBetter prediction score is shown by filled circles and a solid line, and DiaRem by open squares and a dotted line. Area under the DiaBetter ROC curve and DiaRem ROC curve with 95% confidence intervals and p value for the test of equality of ROC areas is shown. The diagonal line is the line of no discrimination, it divides the ROC space into two, the points above the diagonal represent good classification results (better than random), points below the line represent poor results (worse than random).
Mean per cent weight loss was higher in the RYGB group than the sleeve gastrectomy group [26.6 (95% CI: 24.8–28.4) vs 20.6 (95% CI: 18.3–22.8), P < 0.001]. Combining the RYGB and sleeve gastrectomy groups showed 2‐year post‐surgery remission was associated with younger age, female gender, higher BMI, lower HbA1c and lower DiaRem and DiaBetter scores at baseline. When stratifying by procedure type some of these associations were attenuated or became statistically insignificant due to low numbers (Table 4).
At 2 years post surgery, the sleeve gastrectomy group had a higher HbA1c level, 0.37% (95% CI: 0.09–0.65, P = 0.009) than the RYGB group after controlling for baseline DiaBetter score. However, after adjusting for 2‐year per cent weight loss, the association with surgery type was no longer statistically significant (P = 0.199). We obtained the same results when controlling for the DiaRem score [0.43% higher HbA1c in the sleeve gastrectomy group (95% CI: 0.14–0.75, P = 0.003), not significant after adjusting for per cent weight loss (P = 0.103)]. When comparing people with Type 2 diabetes with combined remission vs no remission and adjusting for DiaBetter score we found that people who underwent RYGB had 151% higher odds of Type 2 diabetes combined remission vs no remission compared to sleeve gastrectomy [odds ratio (OR) 2.51, 95% CI: 1.12–5.60, P = 0.025] (Table 5). When considering complete remission only, the odds of Type 2 diabetes combined remission where 193% higher (OR 2.93, 95% CI: 1.26–6.81, P = 0.012). However, when per cent weight loss was adjusted for, this association became non‐significant both for combined remission (OR 1.69, 95% CI: 0.72–3.97, P > 0.05, Table 5) and complete remission (OR 1.74, 95% CI: 0.70–4.30, P > 0.05). When adjusting for DiaRem score we found that the odds of combined remission where 175% higher in RYGB (OR 2.75, 95% CI: 1.25–6.05, P = 0.012, Table 5). Also in this case, when per cent weight loss was adjusted for, this association became non‐significant (OR 1.82, 95% CI: 0.79–4.22, P > 0.05, Table 5).
Table 5.
Comparison of Type 2 diabetes combined remission vs no remission in the primary cohort
| Odds ratio | se | 95% confidence interval | P‐value | |
|---|---|---|---|---|
| Basic model adjusting for DiaBetter score | 2.51 | 1.03 | 1.12–5.59 | 0.025 |
| + age (years) | 2.68 | 1.11 | 1.19–6.06 | 0.018 |
| + gender (women vs men) | 2.41 | 1.00 | 1.07–5.44 | 0.034 |
| + BMI (kg/m2) | 2.78 | 1.19 | 1.20–6.44 | 0.017 |
| + % weight loss at 2 years’ post surgery | 1.69 | 0.74 | 0.72–3.97 | 0.232 |
| Basic modela adjusting for DiaRem score | 2.75 | 1.10 | 1.25–6.05 | 0.012 |
| + gender (women vs men) | 2.68 | 1.09 | 1.20–5.97 | 0.016 |
| + BMI (kg/m2) | 3.09 | 1.30 | 1.35–7.05 | 0.007 |
| + % weight loss at 2 years’ post surgery | 1.82 | 0.77 | 0.79–4.22 | 0.157 |
Age is included in DiaRem.
