Abstract
Background
We determined whether high-sensitivity cardiac troponin I can improve the estimation of the pre-test probability for obstructive coronary artery disease in patients with suspected stable angina.
Methods and Results
In a pre-specified sub-study of the Scottish COmputed Tomography of the Heart (SCOT-HEART) trial, plasma cardiac troponin was measured using a high-sensitivity single molecule counting assay in 943 adults with suspected stable angina who had undergone coronary computed tomography angiography. Rates of obstructive coronary artery disease were compared with the pre-test probability determined by the Coronary Artery Disease Consortium (CADC) risk model with and without cardiac troponin concentrations. External validation was undertaken in an independent study population from Denmark comprising 487 patients with suspected stable angina. Higher cardiac troponin concentrations were associated with obstructive coronary artery disease with a 5-fold increase across quintiles (9 to 48%, p<0.001) independent of known cardiovascular risk factors (odds ratio [OR] 1.35 [95% confidence interval (CI) 1.25-1.46] per doubling of troponin). Cardiac troponin concentrations improved the discrimination and calibration of the CADC model for identifying obstructive coronary artery disease (c-statistic 0.788 to 0.800, p=0.004; χ2 16.8 [p = 0.032] to 14.3 [p = 0.074]). The updated model also improved classification of the American College of Cardiology/American Heart Association pre-test probability risk categories (net reclassification improvement 0.062 [95% CI, 0.035-0.089]). The revised model achieved similar improvements in discrimination and calibration when applied in the external validation cohort.
Conclusions
High-sensitivity cardiac troponin I concentration is an independent predictor of obstructive coronary artery disease in patients with suspected stable angina. Use of this test may improve the selection of patients for further investigation and treatment.
Clinical Trial Registration
Clinicaltrials.gov; Unique identifier NCT: 01149590
Keywords: cardiac troponin, high-sensitivity assays, stable angina, coronary artery disease, coronary computed tomography angiography
Journal Subject Terms: Biomarkers, Computerized Tomography, Chronic Ischemic Heart Disease, Clinical Studies
Introduction
Presentations with suspected stable angina are common yet determining an accurate diagnosis is frequently challenging. Patients and clinicians alike are understandably keen to identify the cause of the symptoms in order that these can be treated and hopefully ameliorated. Of equal importance, is the concern that these symptoms may reflect prognostically significant atherosclerotic disease with the associated risk of future cardiovascular events. These concerns are appropriate given that 1 in 6 patients will suffer coronary death or non-fatal acute coronary syndrome in the 3 years following a diagnosis of stable angina (1). Importantly, this risk remains substantial even in those patients with symptoms deemed non-cardiac in origin (1). Consequently, despite the central role of the clinical history and cardiovascular risk factor ascertainment in the assessment process, supplementary investigations are frequently required to provide additional certainty related to the presence or absence of obstructive coronary artery disease (2). A number of national and international bodies have proposed standardised pathways that employ risk models to estimate the pre-test probability (PTP) of obstructive coronary artery disease and guide decision-making with regards to appropriate use of investigations (3–5). However, there is evidence both that these models may over-estimate risk (6–8) and that clinician use of stratification tools remains sub-optimal (9, 10).
In light of these challenges, there is widespread interest in identifying suitable biomarkers that may improve diagnostic accuracy in patients with suspected stable coronary artery disease. As yet, no novel circulating biomarker has been shown to improve diagnostic classification (3). It is in this context that a role may emerge for the most recent generation of high-sensitivity cardiac troponin assays. These tests offer the ability to reliably measure troponin in the majority of the healthy population and have already had a significant impact on the assessment of suspected acute coronary syndromes (11). Meanwhile, evidence is emerging of potential roles in the context of stable cardiovascular diseases (12, 13).
This study aimed to determine if routine quantification of plasma high sensitivity cardiac troponin I concentrations could improve estimation of the pre-test probability of obstructive coronary artery disease in patients with suspected stable angina.
Methods
The data, analytic methods, and study materials will be made available to other researchers for purposes of reproducing the results or replicating the procedure upon reasonable request to the corresponding author.
Study design
The Scottish COmputed Tomography of the Heart (SCOT-HEART) trial was a prospective, multi-centre, randomised controlled study that investigated the role of coronary computed tomography angiography (CCTA) in patients referred to a specialist clinic with suspected angina due to coronary heart disease. The study design (14) and principal findings (15) have previously been reported. Briefly, participants were recruited from 12 cardiology chest pain clinics across Scotland and those randomised to the intervention arm underwent CCTA imaging at one of 3 sites in addition to routine clinical assessment. There was a pre-specified biomarker sub-study which obtained blood samples from those participants where the CCTA was performed at the Clinical Research Imaging Centre in Edinburgh, UK. Recruitment began November 18, 2010 and follow-up of clinical outcomes continued until June 30, 2016. The study was performed in accordance with the Declaration of Helsinki and with research ethics committee approval. Written informed consent was obtained from all individuals prior to study participation.
High-sensitivity cardiac troponin I measurement
Venous blood samples for biomarker testing were obtained immediately prior to CCTA imaging. Blood was processed and stored at –80°C until analysed. Plasma high-sensitivity cardiac troponin I concentrations were measured using a high-sensitivity single molecule counting assay on the Erenna platform (Singulex Inc, Alameda, California, USA) which has a limit of detection (LoD) of 0.1 ng/L, a limit of quantification (LOQ, co-efficient of variation <10%) of 0.4 ng/L and a 99th centile upper reference limit (URL) of 10.9 ng/L (16, 17). To facilitate internal validation of this measurement with a clinically available assay, a secondary analysis was performed wherein the samples were analysed using the ARCHITECTSTAT high-sensitive troponin I assay (Abbott Laboratories, Abbott Park, Illinois, USA) which has a limit of detection of 1.2 ng/L and coefficient of variation <10% at 3.0 ng/L and sex-specific 99th centile upper reference limits of 16 ng/L and 34 ng/L in women and men respectively (17, 18).
