Abstract
Purpose of Review
Despite signs of cortical and subcortical loss, patients with prodromal and early stage neurodegenerative disease are able to perform at a level comparable to the normal population. It is presumed that the onset of compensatory processes, that is, changes in brain activation within a function-specific network or in the recruitment of a region outside of the task-network underlies this maintenance of normal performance. However, in most studies to date increased brain activity is not correlated with indices of both pathology and performance and what appears to be compensation could simply be a symptom of the disease.
Recent Findings
MRI studies have explored compensation in neurodegenerative disease, claiming that compensation is evident across a number of disorders, including Alzheimer’s and Parkinson’s disease, but generally always in early stages; after this point compensation is generally no longer able to operate under the severe burden of disease pathology. However, none of these studies explicitly adopted a particular model of compensation. Thus, we also discuss our recent attempts to operationalise compensation for empirical testing.
Summary
There is clear evidence of compensatory processes in the early stages of neurodegenerative disease. However, for a more complete understanding, this requires more explicit empirical modelling.
Keywords: Compensation, Neurodegeneration, Magnetic Resonance Imaging
Introduction
During the early stages of neurodegeneration, normal performance levels are maintained despite neuronal loss and/or the presence of neurodegenerative pathologies. It has been suggested that this is due to compensatory processes, i.e. the adaptation of neural networks that allow the affected individual to exhibit normal behaviour in the presence of neuronal loss (1–3). Although plausible, compensation as a mechanism is likely to be highly complex and multi-faceted. To test confidently for the presence of compensatory processes in brain structure or activity requires full characterisation and modelling for explicit hypothesis testing. Here, we discuss the concept of compensation in neurodegeneration; examine recent studies that propose compensation in neurodegenerative populations using MRI; and finally, consider our recent attempts to operationalise compensation in Huntington’s disease (HD), a model neurodegenerative disorder.
Defining compensation
As yet, there is no established definition of compensation in neurodegeneration. Consequently, the term is often used indiscriminately to represent a diverse range of processes (indexed by often poorly defined changes in brain activity/connectivity) that could potentially represent compensation. For example, increased brain activity in a region within a task network in the presence of pathology is often deemed evidence of ongoing compensation. So too is activity in a brain region not typically associated with a particular function during task performance in an individual with neurodegeneration. However, such qualitative descriptions are often post-hoc and only support a partial characterisation of neural processes underlying compensation.
To describe compensation fully, brain activity or connectivity needs to be considered in the context of a larger model incorporating two additional key factors. The first is behaviour. Compensating brain activity/connectivity should assist in maintaining a normal level of behaviour; if it is below standard norms then it cannot be said that compensation is present, irrespective of changes in brain activity. Second is pathology; neuronal loss or indirect markers of neuronal loss due to neurodegenerative pathology. Much discussion has centred on compensation in the normal ageing population, which could be extrapolated to that within neurodegeneration cohorts (1, 4) (Figure 1). However, in healthy groups, these accounts cannot provide a complete characterisation of compensation as they focus only on the relationships between brain activity and behaviour, and do not also account for the structural change characteristic of neurodegeneration. As neurodegeneration is more prevalent in older individuals, disentangling the potential effects of ageing and neurodegeneration on putative effects of compensation requires an explicit model. In the studies discussed here, evidence of compensation mainly rests on increases in brain activity and/or behaviour.
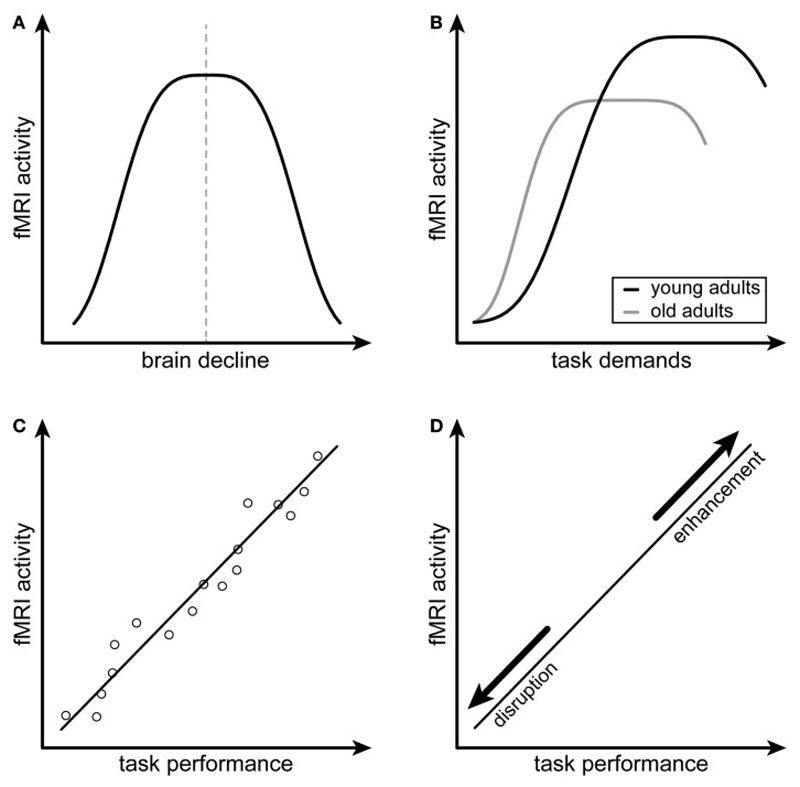
Figure 1. Attempted and Successful Compensation.
