Abstract
Fibroblasts are primary cellular protagonists of wound healing. They also exhibit circadian timekeeping which imparts a ~24-hour rhythm to their biological function. We interrogated the functional consequences of the cell-autonomous clockwork in fibroblasts using a proteome-wide screen for rhythmically expressed proteins. We observed temporal coordination of actin regulators that drives cell-intrinsic rhythms in actin dynamics. In consequence the cellular clock modulates the efficiency of actin-dependent processes such as cell migration and adhesion, which ultimately impact the efficacy of wound healing. Accordingly, skin wounds incurred during a mouse’s active phase exhibited increased fibroblast invasion in vivo and ex vivo, as well as in cultured fibroblasts and keratinocytes. Our experimental results correlate with the observation that the time of injury significantly affects healing after burns in humans, with daytime wounds healing ~60% faster than night-time wounds. We suggest that circadian regulation of the cytoskeleton influences wound healing efficacy from the cellular to the organismal scale.
Introduction
Circadian rhythms allow organisms to organise behaviour and physiology to an approximately 24-hour rhythm, facilitating adaptation to the environmental cycle of day and night. Whilst circadian rhythms in mammals are most evident at an organismal level, circadian timekeeping occurs cell-autonomously (1). The clock in every cell and tissue is synchronised in vivo by systemic cues such as body temperature and glucocorticoid signalling, which are themselves co-ordinated by a master clock in the hypothalamic suprachiasmatic nuclei (2). There is mounting evidence that circadian disruption, associated with modern lifestyles as well as aging, contributes to morbidities as diverse as cancer, cardiovascular disease and diabetes (3, 4). A major knowledge gap exists however, between the well-characterized circadian gene expression rhythms that occur in healthy peripheral tissues in vivo and the way in which different cell types exploit their innate clockwork to achieve beneficial circadian regulation of cell type-specific functions. The specific advantage conferred by the cell-intrinsic clockwork upon cellular function has not been explored for most cell types, but potentially holds the key to ameliorating the adverse effects of chronic circadian clock disruption.
The cellular clockwork is underpinned by cycles of ‘clock gene’ expression, wherein complexes containing Aryl hydrocarbon receptor nuclear translocator-like protein 1 (ARNTL/BMAL1) drive expression of E-box regulated genes, including the transcriptional co-repressor proteins Period (PER) and Cryptochrome (CRY), which eventually repress their own transcription. Beyond this core loop, BMAL1/PER/CRY-dependent expression of myriad ‘clock-controlled genes’ facilitates differential control of cellular activity across each circadian cycle (5). Indeed, in vivo the core clockwork orchestrates a considerable proportion of the transcriptome (3-16%) to a ~24-hour program, with the identity of specific ‘clock-controlled genes’ varying in a tissue-specific fashion (6).
Fibroblasts are a well-established model of the cell-autonomous clock and possess robust circadian rhythms in clock gene expression (1). However, transcriptomic analyses of cultured fibroblasts have revealed very few circadian-regulated transcripts compared with the number observed in tissues from mice (>3000 in liver compared with 11 in fibroblast cultures) (7). This indicates that in vivo a considerable part of circadian transcriptional regulation is driven systemically (7). Proteomic analyses, which additionally incorporate post-transcriptional circadian regulation, have been applied to whole tissues from mice; however, the extent to which the cell-autonomous clock impacts upon cellular protein abundance has not been investigated. The capacity of most cell types to keep time in isolation of systemic cues suggests functionality, but the advantage that it confers upon fibroblast-specific cellular functions has not been interrogated (2).
Within the body, fibroblasts are mesenchymal cells that serve to secrete extracellular matrix, a function that is especially salient during wound healing. Upon wounding, fibroblasts respond to chemotactic cues that stimulate proliferation and migration into the affected area (8). Fibroblast motility is driven by actin polymerisation at the leading edge of the cell to form protrusive lamellipodia and filopodia (9), and by disassembly of actin filaments at the trailing edge. Mutation of the actin binding protein CAP2, for example, causes wound healing defects in mice and reduced cell motility in scratch assays, associated with altered actin cytoskeletal dynamics and abnormal cell morphology (10).
Separately, it has been reported that certain parenchymal cell types exhibit circadian rhythms in actin polymerisation, and this was suggested to be controlled by systemic circadian cues rather than the cell-autonomous clockwork (11). The putative driver of these actin rhythms has not been identified however, and neither is it clear whether circadian regulation of the actin cytoskeleton has any functional consequence. One might anticipate that if circadian regulation of the actin cytoskeleton also occurs in mesenchymal cells, then this would confer an adaptive advantage upon processes that are especially reliant upon actin dynamics, such as cell motility during wound-healing. It is established that clock gene mutant rodents exhibit impaired wound healing phenotypes, but because clock genes have multiple functions beyond timekeeping, wound healing defects cannot confidently be ascribed to circadian dysfunction and whether the actual capacity to heal shows a daily rhythm has not been investigated (12–14).
Here we demonstrate that the cell-autonomous clock in fibroblasts drives a temporal proteomic program that imposes rhythmic regulation upon the actin cytoskeleton. We explore the functional consequences of this rhythm and reveal that the cellular clock regulates wound healing in vitro, ex vivo and in vivo through circadian control of cytoskeletal dynamics. Finally, we show that our findings are mirrored by a daily rhythm in the efficacy of healing in a post hoc analysis of human clinical burn data.
Results
Defining the cell-intrinsic circadian proteome
Although previous studies have effectively shown the extent of cell-autonomous transcriptional rhythms, it has become increasingly apparent that post-transcriptional mechanisms contribute to determining how the cellular clockwork asserts control of biological function (15–17). Primary fibroblasts exhibit well-characterised circadian rhythms in clock gene activity which persist under constant conditions for at least 6 weeks in vitro (18). To characterise the cell-autonomous circadian biology of fibroblasts we performed a proteome-wide screen for proteins where abundance changes as a function of cellular circadian timing.
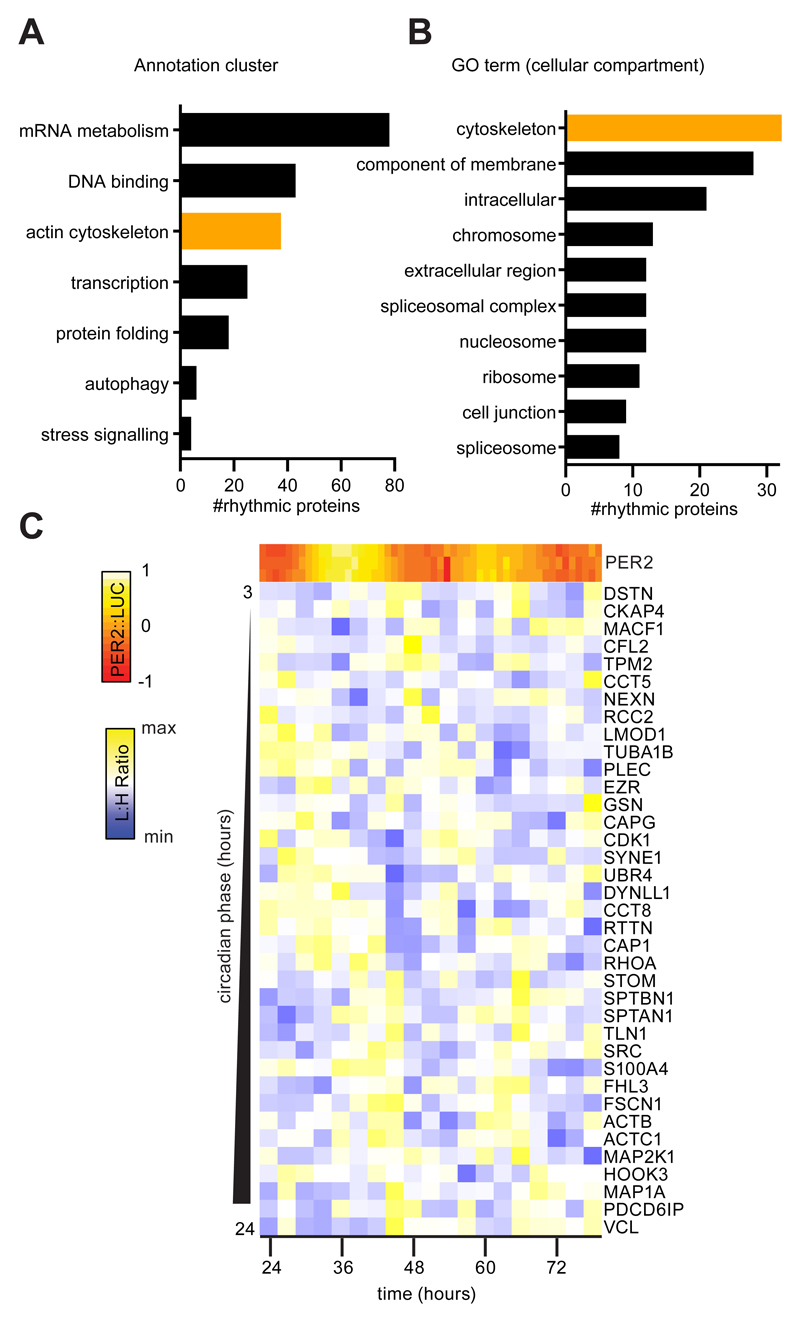
We quantified proteins in extracts from quiescent, confluent primary fibroblast cultures collected over two circadian cycles using Stable Isotope Labelling by Amino acids in Cell culture (SILAC) followed by mass spectrometry. The primary fibroblasts were isolated and expanded from mice expressing PER2 fused with Luciferase (PER2::LUC) which served as a parallel reporter of cellular timekeeping (19). Of 1608 proteins identified across the timecourse, 237 exhibited a robust circadian rhythm in abundance (P < 0.010, Fig. 1A and fig. S1 and (20)). Gene set enrichment analysis using the Database for Annotation, Visualization and Integrated Discovery (DAVID) yielded several gene annotation clusters containing enriched terms (Fig. 1A-B and table S1) that related to mRNA metabolism, DNA binding, the actin cytoskeleton, transcription, protein folding, autophagy and stress signalling (21).
