Abstract
Background
We have previously reported on a novel organ-specific immunomodifying therapy that provides protection from early allograft rejection in the absence of systemic immunosuppressive drugs. This novel therapy is a nano-barrier membrane called ImmunoCloak, consisting of a matrix of laminin, proteoglycans, fibronectin and collagens. The membrane “immunocloaks” the luminal surfaces within the renal vasculature by covering the point of contact between donor vascular endothelial cells and the recipient’s immune cells; without adversely affecting renal function. The resulting nonthrombogenic and nonimmunogenic apical surface significantly delays the onset of rejection 5-fold over untreated controls. Currently, our focus is to elucidate the mechanisms of protection provided by placement of the membrane.
Methods
The mechanisms underlying the protective effect of the ImmunoCloak treatment was evaluated using human peripheral blood mononuclear cells and by testing for antigen presentation by cytokine/chemokine analysis using the Luminex platform, T cell allogeneic responses were measured by flow cytometry and diapedesis was assessed using transwell plates.
Results
We now report that ImmunoCloak interrupts antigen presentation thereby preventing early T cell activation and interferes with diapedesis. There was significant inhibition in the synthesis of proinflammatory cytokines with a concordant blockade of T cell mediated responses. The placement of the ImmunoCloak also significantly reduced leukocyte migration through the endothelial cell layer by 93%.
Conclusion
Eliminating the need for nephrotoxic immunosuppressive drugs during the early posttransplant period could help to ameliorate the severity of delayed graft function and could provide a path to utilizing more ischemically damaged renal allografts.
Background
The development of an organ-specific immunomodifying therapy that prevents the initial allorecognition that occurs upon reperfusion, would help to revolutionize transplantation. More importantly, it would minimize, or ideally eliminate, the toxic side effects of today’s immunosuppressive regimens. A logical target for an organ-specific treatment is the layer of endothelial cells lining the blood vessels within a vascularized allograft, because this represents the primary target of immune mediated rejection. Such an approach constitutes the tissue engineering of the vasculature within an allograft and can be referred to as “immunocloaking”.
The initial point of contact between the recipient immune cells in the circulation and the donor tissue is the vascular endothelium. It has been long recognized that vascularized allografts are rejected by both cellular-mediated and humoral mechanisms that are directed against the alloantigens expressed on the endothelial cells lining the vasculature within an allograft.1-3 During reperfusion when recipient immune cells encounter the mismatched antigens within an allograft, allorecognition occurs and from that point onward there develops the up-hill battle to prevent immunologic rejection of the tissue.
While the direct and semi-direct pathways of allorecognition are well known to play an important role in the initiation of acute allograft rejection, it has also been recognized that the indirect pathway of allorecognition plays an important role clinically in acute rejection as well as in chronic rejection.4-7 The natural turnover of graft tissues over time with the corresponding continual uptake of donor alloantigen by the recipient antigen presenting cells fuels the indirect immune response. In addition, the development of donor-specific antibody is dependent upon the indirect pathway of alloreactivity. The T cells that become activated by indirect allorecognition following engraftment become the major mediator initiating subsequent alloresponses.8 In addition, the MHC antigens expressed on the surface of the vascular endothelium are also able to activate naïve CD8+ T cells with direct allospecificity sufficient to result in acute allograft rejection.9 The findings of this experimental study are supported by the case report where a human renal allograft underwent immunologic rejection despite the absence of allogeneic passenger leukocytes.4 Therefore, the development of an effective organ-specific therapy protecting the allograft vasculature from immune mediated rejection will have to prevent the initial allorecognition that normally occurs upon reperfusion.