The first (inverted U-shaped relationship between brain activity and neuronal loss) and second (relationship between brain activity and task demands) criteria of attempted compensation are depicted in A and B respectively. The first (positive correlation between brain activity and task performance) and second (altered relationship between brain activity and task performance following disruption or enhancement of the compensating brain region) criteria of successful compensation are depicted in C and D respectively. (A,B) are adapted from Figure 37-3, p. 635, Dennis and Cabeza. Figure originally published in Frontiers in Psychiatry, 2014: Scheller E et al., Attempted and successful compensation in preclinical and early manifest neurodegeneration - a review of task FMRI studies 2014. Ref 2.
Recent Studies of Compensation
Given the extensive investigation into neurodegeneration, there are only a limited number of studies providing evidence of compensation. This is testament to the complexity in both defining and testing compensation empirically. Of note in the studies discussed here, compensation is only evident in prodromal or mild cases of neurodegeneration; diminishing once neurodegenerative pathology becomes too severe. This supports the notion of a trajectory of compensation across neurodegenerative disorders, whereby the onset of the compensatory mechanism is triggered but eventually desists once disease reaches a certain level of pathological severity.
Compensation and fMRI
Changes in brain activity measured using task-related fMRI have been proposed as evidence of compensation in neurodegeneration. Most studies that have identified compensatory brain activity have highlighted task-based networks showing increased activation comparing either between patient and control groups or between two patient groups at different stages of neurodegenerative disease (5, 6). Such findings do not unequivocally indicate compensation; they could simply represent pathology-related change, particularly in cases where there is no congruent maintenance of performance. For example, in a recent study, a combined group of patients with Mild Cognitive Impairment (MCI) and Alzheimer’s disease (AD) displayed enhanced left prefrontal and amygdala activity compared to controls during emotionally-salient verbal working-memory (7). However, as task difficulty increased their task response times were significantly slower than that of controls. Equally, a similar group of MCI patients presented with increased resting-state functional connectivity between the parahippocampal gyrus and prefrontal cortex compared to controls, but this change was correlated with worsening episodic memory performance (8). In both cases, the absence of maintained performance suggests that increased brain activity/connectivity could be either partial/incomplete compensation or the effects of pathology.
In contrast, Amyotrophic Lateral Sclerosis patients demonstrate increased activation in the left superior frontal gyrus (SFG) while maintaining typical levels of memory filtering during a non-verbal working-memory task and despite frontal lobe atrophy (9). Similarly, non-medicated, cognitively-unimpaired Parkinson’s Disease (PD) patients exhibited increased activation bilaterally in the putamen and posterior insula while maintaining performance levels close to those of controls during working memory (10). Putaminal activation can be used successfully in such situations to distinguish PD patients from controls. While in both studies augmented brain activity is likely evidence of compensation, this is a post-hoc interpretation due to a lack of direct association between maintained performance and increased brain activity as a function of pathology.
The same absence of mechanistic characterisation is evident in a series of studies performed in early-stage non-medicated PD patients when compared to controls (11, 12). Increased activity in the bilateral parietal cortex and right SFG during set-shifting was presumed compensation for reduced ventrolateral prefrontal cortex activity (11). Similarly, increased putaminal and insular activation during working memory was presumed compensation for reduced dorsolateral prefrontal (DLPFC) connectivity (12). Although patient performance was maintained across both cognitive domains, there were some aspects where patients performed less well than controls. To understand fully the impact of this in light of the apparent compensatory behaviour requires more explicit investigation of the relationship between performance and brain activity.
Functional Connectivity and Compensation
Recently, there has been a move from characterising changes in task-related activity associated with a single network to examining a series of networks in the brain at rest. Functional connectivity analysis of resting-state fMRI data allows the investigation of some task-related networks in the brain at-rest, probing network connectivity changes related to neuronal loss. There has been particular focus on subsystems within the task-negative default mode network (DMN), a group of midline regions, most robust in the brain at-rest, and associated with self–referential thinking and memory (13–15). The DMN is affected early in AD with disease-related reductions in network connectivity (16–18) and investigation of the DMN in AD could provide insight into early systems-level changes that may occur. For example, patients from across the AD spectrum display reduced DMN connectivity beginning in the most highly-connected posterior regions, leading to the emergence of increased connectivity between posterior and anterior and ventral DMN subsystems respectively. Increased connectivity between posterior and ventral subsystems correlates with pathology: amyloid deposits and hippocampal volume and predicts AD onset (19). These purported compensatory processes may mark the beginning of a cascading network-wide failure that occurs prior to measurable structural and functional decline in AD. This is particularly interesting in terms of the trajectory of compensation – its onset and its cessation. However, there was no explicit testing of the correspondence between network subsystem connectivity and cognitive performance.