Fig. 1. The cell-intrinsic fibroblast circadian proteome contains numerous cytoskeletal regulators.
A. Protein annotation clusters generated by DAVID containing terms enriched (P <0.10) with rhythmic protein abundances identified by Rhythmicity Analysis Incorporating Nonparametric methods (RAIN) (P<0.01) from analysis of primary lung fibroblasts from PER2::LUC mice. B. The 10 largest Gene Ontology (GO) (cellular compartment) terms within the rhythmic dataset by protein number. C. Mean abundance (Light:Heavy (L:H) ratio) of rhythmic proteins from the ‘actin cytoskeleton’ cluster determined by 3 SILAC experiments with 3 parallel PER2::LUC measurements indicating the circadian phase (heat map).
Cellular processes associated with several of these clusters have been previously characterised as circadian, particularly transcription and DNA binding (22). Given the importance of the cytoskeleton in directing cell motility and thus fibroblast function, we were intrigued by the number of cytoskeletal regulators, specifically actin regulators such as Cofilin 2 and RhoA, that were identified as rhythmic (Fig. 1C and fig S2). Individual terms which make up the ‘actin cytoskeleton’ cluster were not enriched (P=0.071 to 0.168, table S1). This indicates that, although certain proteins show abundance rhythms, there is not wholesale regulation of the pathway. However, the number of rhythmic cytoskeletal regulators is highly suggestive that activity may be clock-regulated, given that rhythmic abundance of a single control node could be sufficient to render cytoskeletal activity circadian as a result.
Cell intrinsic clock-control of actin dynamics
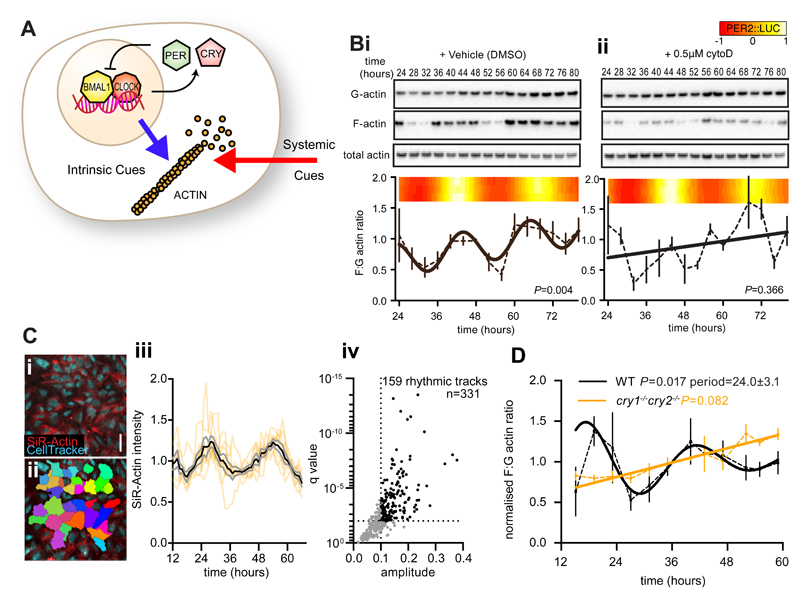
Actin dynamics exhibit circadian regulation in some peripheral tissues in vivo, and are proposed to regulate clock gene expression in response to systemic cues (11). In light of our proteomics data we asked whether actin dynamics might also be driven by the cell-intrinsic clockwork, in the absence of systemic timing cues (Fig. 2A). To test this, actin polymeric state was assayed over two circadian cycles in confluent (quiescent) monolayers of PER2::LUC fibroblasts. We observed a circadian rhythm in F:G actin ratio, with G actin generally in excess of F actin, and without any consistent rhythm in total actin abundance (Fig. 2B).
Fig. 2. CRY-dependent cell-intrinsic rhythms in actin polymerisation.
A. Schematic depicting rhythms in actin polymerisation, which may be cell intrinsic (blue arrow; driven by circadian gene expression) in addition to systemic cues (red arrow). B. Immunoblots using anti-actin antibody against fractionated and total protein from PER2::LUC fibroblasts at the indicated time after synchronisation in the presence of DMSO (i) or cytoD (0.5 μM) (ii). F:G actin ratio is quantified below, with best-fit curves from a comparison of fits (n=3 mean±Standard Error (SEM)). 3 parallel bioluminescent measurements (heat map) are included as a marker for the circadian clock. As G actin was in excess, exposures between western panels are not equivalent. C. Live cell recordings of actin abundance (SiR-actin) in cells labelled with Celltracker Green (i,ii, scale bar =100 μm). iii. SiR-actin intensity over time for 8 individual tracks (orange lines) with mean (black) ±SEM (grey) overlayed. Tracks with a circadian harmonic regression FDR (q value) <1% and amplitude >10% of the mean are highlighted and quantified (iv). D. F:G actin ratios from wild type (WT, black line) or cry1-/- cry2-/- (orange line) fibroblasts at the indicated time after synchronisation, with best-fit curves from a comparison of fits (n=3, mean±SEM, RAIN p-values indicated).
Cytochalasin D (cytoD) (Fig. 2B) is a drug which binds both F and G actin, preventing their interaction with cofilin, thereby decreasing the rates of both actin polymerisation as well as depolymerisation (23). We found that the rhythm in F:G actin ratio was disrupted by cytoD, indicating that fibroblast actin dynamics are normally under clock control. By phalloidin staining we confirmed that cells treated with cytoD showed disruption of the actin cytoskeleton, and noticed an increased incidence of binuclearity, but no other major morphological defects were apparent. CytoD treatment had no effect on cellular circadian rhythms, reported by PER2::LUC bioluminescence (Fig. 2B, fig. S3), and the fibroblast circadian clock was similarly resistant to other actin modulating drugs (jasplakinolide and latrunculin A). This indicates that the circadian rhythms of actin dynamics do not strongly feed back into the cell-intrinsic circadian clock mechanism; rather, they are an output from it (fig. S3), and circadian bioluminescent reporter rhythms were therefore unaffected. The periods of the F:G actin ratio rhythm and PER2:LUC were accordant, although the precise period of the F:G actin rhythm could not be determined as accurately as the PER2::LUC continuous recording, due to the comparatively lower sampling frequency (four-hourly vs. half-hourly) and its non-sinusoidal damping waveform.
