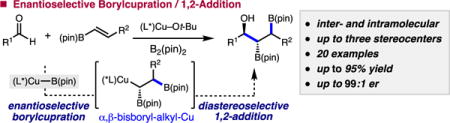
Abstract
Catalytic enantioselective synthesis of 1-hydroxy-2,3-bisboronate esters through multicomponent borylation/1,2-addition is reported. Catalyst and substrates are readily available, form both a C–B and C–C bond, and generate up to three contiguous stereocenters. The reaction is tolerant of aryl, vinyl, and alkyl aldehydes and ketones in up to 95% yield, >20:1 dr, and 99:1 er. Intramolecular additions to aldehydes and ketones result in stereodivergent processes. The hydroxy bis(boronate) ester products are amenable to site-selective chemical elaboration.
Keywords: Cu-catalyzed, tandem borylation
Graphical abstract
Enantioselective methods for the preparation of alcohols and sp2-organoborons are important in the chemical synthesis of chiral bioactive molecules. Consequently, stereoselective reactions that render the syntheses of these important functional groups more efficient are highly desirable.1 Addition of chiral non-racemic alkyl-metal reagents to carbonyls provides a direct approach for the enantioselective generation of chiral alcohols bearing a vicinal stereogenic center.2 However, chiral organometallic sp2-carbon nucleophiles (e.g., Grignards and organolithiums)3 are generally preformed, air and moisture sensitive reagents that carry limited functionality, and are generated under cryogenic conditions to minimize enantiomerization.4,5 Catalytic enantioselective in situ generation of chiral C(sp3)-metal reagents from achiral starting materials provides an effective solution to generating such classes of non-racemic alkyl nucleophiles.6,7 One class of versatile chiral nucleophiles are enantioenriched α-borylalkyl-Cu reagents; such intermediates undergo stereoselective C–C bond formations while simultaneously incorporating an organoboron moiety that can be further elaborated into a number of useful functional groups.8
Our group and others have recently developed catalytic methods for generating chiral α-borylalkyl organometallics via transmetallation of bench-stable 1,1-diborylalkanes.9 Morken10a-b and Hall[11c] independently reported the enantioselective Pd-catalyzed cross coupling of substituted 1,1-diborylalkanes, while recent advances in catalytic enantioselective allylic substitution and diastereoselective additions to imines have focused on achiral diborylmethane.11,12 We reported the enantio- and diastereoselective Cu-catalyzed addition of 1,1-diborylalkanes to aldehydes and α- Cu-catalyzed transmetalation of a 1,1-diborylalkane to generate an α-borylalkyl-Cu intermediate, which diastereoselectively adds to aldehydes or α-ketoesters (Scheme 1A). The transformation generates both a new C(sp3)–C(sp3) bond, and up to two stereogenic centers, including an enantioenriched alkylboron group.
Scheme 1.
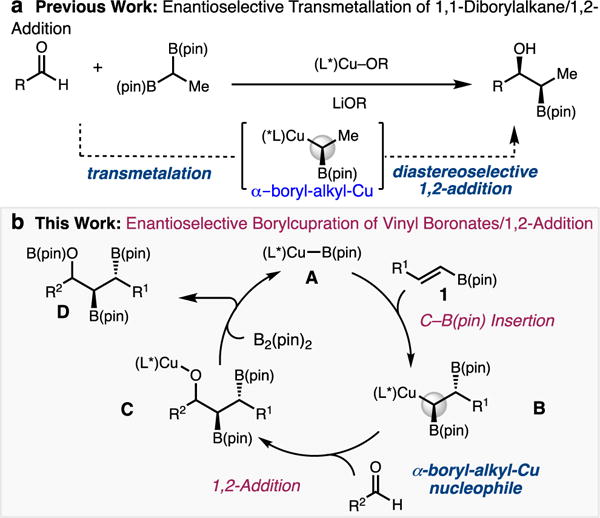
Development of Multicomponent Strategy for the Synthesis of Hydroxy Bis(boronates).
