Abstract
Background
Neonatal herpes is a rare but potentially devastating condition (60% fatality without treatment). Transmission usually occurs during delivery from mothers with herpes simplex virus type 1 (HSV-1) or HSV-2 genital infection. The global burden has never been quantified. We developed a novel methodology for burden estimation and present first WHO global and regional estimates of the annual number of neonatal herpes cases during 2010–2015.
Methods
Previous estimates of HSV-1 and HSV-2 prevalence and incidence in women aged 15–49 years were applied to 2010–2015 birth rates to estimate infections during pregnancy. Published risks of neonatal HSV transmission were then applied according to whether maternal infection was incident or prevalent with HSV-1 or HSV-2 to estimate neonatal herpes cases.
Findings
Globally the overall rate of neonatal herpes was estimated to be ~10 cases per 100,000 births, equivalent to a best-estimate of ~14,000 cases annually (HSV-1: ~4,000; HSV-2: ~10,000). We estimated that the most neonatal herpes cases occurred in Africa, due to high maternal HSV-2 infection and high birth rates. HSV-1 contributed more cases than HSV-2 in the Americas, Europe and Western Pacific. High rates of genital HSV-1 infection and moderate HSV-2 prevalence meant the Americas had the highest overall rate. However, our estimates are highly sensitive to the core assumptions, and considerable uncertainty exists for many settings given sparse underlying data.
Interpretation
These neonatal herpes estimates mark the first attempt to quantify the global burden of this rare but serious condition. Better primary data collection on neonatal herpes is critically needed to reduce uncertainty and refine future estimates. This is particularly important in resource-poor settings where we may have underestimated cases. Nevertheless, these first estimates suggest development of new HSV prevention measures such as vaccines could have additional benefits beyond reducing genital ulcer disease and HSV-associated HIV transmission, through prevention of neonatal herpes.
Funding
World Health Organization
INTRODUCTION
Neonatal infection with herpes simplex virus (HSV) is a potentially devastating complication of genital herpes during pregnancy. Neonatal herpes is rare, but associated with considerable morbidity and mortality: untreated, the case-fatality rate is estimated to be 60%1,2. Even with antiviral treatment, mortality rates and lasting neurological impairment remain substantial, especially for neonates with central nervous system (CNS) disease (about 30% of cases) and disseminated disease (25% of cases) compared with skin, eyes and mucosa (SEM) disease (around 45% of cases)1,2. Neonatal herpes is a costly condition since it typically involves a hospital stay, intensive monitoring, intravenous drug treatment, and extensive laboratory testing, and often results in longer-term costs associated with disability due to severe neurological sequelae3–5.
The majority (>85%) of neonatal herpes infections occur from exposure to HSV type 1 (HSV-1) or type 2 (HSV-2) shed in the genital tract during delivery1,6. Neonatal herpes infection due to a prevalent maternal infection is possible but the risk is low due to the presence of protective maternal IgG antibodies which are able to cross the placenta to afford immunity to the neonate1,7. The risk of neonatal herpes infection is considerably greater for incident maternal infections close to term, when virus is shed from the genital tract but maternal IgG antibodies have yet to be produced1,7. Intrauterine infection, although highly morbid, accounts for less than 5% of neonatal herpes infections1,6. Postpartum infection (around 10% of cases) is thought to be acquired through contact with oral HSV-1 shed by caregivers1,6.
Worldwide, HSV-1 and HSV-2 are both highly prevalent8–12. HSV-2 is predominantly sexually-transmitted, causing genital herpes. HSV-1 is predominantly orally-transmitted, causing oro-labial herpes (“cold sores”); however genital HSV-1 infection is possible. In 2012 there were an estimated 417 million people aged 15–49 years with prevalent HSV-2 infection globally8. Since serological tests do not distinguish between oro-labial and genital infection, it is difficult to accurately estimate the global number of prevalent genital HSV-1 infections. Estimates for 2012 put the figure among 15–49 year olds at 140–239 million, depending on the value taken for the proportion of incident HSV-1 infections after age 15 that are genital9. There is evidence that genital HSV-1 is increasing in prevalence in some high-income settings, becoming an important cause of genital herpes13, which may increase rates of neonatal herpes.
The occurrence of neonatal herpes has been difficult to quantify, and the worldwide annual number of cases has never been estimated. Most countries do not require case reporting of neonatal herpes infections4, although a few areas have implemented active surveillance efforts for neonatal herpes14,15. Prospective cohort studies to measure incidence have been conducted only rarely7. Without estimates of the numbers of cases of neonatal herpes occurring each year, it is challenging to raise awareness of this devastating infection. In addition, global estimates are crucial for stimulating efforts to develop HSV vaccines, microbicides, and improved diagnostics and treatment, and modelling more precisely their potential benefits. Therefore, we present the first set of World Health Organization (WHO) global estimates of the annual number of incident cases of neonatal herpes infection from HSV-1 or HSV-2 infection in mothers aged 15–49 years during 2010–2015.
METHODS
To generate estimates of incident neonatal herpes cases worldwide, we used as our starting point the latest WHO global and regional estimates of HSV-1 and HSV-2 prevalence and incidence in women, which were done for 2012 and published in 2015. These estimates were informed by comprehensive literature reviews conducted through February 2014; full details of the search strategy, methods and results are reported in the corresponding papers8,9. After applying live birth rates by maternal age group for each WHO region for 2010–201516 to determine estimates of prevalent and incident maternal HSV infections during pregnancy, we applied published risks of neonatal transmission according to whether the maternal infection was incident or prevalent and type 1 or type 27,17,18, to generate annual numbers of incident neonatal infections according to the following equation:
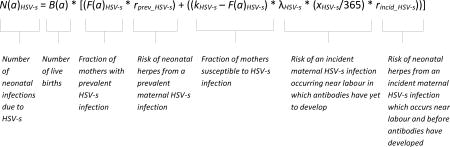 |
where:
N(a)HSV-s is the annual number of incident neonatal HSV infections corresponding to maternal year of age a due to HSV type s where s=1 or 2;
B(a) is the annual number of live births at maternal age a16;
F(a)HSV-s is the proportion of women with prevalent HSV-s infection at age a8,9;
rprev_HSV-s is the per-birth risk of neonatal infection from a prevalent maternal HSV-s infection7;
kHSV-s is the maximum proportion of women that can be expected to be infected with HSV-s over a lifetime of exposure8,9;
λHSV-s is the incidence of HSV-s infection per year among (uninfected) women8,9;
xHSV-s is the average number of days between HSV-s infection and the development of protective IgG antibodies (i.e., the window for transmission associated with an incident maternal HSV-s infection)19,20;
rincid_HSV-s is the per-birth risk of neonatal infection from an incident maternal HSV-s infection which occurs near labour and before antibodies have developed7,17,18.
Table 1 displays the key parameter values used in the estimates. Estimates of numbers of incident neonatal infections were done for each single year of maternal age (15–49 years) and then summed across each 5-year maternal age group. Separate estimates were produced for each WHO region (the Americas, Africa, Eastern Mediterranean, Europe, South-East Asia and Western Pacific) and then summed to obtain global estimates of the number of neonatal herpes infections. A sensitivity analysis was carried out varying the assumed risks of neonatal transmission (Table 1). For full details of the Methods see Supplementary appendix.
Table 1.