Next, we evaluated the odds of combined remission by per cent weight loss categorized in quintiles, 5% weight loss groups and as a continuous variable (Tables S6 and S7). When testing the odds of Type 2 diabetes remission by quintiles of per cent weight loss and adjusting for DiaBetter score, we found that those in either the third, fourth or fifth highest quintile (per cent weight loss > 21.15) had significantly higher odds of Type 2 diabetes combined remission than subjects in the first quintile (per cent weight loss < 16.51) (Table S8). When considering complete remission only, we found that those in the second, third, fourth and fifth quintiles (per cent weight loss > 16.51%) had significantly higher odds of remission than people in the first quintile (per cent weight loss < 16.51). When testing the odds of Type 2 diabetes remission adjusting for DiaRem score we found similar results (Table S8); when adjusting for DiaBetter score, for every 1% weight loss, the odds of Type 2 diabetes combined remission increased by 7% (OR 1.07, 95% CI: 1.03–1.12, P < 0.001). This association remained after adjustments for age, gender, BMI and surgery type. Furthermore, the odds of Type 2 diabetes combined remission increased by 54% with each 5% of weight lost (OR 1.54, 95% CI: 1.21–1.96, P < 0.001), age, gender, BMI and surgery type had no effect on these associations (Table S9). Adjusting for DiaRem, for every 1% weight loss, odds of Type 2 diabetes combined remission increased by 8% (OR 1.08, 95% CI: 1.04–1.13, P < 0.001) and with every unit increase in the grouping of 5% weight loss by 63% (OR 1.63, 95% CI: 1.29–2.07, P < 0.001) with no effect on these associations from gender, BMI and surgery type (Table S9).
Discussion
Bariatric surgery is established as a treatment option for people with obesity and Type 2 diabetes, with many people experiencing Type 2 diabetes remission postoperatively 2. However, access to bariatric surgery is limited and surgery carries a mortality risk, albeit small, together with the risk of post‐surgery complications and the need for life‐long nutritional supplementation and follow‐up 1. Furthermore, weight loss and glycaemic improvements following RYGB and sleeve gastrectomy are highly variable 2. Adopting a personalized approached to bariatric surgery by identifying which people with Type 2 diabetes will benefit most and maximizing their postoperative care to reduce their future risk of Type 2 diabetes‐related complications will increase the benefit to risk ratio for people with Type 2 diabetes. The DiaRem score does not include Type 2 diabetes duration, which associates with the probability of Type 2 diabetes remission and is a key determinant of access to surgery in the UK 16. The number of people developing Type 2 diabetes at a younger age is increasing, particularly within those of Asian origin 17, 18. Thus, we developed the DiaBetter score to include Type 2 diabetes duration. Sleeve gastrectomy is now the most common surgical procedure undertaken globally, but there are no studies reporting the predictive value of the DiaRem score in people with Type 2 diabetes following sleeve gastrectomy. Hence, we undertook a retrospective study to compare the ability of the DiaRem and DiaBetter scores to predict Type 2 diabetes remission at 2 years following RYGB or sleeve gastrectomy. The DiaBetter and DiaRem scores had comparable predictive value in both RYGB and sleeve gastrectomy cohorts, with people in the lowest score group exhibiting the highest rates of Type 2 diabetes remission and those in the highest score the lowest Type 2 diabetes remission rates. Our primary cohort was predominantly Caucasian and had an average age of 50.6 ± 8.4 years, future studies are needed to compare the DiaBetter and DiaRem scores in younger cohorts and in mixed ethnic groups.
Currently, it is unclear whether people with Type 2 diabetes should be offered RYGB or sleeve gastrectomy. In agreement with a meta‐analysis including 16 studies of > 9000 obese people undergoing either RYGB or sleeve gastrectomy 19, we found that those with Type 2 diabetes who underwent RYGB obtained greater weight loss outcomes than those who underwent sleeve gastrectomy. Although several on‐going randomized controlled trials comparing RYGB to sleeve gastrectomy have reported no difference in weight loss between these two procedures, these studies have relatively small numbers of people with Type 2 diabetes and are not powered to detect differences between procedures in those with Type 2 diabetes 6, 7, 20.
Importantly, our findings show that at 2 years, per cent weight loss rather than procedure type, is the key determinant of Type 2 diabetes remission. Several other studies have reported the importance of weight loss as a determinant of improved glycaemic control following bariatric surgery 21, 22. For example, the 10‐year post‐surgery data from the Swedish Obese Study also reported that the degree of weight loss was a more important determinant of reduced fasting insulin and glucose than the procedure choice 23. Furthermore, in the STAMPEDE randomized trial comparing medical intervention alone or with RYGB or sleeve gastrectomy, change in BMI was the only independent predictor of achieving the primary outcome measure of the study [HbA1c < 42 mmol/mol (6.0%) with or without medical therapy] 7.