Coronary computed tomography angiography
Participants underwent coronary artery calcium scoring and CCTA using a 320-detector scanner (Aquilion One, Toshiba Medical Systems, Nasushiobara, Japan). Computed tomography images were analysed by 2 trained observers with excellent reproducibility (19). Differences in categorisation were resolved by consensus. Coronary artery calcium scoring was performed using dedicated software (VScore, Vital Images, Minnetonka, USA). Agatston score was calculated using a threshold of 130 HU (Hounsfield units) for each vessel and summed to give a total score. The coronary arteries were assessed using a 15-segment model with each segment classified into one of five categories dependent on the degree of luminal cross-sectional area stenosis: normal (<10% stenosis), mild non-obstructive (10-49% stenosis), moderate non-obstructive (50-69% stenosis), obstructive (70-99% stenosis) or total/sub-total occlusion (100% stenosis). For the purposes of the primary outcome, obstructive coronary artery disease was defined prior to this analysis within the published SCOT-HEART trial protocol, as a luminal cross-sectional area stenosis of ≥70% (approximating to a 50% diameter stenosis) in at least one major epicardial vessel or ≥50% in the left main stem (14). Using previously described methods (20), the segment stenosis score (SSS) was quantified as a measure of overall atherosclerotic burden. All image analysis was performed blinded to the biomarker results.
The Coronary Artery Disease Consortium (CADC) model
The CADC is part of the European network for the Assessment of Imaging in Medicine (EuroAIM). In 2011 and 2012 the CADC updated and extended the earlier Diamond-Forrester model to estimate more accurately the pre-test probability (PTP) of obstructive coronary artery disease identified on invasive coronary angiography in patients with suspected stable angina (8, 21). The CADC model incorporates age, sex and chest pain characteristics and underpins the risk tables included in the current European Society of Cardiology guideline on the management of stable coronary artery disease (3). Furthermore, it has recently been shown to provide more accurate estimates of the probability of obstructive coronary artery disease than the modified Diamond-Forrester model currently endorsed by the American College of Cardiology/American Heart Association (ACC/AHA) guidelines and appropriate use criteria for the diagnosis of stable CAD (22–24). The ACC/AHA guideline uses two thresholds in order to stratify patients into three risk groups. Patients with a PTP less than 10% are deemed low-risk, those with a PTP between 10% and 90% are classed as intermediate risk, whilst those with a PTP ≥90% are high risk for CAD. It is widely recognised that the diagnostic utility of non-invasive testing is greatest in patients with intermediate pre-test probability of obstructive coronary disease and the benefits of further testing are limited in low-risk individuals. High-risk patients may warrant invasive coronary angiography for the purposes of prognostic stratification or to facilitate therapeutic revascularisation.
Validation cohort
External validation of the revised model was performed in a previously described study population (25, 26) comprising 487 patients with suspected stable angina who underwent biomarker sampling in addition to coronary imaging (CCTA in 336 invasive angiography in 151 ) at the Odense University Hospital, Denmark. Troponin concentrations were determined using the Abbott Architect assay.
Statistical analysis
Statistical analysis was performed using R version 3.3.0 (R Foundation for Statistical Computing, Vienna, Austria). Summary statistics for patient characteristics were estimated, by quintile of cardiac troponin concentration, with Chi-squared and ANOVA tests being used to compare categorical and continuous variables, respectively. In logistic regression models, the probability of each patient having obstructive coronary artery disease was estimated. Cardiac troponin concentration and coronary artery calcium scores were log-transformed as linearising transformations. Associations were estimated unadjusted, and after adjusting for age, sex, chest pain characteristics, cardiovascular risk factors and non-invasive test results. The baseline CADC model and CADC model with the addition of cardiac troponin were also fitted. In both cases the model intercept was estimated from the sample data (with the coefficients for age, sex and chest pain typicality fixed) to allow fair comparison of model performance. Discrimination and calibration were compared for the current CADC model, and the CADC model with troponin, using the Delong method (27) and the Hosmer-Lemeshow (H-L) goodness of fit test (p-value <0.05 defined as poor calibration) respectively. The coefficient of discrimination (D) was calculated according to the method proposed by Tjur (28). The categorical net reclassification improvement index was estimated using the ACC/AHA-recommended PTP threshold of 10% to distinguish low risk from intermediate or high risk. The association between troponin assays was assessed using the Pearson correlation coefficient.
The performance, in terms of discrimination and calibration, of the new model incorporating troponin concentration was also compared to the existing CADC model in an independent cohort. Neither the intercept nor the coefficients were re-estimated for either model.
Results
Data collection and study population
The study population of the SCOT-HEART trial has previously been described (15). Between November 18th 2010 and September 24th 2014, 4,146 participants were recruited of whom 2,073 were randomly assigned to standard care plus CCTA and 1,778 of these underwent CCTA at one of three sites. Blood samples were obtained from 987 participants at the time of CCTA imaging at a single centre and 943 had plasma cardiac troponin I concentrations measured. CCTA image quality was non-diagnostic in 6 cases resulting in an analysis set comprising 937 participants (Supplementary Figure 1). The baseline characteristics were similar between trial participants with and without estimations of troponin concentrations (Supplementary Table 1).
Coronary computed tomography angiography
The median interval between randomisation and CCTA was 13 days (interquartile range [IQR] 7 to 18 days). The median coronary artery calcium score was 31 (IQR 0 to 281) Agatston Units (AU). CCTA demonstrated normal coronary arteries in 322 (34%), mild to moderate non-obstructive disease in 348 (37%), and obstructive disease in 267 (28%) participants.