Other studies have investigated resting-state connectivity within the DMN in MCI and AD. As part of a comprehensive exploration of inferior parietal lobe (IPL) subnetwork connectivity, moderate AD patients with robust grey matter reductions compared to healthy controls display increased connectivity between the IPL and the posterior DMN, putatively compensating for the reductions in connectivity within DMN subnetworks and other IPL networks (20). Similarly, using Granger Causality, increases in directed connectivity from the posterior cingulate cortex (PCC) to the right temporal lobe and to the PCC from temporal regions might indicate compensatory activity in MCI patients (21), while high-performing AD patients also demonstrated increased occipital connectivity with three separate functional connectivity patterns including that of the anterior DMN and bilateral executive network when compared to low-performing AD patients; and with no comparable increase in the control group (22). Connectivity changes within the DMN are also indicative of PD pathology. A recent meta-analysis of the ReHo (regional homogeneity) method of analysing resting-state fMRI data - a similar approach to seed-based connectivity - showed in over 11 comparisons that it was within regions of the DMN that most changes were seen: bilateral IPL and medial prefrontal cortices when compared to controls (23).
The striatum is affected in the early stages of neurodegenerative disorders such as PD and HD and accordingly is a region of interest for connectivity analyses. There is evidence for increased putaminal connectivity with the cerebellum in mild to moderate PD patients, which correlates with motor performance improvement (24) and increased connectivity between the basal ganglia and the motor cortex in cognitively-unimpaired PD patients (25). Interestingly, one recent study investigated both PD and AD patients showing that in both cases reduced striatal connectivity was associated with improved cognitive performance. However, while plausible, it cannot be confirmed that this reduction necessarily contributed to improved cognitive performance, particularly given that similar patterns of connectivity were found in controls and therefore, may simply represent ageing (26).
Different mechanisms, i.e. potential compensation versus disease-related effects may underlie increased brain connectivity in subsystems of brain networks. For example, in a group of prodromal AD patients, increased connectivity between the retrosplenial cortex and the lateral occipital cortex compared to both controls and a subjective cognitively-impaired (SCI) group correlated with verbal memory performance, even when accounting for cognitive reserve factors (27). However, increased connectivity between the PCC and lingual gyrus correlates only negatively with attention suggesting a compensatory versus disease-effect dissociation in the two DMN subsystems.
In the same way, different mechanisms may underlie changes in connectivity in patients with varying disease subtypes. Heterozygous PD associated-gene-carriers, most of whom were not affected by the disease, for example, show increased connectivity between the salience network and DMN, which correlates with improved working memory performance (28). More severely affected homozygous PD gene-carriers, however, also show increased network connectivity between the salience and the right fronto-parietal network. This, however, correlates with a worsening of short term working memory performance signalling compensation onset in the early or mild to moderate stages of PD, which diminishes as pathology worsens. Similarly, using the putamen and the caudate as seeds for functional connectivity analyses, early-onset PD patients show increased connectivity between the striatum and parietal and frontal regions with that between the caudate and somatosensory cortex negatively correlated with clinical score. Late-onset PD patients correspondingly showed increased connectivity in the cerebello-striatal circuit and in this subgroup connectivity change between the putamen and cerebellum is associated with lower clinical scores (29). Finally, recent studies have examined two other PD subtypes: postural instability and gait difficulty (PIGD) and tremor-dominant (TD). Here, there was a differentiation in patterns of increased connectivity from the subthalamic nucleus to the cerebellum in TD and the visual cortex in PIGD (30) with ‘hyperconnectivity’ between the motor cortex and IPL correlated with reduced behavioural impairment in TD compared to PIGD patients (31).
Structural compensation
Generally, MRI-based compensation is explored by examining changes in brain activity. Changes in anatomical connectivity or underlying white matter microstructure, as measured by diffusion-weighted imaging may (with caution in terms of biological interpretation), also give some clue as to the biological changes, such as demyelination and axonal degeneration that occur during neurodegeneration (32–34). Increased fractional anisotropy (FA; measure of white matter integrity in the main fibre direction), reduced diffusivity and increased density in callosal, projection and association tracts in low-disease load PD patients, for example, suggest considerable improvements in widespread white matter organisation (35). Furthermore, these changes are weakly correlated with motor symptom severity; i.e. greater white matter organisation means lower levels of motor dysfunction. As the substantia nigra (SN) is central to PD pathology, potential increased integrity in white matter tracts originating and projecting outside of this area could indicate compensation in the presence of SN degeneration; this is further supported by the absence of such changes in the severely affected group. Similar alterations were noted in white matter motor pathways including corticospinal and putaminal tracts in a different PD cohort (36). However, here there was increased diffusivity in the main direction of the principal fibre, indicating increased disorganisation and potentially axonal degeneration. It is possible that diffusivity in the pathways parallel to the main underlying fibre is simply higher than that in those perpendicular to it or alternatively, that increases in the number of streamlines (volume) represent reorganisation within the principal fibres leading to increased axial diffusivity. However, the lack of volumetric differences between controls and PD patients, plus an absence of correlations with motor severity makes the idea of ‘compensatory’ axonal sprouting unlikely.