Using the actin-binding silicon-rhodamine dye, SiR-Actin (24), we validated the rhythm in the total abundance of F actin in individual fibroblasts within a monolayer over 48 hours (Fig. 2C). Using harmonic regression analysis we found that 48% of cells had significant rhythms in F actin (false discovery rate <0.01 and amplitude >10% mean) (25).
Transcriptionally arrhythmic CRY-deficient fibroblasts displayed no rhythm in F:G actin ratio (Fig. 2D). Furthermore, we also observed F:G actin rhythms in another fibroblast line (NIH3T3) derived from mouse embryonic tissue (fig. S4).
To summarise, using two independent methods we detected cell-intrinsic circadian regulation of actin polymeric state that was dependent on cycling clock gene activity. We conclude that in fibroblasts, circadian regulation of actin dynamics is driven by cell-autonomous rhythms of clock gene expression.
A circadian rhythm in fibroblast wound healing
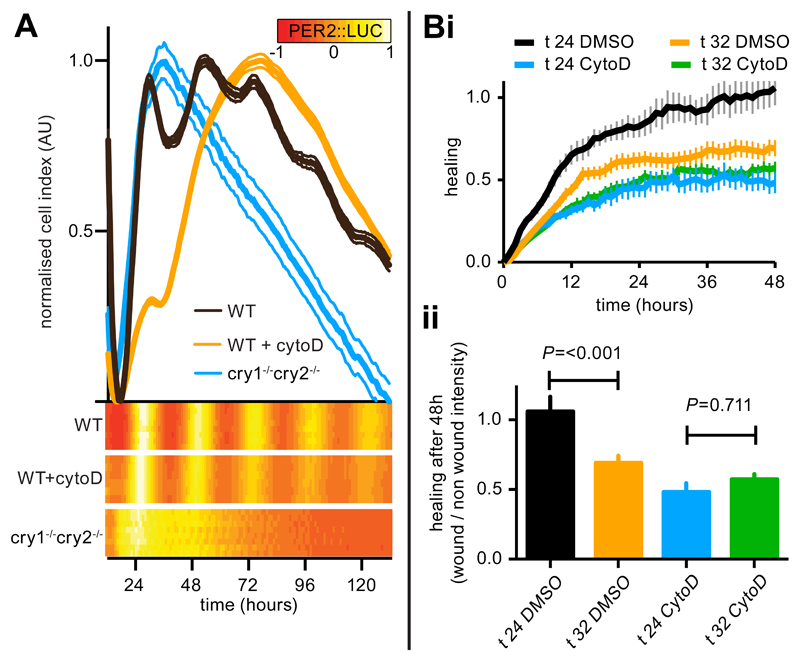
In vivo, the rapid fibroblast expansion at tissue wounds is known to result from both migration and proliferation of fibroblasts derived from local mesenchymal cells (8). To investigate the impact of actin cytoskeletal rhythms on cell migration we performed wound-healing assays on synchronised monolayers of immortalised skin fibroblasts at different circadian phases over 2 days under constant conditions (Fig. 3A). The fibroblasts showed striking circadian variations in the residual wound area after 16 hours of healing, with minimal healing elicited by wounds inflicted just after the nadir of PER2 expression, at 32-36 and then at 56-60 hours after synchronisation (Fig. 3A). In contrast, wounding at peak PER2 expression (20-24 or 44-48 hours after synchronisation) was followed by efficient, near complete healing of cell monolayers.
Fig. 3. A circadian rhythm in fibroblast wound healing response.
A. Fibroblast monolayers derived from adult PER2::LUC mouse skin were entrained and wounded after 20-64 hours in free run (i). (ii) Images of wound healing assays; time at wounding is indicated, residual wound is indicated by pink highlighting (scale bar = 500 μm). (iii) Quantification of the residual wound after 16 hours of wound healing (line, mean±SEM, n=4) with 3 parallel PER2::LUC measurements (heat maps). RAIN p-value indicated. Bi. Fibroblasts labelled with CellTracker Red healing after wounding at the indicated time after synchronisation (t). Scale=100 μm. Wound healing (ii) and leading cell velocity (iii) are quantified (n=4 or 5, ±SEM). P values from Tukey’s multiple comparisons test after 60 hours of healing (tH60) (ii) or tH0 (iii) are indicated. Ci Fibroblasts labelled with SiR-actin (red) and CellTracker Green (cyan) treated as in B (scale bar = 50 μm). A single cell for each condition has been highlighted in white with time healing indicated. The centre of mass for each label (ii) was determined over 9 hours of healing and the mean (±SEM) degree of polarisation (Δx) is indicated (iii). The P value from a two-way analysis of variance (ANOVA) is indicated.
To monitor cell motility more closely, we followed fibroblast migration into wounds by confocal microscopy in cultures of fibroblasts at phases where we observed the maximal and minimal healing responses (24 and 32 hours after synchronisation, respectively). Monolayers wounded after 24 hours re-established more efficiently than those wounded after 32 hours, and this difference remained apparent after 60 hours of healing (Fig. 3B, movies S1 and S2). Cell division occurred infrequently in these cultures and had no circadian organisation (fig. S5), suggesting that differential cell motility underpins the time-of-wounding effect. Indeed, the initial velocity of the most motile 10% of cells was significantly greater for wounds inflicted 24 hours after synchronisation compared with those wounded 32 hours after synchronisation (3.68±0.04 μm.sec-1 vs 2.78±0.03 μm.sec-1, P<0.0001) (Fig. 3B). There was no discernable circadian rhythm in cell velocity during healing, although circadian rhythms in PER2 expression remained evident throughout (fig. S6). Cell motility, cortical actin distribution and cell size lacked any discernable circadian organisation in the absence of wounding (fig. S6) indicating that the circadian variation in initial cell motility we observed is only unmasked upon the insult of wounding. The circadian variation in F:G actin and wound healing response was absent in arrhythmic control fibroblasts lacking CRY proteins (fig. S7).
To assess how the circadian rhythm in actin dynamics might contribute to the time-of-wounding effect on healing we used SiR-actin to dynamically follow actin distribution in cells undergoing healing. Wound-oriented polarisation of F-actin in cells undergoing healing was consistently greater when monolayers were wounded 24 hours after synchronisation rather than 32 hours after synchronisation (Fig. 3C). This is indicative of more efficient F actin enrichment at lamellipodia, the primary means by which fibroblasts effect migration (9). The time-dependent difference in F actin polarisation was sustained for at least the first 9 hours of healing. This is consistent with the migratory capacity of cells, adjacent to a nascent wound, being determined by the actin dynamical state at the circadian phase when the wound is incurred.
Uncoupling wound healing from the circadian clock
Cell adhesion is also dependent on the actin cytoskeleton. We thus thought it likely that BMAL1/PER/CRY-dependent circadian regulation of actin dynamics would impact similarly on cell adhesion, which can be detected directly through measurement of cellular impedance (26). Accordingly, continuous recording of cellular impedance in PER2::LUC cultures revealed a CRY-dependent circadian oscillation, with greatest adhesion in-phase with PER2 expression (Fig. 4A).
Fig. 4. Actin polymerisation rhythms are required for circadian regulation of adhesion and wound healing efficacy by fibroblasts.
A. Impedance measurements from cry1-/-cry2-/- (blue) or WT fibroblasts treated with DMSO (black) or cytoD (orange) with simultaneous PER2::LUC measurements (heat maps) (mean±SEM, n= 6-8). B. Quantification of mean fibroblast monolayer healing after wounding at the indicated time post-synchronisation (t) in the presence of 0.5 μM cytoD or vehicle (n=6-12, ±SEM). p-values from an ANOVA with Tukey’s test for multiple comparisons are indicated.
