Abstract
Hypertension control may offer less protection from incident cardiovascular disease (CVD i) in adults with than without apparent treatment‐resistant hypertension (aTRH), ie, blood pressure uncontrolled while taking three or more antihypertensive medications or controlled to <140/<90 mm Hg while taking four or more antihypertensive medications. Electronic health data were matched to health claims for 2006–2012. Patients with CVD i in 2006–2007 or with untreated hypertension were excluded, leaving 118,356 treated hypertensives, including 40,690 with aTRH, and 460,599 observation years. Blood pressure and medication number were determined by all clinic visit means from 2008 to CVD i or end of study. Primary outcome was first CVD i (stroke, coronary heart disease, heart failure) from hospital and emergency department claims. Controlling for age, race, sex, diabetes, chronic kidney disease, and statin use, hypertension control afforded less CVD i protection in patients with aTRH (hazard ratio, 0.87; 95% confidence interval, 0.82–0.93) than without aTRH (hazard ratio, 0.69; 95% confidence interval, 0.65–0.74; P<.001). Strategies beyond hypertension control may prevent more CVD i in patients with aTRH.
Hypertension control in the United States nearly doubled from 27% in 1988–1994 to 53% in 2007–2010.1, 2 Better hypertension control is reflected in increases of hypertension awareness and the proportions of aware patients who are treated and treated patients who have controlled blood pressure (BP).1, 2, 3, 4 Better control among treated patients is partially driven by an increase in the number of antihypertensive medications3, 4 given unfavorable trends in prevalent obesity and regression or minimal improvement in nutritional patterns over time.5, 6, 7 As the intensity of antihypertensive pharmacotherapy escalates, the proportion increases of hypertensive patients with uncontrolled BP taking three or more antihypertensive medications or with controlled BP taking four or more different classes of medication, ie, apparent treatment‐resistant hypertension increase.3, 4, 8 The term aTRH is used3 when out‐of‐office blood pressure, medication adherence, and medication dose are unavailable. Among treated, uncontrolled hypertensive patients, aTRH increased from 16% in 1988–1994 to 28% in 2005–2008.3
Hypertension guidelines generally recommend adding and uptitrating antihypertensive medications until goal BP is achieved.9, 10, 11, 12 The implied assumption is that benefits of treating hypertension are mainly related to BP reduction irrespective of medication number required. In patients with uncontrolled TRH, the subset with hypertension outside the office have more cardiovascular events than those with nonhypertensive readings outside the office, ie, office‐resistant hypertension has a better prognosis.13, 14 These findings indicate that BP outside the office is an important determinant of outcomes in resistant hypertension. However, patients with aTRH have worse outcomes than uncontrolled hypertensive patients without aTRH.15 In the Treating to New Targets (TNT) cholesterol study,16 patients with both controlled and uncontrolled aTRH had a similar greater risk for cardiovascular events than uncontrolled patients without aTRH. Two other reports indicate that patients with aTRH, including controlled aTRH, are at greater risk for one or more cardiovascular disease (CVD) events or death than patients without aTRH.17, 18
The literature confirms that (a)TRH is associated with more CVD events and suggests that BP control may be less beneficial for reducing adverse outcomes than hypertension that is not resistant. This observation has important implications for the large and growing population of patients with aTRH. Thus, the principal aim of our study was to assess the relative benefit of hypertension control in reducing CVD events in patients with and without aTRH.
Methods
The study was approved by the Office of Research Protection and Integrity at the Medical University of South Carolina and the University of South Carolina School of Medicine–Greenville. Data were obtained from various electronic health record system (EHRS) used by 187 clinical sites in the Care Coordination Institute quality improvement network during 2006–2012.8 Each clinic signed an agreement establishing the Care Coordination as a Business Associate for quality improvement. The Business Associate Agreement also authorized use of de‐identified data for research.
Inclusion and Exclusion Criteria
Adults 18 years and older with a diagnosis of hypertension who had two or more clinical visits with a valid BP in calendar years 2006–2012 and at least one prescription medication for any disease state were eligible. A valid BP was defined as systolic BP (SBP) in the range of 60 mm Hg to 300 mm Hg, diastolic BP (DBP) 40 mm Hg to 200 mm Hg, and SBP greater than DBP. To facilitate generalization of study findings, exclusion criteria were limited to: (1) CVD events on a billing claim prior to 2008 or prior to first appearance in the EHRS of a participating clinic; (2) estimated glomerular filtration rate <30 mL/1.73 m2/min; or (3) International Classification of Diseases, Ninth Revision (ICD‐9) codes 403, 585, or 586; active drug or alcohol abuse (ICD‐9 303, 303.9X, 304.XX), major psychiatric illness (ICD‐9 295.XX, 296.3, 297.X, 298.X), and malignancy (ICD‐9 140–209).
To be eligible for the current study, hypertensive patients were required to have at least one match to all payer Universal Billing 92/04 claims database at the South Carolina Office of Research and Statistics. Matching EHRS data to claims data required at least two of three personal identifiers including name, date of birth, and social security number. Only the list of identifiers was sent together with a one‐way masked identifier to the South Carolina Office of Research and Statistics, which performed the match. Only the masked identifier was returned together with paid claims for individuals with a match. The EHRS and paid claims data were combined using only the masked identifier.