Herein, we outline an alternative strategy for the generation of chiral non-racemic α-borylalkyl–Cu nucleophiles (B) (Scheme 1B) through an enantioselective borylcupration of vinyl boronate esters (e.g., 1) by a (L)Cu–B(pin) species (A).13 The resulting nucleophile can then undergo 1,2-addition to an aldehyde to generate 1-hydroxy bis(boronate) esters D.14 This multicomponent process sequentially forms a C–C and a C–B bond in addition to installing two alkyl organoboron groups that may be further functionalized.
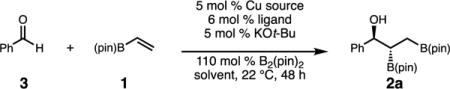
To determine the feasibility of the proposed multicomponent process, we obtained an initial result that established the ability of racemic phosphine-copper complexes to promote the sequential C–B/C–C bond forming sequence. As depicted in Equation 1, treatment of vinyl-B(pin), benzaldehyde, and B2(pin)2 with 5 mol % [Cu(MeCN)4]PF6, 6 mol % rac-binap, and 5 mol % KOt-Bu results in the multicomponent coupling to produce rac-2a in >98% conversion, and 9:1 dr favoring the anti diastereoisomer. Notably, it was discovered that the minor syn diastereoisomer of the product undergoes boron-Wittig type elimination during silica gel chromatography,15 and after purification of the crude reaction mixture rac-2a is isolated in 77% yield as a single diastereomer. This is advantageous as the careful separation of diastereoisomers is not required for less selective substrates.
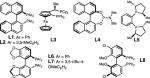
Having established the in situ preparation and diastereoselective reaction of α-borylalkyl-Cu reagents with vinyl-B(pin), we sought to render the process enantioselective. Table 1 summarizes our optimization studies with chiral phosphines in the Cu-catalyzed multicomponent reaction. Under identical reaction conditions to those in Equation 1, L1 afford 2a in 87% conv, 6.7:1 dr, and 77:23 er. Changing the solvent to toluene led to an increase in conversion (>98% conv) and enantioselectivity (82:18 er), with comparable diastereoselectivity. With chiral bidentate phosphines L2 or L3, 2a is formed in increased enantioselectivity (up to 95:5 er), albeit in low diastereoselectivity (entries 3–4). In order to improve the diastereoselectivity of the reaction while maintaining high enantioselectivity, other mono- and bidentate chiral phosphines were screened (L4–L7), however, 2a is formed in <12% conversion in all cases (Entries 5–8, Table 1). Biphenyl phosphine L8 proved to be the most effective ligand for the Cu-catalyzed reaction, affording 2a in >98% conv, 3.2:1 dr, and 95:5 er. These conditions, however, proved variable for other aldehyde substrates, and provided inconsistent conversion and enantioselectivities. We hypothesized that efficiently generating the phosphine-copper(alkoxide) complex in situ could be in part responsible, as such we employed CuOt-Bu as the copper source. Application of 10 mol % CuOt-Bu, in addition to benzene as solvent, delivers 2a in >98% conv, 3.5:1 dr, and 95.5:4.5 er (Entry 11, Table 1).
Table 1.
Evaluation of Chiral Copper Complexesa
| ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| entry | ligand | Cu source | solvent | NMR yield (%)b | drb | erc,d |
| 1 | L1 | Cu(MeCN)4PF6 | thf | 87 | 6.7:1 | 77:23 |
| 2 | L1 | Cu(MeCN)4PF6 | toluene | >98 | 4.6:1 | 82:18 |
| 3 | L2 | Cu(MeCN)4PF6 | toluene | 98 | 1.7:1 | 92:8 |
| 4 | L3 | Cu(MeCN)4PF6 | toluene | 88 | 1:1 | 95:5 |
| 5 | L4 | Cu(MeCN)4PF6 | toluene | <2 | – | – |
| 6 | L5 | Cu(MeCN)4PF6 | toluene | <2 | – | – |
| 7 | L6 | Cu(MeCN)4PF6 | toluene | 12 | 11.5:1 | – |
| 8 | L7 | Cu(MeCN)4PF6 | toluene | <2 | – | – |
| 9 | L8 | Cu(MeCN)4PF6 | toluene | >98 | 3.2:1 | 95:5 |
| 10 | L8 | CuOt-Bu | toluene | 87 | 3:1 | 95:5 |
| 11e | L8 | CuOt-Bu | C6H6 | >98 | 3.5:1 | 95.5:4.5 |
|
| ||||||
| Ligands: | ||||||
| ||||||
Reactions performed under N2 atmosphere.
