Abstract
Despite its basic and translational importance, the neural circuitry supporting the perception of emotional faces remains incompletely understood. Functional imaging studies and chronic lesion studies indicate distinct roles of amygdala and insula in recognition of fear and disgust in facial expressions, whereas intracranial encephalography studies, which are not encumbered by variations in human anatomy, indicate a somewhat different role of these structures. In this paper, we leveraged lesion mapping techniques in individuals with acute right hemisphere stroke to investigate lesions associated with impaired recognition of prototypic emotional faces before significant neural reorganization can occur during recovery from stroke. Right hemisphere stroke patients were significantly less accurate than controls on a test of emotional facial recognition for both positive and negative emotions. Patients with right amygdala or anterior insula lesions had significantly lower scores than other RH stroke patients on recognition of angry and happy faces. Lesion volume within several regions, including right amygdala and anterior insula, each independently contributed to the error rate in recognition of individual emotions. Results provide additional support for a necessary role of right amygdala and anterior insula within a network of regions underlying recognition of facial expressions, particularly those that have biological importance or motivational relevance and have implications for clinical practice.
Keywords: emotion perception, facial recognition, stroke, magnetic resonance imaging, brain mapping
Introduction
The ability to recognize another’s emotion from their facial expression is crucial for effective human interactions in both social and professional realms. Visual recognition of facial expression is an important means to judge emotional tenor and thus evoke an appropriately empathic response1,2. An extensive literature addresses the neurologic basis of emotional facial recognition, using functional imaging studies and chronic lesion studies3–7. However, this literature does not yield entirely consistent conclusions. Several chronic lesion studies demonstrate right hemisphere dominance for emotional facial recognition, or at least processing of certain emotions8,9 (but see10). Functional imaging studies reveal a bilateral network of neural regions that are engaged during emotional facial recognition tasks, and show that distinct areas are specifically activated in response to certain emotions (e.g., anger or disgust)11,12. However, functional imaging studies only show that blood oxygen dependent (BOLD) signal in an area (i.e., areas where blood flow exceeds oxygenation, corresponding to activation of neurons) is correlated with performance on a task, whereas lesion studies are needed to show which of those areas are essential for the task.
Role of the Right Hemisphere
The concept of cortical asymmetry of emotion dates to the nineteenth century work of Hughlings-Jackson (1874/1915)13 who proposed that emotion is lateralized to the right hemisphere. The “right hemisphere hypothesis” traditionally ascribes a greater role in emotion processing, regardless of valence, to the right, rather than the left, hemisphere9,14. The “valence hypothesis” invokes the right hemisphere for negative or unpleasant emotions, and the left hemisphere for positive or pleasant emotions15,16. A somewhat different hypothesis is that primary emotions (e.g., anger, fear, sadness) are preferentially modulated by the right hemisphere, whereas social emotions (e.g., affection, pride, embarrassment) are preferentially processed by the left hemisphere17,18. More recently, Abbott et al.19 proposed that the right hemisphere processes emotional facial expressions from both configural information (global facial qualities) and featural information (constituent aspects of faces, that is eyes, mouth depicted in the partial faces), while the left hemisphere processes emotional facial expressions from primarily featural facial information. Interestingly, using eye tracking, Thomas, Wignall, Loetscher, and Nicholls20 found participants fixated more on right side of the mouth when judging happiness, and fixated more on the left eye when judging sadness. A recent study combining behavioral categorization of whole and half faces displaying anger, sadness or surprise, computational modeling, and event-related potentials (ERP) revealed additional evidence that expression encoding and emotional assessment require holistic processing, mainly in the right hemisphere21. Together, these studies indicate a critical role of the right hemisphere in (1) global or configural processing of faces, and (2) faces expressing negative emotions, like fear and anger.
Fear and the Amygdala
Both right and left amygdalae are implicated in fear conditioning in animals22 and are activated in humans when fearful faces are viewed11,23,24. A meta-analysis of neuroimaging studies in 1600 healthy individuals revealed bilateral amygdala engagement during processing of fearful faces, and to a lesser degree happy faces and sad faces (right amygdala only)12. A recent fMRI study of 235 male and 235 female adolescents matched for age and handedness revealed significantly stronger right amygdala activation in males compared to females during emotional face perception25. One recent study found that the right amygdala was activated in response to threatening faces, but only in central vision, while the striatum (caudate and putamen) were preferentially activated by threatening faces in peripheral vision26. In single-subject case studies and small case series of individuals with bilateral amygdala damage, impaired recognition of the facial expression of fear was documented27–30. A follow-up study of one patient indicated that the impairment in fear processing can be attributed to inability to use information from the eye region in processing emotional faces31. A recent eye tracking study of three patients with bilateral ventromedial prefrontal cortex damage showed that this damage disrupted attention to the eye region, and interfered with recognition of emotional faces, particularly fear32. In individuals with frontotemporal dementia, atrophy of the right amygdala and anterior cingulate were associated with impaired fear recognition33; however, redirecting attention to the eyes did not improve recognition of emotion34, as it does in some patients with more selective amygdala dysfunction or those with Alzheimer’s disease who have early bilateral amygdala atrophy35.
Although the involvement of the amygdala in processing of fear is replicated in functional imaging studies and studies of neurodegenerative disease, the role of this structure in the processing of other emotions requires further clarification36,37. Studies that specifically investigate the role of the amygdala in other emotions, particularly intense emotions or those that might stimulate an autonomic response, confirm other roles, such as processing anger and joy38–41. Together, these studies indicate that while right and left amygdalae are likely to be components of a network normally engaged in processing fear (also including orbitofrontal, ventromedial prefrontal cortex) and other emotions in faces, the amygdalae are not sufficient for, nor specific to, fear recognition. Some of the inconsistent results between studies regarding the critical role of the right or left amygdala in recognizing fearful faces could be due to: (1) studying patients with lesions at variable times in the course of recovery or adaptation to the lesion42; (2) studying controls with varying degrees of attention to the eyes or use of stimuli that draw varying attention to the eyes43.
Disgust and the Insula
Disgust recognition involves the insula and perhaps components of the basal ganglia. In normal volunteers, one study found significant activation of the right anterior insula associated with processing facial expressions of disgust44. In contrast, other studies of normal volunteers have revealed activation in the left anterior insula, bilateral putamen, and right globus pallidus, caudate nucleus, and superior, middle and inferior posterior temporal gyri45 or bilateral insula, occipital, and fronto-orbital cortex46 in response to facial expressions of disgust. A meta-analysis of healthy participants revealed bilateral insular activation during processing of disgusted faces12. An individual with a stroke involving left anterior insula, posterior insula, putamen and globus pallidus was selectively impaired in recognizing facial expressions of disgust47. In individuals with Huntington’s disease, left insula volume positively correlated with accuracy in disgust recognition48. Similarly, in individuals with frontotemporal dementia, volume of the left insula and left temporal pole correlated with disgust recognition33. However, insula is not only important for recognition of disgust. Accuracy in recognition of angry facial expressions was associated with bilateral posterior insular cortex volume in patients with frontotemporal lobar degeneration49. Furthermore, the insula is not the only area important for recognition of disgust. Impaired recognition of disgusted faces in patients with Huntington’s and Parkinson’s disease was attributed to disruption to impaired subcortical-cortical circuits that include basal ganglia, thalamus, and right cortical regions50,51.