Key parameter values used in the estimates, and accompanying range used in the sensitivity analysis
| Parameter | Symbol | Default value |
Range used in sensitivity analysis |
Reference(s) |
|---|---|---|---|---|
| HSV-2 | ||||
| Average number of days between HSV-2 infection and the development of protective IgG antibodies (=transmission window) | xHSV-2 | 21 days | -- | 19,20 |
| Risk of neonatal infection from a prevalent maternal HSV-2 infection | rprev_HSV-2 | 0·02% | 0·0045% and 0·064% | 7 |
| Risk of neonatal infection from an incident maternal HSV-2 infection which occurs near labour and before antibodies have developed | rincid_HSV-2 | 7.7% | 2·7% and 15·4% | 7,17 |
| HSV-1 | ||||
| Average number of days between HSV-1 infection and the development of protective IgG antibodies (=transmission window) | xHSV-1 | 25 days | -- | 20 |
| Risk of neonatal infection from a prevalent maternal HSV-1 infection (any HSV-1infection) | rprev_HSV-1 | 0·0063% | 0·00077% and 0·023% | 7 and Stacy Selke, personal communication |
| Risk of neonatal infection from an incident maternal HSV-1 infection (any HSV-1 infection) which occurs near labour and before antibodies have developed | rincid_HSV-1 | 11% | 3·1% and 26·1% | 7 and Amalia Magaret, personal communication, based on data described in18 |
For full details see Supplementary appendix.
Role of the funding source
This work was funded by the UNDP/UNFPA/UNICEF/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction. WHO commissioned the study, advised as required, co-ordinated data requests, helped with redrafts, and approved manuscript submission. KJL had full access to all data in the study and had final responsibility for the decision to submit for publication.
RESULTS
Findings of the previous HSV estimates which are relevant to the current estimates of neonatal herpes cases are presented in additional tables (Table S1 and Table S2). Table S1 summarizes the number of studies contributing to the female estimates of HSV-1 and HSV-2 infection in the previous papers8,9 by WHO region and 5-year age-band, and the individual countries which reported HSV-1 or HSV-2 prevalence to inform these estimates. (Studies may be represented for more than one age group, and studies reporting HSV-2 often reported HSV-1.) Table S2 shows the estimated prevalence and incidence of HSV-2, any HSV-1 and genital HSV-1 infection in women in 2012 found in the previous papers8,9. Globally, among the 139 million live births among women aged 15–49 years each year during 2010–2015 on average, an estimated 24 million births occurred to women who had either prevalent or incident HSV-2 infection during pregnancy, and 108 million births occurred to women who had either prevalent or incident HSV-1 infection (at any site) during pregnancy (some of which – i.e. those births in dually-infected mothers – will be counted among the numbers with HSV-2 infection).
INCIDENT NEONATAL HERPES CASES
Globally, the annual number of incident neonatal herpes cases during 2010–2015 was estimated to be 14,257, of which approximately two-thirds (9,911 cases) were due to HSV-2, and one-third (4,346 cases) due to HSV-1 (Table 2). The global rate of neonatal herpes when averaged across all regions was estimated to be 10·3 per 100,000 births (Table 3).
Table 2.
Global and regional estimates of the annual number of cases of neonatal herpes during 2010–2015, by HSV type and maternal age group
| Any neonatal herpes | ||||||||
| Region | Maternal age group (years) | Total | ||||||
| 15–19 | 20–24 | 25–29 | 30–34 | 35–39 | 40–44 | 45–49 | ||
| Americas | 484 | 864 | 818 | 565 | 278 | 72 | 10 | 3091 |
| Africa | 934 | 1521 | 1282 | 847 | 460 | 168 | 59 | 5270 |
| Eastern Mediterranean | 152 | 307 | 269 | 161 | 78 | 26 | 6 | 1000 |
| Europe | 79 | 256 | 312 | 232 | 99 | 20 | 1 | 999 |
| South-East Asia | 103 | 459 | 402 | 212 | 96 | 30 | 10 | 1313 |
| Western Pacific | 131 | 1123 | 848 | 319 | 119 | 37 | 6 | 2583 |
| Global total | 1884 | 4530 | 3930 | 2336 | 1131 | 353 | 92 | 14257 |
| Neonatal herpes due to a maternal HSV-1 infection | ||||||||
| Region | Maternal age group (years) | Total | ||||||
| 15–19 | 20–24 | 25–29 | 30–34 | 35–39 | 40–44 | 45–49 | ||
| Americas | 358 | 587 | 507 | 320 | 143 | 34 | 4 | 1954 |
| Africa | 11 | 4 | 2 | 1 | 1 | 0 | 0 | 19 |
| Eastern Mediterranean | 74 | 113 | 76 | 38 | 17 | 5 | 1 | 325 |
| Europe | 62 | 174 | 179 | 113 | 42 | 8 | 0 | 577 |
| South-East Asia | 18 | 20 | 8 | 3 | 1 | 0 | 0 | 51 |
| Western Pacific | 99 | 706 | 430 | 131 | 41 | 11 | 2 | 1420 |
| Global total | 622 | 1604 | 1202 | 606 | 246 | 59 | 8 | 4346 |
| Neonatal herpes due to a maternal HSV-2infection | ||||||||
| Region | Maternal age group (years) | Total | ||||||
| 15–19 | 20–24 | 25–29 | 30–34 | 35–39 | 40–44 | 45–49 | ||
| Americas | 126 | 277 | 310 | 245 | 134 | 38 | 6 | 1137 |
| Africa | 924 | 1517 | 1280 | 846 | 460 | 167 | 59 | 5252 |
| Eastern Mediterranean | 78 | 194 | 193 | 123 | 61 | 21 | 5 | 675 |
| Europe | 17 | 82 | 133 | 119 | 57 | 12 | 1 | 422 |
| South-East Asia | 86 | 439 | 394 | 209 | 95 | 30 | 10 | 1262 |
| Western Pacific | 32 | 417 | 418 | 188 | 78 | 26 | 4 | 1163 |
| Global total | 1262 | 2927 | 2729 | 1730 | 885 | 294 | 84 | 9911 |
Totals may be slightly different due to rounding. Numbers of cases are given to the nearest integer. It should be noted that all numbers are model estimates. Measurement resolution should not be interpreted as indicative of precision.
Table 3.