High-sensitivity cardiac troponin I concentrations
Using the Singulex assay, cardiac troponin I concentrations were above the LoD in 934/937 (99.6%) patients. The three samples with concentrations below this limit were assigned a value of 0.1 ng/L. The median concentration of hs-cTnI in all patients was 1.41 (IQR 0.89 to 2.28) ng/L with 907 (96.8%) and 27 (2.9%) of patients above the limit of quantification (0.4 ng/L) and 99th centile URL (10.9 ng/L) respectively. The median concentration of hs-cTnI in patients with obstructive coronary disease was 1.9 (IQR 1.3 to 3.1) ng/L whilst the median concentration of hs-cTnI in those without coronary obstruction was 1.2 (IQR 0.8 to 1.9) ng/L, p<0.001.
Higher cardiac troponin quintiles were associated with increasing age, male sex and a number of cardiovascular risk factors (Table 1). The majority (82.3%) of patients underwent exercise electrocardiography and this test was more likely to demonstrated inducible ischaemia in those with higher cardiac troponin concentrations (Table 2).
Table 1. Baseline characteristics of patients with suspected angina stratified by cardiac troponin.
| Cardiac troponin I concentrations by quintile (range [ng/L]) | |||||
|---|---|---|---|---|---|
| Q1 (≤0.82) |
Q2 (0.83-1.16) |
Q3 (1.17-1.61) |
Q4 (1.62-2.66) |
Q5 (>2.66) |
|
| n | 193 | 186 | 183 | 192 | 183 |
| Age, years | 51.8 (9.4) | 57.0 (8.3) | 59.0 (9.5) | 60.7 (8.8) | 60.7 (8.8) |
| Male, % | 60 (31.1) | 93 (50.0) | 113 (61.7) | 132 (68.8) | 135 (73.8) |
| Chest pain symptom, % | |||||
| Typical angina | 54 (28.0) | 64 (34.4) | 81 (44.3) | 101 (52.6) | 99 (54.1) |
| Atypical angina | 65 (33.7) | 39 (21.0) | 46 (25.1) | 32 (16.7) | 39 (21.3) |
| Non-anginal | 74 (38.3) | 83 (44.6) | 56 (30.6) | 59 (30.7) | 45 (24.6) |
| BMI | 29.6 (6.2) | 29.1 (5.5) | 30.1 (5.1) | 30.0 (5.5) | 29.4 (5.4) |
| Pre-existing CHD, % | 12 (6.2) | 6 (3.2) | 19 (10.4) | 20 (10.4) | 21 (11.5) |
| Hypertension, % | 36 (18.7) | 54 (29.3) | 70 (38.5) | 92 (48.7) | 84 (46.4) |
| Hyperlipidemia, % | 95 (49.2) | 109 (58.6) | 117 (63.9) | 126 (65.6) | 118 (64.5) |
| Diabetes mellitus, % | 20 (10.4) | 17 (9.1) | 13 (7.1) | 23 (12.0) | 25 (13.7) |
| Current smoker, % | 46 (23.8) | 45 (24.2) | 33 (18.0) | 31 (16.2) | 30 (16.4) |
| Family history of CHD, % | 95 (50.0) | 85 (46.4) | 81 (44.8) | 71 (37.4) | 62 (34.1) |
| 10-year CHD risk* | 11.0 [6.0, 16.0] | 15.0 [9.0, 22.8] | 17.0 [11.0, 24.0] | 19.0 [14.0, 27.0] | 19.0 [14.0, 27.5] |
Data are mean (standard deviation), median [IQR], or value (%); BMI, body mass index; CHD, coronary heart disease.
This value is determined through calculation of the ASSIGN Score, a risk model derived and validated within Scotland for the determination of cardiovascular risk in patients without known coronary heart disease (29)(see http://assign-score.com/)
Table 2. Exercise electrocardiography and coronary computed tomography findings by troponin quintile.
| Cardiac troponin I concentrations by quintile (ng/L) | ||||||
|---|---|---|---|---|---|---|
| Q1 (≤0.82) |
Q2 (0.83-1.16) |
Q3 (1.17-1.61) |
Q4 (1.62-2.66) |
Q5 (>2.66) |
p-value | |
| Exercise ECG performed, % | 162 (83.9) | 161 (86.6) | 153 (84.1) | 149 (77.6) | 145 (79.2) | 0.129 |
| Exercise ECG outcome | <0.001 | |||||
| Normal, % | 123 (84.2) | 109 (71.2) | 93 (64.1) | 86 (62.3) | 64 (47.1) | |
| Inconclusive, % | 11 (7.5) | 24 (15.7) | 23 (15.9) | 26 (18.8) | 29 (21.3) | |
| Abnormal, % | 12 (8.2) | 20 (13.1) | 29 (20.0) | 26 (18.8) | 43 (31.6) | |
| Coronary calcium score | 0.0 [0.0, 31.0] | 10.0 [0.0, 123.00] | 49.0 [0.0, 316.5] | 118.0 [1.8, 629.3] | 140.0 [4.0, 739.5] | <0.001 |
| Coronary disease on CT, % | <0.001 | |||||
| No significant CHD, % | 107 (55.4) | 78 (41.9) | 55 (30.1) | 44 (22.9) | 37 (20.2) | |
| Non-obstructive CHD, % | 67 (34.7) | 69 (37.1) | 75 (41.0) | 78 (40.6) | 59 (32.2) | |
| Obstructive CHD, % | 18 (9.3) | 39 (21.0) | 53 (29.0) | 70 (36.5) | 87 (47.5) | |
| SSS Score | 0.0 [0.0, 2.0] | 1.0 [0.0, 6.0] | 3.0 [0.0, 10.0] | 5.0 [1.0, 12.0] | 7.0 [1.0, 14.0] | <0.001 |
Data are median [IQR], or value (%); ECG, electrocardiography; IQR, interquartile range; CHD, coronary heart disease; SSS, segment stenosis score.