Consistent with the lesions studies, the insula is also activated in response to other emotions37. One meta-analysis indicated left insula showed activation in response to fearful faces, but was more sensitive to disgust than fear12. These studies indicate that both left and right insula are likely to be involved in processing facial expression of disgust, but the relationship is neither specific to the insula (other lesions can cause impaired processing of disgust, as noted for Huntington’s disease and Parkinson’s disease), nor specific to disgust (insula is involved in processing other emotions). Again, some of the inconsistencies across lesion studies could be due to studying patients at variable times after onset of the lesion, with variable opportunity for structure-function reorganization or accommodation to the lesion.
Evidence against Specificity of Amygdala and Insula for Fear and Disgust
Additional evidence against specificity of these structures for fear as well as disgust, comes from intracranial electroencephalography (iEEG), in which activity from individual neurons or groups of neurons is recorded, indicating that the amygdala activation observed in response to fearful faces may reflect the amygdala’s role in encoding emotionally relevant stimuli52. Unlike imaging, iEEG is not encumbered by normal variations in human anatomy, a limitation of imaging studies. One iEEG study demonstrated that single-neurons in the amygdala spiked in response to emotional faces, but not exclusively fearful faces53. Likewise, Rutishauser et al.54 did not find differential spiking to fearful faces versus other emotional faces in the amygdala. An ERP study also showed late differential responses in the amygdala to both fearful and disgusted faces, compared to neutral and happy faces55. Together, these results indicate that the amygdala may be critical to encoding the emotional relevance or biological importance of facial expressions (see also11), and the right amygdala may be particularly critical for aversive emotional faces, important in the recognition of imminent danger. The insula, which has many connections to the limbic system, including the amygdala, may similarly be part of a complex cortico-limbic-autonomic network underlying recognition of the emotional relevance of facial expression. However, there have been inconsistencies across studies regarding the necessary role of the insula and the amygdala in recognizing emotional faces other than disgust and fear, and less consistent areas of activation associated with processing of anger, sadness, and happiness in functional imaging studies11,37,56,57 (see Table 1 for summary).
Table 1.
Summary of Associations between Amygdala and Insula and Emotional Facial Expressions
| Happy | Joy | Angry | Disgust | Fear | Sad | |
|---|---|---|---|---|---|---|
| Right Amygdala | Fusar-Poli et al, 200912 | Kumfor et al, 201333 | Fusar-Poli et al, 200912 | |||
| Bilateral Amygdala | Milesi et al, 201440 | Milesi et al, 201440 | Krolak-Salmon et al, 200455 | Breiter et al, 199611; Fusar-Poli et al, 200912; Pare et al, 200423; Lindquist et al, 201224; Adolphs et al, 199427; 199528; Calder et al, 199629; Sprengelmeyer atl, 199030; Milesi et al, 201440; Krolak-Salmon et al, 200455 | ||
| Right Insula | Philippi et al, 200944 | |||||
| Left Insula | Kumfor et al, 201333; Phillips et al, 199845; Calder et al, 200047; Kipps et al, 200748 | Fusar-Poli et al, 200912 | ||||
| Bilateral Insula | Omar et al, 201049 | Fusar-Poli et al, 200912; Jehna et al, 200146 |
One problem in studying emotional facial recognition with iEEG or functional imaging is that these studies can only reveal areas that are engaged in the task, not areas that are critical for the task58. Small changes in task demands or control conditions can result in differences in the areas where activity is significantly associated with a particular task, which may account for conflicting results. Lesion studies are needed to determine if a particular area is essential for the function. However, chronic lesion studies may fail to reveal regions necessary for recognition of basic emotions such as happiness and sadness, because these functions may recover quickly after unilateral lesions.
In this paper, data are presented to show brain lesions associated with impaired recognition of emotional facial expressions before significant neural reorganization can occur during recovery from acute right hemisphere stroke. Participants viewed faces of prototypic emotions and were asked to identify the emotional label in a seven-forced-choice response format. MRI images were analyzed to investigate lesions in the right hemisphere that contributed to impaired performance on recognition of particular emotions in facial expression. We hypothesized that acute lesions in right amygdala and right anterior insula are associated with impaired recognition of motivationally relevant (including aversive) emotional facial expressions, and that other right cortical lesions differentially contribute to recognition of distinct emotions in facial expression.
Materials and Methods
Participants
Thirty patients with right hemisphere (RH) stroke (mean age = 52.8 ± 12.1 years; 13 female; mean education = 14.4 ± 2.5 years) and 30 healthy controls (mean age = 50.5 ± 14.6 years; 15 female; mean education = 15.6 ± 2.6 years) were enrolled. Patients and controls were not significantly different in age (t(60) = −0.7, p = 0.49), education (t(60) = +1.43, p = 0.16), or gender (Fisher’s Exact (FE): p = 0.80). Patients and controls provided informed consent to participate in the study under a protocol approved by the Institutional Review Board of the Johns Hopkins University. Participants had none of the following exclusion criteria: (1) prior neurological disease; (2) reduced level of consciousness or on-going sedation; (3) uncorrected hearing or vision impairment; (4) lack of premorbid competency in English; and (5) failure to follow task directions. Patients were also excluded if they were unable to have MRI due to claustrophobia, implanted ferrous metal, or weight >300 lb. Stroke patients were recruited from the inpatient service in the hospital. Controls were recruited from a convenience sample in the community.
Imaging and Image Processing
For each participant, we obtained an MRI within 24 hours of admission to the hospital for acute ischemic stroke. Images were processed according to procedures published previously59–62.
Facial Expression Task
Integrity of recognition of emotions was examined from static facial expressions in an emotion categorization task as described in an earlier study from our lab63. Faces expressing one of seven basic emotions (happy, surprise, angry, disgust, fear, sad, neutral) were presented centrally one at a time using color photographs. Participants viewed these faces of prototypic emotions and were asked to identify the emotional label in a seven-forced-choice response format (alternatives: happy, surprise, angry, disgust, fear, sad, neutral). There were eight exemplars of each emotion (each emotion depicted by each of eight actors/actresses), for a total of 56 trials. The stimuli for facial expressions were selected from a set of perceptually validated pictures including individuals of different genders and races64. Understanding of the emotional labels and ability to use the computerized response box were confirmed prior to testing. Response time was unlimited, but participants were encouraged to respond as quickly as possible.
Statistical Analysis
Patterns of performance on the emotional face recognition test by RH stroke patients and controls were compared quantitatively and qualitatively. We compared performance of RH stroke patients and controls using unpaired t-tests (STATA version 12) and Fisher’s Exact Test (Social Science Statistics, http://www.socscistatistics.com). We compared recognition of specific emotions of patients with lesions including right amygdala and right anterior insula to RH stroke patients without lesions in the right amygdala or right anterior insula also using unpaired t-tests and Fisher’s Exact Test. We identified cut-off scores for normal performance on the facial recognition task based on the performance of our 30 controls who were of comparable age, gender, and education as our stroke patients. The cut-off scores were >2 SD below the mean for controls, which would be outside the range of normal for this population. Multiple regression analyses were carried out to investigate whether additional gray or white matter lesions in the right hemisphere contribute to impaired performance on recognition of particular emotions in facial expression.