Operational Definitions
BP was defined by mean values from all clinic visits between first entry in the database to: (1) the time of but not including the day of an incident CVD event (CVDi), or (2) the last entry during the study period for individuals without an event.9, 10 BP control was defined by the mean of all visit values <140/<90 mm Hg and uncontrolled as SBP ≥140 mm Hg or DBP ≥90 mm Hg for all patients including those with diabetes mellitus and/or chronic kidney disease (CKD).
Apparent Treatment‐Resistant Hypertension
aTRH was defined as ≥140/≥90 mm Hg while prescribed three or more antihypertensive agents or <140/<90 mm Hg while prescribed four or more different classes of antihypertensive medications.3, 19 For this report, the mean number of antihypertensive medications was determined from an average of visits using the same approach as for assessing mean BP values. An absolute diuretic requirement was not required in defining TRH19 and was not required for inclusion in our aTRH population. Visit weighted means for BP and medication number rather than time‐varying covariates were used given wide intra‐individual variation in visit frequency in this observational study, which differs from most clinical trials and epidemiological studies.
Definition of Outcomes
The primary outcome was a composite of first emergency department or hospital admission for myocardial infarction, unstable angina, ischemic or hemorrhagic stroke, or congestive heart failure (CHF). To avoid duplication, if an emergency department admission led to a hospital admission, only the latter was counted as an outcome. Secondary outcomes were defined by the first occurrence of the components of the primary outcome. Events occurring after a primary outcome during the study were also included in the count of secondary outcomes. CVD was defined by primary diagnoses on claims, which included ICD‐9 codes for coronary heart disease (CHD), myocardial infarction (410), unstable angina (411.1, 411.8), congestive heart failure (428.0–428.9), and stroke (431 [hemorrhagic], 433–434 [nonhemorrhagic]). The claims data did not include vital status of the patient and death files were unavailable for this analysis.
Data Reporting and Analysis
Baseline descriptive data are presented as mean and two standard errors of the mean, given the comparatively large number of patients and small values for one standard error. Data analyses were conducted with SAS software version 9.03 (SAS Institute Inc, Cary, NC). Descriptive statistics for group comparisons included t tests for continuous variables and chi‐square for proportions.
The relationship between the dependent variable (CVDi) and various risk factors or independent variables was examined as a function of time using survival analysis. Variables with bivariate association P values ≤.20 were included in the multivariable model. Multicollinearity among covariates was evaluated by assessing deviations of regression coefficients and their standard errors in the fitted univariate and multivariate models,20 and none was detected. Covariates were entered simultaneously into the model. Age‐adjusted Kaplan‐Meier CVD event‐free survival curves were generated. A log‐rank test was used to test the homogeneity of survival curves across racial strata.20 P values <.05 were considered significant.
Cox proportional hazards regression was used to estimate effects of hypertension control on the primary outcome of CVDi, while controlling for age, sex, race, statin use, diabetes mellitus, CKD, and aTRH. Secondary outcomes in the multivariable hazards regression analysis included stroke, CHD, and CHF separately. Low‐density lipoprotein cholesterol (LDL‐C) and smoking status were not included in the hazard regression analysis given >50% missing data for both. The proportional hazard assumption was tested with the goodness‐of‐fit chi‐square test, which compares the observed and expected survival probabilities, and by graphical means using log‐log Kaplan‐Meier curves.21 Proportional hazards assumptions for untreated hypertension did not meet goodness‐of‐fit assumptions, so these data are reported separately. The heterogeneity of the stratum‐specific hazard ratios (HRs) between SBP and conversion across the various stages of DBP as proposed by Breslow‐Day22 for analysis of cohort data was used.23 Adjusted HRs and 95% confidence intervals are reported. P values <.01 were accepted as statistically significant.
Results
Descriptive characteristics are provided for 181,755 hypertensive patients including 57,749 with uncontrolled (31.8%) and 124,006 (68.2%) with controlled hypertension (Table 1). These two groups are further subdivided by the number of antihypertensive medications prescribed with uncontrolled and controlled aTRH as previously defined.
Table 1.