Higher cardiac troponin quintiles were associated with greater coronary atherosclerotic burden as determined by coronary artery calcium score or segment stenosis score. They were also more likely to have obstructive coronary disease with a five-fold increase between the first and fifth quintiles (9.3% to 47.5%; Table 2). Each 2-fold increment in troponin concentration was associated with a 1.71-fold increment (95% confidence intervals (CI), 1.60-1.83) in the odds of identifying obstructive coronary artery disease on CCTA. This association was moderately attenuated after adjusting for age and sex (OR 1.39; 95% CI, 1.29-1.49), but persisted on further adjustment for chest pain description, cardiovascular risk factors, exercise ECG findings and the coronary calcium score (OR 1.27; 95% CI, 1.17-1.39; Supplementary Table 2).
Troponin testing with a second high-sensitivity cardiac troponin I assay (Abbott Diagnostics) was performed on 931 samples and demonstrated good agreement with the Singulex assay (r=0.88). The median troponin concentration was 2.1 ng/L (95% CI, 1.3-3.5 ng/L) and a number of samples reported results below the LoD (200, 21.5%). Despite this, the overall findings were consistent with the primary analysis (Supplementary Tables 4-8, Supplementary Figures 2-3).
Update and extension of the CADC model
Compared to the cohort used to develop the CADC model, participants in our cohort were of similar age and more likely to have typical angina or obstructive disease on coronary imaging (Supplementary Table 3). Goodness-of-fit for the baseline CADC model was inadequate (χ2 = 20.2, HL p = 0.0095), although improved following re-estimation of the model intercept (difference in deviance 7.1, 1 degrees of freedom, p=0.0076). Adding cardiac troponin concentrations, further improved model fit (difference in deviance 16.3, 1 degrees of freedom, p<0.0001).
The addition of cardiac troponin concentration improved overall model performance (D 0.230 to 0.257; Table 3) including discrimination (c-statistic: 0.788 to 0.800, p=0.0039; Supplementary Figure 4). The addition of cardiac troponin concentration also improved classification of patients into ACC/AHA risk categories (Table 4, Figure 1). There was a net 10.5% (95% CI 7.7-13.8) reduction in the number of patients determined to be at intermediate or high risk according to the CADC model but without obstructive coronary disease on CCTA. One additional patient was inappropriately reclassified as low risk who had been determined to have intermediate pre-test probability of CAD on the original CADC model (net reclassification index (NRI), 0.062 [95% CI, 0.035-0.089]).
Table 3. Coronary Artery Disease Consortium (CADC) model statistics.
| Performance measure | CADC Model* | CADC Model with troponin* |
|---|---|---|
| Overall | ||
| Coefficient of Discrimination | 0.230 | 0.257 |
| Brier score | 0.163 | 0.159 |
| Discrimination | ||
| C-statistic [95% CI] | 0.788 [0.758-0.819] | 0.800 [0.770-0.830] † |
| Calibration (Hosmer-Lemeshow Test) | ||
| Chi-square | 16.84 | 14.30 |
| P-value | 0.032 | 0.074 |
| NRI (Categorical) [95% CI] | 0.062 [0.035 - 0.089] | |
| Statistics at 10% PTP threshold | ||
| Sensitivity [95% CI] | 0.944 [0.916-0.971] | 0.940 [0.912-0.969] p=0.655 |
| Specificity [95% CI] | 0.375 [0.337-0.411] | 0.440 [0.403-0.478] p<0.001 |
| PPV [95% CI] | 0.376 [0.339-0.412] | 0.401 [0.363-0.439] p<0.001 |
| NPV [95% CI] | 0.944 [0.916-0.971] | 0.949 [0.924-0.973] p=0.518 |
CADC, Coronary Artery Disease Consortium; CI, confidence interval; NRI, net reclassification improvement; PTP, pre-test probability; PPV, positive predictive value; NPV, negative predictive value;
both models apply updated intercept determined from SCOT-HEART population
p = 0.0039 that true difference in AUC is not equal to 0.
Table 4. Net reclassification with the addition of cardiac troponin I to the CADC model.
| CADC Model with cardiac troponin | ||||
|---|---|---|---|---|
| Low Risk (<10%) |
Intermediate/High Risk (≥10%) |
% Reclassified | ||
| Outcome: No Obstructive Disease | ||||
| CADC Model | Low Risk (<10%) |
245 | 6 | 2 |
| Intermediate/High Risk (≥10%) |
50 | 369 | 12 | |
| Outcome: Obstructive Disease | ||||
| CADC Model | Low Risk (<10%) |
13 | 2 | 13 |
| Intermediate/High Risk (≥10%) |
3 | 249 | 1 | |
| NRI(Categorical) [95% CI]: 0.062 [0.035 - 0.089] | ||||
CADC, Coronary Artery Disease Consortium; NRI, net reclassification improvement; CI, confidence interval.
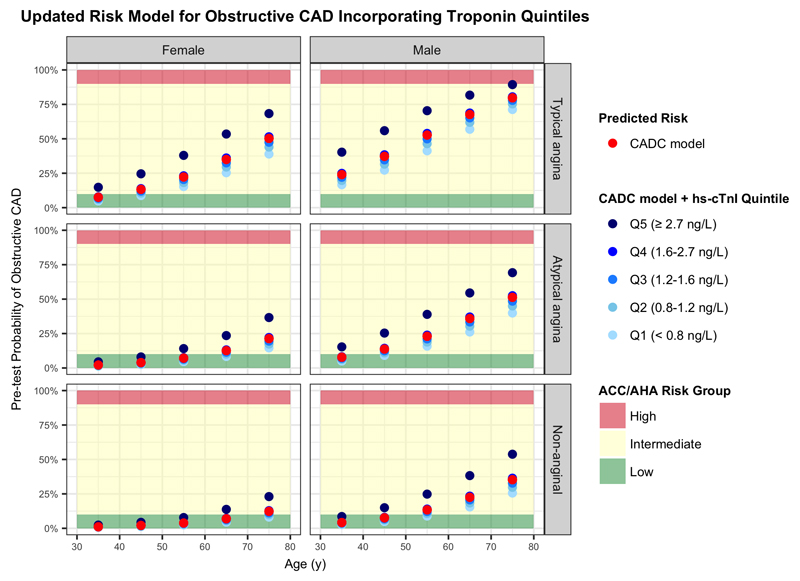
Figure 1. Cardiac troponin improves predicted risk of obstructive coronary artery disease in patients with suspected angina.