Global and regional estimates of the annual incidence of neonatal herpes per 100,000 births during 2010–215, by HSV type and maternal age group
| Any neonatal herpes | ||||||||
| Region | Maternal age group (years) | Overall rate per 100,000 births | ||||||
| 15–19 | 20–24 | 25–29 | 30–34 | 35–39 | 40–44 | 45–49 | ||
| Americas | 22·1 | 20·6 | 19·5 | 18·8 | 18·4 | 18·1 | 18·1 | 19·9 |
| Africa | 18·4 | 16·2 | 14·9 | 14·0 | 13·5 | 13·1 | 12·8 | 15·4 |
| Eastern Mediterranean | 12·4 | 7·9 | 5·9 | 5·0 | 4·7 | 4·5 | 4·4 | 6·5 |
| Europe | 14·9 | 10·9 | 8·7 | 7·6 | 7·1 | 6·9 | 6·8 | 8·9 |
| South-East Asia | 3·2 | 3·3 | 3·6 | 4·1 | 4·5 | 4·9 | 5·3 | 3·6 |
| Western Pacific | 15·3 | 1··3 | 9·3 | 8·4 | 8·1 | 8·0 | 8·1 | 10·0 |
| Global total | 14·4 | 10·3 | 9·6 | 9·6 | 9·7 | 9·7 | 9·8 | 10·3 |
| Neonatal herpes due to a maternal HSV-1 infection | ||||||||
| Region | Maternal age group (years) | Overall rate per 100,000 births | ||||||
| 15–19 | 20–24 | 25–29 | 30–34 | 35–39 | 40–44 | 45–49 | ||
| Americas | 16·3 | 14·0 | 12·1 | 10·6 | 9·5 | 8·6 | 7·9 | 12·6 |
| Africa | 0·2 | 0·04 | 0·02 | 0·02 | 0·02 | 0·02 | 0·02 | 0·05 |
| Eastern Mediterranean | 6·1 | 2·9 | 1·7 | 1·2 | 1·0 | 0·9 | 0·9 | 2·1 |
| Europe | 11·7 | 7·4 | 5·0 | 3·7 | 3·0 | 2·6 | 2·4 | 5·2 |
| South-East Asia | 0·5 | 0·1 | 0·07 | 0·06 | 0·06 | 0·06 | 0·06 | 0·1 |
| Western Pacific | 11·6 | 7·1 | 4·7 | 3·5 | 2·8 | 2·5 | 2·3 | 5·5 |
| Global total | 4·7 | 3·7 | 2·9 | 2·5 | 2·1 | 1·6 | 0·9 | 3·1 |
| Neonatal herpes due to a maternal HSV-2 infection | ||||||||
| Region | Maternal age group (years) | Overall rate per 100,000 births | ||||||
| 15–19 | 20–24 | 25–29 | 30–34 | 35–39 | 40–44 | 45–49 | ||
| Americas | 5·7 | 6·6 | 7·4 | 8·2 | 8·9 | 9·6 | 10·2 | 7·3 |
| Africa | 18·2 | 16·2 | 14·9 | 14·0 | 13·4 | 13·1 | 12·8 | 15·3 |
| Eastern Mediterranean | 6·4 | 5·0 | 4·2 | 3·9 | 3·6 | 3·5 | 3·5 | 4·4 |
| Europe | 3·2 | 3·5 | 3·7 | 3·9 | 4·1 | 4·3 | 4·4 | 3·8 |
| South-East Asia | 2·7 | 3·1 | 3·6 | 4·0 | 4·4 | 4·9 | 5·3 | 3·5 |
| Western Pacific | 3·7 | 4·2 | 4·6 | 4·9 | 5·3 | 5·6 | 5·9 | 4·5 |
| Global total | 9·6 | 6·7 | 6·6 | 7·1 | 7·6 | 8·1 | 9·0 | 7·2 |
Rates are given to 1 decimal place, or 2 decimal places for very low rates, in order to demonstrate trends. It should be noted that all rates are model estimates. Measurement resolution should not be interpreted as indicative of precision.
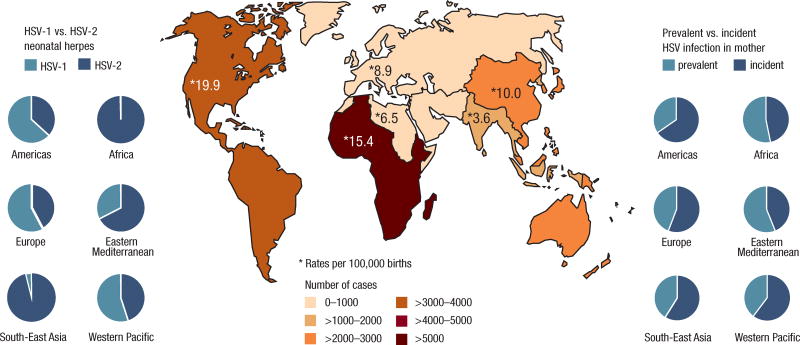
Our results showed that Africa contributed the largest number (around one-third) of neonatal herpes cases to the global total (Table 2; Figure 1). This was a consequence of the much higher incidence and prevalence of adult female HSV-2 infection in this region (Table S2), combined with high number of births (Table S1). Our calculations showed that HSV-1 is currently not a significant cause of neonatal herpes in Africa (Table 2; Figure 1). This is based on available data showing a high modelled rate of (oral) HSV-1 infection during childhood and saturation in prevalence by adolescence at almost 100% prevalence in Africa, thus removing potential for further genital HSV-1 infection in adulthood (Table S2). HSV-1 does not seem to be a significant cause of neonatal herpes in South-East Asia either (Table 2; Figure 1), again based on available data which seem to show saturation in HSV-1 prevalence by adolescence, although the modelled level of saturation is much lower than in Africa (Table S2).
Figure 1.
Estimates of the annual number of cases, and rate per 100,000 births, of neonatal herpes during 2010–2015, and relative contribution of HSV-1 versus HSV-2 and prevalent versus incident HSV infection in the mother to the numbers of cases, by WHO region
In contrast, HSV-1 was estimated to cause more neonatal herpes cases than HSV-2 in the Americas, and also in Europe and Western Pacific (Table 2; Figure 1). The high numbers of neonatal herpes cases due to HSV-1 in the Americas were due to relatively low rates of childhood HSV-1 infection, with new HSV-1 infections continuing to occur during adulthood (Table S2), and the attendant risk to the neonate from genital HSV-1. High rates of genital HSV-1 relative to other regions, combined with moderately high HSV-2 prevalence among women, meant that the Americas was estimated to have the highest overall rate of neonatal herpes in the world: 19·9 per 100,000 births (all births, not just those of infected women) (Table 3).
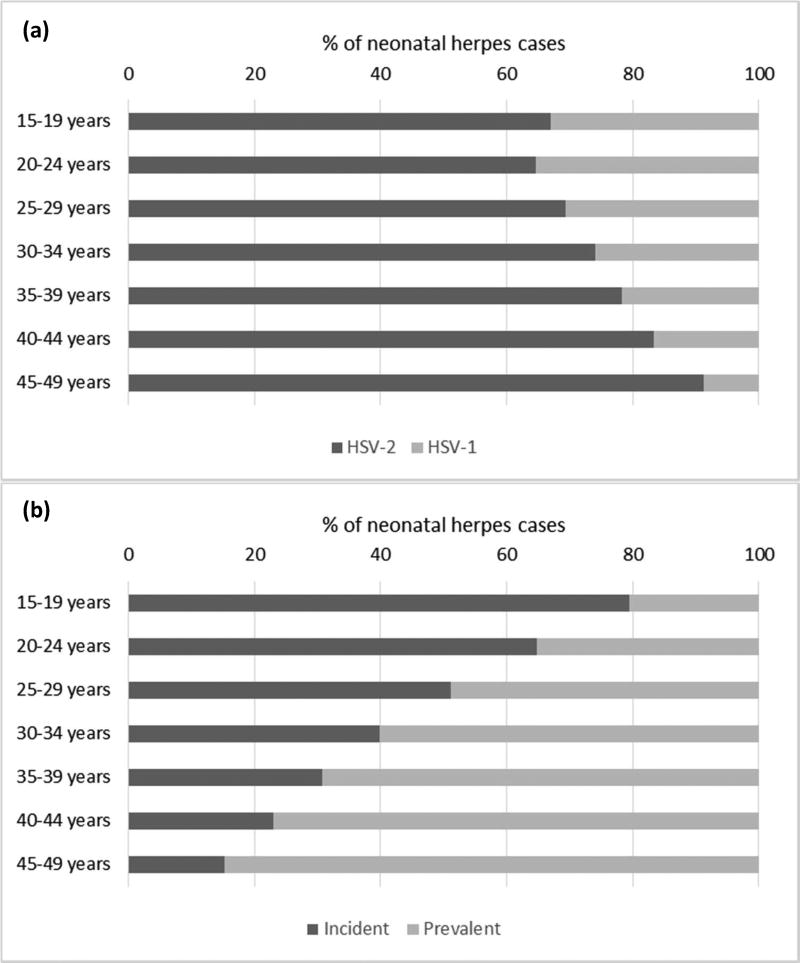
The number of neonatal herpes cases by maternal age group increased between the 15–19 and 20–24 age groups (1,884 to 4,530 cases) and decreased thereafter (Table 2). This was largely due to the steep rise in number of births by maternal age group. Neonatal herpes incidence (rate) decreased with increased maternal age for HSV-1, while incidence decreased with increased age and then increased again for HSV-2 (Table 3). These patterns were reflected in an overall trend of increasing proportion of cases due to HSV-2 with maternal age (Figure 2).