Results
Controls versus RH Patients
RH stroke patients were significantly less accurate than controls in identifying all positive and negative emotional facial expressions and neutral expressions (i.e., happy, surprise) (mean 82.9% vs. 92.9% correct; t(58) = 3.1760; p = 0.0024), negative or aversive emotions (i.e., angry, disgust, fear, sad) (mean 58.9% vs. 77.5% correct; t(58) = 4.5; p < 0.0001), and neutral emotional faces (mean 82.1 % vs. 98.4 % correct; t(58) = 2.7; p = 0.0094). Positive facial expressions were identified correctly more frequently than negative facial expressions for 28/30 (93%) patients and all controls. (Table 2).
Table 2.
Mean Percent Correct for RH Stroke Patients and Controls on Facial Recognition
| Facial Expressions | Mean (SD) | T value | df | P value | |
|---|---|---|---|---|---|
| RH Stroke N = 30 |
Controls N = 30 |
||||
| Positive | 0.83 (0.16) | 0.93 (0.05) | 3.1760 | 58 | 0.0024* |
| Negative | 0.59 (0.21) | 0.78 (0.10) | 4.478 | 58 | 0.0000* |
| Happy | 0.94 (0.14) | 0.99 (0.03) | 2.0371 | 58 | 0.046* |
| Surprise | 0.72 (0.27) | 0.86 (0.11) | 2.6589 | 58 | 0.010* |
| Angry | 0.63 (0.29) | 0.83 (0.17) | 3.2183 | 58 | 0.0021* |
| Disgust | 0.75 (0.28) | 0.88 (0.18) | 2.0655 | 58 | 0.043* |
| Fear | 0.34 (0.25) | 0.54 (0.21) | 3.3163 | 58 | 0.0016* |
| Sad | 0.63 (0.27) | 0.85 (0.12) | 4.1451 | 58 | 0.0001* |
| Neutral | 0.82 (0.31) | 0.98 (0.07) | 2.6856 | 58 | 0.0094* |
The mean score for healthy controls on positive faces was 92.9%±5.4 correct. All controls and 19/30 (63%) RH stroke patients scored within 2 SD of the mean for healthy controls (i.e., ≥ 82.1%) for positive emotions. The mean score for healthy controls on aversive faces was 77.5%±9.8 correct. All but one of the controls and 15/30 (50%) RH stroke patients scored within 2 SD of the mean for healthy controls (i.e., ≥ 57.9%). RH stroke was strongly associated with significant impairment in recognizing both positive and negative/aversive faces (Fisher’s Exact (FE) = 0.0001 for both, p < 0.05).
Effects of Right Amygdala Lesions
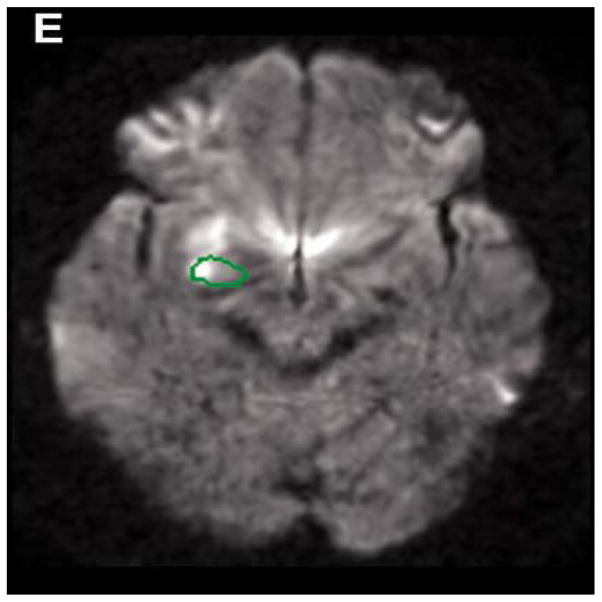
Patients with right amygdala lesions (Figure 1) had significantly lower scores than RH stroke patients without lesions in the amygdala and insula on recognition of happiness (mean 82.1% vs. 98.6% correct; t(23) = 3.1; p = 0.0028) and anger (mean 48.2% vs. 73.6% correct; t(23) = 2.1; p = 0.023). Furthermore, more patients with right amygdala lesions compared to patients without right amygdala lesions were impaired in recognizing happy faces. The mean score for healthy control on happy faces was 98.6%±4.0. In patients with right amygdala lesions, 4/7 (57%) scored more than 2 SD below the mean for healthy controls on happy faces (i.e., scored below 94.6%), whereas only 2/18 (11%) RH stroke patients without right amygdala or insula lesions scored more than 2 SD below the mean score for healthy controls on happy faces (FE = 0.03, p < 0.05).
Figure 1.
Infarction in the Right Amygdala
Also, more patients with right amygdala lesions were significantly impaired in recognizing angry faces than patients without amygdala lesions. The mean score for healthy controls on angry faces was 83.2%±17.3. Among patients with right amygdala lesions, 4/7 (57%) scored more than 2 SD below the mean for healthy controls on angry faces (i.e., < 48.6%) (FE: = 0.03; p < 0.05), whereas 2/18 (11%) RH stroke patients without right amygdala or insula lesions scored more than 2 SD below the mean score for healthy controls on angry faces.
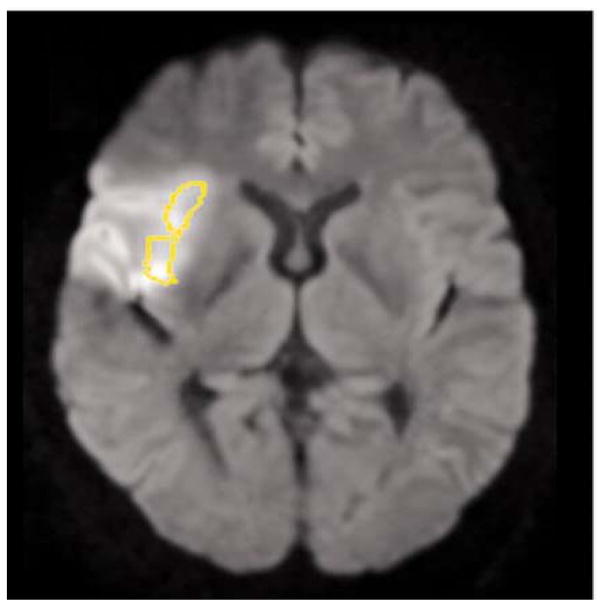
Effects of Right Anterior Insular Lesions
Patients with right anterior insula lesions (Figure 2) had significantly lower scores than RH stroke patients without lesions in the amygdala and insula on recognition of happiness (mean 87.5% vs. 98.6% correct; t(27) = 2.2; p = 0.035) and anger (mean 47.7% vs. 73.6% correct; t(27) = 2.6; p = 0.0083). Furthermore, more patients with right anterior insula lesions were associated with significant impairment in angry faces, compared to patients without insular lesions. The mean score for healthy controls on angry faces was 83.2%±17.3. Among patients with right anterior insula lesions, 6/11 (55%) scored more than 2 SD below the mean for healthy controls on angry faces (i.e., below 48.6%), whereas 2/18 (11%) RH stroke patients without right amygdala or insula lesions scored more than 2 SD below the mean score for healthy controls on angry faces (FE = 0.0281; p < 0.05).
