Abstract
Fc gamma receptors (FcγR) are involved in multiple aspects of immune cell regulation, are central to the success of monoclonal antibody (mAb) therapeutics and underpin the pathology of several autoimmune diseases. However, reliable assays capable of accurately measuring FcγR interactions with their physiological ligands, immunoglobulin G (IgG) immune complexes (IC), are limited. A method to study and detect IC interactions with FcγRs was therefore developed. This method, designed to model the signalling pathway of the inhibitory FcγRIIB (CD32B), utilised NanoLuc® Binary Interaction Technology (NanoBiT™) to measure recruitment of the Src homology 2 (SH2) domain-containing inositol phosphatase 1 (SHIP-1) to the immunoreceptor tyrosine-based inhibitory motif (ITIM) of this receptor. Such recruitment required prior crosslinking of an immunoreceptor tyrosine-based activation motif (ITAM)-containing activatory receptor, and evoked luciferase activity in discrete clusters at the cell surface, recapitulating the known biology of CD32B signalling. The assay detected varying forms of experimental IC, including heat-aggregated IgG, Rituximab:anti-idiotype complexes and anti-trinitrophenol (TNP)-TNP complexes in a sensitive manner (≤1µg/ml), and discriminated between complexes of varying size and isotype. Proof-of-concept for the detection of circulating ICs in autoimmune disease was provided, as responses to sera from patients with systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA) were detected in small pilot studies. Finally, the method was translated to a stable cell line system. In conclusion, a rapid and robust method for the detection of IC was developed, which has numerous potential applications including the monitoring of IC in autoimmune diseases and the study of underlying FcγR biology.
Keywords: FcγRIIB, SHIP-1, immune complex, SLE
Introduction
The interaction between IgG molecules and their receptors, FcγRs, is involved in maintaining immune homeostasis, by mediating processes such as pathogen clearance(1), and providing regulation to FcγR-expressing immune cells including B cells(2) and myeloid cells(3). However, in autoimmune disease, this balance is lost, resulting in an increased production of autoantibodies(4) and consequently IC, which are capable of immune activation(5, 6). Conversely, recent studies have indicated that an excess of IC may inhibit FcγR-mediated effector mechanisms(7, 8).
Human immune cells differentially express four ITAM-containing activatory FcγRs: CD16A (FcγRIIIA), CD32A (FcγRIIA), CD32C (FcγRIIC) and CD64 (FcγRI); a postulated “neutral”, glycosylphosphatidyl inositol-linked receptor, CD16B (FcγRIIIB); and a single ITIM-containing inhibitory FcγR, CD32B (FcγRIIB)(9). The ligands of FcγRs include cell-bound or cell-free IC, and with the exception of the high-affinity FcγR CD64 (and in some cases CD16A), do not appreciably bind monomeric IgG(10).
In order to study the biology of antibody-FcγR interactions and potentially compare next-generation Fc-modified therapeutic mAb, suitable assays are required to probe physiologically-relevant FcγR interactions. However, biophysical assays such as surface plasmon resonance (SPR) frequently used to measure IgG binding to FcγRs fail to fully recapitulate the biology of FcγRs and lack key properties such as fluidity within the cell membrane and receptor clustering following ligand interaction. Furthermore, we recently emphasized the importance of considering the multimeric nature and size of IgG IC(11). Assay methods capable of accurately measuring these interactions and recapitulating the known FcγR receptor biology are therefore essential.
There has been recent progress in the development of cell-based assays to detect the interactions between antibody-opsonised target cells and FcγRs, in the context of screening therapeutic mAbs for potential to induce antibody-dependent cell-mediated cytotoxicity (ADCC)(12) or antibody-dependent cell-mediated phagocytosis (ADCP)(13). These reporter-based assays do not require primary human immune cells as an effector cell source, are highly reproducible and so ideal for high-throughput screening purposes. There have also been recent advances in technologies for the detection of IC, focused on either the use of cell-free tetrameric(14) or dimeric(15) FcγRs to detect IgG-antigen-coated beads or surface immobilized IgG/IC, respectively, or cell-based methods to detect plate-bound IgG/IC(16, 17). Although potentially useful, these assays are designed to detect surface-immobilised IC binding to FcγRs, as opposed to IC in solution. The detection of cell-free IC is likely to be highly relevant in monitoring autoimmune patients and for predicting how antibody-virus complexes or developmental therapeutic mAbs bind to and/or activate FcγRs. In relation to the latter, IC-loaded dendritic cells (DCs) were shown to be capable of inducing immune responses to tumours expressing cognate antigens(18, 19), and there have been indications that antigen-antibody IC form in vivo following mAb therapy, and stimulate anti-tumour immune responses via FcγRIIA(20) on DCs. Similarly, from a basic immunology perspective, the exact requirements for FcγR activation versus blocking in terms of IC size/orientation is incompletely understood, with a recent study suggesting that multimers containing at least 5 Fc domains favour immune cell activation(21). Assays capable of discriminating these activities may therefore contribute to a broader understanding of FcγR biology.
A model system for the detection of IgG IC was therefore devised, based upon the known interaction of the inhibitory FcγR CD32B with SHIP-1(22). CD32B was chosen as the FcγR as it is known to have low affinity for monomeric IgG(10), binds IC(11), is the sole inhibitory FcγR with well-defined roles in immune regulation (3, 4, 23), and has a well-validated signalling pathway. Specifically, following CD32B crosslinking with activating receptors such as the B cell receptor (BCR)(24) (B cells), the Fc epsilon receptor (FcεRI)(22, 25) (mast cells/basophils) or FcγRIIA(25, 26) (myeloid cells), a Src kinase phosphorylates the ITIM of CD32B, allowing docking and activation of SHIP-1, which mediates the majority of the negative regulation deriving from CD32B(22, 27). SHIP-1 attenuates activatory receptor signalling by dephosphorylating phosphatidyl inositol-3,4,5-triphosphate (PIP3) to phosphatidyl inositol-3,4-bisphosphate (PIP2), which consequently limits recruitment of pleckstrin homology (PH) domain-containing proteins such as Bruton’s tyrosine kinase (Btk) to the cell membrane(28). One functional consequence of SHIP-1 activity is the inhibition of FcγR-mediated phagocytosis(29), although it should be noted that SHIP-1 may also function independently of CD32B to limit activity (30, 31) and also that SHIP-1 is also able to inhibit signalling outside of its immediate signalling complex, so-called ‘trans-inhibition’(32), which is not necessarily dependent on CD32B ligation.
Nevertheless, in order to detect IC, CD32B interaction with SHIP-1 was assessed using NanoBiT™ technology(33). This involved the genetic fusion of complementary small (SmBiT, 11 amino acid) and large (LgBiT, 156 amino acid) fragments of the NanoLuc® luciferase enzyme to the coding regions of CD32B or SHIP-1, respectively. Interaction between the partner proteins results in the coincident interaction of the complementary SmBiT and LgBiT fragments, forming a complete functional luciferase enzyme that can be detected with a cell-permeable substrate. Here, we report the characterisation and validation of this system for the detection of distinct experimental IC, and also provide proof-of-principle for the detection of IC in autoimmune disease sera in small pilot studies.
Materials and methods
Antibodies and reagents
The following mAbs were utilised: CD32 Alexa Fluor®647 (Fun-2, mouse IgG2b, BioLegend), CD32B (6G11, human IgG1, BioInvent), CD32A (E08, F(ab’)2, BioInvent), CD79B (AT105-1, mIgG1; ZL9-3, mIgG1/F(ab’)2, in-house), CD79A (ZL7-4, mIgG1, in-house), CD20 (rituximab, chimeric hIgG1, Southampton General Hospital pharmacy; rituximab, chimeric hIgG2 and 4, in-house), SHIP-1 Alexa Fluor® 647 (P1C1-A5, mIgG1, BioLegend), rituximab idiotype (MB2A4, rat IgG2a, in-house), human IgM µ chain (m15-8, mIgG1/F(ab’)2, in-house) and TNP (7B4, human IgG1-4, in-house). Conjugations with Alexa Fluor®-488 5-TFP (Invitrogen) or allophycocyanin (Europa Bioproducts) were performed in-house as required. The following polyclonal antibodies were utilised: human IgG purified from pooled human plasma (in-house), goat F(ab’)2 anti-human IgG (H+L), and phycoerythrin-conjugated goat F(ab’)2 anti-human or anti-mouse IgG Fc fragment-specific (all Jackson ImmunoResearch). The Src family kinase inhibitors PP2 and Dasatinib Monohydrate were from Selleckchem. Human AB serum was from Sigma.
Cells
HEK293F suspension cells (Invitrogen) were cultured in Erlenmeyer flasks in Freestyle™ 293 Expression Medium (Gibco) in a shaking incubator at 37°C/8% CO2. Adherent HEK293 landing pad cells were provided by Promega, cultured in DMEM containing 4.5g/L D-glucose, L-glutamine and 110mg/L Sodium Pyruvate (Gibco), 10% FBS (HyClone or Sigma), 200µg/ml hygromycin B (Sigma) and passaged using Trypsin-EDTA (Gibco or Lonza).
The human monocytic cell line, THP-1 and B-cell-line, Ramos (both ATCC), were cultured at 37°C/5% CO2 in RPMI-1640 medium (Gibco) supplemented with 2 mM L-glutamine, 1 mM sodium pyruvate (both Gibco) and 20 or 10% fetal calf serum (Sigma), respectively.
Generation of IC
Heat-aggregated IgG
Human IgG, pooled from healthy donor human plasma and containing all isotypes of IgG was buffer-exchanged into PBS and aggregated by heating at 63°C for 30 minutes. Analytical HPLC (Zorbax-GF-250 or Superose 6 Increase columns) was used to confirm aggregation. The resulting monomeric and aggregated IgG fractions were separated by preparative size exclusion chromatography (Superdex 200 column), pooled, concentrated (Vivaspin®20 units, Sartorius AG), buffer-exchanged into PBS and 0.2µm filtered.
TNP-BSA IC
IC consisting of anti-TNP IgG and TNP-BSA were generated as previously described(11). Briefly, mixtures of 10µg/ml anti-TNP and 5µg/ml of BSA-TNP (2:1 ratio) were incubated for 3 hours at room temperature and stored at 4°C until use. BSA coated with 4 (for the generation of small IC) or 33 (for the generation of large IC) molecules of TNP (BioSearch Technologies) was used.
Rituximab: anti-idiotype IC
Rituximab variants were mixed with anti-idiotype MB2A4(34) at a 1:1 ratio (0.5mg/ml of each), and incubated as above for TNP IC. Complexes were stored on ice until used, and the formation of higher molecular weight complexes confirmed by analytical HPLC as above.
Patient serum samples
Serum samples from SLE patients were obtained through a routine clinic. Ethical approval for the study was obtained from the National Research Ethics Committee. Samples from all participants were obtained with their informed consent, adhering to the Declaration of Helsinki, and the demographic features are summarized elsewhere(35). Peripheral blood was collected into tubes lacking heparin, serum taken and stored at -20°C or below. Anti-dsDNA antibodies were measured by ELISA (Euro Diagnostica). A threshold of 50 IU/ml distinguished between positive (>50 IU/ml) and negative (<50 IU/ml) anti-dsDNA measurements.
DNA
Constructs expressing human CD79B(36), CD32A (H131 and R131) or CD32A with a stop codon introduced to remove the cytoplasmic domain(37) were generated previously. Vectors encoding R4 integrase (R4I-4A), or CD32B-SmBiT, SHIP-1-LgBiT (N-terminus) and CD32A in a bidirectional promoter vector were provided by Promega. Herring sperm DNA (Promega) was used as a carrier in transient transfection experiments, and pmax-GFP® (Lonza) as a positive control.