Here, we demonstrated that for every 1% weight loss, the odds of Type 2 diabetes remission increased by 7%, and for each 5% weight loss by 54%. This observation supports the need to maximize weight loss outcomes after bariatric surgery. In a recent study, we observed in a population with poor weight loss post surgery how an intervention programme combining exercise training with nutritional–behavioural counselling contributed to a greater weight loss at 12 months compared with a matched control group 24.
The strengths of this study include: using the updated and more rigorous consensus definition of Type 2 diabetes remission; examining 2‐year post‐surgery weight loss and Type 2 diabetes outcomes in people with Type 2 diabetes following RYGB and sleeve gastrectomy with > 100 people per group; use of a separate validation group; and correcting for baseline confounding factors, which enabled us to examine the impact of per cent weight loss per se. Our retrospective cohort study has several limitations. First, people were not randomized to RYGB or sleeve gastrectomy, leading to a potential selection bias and differences in baseline risk for Type 2 diabetes remission. Second, the date of Type 2 diabetes onset may be inaccurate due to a long period of unrecognized hyperglycaemia preceding clinical diagnosis, with the effect of underestimating Type 2 diabetes duration. Third, we examined 2‐year post‐surgery outcomes, which have been shown to impact subsequent microvascular complication rates. However, longer term studies are required. Our sample size was limited and data collection retrospective. A large multicentre randomized controlled prospective trial with prospective evaluation of the DiaRem and DiaBetter scores comparing per cent weight loss and Type 2 diabetes remission in people with Type 2 diabetes undergoing RYGB or sleeve gastrectomy encompassing a wider age range and different ethnicities is now warranted.
In conclusion, our single‐centre retrospective study comparing the impact of sleeve gastrectomy and RYGB upon combined remission of Type 2 diabetes at 2 years post surgery found that the DiaBetter and DiaRem scores had comparable predictive value in our population. Per cent weight loss rather than procedure type was associated with combined remission. Our findings suggest that per cent weight loss plays a major role in determining glycaemic improvements after metabolic bariatric procedures and provides a strong argument for management strategies focused upon optimizing weight loss following bariatric surgery.
Funding sources
RLB is funded by the National Institute for Health Research (NIHR), Sir Jules Thorn Charitable Trust, Rosetrees Trust, Robert Luff Foundation and Stoneygate Trust. AP is funded by Rosetrees Trust, Robert Luff Foundation, Stoneygate Trust. JMM is funded by NIHR.
Competing interests
None declared.
Supporting information
Figure S1. Flow diagram of people with Type 2 diabetes and obesity who underwent primary Roux‐en‐Y gastric bypass and sleeve gastrectomy.
Table S1. Distribution of the predictor variables used to derive DiaBetter score in each score group.
Table S2. The odds of Type 2 diabetes remission by predictor variables used to derive DiaBetter score.
Table S3. Comparison between baseline characteristics and weight loss outcomes of people undergoing Roux‐en‐Y gastric bypass or sleeve gastrectomy with complete data and incomplete data.
Table S4. Sensitivity, specificity, positive predictive values and negative predictive values of each DiaRem, and DiaBetter group.
Table S5. Baseline characteristics of the population used for the validation of the DiaBetter score including 2‐year post‐surgery per cent weight loss and percentage of combined remission for DiaBetter and DiaRem scores.
Table S6. Stratification of primary cohort of Roux‐en‐Y gastric bypass and sleeve gastrectomy into quintiles of per cent weight loss at 2 years post surgery.
Table S7. Stratification of primary cohort of Roux‐en‐Y gastric bypass and sleeve gastrectomy group into 5% weight loss groups at 2 years post surgery with frequency and percentages for each group.
Table S8. Odds of combined remission vs no remission when stratifying for quintiles of per cent weight loss at 2 years post surgery for Roux‐en‐Y gastric bypass and sleeve gastrectomy groups in the primary cohort.
Table S9. Odds of combined remission vs no remission when stratifying for groups of 1% and 5% weight loss at 2 years post surgery for Roux‐en‐Y gastric bypass and sleeve gastrectomy groups in the primary cohort.
Acknowledgements
We would like to thank all members, past and present, of the UCLH Bariatric Group.