Compensation and Cognitive Reserve
The difference between compensation and cognitive reserve is a complex one and often not explicitly characterised (1–4). Cognitive reserve refers to brain resilience in the presence of neuropathology and is largely influenced by education, lifestyle and socio-economic status (1, 3). It has been suggested that cognitive reserve is marked by augmented neuronal reserve allowing for increased efficiency in brain task-processing and potentially more activity in a task-network region; while compensation represents the brain’s ability to recruit task-unrelated regions to account for neuronal loss (37). Other accounts, however, have suggested that compensation can also be present simply when activity in task-related regions increases (1, 38). In a recent study, the effects of cognitive reserve, measured by number of years in formal education, were explicitly tested in MCI and AD patients (39). Those with MCI and high levels of cognitive reserve displayed equivalent levels of verbal and short-term memory as controls, despite higher levels of AD pathology, i.e. medial temporal lobe atrophy. This performance was putatively sustained by a system of increased connectivity in fronto-parietal networks together with decreased connectivity in fronto-temporo-cerebellar networks and reduced posterior and thalamic efficiency. Those with AD diagnosis, however, display no such evidence of compensatory processes regardless of cognitive reserve levels. Cognitive reserve can have a profound effect on the recruitment of neural networks to facilitate normal behaviour in the presence of neuronal degeneration. However, once again in this example it is also possible that the extra connectivity could simply be symptomatic of MCI pathology.
Operationalising Compensation
Given the absence of an agreed way of formally characterising and thus determining the presence of compensation in neurodegeneration using brain imaging, we recently endeavoured to operationalise compensation and create a model that can explicitly test for the presence of compensatory processes (40). The model incorporated the three components that we consider important in fully characterising compensation; pathology, brain activity and behaviour and we investigated the interactions of these three components in premanifest HD (preHD) (Figure 2). HD may be thought of as a model neurodegenerative disorder for studying compensation. The certainty of onset allows investigation of biological and clinical changes in preHD many years prior to disease onset. Large observational follow-up studies allow us to examine our compensation model in a prospective preHD cohort with participants ranging from 15 years to one year before clinical diagnosis (Figure 2). We focussed exclusively on those with a high level of pathology, measured by volumetric change, a proven and very robust marker of HD progression, but who also demonstrated increased brain activity and maintained a normal level of behaviour. Using both task and resting-state fMRI, we demonstrated a pattern of asymmetrical compensation in the cognitive network (40). Specifically, in preHD gene-mutation carriers with the highest levels of pathology, we identified increased activity in the right parietal network during working memory and increased resting-state connectivity between the right DLPFC and left-sided regions coupled with normal performance levels in the n-back task and global cognition respectively (Figure 2). These apparent compensatory effects were absent for the left (or dominant) hemisphere, which appeared more susceptible to pathology compared to the right where compensatory processes facilitated normal cognitive function.
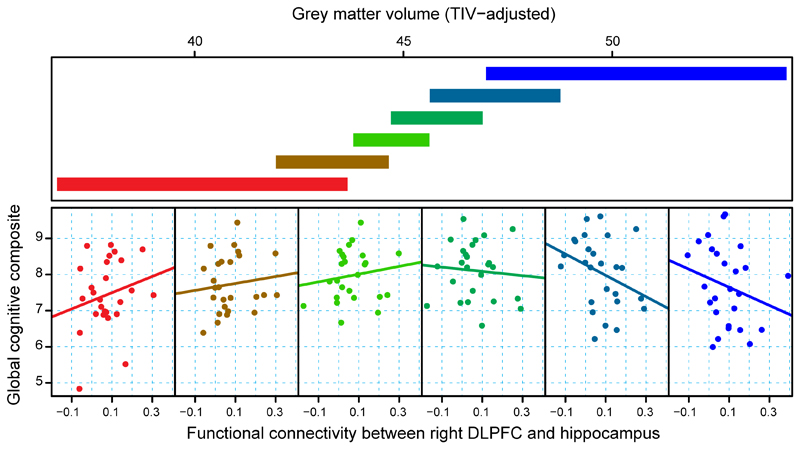
Figure 2. Cross-sectional Compensation in Huntington’s Disease.
Conditioning plot which illustrates global cognitive performance as a function of connectivity between the right dorsolateral prefrontal cortex and the left hippocampus, conditional on a structural measure of disease load (grey matter volume). The upper panel depicts overlapping ranges of structural disease load that determine the subsample for which observed points are plotted for each associated scatterplot. A linear regression line is fit within each panel. The extreme left scatterplot (red) includes the smallest brain volume (highest structural disease load) range from the data set (lower left red slab). The extreme right scatterplot (blue) includes the largest volume (lowest disease load) range from the data set (upper right blue slab). Figure originally published in EBioMedicine, 2015: Kloppel S, Gregory S et al., Compensation in Preclinical Huntington's Disease: Evidence From the Track-On HD Study. Ref 40.