Figure 2.
Infarction in the Right Anterior Insula
Contribution of Specific Gray and White Matter Structures
We tested the hypothesis that acute lesions to distinct areas of the brain differentially affect recognition of facial expression of individual emotions by running multivariable regression analyses, with accuracy (percent correct) in recognition of facial expressions of individual emotions (e.g., happiness) as the dependent variable, and the percent damage to individual gray and white matter structures in the JHU-MNI atlas as the independent variables.
The model that best accounted for recognition of happy facial expressions included percent damage to thalamus, caudate, superior temporal gyrus (STG), orbitofrontal gyrus, STG pole, middle temporal gyrus, middle temporal gyrus pole, inferior temporal gyrus, anterior insula, amygdala, putamen, globus pallidus, genu of the corpus callosum, inferior fronto-occipital fasciculus, sagittal stratum, and uncinate (r2 = 0.93; adjusted r2 = 0.90; p < 0.0001). Medial orbitofrontal gyrus, anterior cingulate cortex, and fusiform gyrus were omitted because of collinearity. All variables independently contributed to accuracy rate in recognition of happy faces except thalamus, caudate, and STG. The percent of damage to the same areas accounted for recognition of surprised faces (r2 = 0.41; adjusted r2 = 0.19; p = 0.049), neutral faces (r2 = 0.66; adjusted r2 = 0.53; p < 0.0001), angry faces (r2 = 0.50; adjusted r2 = 0.31; p = 0.0051), and sad faces (r2 = 0.47; adjusted r2 = 0.28; p = 0.01), although the areas each carried a different “weight” in recognizing each emotion.
There were no models in which higher percent damage to individual areas in combination or alone accounted for lower accuracy in recognition of facial expressions of disgust or fear. However, a significant impairment (>2 SD below the mean for normal controls) in recognizing disgusted facial expressions was associated with lesion to the anterior insula (FE: p = 0.049) or inferior fronto-occipital fasciculus (FE: p = 0.03)
Discussion
Overall, and as expected, RH stroke patients performed significantly more poorly than controls in recognizing emotional faces. This result is consistent with functional imaging studies and lesion data showing a dominant role of the right hemisphere in processing (at least some) emotional facial expressions65. Negative or aversive emotions were identified with lower accuracy than positive or neutral emotions by both RH stroke patients and controls (as shown in Table 1). Moreover, there was a greater difference between RH stroke patients and controls on aversive faces than positive and neutral faces, even though there was higher power for (more instances of) positive than negative emotions.
The finding that some facial expressions are more difficult to identify than others is consistent with previous behavioral research, showing more accurate identification of happy expressions than negative expressions66,67. This phenomenon is attributed to happy facial expressions being promptly identified based on its unique feature, the smile; whereas other non-happy facial expressions have less distinctive or confusing features complicating their identification68,69, or require more global or configural processing which is particularly challenging after right hemisphere lesions.
Importantly, not all patients with right hemisphere lesions were impaired in recognition of emotional faces. Nine patients with right hemisphere lesions scored less than 2SD below the mean for controls. Only the subset with focal lesions in critical areas, such as amygdala or anterior insula, had significantly impaired in recognition of emotions from faces. Our results are consistent with iEEG results indicating that the amygdala and insula are especially important in recognition of aversive facial expressions or perhaps facial expressions that have behavioral relevance. In our study, patients with amygdala or anterior insula damage had significantly more difficulty than other RH stroke patients in recognition of anger. Multivariable regression analysis also confirmed that the degree of damage to the right amygdala and right anterior insula was independently associated with error rate in recognition of certain aversive faces. Our results are consistent with some fMRI, ERP, and lesion studies indicating a role of the amygdala (and anterior insula) in discriminating the emotional relevance of the stimuli, rather than recognizing only specific emotions37,52.
Patients with amygdala or anterior insular damage were also impaired in recognition of happy faces, and the degree of damage to these areas was independently associated with the severity of impairment. These findings are in line with lesion study showed that patients with anterior temporal lobectomy confused joyful faces with neutral faces40. An fMRI study showed that execution of happy facial expressions led to significantly stronger right amygdala activation than execution of the non-emotional or neutral facial expressions70. However, most studies have found it difficult to identify lesions associated with impaired recognition of happy faces, or areas activated specifically with recognition of happy faces. Our study may be novel because we studied patients acutely after stroke, before the opportunity for recovery, or before other areas of the brain assume the function of the right amygdala in this critical social function. We identified other gray and white matter areas where acute damage independently contributed to error rate in recognition of happy faces, including right superior and middle temporal pole, uncinate fasciculus, and inferior fronto-occipital fasciculus. It is possible that if some of these areas are spared, they may be able to rapidly assume the role of the right amygdala in recognition of happy faces (accounting for variability across chronic lesion studies).
We confirmed a role of the anterior insula in recognition of disgust, but also found that anterior insula was critical in recognition of other emotional facial expressions, including anger. Similar results have been reported in preclinical Huntington’s disease, in which volume of insula was associated with accuracy in recognition of negative emotional faces, but not limited to faces of disgust71. The anterior insula has widespread connections to orbitofrontal and other limbic areas, making it a plausible critical link in emotional processing. Again, its role may be duplicated by the left anterior insula, or other regions, such that other areas can quickly assume its function in recovery after unilateral stroke. The strong association we and others have identified between lesions in right anterior insula and emotional face processing could be at least partly responsible for its role in emotional empathy62.
We were not able to show a strong association between damage to right amygdala and fear, likely because all right hemisphere stroke patients showed low accuracy in recognizing fear from photographs of faces. Other studies have shown that it is difficult to differentiate fear and surprise in facial expressions, particularly out of context72. Our negative results should not be taken as evidence against the role of right amygdala and fear recognition, but further evidence of the complexity of showing fear through facial expression alone.
We showed that the percent damage to the right temporal pole and orbitofrontal cortex independently contributed to predicting lower accuracy in recognizing happy, surprise, and neutral faces, consistent with a role of these areas in emotional recognition from faces reported in lesion studies33,73 and functional imaging studies56. Likewise, we confirmed a role of the right inferior fronto-occipital fasciculus and uncinate fasciculus in recognizing happy, surprised, sad, and neutral emotion from facial expressions44,74. We are not claiming these structures are important only for a subset of emotions. The significant results for a subset of emotions may reflect the range of performance across patients with and without damage to these structures. Greater power might reveal associations between damage to these structures and impairment in recognizing other emotions.