To establish causality, we reasoned that if PER:CRY-dependent oscillations in the abundance of actin regulators drive the rhythmic microfilament dynamics that underlie the rhythm in F:G actin ratio, which in turn direct rhythmic adhesion and the time-of-wounding effect on migration, then cytoD disruption of the rhythm in actin dynamics (Fig. 2B) would attenuate both rhythmic outputs (27). To test this we added 0.5 μM cytoD to PER2::LUC fibroblast monolayers and monitored impedance and the response to healing 24 or 32 hours after synchronisation, as well as PER2 abundance in parallel. Compared with controls, we observed that cellular impedance rhythms were more severely damped and no longer showed a circadian rhythm in the presence of cytoD (Fig. 4A). Whilst cells still migrated after wounding in the presence of cytoD, they became insensitive to the time of wounding (Fig. 4B and fig. S8). Therefore, rhythmic wound healing and adhesion result from circadian control of actin dynamics, but do not affect the cellular clock mechanism itself. Both circadian actin rhythms (Fig. 2), and their consequences (Fig. 4), can be uncoupled from clock-control by cytoD treatment, without affecting cellular timekeeping.
We considered that Rho activity might also contribute to the circadian differences in F:G actin ratio, wound healing and cell adhesion. Using the Rho inhibitor CT04 we observed damped rhythms in cell adhesion in PER2::LUC fibroblasts with only subtle effects on circadian rhythms in PER2 abundance (fig. S9). We performed wound-healing assays in cells treated with CT04 and observed abrogation of the time-of-wounding effect (fig. S9). The effect of Rho inhibition on impedance and wound healing rhythms suggests that changes in Rho activity might also contribute towards transmitting circadian timing information to the cytoskeleton.
Diurnal fibroblast mobilisation in vivo
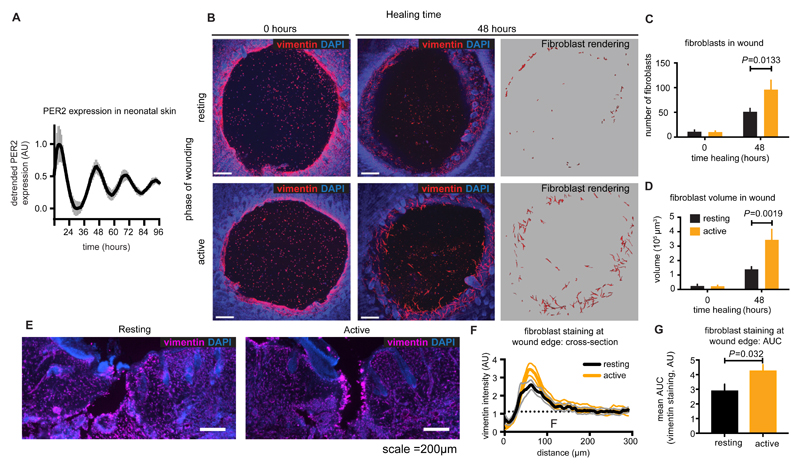
To investigate the functional consequences of rhythmicity within the fibroblast actin cytoskeleton in the setting of an intact tissue, we measured fibroblast mobilisation in an ex vivo murine skin explant model. Circadian rhythms in the skin of freely behaving mice have been demonstrated previously, and our in vitro experiments suggested that these would persist after wounding (28). Skin explants from PER2::LUC pups were mounted onto membranes and bioluminescence was monitored to confirm that the peripheral circadian clock continues to function in cultured skin ex vivo (Fig. 5A). Subsequently, skin from 5-day old mouse pups was harvested in the middle of the resting period or early active phase and the explant was wounded by biopsy punch and then mounted onto culture membranes. These times of day were chosen on the basis of our cell culture assays, being the times when we would expect to observe the greatest difference in cell migration. The migration of fibroblasts into heterologous blood clots filling the biopsy wounds was assayed by immunofluorescent staining against vimentin. We found that the number and total volume of fibroblasts invading the wound area was roughly two-fold greater in skin explants collected during the active phase than those made during the resting period (Fig. 5B-D, Movies S3 and S4). Finally, we wounded freely behaving adult mice by incision, again during the mid-resting or early active phases, and allowed them to heal for 48 hours (Fig. 5E). Fibroblast enrichment at the wound edge was again quantified using vimentin staining in transverse sections. Once more we observed that fibroblast mobilisation to wounds was significantly greater when wounds were inflicted during the active phase compared with the rest phase (P< 0.032, Fig. 5F & G). This indicates that the degree of fibroblast mobilisation in monolayers, skin explants and mice is dependent on the time-of-wounding.
Fig. 5. Diurnal variation in wound healing outcome and fibroblast mobilisation.
A. Bioluminescent recording of PER2 expression in neonatal (P5) skin explants from PER2::LUC mice (mean±SEM n=6). B. Mouse skin wounds before and after 48 hours of healing. Fibroblasts were identified by anti-vimentin reactivity (red) and morphology, and quantified by number (C ) and volume (D) (mean±SEM, n=6-7, Holm-Sidak’s adjusted P value is indicated). Scale bar =200 μm. E. 60 μm transverse sections of mouse wounds made during the active and resting phases stained using anti-vimentin (magenta) and Hoescht (blue). Cross-sectional vimentin staining across wound edges was quantified (F, mean±SEM) and Area Under Curve (AUC) was calculated using distal vimentin as a baseline (G) (mean±SEM, n=16 (active) or 20 (resting), P from a student’s t test is indicated).
Within healing tissue, fibroblasts synthesise new extracellular matrix by deposition of collagen and fibronectin (29). Active phase wounds might exhibit enhanced scar tissue formation due to increased collagen deposition, associated with more efficient fibroblast recruitment at this time. To test this we inflicted bilateral wounds to the upper dorsal skin of mice by full-depth biopsy punch, during the resting or active phase. We then allowed the skin to heal completely over 14 days, before measuring collagen distribution around the healed volume. Epidermal collagen deposition was significantly increased above the sites of wounds that were incurred during the active phase compared with the rest phase (P< 0.001, fig. S10). In contrast to our observations at 2 days post-wounding, after 14 days of healing fibroblasts no longer showed any significant time-of-wounding difference (fig. S10). This is consistent with the expectation that fibroblasts numbers plateau at the wound site within 14 days (30).