Figure 2.
Percentage of neonatal herpes cases due to (a) HSV-1 and HSV-2; and (b) prevalent versus incident maternal HSV infection during 2010–2015, by age group of the mother
Patterns in rates are a function of the proportion of women with incident versus prevalent infection, and the risks of transmission associated with each. Neonatal herpes rates due to HSV-1 declined with increased maternal age because the number of women able to be newly infected with HSV-1 decreased with age, and the risk associated with prevalent maternal HSV-1 infection is low relative to that for incident maternal infection. For HSV-2, global trends masked quite different regional trends. Neonatal herpes incidence increased with maternal age where maternal incident HSV-2 infections continued and prevalence increased with age (Americas, Europe, South-East Asia, Western Pacific), but decreased if new infections slowed and maternal HSV-2 infection reached saturation (Africa and Eastern Mediterranean) (Table 3).
We calculated that the proportion of neonatal herpes cases was split roughly equally between prevalent versus incident maternal HSV infections, although some regional differences were seen, with most cases attributable to incident maternal infection in Europe, South-East Asia, Western Pacific, and, most markedly, the Americas (Figure 1). However, the relative contribution of prevalent versus incident HSV infection to neonatal herpes cases showed a strong association with maternal age (Figure 2).
SENSITIVITY ANALYSIS
The number and rate of neonatal herpes is sensitive to the assumed risks of neonatal herpes from a maternal infection (HSV-1 versus HSV-2; incident versus prevalent infection), reflecting the underlying uncertainty in the values attached to these risks (Table 4 and Table 5). The variation in numbers of cases and rates between the lowest and highest assumed values was an order of magnitude of approximately 10. When the lowest values were used across all assumptions, the total annual number of cases of neonatal herpes globally during 2010–2015 was estimated to be 3,703 (2·7 cases per 100,000 births), and when the highest values were used across all assumptions, the total annual number of cases worldwide in 2010–2015 was estimated to be 36,415 (26·3 cases per 100,000 births).
Table 4.
Sensitivity analysis for estimates of annual neonatal herpes cases during 2010–2015, varying neonatal herpes transmission risk (presented as lowest estimate; highest estimate)
| Any neonatal herpes | ||||||||
| Region | Maternal age group (years) | Total | ||||||
| 15–19 | 20–24 | 25–29 | 30–34 | 35–39 | 40–44 | 45–49 | ||
| Americas | 130; 1193 | 223; 2195 | 204; 2135 | 136; 1512 | 65; 759 | 16; 200 | 2; 28 | 777; 8022 |
| Africa | 291; 2066 | 433; 3645 | 336; 3264 | 208; 2252 | 107; 1263 | 38; 469 | 13; 167 | 1425; 13126 |
| Eastern Mediterranean | 43; 360 | 80; 784 | 63; 736 | 35; 465 | 16; 233 | 5; 78 | 1; 19 | 243; 2674 |
| Europe | 21; 195 | 65; 657 | 75; 836 | 53; 646 | 22; 285 | 4; 58 | 0; 4 | 241; 2680 |
| South-East Asia | 32; 231 | 135; 1070 | 113; 971 | 58; 526 | 25; 243 | 8; 78 | 3; 26 | 374; 3146 |
| Western Pacific | 38; 321 | 326; 2863 | 243; 2250 | 89; 874 | 33; 334 | 10; 107 | 2; 17 | 741; 6767 |
| Global total | 552; 4365 | 1225; 11213 | 1000; 10193 | 564; 6275 | 262; 3117 | 79; 991 | 20; 262 | 3703; 36415 |
| Neonatal herpes due to a maternal HSV-1 infection | ||||||||
| Region | Maternal age group (years) | Total | ||||||
| 15–19 | 20–24 | 25–29 | 30–34 | 35–39 | 40–44 | 45–49 | ||
| Americas | 90; 918 | 141; 1554 | 116; 1388 | 70; 904 | 30; 418 | 7; 102 | 1; 14 | 454; 5928 |
| Africa | 3; 26 | 1; 11 | 0; 7 | 0; 4 | 0; 3 | 0; 1 | 0; 0 | 4; 53 |
| Eastern Mediterranean | 19; 187 | 26; 308 | 15; 229 | 6; 125 | 2; 59 | 1; 19 | 0; 5 | 69; 932 |
| Europe | 16; 157 | 41; 466 | 38; 512 | 21; 346 | 7; 136 | 1; 25 | 0; 2 | 124; 1644 |
| South-East Asia | 5; 44 | 4; 58 | 1; 27 | 0; 11 | 0; 5 | 0; 1 | 0; 0 | 11; 146 |
| Western Pacific | 25; 251 | 166; 1896 | 91; 1238 | 24; 405 | 7; 135 | 2; 39 | 0; 6 | 315; 3972 |
| Global total | 157; 1584 | 379; 4293 | 262; 3401 | 122; 1796 | 46; 756 | 10; 188 | 1; 26 | 977; 12045 |
| Neonatal herpes due to a maternal HSV-2 infection | ||||||||
| Region | Maternal age group (years) | Total | ||||||
| 15–19 | 20–24 | 25–29 | 30–34 | 35––39 | 40–44 | 45–49 | ||
| Americas | 40; 275 | 82; 641 | 88; 747 | 67; 608 | 35; 341 | 10; 98 | 1; 15 | 323; 2724 |
| Africa | 289; 2040 | 432; 3633 | 336; 3257 | 207; 2248 | 107; 1260 | 38; 468 | 13; 166 | 1421; 13073 |
| Eastern Mediterranean | 24; 173 | 54; 476 | 48; 508 | 29; 339 | 14; 174 | 4; 59 | 1; 14 | 173; 1742 |
| Europe | 5; 37 | 24; 191 | 37; 324 | 32; 301 | 15; 148 | 3; 32 | 0; 2 | 116; 1036 |
| South-East Asia | 27; 187 | 131; 1012 | 112; 944 | 57; 514 | 25; 239 | 8; 77 | 3; 26 | 363; 2999 |
| Western Pacific | 10; 70 | 123; 967 | 118; 1012 | 51; 468 | 20; 199 | 7; 68 | 1; 11 | 329; 2795 |
| Global total | 395; 2781 | 846; 6920 | 738; 6792 | 442; 4479 | 216; 2361 | 69; 802 | 19; 235 | 2726; 24370 |
Estimates are presented by HSV type and maternal age group. Totals may be slightly different due to rounding. Numbers of cases are given in integers. It should be noted that all numbers are model estimates. Measurement resolution should not be interpreted as indicative of precision.
Table 5.