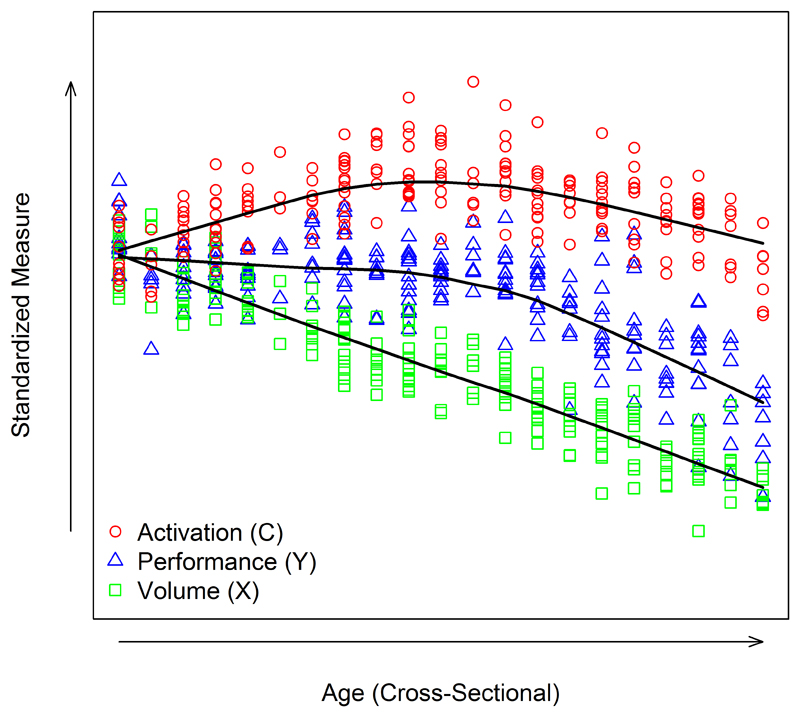
However, given the complexity of compensation, our initial approach was perhaps too simplistic, with a focus solely on preHD patients with the highest levels of pathology. Therefore, we modified our model, moving away from single interactions between brain activity and performance to the long-term trajectory of compensation, modelling the different putative phases of disease progression that may incorporate both the initial onset of compensation and then eventually cessation (41). Using age as our time metric, we proposed three time phases with progressively increasing pathology (Figure 3). Initially, brain activation increases as performance is maintained; then as the disease progresses brain activation plateaus and performance levels begin to deteriorate; finally, with pathology at high levels brain activation and performance both decrease rapidly. In this case, a premanifest cohort as discussed above would likely fall within the first phase of this model, where neuronal loss is ongoing, but activation increased and performance maintained. By eliminating the examination of single interactions between disease load and brain activation, we can place individuals on the compensation trajectory and by modelling as a function of age or time, we can extrapolate this cross-sectional model to look at compensation changes over time. Observational studies typically do not have more than a few years of follow-up, so we make inference about age patterns from both within-subject changes and between-subject differences.
Figure 3. Operationalisation of Compensation in Neurodegeneration.
Visualisation of simulated cross-sectional data modelling the three key components including: pathology(volume), compensation (brain activity) and behaviour. Scatterplot of values by age measured at one time point per person. Figure originally published in Brain, 2017: Gregory S, Long JD, Kloppel S, Razi A, Scheller E, Minkova L, et al. Operationalizing compensation over time in neurodegenerative disease. Ref 41.
Conclusion
Patients performing at normal levels in the presence of structural degeneration and/or pathology is a common feature of neurodegenerative disorders. Recent studies have accordingly identified evidence of such compensation using multimodal MRI, including increased brain activity using task-fMRI, functional connectivity in brain networks using resting-state fMRI and structural connectivity using diffusion imaging in those with mild to moderate levels of disease which desists once pathology becomes too severe. However, no studies have explicitly tested changes in brain activity/connectivity and these changes could simply be related to disease. It is necessary to operationalise compensation in a way that explicitly tests performance and brain changes in the presence of pathology.
Key points.
Compensation has been used to explain maintenance of normal behaviour in the presence of neurodegenerative pathology
Potential compensatory mechanisms using MRI have been identified in a number of neurodegenerative disorders predominantly in patients with mild to moderate pathology.
Potential compensation is evident in increased task activation, increased functional network connectivity and anatomical connectivity using a number of imaging modalities.
Characterisation of compensation for empirical testing requires models that explicitly examine brain activity/connectivity changes, performance and neurodegenerative pathology.
Acknowledgements
The authors wish to thank Rachael Scahill and Eileanoir Johnson for their input.
Financial support and sponsorship
Sarah Gregory, Sarah Tabrizi and Geraint Rees are funded by the Wellcome Trust (Grant reference 200181/Z/15/Z). Jeffrey Long is funded by the CHDI foundation.