Generation of chimeric fusion proteins
Open reading frames of interest were amplified by PCR using Pfu DNA polymerase (Promega) prior to cloning into NanoBiT™ expression vectors with C- or N-terminal large or small BiT (Lg/SmBiT) fusions (pNB1k, pNB2k or pNB3k (Promega)). With the exception of human SHIP-1, whereby human cell line (SUDHL4) cDNA was used as a template, in-house plasmid constructs were used as templates for PCR. The primers used for PCR cloning were as follows: huCD32B forward: 5’-ATATGCGATCGCATGGGAATCCTGTCATTCTTAC-3’, huCD32B reverse: 5’-ATATGTTTAAACAATACGGTTCTGGTCATCAGG-3’; huSHIP-1 forward: 5’-ATATGCGATCGCCATGGTCCCCTGCTGGAA-3’, huSHIP-1 reverse: 5’-ATATGTTTAAACCTGCATGGCAGTCCTGCC-3’; huCD40 forward: 5’- ATATGCGATCGCATGGTTCGTCTGCCTCTGCAG-3’, huCD40 reverse: 5’- ATATGTTTAAACCTGTCTCTCCTGCACTGAGATG-3’.
Initial PCR products were cloned into pCR®-BluntII-TOPO® vectors using Zero Blunt TOPO PCR Cloning Kit (Invitrogen), sequenced and re-cloned into NanoBiT™ expression vectors with appropriate restriction enzyme sites, followed by ligation with T4 DNA ligase (Promega) overnight at 4°C. Plasmids were propagated using standard molecular biology techniques, and purified using QIAprep Spin Miniprep and HiSpeed Plasmid Maxi kits (Qiagen). Mutation of the CD32B ITIM was achieved via site-directed mutagenesis using the Quikchange Lightning Multi Site-Directed Mutagenesis Kit (Agilent), according to the manufacturers’ instructions, and confirmed by sequencing. The mutagenesis primer used was: 5’-GCTGAGAACACAATCACCTTTTCACTTCTCATGCACCCG-3’.
Transient transfections
HEK293F cells were transiently transfected with plasmid constructs using 293fectin™ transfection reagent (Invitrogen) according to manufacturer’s guidelines. THP-1 and Ramos cells were transfected using Nucleofection kit V (Lonza), in advance of luminescence assays on d1 post-transfection, according to the manufacturer’s guidelines. Nucleofection programmes U-002 and O-006 were used for THP-1 and Ramos cells, respectively.
Stable transfections
HEK293 landing pad cells containing an AttPR4 (acceptor) landing pad integration site (Promega) were transfected with a vector encoding the open reading frames of interest and containing a AttB R4 (donor) site. AttPR4 landing pad HEK293 cells were co-transfected with a donor AttBR4 retargeting vector (encoding CD32B-SmBiT, SHIP-1-LgBiT, CD32A) and an R4 integrase vector (R4I-41) (~3.4:1 ratio) using FuGENE HD transfection reagent (Promega). Cells were selected in 2mg/ml G-418 (Promega) from day 3 and the highest CD32A/B expressing cells sorted using a BD FACS AriaII Cell Sorter (BSL-1) following staining with Fun-2. Single colonies were then expanded.
Flow cytometry
Cells were stained with fluorophore-conjugated antibody (10µg/ml) for 30 minutes on ice, washed in FACS buffer (1% bovine serum albumin (w/v), 0.025% azide (v/v) in PBS) and samples acquired on a flow cytometer (BD FACSCalibur™) or labelled with unlabelled primary antibody, as above, washed in FACS buffer, and stained with a fluorescently labelled secondary anti-IgG F(ab’)2. To detect intracellular SHIP-1 expression, cells were first fixed with 1% formaldehyde (in PBS) for 10-15 minutes at room temperature, stained with mAbs diluted in 0.3% (w/v) Saponin (Sigma) in PBS, washed sequentially with 0.03% and 0% Saponin in FACS wash, and acquired as above. FCS Express V3 (De Novo Software) was used to analyse flow cytometry data.
Live cell SHIP-1 recruitment assay - transient
HEK293F cells were transfected as above at a cell density of 1x106 cells/ml. 150ng or 500ng of each DNA vector was transfected depending on cell volume, adjusted to 1.5µg or 5µg total DNA with Herring Sperm DNA (Promega). Transfected cells were typically plated at 100μl/well in white and flat-bottomed, 96-well assay plates on the day following transfection. SHIP-1 recruitment assays were performed in either kinetics or end-point format: For kinetics Nano-Glo® Live Cell Substrate was diluted in Nano-Glo® LCS Dilution Buffer (both Promega) and added at 25µl/well. Baseline luminescence was measured using a Varioskan flash plate reader (Thermo Electron 3001, SkanIt software v2.4.3). Stimuli were typically added at 10µl/well for a final concentration of 10µg/ml. Responses to stimuli were measured at 5-minute intervals. In endpoint format, cells were stimulated for 15 minutes at room temperature, followed by substrate addition and measurement of luminescence. Fold inductions of response were calculated as follows:
When comparing responses to serum samples between different experiments, results were normalised to a positive control (10µg/ml heat-aggregated IC) as follows:
Live cell SHIP-1 recruitment assay - stable
In assays with stably transfected CD32B-SmBiT, SHIP-1-LgBiT and CD32A-expressing HEK293 cells, cells were harvested with trypsin-EDTA or Accutase (Innovative Cell Technologies) and plated at 2 or 5x104 cells/well in Opti-MEM-1® and assays performed as above, or using a Glomax® Discover System (Promega).
Luminescence microscopy
Stable CD32B-SmBiT-, SHIP-1-LgBiT-, CD32A-expressing HEK293 landing pad cells were seeded at 7.5x104 cells/dish in DMEM + 10% FBS medium in µ-Dish 35 mm, high plates (ibidi) and allowed to adhere overnight. The following day, medium was changed to Opti-MEM-I (containing 100µM of fumarizine substrate), and luminescence was visualised using an LV200 Bioluminescence imaging system (Olympus) at time 0 and at 1 minute intervals post treatment with monomeric IgG or IC (10µg/ml).
Statistics
Data was analysed using GraphPad Prism (v6.07). When appropriate, statistical tests were utilised (detailed in Figure legends) to assess significance. Statistically significant results were judged to have p values ≤ 0.05.
Results
Development of a SHIP-1 recruitment assay for the detection of IgG IC
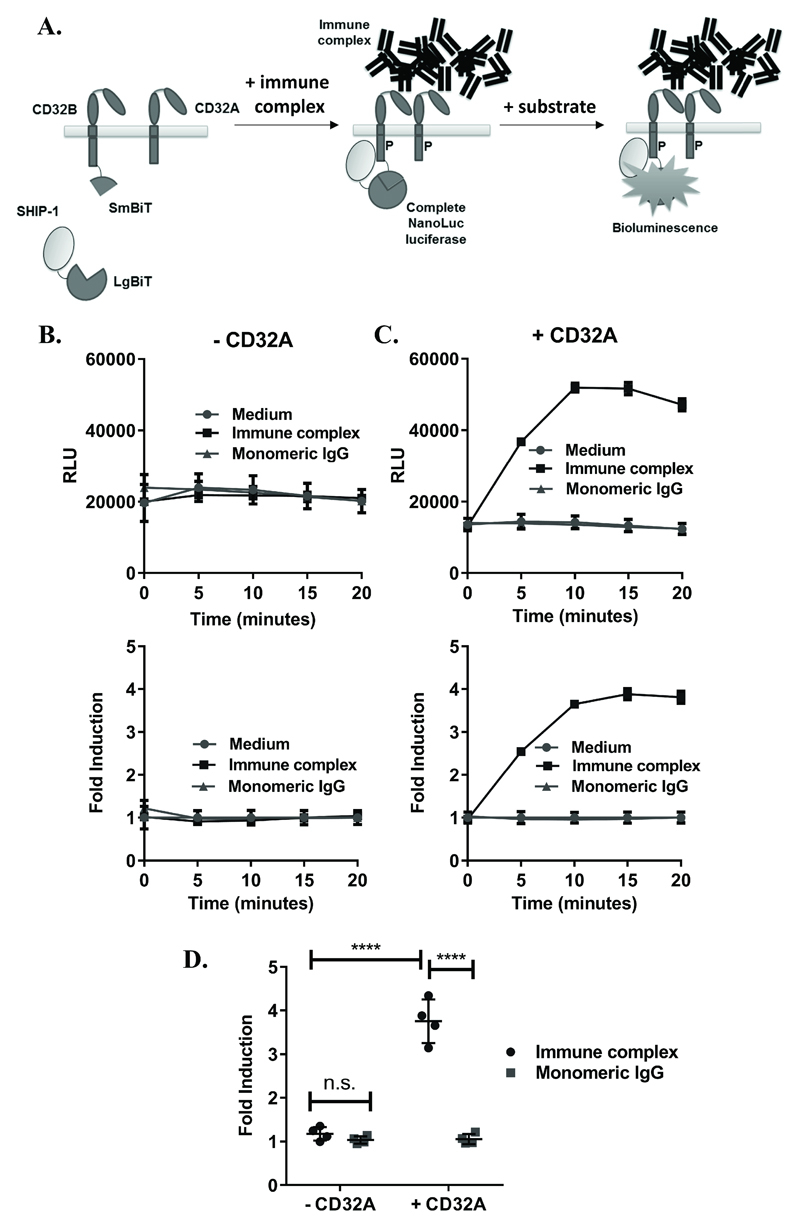
To develop an assay to detect IgG IC binding to FcγRs, the NanoBiT™ technology was applied as outlined in Figure 1A. Constructs expressing CD32B-SmBiT, SHIP-1-LgBiT and CD32A-H131 were first individually transfected into HEK293F cells and expression confirmed by flow cytometry (Supplementary Figure 1A-C). Binding of IC to CD32B-SmBiT was also confirmed, as a measure of binding to physiological ligand (Supplementary Figure 1D). HEK293F cells were then transiently co-transfected with these constructs to achieve expression of CD32B-SmBiT and SHIP-1-LgBiT, in the absence (Figure 1B) or presence (Figure 1C) of the activatory FcγR CD32A. Heat-aggregated pooled normal IgG was used as a mimic of IC(10) (Supplementary Figure 1E) and compared with monomeric IgG or medium alone as negative controls. No luminescence increases were observed in any case following the treatment of cells with monomeric IgG, or medium alone (Figure 1B-C) indicating the absence of SHIP-1 recruitment to CD32B under these conditions. Similarly, in the absence of CD32A, negligible responses were detected following IC stimulation (Figure 1B). However, when CD32A was co-expressed with CD32B, clear increases in luminescence in response to IC were observed (Figure 1C), which peaked within 10-15 minutes and were significantly higher than cells treated with monomeric IgG or lacking CD32A (Figure 1D).
Figure 1. Development of an assay for detection of IC-mediated SHIP-1 recruitment.
(A) Assay concept: (Left) cells expressing CD32B and SHIP-1 with complementary SmBiT and LgBiT fusions, respectively, and CD32A with no fusion. (Middle) Following stimulation with IC, SHIP-1 is recruited to the phosphorylated (P) ITIM of CD32B, leading to interactions between LgBiT and SmBiT fragments to form complete NanoLuc enzymes. (Right) In the presence of a NanoLuc substrate, complete luciferase enzymes produce luminescence, reflecting SHIP-1 recruitment to CD32B. NB: Monomeric IgG, being unable to crosslink FcγRs, is not expected to induce SHIP-1 recruitment, resulting in no increase in luminescence. (B-C) HEK293F cells, transiently co-transfected with CD32B-SmBiT and SHIP-1-LgBiT (C-terminus) alone (B) or with CD32A (C) on d0, were stimulated with medium, IC or monomeric IgG (10µg/ml) on d1. Luminescence readings were taken at time 0 following substrate addition, and at 5 minute intervals following stimulation. Responses are shown as (top) relative luminescence units (RLU) or (bottom) fold inductions of response relative to medium control (fold induction). Means ± S.D. of technical replicates are shown. (D) Mean fold inductions (at 15 minutes) from repeat experiments where cells were co-transfected as in (C) and treated with monomeric IgG or IC on d1, as above (n=4 experiments). Statistical significance between groups was assessed using a 2-way ANOVA with Tukey’s multiple comparisons test. **** – p<0.0001; n.s. – not-significant.