Sensitivity analysis for estimates of annual neonatal herpes incidence per 100,000 births during 2010–2015, varying neonatal herpes transmission risk (presented as lowest estimate-highest estimate)
| Any neonatal herpes | ||||||||
| Region | Maternal age group (years) | Overall rate per 100,000 births | ||||||
| 15–19 | 20–24 | 25–29 | 30–34 | 35–39 | 40–44 | 45–49 | ||
| Americas | 5·9–54·4 | 5·3–52·2 | 4·9–50·9 | 4·5–50·3 | 4·3–50·2 | 4·1–50·5 | 4·0–51·1 | 5·0–51·6 |
| Africa | 5·7–40·7 | 4·6–38·9 | 3·9–37·9 | 3·4–37·3 | 3·1–36·9 | 2·9–36·7 | 2·8–36·5 | 4·2–38·3 |
| Eastern Mediterranean | 3·5–29·5 | 2·0–20·1 | 1·4–16·2 | 1·1–14·5 | 0·9–13·8 | 0·9–13·5 | 0·8–13·3 | 1·6–17·5 |
| Europe | 4·0–36·8 | 2·8–27·9 | 2·1–23·4 | 1·7–21·3 | 1·6–20·4 | 1·5–20·1 | 1·4–20·2 | 2·1–23·9 |
| South-East Asia | 1·0–7·1 | 1·0–7·6 | 1·0–8·8 | 1·1–10·1 | 1·2–11·4 | 1·3–12·6 | 1·3–13·9 | 1·0–8·6 |
| Western Pacific | 4·5–37·6 | 3·3–28·7 | 2·7–24·7 | 2·4–23·1 | 2·2–22·7 | 2·2–23·0 | 2·1–23·5 | 2·9–26·3 |
| Global total | 4·2–33·3 | 2·8–25·5 | 2·4–24·8 | 2·3–25·8 | 2·3–26·8 | 2·2–27·3 | 2·2–27·9 | 2·7–26·3 |
| Neonatal herpes due to a maternal HSV-1 infection | ||||||||
| Region | Maternal age group (years) | Overall rate per 100,000 births | ||||||
| 15–19 | 20–24 | 25–29 | 30–34 | 35–39 | 40–44 | 45–49 | ||
| Americas | 4·1–41·9 | 3·4–37·0 | 2·8–33·1 | 2·3–30·1 | 2·0–27·7 | 1·7–25·8 | 1·5–24·3 | 2·9–34·1 |
| Africa | 0·06–0·5 | 0·01–0·1 | 0·00–0·08 | 0·00–0·07 | 0·00–0·07 | 0·00–0·07 | 0·00–0·07 | 0·01–0·2 |
| Eastern Mediterranean | 1·6–15·3 | 0·7–7·9 | 0·3–5·0 | 0·2–3·9 | 0·1–3·5 | 0·1–3·3 | 0·1–3·3 | 0·5–6·1 |
| Europe | 3·0–29·8 | 1·7–19·8 | 1·1–14·3 | 0·7–11·4 | 0·5–9·8 | 0·4–8·9 | 0·3–8·4 | 1·1–14·7 |
| South-East Asia | 0·1–1·4 | 0·03–0·4 | 0·01–0·2 | 0·01–0·2 | 0·01–0·2 | 0·01–0·2 | 0·01–0·2 | 0·03–0·4 |
| Western Pacific | 2·9–29·4 | 1·7–19·0 | 1·0–13·6 | 0·6–10·7 | 0·5–9·2 | 0·4–8·4 | 0·3–8·0 | 1·2–15·4 |
| Global total | 1·2–12·1 | 0·9–9·8 | 0·6–8·3 | 0·5–7·4 | 0·4–6·5 | 0·3–5·2 | 0·1–2·8 | 0·7–8·7 |
| Neonatal herpes due to a maternal HSV-2 infection | ||||||||
| Region | Maternal age group (years) | Overall rate per 100,000 births | ||||||
| 15–19 | 20–24 | 25–29 | 30–34 | 35–39 | 40–44 | 45–49 | ||
| Americas | 1·8–12·5 | 2·0–15·2 | 2·1–17·8 | 2·2–20·2 | 2·3–22·5 | 2·5–24·7 | 2·6–26·8 | 2·2–17·5 |
| Africa | 5·7–40·2 | 4·6–38·8 | 3·9–37·9 | 3·4–37·2 | 3·1–36·8 | 2·9–36·6 | 2·8–36·4 | 4·1–38·2 |
| Eastern Mediterranean | 2·0–14·1 | 1·4–12·2 | 1·1–11·1 | 0·9–10·6 | 0·8–10·3 | 0·8–10·2 | 0·7–10·1 | 1·1–11·4 |
| Europe | 1·0–7·0 | 1·0–8·1 | 1·0–9·1 | 1·0–9·9 | 1·1–10·6 | 1·1–11·3 | 1·1–11·8 | 1·0–9·3 |
| South-East Asia | 0·8–5·8 | 0·9–7·2 | 1·0–8·5 | 1·1–9·9 | 1·2–11·2 | 1·3–12·4 | 1·3–13·7 | 1·0–8·2 |
| Western Pacific | 1·2–8·2 | 1·2–9·7 | 1·3–11·1 | 1·3–12·4 | 1·4–13·5 | 1·4–14·6 | 1·4–15·5 | 1·3–10·9 |
| Global total | 3·0–21·2 | 1·9–15·8 | 1·8–16·5 | 1·8–18·4 | 1·9–20·3 | 1·9–22·1 | 2·0–25·1 | 2·0; 17·6 |
Estimates are presented by HSV type and maternal age group. Rates are given to 1 decimal place, or 2 decimal places for very low rates, in order to demonstrate trends. It should be noted that all rates are model estimates. Measurement resolution should not be interpreted as indicative of precision.
DISCUSSION
This is the first attempt to quantify the global number of incident neonatal herpes cases. We estimated that each year during 2010–2015 there were over 14,000 cases of neonatal herpes arising from HSV infection in mothers aged 15–49 years worldwide (HSV-1: ~4,000; HSV-2: ~10,000), which is equivalent to an annual rate of neonatal herpes of 10·3 per 100,000 births. Our estimates of neonatal herpes cases are highly sensitive to the assumptions made. For example, the numbers of annual cases could be as low as ~4,000, or as high as ~36,000, if the lowest or highest plausible values for all components of neonatal transmission risk are used. Nonetheless, these estimates enable us to gain a first insight into the global picture of neonatal herpes, to compare burden of cases between regions, including the impact of HSV-1 versus HSV-2 and prevalent versus incident maternal infection, and to understand where further data collection is needed. For example, the Americas had the highest estimated regional rate of neonatal herpes, in large part because of the role of HSV-1 infection, which contributed two-thirds of cases to the regional total. This is consistent with recent surveillance data from Canada showing that HSV-1 caused 63% of neonatal herpes cases15. By contrast, in Africa, virtually all cases were due to HSV-2, and high HSV-2 infection rates combined with high birth rates in this region led it to have the highest estimated number of cases globally.
Our global estimated neonatal herpes rate of 10.3 per 100,000 births is consistent with recent estimates from North America, Europe, and Australia using surveillance and administrative data, which have ranged from between 2.5–13.3 per 100,000 live births1,5,14,15,21–26. The global number of cases we estimated is similar to what would be expected if neonatal herpes rates from the largest recent population-based estimates from USA hospital discharge data (9.6 per 100,000 births) were applied to global births5. Our higher estimated rate of 19.9 cases per 100,000 births for the Americas may reflect the challenges of retrospective reviews and difficulty capturing all cases for a condition that has not always had a single clear diagnosis code, and the overall uncertainty inherent in our estimates. A rate of 30.8 per 100,000 live births was found in the only large multi-centre prospective study of neonatal herpes acquisition, which was the study which informed our underlying neonatal transmission risks7. Globally, comparisons with other region-specific rates are made difficult by a general lack of data with regard to neonatal herpes27.