Circadian rhythms in keratinocyte wound healing
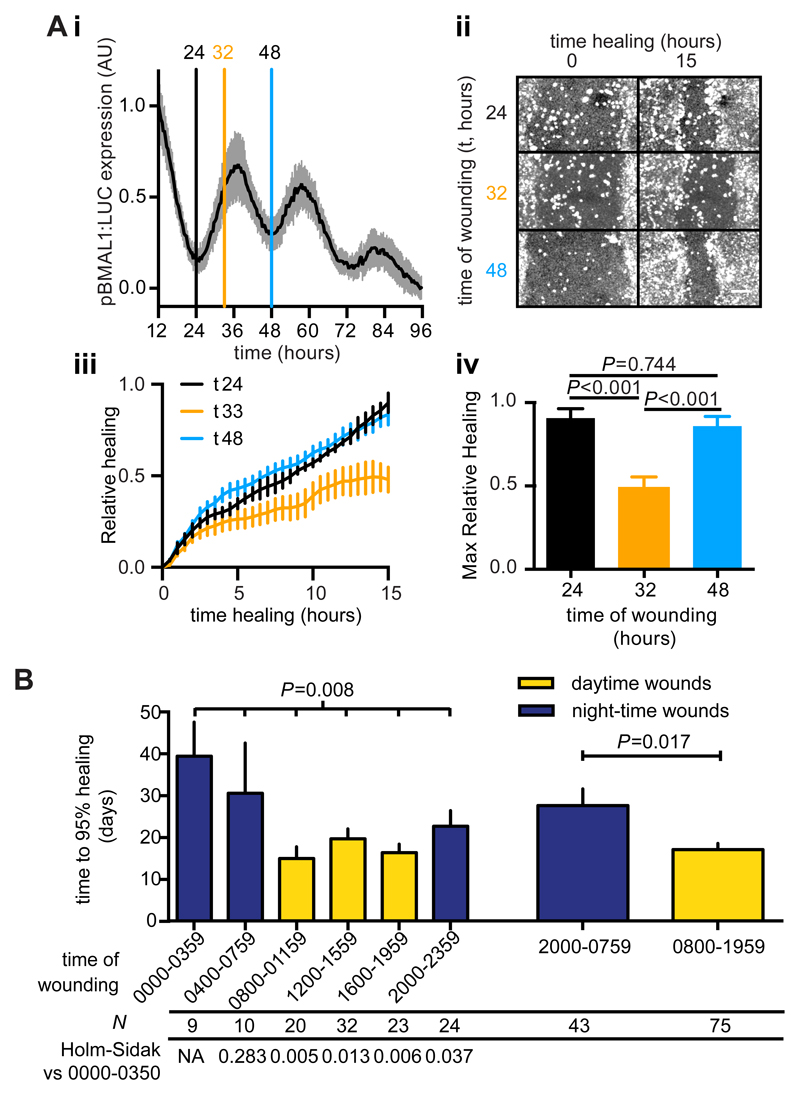
Wound healing is a complex process employing multiple cell types in addition to fibroblasts. Keratinocytes migrate into nascent dermal wound areas, and any circadian modulation of this response would be expected to contribute to circadian regulation of wound healing in vivo (31). To test whether keratinocytes also display circadian wound healing responses we wounded synchronised monolayers of human keratinocytes (HaCaT) at times when fibroblast motility was at its peak or nadir, corresponding to the minimum and near maximal BMAL1 promoter activity, respectively (Fig. 6A). We observed a marked reduction in healing when cells were wounded at 32 hours after synchronisation versus those wounded after 24 or 48 hours. There was no significant difference between the healing response when monolayers were wounded 24 or 48 hours after synchronisation, consistent with keratinocyte migration also being regulated by the circadian clock (Fig. 6A).
Fig. 6. A circadian rhythm in keratinocyte wound healing and a diurnal variation in human burn healing outcome.
A. Synchronised human HaCaT keratinocyte monolayers expressing luciferase under control of the BMAL1 promoter (i, mean±SD, n=24) were wounded at the indicated times (vertical lines) and healing monitored by confocal microscopy (ii). Relative fluorescence in the wound area (iii, mean±SEM n=4) was calculated and maximal healing after 15 hrs was compared by Tukey’s multiple comparisons test (iv, P values are indicated) B. Mean time to 95% healing ±SEM from 118 human burn incidents separated by time of burn occurrence in 4 (left) or 12 hour (right) bins. ANOVA P value is indicated, as is the P value for Welch’s t-test comparing daytime vs night-time wounds. P values from Holm-Sidak’s test versus the 0000-0359 bin are indicated below.
A time-of-wounding effect in human burn injury outcome
The observation that migration of at least two major cell types involved in wound healing is circadian regulated suggests that wound healing efficiency overall might be influenced by the biological time of wounding. Although it is unknown whether wound healing is under circadian regulation in vivo, it has been reported that mice carrying mutations of circadian clock genes have impaired wound healing phenotypes (12). If rather than being simply permissive for normal healing the circadian clock instead actively regulates tissue repair, thereby timing the most effective healing to when wounds are more likely to occur, we would expect to see time-of-day effects on wound healing. To this end, we analysed historical clinical data from the Burn Injury Database (iBID) and calculated the time required for human burn injuries to heal to 95% as a function of the time of day when the burn occurred (32). We observed an ~60% increase in healing time when burns occurred during the night compared with during the day (Fig. 6B). Whilst this post hoc analysis cannot prove that human wound healing is under clock control, we note that the time-of-day associated with optimal healing for human burns patients is consistent with our results from rodent and cellular models: all occurred at biological times when mammals would be most active, when they are most likely to incur a wound (fig. S11).
Discussion
Using a proteome-wide screen for rhythmic protein abundance we identified a concerted regulation of 32 cytoskeletal proteins (GO:0005856). Mapping these onto the ‘Regulation of Actin Cytoskeleton’ pathway (as defined by the Kyoto Encyclopaedia of Genes and Genomes (KEGG)) revealed that several actin effector proteins, such as Cofilin2, and the key control node, RhoA, are rhythmically expressed (fig. S12). This concerted circadian regulation of the actin cytoskeleton generates rhythmic actin dynamics, which in turn modulate the ability of cells to respond appropriately at the moment of wounding – identifying a potentially important function of the cellular clock in a specific peripheral cell type. Our findings build upon an earlier link between wound healing and the circadian clock identified by Kowalska et al (12). Kowalska et al utilised per1-/-/per2brdm/brdm and bmal1-/- mutant mice, well established genetic models that do not express circadian rhythms in behaviour or gene expression. In addition to their molecular clock function however, PER1 and PER2 are immediate early genes and tumour suppressors, whereas BMAL1 is a translation factor and regulator of the antioxidant response; consequently, some elements of each mutant’s phenotype are now known to be unrelated to timekeeping function (33–35). Kowalska et al quite reasonably focused on circadian gating of cell division as a basis for understanding circadian regulation of wound healing; we propose that circadian control of migration also plays a critical role. Using a combined in vitro and ex vivo approach we demonstrate a circadian rhythm in the efficacy of wound healing, allowing us to rule out any substantial contribution of circadian gating of cell division to the differential ability of fibroblasts to enact wound healing in this context.
Our functional data show that circadian regulation of wound-evoked cell motility likely largely contributes to wound healing and epidermal collagen deposition in vivo, in skin and other tissues. Cell-autonomous circadian regulation of migration was only unmasked in response to wounding and was underpinned by clock gene-dependent temporal organisation of actin dynamics, and associated with rhythmic cellular adhesion (fig. S11). That F actin is conducive to efficient wound healing is explained by its role in protrusive force production at lamellipodia in migrating cells (9). Rho and Rac-mediated signalling to the ARP2/3 complex and resulting cytoskeletal changes modulate cell adhesion and migration (36). As expected, the adhesion rhythms we observed were attenuated by both Cofilin and Rho inhibition, without disrupting clock gene expression cycles (Fig. 2 and fig. S9). Therefore, circadian regulation of the activity of Cofilin, Rho and other actin regulators is likely to be directly responsible for circadian actin dynamics in mammalian cells. Our findings complement reports of rhythmic Rho1 activity in the neurons of fruit flies, and lead us to speculate that circadian regulation of the cytoskeleton may be a conserved phenomenon in metazoans (37). However, rather than circadian control of a single Rho activator, we observed distributed control of actin regulatory pathways (fig. S12), incorporating multiple regulators.
The observation that wound healing is more efficient during the active phase could inform future clinical practice and has clear translational potential. Unfortunately we are currently unable to perform more in-depth analyses of the clinical dataset, to rule out severity changes over time for instance, due to the limitations of data-collection and sample size. In future, larger datasets should be gathered with circadian factors included. An experimental approach where wounds are produced in a controlled manner in a regulated environment would provide unequivocal evidence as to whether or not human wound healing is influenced by the circadian clock. We speculate that maximal healing could be promoted by pharmacological resetting of local cellular clocks prior to surgery, such as through topical application of chronoactive drugs (38).
Beyond adhesion and migration, the actin cytoskeleton is fundamental to eukaryotic cell biology; being essential to cell division, signal transduction, and pathogenesis (39–41). Its circadian regulation would therefore be likely to affect other aspects of biology of broad relevance to human health and disease.
Materials and Methods
Study design
The primary objective was to investigate the role of circadian rhythms in controlling actin dynamics and fibroblast mobilisation during wound healing. Data was generated by mass spectrometry and western blotting on cell extracts, longitudinal bioluminescent assays, microscopic analysis of cultured cells, cellular electrical impedance assays, and immunofluorescence on fixed skin sections. For all experiments replicate numbers are outlined in the methods or figure legend. Minimum sample size was determined by power analysis based on an estimated (from preliminary work) effect size with 15% sigma and 5% type 1 error rate, replicates were included such that 1-ß=0.9. Mice in all experiments were age-matched and randomised into groups. Experimenters were not blinded to experimental groups but where possible automated analysis was used to remove bias. Adverse animal welfare issues were sufficient to halt experiments, but did not arise during this work.