This study has several limitations. We studied acute stroke patients, allowing us the opportunity to investigate performance before recovery occurs. While the study of individuals with chronic stroke is complicated by the influence of neural reorganization and compensations, the study of individuals with acute stroke may be complicated by the influence of diaschisis. Convergence of evidence is vital in clarifying controversial issues. As noted previously, the results of this study were consistent with prior functional imaging studies. Another limitation of this study was the fact that we did not evaluate performance by left hemisphere stroke patients (because patients with acute lesions involving left amygdala and anterior insula typically have impaired comprehension and cannot reliably perform the task). Future studies will evaluate emotional facial recognition using nonverbal tasks (e.g., skin conductance response and priming tasks) with left and right left hemisphere stroke and controls, so that we do not have to exclude patients with verbal comprehension deficits (and the lesions of interest in the left hemisphere). An additional limitation is that we did not evaluate patients’ assessments of the valence or emotional relevance of the stimuli, or assess our participants at later time points to investigate change over time. Future research will also address these limitations. We also included relatively small numbers of patients with specific lesions (8 with amygdala and 11 with anterior insular damage), which limited the types of analyses we could conduct. Finally, we measured accuracy in facial expression rather than reaction time. Reaction time is very variable in acute stroke, and measuring reaction time would not have allowed the clinician to help the patient find the button corresponding to the spoken name of the emotion (when patients named aloud the emotion spontaneously), to compensate for any hemispatial neglect.
Clinical Implications
Despite its limitations, results of our study have implications for clinical practice and research. Deficits in recognition of facial expression caused by particular right hemisphere lesions have implications for interpersonal interactions. Individuals with lesions in amygdala, insula, temporal pole, orbitofrontal cortex, fronto-occipital fasciculus or uncinate may make incorrect assessments of another’s affective state and respond inappropriately, resulting in social isolation, as demonstrated in a variety of disease states75–77. Blonder et al.7 showed that after RH stroke, the inability to recognize facial expressions is associated with decreased marital satisfaction. Behavioral therapy to promote social cognition, such as facial expression recognition, has received little attention in the stroke population. Therapy to improve facial expression recognition may be indicated in the right hemisphere stroke population, particularly those with amygdala and insula damage. Adolphs et al.31 reported that simply instructing a patient with bilateral amygdala damage to attend to the eyes improved recognition of fear in faces. Patients with other lesions may need other interventions; future studies must identify the specific roles of individual structures in the complex network underlying recognition of emotions from faces78. This study contributes some novel evidence regarding the critical structures within this network.
Acknowledgments
This work was supported by the National Institutes of Health through NINDS award RO1NS047691 (to AEH), and through NICHD award R01 HD065955 (to KO). The content is solely the responsibility of the authors and does not necessarily represent the views the National Institutes of Health. Salary support was provided to DCT through NIDCD R01DC005375 and R01DC011317; CD through NIDCD R01DC005375; YG through NINDS RO1NS047691; Salary support was provided to AEH by NIDCD R01 DC005375, NINDS R01NS047691, and NIDCD P50DC 014664.
Biographies
Donna C. Tippett, MA, MPH, CCC-SLP is an Associate Professor in the Department of Neurology, Department of Otolaryngology—Head and Neck Surgery, Neurology, and Department of Physical Medicine and Rehabilitation at the Johns Hopkins University School of Medicine. She earned a Master of Arts in Speech-Language Pathology from the University of Maryland and a Master of Public Health from the Johns Hopkins Bloomberg School of Public Health. She is an ASHA-certified speech-language pathologist and sees patients in the Johns Hopkins Outpatient Center. She is also an adjunct faculty member at the University of Maryland, Towson University, and the University of the District of Columbia where she teaches neuroanatomy, medical speech-language pathology, and dysphagia courses. Her research interests include patterns of longitudinal decline in primary progressive aphasia, treatment of word finding deficits in this population, and brain-behavior relationships in emotional empathy.
Brittany R. Godin, MS, CCC-SLP is an ASHA-certified speech-language pathologist licensed in Maryland and the District of Columbia. She works at the University of Maryland Charles Regional Medical Center in La Plata and Medstar Georgetown University Hospital, in Washington, DC, specializing in diagnostic and treatment of adults with neurogenic speech, language, cognitive, voice, and swallowing disorders in both acute inpatient and outpatient settings. Her research interests include management of voice disorders in professional voice users and dysphagia management in critical care patients.
Kumiko Oishi, PhD is an Assistant Research Scientist in the Center for Imaging Science, Whiting School of Engineering, Johns Hopkins University. She earned a Bachelor of Science from the Nagoya City University, a BMS from Kobe University School of Medicine, and a PhD from the Kobe University School of Science and Technology, Kobe, Japan.
Kenichi Oishi, MD, PHD is an Associate Professor in the Department of Radiology and Radiological Science at the Johns Hopkins University School of Medicine. He specializes in magnetic resonance research. Dr. Oishi completed two residencies, one in internal medicine at Kobe City General Hospital, Kobe, Japan, and another in neurology at the National Center of Neurology and Psychiatry, Tokyo, Japan. After working as a research and teaching assistant in the Department of Clinical Molecular Medicine at Kobe University, he joined the faculty there as an Assistant Professor of Neurology in 2005. He accepted a position as a Research Associate in the Department of Radiology and Radiological Science at Johns Hopkins in 2008, and in 2009 he joined the faculty as an Assistant Professor. Dr. Oishi has published more than 70 journal articles, two book chapters and one book. His research has garnered several grants from the National Institutes of Health, and he holds three patents. He is a member of several professional organizations, including the American Academy of Neurology and the International Society of Magnetic Resonance in Medicine.
Cameron Davis, MS is a Clinical Research Associate at PRA Health Sciences and Merck Pharmaceuticals. She earned a Masters of Biotechnology from the Johns Hopkins University, a Graduate Certificate in Principles of Public Health from the University of Maryland, College Park, and Bachelor of Science from Delaware State University.
Yessenia Gomez, MIPH is a Clinical Trials Assistant at The George Institute for Global Health in Newtown, Australia. She earned a Masters of International Public Health from the University of Sydney, a Bachelor of Science in Biological Sciences from Florida International University, and a certificate in global health from the University of Maryland.
Lydia Trupe, MPH is an Associate at ideas42, where she is currently working on applying behavioral science to international health issues, including family planning and reproductive health and the prevention of intimate partner violence. She completed her Master in Public Health at the University of Cape Town, where her research focused on the use of environmental design to prevent community violence. She earned a Bachelor of Art in political science from the George Washington University with a minor in public health, and she has conducted research on a diverse range of topics from stroke risk awareness in Samoa to uptake of breast cancer screening in South Africa.
Eun Hye Kim, BS, BA earned a Bachelor of Science in Biochemistry and Bachelor of Art in Music, and is currently pursuing her MD at The Ohio State University College of Medicine.
Argye E. Hillis, MD, MA, is a Professor of Neurology, with joint faculty appointments in Physical Medicine and Rehabilitation and in Cognitive Science at Johns Hopkins University. Dr. Hillis serves as the Executive Vice Chair of the Department of Neurology, and Director of the Cerebrovascular Division of Neurology Prior to medical training and neurology residency, Argye worked as a speech-language pathologist, and conducted clinical research focusing on understanding and treating aphasia and hemispatial neglect. She has brought these areas of experience to impact on her clinical research in neurology, which involves cognitive and neuroimaging studies of aphasia and cognitive deficits due to right hemisphere stroke and focal dementias. Her current research combines longitudinal task-related and task-free functional imaging and structural imaging from the acute stage of stroke through the first year of recovery, with detailed cognitive and language assessments to improve our understanding how language and other cognitive and emotional functions recover after stroke.