Author contributions
A.Pucci, Finer and R.L. Batterham made substantial contributions to conception and design of the study. A. Pucci, W.H. Cheung and J.M Makaronidis performed the data collection. A. Pucci, U. Tymoszuk, S. Scholes and R.L. Batterham made substantial contributions to analysis and interpretation of data. M. Elkalaawy, M. Hashemi, A. Jenkinson, M. Adamo, N. Finer and R.L. Batterham were directly involved in the management of the patients. G. Tharakan, M. Guimaraes, M. Nora and M.P. Monteiro provided the validation cohort. A. Pucci and R.L. Batterham drafted the paper. All of the authors contributed to and approved the final draft of the report.
Diabet. Med. 35, 360–367 (2018)
References
- 1. Angrisani L, Santonicola A, Iovino P, Formisano G, Buchwald H, Scopinaro N. Bariatric surgery worldwide 2013. Obes Surg 2015; 25: 1822–1832. [DOI] [PubMed] [Google Scholar]
- 2. Schauer PR, Mingrone G, Ikramuddin S, Wolfe B. Clinical outcomes of metabolic surgery: efficacy of glycemic control, weight loss, and remission of diabetes. Diabetes Care 2016; 39: 902–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Adams TD, Arterburn DE, Nathan DM, Eckel RH. Clinical outcomes of metabolic surgery: microvascular and macrovascular complications. Diabetes Care 2016; 39: 912–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Manning S, Pucci A, Carter NC, Elkalaawy M, Querci G, Magno S et al Early postoperative weight loss predicts maximal weight loss after sleeve gastrectomy and Roux‐en‐Y gastric bypass. Surg Endosc 2015; 29: 1484–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yousseif A, Emmanuel J, Karra E, Millet Q, Elkalaawy M, Jenkinson AD et al Differential effects of laparoscopic sleeve gastrectomy and laparoscopic gastric bypass on appetite, circulating acyl‐ghrelin, peptide YY3‐36 and active GLP‐1 levels in non‐diabetic humans. Obes Surg 2014; 24: 241–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Keidar A, Hershkop KJ, Marko L, Schweiger C, Hecht L, Bartov N et al Roux‐en‐Y gastric bypass vs sleeve gastrectomy for obese patients with type 2 diabetes: a randomised trial. Diabetologia 2013; 56: 1914–1918. [DOI] [PubMed] [Google Scholar]
- 7. Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Brethauer SA, Navaneethan SD et al Bariatric surgery versus intensive medical therapy for diabetes – 3‐year outcomes. N Engl J Med 2014; 370: 2002–2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mingrone G, Panunzi S, De Gaetano A, Guidone C, Iaconelli A, Nanni G et al Bariatric–metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow‐up of an open‐label, single‐centre, randomised controlled trial. Lancet 2015; 386: 964–973. [DOI] [PubMed] [Google Scholar]
- 9. Rubino F, Nathan DM, Eckel RH, Schauer PR, Alberti KG, Zimmet PZ et al Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by International Diabetes Organizations. Diabetes Care 2016; 39: 861–877. [DOI] [PubMed] [Google Scholar]
- 10. Lee WJ, Hur KY, Lakadawala M, Kasama K, Wong SK, Chen SC et al Predicting success of metabolic surgery: age, body mass index, C‐peptide, and duration score. Surg Obes Relat Dis 2013; 9: 379–384. [DOI] [PubMed] [Google Scholar]
- 11. Still CD, Wood GC, Benotti P, Petrick AT, Gabrielsen J, Strodel WE et al Preoperative prediction of type 2 diabetes remission after Roux‐en‐Y gastric bypass surgery: a retrospective cohort study. Lancet Diabetes Endocrinol 2014; 2: 38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tharakan G, Scott R, Szepietowski O, Miras AD, Blakemore AI, Purkayastha S et al Limitations of the DiaRem Score in predicting remission of diabetes following Roux‐en‐Y gastric bypass (RYGB) in an ethnically diverse population from a single institution in the UK. Obes Surg 2017; 27: 782–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Manning S, Carter NC, Pucci A, Jones A, Elkalaawy M, Cheung WH et al Age‐ and sex‐specific effects on weight loss outcomes in a comparison of sleeve gastrectomy and Roux‐en‐Y gastric bypass: a retrospective cohort study. BMC Obes 2014; 1: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): the TRIPOD statement. Ann Intern Med 2015; 162: 55–63. [DOI] [PubMed] [Google Scholar]