Determined by analysis of 400 or 600 MHz 1H NMR spectra of crude reactions with hexamethyldisiloxane as internal standard.
Determined by HPLC analysis; see the Supporting Information for details.
The er of the syn diastereoisomer is not reported due to its propensity to undergo elimination.
[0.167 M] in benzene with 2 equivalents of 1.
With optimized conditions in hand, we set out to explore the scope of the Cu-catalyzed multicomponent borylation/1,2-addition reaction of 1 to various aryl and alkenyl aldehydes (Table 2).16 Of note, the crude dr of the reactions varies from 1:1–>20:1, however, in general the products are isolated as the anti diastereoisomer after purification on silica gel due syn isomer elimination. In the presence of 10 mol % CuOt-Bu, 11 mol % L8, and 110 mol % B2(pin)2, 1 and benzaldehyde reacted to produce 1-hydroxy-2,3-bisboronate 2a in 74% yield, and 96:4 er (Entry 1). A variety of substituted aryl aldehydes containing halogens, methoxy, or alkyl groups undergo efficient 1,2-addition to afford 2b–g in 68–83% yield (5.7:1–>20:1 dr, 94:6–96:4 er) (entries 2–7). The catalytic protocol is tolerant of heteroaromatic aldehydes; for example, N-Boc indole 2h and furan 2i are generated in good yield and high enantioselectivity (entries 8–9). Bis-halogenated electron-deficient aryl aldehyde, 2j is formed in 71% yield, and 95:5 er. 3-Pyridinecarboxaldehyde reacts sluggishly under the catalytic protocol, generating 2k in 18% 1H NMR yield (Entry 11). Alkenyl aldehydes effectively participate in the Cu-catalyzed multicomponent process delivering 2l in 78% yield, and 93:7 er (entries 12). Alkyl aldehydes, which were previously found to be incompatible in the diastereoselective Cu-catalyzed 1,2-additions with 1,1-diborylalkanes, except in the case of n-BuLi as an irreversible activator,17 were also found to function as effective substrates (Entries 13–15). For example, secondary alcohols 2m–2o are furnished with moderate efficiency in 48–70% yield (crude 1:1–5.7:1 dr; 75:25–92:8 er).
Table 2.
Enantioselective Cu-Catalyzed Multicomponent Borylation/1,2-Addition of Vinyl-B(pin) to Aryl, Alkenyl and Alkyl Aldehydesa
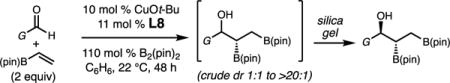
| |||||
|---|---|---|---|---|---|
|
| |||||
| entry | product | isolated yield (%)d | isolated dr (anti syn)b | erc | |
| 1 | 2a | G = Ph | 74 | >20:1 | 96:4 |
| 2 | 2b | G=p-FC6H4 | 70 | >20:1 | 96:4 |
| 3 | 2c | G = p-BrC6H4 | 68 | 5.7:1 | 95:5 |
| 4 | 2d | G = p-OMeC6H4 | 70 | >20:1 | 94:6 |
| 5e | 2e | G =m-MeC6H4 | 75(77) | >20:1 (4:1) | 95:5 |
| 6 | 2f | G = o-MeC6H4 | 81 | >20:1 | 96:4 |
| 7 | 2g | G = o-BrC6H5 | 83 | >20:1 | 96:4 |
| 8 | 2h |
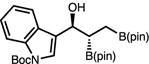
|
63 | 3:1 | 94:6 |
| 9e | 2i |
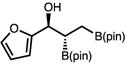
|
52 (57) | >20:1 (3.5:1) | 97:3 |
| 10 | 2j |
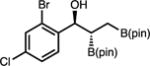
|
71 | >20:1 | 95:5 |
| 11 | 2k |
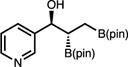
|
18t | – | – |
| 12 | 2I |
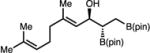
|
78 (36) | >20:1 (>20:1 dr) | 93:7 |
| 13 | 2m |
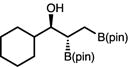
|
63 | >20:1 | 92:8 |
| 14 | 2n |
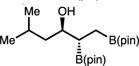
|
48 | >20:1 | 75:25 |
| 15e | 2o |
|
70t (52) | 3:1 (3:1) | 90:10 |
See Table 1.