Refinement and validation of the assay
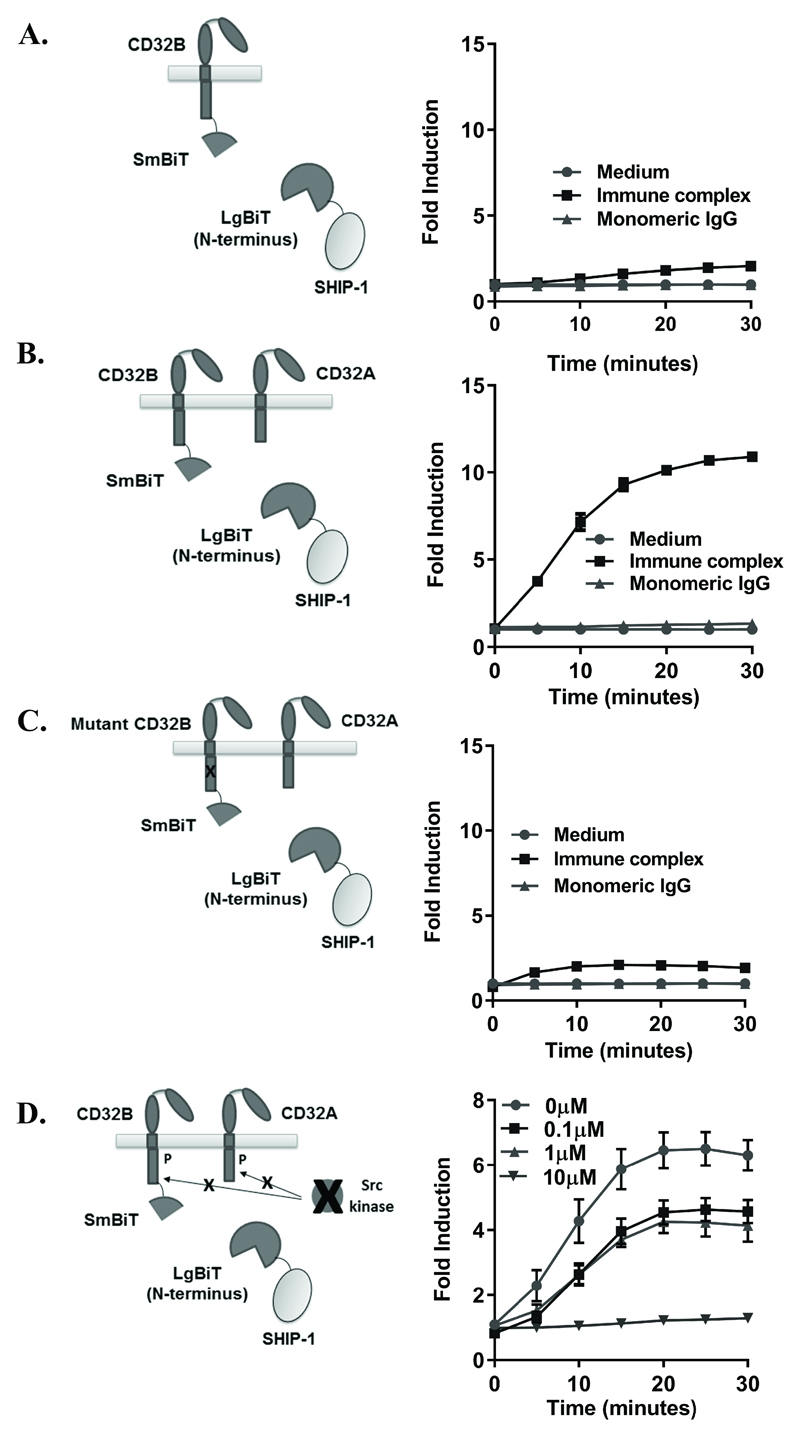
Initial experiments were performed using a fusion protein displaying LgBiT at the C-terminus of SHIP-1. To explore if this was the optimal orientation we performed equivalent experiments with a SHIP-1 variant expressing LgBiT at the N-terminus. Similar responses were observed, indicating that the orientation of LgBiT on SHIP-1 did not affect CD32B recruitment. Consistent with the experiments above, the activatory CD32A was still required for recruitment (compare Figure 2A, where CD32A was absent, with Figure 2B, where CD32A was present). The luminescence values (and fold inductions) with the N-terminal SHIP-1-LgBiT fusion were routinely greater than with the C-terminal LgBiT fusion and so the N-terminal variant was used in all subsequent experiments.
Figure 2. Validation of IC detection.
(A-C) HEK293F cells, transiently co-transfected with CD32B-SmBiT, SHIP-1-LgBiT (N-terminus) alone (A), with CD32A (B) or ITIM-mutated CD32B-SmBiT, SHIP-1-LgBiT and CD32A (C) on d0, were stimulated with medium, IC or monomeric IgG (10µg/ml) on d1. Luminescence was measured at time 0 following substrate addition, and at 5-minute intervals. (D) HEK293F cells, transfected as in (B) were pre-treated with the Src family kinase inhibitor PP2 at the indicated concentrations, or DMSO control, on d1 following transfection, followed by stimulation with medium, IC or monomeric IgG (10µg/ml). Luminescence was measured as above. (A-D) Left - schematic diagrams; right - fold inductions, with means ± S.D. of technical replicates, representative of 3 (A), at least 3 (B) and 3 (C) independent experiments. (D) No responses to medium control or monomeric IgG were detected (data not shown). The absence of response to IC with prior 10µM PP2 treatment is representative of 3 independent experiments.
SHIP-1 is known to bind to the phosphorylated ITIM of CD32B(22). Therefore, to assess whether the CD32B ITIM was required for SHIP-1 recruitment, wildtype CD32B-SmBiT was replaced with a mutant CD32B-SmBiT molecule lacking the intrinsic ITIM tyrosine. Cells transfected with this construct displayed greatly diminished, albeit measurable, responses indicating that CD32B ITIM tyrosine phosphorylation is required for efficient triggering of these responses (Figure 2C). Similarly, when CD32A was replaced with a truncated variant lacking the ITAM-containing cytoplasmic domain, responses were abrogated (data not shown), indicating that the intracellular domains of both CD32A and CD32B are required for SHIP-1 recruitment.
The above indicates a requirement for phosphorylation of the cytoplasmic domains of CD32B and CD32A in generation of the responses. Activatory FcR signalling triggers CD32B ITIM phosphorylation via Src kinases such as Lyn(38). To confirm that Src kinases were responsible for SHIP-1 recruitment downstream of phosphorylation in our assay, cells were pre-treated with an inhibitor (PP2) (39). Responses were abrogated in a dose-dependent manner with complete inhibition at 10µM (Figure 2D), indicating that Src kinase-mediated phosphorylation of CD32A/B is required for SHIP-1 recruitment to CD32B. Similar dose-dependent inhibition was also achieved with a second Src inhibitor, dasatinib (data not shown). As a final control, the SmBiT was fused to CD40, a receptor that is not expected to interact with IC or CD32A in the cell membrane, to prove that crosslinking of a relevant activatory receptor with CD32B is required for the observed SHIP-1 recruitment (data not shown). Together, these control experiments demonstrated that the IC-mediated recruitment of SHIP-1 detected in this assay is specific to CD32B and requires a relevant activatory-ITAM bearing receptor to be phosphorylated by Src-kinases.
Determining the sensitivity of IC detection
Having defined the capacity of the assay to detect SHIP-1 recruitment to CD32B, its sensitivity was subsequently explored. To test sensitivity, IC was spiked into monomeric IgG samples at a range of concentrations and used to stimulate cells transfected with CD32B-SmBiT, SHIP-LgBiT and CD32A. A dose-response was observed, with higher ratios of IC:monomeric IgG inducing greater responses, representing more SHIP-1 recruitment to CD32B (Supplementary Figure 2A). This indicates that the level of IC correlates with response, as expected. Small increases in luminescence, and hence SHIP-1 recruitment to CD32B, could be detected at IC:monomeric IgG ratios as low as 0.25:9.75 in a 10μg/ml solution, (0.25μg/ml IC), indicating high sensitivity of the assay to low concentrations of IC.
In addition to IC concentration, it was questioned whether the assay was sensitive to additional variables such as isotype content and size of IC. To assess this, anti-trinitrophenol (TNP)-TNP-BSA complexes generated from different isotypes (human IgG1, 2, 3 and 4) and sizes (small versus large) were generated as before(11). Binding of the complexes to stably-transfected FcγRIIb-expressing CHO-K1 cells(40) was confirmed, with a hierarchy of hIgG3>hIgG1>hIgG4>hIgG2, and with large complexes binding at a greater level than small complexes in all cases (data not shown). When applied to the SHIP-1 recruitment assay, responses above the negative control were observed with all complexes (Supplementary Figure 2B), showing that detection of IC using this assay is not limited to heat-aggregated IgG IC. Furthermore, sensitivity of the assay to changes in isotype was indicated, with a hierarchy of response of hIgG3=hIgG1>hIgG2>hIgG4 (Supplementary Figure 2B and data not shown). Large IC also induced significantly greater responses than small IC (Supplementary Figure 2B).
To confirm these results, and measure sensitivity to IgG isotype in an additional setting, IC were generated by incubating variants of the anti-CD20 mAb Rituximab (hIgG1, 2 and 4) with an anti-idiotype antibody (MB2A4) recognizing the F(ab) domain of Rituximab(34). This method produces a heterogeneous mix of IC of varying molecular weights (Supplementary Figure 2C). The generation of higher molecular weight complexes for all isotypes of rituximab was confirmed by HPLC (data not shown). Considering known differences in binding of different isotypes of human IgG to isoforms of CD32A (namely H131 versus R131)(10), cells transfected with CD32A-H131 or R131 were also compared.
Responses to all isotypes of rituximab:anti-iditoype complexes were detected (Supplementary Figure 2D), but not monomeric, uncomplexed rituximab (data not shown). Similar to the detection of TNP:anti-TNP complexes (Supplementary Figure 2B), this suggests that the assay is capable of detecting IC of distinct isotypes but not monomeric IgG. Also, as indicated in experiments with TNP:anti-TNP complexes, the assay was sensitive to different sizes of IC following the fractionation and purification of rituximab complexes by size exclusion chromatography (data not shown). However, a different hierarchy of response was observed with CD32A-H131 (hIgG1≥hIgG2>hIgG4) versus CD32A-R131 (hIgG1=hIgG4>hIgG2) (Supplementary Figure 2D).