POTENTIAL FOR UNDERESTIMATION IN RESOURCE-POOR SETTINGS
These global neonatal herpes estimates provide a starting point for understanding the burden of neonatal herpes worldwide; however, it is likely that we have underestimated the numbers of cases in resource-poor settings. Our estimates rely heavily on data from the USA for parameterising transmission risks. We used numbers from a large, multi-centre prospective study in the USA of the effect of maternal HSV shedding and serological status on risk of transmission to the neonate7, but this study may not be generalizable to other settings. For example, the overall neonatal transmission risks in this study incorporated routine use of caesarean section when genital lesions were present as well as for other indications, which was shown to substantially reduce the risk of neonatal herpes infection7. Thus, the risks and corresponding number of cases could be much higher in settings where caesarean section is not frequently performed. Studies have also shown that HIV infection increases genital HSV-2 shedding frequency and quantity28,29. A recent study in South Africa among women in labour found high rates of HSV-2 shedding at delivery, especially in women co-infected with HIV27. Neonatal herpes rates could therefore be even higher in regions with substantial HIV burden in women of reproductive age27.
In addition, these estimates are an attempt to quantify only the number of cases of neonatal herpes, and do not tell us anything about the severity of infection. The clinical course of neonatal herpes, and the case-fatality rate, depend much on whether or not antivirals are given and how promptly, and thus will vary substantially by setting. In areas with less-developed medical infrastructure and limited diagnostic testing, neonatal herpes may be missed or mistaken for other serious illnesses, resulting in a higher burden of death and neurologic sequelae2. If we use a value of 60% for the proportion of neonatal cases that are fatal if left untreated1,2, then a rough estimate of the upper limit of the mortality rate due to neonatal herpes is 0·062 per 1,000 births, or 8,554 neonatal deaths annually given our base case scenario. This number does not of course consider those infants left with life-long disability, which is also likely to reach the thousands.
Collecting primary data on the incidence of neonatal herpes in resource-poor settings, and especially in sub-Saharan Africa, is therefore crucial. Preliminary data from a validation study of minimally invasive autopsy for evaluating neonatal deaths in Mozambique showed that HSV was the final cause of death in two of 41 neonatal deaths, and was a significant contributing factor in one of 18 stillbirths evaluated (Clara Menendez, personal communication). While these are small numbers, these data indicate that neonatal herpes could be much underappreciated as a cause of neonatal mortality in resource-poor settings. Expanded evaluations of neonatal deaths in these settings through the Child Health and Mortality Prevention Surveillance (CHAMPS) network will include HSV testing and will provide critical new data to understand the global impact of neonatal herpes30.
ADDITIONAL LIMITATIONS
There are a number of other important limitations to our estimates relevant to all regions. First, since these estimates of neonatal herpes cases are in turn based on the most recent estimates of HSV-1 and HSV-2 prevalence and incidence in women aged 15–49 years, the neonatal herpes estimates are affected by the same data availability, generalizability and quality issues affecting the adult estimates8,9. Individual studies can have a substantial influence on the estimated burden of maternal infection by region, and, in turn, on the estimates of neonatal herpes cases. Our estimates of genital HSV-1 are particularly uncertain. We assumed a value for the proportion of incident adult HSV-1 infections that are genital of 50%31. To our knowledge there have been no studies which have estimated this proportion in settings outside of the USA, however Africa, Eastern Mediterranean and South-East Asia appear to have little new HSV-1 infection in adults9, so choice of parameter values for HSV-1 is less influential in these regions.
Second, although the large, multi-centre prospective study in the USA from which our transmission risks were taken followed over 58,000 pregnant women, and represents the best available estimates of risk, the numbers of neonatal herpes cases in this study were extremely small: just 14 cases, which were used to inform our regional and global estimates. Our sensitivity analysis, which incorporated the confidence intervals around the risks from this source study, showed that varying the risks of neonatal transmission due to incident and prevalent maternal infection had a substantial impact on the estimated numbers of neonatal herpes cases.
Third, HSV incidence could be different among pregnant women compared with non-pregnant women; however this is not well understood32,33. Acquisition of genital herpes could be lower in pregnant women as a consequence of less frequent sexual activity, particularly during late-stage pregnancy, and lower partner change rates. However, changes in the maternal immune system may increase susceptibility to genital herpes during pregnancy34, while lower rates of condom use may expose pregnant women to a higher risk of infection.
IMPACT
Genital HSV infections among adolescents and adults are a global public health problem, estimated to affect over half a billion people worldwide8,9. This is the first attempt to quantify and thus better understand the global burden of neonatal herpes. However, data on mother-to-child HSV transmission rates in less-industrialized settings are absent, and we have instead relied on single studies of risk from the USA to generate estimates across all regions. In so doing, we may have underestimated neonatal herpes cases in resource-poor settings, perhaps severely. By highlighting the various limitations of these estimates, we hope to stimulate better and more coordinated data collection efforts to improve future estimates. Enhanced case reporting and surveillance where feasible and focused studies to collect prospective data on neonatal herpes incidence, mortality, and transmission risks will be extremely valuable. This is particularly important for settings in sub-Saharan Africa, since low rates of caesarean section and generalised HIV epidemics have the potential to increase the number of neonatal herpes cases well above that estimated here. Additional assessments of the incidence and prevalence of HSV-2 and genital HSV-1 among women, especially in countries outside of North America and Europe, are also needed.
Neonatal herpes has high fatality rates and potential for long-term neurologic disability among surviving neonates, but it is rare. This leaves a quandary for appropriate targeting of prevention efforts, and at what cost, for the tens of millions of women who have or are at risk of genital HSV during pregnancy. Prevention efforts have included visual inspection for herpetic lesions at delivery, selective use of caesarean section, potential use of suppressive antiviral therapy in late pregnancy, and behavioural primary prevention messages to reduce transmission of HSV to a susceptible mother in late pregnancy35. However, available prevention and treatment options are imperfect, are often expensive, and typically depend on good existing medical infrastructure. Prevention efforts are hampered by the often asymptomatic presentation of maternal HSV infection and the preponderance of cases caused by incident rather than prevalent maternal infection in some settings, as we highlight in these estimates. In addition, caesarean section has associated risks in itself, especially in settings with poor medical infrastructure. Thus, increasing these procedures in resource-poor settings without clearly defined prevention benefits may do more harm than good.
For these reasons, an effective new vaccine or microbicide developed against genital herpes in adults could have an important and needed benefit in preventing neonatal herpes. Recent scientific advances hold real promise for new HSV vaccine development36. The primary targets of such vaccines are prevention of painful genital ulcer disease (GUD) in tens of millions of adults8, reduction in the negative impact on sexual relationships, and reduction in the increased HIV risk associated with genital HSV infection37,38. Within the scope of all conditions affecting neonatal health, the current estimates suggest that HSV is not a major contributor, although its impact may be considerably underappreciated in some settings. However, if a vaccine or microbicide in adults could indirectly reduce neonatal transmission, this would not only expand the reach of these interventions, but could partly mitigate the difficulties in preventing this condition through existing management. Moreover, the high mortality and long-term disability in surviving infants due to neonatal herpes could actually translate into a considerable number of disability-adjusted life years and costs that could be prevented with a vaccine despite low incidence39,40. These global estimates provide a first insight into the potential magnitude of this added benefit. Better primary data on neonatal herpes, particularly in low-resource settings, will help define more precisely the potential global health impact of critically needed new primary prevention measures against HSV infection36,41.
Supplementary Material
RESEARCH IN CONTEXT.
Evidence before this study
Neonatal herpes is rare but often leads to death or life-long disability. Although some surveillance and other studies have assessed neonatal herpes incidence in selected high-income country settings, the global burden has never been estimated. In addition, no estimates of neonatal herpes incidence exist for resource-poor settings. Particularly in areas with high rates of genital infection with herpes simplex virus (HSV) in adults and poor medical infrastructure for prevention, diagnosis, and management, neonatal herpes could be an important under-recognised cause of neonatal morbidity and mortality. Following completion of the first global estimates of HSV-1 infection and updated global estimates of HSV-2 infection in 2015, we estimated the global burden of neonatal herpes incorporating the underlying epidemiology of HSV infections in the population.