Isolated yield of the anti diastereoisomer unless otherwise stated.
Values in parentheses correspond to the TBS protected hydroxydiboronate, resulting from protection of the crude reaction mixture (see Supporting Information for details).
NMR yield.
We next sought to extend the methodology to substituted 1-alkenyl-B(pin) substrates, which possess the ability to generate three contiguous stereogenic centers. Initially, our investigations focused on intramolecular variants of the enantioselective reaction between 1 and several aldehydes. As illustrated in Scheme 2, treatment of E-3a with 10 mol % chiral Cu catalyst in toluene at 4 °C resulted in the desired cyclized product 4 in 49% yield, >20:1 dr, and 98.5:1.5 er. Although the multi-component process proceeds with very high selectivity, competitive 1,2-addition of Cu–B(pin) to the aldehyde is problematic, and could not be minimized.18 To minimize this unfavorable pathway, we switched to more sterically encumbered ketones as substrates. Treatment of methyl ketone E-3b to the catalytic protocol but with L1 as ligand and t-BuOH (100 mol %) in dioxane at 22 °C, affords tertiary alcohol 5 in 95% yield, >20:1 dr, and 94:6 er. In addition, three other intramolecular ketone substrates were evaluated, which afford aryl (6), alkyl (7), and N-heterocyclic (8) containing tertiary alcohols. Several features of these intramolecular studies are notable: (1) Congested tertiary alcohol hydroxydiboronates can be formed in good yields, and excellent diastereo- (>20:1) and enantioselectivites (up to 97:3 er);19 (2) The catalytic method is amenable to the enantioselective synthesis of alkyl and N-heterocyclic structures; (3) tert-Butanol serves to turn over the catalyst.
Scheme 2.
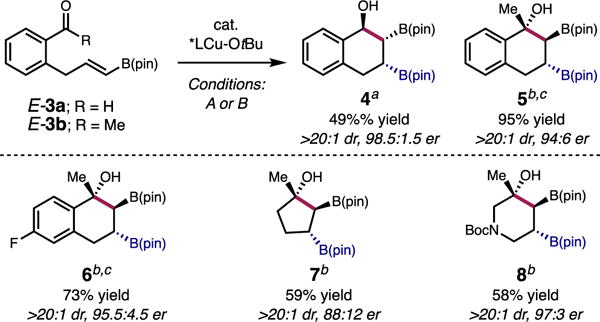
Diastereo- and Enantioselective Intramolecular Cyclizations to Form Three Stereogenic Centers.
aConditions A: 10 mol % CuOt-Bu, 12 mol % L8, 110 mol % B2(pin)2, toluene, 4 °C, 48 h. bConditions B: 10 mol % CuOt-Bu, 12 mol % L1, 110 mol % B2(pin)2, 100 mol % t-BuOH, dioxane, 22 °C, 18 h. cIsolated yield of the corresponding triol.