Cell culture and entrainment
Skin tissue from Mus musculus strain PER2::LUC was used to establish an immortalised fibroblast cell line using the method detailed in Seluanov et al (19, 42, 43, 45). cry1-/- cry2-/- fibroblast cells were similarly derived from otherwise isogenic cry1-/- cry2-/- mice (46). cry1-/- cry2-/- mouse embryonic fibroblasts (MEF) and isogenic wild-type MEFs were obtained from Akhilesh Reddy. All mouse strains were gifts from Michael Hastings and were kept under the auspices of Home Office Project Licence 70/7903, under UK Animals (Scientific Procedures) Act, 1986. NIH3T3 fibroblasts and human keratinocyte (HaCaT) cells stably expressing pBMAL1:LUC were made by transfecting cells (ATCC-CRL-1658, or HaCaT cells kindly provided by Prof. Achim Kramer (47)) with a construct encoding firefly luciferase downstream of the BMAL1 promoter in pGL4.22 (Promega) and selected for puromycin resistance. Circadian entrainment was by 12h:12h 32°C:37°C temperature cycling for 3 days. For end-point analysis, plates of cells were washed with phosphate buffered saline (PBS) and lysed at the specified time after the last entraining stimulus. Details of lysis and processing are included in Supplemental Methods. Circadian rhythmicity was assessed either by RAIN, harmonic regression or by non-linear regression to a damped sine wave (see Supplemental Methods). Cells were maintained under absolutely identical conditions to the controls used for circadian bioluminescence recording in every case - see supplementary methods and Feeney et al for additional details (44).
Live cell actin analysis
Cells were labelled with 1 μM CellTracker Green (Thermo) and 100 nM Silicon-Rhodamine (SiR)-actin (Cytoskeleton.org) was included in the media throughout entrainment and image acquisition. A Leica SP8 confocal microscope with a 10x/0.40 objective was used to capture 7 μm z-sections covering the entire monolayer thickness every 60 minutes. Average z-projections were generated for each time-point and individual cells tracked using the Nikon Elements General Analysis suite. The SiR-actin signal from each cell track over 24 hours long was subjected to harmonic regression analysis to determine rhythmicity (25).
For analysis of F-actin distribution during healing, cells were delimited manually. The centres of mass for CellTracker Green (cell body) and SiR-actin (F-actin) were calculated using ImageJ (National Institutes of Health). The difference in position was calculated for each time-point and used as a measure of the degree of actin polarisation in the cell. We were not able to conduct a detailed analysis of stress fibres in these cultures due to limitations in spatial resolution and signal:noise ratio, both of which are essential to reliable identification of bona fide stress fibres.
Monolayer healing assays
Live-cell and endpoint scratch assays were performed by wounding synchronised monolayers at defined timepoints with a 200 μL plastic pipette tip and allowing healing at constant 37°C. Healing was assessed by the relative size of residual wound areas or using CellTracker dye-based assays on a confocal microscope as detailed in our supplementary methods.
Ex vivo wound healing assays
PER2::LUC pups were raised in 12hr:12hr L:D cycles from birth. For ex vivo assays, on P5 they were sacrificed by cervical dislocation at Zeitgeber Time (ZT) 5 or ZT13 where ZT0 is ‘lights on’. Skin explants ~0.5 cm2 were obtained from each mouse and 1.5 mm circular holes made in the explants using a biopsy punch. The explants were then mounted onto 0.4 μm millicell cell culture inserts (Millipore) and 4 μL of platelet rich plasma (PRP) +10% (see supplemental methods) calcium carbonate was added to each wound area and allowed to clot for 5 minutes. The explants were grown in Dulbecco’s Modified Eagle’s Medium (DMEM) + 10% Hyclone III serum at 37 °C, 5% CO2 for 48 hours prior to fixation with 3% paraformaldehyde and immunostaining for vimentin to identify fibroblasts
In vivo wound healing
PER2::LUC mice were raised in 12hr:12hr L:D cycles from birth. Age-matched (3-5 month-old) mice were shaved and wounded by a 1 cm incision at ZT5 or ZT13 where ZT0 is ‘lights on’ and allowed to heal for 48 hours in 12hr:12hr L:D cycles. Age-matched (1-2 month-old) mice were subjected to two bilateral full-thickness 1.5mm circular biopsy punches to the upper back and allowed 14 days of healing in 12hr:12hr L:D cycles. Mice were sacrificed and wounded skin areas were excised and fixed with 4% PFA on 0.4 μm millicell cell culture inserts (Millipore). 60 μm transverse sections were made in frozen gelatin prior to immunostaining. Fibroblasts were identified by immunostaining with rabbit anti-vimentin, mouse anti-PDGFRα or rabbit anti-CD26 antibody (Abcam) and Alexa647 conjugated donkey anti-rabbit/mouse IgG (Thermo). Fibroblast enrichment at the wound edge after 48 hours was measured as follows. The longest continuous wound edge from each section was manually traced. The mean vimentin signal from the edge to 300 μm into the tissue was quantified along the length of the wound giving vimentin signal versus distance from wound edge (Fig.5F). The enrichment of vimentin was defined as the area under the curve (AUC) using the mean vimentin signal between 200-300 μM from the edge as the baseline (Fig.5G). Fibroblast distribution after 14 days of healing was analysed by mean fluorescent intensity from the epical edge of the signal at the centre of the wound to a depth of 200 μm. Vimentin and PDGFRα stained the dermis, CD26 stained the epidermis and dermis. The total signal for each fibroblast marker was quantified by AUC to a depth of 100 or 200 μm.
Collagen deposition was measured after 14 days by staining the sections for 3 hours with Col-F (ImmunoChemistry laboratories). The Col-F signal was quantified along the full thickness of the epidermis for >2 mm, centred on the wound. The Col-F was quantified by calculating the AUC using the distal signal as a baseline.
Human data
Anonymised records were obtained from a specialized burn database (iBID) which records outcomes from all the major burns units in England and Wales (32). In 2012, time of injury and number of days until 95% healing began to be recorded. Burns were included if a 95% heal time was recorded on the database and the subject had been admitted via the emergency department for their burn. Subject inclusion criteria were 18-60 years old with a body mass index (BMI) between 20-30 kg/m2. Subject records were excluded if they had any previous diseases or a skin graft was used in the treatment of their burn, leaving 118 patients in total (2012-2015).
Statistical analysis
For this study we utilised the following statistical methods; GO enrichment analysis using DAVID, RAIN, the extra sum-of-squares F test for comparison of nonlinear regression fits, harmonic regression, two-way ANOVA with and without Tukey’s multiple comparisons correction, two-way ANOVA with the Holm-Sidak adjustment, one-way ANOVA, Student’s and Welch’s t-test. They are each detailed along with the relevant parameters in our methods and supplemental methods and figure legends. In general P <0.05 was considered significant and P <0.01 highly significant.
Supplementary Material
table S2. Individual subject-level data for N < 20
Healing of fibroblast monolayers wounded 24 hours post-synchronisation
Healing of fibroblast monolayers wounded 32 hours post-synchronisation
Fibroblast invasion of wounds made in the active phase after 48 hours of healing
Fibroblast invasion of wounds made in the inactive phase after 48 hours of healing
One Sentence Summary.
The circadian clock in fibroblasts determines the efficiency of wound healing through rhythmic regulation of actin cytoskeletal dynamics.
Acknowledgments
At the MRC LMB, we are grateful to the Biomedical Services Group for animal care, Mark Skehel and his team for mass spectrometry, and Stefano Giandomenico for help with tissue sectioning. We would like to thank Priya Crosby, Michael Hastings, Liz Maywood, Simon Bullock, Emmanuel Derivery and Phil Jones for valuable discussion. We thank Achim Kramer for the gift of HaCaT cells, and Akhilesh Reddy for MEFs. Funding: JSO is supported by the Medical Research Council (MC_UP_1201/4) and the Wellcome Trust (093734/Z/10/Z). MP is funded by KWF BUIT 2014-6637. JB holds a MRC clinician scientist award (MR/L006499/1).