The red dots represent the risk of obstructive CAD as estimated by the established CAD Consortium model accounting for age, sex and symptom description. The blue dots represent the revised risk estimates with the addition of cardiac troponin quintiles. The shaded regions correspond to the risk groups and associated recommendations for further investigations as described in the ACC/AHA guidelines on the management of stable CAD.
CAD, coronary artery disease; ACC, American College of Cardiology; AHA, American Heart Association; hs-cTnI, high-sensitivity cardiac troponin I; ECG, electrocardiography; y, years.
External Validation
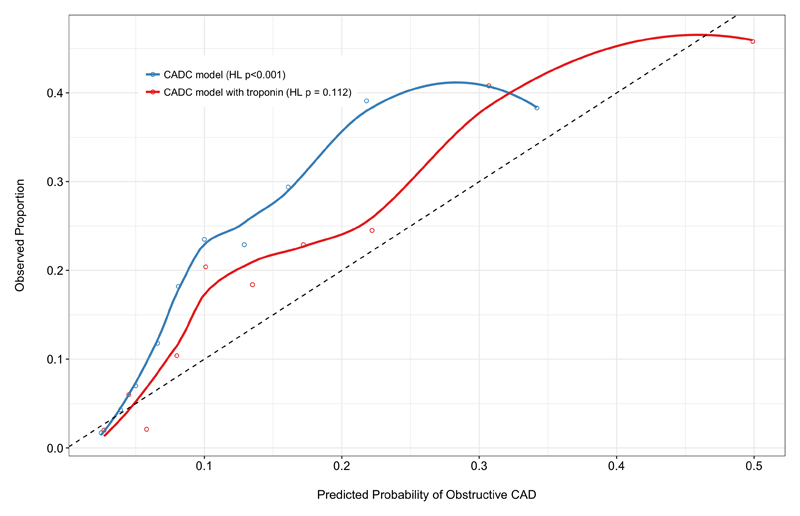
The validation cohort has been previously described (25, 26) and a summary of baseline characteristics is provided in Supplementary Table 8. The overall prevalence of obstructive coronary disease was 19.3% and again, a five-fold increase was seen across troponin quintiles. The addition of cardiac troponin concentration improved overall model performance (D 0.071 to 0.121) including discrimination (c-statistic: 0.738 to 0.757, p=0.025; Supplementary Figure 5), and model calibration (CADC model: χ2 = 38.4, HL p < 0.0001; CADC model including troponin: χ2 = 13.0, HL p = 0.1123) (Figure 2).
Figure 2. Model calibration within the external validation cohort.
Plot demonstrates poor calibration of predicted probability vs observed proportion of obstructive coronary artery disease using the established CADC model (blue). Good model calibration is demonstrated within the validation cohort following the addition of troponin to the CADC model (red). The dashed line represents perfect calibration.
CADC, Coronary Artery Disease Consortium; HL, Hosmer-Lemeshow.
Discussion
In the assessment of suspected stable angina, measurement of high-sensitivity cardiac troponin I improves the accuracy of the pre-test probability of obstructive coronary artery disease as estimated using the existing guideline-endorsed CAD Consortium risk model. Applied in this manner, high-sensitivity troponin testing can appropriately reclassify one in 10 intermediate or high risk patients without obstructive disease into a low-risk category. Consequently, this simple investigation has potential to improve the appropriate use of diagnostic stress imaging tests by reducing unnecessary testing in 10.5% of those without disease. Alternatively, if the test was applied to all individuals with suspected CAD, 21 troponin tests would be required to avoid 1 unnecessary CCTA. Reassuringly, this reduction in unnecessary imaging is achieved without any decrease in the negative predictive value of the model, thereby confirming the safety of our new diagnostic approach. We have developed a risk estimation tool that incorporates cardiac troponin I concentrations to allow clinicians to improve their estimation of pre-test probability for coronary disease (available at https://scotheart-mobile.herokuapp.com/)
Our study has a number of notable strengths. First, we chose to use a troponin assay with exceptional analytical characteristics (17), including a diagnostic sensitivity that outperforms other available platforms and that was able to detect cardiac troponin concentrations in 99.6% of our population, and to accurately quantify cardiac troponin concentrations in 96.8% of patients. Second, as this study was nested within a larger randomised trial of CCTA imaging in patients with suspected angina, we were able to minimise the potential for case ascertainment bias that can arise when the decision to proceed to coronary imaging is dependent on clinician perception of coronary disease risk. Third, we made use of state-of-the-art CT imaging using a 320-slice scanner to define the presence and extent of coronary artery disease in all patients. Fourth, the prospective nature of this study enabled detailed and accurate phenotypic characterisation of patients at baseline and comprehensive clinical follow-up. Finally, we demonstrated the externally validity of the derived model in an international and independent cohort.
Current guidelines recommend a routine full blood count and measurement of renal function to identify drivers of myocardial ischaemia and improve risk prediction. They also encourage analysis of lipid profiles and glycaemic indices as these represent important cardiovascular risk factors. Whilst acknowledging that elevations in troponin have some prognostic value in stable patients, the consensus opinion in 2013 (3) was that there was insufficient independent prognostic value to warrant routine measurement. This viewpoint is now being challenged by a growing body of evidence that demonstrates cardiac troponin to have independent prognostic value regarding several cardiovascular disorders including heart failure and myocardial infarction, and may even be a useful indicator of therapeutic response (12, 30–32).