Detection of CD32B signalling following co-localisation with other ITAM-bearing receptors
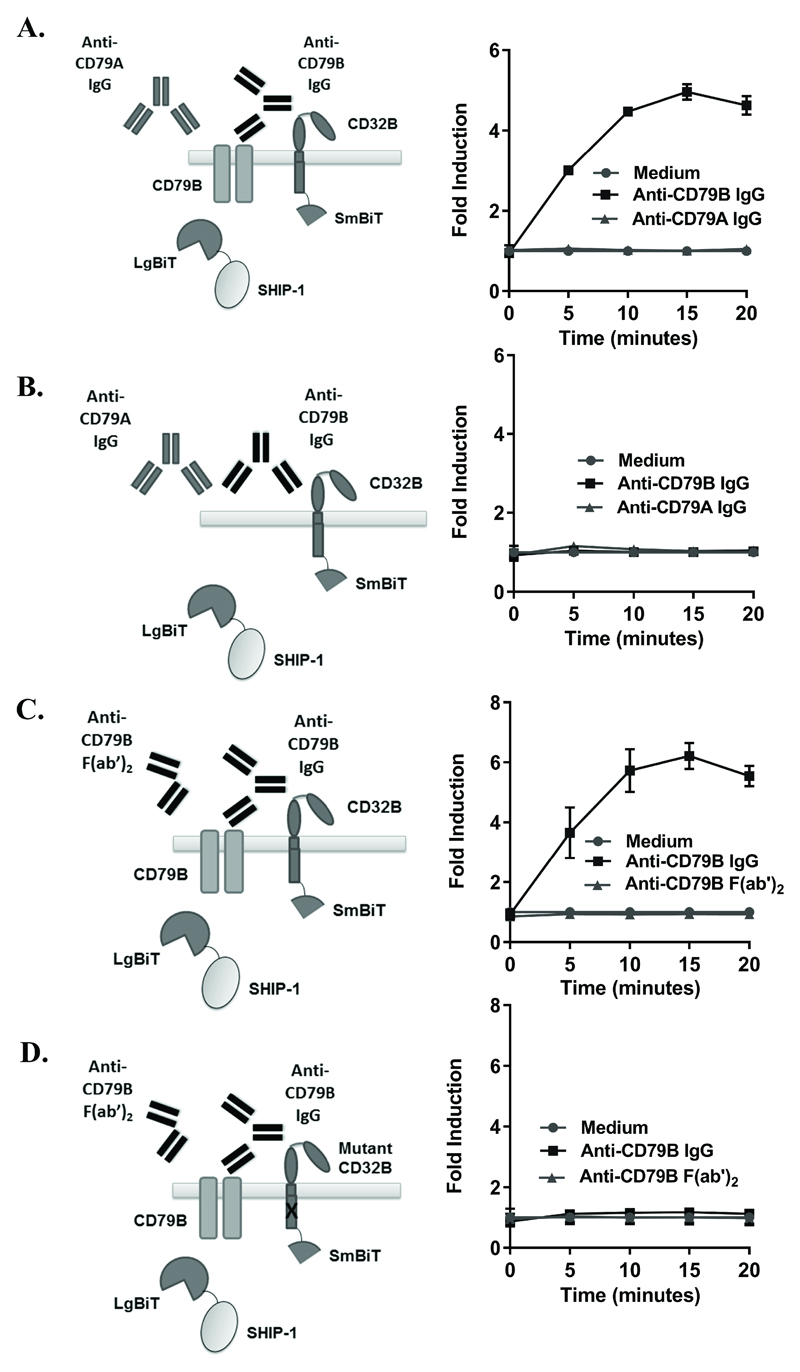
Considering the requirement of an activatory receptor for the detection of SHIP-1 recruitment to CD32B (Figures 1-2 and data not shown), it was next assessed whether this phenomenon was specific for activatory FcγR, or whether crosslinking with a non-FcγR ITAM-containing receptor could mediate such trans-phosphorylation/licensing of CD32B in the assay. Anti-BCR antibodies have previously been used to crosslink CD32B in cis as a mimic of IC(24, 41), and crosslinking of a chimeric IgM molecule (containing intracellular CD79B) with CD32B inhibited BCR signalling, as measured by an attenuation in Ca2+ flux(24). Moreover, agents capable of crosslinking CD32B with CD79B are in clinical development with the aim of inhibiting autoimmune B cells(42). The ITAM-containing CD79B (Igβ subunit of the BCR) was therefore chosen as an alternative activatory receptor. Following confirmation of robust expression (data not shown), co-localisation of CD79B with CD32B was assessed using anti-CD79B IgG mAbs in cells transiently transfected with CD32B-SmBiT, SHIP-1-LgBiT and CD79B. Responses to anti-CD79B IgG were detected (Figure 3A), indicating that co-localisation of CD32B with an alternative ITAM containing non-FcγR activatory receptor can mediate the phosphorylation of CD32B and subsequent SHIP-1 recruitment. Specificity of the response for CD79B was also shown, as no response was seen with mAb recognising CD79A (the Igɑ subunit of the BCR) (Figure 3A), or cells transfected with CD32B-SmBiT and SHIP-1-LgBiT alone (Figure 3B), showing these cells were not responding to monomeric IgG and required co-expression of an activatory receptor.
Figure 3. Detection of SHIP-1 recruitment following CD32B-CD79B crosslinking.
(A,B) HEK293F cells, transiently co-transfected with CD32B-SmBiT, SHIP-1-LgBiT and CD79B (A) or CD32B-SmBiT, SHIP-1-LgBiT alone (B) on d0 were stimulated with anti-CD79B IgG (AT105-1) or anti-CD79A IgG (ZL7-4) (20 µg/ml) on d1. Luminescence was measured at time 0 following substrate addition, and at 5 minute intervals following stimulation. (C, D) HEK293F cells, transiently co-transfected as in A) (C), or with ITIM-mutated CD32B-SmBiT, SHIP-1-LgBiT and CD79B (D) on d0 were stimulated with anti-CD79B IgG (ZL9-3 IgG) or F(ab’)2 (ZL9-3 F(ab’)2) (10 µg/ml) on d1. Luminescence was measured as above. (A-D) Left – schematic diagrams; right – fold inductions. Means ± S.D. of technical replicates are shown, representative of 5 (A) and 4 (C) independent experiments. (B, D) Control experiments performed at the same time as A) and C) respectively, representative of 3 independent experiments.
To confirm these observations and study whether co-localisation of CD79B with functional CD32B is required for the observed response, control experiments utilising CD32B with a mutated ITIM (as in Figure 2C), and F(ab’)2 fragments (which cannot bind to CD32B in cis) compared to IgG of a second CD79B-specific antibody were performed. Similar binding of the anti-CD79B whole IgG and F(ab’)2 fragments were confirmed by flow cytometry (data not shown), and SHIP-1 recruitment was detected with whole IgG anti-CD79B in the setting of wildtype CD32B (Figure 3C). However, SHIP-1 recruitment was not observed in the setting of CD32B with a non-functional ITIM (Figure 3D), or following stimulation with F(ab’)2 anti-CD79B (Figures 3C-D). This shows that crosslinking of CD79B with functional CD32B is responsible for the observed SHIP-1 recruitment in the assay and further indicates that SHIP-1 is recruited downstream of CD32B ‘licensing’ by ITAM-containing activating receptors.
Detection of SHIP-1 recruitment in immune cell lines
HEK293F cells do not natively express FcγR and so may lack relevant cell-signalling pathways inherent to relevant immune cells. To address this, experiments were performed in the human monocytic cell line (THP-1) to provide evidence that the recruitment of SHIP-1 to CD32B could also be detected in relevant immune cells, natively expressing FcγR. Considering the endogenous expression of CD32A in THP-1 cells, CD32B-SmBiT and SHIP-1-LgBiT only were transfected. Responses to immune complex but not monomeric IgG were detected (Supplementary Figure 3A), although luminescence values and fold inductions of response were low, presumably due to the low transfection efficiency. However, responses detected in the setting of CD32B-SmBiT/SHIP-1-LgBiT were not observed when a GFP control was transfected (Supplementary Figure 3B), showing that the responses to immune complex required transfected CD32B/SHIP-1. This indicates that the method can be equally applied to immune cells expressing endogenous FcγR and signalling pathways. Similar proof-of-principle experiments were performed with the B-cell-line Ramos, which has constitutive expression of an IgM BCR and low-negligible expression of CD32B (data not shown). Responses were detected in Ramos cells transfected with CD32B-SmBiT and SHIP-1-LgBiT and treated with a full-length anti-IgM antibody to crosslink IgM with CD32B (Supplementary Figure 3C). Similar responses were not detected when cells were treated with an anti-IgM F(ab’)2 variant that is unable to bind CD32B (Supplementary Figure 3D), indicating that Fc-mediated crosslinking of IgM and CD32B is required to license CD32B and subsequent SHIP-1 recruitment in this setting. This provides further evidence that the method can be applied to immune cell lines if required.
Detection of IC in autoimmune disease sera
Considering the sensitivity of the assay to varying levels of IC, size, and isotype (Supplementary Figure 2), its ability to detect IgG IC in the serum of autoimmune patients was assessed. Considering that SLE is a disease characterized by circulating IC(43), sera from three SLE patients was investigated in a preliminary assay. To assay for the presence of IC the sera were diluted and applied to cells transfected with CD32B-SmBiT, SHIP-1-LgBiT and CD32A. Increases in luminescence were detected above baseline in response to all three SLE patient sera, indicative of the presence of IC (Supplementary Figure 4A). In contrast, no responses were detected with human AB serum pooled from ‘normal’ donors. Responses to sera from patients with RA were also detected (Supplementary Figure 4B), indicating that the responses are not restricted to SLE alone.
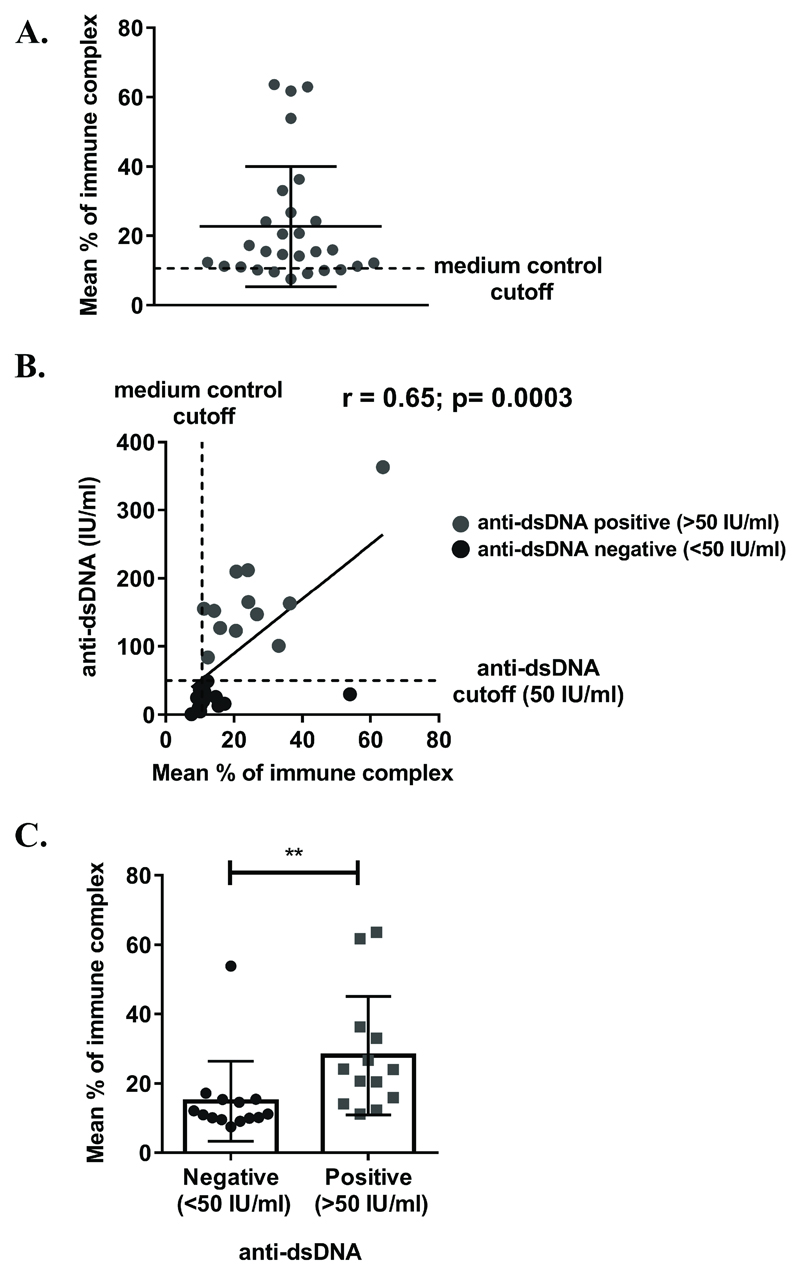
The assay was subsequently applied to a larger (albeit still small) series of 28 SLE samples from patients with known clinical data (levels of anti-dsDNA and BILAG (British Isles Lupus Assessment Group 2004) disease severity score). As all samples were not screened simultaneously, measurements from different assays were normalised to a standard positive control (10µg/ml of heat-aggregated IgG IC) and expressed as percentage of this response. Responses above medium-only controls were detected with 19/28 serum samples indicative of the presence of IC (Figure 4A). Following the exclusion of an outlier with an extremely high anti-dsDNA measurement (3288 IU/ml), and a sample with no anti-dsDNA result, analysis revealed a statistically significant positive correlation between assay response and previously determined anti-dsDNA levels (Figure 4B). Moreover, a statistically significant difference in response between patients with positive (>50 IU/ml) versus negative (<50 IU/ml) anti-dsDNA measurements was observed (Figure 4C). These results provide proof-of-principle that the assay is capable of detecting varying levels of IC in sera from patients with autoimmune disease, and provide the background for application of the assay in larger clinical cohorts.