Characteristics of Uncontrolled and Controlled Hypertensive Patients by Number of BP Medications Prescribed
| Variable | Uncontrolled (n=57,749, 31.8%) | Controlled (n=124,006, 68.2%) | ||||
|---|---|---|---|---|---|---|
| BP Medication Number | BP Medication Number | |||||
| 0 | 1–2 | ≥3 | 0 | 1–3 | ≥4 | |
| No., (%) | 16,363 (28.3) | 18,329 (31.7) | 23,057 (39.9) | 47,036 (37.9) | 59,348 (47.9) | 17,622 (14.2) |
| Age, y | 49.5±0.2*‡ | 54.6±0.2† | 58.1±0.2 | 47.1±0.1*‡ | 54.8±0.1 | 61.8±0.2 |
| Male, % | 45.6‡ | 45.1† | 43.4 | 38.6*‡ | 42.9† | 48.9 |
| Race, % | ||||||
| White | 71.3*‡ | 65.3† | 51.7 | 70.4*‡ | 69.8† | 66.1 |
| Black | 26.5*‡ | 32.9 | 46.8 | 26.7*‡ | 27.9† | 32.4 |
| Other, unknown | 2.2*‡ | 1.8 | 1.5 | 3.0*‡ | 2.3† | 1.6 |
| BMI, kg/m2 | 31.3±0.2*‡ | 31.7±0.1† | 32.2±0.1 | 30.6±0.1‡ | 30.7±0.1† | 31.5±0.1 |
| <25 | 29.8*‡ | 27.6† | 25.9 | 29.9*‡ | 29.0† | 25.1 |
| 25 to <30 | 25.6‡ | 25.2† | 24.0 | 27.3‡ | 27.2† | 26.3 |
| ≥30 | 44.6*‡ | 47.2† | 50.1 | 42.8*‡ | 43.8† | 48.6 |
| Visit frequency, No./year | 3.0±0.04*‡ | 3.3±0.04† | 3.9±0.04 | 3.4±0.02*‡ | 3.7±0.02† | 4.1±0.04 |
| SBP, mm Hg (last visit) | 144.6±0.3*‡ | 143.5±0.3† | 145.8±0.3 | 126.9±0.1*‡ | 125.5±0.1† | 127.7±0.2 |
| DBP, mm Hg (last visit) | 83.5±0.2*‡ | 81.9±0.2† | 81.3±0.2 | 77.2±0.1*‡ | 74.8±0.1† | 73.9±0.2 |
| SBP, mm Hg (all visits) | 148.7±0.2‡ | 148.8±0.2† | 150.6±0.2 | 126.5±0.1*‡ | 126.1±0.1† | 128.3±0.1 |
| DBP, mm Hg (all visits) | 86.0±0.2*‡ | 85.3±0.1† | 84.3±0.1 | 77.5±0.1*‡ | 75.8±0.1† | 74.9±0.1 |
| BP <120/<80 mm Hg, % | 0 | 0 | 0 | 15.0*‡ | 19.7† | 12.8 |
| BP 120–139/80–89 mm Hg, % | 0 | 0 | 0 | 85.0*‡ | 80.3† | 87.2 |
| BP 140–159/90–99 mm Hg, % | 82.1*‡ | 84.8† | 80.8 | 0 | 0 | 0 |
| BP ≥160/≥100 mm Hg, % | 17.9*‡ | 15.2† | 19.2 | 0 | 0 | 0 |
| BP medication count, mean±SD | 0*‡ | 1.5±0.01† | 4.2±0.01 | 0*‡ | 1.9±0.01† | 4.8±0.02 |
| LDL‐C, mg/dL | 111.3±0.6*‡ | 107.6±0.5† | 105.6±0.5 | 110.3±0.3*‡ | 102.4±0.3† | 97.0±0.5 |
| Statin, % | 24.8*‡ | 48.3† | 56.7 | 24.8*‡ | 48.3† | 66.8 |
| Diabetes mellitus, % | 13.8*‡ | 26.0† | 38.3 | 13.1*‡ | 26.7† | 41.5 |
| CKD, % | 3.8*‡ | 4.6† | 7.5 | 2.9*‡ | 4.6† | 8.8 |
| Current smoker, % | 4.0 | 4.0 | 3.6 | 5.4*‡ | 4.8 | 4.4 |
| 10‐y CHD risk | ||||||
| >20%, % | 21.6*‡ | 37.6† | 48.4 | 16.6*‡ | 32.5† | 48.3 |
| 10%–20%, % | 19.1* | 21.1† | 18.8 | 11.6*‡ | 17.1† | 18.4 |
| <10%, % | 59.3*‡ | 41.3† | 32.8 | 71.8*‡ | 50.4† | 33.3 |
Abbreviations: BP, blood pressure; CHD, coronary heart disease; CKD, chronic kidney disease; DBP, diastolic blood pressure; LDL‐C, low‐density lipoprotein cholesterol; SBP, systolic blood pressure; SD, standard deviation. Values are expressed as mean±2 standard error of the mean or percentage. *P<.01 for column 1 vs 2 within the controlled and uncontrolled groups, respectively, ie. 0 vs 1‐2 or 0 vs 1‐3 BP medications. †P<.01 for column 2 vs 3. ‡p<.01 for column 1 vs 3.
Irrespective of BP control, untreated patients were younger than those on treatment, and treated patients without aTRH were younger than those with aTRH. The proportion of white patients decreased and the proportion of black patients increased as the number of antihypertensive medications rose from untreated to non‐aTRH and aTRH. This pattern of race vs the number of antihypertensive medications was more evident for uncontrolled than controlled hypertension. Body mass indices and prevalent obesity increased with the number of antihypertensive medications. Visit frequency also increased with the number of antihypertensive medications prescribed and was greater in patient groups with controlled than uncontrolled hypertension.
SBP and DBP values were higher in patients with uncontrolled than controlled hypertension, as expected. While statistically significant differences were seen within the uncontrolled and controlled groups subdivided by number of antihypertensive medications, the last visit and all visit means were generally within ≤2 mm Hg. Among uncontrolled patients, last visit mean BPs were approximately 4 to 5/2 to 4 mm Hg lower than all visit means. In controlled hypertensive patients, the last visit mean was lower than the all visits mean by ≤1 mm Hg for SBP and DBP. Among uncontrolled hypertensive patients, ≥80% had BP in the stage 1 range, ie, SBP 140–159 and/or DBP 90–99 mm Hg and <160/<100 mm Hg, whereas ≥80% of controlled hypertensive patients had values in the prehypertensive range. The number of different classes of antihypertensive medications prescribed was consistent with categorical definitions. The prevalence of diabetes and CKD increased in both groups with the number of antihypertensive medications. The prevalence of cigarette smoking was low and consistent with a high level of missing data for this variable.