The divergence in the relative stereochemistry (anti-, syn- vs anti-, anti-) of the hydroxyl and B(pin) groups in 4 and 5 can be explained by the Cu–alkyl additions occurring via two different modes (Scheme 3): (1) The more reactive and sterically unhindered aldehyde is able to directly engage with the Cu–alkyl through a stereoretentive pathway (I) resulting in anti-,syn-4. (2) Conversely, the more sterically congested methyl ketone reacts with the α-boryl-Cu-alkyl through a stereoinvertive back-side addition (II) to afford anti-,anti-5. A similar invertive Cu–alkyl 1,2-addition mechanism was recently proposed in the enantioselective 1,2-addition of benzylic copper nucleophiles to imines.7g
Scheme 3.
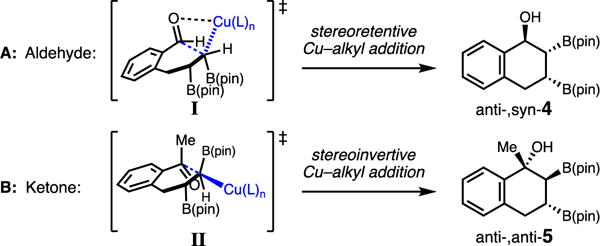
Proposed Transition States for Aldehydes and Ketones Involving Stereoretentive and Stereoinvertive Additions.
The 1-hydroxy-2,3-bisboronate products synthesized via the Cu-catalyzed method may be further functionalized into synthetically useful molecules (Scheme 4). For example, oxidation to the corresponding 1,2,3-triols; treatment of 2p with aqueous NaOH and 30% H2O2 to generate triol 9 in 93% yield is illustrative (Scheme 4a). Site-selective cross couplings of TBS-protected diboronate 10 (isolated in 62% yield from 2a)20 with isocrotyl bromide 11 catalyzed by 1 mol % Pd-RuPhos,15c results in efficient formation of homoallyl boronate 12 in 59% yield (Scheme 4b). The secondary boronate in 12 can also be further functionalized into amines (e.g., 14)21 and aryl groups (e.g., 13), however, the congested nature of the B(pin) unit results in 30–52% yield.22 Silyl ether protected cyclic hydroxy-diboronates, also undergo efficient stereospecific functionalization, however, both secondary alkyl-B(pin) units were found to react with equal efficiency. The bishomologation to form 16 in 78% yield in Scheme 4c is illustrative.
Scheme 4.
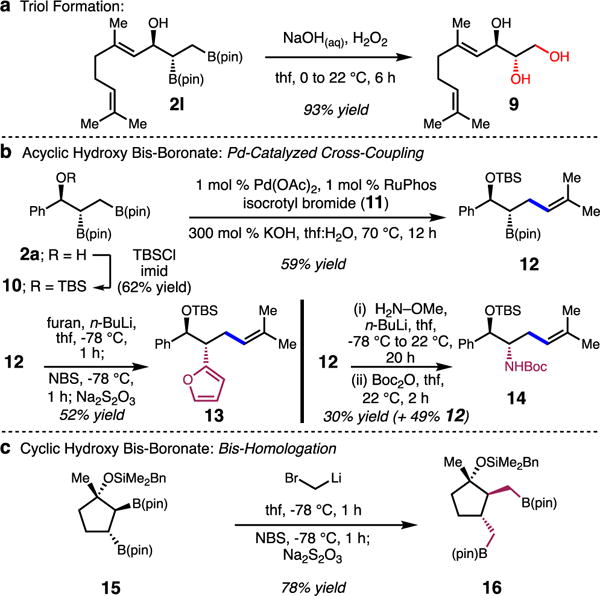
Functionalizations of 1-Hydroxy-2,3-Bisboronate Esters.
In conclusion, we have developed an efficient, enantioselective multicomponent borylation/1,2-addition reaction that generates in situ α,β-bisboryl-copper-alkyl nucleophiles, which add diastereoselectively inter- and intramolecular to aldehydes and ketones. Notably, intramolecular additions to ketones proceed via a stereoinvertive pathway. The hydroxy(bis)boronates formed by this method are amenable to stereospecific and site-selective functionalizations including oxidation, cross-coupling, and homologation. Mechanistic studies, as well as development of other enantioselective multicomponent reactions are in progress.