Figure 4. Detection of IC in autoimmune serum samples.
(A-C) HEK293F cells, transiently transfected with CD32B-SmBiT, SHIP-1-LgBiT and CD32A-H131 on d0 were stimulated with serum from 28 SLE patients (diluted 1:20) on d1 in a kinetics assay. Data cumulated from 3 separate experiments were normalised to 10µg/ml of heat-aggregated IgG IC control (% of immune complex). Each circle represents the mean response (of technical replicates) for an individual serum sample 30 minutes post-stimulation. Medium control cut-off represents the mean response of cells treated with medium from all experiments. Means ± S.D. of all samples are shown in black. (B) A correlation of mean responses (from (A)) and anti-dsDNA test result. Black – serum samples from patients with a negative anti-dsDNA measurement; grey – serum samples from patients with a positive anti-dsDNA measurement. Anti-dsDNA cutoff (50 IU/ml) is used to distinguish between negative (<50 IU/ml) and positive (>50 IU/ml) anti-dsDNA measurements. Correlation was assessed using a non-parametric Spearman rank test (p=0.0003). 2x samples from (A) were excluded (unknown anti-dsDNA measurement and high (3288) anti-dsDNA measurement). (C) A comparison of mean responses (from (A)) between serum samples from patients with a negative anti-dsDNA measurement (<50 IU/ml) versus patients with a positive anti-dsDNA measurement (>50 IU/ml). Each symbol represents the mean response at 30 minutes for an individual serum sample (black – anti-dsDNA negative; grey – anti-dsDNA positive). Means of all samples ± S.D. are shown in black. Statistical significance was assessed using an unpaired Mann Whitney U test. ** - p<0.01. 1x sample from (A) was excluded (unknown anti-dsDNA measurement).
Generation of a stable cell line capable of detecting IgG IC
The above details validation of the assay concept and evidences its potential utility in the measurement of experimentally and clinically relevant IgG IC. However, to increase reproducibility and to obviate the need for continual transient expressions and validations, we sought to generate a stable source of cells for IC detection. Site-specific stable integration of a bidirectional construct encoding CD32B-SmBiT, SHIP-1-LgBiT (N-terminus) and CD32A-H131 was performed using adherent HEK293 landing pad cells. The initial stable pool responded in a similar fashion to transiently-transfected cells, with fold inductions of response to IC of between 5 and 10 (Figure 5A), and a clear distinction between responses to monomeric IgG and IC. Moreover, the response to IC was blocked with F(ab’)2 fragments of anti-human IgG (Figure 5A), showing that the IgG-Fc:FcγR interaction was required for SHIP-1 recruitment.
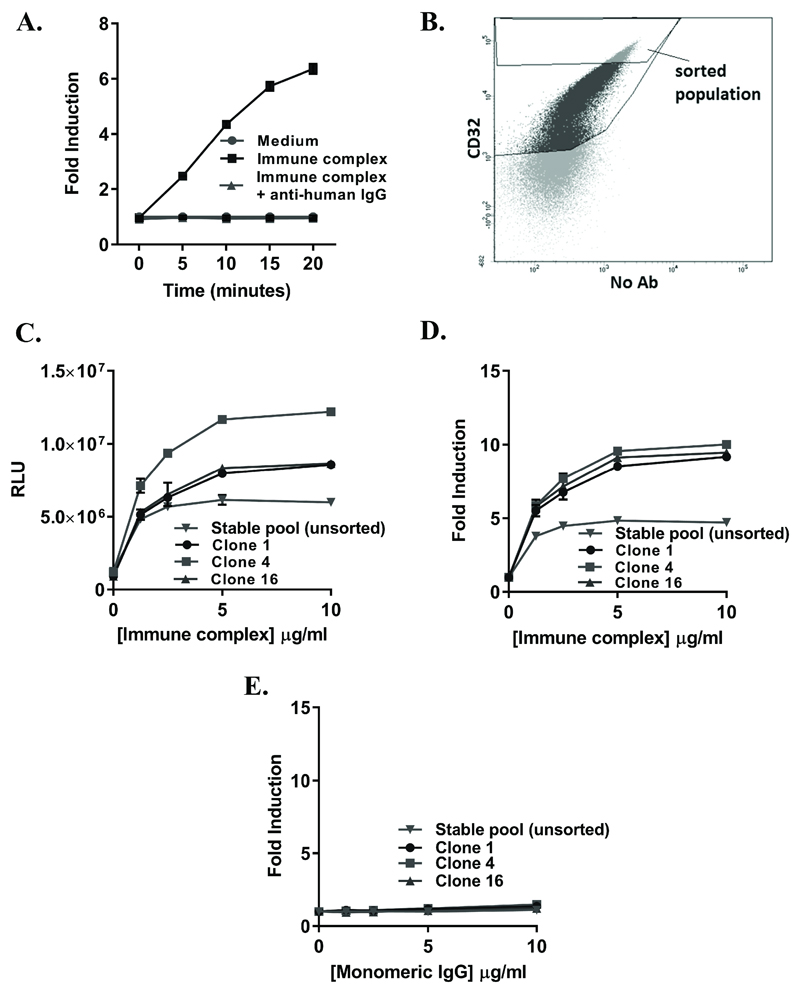
Figure 5. Validation of a stable cell line for IC detection.
(A) Stable pool (unsorted) HEK293 landing pad cells stably-transfected with CD32B-SmBiT, SHIP-1-LgBiT (N terminus) and CD32A and selected in 2mg/ml G-418 were plated at 5x104 cells/well and stimulated with medium, IC (10µg/ml) or IC + anti-human IgG (H+L) F(ab’)2 (both 10µg/ml). Luminescence was measured following substrate addition at time 0 and at 5 minute intervals following stimulation. (B) The stable pool of HEK293 CD32B-SmBiT/SHIP-1-LgBiT (N terminus)/CD32A landing pad cells (selected in 2mg/ml G-418) were stained for CD32 expression and the highest expressing cells FACSsorted, followed by the expansion of single clones. Untransfected HEK293 cells were used to gate on CD32+ cells, and the sorting gate used was positioned arbitrarily on the highest 8% of cells expressing CD32. (C-D) Stable CD32B-SmBiT/SHIP-1-LgBiT/CD32A HEK293 cells (clones 1, 4, 16 or stable pool (unsorted) cells) were stimulated with IC at the indicated concentrations. Luminescence was measured in endpoint format at 1h post-stimulation. (E) The same stable cells (clones 1, 4, 16 or stable pool (unsorted) cells) were stimulated with monomeric IgG at the indicated concentrations. Luminescence was measured as in (C-D). (A, C-E) Responses are presented as RLU (C), or fold inductions (A,D,E). Means ± S.D. of technical replicates are shown.
However, as expression analysis indicated multiple populations within the stable pool, FACS sorting was performed to select cells expressing the highest levels of CD32 (Figure 5B), and single colonies were expanded. When expanded, clones 1, 4 and 16 were compared alongside the unsorted stable pool in SHIP-1 recruitment assays. All clones responded to IC in a dose-dependent manner (Figure 5C-D), with negligible responses to monomeric IgG (Figure 5E; compare Figure 5D and 5E)). Moreover, although the unsorted stable pool gave a robust response, there were improvements in RLU (Figure 5C) and fold inductions (Figure 5D) in response to IC in all sorted clones, with fold inductions of response to IC >5 at an IC concentration of ~1µg/ml (Figure 5D), indicating sensitivity to low concentrations. These clones therefore represent robust, reliable and sensitive cells that can be utilized for IC detection.
The assessment of SHIP-1 recruitment to CD32B using luminescence microscopy
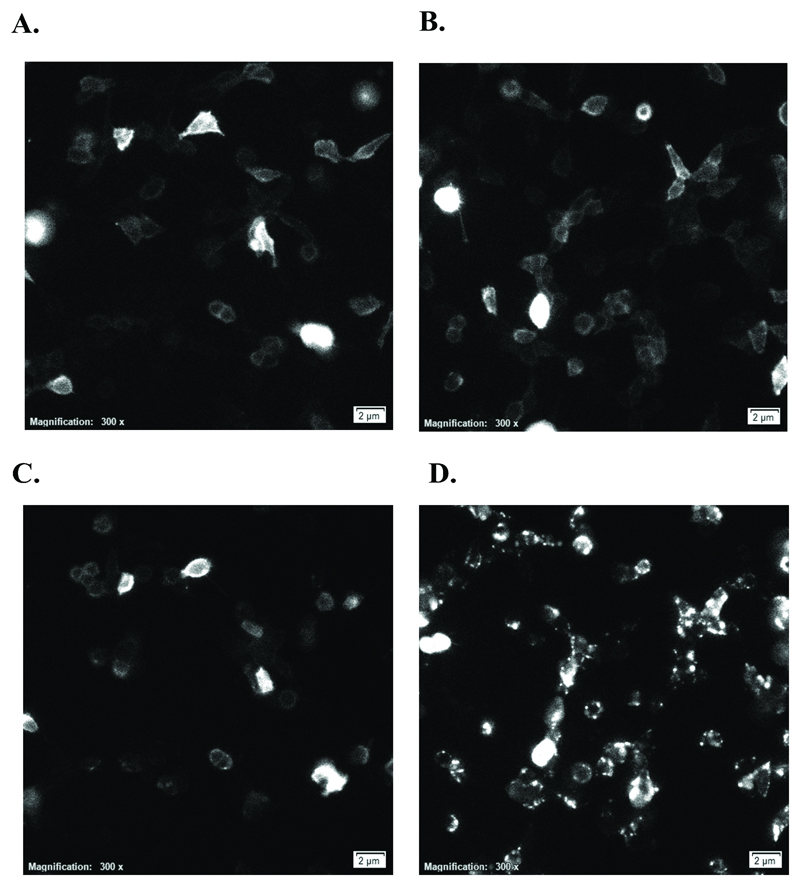
The above experiments demonstrate that SHIP-1 is recruited to CD32B following a stimulus that crosslinks CD32B with an activatory receptor. To provide evidence for where this interaction occurs we performed luminescence microscopy. Stable HEK293 cells expressing CD32B-SmBiT, SHIP-1-LgBiT and CD32A (Figure 5C-D; clone 16) were utilised in these experiments. Cells were adhered to glass slides, and photographs taken using a luminescence microscope at 1 minute intervals over 30 minutes following stimulation with monomeric IgG or IC. At time 0, some baseline luminescence was visualised by luminescence microscopy (Figure 6A-B). This recapitulates the findings in luminescence assays with these cells (Figure 5C), whereby a high baseline luminescence is detected, presumably due to fusion protein overexpression and baseline interactions between LgBiT and SmBiT independent of a stimulus. However, treatment with monomeric IgG led to no obvious change in the luminescence visualised (Figure 6C and Supplementary Video 1). This is in stark contrast to IC-treated cells, whereby distinct membrane clusters of luminescence were identified, representing SHIP-1 recruitment to CD32B (Figure 6D and Supplementary Video 2). These results provide confirmatory evidence of the recruitment of SHIP-1 to membrane clusters of CD32B, in response to IC but not monomeric IgG.