Unadjusted values for primary and secondary outcomes are provided in Table 2 for patient groups divided by hypertension control status and number of antihypertensive medications prescribed. The total years of observations were 708,381 with numbers in each of the subgroups provided. There were 14,457 primary events ranging from a low of 1357 in the untreated, uncontrolled group to 3876 in the controlled group who took one to three antihypertensive medications. Primary and all secondary event rates per 1000 patient‐years were lower in controlled than uncontrolled, untreated patients and treated patients without aTRH. The unadjusted primary CVDi outcome was higher in controlled than uncontrolled aTRH patients and was accounted for mainly by more unstable angina and CHF. There were fewer unadjusted stroke events among patients with controlled than uncontrolled aTRH.
Table 2.
Unadjusted Primary and Secondary Outcomes in Uncontrolled and Controlled Hypertensives by Medication Number
| Variable | Uncontrolled (n=57,749, 31.8%) | Controlled (n=124,006, 68.2%) | ||||
|---|---|---|---|---|---|---|
| BP Medication Number | BP Medication Number | |||||
| 0 | 1–2 | ≥3 | 0 | 1–3 | ≥4 | |
| No., (%) | 16,363 (28.3) | 18,329 (31.7) | 23,057 (39.9) | 47,036 (37.9) | 59,348 (47.9) | 17,622 (14.2) |
| Patient‐years | 61,314 | 72,569 | 89,438 | 186,468 | 231,296 | 67,296 |
| Primary outcome, No./1000 patient‐years | 22.1 | 22.8 | 34.4 | 10.7 | 16.8 | 37.3 |
| Secondary outcomes, No./1000 patient‐years | ||||||
| Stroke, hemorrhagic | 1.9 | 1.4 | 1.8 | 0.7 | 0.8 | 1.4 |
| Stroke, ischemic | 6.9 | 4.8 | 7.0 | 2.5 | 2.6 | 5.3 |
| CHD, unstable angina | 3.5 | 4.0 | 6.0 | 2.2 | 3.5 | 8.4 |
| CHD, myocardial infarction | 6.8 | 5.3 | 7.3 | 3.4 | 3.8 | 6.9 |
| Congestive heart failure | 13.7 | 13.1 | 23.3 | 7.1 | 10.9 | 28.4 |
| Events, No. | ||||||
| Primary outcome | 1357 | 1653 | 3076 | 1988 | 3876 | 2507 |
| Secondary outcomesa | ||||||
| Stroke, hemorrhagic | 115 | 98 | 164 | 136 | 191 | 94 |
| Stroke, ischemic | 426 | 345 | 630 | 466 | 600 | 355 |
| CHD, unstable angina | 212 | 288 | 537 | 407 | 812 | 563 |
| CHD, myocardial infarction | 420 | 388 | 650 | 634 | 868 | 461 |
| Congestive heart failure | 838 | 952 | 2085 | 1324 | 2516 | 1913 |
Abbreviations: BP, blood pressure; CHD, coronary heart disease. aSecondary outcomes include components of the primary outcome events and events occurring after the primary outcome and during the study period.
Age‐adjusted Kaplan‐Meier survival curves are provided in Figure 1. Among adults prescribed antihypertensive medications, patients with hypertension controlled on one to three BP medications had the best event‐free survival rates, followed by patients with uncontrolled hypertension on one or two BP medications. Both aTRH groups had similar age‐adjusted, event‐free survival but significantly lower than patients with uncontrolled hypertension prescribed one or two BP medications. In adults with diagnosed but untreated hypertension, the subset with BP <140/<90 mm Hg had better event‐free survival than the group with higher BP.
Figure 1.
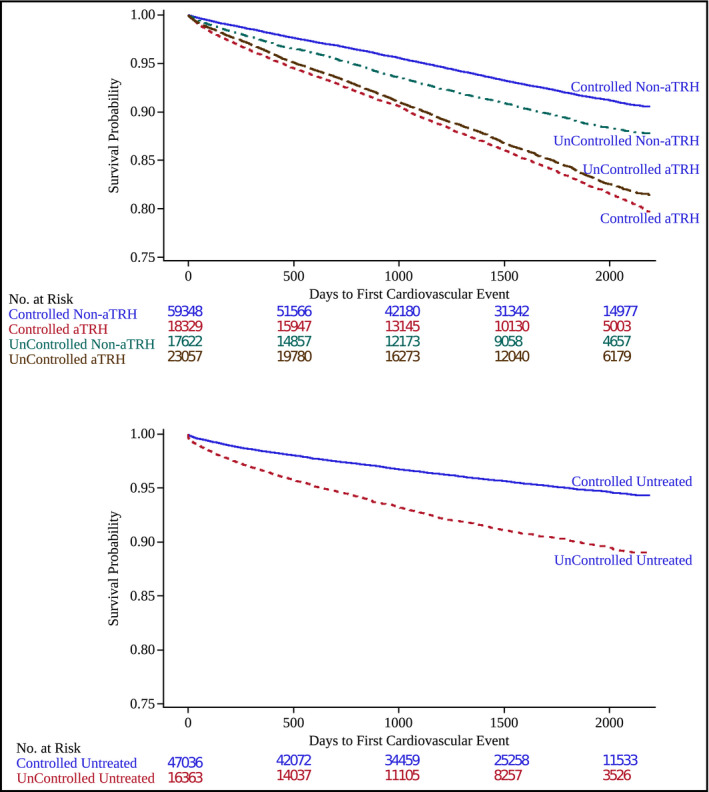
Age‐adjusted cardiovascular disease event‐free survival (Kaplan‐Meier survival curves) is shown for treated patients by hypertension control and apparent treatment‐resistant hypertension (aTRH) status (top panel). Comparable data for untreated patients by control status (bottom panel).