Supplementary Material
Acknowledgments
Financial support was provided by the National Institutes of Health (R01GM116987, 3R01GM116987-01S1) and the University of North Carolina at Chapel Hill. AllyChem is acknowledged for donations of B2(pin)2.
Footnotes
Supporting Information. Experimental procedures and spectral and analytical data for all products. This material is available free of charge via the Internet at http://pubs.acs.org.
ORCID
Simon J. Meek: 0000-0001-7537-9420
Notes Authors declare no competing financial interests.
References
- 1.(a) Hall DG, editor. Boronic Acids. Wiley-VCH; Weinheim, Germany: 2011. [Google Scholar]; b Chinnusamy T, Feeney K, Watson CG, Leonori D, Aggarwal VK. Comprehensive Organic Synthesis II; Elsevier. 2014:692–718. [Google Scholar]
- 2.(a) Hoffmann RW, Hölzer B. Chem Commun. 2001:491–492. [Google Scholar]; (b) Ros A, Aggarwal VK. Angew Chem, Int Ed. 2009;48:6289–6292. doi: 10.1002/anie.200901900. [DOI] [PubMed] [Google Scholar]; (c) Rayner PJ, O’Brien P, Horan RAJ. J Am Chem Soc. 2013;135:8071–8077. doi: 10.1021/ja4033956. [DOI] [PubMed] [Google Scholar]
- 3.For examples of synthesis and reaction of chiral alkyl lithiums, see:; (a) Hoppe D, Hense T. Angew Chem, Int Ed. 1997;36:2282–2316. [Google Scholar]; (b) Beak P, Basu A, Gallagher DJ, Park YS, Thayumanavan S. Acc Chem Res. 1996;29:552–560. [Google Scholar]; (c) Basu A, Thayumanavan S. Angew Chem, Int Ed. 2002;41:716–738. doi: 10.1002/1521-3773(20020301)41:5<716::aid-anie716>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 4.For a recent review that discusses the configurational stability of enantioenriched alkyl nucleophiles, see:; (a) Swift EC, Jarvo ER. Tetrahedron. 2013;69:5799–5817. doi: 10.1016/j.tet.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]; For examples of configurational stability of Grignards, see:; (b) Whitesides GM, Witanowski M, Roberts JD. J Am Chem Soc. 1965;87:2854–2862. [Google Scholar]; (c) Whitesides GM, Roberts JD. J Am Chem Soc. 1965;87:4878–4888. [Google Scholar]; (d) Hoffmann RW. Chem Soc Rev. 2003;32:225–230. doi: 10.1039/b300840c. [DOI] [PubMed] [Google Scholar]
- 5.For examples of enantiospecific reactions of non-racemic alkylcuprates, see:; (a) Dieter RK, Topping CM, Chandupatla KR, Lu K. J Am Chem Soc. 2001;123:5132–5133. doi: 10.1021/ja0156587. [DOI] [PubMed] [Google Scholar]; (b) Dieter RK, Oba G, Chandupatla KR, Topping CM, Lu K, Watson RT. J Org Chem. 2004;69:3076–3086. doi: 10.1021/jo035845i. [DOI] [PubMed] [Google Scholar]
- 6.For recent examples of catalytic enantioselective C–C bond forming reactions of chiral C(sp3)–Cu nucleophiles generated from olefins, see: 1,2-Addition:; (a) Yang Y, Perry IB, Lu G, Liu P, Buchwald SL. Science. 2016;353:144–150. doi: 10.1126/science.aaf7720. [DOI] [PMC free article] [PubMed] [Google Scholar]; Alkylation:; (b) Ito H, Kosaka Y, Nonoyama K, Sasaki Y, Sawamura M. Angew Chem, Int Ed. 2008;47:7424–7427. doi: 10.1002/anie.200802342. [DOI] [PubMed] [Google Scholar]; (c) Zhong C, Kunii S, Kosaka Y, Sawamura M, Ito H. J Am Chem Soc. 2010;132:11440–11442. doi: 10.1021/ja103783p. [DOI] [PubMed] [Google Scholar]; (d) Wang YM, Bruno NC, Placeres ÁL, Zhu S, Buchwald SL. J Am Chem Soc. 2015;137:10524–10527. doi: 10.1021/jacs.5b07061. [DOI] [PMC free article] [PubMed] [Google Scholar]; Allylation:; (e) Jia T, Cao P, Wang B, Lou Y, Yin X, Wang M, Liao J. J Am Chem Soc. 2015;137:13760–13763. doi: 10.1021/jacs.5b09146. [DOI] [PubMed] [Google Scholar]; (f) Wang YM, Buchwald SL. J Am Chem Soc. 2016;138:5024–5027. doi: 10.1021/jacs.6b02527. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Yang Y, Perry IB, Buchwald SL. J Am Chem Soc. 2016;138:9787–9790. doi: 10.1021/jacs.6b06299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.For recent examples of catalytic enantioselective reactions of allyl-, propargyl-, and allenyl-metal nucleophiles generated from olefins, see: Hydrogenation or transfer hydrogenation:; (a) Jang HY, Krische MJ. Acc Chem Res. 2004;37:653–661. doi: 10.1021/ar020108e. [DOI] [PubMed] [Google Scholar]; (b) Skucas E, Ngai MY, Komanduri V, Krische MJ. Acc Chem Res. 2007;40:1394–1401. doi: 10.1021/ar7001123. [DOI] [PubMed] [Google Scholar]; (c) Bower JF, Kim IS, Patman RL, Krische MJ. Angew Chem, Int Ed. 2009;48:34–46. doi: 10.1002/anie.200802938. [DOI] [PMC free article] [PubMed] [Google Scholar]; Cu–Borylation:; (d) Meng F, Jang H, Jung B, Hoveyda AH. Angew Chem Int Ed. 2013;52:5046–5051. doi: 10.1002/anie.201301018. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Meng F, Haeffner F, Hoveyda AH. J Am Chem Soc. 2014;136:11304–11307. doi: 10.1021/ja5071202. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Meng F, McGrath KP, Hoveyda AH. Nature. 2014;513:367–374. doi: 10.1038/nature13735. [DOI] [PMC free article] [PubMed] [Google Scholar]; Cu–H: (g) see ref 5(a)
- 8.(a) Knochel P. J Am Chem Soc. 1990;112:7431–7433. [Google Scholar]; (b) Suzuki A, Miyaura N, Sakai M, Saito S. Tetrahedron. 1995;52:915–924. [Google Scholar]; (c) Waas JR, Sidduri AR, Knochel P. Tetrahedron Lett. 1992;33:3717–3720. [Google Scholar]
- 9.(a) Endo K, Ohkubo T, Hirokami M, Shibata T. J Am Chem Soc. 2010;132:11033–11035. doi: 10.1021/ja105176v. [DOI] [PubMed] [Google Scholar]; (b) Endo K, Ohkubo T, Shibata T. Org Lett. 2011;13:3368–3371. doi: 10.1021/ol201115k. [DOI] [PubMed] [Google Scholar]; (c) Endo K, Ohkubo T, Ishioka T, Shibata T. J Org Chem. 2012;77:4826–4831. doi: 10.1021/jo3004293. [DOI] [PubMed] [Google Scholar]
- 10.(a) Sun C, Potter B, Morken JP. J Am Chem Soc. 2014;136:6534–6537. doi: 10.1021/ja500029w. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Potter B, Szymaniak AA, Edelstein EK, Morken JP. J Am Chem Soc. 2014;136:17918–17921. doi: 10.1021/ja510266x. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Sun HY, Kubota K, Hall DG. Chem - Eur J. 2015;21:19186–19194. doi: 10.1002/chem.201406680. [DOI] [PubMed] [Google Scholar]
- 11.(a) Shi Y, Hoveyda AH. Angew Chem, Int Ed. 2016;55:3455–3458. doi: 10.1002/anie.201600309. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Zhan M, Li RZ, Mou ZD, Cao CG, Liu J, Chen YW, Niu D. ACS Catal. 2016;6:3381. [Google Scholar]; For an enantioselective example up to 82:18 er, see:; (c) Zhang ZQ, Zhang B, Lu X, Liu JH, Lu XY, Xiao B, Fu Y. Org Lett. 2016;18:952. doi: 10.1021/acs.orglett.5b03692. [DOI] [PubMed] [Google Scholar]; For non-enantioselective allylic substitutions, see:; (d) Kim J, Park S, Park J, Cho SH. Angew Chem, Int Ed. 2016;55:1498–1501. doi: 10.1002/anie.201509840. [DOI] [PubMed] [Google Scholar]