- 15. DeLong ER, DeLong DM, Clarke‐Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988; 44: 837–845. [PubMed] [Google Scholar]
- 16. National Institute for Health and Care Excellence (NICE) . Obesity Identification Assessment and Management. Clinical guideline 189. Available at https://www.nice.org.uk/guidance/cg189/chapter/1-Recommendations#bariatric-surgery-for-people-with-recent-onset-type-2-diabetes Last accessed 21 November 2016. [Google Scholar]
- 17. Ma RC, Chan JC. Type 2 diabetes in East Asians: similarities and differences with populations in Europe and the United States. Ann NY Acad Sci 2013; 1281: 64–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mayer‐Davis EJ, Lawrence JM, Dabelea D, Divers J, Isom S, Dolan L et al Incidence trends of Type 1 and Type 2 diabetes among youths, 2002–2012. N Engl J Med 2017; 376: 1419–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang C, Yuan Y, Qiu C, Zhang W. A meta‐analysis of 2‐year effect after surgery: laparoscopic Roux‐en‐Y gastric bypass versus laparoscopic sleeve gastrectomy for morbid obesity and diabetes mellitus. Obes Surg 2014; 24: 1528–1535. [DOI] [PubMed] [Google Scholar]
- 20. Peterli R, Borbely Y, Kern B, Gass M, Peters T, Thurnheer M. et al Early results of the Swiss Multicentre Bypass or Sleeve Study (SM‐BOSS): a prospective randomized trial comparing laparoscopic sleeve gastrectomy and Roux‐en‐Y gastric bypass. Ann Surg 2013; 258: 690–694; discussion 695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Edelman S, Ng‐Mak DS, Fusco M, Ashton D, Okerson T, Liu Q et al Control of type 2 diabetes after 1 year of laparoscopic adjustable gastric banding in the helping evaluate reduction in obesity (HERO) study. Diabetes Obes Metab 2014; 16: 1009–1015. [DOI] [PubMed] [Google Scholar]
- 22. Steven S, Carey PE, Small PK, Taylor R. Reversal of Type 2 diabetes after bariatric surgery is determined by the degree of achieved weight loss in both short‐ and long‐duration diabetes. Diabet Med 2015; 32: 47–53. [DOI] [PubMed] [Google Scholar]
- 23. Sjoholm K, Sjostrom E, Carlsson LM, Peltonen M. Weight change‐adjusted effects of gastric bypass surgery on glucose metabolism: two‐ and 10‐year results from the Swedish Obese Subjects (SOS) Study. Diabetes Care 2016; 39: 625–631. [DOI] [PubMed] [Google Scholar]
- 24. Jassil FC, Manning S, Lewis N, Steinmo S, Kingett H, Lough F et al Feasibility and impact of a combined supervised exercise and nutritional–behavioral intervention following bariatric surgery: a pilot study. J Obes 2015; 2015: 693829. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Flow diagram of people with Type 2 diabetes and obesity who underwent primary Roux‐en‐Y gastric bypass and sleeve gastrectomy.
Table S1. Distribution of the predictor variables used to derive DiaBetter score in each score group.
Table S2. The odds of Type 2 diabetes remission by predictor variables used to derive DiaBetter score.
Table S3. Comparison between baseline characteristics and weight loss outcomes of people undergoing Roux‐en‐Y gastric bypass or sleeve gastrectomy with complete data and incomplete data.
Table S4. Sensitivity, specificity, positive predictive values and negative predictive values of each DiaRem, and DiaBetter group.
Table S5. Baseline characteristics of the population used for the validation of the DiaBetter score including 2‐year post‐surgery per cent weight loss and percentage of combined remission for DiaBetter and DiaRem scores.
Table S6. Stratification of primary cohort of Roux‐en‐Y gastric bypass and sleeve gastrectomy into quintiles of per cent weight loss at 2 years post surgery.
Table S7. Stratification of primary cohort of Roux‐en‐Y gastric bypass and sleeve gastrectomy group into 5% weight loss groups at 2 years post surgery with frequency and percentages for each group.
Table S8. Odds of combined remission vs no remission when stratifying for quintiles of per cent weight loss at 2 years post surgery for Roux‐en‐Y gastric bypass and sleeve gastrectomy groups in the primary cohort.
Table S9. Odds of combined remission vs no remission when stratifying for groups of 1% and 5% weight loss at 2 years post surgery for Roux‐en‐Y gastric bypass and sleeve gastrectomy groups in the primary cohort.