Overall, our findings expand on this research demonstrating that troponin concentrations predict the presence of obstructive coronary artery disease in patients with suspected stable angina. The mechanisms behind this association, including ventricular strain (33), and myocardial ischaemia (34) are now emerging. Additionally, it seems apparent from our study that atherosclerotic burden plays an important role. Whether these low concentrations of troponin reflect subclinical myocardial necrosis related to coronary plaque disruption and microvascular disease, or increased myocardial cell turnover remains to be determined. To our knowledge this is the first time a single, non-genetic (35) circulating biomarker has been shown to provide improved discrimination for the diagnosis of stable obstructive coronary artery disease beyond established risk factors. Importantly, this improvement results in successful reclassification of patients into more appropriate diagnostic probability groups which could enable more rational use of subsequent investigations.
The high-sensitivity assay used in this study has particularly robust analytical characteristics but is presently available for research use only. We were able to measure troponin concentrations in more than 99% of the population across both sexes and a wide range of ages. Our internal validation demonstrated consistent results when using a commercially available test, but it is important to note the risk calculation will be assay specific. Whether our findings can be extrapolated to alternative clinical assays is unclear, but it would be prudent for manufacturers to validate each testing platform individually before considering use in this setting where troponin concentrations are approaching the limits of detection. Furthermore, we cannot be certain of how knowledge of troponin concentration may influence clinical management decisions as treating clinicians did not have access to the biomarker results during the conduct of the trial.
We made use of the latest generation of CT scanners developed with a focus on advancing the performance of coronary computed tomography angiography. Although some authors may suggest that invasive coronary angiography remains the reference standard, it seems unlikely that troponin would be related to CT-defined coronary artery disease independent of the presence and extent of true coronary artery disease. As such, any misclassification is likely to be non-differential with respect to troponin, and hence to cause us to underestimate the association between troponin and stable coronary artery disease, and the predictive performance of the model. Moreover, the chosen criteria for defining significant coronary disease on CCTA has previously been shown to correlate well with invasive angiographic findings and with non-invasively determined myocardial ischaemia (36). Indeed, in the SCOT-HEART trial, CCTA was associated with a >60% reduction in the rate of normal coronary angiography and a 30% increase in obstructive disease when downstream invasive coronary angiography was performed (37). We also contend that a particular strength of this study arises from it being nested within a larger trial which randomised patients to coronary imaging, thereby minimising the case ascertainment bias inherent in earlier trials that only included patients referred for invasive coronary angiography. This applicability to the general population is reflected in the relatively lower rates of obstructive disease identified compared with previous reports.
We added a single additional continuous variable to an existing model. As such, the improvement in model performance by adding cardiac troponin is unlikely to have been substantially inflated by overfitting. Confirmation of this is demonstrated by our findings on applying the model to the external validation cohort. Indeed, it appears increasingly likely, given the potential prognostic and diagnostic information cardiac troponin offers, that indications for testing outside the acute coronary syndrome setting now exist.
Conclusions
Plasma high-sensitivity cardiac troponin I concentrations independently predict the presence of obstructive coronary disease in patients with suspected stable angina. Employing this test within the chest pain clinic may improve the selection of patients for further investigation and treatment of coronary artery disease.
Supplementary Material
What is known.
Most patients presenting with suspected stable angina do not ultimately have obstructive coronary artery disease identified as a cause for their symptoms.
Despite this, current guideline-endorsed, risk-based approaches to the assessment of these patients result in the majority having to undergo non-invasive cardiac imaging tests to exclude this diagnosis
What the study adds.
Measuring high-sensitivity cardiac troponin concentrations in patients with suspected stable angina can safely increase the proportion of patients determined to be at low risk of coronary disease and therefore reduce the need for more costly imaging investigations.
Acknowledgments
We would like to Edwin Carter for his assistance in the preparation of samples.
Sources of Funding
The SCOT-HEART trial was funded by The Chief Scientist Office of the Scottish Government Health and Social Care Directorates (CZH/4/588), with supplementary awards from Edinburgh and Lothian’s Health Foundation Trust and the Heart Diseases Research Fund. DN (CH/09/002; RE/13/3; RG/16/10/32375), MW (FS/11/014) and NM (FS/16/14/32023) are supported by the British Heart Foundation. DMcA is the recipient of a Wellcome Trust Intermediate Clinical Fellowship (201492/Z/16/Z). DEN is the recipient of a Wellcome Trust Senior Investigator Award (WT103782AIA). EvB is supported by the Scottish Imaging Network: A Platform of Scientific Excellence (SINAPSE). The Royal Bank of Scotland supported the provision of 320-multidetector CT for NHS Lothian and the University of Edinburgh. The Clinical Research Imaging Centre (Edinburgh) is supported by the National Health Service Research Scotland (NRS) through National Health Service Lothian Health Board.
The research study that recruited the external validation cohort was supported by the Faculty of Health Sciences, University of Southern Denmark; and the Aase og Ejnar Danielsens Fond, Denmark.
Footnotes
Disclosures
Singulex provided reagent, calibrators and controls without charge and undertook the analysis of cardiac troponin I. NLM has acted as a consultant for Abbott Laboratories, Beckman-Coulter, Roche and Singulex. All other authors have no conflicts of interest.