Figure 6. Visualisation of the formation of CD32B membrane clusters following immune complex binding.
Stable CD32B-SmBiT/SHIP-1-LgBiT/CD32A HEK293 cells (clone 16) were allowed to adhere overnight, medium replaced with Opti-MEM-1 containing 100µM fumarizine and luminescence microscopy utilised to visualise luminescence post-stimulation with 10µg/ml monomeric IgG (A – 0 minutes, C – 30 minutes) or immune complex (B – 0 minutes, D – 30 minutes). Also see Supplementary Videos 1 and 2.
Discussion
IC have been implicated in the pathophysiology of multiple diseases, such as SLE and rheumatoid arthritis, and their importance has recently been demonstrated in a model of IC-induced kidney inflammation(6). It has also been indicated that IC composed of tumour-specific mAb and antigen, following tumour cell death, may stimulate adaptive immune responses(20) (44). Accurate, robust assays to detect IC and understand their biology are therefore highly desirable.
Recently developed assays(14–17) designed to detect autoantigen or viral IC interactions with FcγRs, with the exception of the use of those employing tetrameric FcγRs(14), are plate-bound methodologies limited by the fact that antibodies require immobilization and are captured in all orientations (i.e. via both F(ab) and Fc regions). Although surface-immobilised IgG can be found in some pathological conditions, such as anti-collagen II in rheumatoid arthritis(45), plate binding does not reflect a physiological situation, and cannot be extrapolated to the detection of circulating, soluble IC in disease. Therefore, we developed a cell-based method capable of detecting soluble IC downstream of biologically-relevant FcγR interaction. This is pertinent in the setting of small IC, such as autoimmune or viral IC, which bind and crosslink FcγRs on immune cells independently of antibody binding to a cell expressing the cognate antigen. Responses are also quick (within minutes) to develop, further highlighting the sensitivity of the assay. This contrasts to reporter assays(12), which typically require incubation times of several hours, reflecting the time required for luciferase synthesis, as opposed to rapid detection of NanoLuc enzymatic activity in NanoBiT™ assays through protein:protein interactions.
Experiments with both N- and C-terminal LgBiT variants of SHIP-1 showed that optimal SHIP-1 recruitment to CD32B in response to IC requires the presence of an ITAM-containing activatory receptor, such as the activatory FcγR CD32A (Figures 1-2 and data not shown), or the non-FcγR CD79B (Figure 3). However, replacing CD32B-SmBiT with CD40-SmBiT obviated responses to IC (data not shown). This was also observed when the activatory CD32A was replaced with inhibitory CD32B (data not shown), showing that crosslinking of both inhibitory and activatory receptors is required for SHIP-1 recruitment. Furthermore, experiments with signalling-defective CD32B/A mutants (Figure 2C and data not shown), or Src kinase inhibition (Figure 2D), demonstrate that functional ITAM/ITIM domains and phosphorylation are required. This represents a mechanism whereby the ITIM domains of CD32B molecules are phosphorylated secondary to CD32A ITAM phosphorylation. Presumably in this way, SHIP-1 is optimally recruited only when required to downregulate activatory receptor signalling. The activatory receptor can therefore be said to be “participating in its own inhibition”(38). These results recapitulate the known biology of CD32B, in that inhibitory signalling, typified by SHIP-1 recruitment/activation, is induced following co-localisation with an activatory receptor such as CD32A(26), FcεRI(22, 38) or the BCR(22).
In addition to FcγRs, CD32B/SHIP-1 has been reported to regulate signalling from other receptors such as FcεRI(22) or Dectin-1, the latter apparently dependent on the galactose component of IgG IC (46). Although FcεRI has not been studied to date, our preliminary analysis indicates that SHIP-1 association with CD32B is not observed following CD32B/Dectin-1 crosslinking in comparison to CD32B/CD32A. Possible reasons for this include a relative inability of Dectin-1 to bind IC in comparison to classical activatory FcγRs, and the presence of an ITAM-like motif (not a canonical ITAM motif) in the cytoplasmic tail of Dectin-1 (http://www.uniprot.org/uniprot/Q9BXN2). It is therefore reasonable to suggest that Dectin-1 binds IC and activates CD32B signalling to a lesser extent than the activatory FcγR CD32A. However, a detailed comparison of hyper- versus hypo-galactosylated IC would be required to test this.
Despite the known ability of SHIP-1 to bind the ITIM of CD32B, alternative roles of activatory receptors in this context(47) should be considered. SHIP-1 was previously shown to bind phosphorylated ITAM motifs of the FcεRI β and γ chains and the T cell receptor-associated CD3 (ε,γ,δ) and ζ chains(48). Moreover, hyper-clustering of FcεRI alone was capable of ‘trans-inhibition’ of signaling from other receptors (32). This reflects similar findings of CD32B-independent SHIP-1 localisation with the BCR(39) and CD32A(30, 49). Such SHIP-1 recruitment to CD32A may act to inhibit phagocytosis independently of CD32B(30, 31), which contrasts with the known ability of CD32B to attenuate CD32A-mediated phagocytosis(26, 50). Nevertheless, this function of activatory receptors could easily be tested by generating the relevant SmBiT fusions (i.e. CD32A-SmBiT or CD79B-SmBiT), crosslinking the activatory receptor alone in the absence of CD32B (with specific, agonistic mAbs), and measuring SHIP-1 recruitment.
Interestingly, luminescence increases following IC stimulation were not completely abrogated with ITIM mutant CD32B-SmBiT (Figure 2C). This may indicate a minor role of other regions in the intracellular domain of CD32B in mediating SHIP-1 binding, as was shown for mouse CD32B(51). An alternative explanation is that, as indicated above(30, 49), CD32A may recruit SHIP-1 independently of CD32B, the LgBiT of which may be capable of binding to the SmBiT of CD32B which is in close proximity with CD32A (following crosslinking by IC).
An additional differentiating feature of this assay compared to others is that it inherently requires the co-expression of multiple FcγRs, namely CD32B and CD32A. This is more representative of a physiological situation whereby activatory and inhibitory receptors are co-expressed. An example of this is CD32A+CD32B+ DCs, which may be involved in the generation of vaccinal responses to mAb therapy as part of a mechanism proposed to involve IC(20). Moreover, the readout represents the sum of interactions between 2 FcγRs. Consequently, the assay may be used to provide information on how a change in the FcγRs co-expressed on a given cell influences FcγR activation. As an example, complexes of rituximab were used to stimulate cells expressing CD32B-SmBiT and CD32A-H131 or R131 (Supplementary Figure 2D). The trend in assay responses broadly followed the binding affinities for different isotypes of human IgG to these CD32A variants(10), with a notably greater response of CD32A-R131 cells to human IgG4 complexes.
Of particular interest is whether this assay can be applied to the detection of IC in the sera of autoimmune disease patients. Responses to sera from most, but not all, SLE patients were detected in a small pilot study, indicating the ability of the assay to detect IC in sera from patients with autoimmune diseases in an antigen non-specific manner (Figure 4 and Supplementary Figure 4). This is a significant advantage of the assay as multiple types of IC (between patient cohorts and longitudinally within a single patient) can theoretically be detected and knowledge of the specificity of the autoantibodies is not required. It is noteworthy, however, that the responses appeared to correlate with the level of autoantibodies to dsDNA (Figure 4B), and that serum samples from patients with positive anti-dsDNA measurements induced statistically greater responses (Figure 4C). This suggests that the assay may be sensitive to detect an increase in autoantibody ICs containing dsDNA and anti-dsDNA. However, as other autoantibody systems exist in SLE patients (i.e. nucleosomal), whether assay responses correlate with the detection of autoantibodies to other autoantigens is of interest.
The clear responses to autoimmune disease sera warrants further study. To this end, one area of current focus is determining where the test may be of value in a clinical setting. It can be envisaged that the assay may aid prognosis, by providing a rapid estimation of the presence of IC in patient sera. Another possibility is that the assay may be used to monitor responses to treatments, such as corticosteroids, if they cause a drop in circulating IC. It is of interest that responses to sera were variable between SLE patients in this preliminary assessment (Figure 4A), even within the anti-dsDNA positive population (Figure 4B-C), highlighting inter-patient variability. Similarly, responses to SLE patient sera did not correlate with disease severity as assessed using the BILAG scoring system (data not shown). Potential explanations for this include heterogeneity of the disease, and/or the timing of sample capture and BILAG scoring. In relation to the latter, a change in clinical disease may lag behind a change in circulating IC. Alternatively, there may be an inherent lack of correlation between circulating IC and disease/disease severity. Therefore, the measurement of circulating IC may be more useful to predict specific clinical manifestations in individual organs rather than a change in global disease as measured by BILAG. This is a key focus of future work. For example, because anti-dsDNA antibodies are of limited value as markers of disease activity(52), the assay may serve as a surrogate marker for the efficiency of IC interaction with FcγRs, and a drop in IC detection may help predict a lower risk of IC-mediated sequelae, such as renal damage(6). Similarly, the assay may be useful to predict risk of disease flare, for example in patients with anti-dsDNA and complement (C3) levels suggestive of active disease. Finally, considering the existence of ‘preclinical SLE’(53), namely the presence of autoantibodies prior to disease diagnosis or fulfilment of criteria necessary for diagnosis, it would also be interesting to determine whether ICs can be detected in ‘healthy’ patient sera prior to disease onset. This knowledge may be useful if able to predict future transition to SLE, or determine whether the initiation of earlier treatments is capable of delaying disease onset/progression(53).
Nevertheless, interesting questions remain regarding how variations in the nature of IC in the serum of autoimmune disease patients, or indeed other patients with immune circulating IC, influences FcγR binding and activation. In theory, our assay will preferentially detect larger complexes consisting of isotypes with greater FcγR binding abilities (i.e. hIgG1, 3) (Supplementary Figure 2B). Recent studies have attempted to clarify the relationship between aspects such as IC size and FcγR activation(21). Although more work is required to determine the exact IC size cut-off for detection using this assay, small ICs can still be detected. It is therefore possible that the assay may be useful to detect small, rapidly cleared ICs that are inefficient complement activators and otherwise difficult to detect. Conversely, the assay may have a use in the screening of serum samples from patients with chronic viral infections, and potentially help to explain the surprising link between IC and suppression of FcγR-mediated effector functions(7, 8). This suppressive capacity of ICs has been proposed to be responsible for inefficient B cell depletion by rituximab in animal models of SLE(54), and in this context the assay may help to investigate the effect of ICs on the efficiency of B cell depletion with next-generation anti-CD20 mAb (35), currently being evaluated in clinical trials of patients with refractory SLE (NCT02550652).