Added value of this study
This study presents the first World Health Organization (WHO) global and regional estimates of the annual number of incident neonatal herpes infections during 2010–2015. Our estimation process uses estimates of HSV infection in women by age and WHO region and published mother-to-child transmission risks according to maternal infection characteristics. This enables us to demonstrate important differences in the distribution of cases by geographic region, for example, the proportion of cases caused by HSV-1 and the role of incident maternal infection. However, this study also highlights the lack of epidemiological data to inform and validate the estimates in some settings. In particular, because we extrapolate estimates using transmission risk data from the USA, we have likely underestimated neonatal herpes cases in some low-resource settings.
Implications of all the available evidence
Primary data on neonatal herpes incidence in resource-poor settings are crucial, in order to more accurately quantify the mortality and morbidity attributable to neonatal herpes and guide future prevention efforts. Generating first estimates of the global burden of neonatal herpes is a critical first step in raising awareness of this condition and guiding investment in future interventions such as vaccines and microbicides, by informing the full range and distribution of disease attributable to HSV infection, and therefore, the maximum potential benefit of these interventions.
Acknowledgments
DECLARATION OF INTERESTS
ASM reports grants from NIH during the conduct of the study, and personal fees from Immune Design and AiCuris outside the submitted work. LMN has been a full-time employee of the USA Government Centers for Disease Control and Prevention for the past 15 years, but during the time she worked on this project she was seconded to the World Health Organization.
The authors thank Dr Lauri Markowitz (Centers for Disease Control and Prevention) for providing additional data, Dr Monica Patton (Centers for Disease Control and Prevention) for helpful discussion, Dr Anna Wald and Stacy Selke (University of Washington) for help with parameterisation from the study by Brown et al., Dr Nathalie Broutet (World Health Organization) for commenting on the manuscript drafts, Dr Gretchen Stevens (World Health Organization) for statistical advice, Jessica Ho and Ann-Beth Moller (World Health Organization) for advising on access to population and births data and Janet Petitpierre (World Health Organization) for helping with the figures. KJL, KMET and PV thank the National Institute for Health Research (NIHR) Health Protection Research Unit (HPRU) in Evaluation of Interventions at the University of Bristol for research support.
FUNDING
This work was funded by the UNDP/UNFPA/UNICEF/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction. WHO commissioned the study, advised as required, co-ordinated data requests, helped with redrafts, and approved manuscript submission. KJL had full access to all the data in the study and had final responsibility for the decision to submit for publication. Dr Gottlieb is a staff member of the World Health Organization, and Dr Newman was a WHO staff member during her work on this article. KJL received separate funding from the World Health Organization, USAID/PATH, Health Protection Scotland, Sexual Health 24 and the National Institute for Health Research (NIHR) Health Protection Research Unit (HPRU) in Evaluation of Interventions at the University of Bristol during the course of this study. ASM received funding from the National Institutes of Health (NIH P01-A1-030731-23) during the study. These funders had no role in the writing of the manuscript nor the decision to submit it for publication. The authors alone are responsible for the views expressed in this article and they do not necessarily represent the views, decisions or policies of the institutions with which they are affiliated, the World Health Organization, the NHS, the NIHR, the Department of Health or Public Health England.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONTRIBUTORS
KJL did the literature review, data extraction and estimates calculations and drafted the manuscript. LMN oversaw the study, provided advice as required, and co-ordinated requests for demographic data. ASM provided statistical input and advised on neonatal herpes natural history parameters. MTM provided statistical advice on the sensitivity analysis. KMET assisted with data checking. PV advised on the modelling aspect. SLG gave advice on the study, its parameterisation and the wider context, and helped redraft the manuscript. All authors contributed to the direction of the work, provided technical expertise and commented on the drafts.
References
- 1.Corey L, Wald A. Maternal and neonatal herpes simplex virus infections. The New England journal of medicine. 2009;361(14):1376–85. doi: 10.1056/NEJMra0807633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kimberlin DW. Herpes simplex virus infections of the newborn. Seminars in perinatology. 2007;31(1):19–25. doi: 10.1053/j.semperi.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 3.Ambroggio L, Lorch SA, Mohamad Z, Mossey J, Shah SS. Congenital anomalies and resource utilization in neonates infected with herpes simplex virus. Sexually transmitted diseases. 2009;36(11):680–5. doi: 10.1097/OLQ.0b013e3181aaf54f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Handsfield HH, Waldo AB, Brown ZA, et al. Neonatal herpes should be a reportable disease. Sexually transmitted diseases. 2005;32(9):521–5. doi: 10.1097/01.olq.0000175292.88090.85. [DOI] [PubMed] [Google Scholar]
- 5.Flagg EW, Weinstock H. Incidence of neonatal herpes simplex virus infections in the United States, 2006. Pediatrics. 2011;127(1):e1–8. doi: 10.1542/peds.2010-0134. [DOI] [PubMed] [Google Scholar]
- 6.Gantt S, Muller WJ. The immunologic basis for severe neonatal herpes disease and potential strategies for therapeutic intervention. Clinical & developmental immunology. 2013;2013:369172. doi: 10.1155/2013/369172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown ZA, Wald A, Morrow RA, Selke S, Zeh J, Corey L. Effect of serologic status and cesarean delivery on transmission rates of herpes simplex virus from mother to infant. JAMA : the journal of the American Medical Association. 2003;289(2):203–9. doi: 10.1001/jama.289.2.203. [DOI] [PubMed] [Google Scholar]
- 8.Looker KJ, Magaret A, Turner KME, Vickerman P, Gottlieb SL, Newman LM. Global estimates of prevalent and incident herpes simplex virus type 2 infections in 2012. PloS one. 2015;1(10):e114989. doi: 10.1371/journal.pone.0114989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Looker KJ, Magaret A, May MT, et al. Global and regional estimates of prevalent and incident herpes simplex virus type 1 infections in 2012. PloS one. 2015;10(10):e0140765. doi: 10.1371/journal.pone.0140765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Looker KJ, Garnett GP, Schmid GP. An estimate of the global prevalence and incidence of herpes simplex virus type 2 infection. Bulletin of the World Health Organization. 2008;86(10):805–12. doi: 10.2471/BLT.07.046128. A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Looker KJ, Garnett GP. A systematic review of the epidemiology and interaction of herpes simplex virus types 1 and 2. Sexually transmitted infections. 2005;81(2):103–7. doi: 10.1136/sti.2004.012039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith JS, Robinson NJ. Age-specific prevalence of infection with herpes simplex virus types 2 and 1: a global review. The Journal of infectious diseases. 2002;186(Suppl 1):S3–28. doi: 10.1086/343739. [DOI] [PubMed] [Google Scholar]
- 13.Pena KC, Adelson ME, Mordechai E, Blaho JA. Genital herpes simplex virus type 1 in women: detection in cervicovaginal specimens from gynecological practices in the United States. Journal of clinical microbiology. 2010;48(1):150–3. doi: 10.1128/JCM.01336-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Handel S, Klingler EJ, Washburn K, Blank S, Schillinger JA. Population-based surveillance for neonatal herpes in New York City, April 2006– September 2010. Sexually transmitted diseases. 2011;38(8):705–11. doi: 10.1097/OLQ.0b013e31821b178f. [DOI] [PubMed] [Google Scholar]
- 15.Kropp RY, Wong T, Cormier L, et al. Neonatal herpes simplex virus infections in Canada: results of a 3-year national prospective study. Pediatrics. 2006;117(6):1955–62. doi: 10.1542/peds.2005-1778. [DOI] [PubMed] [Google Scholar]
- 16.United Nations, Department of Economic and Social Affairs, Population Division. [(accessed 23/04/2014)];Population Estimates and Projections Section. http://esa.un.org/unpd/wpp/unpp/panel_indicators.htm.