- 12.For an example of non-enantioselective carboboration of vinyl-B(pin), see:; (a) Yoshida H, Kageyuki I, Takaki K. Org Lett. 2013;15:952–955. doi: 10.1021/ol4001526. [DOI] [PubMed] [Google Scholar]; For a recent examples of enantioselective hydroallylation of vinylboronates, see:; (b) Han JT, Jang WJ, Kim N, Yun J. J Am Chem Soc. 2016;138:15146–15149. doi: 10.1021/jacs.6b11229. [DOI] [PubMed] [Google Scholar]; (c) Lee J, Torker S, Hoveyda AH. Angew Chem, Int Ed. 2017;56:821–826. doi: 10.1002/anie.201611444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.For a recent review of Cu-catalyzed borylative transformations, see:; Semba K, Fujihara T, Terao J, Tsuji Y. Tetrahedron. 2015;71:2183–2197. [Google Scholar]
- 14.For reviews of catalytic enantioselective diboration, see:; (a) Burks HE, Morken JP. Chem Commun. 2007:4717–4725. doi: 10.1039/b707779c. [DOI] [PubMed] [Google Scholar]; (b) Coombs JR, Morken JP. Angew Chem, Int Ed. 2016;55:2636–2649. doi: 10.1002/anie.201507151. [DOI] [PMC free article] [PubMed] [Google Scholar]; For additional selected examples of stereoselective olefin diboration, see: Hydroxyl directed; (c) Blaisdell TP, Caya TC, Zhang L, Sanz-Marco A, Morken JP. J Am Chem Soc. 2014;136:9264–9267. doi: 10.1021/ja504228p. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Blaisdell TP, Morken JP. J Am Chem Soc. 2015;137:8712–8715. doi: 10.1021/jacs.5b05477. [DOI] [PMC free article] [PubMed] [Google Scholar]; Carbohydrate-catalyzed; (e) Fang L, Yan L, Haeffner F, Morken JP. J Am Chem Soc. 2016;138:2508. doi: 10.1021/jacs.5b13174. [DOI] [PMC free article] [PubMed] [Google Scholar]; Cu-catalyzed; (f) Lee Y, Jang H, Hoveyda AH. J Am Chem Soc. 2009;131:18234–18235. doi: 10.1021/ja9089928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coombs JR, Zhang L, Morken JP. Org Lett. 2015;17:1708–1711. doi: 10.1021/acs.orglett.5b00480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.In a number of cases in Table 2, L8 could not be separated from the product; an issue unique to L8. Residual ligand does not interfere with subsequent transformations (see Supplementary Information).
- 17.Joannou MV, Moyer BS, Goldfogel MJ, Meek SJ. Angew Chem, Int Ed. 2015;54:14141–14145. doi: 10.1002/anie.201507171. [DOI] [PubMed] [Google Scholar]
- 18.Laitar DS, Tsui EY, Sadighi JP. J Am Chem Soc. 2006;128:11036–11037. doi: 10.1021/ja064019z. [DOI] [PubMed] [Google Scholar]
- 19.The corresponding intermolecular variant with substituted 1-alkenyl-B(pin) results in <2% conv. under the current catalytic conditions.
- 20.Protection of the alcohol is required to prevent boron-Wittig alkene formation, which occurs under the reactions conditions with the secondary alcohol.
- 21.Mlynarski SN, Karns AS, Morken JP. J Am Chem Soc. 2012;134:16449–16451. doi: 10.1021/ja305448w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.See Supporting Information for details.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