Multivariable hazard ratios and 95% confidence intervals for independent clinical predictors of CVDi, the primary outcome, and the secondary component outcomes of CHD, stroke, and CHF are provided in Table 3. Risk for CVDi and its components increased with age and was greater in patients with than without diabetes mellitus and stage 3 CKD and statin use. LDL‐C and cigarette use were not included in the model given missing data on >50% of patients, which likely confounded the statin analysis.
Table 3.
Multivariable Hazard Ratios (95% Confidence Intervals) of Clinical Factors Associated With Composite CVD Events and the Individual Components in Treated Adults With and Without aTRH
| Clinical Factor | Primary Composite | CHD | CHF | Stroke |
|---|---|---|---|---|
| BP‐controlled aTRH | 0.87 (0.82–0.93) | 0.80 (0.71–0.89) | 1.00 (0.93–1.08) | 0.59 (0.52–0.68) |
| BP‐controlled ∅aTRH | 0.69 (0.65–0.74) | 0.68 (0.62–0.76) | 0.77 (0.71–0.84) | 0.55 (0.48–0.63) |
| Age/year | 1.019 (1.017–1.020) | 1.008 (1.006–1.011) | 1.022 (1.020–1.024) | 1.024 (1.021–1.028) |
| Female sex | 0.78 (0.74–0.81) | 0.58 (0.54–0.63) | 0.85 (0.81–0.90) | 0.95 (0.87–1.05) |
| Black race | 0.88 (0.84–0.92) | 0.62 (0.57–0.74) | 0.93 (0.88–0.99) | 1.12 (1.07–1.30) |
| Diabetes mellitus | 1.55 (1.48–1.62) | 1.56 (1.44–1.69) | 1.73 (1.63–1.82) | 1.34 (1.22–1.48) |
| Chronic kidney disease | 2.43 (2.24–2.64) | 2.21 (1.92–2.54) | 2.72 (2.48–2.98) | 2.09 (1.76–2.45) |
| Statin use | 1.46 (1.39–1.53) | 2.63 (2.23–2.90) | 1.16 (1.09–1.23) | 1.44 (1.30–1.59) |
Abbreviations: aTRH, apparent treatment‐resistant hypertension; BP, blood pressure; CHD, coronary heart disease; CHF, congestive heart failure; CVD, cardiovascular disease.
CVDi, CHD, and CHF occurred less often in women than men and in black than white adults. Black adults were at greater risk for stroke than their white counterparts. Patients with diabetes and CKD were at greater risk for the primary and secondary outcomes than individuals without these conditions.
While CVDi was lower in adults with and without aTRH who had controlled hypertension, the protection afforded by hypertension control was less in patients without than with aTRH (Figure 2). Hypertension control reduced CHD and stroke events in adults with and without aTRH. The reduction in CHF with BP control was seen only in those without aTRH.
Figure 2.
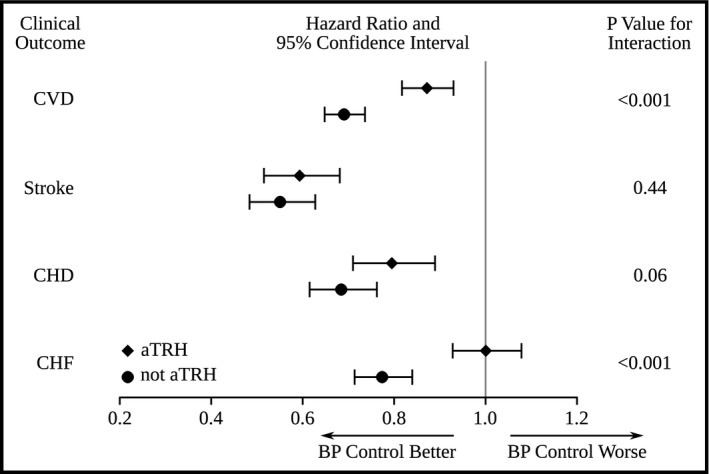
Multivariable hazard ratios (HRs) and 95% confidence intervals (CIs) depicting the relationship of blood pressure (BP) control to various cardiovascular outcomes for patients with and without apparent treatment‐resistant hypertension (aTRH). The primary outcome of incident cardiovascular disease (CVD) includes the separate secondary outcomes of stroke, coronary heart disease (CHD), and congestive heart failure (CHF). The P value for the interaction indicates whether the HRs and 95% CIs were significantly different (P<.05) by BP control in adults with and without aTRH for the outcome indicated.
Discussion
Hypertension control reduced CVDi in adults with and without aTRH but the benefit of control was significantly less in those with aTRH. The difference in BP between the uncontrolled and controlled subsets was similar in those with and without aTRH at 18.1/7.4 vs 18.0/7.1 mm Hg, respectively. Thus, the greater protection from CVDi afforded by hypertension control in adults without aTRH than with aTRH was not explained by office BP.