Footnotes
Author contributions: NPH, MP, ES, JMT and TPK performed the experiments. JC, KAF, and JSON derived the mouse cell lines. KD and JB performed the iBID analysis. NPH and JSON designed the experiments and wrote this manuscript.
Competing Interests: None declared.
Data and materials availability: All data for this study are included in table S2.
References
- 1.Balsalobre A, Damiola F, Schibler U. A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell. 1998;93:929–937. doi: 10.1016/s0092-8674(00)81199-x. [DOI] [PubMed] [Google Scholar]
- 2.Schibler U, Gotic I, Saini C, Gos P, Curie T, Emmenegger Y, Sinturel F, Gosselin P, Gerber A, Fleury-Olela F, Rando G, et al. Clock-Talk: Interactions between Central and Peripheral Circadian Oscillators in Mammals. Cold Spring Harb Symp Quant Biol. 2015;80:223–232. doi: 10.1101/sqb.2015.80.027490. [DOI] [PubMed] [Google Scholar]
- 3.He C, Anand ST, Ebell MH, Vena JE, Robb SW. Circadian disrupting exposures and breast cancer risk: a meta-analysis. Int Arch Occup Environ Health. 2015;88:533–547. doi: 10.1007/s00420-014-0986-x. [DOI] [PubMed] [Google Scholar]
- 4.Gale JE, Cox HI, Qian J, Block GD, Colwell CS, Matveyenko AV. Disruption of circadian rhythms accelerates development of diabetes through pancreatic beta-cell loss and dysfunction. J Biol Rhythms. 2011;26:423–433. doi: 10.1177/0748730411416341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doherty CJ, Kay SA. Circadian control of global gene expression patterns. Annu Rev Genet. 2010;44:419–444. doi: 10.1146/annurev-genet-102209-163432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang R, Lahens NF, Ballance HI, Hughes ME, Hogenesch JB. A circadian gene expression atlas in mammals: implications for biology and medicine. Proc Natl Acad Sci U S A. 2014;111:16219–16224. doi: 10.1073/pnas.1408886111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hughes ME, DiTacchio L, Hayes KR, Vollmers C, Pulivarthy S, Baggs JE, Panda SP, Hogenesch JB. Harmonics of circadian gene transcription in mammals. PLoS Genet. 2009;5 doi: 10.1371/journal.pgen.1000442. e1000442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eming SA, Martin P, Tomic-Canic M. Wound repair and regeneration: mechanisms, signaling, and translation. Sci Transl Med. 2014;6 doi: 10.1126/scitranslmed.3009337. 265sr6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krause M, Gautreau A. Steering cell migration: lamellipodium dynamics and the regulation of directional persistence. Nat Rev Mol Cell Biol. 2014;15:577–590. doi: 10.1038/nrm3861. [DOI] [PubMed] [Google Scholar]
- 10.Kosmas K, Eskandarnaz A, Khorsandi AB, Kumar A, Ranjan R, Eming SA, Noegel AA, Peche VS. CAP2 is a regulator of the actin cytoskeleton and its absence changes infiltration of inflammatory cells and contraction of wounds. Eur J Cell Biol. 2015;94:32–45. doi: 10.1016/j.ejcb.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 11.Gerber A, Esnault C, Aubert G, Treisman R, Pralong F, Schibler U. Blood-borne circadian signal stimulates daily oscillations in actin dynamics and SRF activity. Cell. 2013;152:492–503. doi: 10.1016/j.cell.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 12.Kowalska E, Ripperger JA, Hoegger DC, Bruegger P, Buch T, Birchler T, Mueller A, Albrecht U, Contaldo C, Brown SA. NONO couples the circadian clock to the cell cycle. Proc Natl Acad Sci U S A. 2013;110:1592–1599. doi: 10.1073/pnas.1213317110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fu L, Pelicano H, Liu J, Huang P, Lee C. The circadian gene Period2 plays an important role in tumor suppression and DNA damage response in vivo. Cell. 2002;111:41–50. doi: 10.1016/s0092-8674(02)00961-3. [DOI] [PubMed] [Google Scholar]
- 14.Kondratov RV, Kondratova AA, Gorbacheva VY, Vykhovanets OV, Antoch MP. Early aging and age-related pathologies in mice deficient in BMAL1, the core componentof the circadian clock. Genes Dev. 2006;20:1868–1873. doi: 10.1101/gad.1432206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jang C, Lahens NF, Hogenesch JB, Sehgal A. Ribosome profiling reveals an important role for translational control in circadian gene expression. Genome Res. 2015;25:1836–1847. doi: 10.1101/gr.191296.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robles MS, Cox J, Mann M. In-vivo quantitative proteomics reveals a key contribution of post-transcriptional mechanisms to the circadian regulation of liver metabolism. PLoS Genet. 2014;10 doi: 10.1371/journal.pgen.1004047. e1004047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Atger F, Gobet C, Marquis J, Martin E, Wang J, Weger B, Lefebvre G, Descombes P, Naef F, Gachon F. Circadian and feeding rhythms differentially affect rhythmic mRNA transcription and translation in mouse liver. Proc Natl Acad Sci U S A. 2015;112:E6579–88. doi: 10.1073/pnas.1515308112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leise TL, Wang CW, Gitis PJ, Welsh DK. Persistent cell-autonomous circadian oscillations in fibroblasts revealed by six-week single-cell imaging of PER2::LUC bioluminescence. PLoS ONE. 2012;7 doi: 10.1371/journal.pone.0033334. e33334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoo S-H, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, Siepka SM, Hong H-K, Oh WJ, Yoo OJ, Menaker M, et al. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci U S A. 2004;101:5339–5346. doi: 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thaben PF, Westermark PO. Detecting rhythms in time series with RAIN. J Biol Rhythms. 2014;29:391–400. doi: 10.1177/0748730414553029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang DW, Sherman BT, Tan Q, Kir J, Liu D, Bryant D, Guo Y, Stephens R, Baseler MW, Lane HC, Lempicki RA. DAVID Bioinformatics Resources: expanded annotation database and novel algorithms to better extract biology from large gene lists. Nucleic Acids Res. 2007;35:W169–75. doi: 10.1093/nar/gkm415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koike N, Yoo S-H, Huang H-C, Kumar V, Lee C, Kim T-K, Takahashi JS. Transcriptional architecture and chromatin landscape of the core circadian clock in mammals. Science. 2012;338:349–354. doi: 10.1126/science.1226339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shoji K, Ohashi K, Sampei K, Oikawa M, Mizuno K. Cytochalasin D acts as an inhibitor of the actin-cofilin interaction. Biochem Biophys Res Commun. 2012;424:52–57. doi: 10.1016/j.bbrc.2012.06.063. [DOI] [PubMed] [Google Scholar]
- 24.Lukinavičius G, Reymond L, D’Este E, Masharina A, Göttfert F, Ta H, Güther A, Fournier M, Rizzo S, Waldmann H, Blaukopf C, et al. Fluorogenic probes for live-cell imaging of the cytoskeleton. Nat Methods. 2014;11:731–733. doi: 10.1038/nmeth.2972. [DOI] [PubMed] [Google Scholar]
- 25.Lück S, Thurley K, Thaben PF, Westermark PO. Rhythmic degradation explains and unifies circadian transcriptome and proteome data. Cell Rep. 2014;9:741–751. doi: 10.1016/j.celrep.2014.09.021. [DOI] [PubMed] [Google Scholar]
- 26.Atienza JM, Zhu J, Wang X, Xu X, Abassi Y. Dynamic monitoring of cell adhesion and spreading on microelectronic sensor arrays. Journal of biomolecular screening. 2005;10:795–805. doi: 10.1177/1087057105279635. [DOI] [PubMed] [Google Scholar]