Footnotes
Conflicts of interest
None
References
- 1.Barulli D, Stern Y. Efficiency, capacity, compensation, maintenance, plasticity: emerging concepts in cognitive reserve. Trends in cognitive sciences. 2013;17(10):502–9. doi: 10.1016/j.tics.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scheller E, Minkova L, Leitner M, Kloppel S. Attempted and successful compensation in preclinical and early manifest neurodegeneration - a review of task FMRI studies. Frontiers in psychiatry. 2014;5:132. doi: 10.3389/fpsyt.2014.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Papoutsi M, Labuschagne I, Tabrizi SJ, Stout JC. The cognitive burden in Huntington's disease: pathology, phenotype, and mechanisms of compensation. Movement disorders : official journal of the Movement Disorder Society. 2014;29(5):673–83. doi: 10.1002/mds.25864. [DOI] [PubMed] [Google Scholar]
- 4.Dennis NA, C RE. Frontal lobes and aging: deterioration and compensation. In: Stuss DTK, Knight RT, editors. Principles of Frontal Lobe Function. New York: Oxford University Press; 2013. pp. 628–52. [Google Scholar]
- 5.Malejko K, Weydt P, Sussmuth SD, Gron G, Landwehrmeyer BG, Abler B. Prodromal Huntington disease as a model for functional compensation of early neurodegeneration. PloS one. 2014;9(12):e114569. doi: 10.1371/journal.pone.0114569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kloppel S, Draganski B, Siebner HR, Tabrizi SJ, Weiller C, Frackowiak RS. Functional compensation of motor function in pre-symptomatic Huntington's disease. Brain. 2009;132(Pt 6):1624–32. doi: 10.1093/brain/awp081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berger C, Erbe AK, Ehlers I, Marx I, Hauenstein K, Teipel S. Effects of task-irrelevant emotional stimuli on working memory processes in mild cognitive impairment. Journal of Alzheimer's disease : JAD. 2015;44(2):439–53. doi: 10.3233/JAD-141848. [* This study shows that increased working memory performance with no concurrent increase in compensatory activity indicates a disease-related effect of emotional distractors on working memory performance in patients with mild cognitive impairment and Alzheimer’s disease.] [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y, Simon-Vermot L, Araque Caballero MA, Gesierich B, Taylor AN, Duering M, et al. Enhanced resting-state functional connectivity between core memory-task activation peaks is associated with memory impairment in MCI. Neurobiol Aging. 2016;45:43–9. doi: 10.1016/j.neurobiolaging.2016.04.018. [*This study provides another example of a disease-related effect of increased brain activity, this time in the resting-state functional connectivity between the parahippocampal gyrus and prefrontal cortex compared to controls, which correlated with worsening episodic memory performance.] [DOI] [PubMed] [Google Scholar]
- 9.Vellage AK, Veit M, Kobeleva X, Petri S, Vielhaber S, Muller NG. Working Memory Network Changes in ALS: An fMRI Study. Front Neurosci. 2016;10:158. doi: 10.3389/fnins.2016.00158. [*In this study patients with Amytrophic Lateral Sclerosis displayed hyperactivity in the frontal cortex during the memory filtering condition of a working memory task. This was in the presence of frontal lobe atrophy and suggests the onset of compensatory processes.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poston KL, YorkWilliams S, Zhang K, Cai W, Everling D, Tayim FM, et al. Compensatory neural mechanisms in cognitively unimpaired Parkinson disease. Ann Neurol. 2016;79(3):448–63. doi: 10.1002/ana.24585. [**Cognitively-unimpaired PD patients exhibited increased activation bilaterally in the putamen and posterior insula while maintaining working memory performance levels that were close to those of controls. Machine learning analysis showed that the increase in putaminal activity was able to distinguish the PD patients from the controls.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gerrits NJ, van der Werf YD, Verhoef KM, Veltman DJ, Groenewegen HJ, Berendse HW, et al. Compensatory fronto-parietal hyperactivation during set-shifting in unmedicated patients with Parkinson's disease. Neuropsychologia. 2015;68:107–16. doi: 10.1016/j.neuropsychologia.2014.12.022. [DOI] [PubMed] [Google Scholar]
- 12.Trujillo JP, Gerrits NJ, Veltman DJ, Berendse HW, van der Werf YD, van den Heuvel OA. Reduced neural connectivity but increased task-related activity during working memory in de novo Parkinson patients. Human brain mapping. 2015;36(4):1554–66. doi: 10.1002/hbm.22723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raichle ME. The brain's default mode network. Annual review of neuroscience. 2015;38:433–47. doi: 10.1146/annurev-neuro-071013-014030. [**Excellent overview of the default mode network - its evolution as a target for analysis using resting-state fMRI and how it has come to play a major role in the studies of human brain health and disease.] [DOI] [PubMed] [Google Scholar]
- 14.Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98(2):676–82. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raichle ME, Snyder AZ. A default mode of brain function: a brief history of an evolving idea. NeuroImage. 2007;37(4):1083–90. doi: 10.1016/j.neuroimage.2007.02.041. discussion 97-9. [DOI] [PubMed] [Google Scholar]