References
- 1.Dimberg U, Thunberg M, Elmehed K. Unconscious facial reactions to emotional facial expressions. Psychol Sci. 2000;11(1):86–9. doi: 10.1111/1467-9280.00221. [DOI] [PubMed] [Google Scholar]
- 2.Petrides K, Furnham A. Trait emotional intelligence: behavioural validation in two studies of emotion recognition and reactivity to mood induction. Eur J Pers. 2003;17(1):39–57. doi: 10.1002/per.466. [DOI] [Google Scholar]
- 3.Braun M, Traue H, Frisch S, Deighton R, Kessler H. Emotion recognition in stroke patients with left and right hemispheric lesion: Results with a new instrument—the FEEL Test. Brain Cogn. 2005;58(2):193–201. doi: 10.1016/j.bandc.2004.11.003. S0278-2626(04)00312-4 [pii] [DOI] [PubMed] [Google Scholar]
- 4.Cheung C, Lee T, Yip J, King K, Li L. The differential effects of thalamus and basal ganglia on facial emotion recognition. Brain Cogn. 2006;61(3):262–68. doi: 10.1016/j.bandc.2006.01.008. S0278-2626(06)00024-8 [pii] [DOI] [PubMed] [Google Scholar]
- 5.Pell M. Cerebral mechanisms for understanding emotional prosody in speech. Brain Lang. 2006;96(2):221–34. doi: 10.1016/j.bandl.2005.04.007. S0093-934X(05)00086-6 [pii] [DOI] [PubMed] [Google Scholar]
- 6.Harciarek M, Heilman K. The contribution of anterior and posterior regions of the right hemisphere to the recognition of emotional faces. Journal of Clinical and Experimental Neuropsychology. 2009;31(3):322–30. doi: 10.1080/13803390802119930. [DOI] [PubMed] [Google Scholar]
- 7.Blonder L, Pettigrew L, Kryscio R. Emotion recognition and marital satisfaction in stroke. J Clin Exp Neuropsychol. 2012;34(6):634–42. doi: 10.1080/13803395.2012.667069. [DOI] [PubMed] [Google Scholar]
- 8.Bowers D, Bauer R, Coslett H, Heilman K. Processing of faces by patients with unilateral hemisphere lesions. Brain Cogn. 1985;4(3):258–72. doi: 10.1016/0278-2626(85)90020-x. 0278-2626(85)90020-X [pii] [DOI] [PubMed] [Google Scholar]
- 9.Blonder L, Bowers SD, Heilman K. The role of the right hemisphere in emotional communication. Brain. 1991;114(3):1115–27. doi: 10.1093/brain/114.3.1115. [DOI] [PubMed] [Google Scholar]
- 10.Abbott J, Wijeratne T, Hughes A, Perre D, Lindell A. The influence of left and right hemisphere brain damage on configural and featural processing of affective faces. Laterality: Asymmetries of Body. Brain Cogn. 2013;19(4):455–72. doi: 10.1080/1357650X.2013.862256. [DOI] [PubMed] [Google Scholar]
- 11.Breiter H, Etcoff N, Whalen P, Kennedy W, Rauch S, Buckner R, et al. Response and habituation of the human amygdala during visual processing of facial expression. Neuron. 1996;17(5):875–87. doi: 10.1016/s0896-6273(00)80219-6. S0896-6273(00)80219-6 [pii] [DOI] [PubMed] [Google Scholar]
- 12.Fusar-Poli P, Placentino A, Carletti F, Landi P, Allen P, Surguladze S, et al. Functional atlas of emotional faces processing: A voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. J Psychiatry Neurosci. 2009;34(6):418–32. [PMC free article] [PubMed] [Google Scholar]
- 13.Hughlings-Jackson J. On the nature of duality of the brain. Brain. 1874/1915;38:96–103. [Google Scholar]
- 14.Borod J. The Neurophysiology of Emotion. New York: Oxford University Press; 2000. [Google Scholar]
- 15.Yip JT, Leung KK, Li LS, Lee TM. The role of sub-cortical brain structures in emotion recognition. Brain Inj. 2004;18(12):1209–17. doi: 10.1080/02699050410001719916. [DOI] [PubMed] [Google Scholar]
- 16.Braun M, Traue HC, Frisch S, Deighton RM, Kessler H. Emotion recognition in stroke patients with left and right hemispheric lesion: Results with a new instrument-the FEEL test. Brain Cogn. 2005;58(2):193–201. doi: 10.1016/j.bandc.2004.11.003. S0278-2626(04)00312-4 [pii] [DOI] [PubMed] [Google Scholar]
- 17.Langer S, Pettigrew L, Wilson J, Blonder L. Personality and social competency following unilateral stroke. J Int Neuropsychol Soc. 1998;4(05) doi: 10.1017/s1355617798455048. [DOI] [PubMed] [Google Scholar]
- 18.Davidson RJ, Shackman AJ, Maxwell JS. Asymmetries in face and brain related to emotion. Trends in Cogn Sci. 2004;8(9):389–91. doi: 10.1016/j.tics.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 19.Abbott JD, Wijeratne T, Hughes A, Perre D, Lindell AK. The influence of left and right hemisphere brain damage on configural and featural processing of affective faces. Laterality. 2014;19(4):455–72. doi: 10.1080/1357650X.2013.862256. [DOI] [PubMed] [Google Scholar]
- 20.Thomas NA, Wignall SJ, Loetscher T, Nicholls ME. Searching the expressive face: Evidence for both the right hemisphere and valence-specific hypotheses. Emotion (Washington, DC) 2014;14(5):962–77. doi: 10.1037/a0037033. [DOI] [PubMed] [Google Scholar]
- 21.Calvo MG, Beltrán D. Brain lateralization of holistic versus analytic processing of emotional facial expressions. Neuroimage. 2014;92:237–247. doi: 10.1016/j.neuroimage.2014.01.048. [DOI] [PubMed] [Google Scholar]
- 22.Hitchcock J, Davis M. Lesions of the amygdala, but not of the cerebellum or red nucleus, block conditioned fear as measured with the potentiated startle paradigm. Behav Neurosci. 1986;100(1):11–22. doi: 10.1037/0735-7044.100.1.11. [DOI] [PubMed] [Google Scholar]
- 23.Pare D, Quirk GK, LeDoux JE. New vistas on amygdala networks in conditioned fear. J Neurophysiol. 2004;92(1):1–9. doi: 10.1152/jn.00153.2004. [DOI] [PubMed] [Google Scholar]
- 24.Lindquist K, Wager T, Kober H, Bliss-Moreau E, Barrett L. The brain basis of emotion: A meta-analytic review. Behav Brain Sci. 2012;35(03):121–43. doi: 10.1017/S0140525X11000446. http://doi.org/10.1017/S0140525X11000446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schneider S, Peters J, Bromberg U, Brassen S, Menz M, Miedl S, et al. Boys do it the right way: Sex-dependent amygdala lateralization during face processing in adolescents. Neuroimage. 2011;56(3):1847–53. doi: 10.1016/j.neuroimage.2011.02.019. [DOI] [PubMed] [Google Scholar]
- 26.Almeida I, van Asselen M, Castelo-Branco M. The role of the amygdala and the basal ganglia in visual processing of central vs. peripheral emotional content. Neuropsychologia. 2013;51(11):2120–29. doi: 10.1016/j.neuropsychologia.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 27.Adolphs R, Tranel D, Damasio H, Damasio A. Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature. 1994;372(6507):669–72. doi: 10.1038/372669a0. [DOI] [PubMed] [Google Scholar]