References
- 1.Sekhri N, Feder GS, Junghans C, Hemingway H, Timmis AD. How effective are rapid access chest pain clinics? Prognosis of incident angina and non-cardiac chest pain in 8762 consecutive patients. Heart. 2007;93:458–63. doi: 10.1136/hrt.2006.090894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Euro Heart Survey Investigators. The clinical characteristics and investigations planned in patients with stable angina presenting to cardiologists in Europe: from the Euro Heart Survey of Stable Angina. European heart journal. 2005;26:996–1010. doi: 10.1093/eurheartj/ehi171. [DOI] [PubMed] [Google Scholar]
- 3.European Society of Cardiology Task Force. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. European heart journal. 2013;34:2949–3003. doi: 10.1093/eurheartj/eht296. [DOI] [PubMed] [Google Scholar]
- 4.National Institute for Health and Clinical Excellence. Clinical Guideline 95. NICE; London: 2010. Chest pain of recent onset: assessment and diagnosis of recent onset chest pain or discomfort of suspected cardiac origin. [Google Scholar]
- 5.Fihn SD, Gardin JM, Abrams J, Berra K, Blankenship JC, Dallas AP, Douglas PS, Foody JM, Gerber TC, Hinderliter AL, King SB, 3rd, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: executive summary. Circulation. 2012;126:3097–137. doi: 10.1161/CIR.0b013e3182776f83. [DOI] [PubMed] [Google Scholar]
- 6.Almeida J, Fonseca P, Dias T, Ladeiras-Lopes R, Bettencourt N, Ribeiro J, Gama V. Comparison of Coronary Artery Disease Consortium 1 and 2 Scores and Duke Clinical Score to Predict Obstructive Coronary Disease by Invasive Coronary Angiography. Clinical cardiology. 2016;39:223–8. doi: 10.1002/clc.22515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumamaru KK, Arai T, Morita H, Sekine T, Takamura K, Takase S, Rybicki FJ, Kondo T. Overestimation of pretest probability of coronary artery disease by Duke clinical score in patients undergoing coronary CT angiography in a Japanese population. Journal of cardiovascular computed tomography. 2014;8:198–204. doi: 10.1016/j.jcct.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 8.Genders TS, Steyerberg EW, Hunink MG, Nieman K, Galema TW, Mollet NR, de Feyter PJ, Krestin GP, Alkadhi H, Leschka S, Desbiolles L, et al. Prediction model to estimate presence of coronary artery disease: retrospective pooled analysis of existing cohorts. Bmj. 2012;344:e3485. doi: 10.1136/bmj.e3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mudrick DW, Cowper PA, Shah BR, Patel MR, Jensen NC, Peterson ED, Douglas PS. Downstream procedures and outcomes after stress testing for chest pain without known coronary artery disease in the United States. American heart journal. 2012;163:454–61. doi: 10.1016/j.ahj.2011.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel MR, Peterson ED, Dai D, Brennan JM, Redberg RF, Anderson HV, Brindis RG, Douglas PS. Low diagnostic yield of elective coronary angiography. New England Journal of Medicine. 2010;362:886–95. doi: 10.1056/NEJMoa0907272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shah AS, McAllister DA, Mills R, Lee KK, Churchhouse AM, Fleming KM, Layden E, Anand A, Fersia O, Joshi NV, Walker S, et al. Sensitive troponin assay and the classification of myocardial infarction. The American journal of medicine. 2015;128:493–501 e3. doi: 10.1016/j.amjmed.2014.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Everett BM, Brooks MM, Vlachos HEA, Chaitman BR, Frye RL, Bhatt DL. Troponin and cardiac events in stable ischemic heart disease and diabetes. New England Journal of Medicine. 2015;373:610–20. doi: 10.1056/NEJMoa1415921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Omland T, Pfeffer MA, Solomon SD, de Lemos JA, Rosjo H, Saltyte Benth J, Maggioni A, Domanski MJ, Rouleau JL, Sabatine MS, Braunwald E. Prognostic value of cardiac troponin I measured with a highly sensitive assay in patients with stable coronary artery disease. Journal of the American College of Cardiology. 2013;61:1240–9. doi: 10.1016/j.jacc.2012.12.026. [DOI] [PubMed] [Google Scholar]
- 14.The SCOT-HEART investigators. Role of multidetector computed tomography in the diagnosis and management of patients attending the rapid access chest pain clinic, The Scottish computed tomography of the heart (SCOT-HEART) trial: study protocol for randomized controlled trial. Trials. 2012;13:184. doi: 10.1186/1745-6215-13-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The SCOT-HEART investigators. CT coronary angiography in patients with suspected angina due to coronary heart disease (SCOT-HEART): an open-label, parallel-group, multicentre trial. The Lancet. 2015;385:2383–91. doi: 10.1016/S0140-6736(15)60291-4. [DOI] [PubMed] [Google Scholar]
- 16.Wu AH, Estis J, Helestine P, Bui K, Todd J, Kavsak P. High-sensitivity cardiac troponin I in a large community-based population at risk for cardiovascular disease. Clinical chemistry. 2015;61:S121. [Google Scholar]
- 17.Apple FS, Collinson PO. Analytical characteristics of high-sensitivity cardiac troponin assays. Clinical chemistry. 2012;58:54–61. doi: 10.1373/clinchem.2011.165795. [DOI] [PubMed] [Google Scholar]
- 18.Shah AS, Griffiths M, Lee KK, McAllister DA, Hunter AL, Ferry AV, Cruikshank A, Reid A, Stoddart M, Strachan F, Walker S, et al. High sensitivity cardiac troponin and the under-diagnosis of myocardial infarction in women: prospective cohort study. Bmj. 2015;350:g7873. doi: 10.1136/bmj.g7873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williams MC, Golay SK, Hunter A, Weir-McCall JR, Mlynska L, Dweck MR, Uren NG, Reid JH, Lewis SC, Berry C, van Beek EJ, et al. Observer variability in the assessment of CT coronary angiography and coronary artery calcium score: substudy of the Scottish computed tomography of the heart (SCOT-HEART) trial. Open heart. 2015;2:e000234. doi: 10.1136/openhrt-2014-000234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Min JK, Shaw LJ, Devereux RB, Okin PM, Weinsaft JW, Russo DJ, Lippolis NJ, Berman DS, Callister TQ. Prognostic value of multidetector coronary computed tomographic angiography for prediction of all-cause mortality. Journal of the American College of Cardiology. 2007;50:1161–70. doi: 10.1016/j.jacc.2007.03.067. [DOI] [PubMed] [Google Scholar]