- 16.Simic G, Babic M, Borovecki F, Hof PR. Early failure of the default-mode network and the pathogenesis of Alzheimer's disease. CNS neuroscience & therapeutics. 2014;20(7):692–8. doi: 10.1111/cns.12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Damoiseaux JS, Prater KE, Miller BL, Greicius MD. Functional connectivity tracks clinical deterioration in Alzheimer's disease. Neurobiol Aging. 2012;33(4):828 e19–30. doi: 10.1016/j.neurobiolaging.2011.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang P, Zhou B, Yao H, Zhan Y, Zhang Z, Cui Y, et al. Aberrant intra- and inter-network connectivity architectures in Alzheimer's disease and mild cognitive impairment. Scientific reports. 2015;5:14824. doi: 10.1038/srep14824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones DT, Knopman DS, Gunter JL, Graff-Radford J, Vemuri P, Boeve BF, et al. Cascading network failure across the Alzheimer's disease spectrum. Brain. 2016;139(Pt 2):547–62. doi: 10.1093/brain/awv338. [**Important study demonstrating the potential cascading effects of network failure in Alzheimer’s disease. Dysfunctional connectivity begins within subsystems of the Default Mode Network moving to between-subsystem connectivity changes that are predicitve of disease onset. This study shows how putative compensatory processes could be related to the effects of increasing pathology.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Z, Xia M, Dai Z, Liang X, Song H, He Y, et al. Differentially disrupted functional connectivity of the subregions of the inferior parietal lobule in Alzheimer's disease. Brain Struct Funct. 2015;220(2):745–62. doi: 10.1007/s00429-013-0681-9. [**This is an important study for showing the division of the inferior parietal lobe (IPL) into seven subregions using probablistic cytoarchitectonic atlases and then mapping whole-brain resting-state functional connectivity for each subregion in Alzheimer’s disease. Using hierarchical clustering, three distinct patterns of IPL functional connectivity were identified. AD patients displayed increased connectivity between the IPL and the posterior DMN compared to healthy controls potentially compensating for reductions in connectivity within DMN subnetworks and other IPL networks.] [DOI] [PubMed] [Google Scholar]
- 21.Yang H, Wang C, Zhang Y, Xia L, Feng Z, Li D, et al. Disrupted Causal Connectivity Anchored in the Posterior Cingulate Cortex in Amnestic Mild Cognitive Impairment. Frontiers in neurology. 2017;8:10. doi: 10.3389/fneur.2017.00010. [* This study used Granger Causality measures of effective connectivity to show increased bidirectional connectivity from the posterior cingulate cortex to temporal regions that might indicate compensatory activity in MCI patients.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Marco M, Duzzi D, Meneghello F, Venneri A. Cognitive Efficiency in Alzheimer's Disease is Associated with Increased Occipital Connectivity. Journal of Alzheimer's disease:JAD. 2017 doi: 10.3233/JAD-161164. [*This study showed three distinct patterns of increased functional connectivity between the occipital cortex and the anterior DMN and bilateral executive network in high-performing Alzheimer’s disease patients.] [DOI] [PubMed] [Google Scholar]
- 23.Pan P, Zhan H, Xia M, Zhang Y, Guan D, Xu Y. Aberrant regional homogeneity in Parkinson's disease: A voxel-wise meta-analysis of resting-state functional magnetic resonance imaging studies. Neuroscience and biobehavioral reviews. 2017;72:223–31. doi: 10.1016/j.neubiorev.2016.11.018. [*Meta-analysis of DMN studies using ReHo (regional homogeneity) method of resting-state fMRI data analysis that showed that most DMN connectivity changes in Parkinson’s disease were seen in bilateral IPL and medial prefrontal cortices.] [DOI] [PubMed] [Google Scholar]
- 24.Simioni AC, Dagher A, Fellows LK. Compensatory striatal-cerebellar connectivity in mild-moderate Parkinson's disease. NeuroImage Clinical. 2016;10:54–62. doi: 10.1016/j.nicl.2015.11.005. [*Study showing increased putaminal connectivity with the cerebellum in mild to moderate PD patients, which correlated with improved motor performance improvement.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peraza LR, Nesbitt D, Lawson RA, Duncan GW, Yarnall AJ, Khoo TK, et al. Intra- and inter-network functional alterations in Parkinson's disease with mild cognitive impairment. Human brain mapping. 2017;38(3):1702–15. doi: 10.1002/hbm.23499. [*Study in cognitively-unimpaired Parkinson’s disease patients which shows evidence of increased connectivity between the basal ganglia and the motor cortex when compared to controls (25).] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anderkova L, Barton M, Rektorova I. Striato-cortical connections in Parkinson's and Alzheimer's diseases: Relation to cognition. Movement disorders : official journal of the Movement Disorder Society. 2017 doi: 10.1002/mds.26956. [*Recent study which has shown reduced connectivity from the striatum in both Parkinson’s disease and Alzheimer’s disease patients and which was associated with improved cognitive performance.] [DOI] [PubMed] [Google Scholar]
- 27.Dillen KN, Jacobs HI, Kukolja J, von Reutern B, Richter N, Onur OA, et al. Aberrant functional connectivity differentiates retrosplenial cortex from posterior cingulate cortex in prodromal Alzheimer's disease. Neurobiol Aging. 2016;44:114–26. doi: 10.1016/j.neurobiolaging.2016.04.010. [*This study shows the dissociative effects of between subsystem connectivity within the DMN and the potential respective positive and negative effects on performance in prodromal Alzheimer’s disease suggesting a compensatory versus disease-effect dissociation in the two DMN subsystems.] [DOI] [PubMed] [Google Scholar]
- 28.Makovac E, Cercignani M, Serra L, Torso M, Spano B, Petrucci S, et al. Brain Connectivity Changes in Autosomal Recessive Parkinson Disease: A Model for the Sporadic Form. PloS one. 2016;11(10):e0163980. doi: 10.1371/journal.pone.0163980. [*This study shows functional connectivity changes that may occur in Parkinson’s disease according to level of disease. Less affected heterozygous Parkinson’s disease associated-gene-carriers showed increased connectivity between the salience network and DMN, which correlated with improved working memory performance] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hou Y, Yang J, Luo C, Ou R, Song W, Liu W, et al. Patterns of striatal functional connectivity differ in early and late onset Parkinson's disease. Journal of neurology. 2016;263(10):1993–2003. doi: 10.1007/s00415-016-8211-3. [*Another study showing dissociation within patterns of functional connectivity between early-onset and late-onset Parkinson’s disease patients that may reflect differences in pathology.] [DOI] [PubMed] [Google Scholar]