Before concluding that BP is unrelated to lesser protection with hypertension control in adults with aTRH, additional considerations are important. First, previous studies demonstrated that cardiovascular events are more strongly related to ambulatory than office BP. These observations extend to adults with “office‐resistant” TRH who have significantly fewer CVDi than adults with true TRH.13, 14 Moreover, if anything, the prevalence of the office or white‐coat effect appears greater in adults with aTRH than without aTRH. Thus, a high proportion of office‐resistant hypertension in the uncontrolled aTRH subset could contribute to smaller difference in CVDi outcomes in comparison to the controlled aTRH group.
Nighttime BP appears to have greater prognostic significance than daytime BP.24 If adults with controlled aTRH are more likely to be nondippers, ie, <10% decline in BP during sleep, or reverse dippers, than those with uncontrolled aTRH, this phenomenon could contribute to similar CVDi outcomes in the two groups. Another potential explanation is that adults with controlled aTRH have a significant proportion of masked hypertension, which is associated with a prognosis similar to that of patients with elevated BP in both the office and out‐of‐office settings.25 Ambulatory BP values are unavailable to address these possibilities in our study.
Our findings are similar to the TNT study.16 In that report, patients with controlled and uncontrolled aTRH had a similar increase in CVD events when compared with patients who were uncontrolled but without aTRH. The Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT)17 did not directly compare outcomes in controlled and uncontrolled aTRH, although hazard ratios for cardiovascular outcomes were generally similar for all aTRH patients and the uncontrolled subset.
Incident stroke and CHD were lower in adults with controlled than uncontrolled aTRH. Our findings coincide with primary prevention studies in which hypertension control was associated with less stroke and CHD events.26, 27, 28 Some29, 30, 31 but not all32 evidence suggests a J‐curve in which CHD is not reduced or may increase with more strict hypertension control. The percentage of adults with controlled aTRH who had “normal” office BP <120/<80 mm Hg was relatively low at approximately 13%. It may be beneficial to increase the percentage of controlled aTRH patients without diabetes who have SBP <120 mm Hg if the benefits reported in the Systolic Blood Pressure Intervention Trial (SPRINT)32 extend to patients taking four or more different antihypertensive medications.
Our findings contrast with findings from the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study,18 which showed that adults with uncontrolled vs controlled aTRH had more CHD but not more stroke events. Our study found a CHD and stroke benefit for patients with controlled aTRH in contrast to uncontrolled aTRH. Differences in event classification may explain the discrepancy since the REGARDS trial determined events by patient interview and chart review, whereas we used only ICD‐9 codes on health claims.
The reason that hypertension control did not reduce CHF events in aTRH is unknown. Primary prevention studies document that CHF falls with better BP control.26, 27, 28 Patients with aTRH may have more subclinical heart disease that contributes to clinical CHF events independently of BP. Our analysis did not include classes of antihypertensive medications with proven benefit in heart failure, eg, β‐, renin‐angiotensin system, and aldosterone blockers, which could have confounded the BP‐heart failure relationship.
Increasing age, female sex, diabetes, and stage 3 CKD were associated with increased risk for the primary composite outcome as well as the individual components of CHD, CHF, and stroke. While black compared with white race was associated with greater stroke risk as reported,33 there was a negative association with CHD and CHF. In another report, the risk for nonfatal CHD was lower in black than white adults, whereas the reverse was true for fatal CHD. Since our report did not include death files, most events were probably nonfatal.34 Of note, black adults were more likely than their white counterparts to have uncontrolled aTRH, which was associated with worse outcomes than uncontrolled hypertension that was not treatment resistant. A striking finding of our report was the positive rather than negative association between statin use and CHD and stroke events as evidence strongly indicates the opposite.34 Among the potential confounders, statin use increased with medication number and was greatest in patients with aTRH. Moreover, LDL‐C and cigarette smoking, two well‐established risk factors for CHD and stroke, were not included in the hazards regression analysis because data were missing for >50% of patients.
Our study was not designed to assess differential outcomes by medication number in adults without aTRH. However, re‐analysis of Valsartan Antihypertensive Long‐Term Use Evaluation (VALUE) trial35 data indicated that patients controlled on monotherapy had significantly fewer cardiovascular events than patients controlled on combination therapy, even after adjusting for baseline BP and cardiovascular history. Cardiovascular outcomes were not significantly different between controlled and uncontrolled patients on combination therapy.
Several studies collectively indicate that patients requiring more antihypertensive medications to control BP derive less CVD benefit than those controlled on fewer medications. While the association may be coincidental rather than causal, the requirement for more medications may indicate greater pathophysiological derangements, eg, endothelial dysfunction, insulin resistance, inflammation, or target organ damage. These factors are often unaccounted for in risk prediction models. Another possibility is that complex antihypertensive regimens may adversely affect outcomes. If so, then management strategies that control BP with fewer medications may reduce CVDi. These approaches could include gene‐ and renin‐guided therapeutics as well as device‐based strategies.36, 37, 38, 39, 40
Study Limitations and Strengths
Real and potential limitations of this study include the following: (1) the lack of standardized BP measurements and absence of out‐of‐office BP values; (2) incident CVD events were based on claims data rather than a standardized adjudication process; (3) although an attempt was made to exclude individuals with prior CVD, adults with events prior to 2006 or occurring outside South Carolina would have been included; (4) both emergency department and hospital admissions were included in the endpoints and death files were not available for this analysis; (5) data for LDL‐C and cigarette smoking were missing for >50% of adults; (6) BP and medication were defined as the number from the mean of visits prior to an event rather than a specific, single baseline; and (7) accuracy of medications in electronic health records is limited. Strengths of the study include: (1) large numbers of adults with hypertension and total observation years; and (2) a diverse, practice network, mainly primary care, with privately owned small clinics and large group clinics, hospital‐owned practice networks, federally qualified health centers, and rural health clinics.