All of these aspects are facilitated by the translation of the assay to a stable cell line setting (Figure 5). Additionally, using these cells, luminescence microscopy was used to provide evidence that SHIP-1 was being recruited to membrane CD32B in response to IC, as distinct capping was observed in the presence of IC (Figure 6D and Supplementary Video 2) but not monomeric IgG (Figure 6C and Supplementary Video 1).
In addition to those situations above, the assay could also easily be used to confirm the absence of FcγR activation with candidate therapeutic molecules (such as Fc3Y) designed to bind FcγRs without stimulating downstream signalling(21). Alternatively, the assay could be modified for the screening of therapeutics, such anti-CD32B/CD79B bispecific constructs(42) or anti-CD19 mAbs engineered for increased CD32B binding(55–57), all of which rely on activation of CD32B signalling. Finally, considering the sensitivity of the assay to IC, it may be modified for use in a quality control setting involving the lot-release testing of mAbs for aggregates.
Although not detracting from these main conclusions, this approach has several current limitations. For example, the HEK293F cell line, utilised in the above experiments for reasons of high transfection efficiency, is not representative of an immune cell that constitutively expresses FcγRs and so may differ in its responses. However, proof-of-principle confirmation was provided using the monocytic cell line, THP-1 (Supplementary Figure 3A-B) and B-cell-line Ramos (Supplementary Figure 3C-D), indicating that the assay outputs can be translated to immune cell lines.
Another limitation is that as the assay was designed to detect IgG ICs, it is therefore unlikely to detect complexes of other subclasses, unless in complex with IgG. Moreover, the detection of serum ICs was performed with samples that were not fresh, and had experienced freeze-thaw. A comparison between fresh and freeze-thaw samples would be worthwhile in future studies. Finally, ICs were not purified from serum, due to concerns of disrupting native ICs and forming artefactual ICs, and for want of providing rapid measurements in a clinical setting. It is therefore a possibility that unknown factors present in sera may contribute to the responses observed.
In conclusion, an assay was developed that represents a biologically relevant cell-based model for the study of numerous IC parameters such as size, isotype content and concentration and could be applied to the detection of IC in autoimmune disease and screening of novel therapeutics.
Supplementary Material
Acknowledgements
The authors would like to thank all of the members of the Antibody and Vaccine Group (Cancer Sciences Unit, Faculty of Medicine, University of Southampton, UK) for the discussions relating to the experiments reported herein and particularly to Dr Edd James (Cancer Sciences Unit, Faculty of Medicine, University of Southampton) for discussions relating to additional controls. Dr. Mike Slater and Jim Hartnett (Promega Corporation R&D, Madison, WI) are thanked for technical assistance with HEK293 landing pad cell line, and construction of the CD32B-SmBiT/SHIP-1-LgBiT/CD32A-encoding vector, respectively.
Grant Support – Funding was provided through an iCASE studentship to RJS with Promega from the BBSRC (BB/K011502/1), and Programme Grants from Bloodwise (12050) and Cancer Research UK (A20537).
Footnotes
Conflict of Interest Disclosure
MSC is a retained consultant for Bioinvent and has performed educational and advisory roles for Baxalta and GLG. He has received research funding from Roche, Gilead and GSK. PGH is an employee of Promega UK Ltd. BB, DL and MC are employees of Promega Corporation.
Author Contributions
RJS performed research, analyzed and interpreted data and wrote the manuscript; HTCC, RJO, ALT, PD, CZ and DL performed research; BB, PGH, MC provided materials and expert input on assay designs; VR, MJL, GC, AL and FN provided key materials and clinical and/or experimental input and reviewed the manuscript; MSC designed research, analyzed and interpreted data and wrote the manuscript with RJS.
References
- 1.Huber VC, Lynch JM, Bucher DJ, Le J, Metzger DW. Fc receptor-mediated phagocytosis makes a significant contribution to clearance of influenza virus infections. Journal of immunology (Baltimore, Md. : 1950) 2001;166:7381–7388. doi: 10.4049/jimmunol.166.12.7381. [DOI] [PubMed] [Google Scholar]
- 2.Heyman B. Feedback regulation by IgG antibodies. Immunology letters. 2003;88:157–161. doi: 10.1016/s0165-2478(03)00078-6. [DOI] [PubMed] [Google Scholar]
- 3.Clynes R, Maizes JS, Guinamard R, Ono M, Takai T, Ravetch JV. Modulation of immune complex-induced inflammation in vivo by the coordinate expression of activation and inhibitory Fc receptors. The Journal of experimental medicine. 1999;189:179–185. doi: 10.1084/jem.189.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fukuyama H, Nimmerjahn F, Ravetch JV. The inhibitory Fcgamma receptor modulates autoimmunity by limiting the accumulation of immunoglobulin G+ anti-DNA plasma cells. Nature immunology. 2005;6:99–106. doi: 10.1038/ni1151. [DOI] [PubMed] [Google Scholar]
- 5.Clatworthy MR, Aronin CE, Mathews RJ, Morgan NY, Smith KG, Germain RN. Immune complexes stimulate CCR7-dependent dendritic cell migration to lymph nodes. Nature medicine. 2014;20:1458–1463. doi: 10.1038/nm.3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stamatiades EG, Tremblay ME, Bohm M, Crozet L, Bisht K, Kao D, Coelho C, Fan X, Yewdell WT, Davidson A, Heeger PS, et al. Immune Monitoring of Trans-endothelial Transport by Kidney-Resident Macrophages. Cell. 2016;166:991–1003. doi: 10.1016/j.cell.2016.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wieland A, Shashidharamurthy R, Kamphorst AO, Han JH, Aubert RD, Choudhury BP, Stowell SR, Lee J, Punkosdy GA, Shlomchik MJ, Selvaraj P, et al. Antibody Effector Functions Mediated by Fcgamma-Receptors Are Compromised during Persistent Viral Infection. Immunity. 2015;42:367–378. doi: 10.1016/j.immuni.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamada DH, Elsaesser H, Lux A, Timmerman JM, Morrison SL, de la Torre JC, Nimmerjahn F, Brooks DG. Suppression of Fcgamma-Receptor-Mediated Antibody Effector Function during Persistent Viral Infection. Immunity. 2015;42:379–390. doi: 10.1016/j.immuni.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dahal LN, Roghanian A, Beers SA, Cragg MS. FcgammaR requirements leading to successful immunotherapy. Immunological reviews. 2015;268:104–122. doi: 10.1111/imr.12342. [DOI] [PubMed] [Google Scholar]
- 10.Bruhns P, Iannascoli B, England P, Mancardi DA, Fernandez N, Jorieux S, Daeron M. Specificity and affinity of human Fcgamma receptors and their polymorphic variants for human IgG subclasses. Blood. 2009;113:3716–3725. doi: 10.1182/blood-2008-09-179754. [DOI] [PubMed] [Google Scholar]
- 11.Lux A, Yu X, Scanlan CN, Nimmerjahn F. Impact of immune complex size and glycosylation on IgG binding to human FcgammaRs. Journal of immunology (Baltimore, Md. : 1950) 2013;190:4315–4323. doi: 10.4049/jimmunol.1200501. [DOI] [PubMed] [Google Scholar]
- 12.Cheng ZJ, Garvin D, Paguio A, Moravec R, Engel L, Fan F, Surowy T. Development of a robust reporter-based ADCC assay with frozen, thaw-and-use cells to measure Fc effector function of therapeutic antibodies. Journal of immunological methods. 2014;414:69–81. doi: 10.1016/j.jim.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 13.Tada M, Ishii-Watabe A, Suzuki T, Kawasaki N. Development of a cell-based assay measuring the activation of FcgammaRIIa for the characterization of therapeutic monoclonal antibodies. PloS one. 2014;9:e95787. doi: 10.1371/journal.pone.0095787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown EP, Dowell KG, Boesch AW, Normandin E, Mahan AE, Chu T, Barouch DH, Bailey-Kellogg C, Alter G, Ackerman ME. Multiplexed Fc array for evaluation of antigen-specific antibody effector profiles. Journal of immunological methods. 2017;443:33–44. doi: 10.1016/j.jim.2017.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wines BD, Vanderven HA, Esparon SE, Kristensen AB, Kent SJ, Hogarth PM. Dimeric FcgammaR Ectodomains as Probes of the Fc Receptor Function of Anti-Influenza Virus IgG. Journal of immunology (Baltimore, Md. : 1950) 2016;197:1507–1516. doi: 10.4049/jimmunol.1502551. [DOI] [PubMed] [Google Scholar]
- 16.Kecse-Nagy C, Szittner Z, Papp K, Hegyi Z, Rovero P, Migliorini P, Lorand V, Homolya L, Prechl J. Characterization of NF-kappaB Reporter U937 Cells and Their Application for the Detection of Inflammatory Immune-Complexes. PloS one. 2016;11:e0156328. doi: 10.1371/journal.pone.0156328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Szittner Z, Bentlage AE, Rovero P, Migliorini P, Lorand V, Prechl J, Vidarsson G. Label-free detection of immune complexes with myeloid cells. Clinical and experimental immunology. 2016;185:72–80. doi: 10.1111/cei.12788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rafiq K, Bergtold A, Clynes R. Immune complex-mediated antigen presentation induces tumor immunity. The Journal of clinical investigation. 2002;110:71–79. doi: 10.1172/JCI15640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalergis AM, Ravetch JV. Inducing tumor immunity through the selective engagement of activating Fcgamma receptors on dendritic cells. The Journal of experimental medicine. 2002;195:1653–1659. doi: 10.1084/jem.20020338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DiLillo DJ, Ravetch JV. Differential Fc-Receptor Engagement Drives an Anti-tumor Vaccinal Effect. Cell. 2015;161:1035–1045. doi: 10.1016/j.cell.2015.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ortiz DF, Lansing JC, Rutitzky L, Kurtagic E, Prod'homme T, Choudhury A, Washburn N, Bhatnagar N, Beneduce C, Holte K, Prenovitz R, et al. Elucidating the interplay between IgG-Fc valency and FcgammaR activation for the design of immune complex inhibitors. Science translational medicine. 2016;8:365ra158. doi: 10.1126/scitranslmed.aaf9418. [DOI] [PubMed] [Google Scholar]
- 22.Ono M, Bolland S, Tempst P, Ravetch JV. Role of the inositol phosphatase SHIP in negative regulation of the immune system by the receptor Fc(gamma)RIIB. Nature. 1996;383:263–266. doi: 10.1038/383263a0. [DOI] [PubMed] [Google Scholar]