- 28.Adolphs R, Tranel D, Damasio H, Damasio AR. Fear and the human amygdala. J Neurosci. 1995;15(9):5879–91. doi: 10.1523/JNEUROSCI.15-09-05879.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Calder AJ, Young AW, Rowland D, Perrett DI, Hodges JR, Etcoff NL. Facial emotion recognition after bilateral amygdala damage: Differentially severe impairment of fear. Cogn Neuropsychol. 1996;13(5):699–745. doi: 10.1080/026432996381890. [DOI] [Google Scholar]
- 30.Sprengelmeyer R, Young AW, Schroeder U, Grossenbacher PG, Federlein J, Buttner T, Przuntek H. Knowing no fear. Proc Biol Sci. 1999;266(1437):2451–56. doi: 10.1098/rspb.1999.0945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adolphs R, Gosselin F, Buchanan T, Tranel D, Schyns P, Damasio A. A mechanism for impaired fear recognition after amygdala damage. Nature. 2005;433(7021):68–72. doi: 10.1038/nature03086. nature03086 [pii] [DOI] [PubMed] [Google Scholar]
- 32.Wolf R, Philippi C, Motzkin J, Baskaya M, Koenigs M. Ventromedial prefrontal cortex mediates visual attention during facial emotion recognition. Brain. 2014;137(6):1772–80. doi: 10.1093/brain/awu063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kumfor F, Irish M, Hodges J, Piguet O. Discrete neural correlates for the recognition of negative emotions: Insights from frontotemporal dementia. PLoS ONE. 2013;8(6):e67457. doi: 10.1371/journal.pone.0067457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oliver L, Virani K, Finger E, Mitchell D. Is the emotion recognition deficit associated with frontotemporal dementia caused by selective inattention to diagnostic facial features? Neuropsychologia. 2014;60:84–92. doi: 10.1016/j.neuropsychologia.2014.05.020. [DOI] [PubMed] [Google Scholar]
- 35.Hot P, Klein-Koerkamp Y, Borg C, Richard-Mornas A, Zsoldos I, Paignon Adeline A, et al. Fear recognition impairment in early-stage Alzheimer’s disease: When focusing on the eyes region improves performance. Brain Cogn. 2013;82(1):25–34. doi: 10.1016/j.bandc.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 36.Adolphs R. What does the amygdala contribute to social cognition? Ann N Y Acad Sci. 2010;1191(1):42–61. doi: 10.1111/j.1749-6632.2010.05445.x. http://doi.org/10.1111/j.1749-6632.2010.05445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gorno-Tempini M, Pradelli S, Serafini M, Pagnoni G, Baraldi P, Porro C, et al. Explicit and incidental facial expression processing: An fMRI study. Neuroimage. 2001;14(2):465–73. doi: 10.1006/nimg.2001.0811. [DOI] [PubMed] [Google Scholar]
- 38.Harris R, Young A, Andrews T. Dynamic stimuli demonstrate a categorical representation of facial expression in the amygdala. Neuropsychologia. 2014;56:47–52. doi: 10.1016/j.neuropsychologia.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jansma H, Roebroeck A, Münte T. A network analysis of audiovisual affective speech perception. Neurosci. 2014;256:230–41. doi: 10.1016/j.neuroscience.2013.10.047. [DOI] [PubMed] [Google Scholar]
- 40.Milesi V, Cekic S, Peron J, Fruhholz S, Cristinzio C, Seeck M, et al. Multimodal emotion perception after anterior temporal lobectomy (ATL) Front Hum Neurosci. 2014:8. doi: 10.3389/fnhum.2014.00275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reynaud E, Guedj E, Trousselard M, El Khoury-Malhame M, Zendjidjian X, Fakra E, et al. Acute stress disorder modifies cerebral activity of amygdala and prefrontal cortex. Cogn Neurosci. 2015;6(1):39–43. doi: 10.1080/17588928.2014.996212. [DOI] [PubMed] [Google Scholar]
- 42.Atkinson A, Adolphs R. The neuropsychology of face perception: Beyond simple dissociations and functional selectivity. Philos Trans R Soc Lond B Biol Sci. 2011;366(1571):1726–38. doi: 10.1098/rstb.2010.0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gamer M, Buchel C. Amygdala activation predicts gaze toward fearful eyes. J Neurosci. 2009;29(28):9123–26. doi: 10.1523/JNEUROSCI.1883-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Philippi C, Mehta S, Grabowski T, Adolphs R, Rudrauf D. Damage to association fiber tracts impairs recognition of the facial expression of emotion. J Neurosci. 2009;29(48):15089–99. doi: 10.1523/JNEUROSCI.0796-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Phillips M, Young A, Scott S, Calder A, Andrew C, Giampietro V, et al. Neural responses to facial and vocal expressions of fear and disgust. R Soc Lond B Biol Sci. 1998;265(1408):1809–577. doi: 10.1098/rspb.1998.0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jehna M, Neuper C, Ischebeck A, Loitfelder M, Ropele S, Langkammer C, et al. The functional correlates of face perception and recognition of emotional facial expressions as evidenced by fMRI. Brain Res. 2011;1393:73–83. doi: 10.1016/j.brainres.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 47.Calder AJ, Keane J, Manes F, Antoun N, Young AW. Impaired recognition and experience of disgust following brain injury. NatNeurosci. 2000;3(11):1077–78. doi: 10.1038/80586. [DOI] [PubMed] [Google Scholar]
- 48.Kipps C, Duggins A, McCusker E, Calder A. Disgust and happiness recognition correlate with anteroventral insula and amygdala volume respectively in preclinical Huntington’s disease. J Cogn Neurosci. 2007;19(7):1206–17. doi: 10.1162/jocn.2007.19.7.1206. [DOI] [PubMed] [Google Scholar]
- 49.Omar R, Rohrer J, Hailstone J, Warren J. Structural neuroanatomy of face processing in frontotemporal lobar degeneration. J Neurol Neurosurg Psychiatry. 2010;82(12):1341–43. doi: 10.1136/jnnp.2010.227983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jacobs D, Shuren J, Bowers D, Heilman K. Emotional facial imagery, perception, and expression in Parkinson’s disease. Neurology. 1995;45(9):1696–1702. doi: 10.1212/wnl.45.9.1696. [DOI] [PubMed] [Google Scholar]
- 51.Dogan I, Sass C, Mirzazade S, Kleiman A, Werner C, Pohl A, et al. Neural correlates of impaired emotion processing in manifest Huntington’s disease. Soc Cogn Affect Neurosci. 2013;9(5):671–80. doi: 10.1093/scan/nst029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Murray R, Brosch T, Sander D. The functional profile of the human amygdala in affective processing: Insights from intracranial recordings. Cortex. 2014;60:10–33. doi: 10.1016/j.cortex.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 53.Fried I, MacDonald KA, Wilson CL. Single neuron activity in human hippocampus and amygdala during recognition of faces and objects. Neuron. 1997;18(5):753–65. doi: 10.1016/s0896-6273(00)80315-3. S0896-6273(00)80315-3 [pii] [DOI] [PubMed] [Google Scholar]