- 27.Hayot C, Debeir O, Van Ham P, Van Damme M, Kiss R, Decaestecker C. Characterization of the activities of actin-affecting drugs on tumor cell migration. Toxicology and applied pharmacology. 2006;211:30–40. doi: 10.1016/j.taap.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 28.Hamada T, Sutherland K, Ishikawa M, Miyamoto N, Honma S, Shirato H, Honma K-I. In vivo imaging of clock gene expression in multiple tissues of freely moving mice. Nat Commun. 2016;7:11705. doi: 10.1038/ncomms11705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shaw TJ, Martin P. Wound repair: a showcase for cell plasticity and migration. Curr Opin Cell Biol. 2016;42:29–37. doi: 10.1016/j.ceb.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 30.Grierson I, Joseph J, Miller M, Day JE. Wound repair: the fibroblast and the inhibition of scar formation. Eye (Lond) 1988;2(Pt 2):135–148. doi: 10.1038/eye.1988.27. [DOI] [PubMed] [Google Scholar]
- 31.Pastar I, Stojadinovic O, Yin NC, Ramirez H, Nusbaum AG, Sawaya A, Patel SB, Khalid L, Isseroff RR, Tomic-Canic M. Epithelialization in wound healing: A comprehensive review. Adv Wound Care (New Rochelle) 2014;3:445–464. doi: 10.1089/wound.2013.0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stylianou N, Buchan I, Dunn KW. A model of British in-hospital mortality among burns patients. Burns : journal of the International Society for Burn Injuries. 2014;40:1316–1321. doi: 10.1016/j.burns.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 33.Lipton JO, Yuan ED, Boyle LM, Ebrahimi-Fakhari D, Kwiatkowski E, Nathan A, Güttler T, Davis F, Asara JM, Sahin M. The Circadian Protein BMAL1 Regulates Translation in Response to S6K1-Mediated Phosphorylation. Cell. 2015;161:1138–1151. doi: 10.1016/j.cell.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Musiek ES, Lim MM, Yang G, Bauer AQ, Qi L, Lee Y, Roh JH, Ortiz-Gonzalez X, Dearborn JT, Culver JP, Herzog ED, et al. Circadian clock proteins regulate neuronal redox homeostasis and neurodegeneration. J Clin Invest. 2013;123:5389–5400. doi: 10.1172/JCI70317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang G, Chen L, Grant GR, Paschos G, Song W-L, Musiek ES, Lee V, McLoughlin SC, Grosser T, Cotsarelis G, FitzGerald GA. Timing of expression of the core clock gene Bmal1 influences its effects on aging and survival. Sci Transl Med. 2016;8:324ra16. doi: 10.1126/scitranslmed.aad3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bear JE, Haugh JM. Directed migration of mesenchymal cells: where signaling and the cytoskeleton meet. Curr Opin Cell Biol. 2014;30:74–82. doi: 10.1016/j.ceb.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Petsakou A, Sapsis TP, Blau J. Circadian rhythms in rho1 activity regulate neuronal plasticity and network hierarchy. Cell. 2015;162:823–835. doi: 10.1016/j.cell.2015.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cuesta M, Cermakian N, Boivin DB. Glucocorticoids entrain molecular clock components in human peripheral cells. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2014 doi: 10.1096/fj.14-265686. [DOI] [PubMed] [Google Scholar]
- 39.Esnault C, Stewart A, Gualdrini F, East P, Horswell S, Matthews N, Treisman R. Rho-actin signaling to the MRTF coactivators dominates the immediate transcriptional response to serum in fibroblasts. Genes Dev. 2014;28:943–958. doi: 10.1101/gad.239327.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Welch MD, Way M. Arp2/3-mediated actin-based motility: a tail of pathogen abuse. Cell Host Microbe. 2013;14:242–255. doi: 10.1016/j.chom.2013.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heng Y-W, Koh C-G. Actin cytoskeleton dynamics and the cell division cycle. Int J Biochem Cell Biol. 2010;42:1622–1633. doi: 10.1016/j.biocel.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 42.Feeney KA, Hansen LL, Putker M, Olivares-Yañez C, Day J, Eades LJ, Larrondo LF, Hoyle NP, O’Neill JS, van Ooijen G. Daily magnesium fluxes regulate cellular timekeeping and energy balance. Nature. 2016;532:375–379. doi: 10.1038/nature17407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Putker M, Crosby P, Feeney KA, Hoyle NP, Costa ASH, Gaude E, Frezza C, O’Neill JS. Mammalian circadian period, but not phase and amplitude, is robust against redox and metabolic perturbations. Antioxid Redox Signal. 2017 doi: 10.1089/ars.2016.6911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Feeney KA, Putker M, Brancaccio M, O’Neill JS. In-depth Characterization of Firefly Luciferase as a Reporter of Circadian Gene Expression in Mammalian Cells. J Biol Rhythms. 2016;31:540–550. doi: 10.1177/0748730416668898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seluanov A, Vaidya A, Gorbunova V. Establishing primary adult fibroblast cultures from rodents. J Vis Exp. 2010 doi: 10.3791/2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van der Horst GT, Muijtjens M, Kobayashi K, Takano R, Kanno S, Takao M, de Wit J, Verkerk A, Eker AP, van Leenen D, Buijs R, et al. Mammalian Cry1 and Cry2 are essential for maintenance of circadian rhythms. Nature. 1999;398:627–630. doi: 10.1038/19323. [DOI] [PubMed] [Google Scholar]
- 47.Spörl F, Schellenberg K, Blatt T, Wenck H, Wittern K-P, Schrader A, Kramer A. A circadian clock in HaCaT keratinocytes. J Invest Dermatol. 2011;131:338–348. doi: 10.1038/jid.2010.315. [DOI] [PubMed] [Google Scholar]
- 48.Baeker V, Cahuzac N, Georget V. Montpellier RIO Imaging, ImageJ Macros. available at ( http://www.mri.cnrs.fr).
- 49.Franco D, Franco T, Schettino AM, Filho JMT, Vendramin FS. Protocol for obtaining platelet-rich plasma (PRP), platelet-poor plasma (PPP), and thrombin for autologous use. Aesthetic Plast Surg. 2012;36:1254–1259. doi: 10.1007/s00266-012-9957-3. [DOI] [PubMed] [Google Scholar]
- 50.Burkel BM, von Dassow G, Bement WM. Versatile fluorescent probes for actin filaments based on the actin-binding domain of utrophin. Cell Motil Cytoskeleton. 2007;64:822–832. doi: 10.1002/cm.20226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bosgraaf L, van Haastert PJM, Bretschneider T. Analysis of cell movement by simultaneous quantification of local membrane displacement and fluorescent intensities using Quimp2. Cell Motil Cytoskeleton. 2009;66:156–165. doi: 10.1002/cm.20338. [DOI] [PubMed] [Google Scholar]
- 52.Wiśniewski JR, Zougman A, Nagaraj N, Mann M. Universal sample preparation method for proteome analysis. Nat Methods. 2009;6:359–362. doi: 10.1038/nmeth.1322. [DOI] [PubMed] [Google Scholar]
- 53.Cox J, Mann M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat Biotechnol. 2008;26:1367–1372. doi: 10.1038/nbt.1511. [DOI] [PubMed] [Google Scholar]
- 54.Motulsky HJ, Brown RE. Detecting outliers when fitting data with nonlinear regression - a new method based on robust nonlinear regression and the false discovery rate. BMC Bioinformatics. 2006;7:123. doi: 10.1186/1471-2105-7-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Welsh DK, Yoo S-H, Liu AC, Takahashi JS, Kay SA. Bioluminescence imaging of individual fibroblasts reveals persistent, independently phased circadian rhythms of clock gene expression. Curr Biol. 2004;14:2289–2295. doi: 10.1016/j.cub.2004.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.O’Neill JS, Hastings MH. Increased coherence of circadian rhythms in mature fibroblast cultures. J Biol Rhythms. 2008;23:483–488. doi: 10.1177/0748730408326682. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Healing of fibroblast monolayers wounded 24 hours post-synchronisation
Healing of fibroblast monolayers wounded 32 hours post-synchronisation
Fibroblast invasion of wounds made in the active phase after 48 hours of healing
Fibroblast invasion of wounds made in the inactive phase after 48 hours of healing