- 21.CAD Consortium. A clinical prediction rule for the diagnosis of coronary artery disease: validation, updating, and extension. European heart journal. 2011;32:1316–30. doi: 10.1093/eurheartj/ehr014. [DOI] [PubMed] [Google Scholar]
- 22.Wolk MJ, Bailey SR, Doherty JU, Douglas PS, Hendel RC, Kramer CM, Min JK, Patel MR, Rosenbaum L, Shaw LJ, Stainback RF, et al. ACCF/AHA/ASE/ASNC/HFSA/HRS/SCAI/SCCT/SCMR/STS 2013 multimodality appropriate use criteria for the detection and risk assessment of stable ischemic heart disease: a report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Thoracic Surgeons. Journal of the American College of Cardiology. 2014;63:380–406. doi: 10.1016/j.jacc.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 23.Bittencourt MS, Hulten E, Polonsky TS, Hoffman U, Nasir K, Abbara S, Di Carli M, Blankstein R. European Society of Cardiology-Recommended Coronary Artery Disease Consortium Pretest Probability Scores More Accurately Predict Obstructive Coronary Disease and Cardiovascular Events Than the Diamond and Forrester Score: The Partners Registry. Circulation. 2016;134:201–11. doi: 10.1161/CIRCULATIONAHA.116.023396. [DOI] [PubMed] [Google Scholar]
- 24.Fihn SD, Gardin JM, Abrams J, Berra K, Blankenship JC, Dallas AP, Douglas PS, Foody JM, Gerber TC, Hinderliter AL, King SB, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the diagnosis and management of patients with stable ischemic heart disease. Circulation. 2012;126:e354. doi: 10.1161/CIR.0b013e318277d6a0. [DOI] [PubMed] [Google Scholar]
- 25.Hosbond SE, Diederichsen AC, Saaby L, Rasmussen LM, Lambrechtsen J, Munkholm H, Sand NP, Gerke O, Poulsen TS, Mickley H. Can osteoprotegerin be used to identify the presence and severity of coronary artery disease in different clinical settings? Atherosclerosis. 2014;236:230–6. doi: 10.1016/j.atherosclerosis.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 26.Madsen DM, Diederichsen AC, Hosbond SE, Gerke O, Mickley H. Diagnostic and prognostic value of a careful symptom evaluation and high sensitive troponin in patients with suspected stable angina pectoris without prior cardiovascular disease. Atherosclerosis. 2017;258:131–7. doi: 10.1016/j.atherosclerosis.2016.11.030. [DOI] [PubMed] [Google Scholar]
- 27.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–45. [PubMed] [Google Scholar]
- 28.Tjur T. Coefficients of determination in logistic regression models—A new proposal: The coefficient of discrimination. The American Statistician. 2009;63:366–72. [Google Scholar]
- 29.Woodward M, Brindle P, Tunstall-Pedoe H. Adding social deprivation and family history to cardiovascular risk assessment: the ASSIGN score from the Scottish Heart Health Extended Cohort (SHHEC) Heart. 2007;93:172–6. doi: 10.1136/hrt.2006.108167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zeller T, Tunstall-Pedoe H, Saarela O, Ojeda F, Schnabel RB, Tuovinen T, Woodward M, Struthers A, Hughes M, Kee F, Salomaa V, et al. High population prevalence of cardiac troponin I measured by a high-sensitivity assay and cardiovascular risk estimation: the MORGAM Biomarker Project Scottish Cohort. European heart journal. 2014;35:271–81. doi: 10.1093/eurheartj/eht406. [DOI] [PubMed] [Google Scholar]
- 31.Everett BM, Zeller T, Glynn RJ, Ridker PM, Blankenberg S. High-sensitivity cardiac troponin I and B-type natriuretic Peptide as predictors of vascular events in primary prevention: impact of statin therapy. Circulation. 2015;131:1851–60. doi: 10.1161/CIRCULATIONAHA.114.014522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ford I, Shah AS, Zhang R, McAllister DA, Strachan FE, Caslake M, Newby DE, Packard CJ, Mills NL. High-sensitivity cardiac troponin, statin therapy, and risk of coronary heart disease. Journal of the American College of Cardiology. 2016;68:2719–28. doi: 10.1016/j.jacc.2016.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chin CW, Shah AS, McAllister DA, Joanna Cowell S, Alam S, Langrish JP, Strachan FE, Hunter AL, Maria Choy A, Lang CC, Walker S, et al. High-sensitivity troponin I concentrations are a marker of an advanced hypertrophic response and adverse outcomes in patients with aortic stenosis. European heart journal. 2014;35:2312–21. doi: 10.1093/eurheartj/ehu189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee G, Twerenbold R, Tanglay Y, Reichlin T, Honegger U, Wagener M, Jaeger C, Rubini Gimenez M, Hochgruber T, Puelacher C, Radosavac M, et al. Clinical benefit of high-sensitivity cardiac troponin I in the detection of exercise-induced myocardial ischemia. American heart journal. 2016;173:8–17. doi: 10.1016/j.ahj.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 35.Thomas GS, Voros S, McPherson JA, Lansky AJ, Winn ME, Bateman TM, Elashoff MR, Lieu HD, Johnson AM, Daniels SE, Ladapo JA, et al. A blood-based gene expression test for obstructive coronary artery disease tested in symptomatic nondiabetic patients referred for myocardial perfusion imaging the COMPASS study. Circulation Cardiovascular genetics. 2013;6:154–62. doi: 10.1161/CIRCGENETICS.112.964015. [DOI] [PubMed] [Google Scholar]
- 36.Gaemperli O, Schepis T, Valenta I, Koepfli P, Husmann L, Scheffel H, Leschka S, Eberli FR, Luscher TF, Alkadhi H, Kaufmann PA. Functionally relevant coronary artery disease: comparison of 64-section CT angiography with myocardial perfusion SPECT. Radiology. 2008;248:414–23. doi: 10.1148/radiol.2482071307. [DOI] [PubMed] [Google Scholar]
- 37.The SCOT-HEART investigators. Use of coronary computed tomographic angiography to guide management of patients with coronary disease. Journal of the American College of Cardiology. 2016;67:1759–68. doi: 10.1016/j.jacc.2016.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.