- 54.Rutishauser U, Tudusciuc O, Neumann D, Mamelak A, Heller A, Ross I, et al. Single-unit responses selective for whole faces in the human amygdala. Curr Biol. 2011;21(19):1654–60. doi: 10.1016/j.cub.2011.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Krolak-Salmon P, Hénaff M, Vighetto A, Bertrand O, Mauguière F. Early amygdala reaction to fear spreading in occipital, temporal, and frontal cortex. Neuron. 2004;42(4):665–76. doi: 10.1016/s0896-6273(04)00264-8. S0896627304002648 [pii] [DOI] [PubMed] [Google Scholar]
- 56.Blair R, Morris JS, Frith CD, Perrett DI, Dolan RJ. Dissociable neural responses to facial expressions of sadness and anger. Brain. 1999;122(5):883–93. doi: 10.1093/brain/122.5.883. [DOI] [PubMed] [Google Scholar]
- 57.Harmer CJ, Thilo KV, Rothwell JC, Goodwin GM. Transcranial magnetic stimulation of medial-frontal cortex impairs the processing of angry facial expressions. Nat Neurosci. 2001;4(1):17–18. doi: 10.1038/82854. [DOI] [PubMed] [Google Scholar]
- 58.Fellows LK, Heberlein AS, Morales DA, Shivde G, Waller S, Wu DH. Method matters: An empirical study of impact in cognitive neuroscience. J Cogn Neurosci. 2005;17(6):850–58. doi: 10.1162/0898929054021139. [DOI] [PubMed] [Google Scholar]
- 59.Wittsack H, Ritzl A, Fink G, Wenserski F, Siebler M, Seitz R, et al. MR imaging in acute stroke: Diffusion-weighted and perfusion imaging parameters for predicting infarct size 1. Radiology. 2002;222(2):397–403. doi: 10.1148/radiol.2222001731. [DOI] [PubMed] [Google Scholar]
- 60.Kuhl C, Textor J, Gieseke J, von Falkenhausen M, Gernert S, Urbach H, et al. Acute and subacute ischemic stroke at high-field-strength (3.0-T) diffusion-weighted MR imaging: Intraindividual comparative study1. Radiology. 2005;234(2):509–16. doi: 10.1148/radiol.2342031323. [DOI] [PubMed] [Google Scholar]
- 61.Oishi K, Faria A, Jiang H, Li X, Akhter K, Zhang J, et al. Atlas-based whole brain white matter analysis using large deformation diffeomorphic metric mapping: Application to normal elderly and Alzheimer’s disease participants. Neuroimage. 2009;46(2):486–99. doi: 10.1016/j.neuroimage.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Leigh R, Oishi K, Hsu J, Lindquist M, Gottesman R, Jarso S, et al. Acute lesions that impair affective empathy. Brain. 2013;136(8):2539–49. doi: 10.1093/brain/awt177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dara C, Kirsch-Darrow L, Ochfeld E, Slenz J, Agranovich A, Vasconcellos-Faria A, et al. Impaired emotion processing from vocal and facial cues in frontotemporal dementia compared to right hemisphere stroke. Neurocase. 2013;19(6):521–29. doi: 10.1080/13554794.2012.701641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pell MD, Leonard CL. Facial expression decoding in early Parkinson’s disease. Cogn Brain Res. 2005;23(2–3):327–40. doi: 10.1016/j.cogbrainres.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 65.Gur R, Schroeder L, Turner T, McGrath C, Chan R, Turetsky B, et al. Brain activation during facial emotion processing. Neuroimage. 2002;16(3):651–62. doi: 10.1006/nimg.2002.1097. S1053811902910979 [pii] [DOI] [PubMed] [Google Scholar]
- 66.Calvo M, Lundqvist D. Facial expressions of emotion (KDEF): Identification under different display-duration conditions. Behav Res. 2008;40(1):109–15. doi: 10.3758/brm.40.1.109. [DOI] [PubMed] [Google Scholar]
- 67.Limbrecht-Ecklundt K, Scheck A, Jerg-Bretzke L, Walter S, Hoffmann H, Traue HC. The effect of forced choice on facial emotion recognition: A comparison to open verbal classification of emotion labels. Psychosoc Med. 2013;10:Doc04. doi: 10.3205/psm000094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Calvo M, Beltrán D. Recognition advantage of happy faces: Tracing the neurocognitive processes. Neuropsychologia. 2013;51(11):2051–61. doi: 10.1016/j.neuropsychologia.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 69.Bombari D, Schmid P, Schmid Mast M, Birri S, Mast F, Lobmaier J. Emotion recognition: The role of featural and configural face information. Q J Exp Psychol. 2013;66(12):2426–42. doi: 10.1080/17470218.2013.789065. [DOI] [PubMed] [Google Scholar]
- 70.Pohl A, Anders S, Schulte-Rüther M, Mathiak K, Kircher T. Positive facial affect – An fMRI study on the involvement of insula and amygdala. PLoS ONE. 2013;8(8):e69886. doi: 10.1371/journal.pone.0069886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Johnson S, Stout J, Solomon A, Langbehn D, Aylward E, Cruce C, et al. Beyond disgust: impaired recognition of negative emotions prior to diagnosis in Huntington’s disease. Brain. 2007;130(7):1732–44. doi: 10.1093/brain/awm107. 130/7/1732 [pii] [DOI] [PubMed] [Google Scholar]
- 72.Calvo MG, Avero P, Fernández-Martín A, Recio G. Recognition thresholds for static and dynamic emotional faces. Emotion. 2016 Jun 30; doi: 10.1037/emo0000192. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 73.Takeda A, Kobayakawa M, Suzuki A, Tsuruya N, Kawamura M. Lowered sensitivity to facial emotions in myotonic dystrophy type 1. J Neurol Sci. 2009;280(1–2):35–9. doi: 10.1016/j.jns.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 74.Mike A, Strammer E, Aradi M, Orsi G, Perlaki G, Hajnal A, et al. Disconnection mechanism and regional cortical atrophy contribute to impaired processing of facial expressions and theory of mind in multiple sclerosis: A structural MRI study. PLoS ONE. 2013;8(12):e82422. doi: 10.1371/journal.pone.0082422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sato W, Uono S, Matsuura N, Toichi M. Misrecognition of facial expressions in delinquents. Child Adolesc Psychiatry Ment Health. 2009;3(1):27. doi: 10.1186/1753-2000-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kennedy D, Adolphs R. Perception of emotions from facial expressions in high-functioning adults with autism. Neuropsychologia. 2012;50(14):3313–19. doi: 10.1016/j.neuropsychologia.2012.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Statucka M, Walder D. Efficacy of social cognition remediation programs targeting facial affect recognition deficits in schizophrenia: A review and consideration of high-risk samples and sex differences. Psychiatry Res. 2013;206(2–3):125–39. doi: 10.1016/j.psychres.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 78.Adolphs R. Neural systems for recognizing emotion. Curr Opin Neurobiol. 2002;12(2):169–77. doi: 10.1016/s0959-4388(02)00301-x. S095943880200301X [pii] [DOI] [PubMed] [Google Scholar]