- 30.Wang Z, Chen H, Ma H, Ma L, Wu T, Feng T. Resting-state functional connectivity of subthalamic nucleus in different Parkinson's disease phenotypes. Journal of the neurological sciences. 2016;371:137–47. doi: 10.1016/j.jns.2016.10.035. [*This study showed differentiation in patterns of increased connectivity from the subthalamic nucleus to the cerebellum in tremor-dominant Parkinson’s disease and the visual cortex in postural instability and gait difficulty Parkinson’s disease] [DOI] [PubMed] [Google Scholar]
- 31.Vervoort G, Alaerts K, Bengevoord A, Nackaerts E, Heremans E, Vandenberghe W, et al. Functional connectivity alterations in the motor and fronto-parietal network relate to behavioral heterogeneity in Parkinson's disease. Parkinsonism & related disorders. 2016;24:48–55. doi: 10.1016/j.parkreldis.2016.01.016. [*This study demonstrated ‘hyperconnectivity’ between the motor cortex and inferior parietal lobe which correlated with reduced behavioural impairment in tremor-dominant Parkinson’s disease.] [DOI] [PubMed] [Google Scholar]
- 32.Jones DK. Studying connections in the living human brain with diffusion MRI. Cortex; a journal devoted to the study of the nervous system and behavior. 2008;44(8):936–52. doi: 10.1016/j.cortex.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 33.Jones DK, Knosche TR, Turner R. White matter integrity, fiber count, and other fallacies: the do's and don'ts of diffusion MRI. NeuroImage. 2013;73:239–54. doi: 10.1016/j.neuroimage.2012.06.081. [DOI] [PubMed] [Google Scholar]
- 34.Tournier JD, Mori S, Leemans A. Diffusion tensor imaging and beyond. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2011;65(6):1532–56. doi: 10.1002/mrm.22924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mole JP, Subramanian L, Bracht T, Morris H, Metzler-Baddeley C, Linden DE. Increased fractional anisotropy in the motor tracts of Parkinson's disease suggests compensatory neuroplasticity or selective neurodegeneration. European radiology. 2016;26(10):3327–35. doi: 10.1007/s00330-015-4178-1. [**Important study showing that changes in stuctural connectivity may also be representative of compensatory mechanisms. Increased white matter tract intergiry and reduced diffusivity suggest considerable improvements in white matter organisation across the brain in white matter tracts originating and projecting outside of the substantia nigra (SN). This could indicate compensation in the presence of SN degeneration.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wen MC, Heng HS, Ng SY, Tan LC, Chan LL, Tan EK. White matter microstructural characteristics in newly diagnosed Parkinson's disease: An unbiased whole-brain study. Scientific reports. 2016;6:35601. doi: 10.1038/srep35601. [*Another study showing changes in white matter motor pathways in Parkinson’s disease with increased diffusivity in the main direction of the principal fibres of the corticospinal and putaminal tracts, indicating increased disorganisation and potentially axonal degeneration.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stern Y. Cognitive reserve and Alzheimer disease. Alzheimer disease and associated disorders. 2006;20(2):112–7. doi: 10.1097/01.wad.0000213815.20177.19. [DOI] [PubMed] [Google Scholar]
- 38.Reuter-Lorenz PA, Park DC. How does it STAC up? Revisiting the scaffolding theory of aging and cognition. Neuropsychology review. 2014;24(3):355–70. doi: 10.1007/s11065-014-9270-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Serra L, Cercignani M, Mastropasqua C, Torso M, Spano B, Makovac E, et al. Longitudinal Changes in Functional Brain Connectivity Predicts Conversion to Alzheimer's Disease. Journal of Alzheimer's disease : JAD. 2016;51(2):377–89. doi: 10.3233/JAD-150961. [**Important study demonstrating the effects of cognitive reserve on performance in patients with mild cognitive impairment (MCI). This study showed considerable improved performance in those with MCI and high cognitive reserve compared to comparable MCI patients with low cognitive reserve. However, this did not extend to those patients with diagnoses of probable Alzheimer’s disease, thus emphasising the role of compensation during the early stages of neurodegeneration.] [DOI] [PubMed] [Google Scholar]
- 40.Kloppel S, Gregory S, Scheller E, Minkova L, Razi A, Durr A, et al. Compensation in Preclinical Huntington's Disease: Evidence From the Track-On HD Study. EBioMedicine. 2015;2(10):1420–9. doi: 10.1016/j.ebiom.2015.08.002. [*First explicit modelling of cross-sectional compensation in a Huntington’s disease (HD) cohort. Using task-based and resting state fMRI data, this study showed an asymmetrical pattern of compensation in the cognitive network of premanifest HD patients, whereby the right- hemisphere showed some evidence of compensatory effects compared to the left hemisphere where only disease-related effects were evident.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gregory S, Long JD, Kloppel S, Razi A, Scheller E, Minkova L, et al. Operationalizing compensation over time in neurodegenerative disease. Brain. 2017 doi: 10.1093/brain/awx022. [*First attempt at explicit modelling of longitudinal compensation across the trajectory of neurodegenerative disease.] [DOI] [PMC free article] [PubMed] [Google Scholar]