- 17.Phipps W, Saracino M, Magaret A, et al. Persistent genital herpes simplex virus-2 shedding years following the first clinical episode. The Journal of infectious diseases. 2011;203(2):180–7. doi: 10.1093/infdis/jiq035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Delaney S, Gardella C, Saracino M, Magaret A, Wald A. Seroprevalence of herpes simplex virus type 1 and 2 among pregnant women, 1989–2010. JAMA : the journal of the American Medical Association. 2014;312(7):746–7. doi: 10.1001/jama.2014.4359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ashley RL, Eagleton M, Pfeiffer N. Ability of a rapid serology test to detect seroconversion to herpes simplex virus type 2 glycoprotein G soon after infection. Journal of clinical microbiology. 1999;37(5):1632–3. doi: 10.1128/jcm.37.5.1632-1633.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ashley-Morrow R, Krantz E, Wald A. Time course of seroconversion by HerpeSelect ELISA after acquisition of genital herpes simplex virus type 1 (HSV-1) or HSV-2. Sexually transmitted diseases. 2003;30(4):310–4. doi: 10.1097/00007435-200304000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Xu F, Gee JM, Naleway A, et al. Incidence of neonatal herpes simplex virus infections in two managed care organizations: implications for surveillance. Sexually transmitted diseases. 2008;35(6):592–8. doi: 10.1097/OLQ.0b013e3181666af5. [DOI] [PubMed] [Google Scholar]
- 22.Mark KE, Kim HN, Wald A, Gardella C, Reed SD. Targeted prenatal herpes simplex virus testing: can we identify women at risk of transmission to the neonate? American journal of obstetrics and gynecology. 2006;194(2):408–14. doi: 10.1016/j.ajog.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 23.Morris SR, Bauer HM, Samuel MC, Gallagher D, Bolan G. Neonatal herpes morbidity and mortality in California, 1995–2003. Sexually transmitted diseases. 2008;35(1):14–8. [PubMed] [Google Scholar]
- 24.Mahnert N, Roberts SW, Laibl VR, Sheffield JS, Wendel GD., Jr The incidence of neonatal herpes infection. American journal of obstetrics and gynecology. 2007;196(5):e55–6. doi: 10.1016/j.ajog.2006.10.911. [DOI] [PubMed] [Google Scholar]
- 25.Hemelaar SJ, Poeran J, Steegers EA, van der Meijden WI. Neonatal herpes infections in The Netherlands in the period 2006–2011. J Matern Fetal Neonatal Med. 2015;28(8):905–9. doi: 10.3109/14767058.2014.937691. [DOI] [PubMed] [Google Scholar]
- 26.Jones CA, Raynes-Greenow C, Isaacs D Neonatal HSV Study Investigators, Contributors to the Australian Paediatric Surveillance Unit. Population-based surveillance of neonatal herpes simplex virus infection in Australia, 1997–2011. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2014;59(4):525–31. doi: 10.1093/cid/ciu381. [DOI] [PubMed] [Google Scholar]
- 27.Perti T, Nyati M, Gray G, et al. Frequent genital HSV-2 shedding among women during labor in Soweto, South Africa. Infect Dis Obstet Gynecol. 2014:258291. doi: 10.1155/2014/258291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schacker T, Zeh J, Hu HL, Hill E, Corey L. Frequency of symptomatic and asymptomatic herpes simplex virus type 2 reactivations among human immunodeficiency virus-infected men. The Journal of infectious diseases. 1998;178(6):1616–22. doi: 10.1086/314486. [DOI] [PubMed] [Google Scholar]
- 29.Augenbraun M, Feldman J, Chirgwin K, et al. Increased genital shedding of herpes simplex virus type 2 in HIV-seropositive women. Annals of internal medicine. 1995;123(11):845–7. doi: 10.7326/0003-4819-123-11-199512010-00006. [DOI] [PubMed] [Google Scholar]
- 30. [accessed 25/11/2015];The Bill & Melinda Gates Foundation to Fund Disease Surveillance Network in Africa and Asia to Prevent Childhood Mortality and Help Prepare for the Next Epidemic. http://www.gatesfoundation.org/Media-Center/Press-Releases/2015/05/Child-Health-and-Mortality-Prevention-Surveillance-Network.
- 31.Langenberg AG, Corey L, Ashley RL, Leong WP, Straus SE. A prospective study of new infections with herpes simplex virus type 1 and type 2. Chiron HSV Vaccine Study Group. The New England journal of medicine. 1999;341(19):1432–8. doi: 10.1056/NEJM199911043411904. [DOI] [PubMed] [Google Scholar]
- 32.Brown ZA, Selke S, Zeh J, et al. The acquisition of herpes simplex virus during pregnancy. The New England journal of medicine. 1997;337(8):509–15. doi: 10.1056/NEJM199708213370801. [DOI] [PubMed] [Google Scholar]
- 33.Gardella C, Brown Z, Wald A, et al. Risk factors for herpes simplex virus transmission to pregnant women: a couples study. American journal of obstetrics and gynecology. 2005;193(6):1891–9. doi: 10.1016/j.ajog.2005.07.041. [DOI] [PubMed] [Google Scholar]
- 34.Jamieson DJ, Theiler RN, Rasmussen SA. Emerging infections and pregnancy. Emerging infectious diseases. 2006;12(11):1638–43. doi: 10.3201/eid1211.060152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gardella C, Brown Z. Prevention of neonatal herpes. BJOG : an international journal of obstetrics and gynaecology. 2011;118(2):187–92. doi: 10.1111/j.1471-0528.2010.02785.x. [DOI] [PubMed] [Google Scholar]
- 36.Johnston C, Gottlieb SL, Wald A. Status of vaccine research and development of vaccines for herpes simplex virus prepared for WHO PD-VAC. Vaccine. 2016 doi: 10.1016/j.vaccine.2015.12.076. [DOI] [PubMed] [Google Scholar]
- 37.Freeman EE, Weiss HA, Glynn JR, Cross PL, Whitworth JA, Hayes RJ. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS. 2006;20(1):73–83. doi: 10.1097/01.aids.0000198081.09337.a7. [DOI] [PubMed] [Google Scholar]
- 38.Masese L, Baeten JM, Richardson BA, et al. Changes in the contribution of genital tract infections to HIV acquisition among Kenyan high-risk women from 1993 to 2012. AIDS. 2015;29(9):1077–85. doi: 10.1097/QAD.0000000000000646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fisman DN, Lipsitch M, Hook EWr, Goldie SJ. Projection of the future dimensions and costs of the genital herpes simplex type 2 epidemic in the United State. Sexually transmitted diseases. 2002;29(10):608–22. doi: 10.1097/00007435-200210000-00008. [DOI] [PubMed] [Google Scholar]
- 40.GBD 2013 DALYs and HALE Collaborators. Murray CJ, Barber RM, et al. Global, regional, and national disability-adjusted life years (DALYs) for 306 diseases and injuries and healthy life expectancy (HALE) for 188 countries, 1990–2013: quantifying the epidemiological transition. Lancet. 2015;386(10009):2145–91. doi: 10.1016/S0140-6736(15)61340-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gottlieb SL, Low N, Newman LM, Bolan G, Kamb M, Broutet N. Toward global prevention of sexually transmitted infections (STIs): the need for STI vaccines. Vaccine. 2014;32(14):1527–35. doi: 10.1016/j.vaccine.2013.07.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.