- 23.Clatworthy MR, Harford SK, Mathews RJ, Smith KG. FcgammaRIIb inhibits immune complex-induced VEGF-A production and intranodal lymphangiogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:17971–17976. doi: 10.1073/pnas.1413915111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muta T, Kurosaki T, Misulovin Z, Sanchez M, Nussenzweig MC, Ravetch JV. A 13-amino-acid motif in the cytoplasmic domain of Fc gamma RIIB modulates B-cell receptor signalling. Nature. 1994;368:70–73. doi: 10.1038/368070a0. [DOI] [PubMed] [Google Scholar]
- 25.Daeron M, Latour S, Malbec O, Espinosa E, Pina P, Pasmans S, Fridman WH. The same tyrosine-based inhibition motif, in the intracytoplasmic domain of Fc gamma RIIB, regulates negatively BCR-, TCR-, and FcR-dependent cell activation. Immunity. 1995;3:635–646. doi: 10.1016/1074-7613(95)90134-5. [DOI] [PubMed] [Google Scholar]
- 26.Tridandapani S, Siefker K, Teillaud JL, Carter JE, Wewers MD, Anderson CL. Regulated expression and inhibitory function of Fcgamma RIIb in human monocytic cells. The Journal of biological chemistry. 2002;277:5082–5089. doi: 10.1074/jbc.M110277200. [DOI] [PubMed] [Google Scholar]
- 27.Ono M, Okada H, Bolland S, Yanagi S, Kurosaki T, Ravetch JV. Deletion of SHIP or SHP-1 reveals two distinct pathways for inhibitory signaling. Cell. 1997;90:293–301. doi: 10.1016/s0092-8674(00)80337-2. [DOI] [PubMed] [Google Scholar]
- 28.Bolland S, Pearse RN, Kurosaki T, Ravetch JV. SHIP modulates immune receptor responses by regulating membrane association of Btk. Immunity. 1998;8:509–516. doi: 10.1016/s1074-7613(00)80555-5. [DOI] [PubMed] [Google Scholar]
- 29.Cox D, Dale BM, Kashiwada M, Helgason CD, Greenberg S. A regulatory role for Src homology 2 domain-containing inositol 5'-phosphatase (SHIP) in phagocytosis mediated by Fc gamma receptors and complement receptor 3 (alpha(M)beta(2); CD11b/CD18) The Journal of experimental medicine. 2001;193:61–71. doi: 10.1084/jem.193.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nakamura K, Malykhin A, Coggeshall KM. The Src homology 2 domain-containing inositol 5-phosphatase negatively regulates Fcgamma receptor-mediated phagocytosis through immunoreceptor tyrosine-based activation motif-bearing phagocytic receptors. Blood. 2002;100:3374–3382. doi: 10.1182/blood-2002-03-0787. [DOI] [PubMed] [Google Scholar]
- 31.Huang ZY, Hunter S, Kim MK, Indik ZK, Schreiber AD. The effect of phosphatases SHP-1 and SHIP-1 on signaling by the ITIM- and ITAM-containing Fcgamma receptors FcgammaRIIB and FcgammaRIIA. Journal of leukocyte biology. 2003;73:823–829. doi: 10.1189/jlb.0902454. [DOI] [PubMed] [Google Scholar]
- 32.Malbec O, Cassard L, Albanesi M, Jonsson F, Mancardi D, Chicanne G, Payrastre B, Dubreuil P, Vivier E, Daeron M. Trans-inhibition of activation and proliferation signals by Fc receptors in mast cells and basophils. Science signaling. 2016;9:ra126. doi: 10.1126/scisignal.aag1401. [DOI] [PubMed] [Google Scholar]
- 33.Dixon AS, Schwinn MK, Hall MP, Zimmerman K, Otto P, Lubben TH, Butler BL, Binkowski BF, Machleidt T, Kirkland TA, Wood MG, et al. NanoLuc Complementation Reporter Optimized for Accurate Measurement of Protein Interactions in Cells. ACS chemical biology. 2015;11:400–408. doi: 10.1021/acschembio.5b00753. [DOI] [PubMed] [Google Scholar]
- 34.Cragg MS, Bayne MB, Tutt AL, French RR, Beers S, Glennie MJ, Illidge TM. A new anti-idiotype antibody capable of binding rituximab on the surface of lymphoma cells. Blood. 2004;104:2540–2542. doi: 10.1182/blood-2004-05-1733. [DOI] [PubMed] [Google Scholar]
- 35.Reddy V, Klein C, Isenberg DA, Glennie MJ, Cambridge G, Cragg MS, Leandro MJ. Obinutuzumab induces superior B-cell cytotoxicity to rituximab in rheumatoid arthritis and systemic lupus erythematosus patient samples. Rheumatology (Oxford, England) 2017 doi: 10.1093/rheumatology/kex067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cragg MS, Chan HT, Fox MD, Tutt A, Smith A, Oscier DG, Hamblin TJ, Glennie MJ. The alternative transcript of CD79b is overexpressed in B-CLL and inhibits signaling for apoptosis. Blood. 2002;100:3068–3076. doi: 10.1182/blood.V100.9.3068. [DOI] [PubMed] [Google Scholar]
- 37.Vaughan AT, Chan CH, Klein C, Glennie MJ, Beers SA, Cragg MS. Activatory and inhibitory Fcgamma receptors augment rituximab-mediated internalization of CD20 independent of signaling via the cytoplasmic domain. The Journal of biological chemistry. 2015;290:5424–5437. doi: 10.1074/jbc.M114.593806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Malbec O, Fong DC, Turner M, Tybulewicz VL, Cambier JC, Fridman WH, Daeron M. Fc epsilon receptor I-associated lyn-dependent phosphorylation of Fc gamma receptor IIB during negative regulation of mast cell activation. Journal of immunology (Baltimore, Md. : 1950) 1998;160:1647–1658. [PubMed] [Google Scholar]
- 39.Pauls SD, Ray A, Hou S, Vaughan AT, Cragg MS, Marshall AJ. FcgammaRIIB-Independent Mechanisms Controlling Membrane Localization of the Inhibitory Phosphatase SHIP in Human B Cells. Journal of immunology (Baltimore, Md. : 1950) 2016;197:1587–1596. doi: 10.4049/jimmunol.1600105. [DOI] [PubMed] [Google Scholar]
- 40.Tutt AL, James S, Laversin SA, Tipton TR, Ashton-Key M, French RR, Hussain K, Vaughan AT, Dou L, Earley A, Dahal LN, et al. Development and Characterization of Monoclonal Antibodies Specific for Mouse and Human Fcgamma Receptors. Journal of immunology (Baltimore, Md. : 1950) 2015;195:5503–5516. doi: 10.4049/jimmunol.1402988. [DOI] [PubMed] [Google Scholar]
- 41.Phillips NE, Parker DC. Fc-dependent inhibition of mouse B cell activation by whole anti-mu antibodies. Journal of immunology (Baltimore, Md. : 1950) 1983;130:602–606. [PubMed] [Google Scholar]
- 42.Veri MC, Burke S, Huang L, Li H, Gorlatov S, Tuaillon N, Rainey GJ, Ciccarone V, Zhang T, Shah K, Jin L, et al. Therapeutic control of B cell activation via recruitment of Fcgamma receptor IIb (CD32B) inhibitory function with a novel bispecific antibody scaffold. Arthritis and rheumatism. 2010;62:1933–1943. doi: 10.1002/art.27477. [DOI] [PubMed] [Google Scholar]
- 43.Cano PO, Jerry LM, Sladowski JP, Osterland CK. Circulating immune complexes in systemic lupus erythematosus. Clinical and experimental immunology. 1977;29:197–204. [PMC free article] [PubMed] [Google Scholar]
- 44.Abes R, Gelize E, Fridman WH, Teillaud JL. Long-lasting antitumor protection by anti-CD20 antibody through cellular immune response. Blood. 2010;116:926–934. doi: 10.1182/blood-2009-10-248609. [DOI] [PubMed] [Google Scholar]
- 45.Manivel VA, Sohrabian A, Ronnelid J. Granulocyte-augmented chemokine production induced by type II collagen-containing immune complexes is mediated via TLR4 in rheumatoid arthritis patients. European journal of immunology. 2016;46:2822–2834. doi: 10.1002/eji.201646496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Karsten CM, Pandey MK, Figge J, Kilchenstein R, Taylor PR, Rosas M, McDonald JU, Orr SJ, Berger M, Petzold D, Blanchard V, et al. Anti-inflammatory activity of IgG1 mediated by Fc galactosylation and association of FcgammaRIIB and dectin-1. Nature medicine. 2012;18:1401–1406. doi: 10.1038/nm.2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pauls SD, Marshall AJ. Regulation of immune cell signaling by SHIP1: A phosphatase, scaffold protein and potential therapeutic target. European journal of immunology. 2017;47:932–945. doi: 10.1002/eji.201646795. [DOI] [PubMed] [Google Scholar]
- 48.Osborne MA, Zenner G, Lubinus M, Zhang X, Songyang Z, Cantley LC, Majerus P, Burn P, Kochan JP. The inositol 5'-phosphatase SHIP binds to immunoreceptor signaling motifs and responds to high affinity IgE receptor aggregation. The Journal of biological chemistry. 1996;271:29271–29278. doi: 10.1074/jbc.271.46.29271. [DOI] [PubMed] [Google Scholar]
- 49.Maresco DL, Osborne JM, Cooney D, Coggeshall KM, Anderson CL. The SH2-containing 5'-inositol phosphatase (SHIP) is tyrosine phosphorylated after Fc gamma receptor clustering in monocytes. Journal of immunology (Baltimore, Md.: 1950) 1999;162:6458–6465. [PubMed] [Google Scholar]
- 50.Hunter S, Indik ZK, Kim MK, Cauley MD, Park JG, Schreiber AD. Inhibition of Fcgamma receptor-mediated phagocytosis by a nonphagocytic Fcgamma receptor. Blood. 1998;91:1762–1768. [PubMed] [Google Scholar]
- 51.Fong DC, Brauweiler A, Minskoff SA, Bruhns P, Tamir I, Mellman I, Daeron M, Cambier JC. Mutational analysis reveals multiple distinct sites within Fc gamma receptor IIB that function in inhibitory signaling. Journal of immunology (Baltimore, Md.: 1950) 2000;165:4453–4462. doi: 10.4049/jimmunol.165.8.4453. [DOI] [PubMed] [Google Scholar]
- 52.Isenberg DA, Manson JJ, Ehrenstein MR, Rahman A. Fifty years of anti-ds DNA antibodies: are we approaching journey's end? Rheumatology (Oxford, England) 2007;46:1052–1056. doi: 10.1093/rheumatology/kem112. [DOI] [PubMed] [Google Scholar]
- 53.Robertson JM, James JA. Preclinical systemic lupus erythematosus. Rheumatic diseases clinics of North America. 2014;40:621–635. doi: 10.1016/j.rdc.2014.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ahuja A, Teichmann LL, Wang H, Dunn R, Kehry MR, Shlomchik MJ. An acquired defect in IgG-dependent phagocytosis explains the impairment in antibody-mediated cellular depletion in Lupus. Journal of immunology (Baltimore, Md.: 1950) 2011;187:3888–3894. doi: 10.4049/jimmunol.1101629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chu SY, Vostiar I, Karki S, Moore GL, Lazar GA, Pong E, Joyce PF, Szymkowski DE, Desjarlais JR. Inhibition of B cell receptor-mediated activation of primary human B cells by coengagement of CD19 and FcgammaRIIb with Fc-engineered antibodies. Molecular immunology. 2008;45:3926–3933. doi: 10.1016/j.molimm.2008.06.027. [DOI] [PubMed] [Google Scholar]
- 56.Horton HM, Chu SY, Ortiz EC, Pong E, Cemerski S, Leung IW, Jacob N, Zalevsky J, Desjarlais JR, Stohl W, Szymkowski DE. Antibody-mediated coengagement of FcgammaRIIb and B cell receptor complex suppresses humoral immunity in systemic lupus erythematosus. Journal of immunology (Baltimore, Md.: 1950) 2011;186:4223–4233. doi: 10.4049/jimmunol.1003412. [DOI] [PubMed] [Google Scholar]
- 57.Chu SY, Yeter K, Kotha R, Pong E, Miranda Y, Phung S, Chen H, Lee SH, Leung I, Bonzon C, Desjarlais JR, et al. Suppression of rheumatoid arthritis B cells by XmAb5871, an anti-CD19 antibody that coengages B cell antigen receptor complex and Fcgamma receptor IIb inhibitory receptor. Arthritis & rheumatology (Hoboken, N.J.) 2014;66:1153–1164. doi: 10.1002/art.38334. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.