Conclusions
Patients with aTRH derived less protection from CVD events than adults without aTRH in our study. The failure to reduce CHF accounted for the limited benefit of hypertension control in aTRH, since stroke and CHD were reduced. Previous studies also found that BP control has less benefit for reducing CVD events among adults with aTRH.6, 17 Research on strategies beyond BP control is warranted to improve CVD and especially chronic heart failure outcomes in the growing population with aTRH.
Disclosures
During the previous 3 years, Dr Egan received income as a consultant to AstraZeneca, Blue Cross and Blue Shield South Carolina, Daiichi‐Sankyo, Medtronic, and Novartis; research support from Daiichi‐Sankyo, Medtronic, Novartis, and Takeda; and royalties from UpToDate. None of the other authors have any disclosures to report.
Acknowledgments
None.
Funding Sources
Our study was supported in part by Medtronic, National Institutes of Health HL105880, and the Centers for Disease Control and Prevention, Atlanta, GA (Community Transformation Grant through the South Carolina Department of Health and Environmental Control).
J Clin Hypertens (Greenwich). 2016;18:817–824. DOI: 10.1111/jch.12773. © 2016 Wiley Periodicals, Inc.
References
- 1. Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988–2008. JAMA. 2010;303:2043–2050. [DOI] [PubMed] [Google Scholar]
- 2. Egan BM, Zhao Y. Clinical epidemiology of hypertension in the U.S. 1999–2010: different definitions of prevalent hypertension impact the clinical epidemiology of hypertension and attainment of Healthy People goals. J Clin Hypertens (Greenwich). 2013;15:154–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Egan BM, Zhao Y, Axon RN, Brzezinski WA, Ferdinand KC. Uncontrolled and apparent treatment resistant hypertension in the U.S. 1988–2008. Circulation. 2011;124:1046–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Egan BM, Li J, Shatat IF, Fuller JM, Sinopoli A. Closing the gap in hypertension control between younger and older adults: NHANES 1988 to 2010. Circulation. 2014;129:2052–2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mellen PB, Gao SK, Vitolins MZ, Goff DC. Deteriorating dietary habits among adults with hypertension. Arch Intern Med. 2008;168:308–314. [DOI] [PubMed] [Google Scholar]
- 6. Ford ES, Zhao G, Li C, Pearson WS, Mokdad AH. Trends in obesity and abdominal obesity among hypertensive and nonhypertensive adults in the United States. Am J Hypertens. 2008;21:1124–1128. [DOI] [PubMed] [Google Scholar]
- 7. Wang DG, Leung CW, Li Y, et al. Trends in dietary quality among adults in the United States, 1999 through 2010. JAMA Intern Med. 2014;174:1587–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42:1206–1252. [DOI] [PubMed] [Google Scholar]
- 9. Flack JM, Sica DA, Bakris G, et al. Management of high blood pressure in blacks: an update of the International Society on Hypertension in Blacks Consensus Statement. Hypertension. 2010;56:780–800. [DOI] [PubMed] [Google Scholar]
- 10. James PA, Oparil S, Carter BL, et al. 2014 evidence‐based guideline for the management of high blood pressure in adults. Report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507–520. [DOI] [PubMed] [Google Scholar]
- 11. Go AS, Bauman MA, Coleman King SM, et al. An effective approach to high blood pressure control: a Science Advisory from the American Heart Association, the American College of Cardiology, and the Centers for Disease Control and Prevention. Hypertension. 2013; http://hyper.ahajournals.org/content/early/2013/11/14/HYP.0000000000000003.citation. Accessed July 23, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Weber MA, Schiffrin EL, White SB, et al. Clinical practice guidelines for the management of hypertension in the community: a statement by the American Society of Hypertension and the International Society of Hypertension. J Clin Hypertens (Greenwich). 2014;16:14–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Salles GF, Cardoso CR, Muxfeldt ES. Prognostic influence of office and ambulatory blood pressures in resistant hypertension. Arch Intern Med. 2008;168:2340–2346. [DOI] [PubMed] [Google Scholar]
- 14. Pierdomenico SD, Lapenna D, Bucci A, et al. Cardiovascular outcome in treated hypertensive patients with responder, masked, false resistant, and true resistant hypertension. Am J Hypertens. 2005;18:1422–1428. [DOI] [PubMed] [Google Scholar]
- 15. Daugherty SL, Power JD, Magid DJ, et al. Incidence and prognosis of resistant hypertension in hypertensive patients. Circulation. 2012;125:1635–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bangalore S, Fayyad R, Laskey R, et al. Prevalence, predictors and outcomes in treatment‐resistant hypertension in patients with coronary disease. Am J Med. 2014;127:71–81. [DOI] [PubMed] [Google Scholar]
- 17. Munter P, Davis BR, Cushman WC, et al. Treatment resistant hypertension and the incidence of cardiovascular disease and end‐stage renal disease: results from the Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack (ALLHAT). Hypertension. 2014;65:1012–1021. [DOI] [PubMed] [Google Scholar]
- 18. Irvin MR, Booth JN III, Shimbo D, et al. Apparent treatment‐resistant hypertension and risk for stroke, coronary heart disease, and all‐cause mortality. J Am Soc Hypertens. 2014;8:405–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Calhoun DA, Jones D, Textor S, et al. Resistant hypertension: diagnosis, evaluation, and treatment: a Scientific Statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension. 2008;51:1403–1419. [DOI] [PubMed] [Google Scholar]
- 20. Allison PD. Estimating and comparing survival curves with Proc LIFETEST. In: The SAS Institute I. Survival Analysis Using the SAS System: A Practical Guide, 2nd ed. Cary, NC: SAS Institute, Inc; 2010:29–69. [Google Scholar]
- 21. Kleinbaum DG, Klein M. Evaluating the proportional hazards assumption. In: Survival Analysis—A Self‐Learning Text. 2nd ed. New York, NY: Springer‐Verlag; 2005: 131–167. [Google Scholar]
- 22. Breslow NE, Day NE. Modelling the relationship between risk, dose, and time. In: International Agency for Research on Cancer. Statistical Method in Cancer Research: The Design and Analysis of Cohort Data. Lyon, France: Oxford University Press; 1987:232–270. [Google Scholar]
- 23. Greenland S. Applications of stratified analysis methods. In: Rothman KJ, Greenland S, Lash TL, eds. Modern Epidemiology, 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2008:283–302. [Google Scholar]
- 24. Dolan E, Stanton A, Thijs L, et al. Superiority of ambulatory over clinic blood pressure measurement in predicting mortality: the Dublin Outcome Study. Hypertension. 2005;46:156–161. [DOI] [PubMed] [Google Scholar]
- 25. Bobrie G, Clerson P, Ménard JI, et al. Masked hypertension: a systematic review. J Hypertens. 2008;26:1715–1725. [DOI] [PubMed] [Google Scholar]
- 26. Hebert PR, Moser M, Mayer J, Hennekens CH. Recent evidence on drug therapy of mild to moderate hypertension and decreased risk of coronary heart disease. Arch Intern Med. 1993;153:578–581. [PubMed] [Google Scholar]
- 27. Moser M, Herbert PR. Prevention of disease progression, left ventricular hypertrophy and congestive heart failure in hypertension treatment trials. J Am Coll Cardiol. 1996;27:1214–1218. [DOI] [PubMed] [Google Scholar]
- 28. SHEP Cooperative Research Group . Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension: final results of the Systolic Hypertension in the Elderly Program (SHEP). JAMA. 1991;265:3255–3264. [PubMed] [Google Scholar]
- 29. Weber MA, Bakris GL, Hester A, et al. Systolic blood pressure and cardiovascular outcomes during treatment of hypertension. Am J Med. 2013;126:501–508. [DOI] [PubMed] [Google Scholar]
- 30. Messerli FH, Panjrath GS. The J‐curve between blood pressure and coronary artery disease or essential hypertension. Exactly how essential? J Am Coll Cardiol. 2009;54:1827–1834. [DOI] [PubMed] [Google Scholar]
- 31. Cooper‐Dehoff RM, Gong Y, Handberg E, et al. Tight blood pressure control and cardiovascular outcomes among hypertensive patients with diabetes and coronary artery disease. JAMA. 2010;304:61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. The SPRINT Research Group . A randomized trial of intensive versus standard blood‐pressure control. N Engl J Med. 2015;373:2103–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Howard G, Lackland DT, Klendorfer DO, et al. Racial differences in the impact of elevated systolic blood pressure on stroke risk. JAMA Intern Med. 2013;173:46–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Taylor F, Huffman MD, Macedo TH, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013;1:1–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Weber MA, Julius S, Kjedlsen SE, et al. Cardiovascular outcomes in hypertensive patients comparing single‐agent therapy with combination therapy. J Hypertens. 2012;30:2213–2222. [DOI] [PubMed] [Google Scholar]
- 36. Egan BM, Basile JN, Rehman SU, et al. Renin‐guided algorithm matches clinical hypertension specialist care in uncontrolled hypertension: a randomized‐controlled clinical trial. Am J Hypertens. 2009;22:792–801. [DOI] [PubMed] [Google Scholar]
- 37. Franceschini N, Chasman DI, Cooper‐DeHoff RM, Arnett DK. Genetics, ancestry, and hypertension: implications for targeted antihypertensive therapies. Curr Hypertens Rep. 2014;16:461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bakris GL, Nadim MK, Haller H, et al. Baroreflex activation therapy provides durable benefit in patients with resistant hypertension: results of long‐term follow‐up in the Rheos Pivotal Trial. J Am Soc Hypertens. 2012;6:152–158. [DOI] [PubMed] [Google Scholar]
- 39. Esler MD, Krum H, Schlaich M, et al. Renal sympathetic denervation for treatment of drug‐resistant hypertension: one‐year results from the Symplicity HTN‐2 randomized, controlled trial. Circulation. 2012;126:2976–2982. [DOI] [PubMed] [Google Scholar]
- 40. Bhatt DL, Kandzari DE, O'Neill WW, et al. A controlled trial of renal denervation for resistant hypertension. N Engl J Med. 2014;370:1393–1401. [DOI] [PubMed] [Google Scholar]