Our preclinical kidney transplantation studies demonstrate that it is feasible to apply the immunocloaking membrane to the renal vasculature with efficient coverage of the luminal surfaces.10 The bioengineered membrane is comprised predominantly of a mixture of laminins, glycoaminoglycans, fibronectin and collagens. The components are applied to an allograft during a period of ex vivo acellular and near-normothermic perfusion where they polymerize into a tri-dimensional transparent membrane. The interaction between the vascular endothelium lining the renal allograft vasculature and the recognition domains within the ImmunoCloak membrane are mediated via integrin complexes, including individual α chains with the β1 and αv chain of the β3 families.
We have previously reported that deposition of the ImmunoCloak membrane provides ubiquitous coverage on approximately 90% of the vascular luminal surfaces within a renal allograft including both small and large blood vessels. The ubiquitous coverage of the renal vasculature does not adversely affect subsequent renal function.10 In preclinical studies using a canine model, auto-transplanted kidneys that were pretreated with ImmunoCloak displayed normal serum chemistries accompanied by normal urine with no evidence of proteinuria. The recipients that were followed for several months continued to demonstrate normal renal function throughout the posttransplant period. In allograft studies using the same animal model, immunocloaking was found to protect against early allograft rejection in the absence of systemic immunosuppression.10 In the untreated controls the mean onset of rejection occurs on day six without systemic immunosuppression. However, when the kidney allografts were treated with ImmunoCloak, the mean onset of rejection was delayed approximately 5-fold occurring on day 30. A possible mechanism for the demonstrated efficacy of the ImmunoCloak membrane in preventing early allo-recognition was that leukocyte transmigration into tissue occurs only where there is direct contact with vascular endothelium.10 We hypothesized that the placement of the ImmunoCloak membrane on the luminal surfaces of the renal vasculature functions by interrupting the leukocyte/vascular endothelial interactions required for the transmigration of immune cells into the graft.
We believe the ImmunoCloak provides protection for a period of approximately 21 days. Therefore, in its present form ImmunoCloak is a short-term therapy that could provide the opportunity to introduce effective adjunct therapies during the early posttransplant period by preventing the normal immune responses to engraftment. Inasmuch, the ability to minimize or eliminate systemic immunosuppression during the early posttransplant period could help to address the organ shortage by facilitating the use of ischemically damaged kidneys from the donors that are declared dead by cardiac criteria by reducing drug toxicity. We now report the results of our efforts to elucidate the underlying protective mechanisms provided by ImmunoCloak.
Methods
Application of the ImmunoCloak Membrane to Vascular Endothelial Cells (VEC)
The membrane is formulated from extracellular basement membrane proteins. In our previous studies it was applied to the vasculature of an allograft during a period of acellular and near-normothermic perfusion as previously reported.10 In this study the process involves applying the neutralized soluble membrane to the luminal surfaces of confluent VEC in both 96-well and Transwell plates. The immunologic assays were performed using human VEC that were isolated from umbilical veins. The responding mononuclear cells (MNC) were resuspended at a concentration of 1×106/ml. The interaction between the endothelial cells and the recognition domains within the membrane is receptor specific via the laminin and fibronectin portions of the membrane. The components are polymerized into a tridimensional transparent membrane, where laminin polymers serve as the template for the membrane assembly. The cross-linked collagen provides the structural integrity, and the disulfide bonds stabilize the components in the membrane. The membrane is allowed to fully polymerize at 37°C for 3 hours. By applying the solubilized material prepolymerization, it is feasible to control its administration. Since the ImmunoCloak membrane is permeable to small molecular weight compounds, free transport of nutrients and oxygen is unaffected and the immunocloaked tissue remains viable.
Immunologic Screenings
Immunological assays were performed with peripheral allogeneic blood MNC collected from blood donors. The MNC were isolated from heparinized blood using a Histopaque 1077 (Sigma Aldrich) density gradient. The cells were centrifuged at 400g for 30 minutes at room temperature, and washed multiple times with PBS prior to being resuspended in RPMI 1640 (Sigma Aldrich). The MNC were plated into a 96-well plate in triplicate, groups were MNC alone (negative control) and with untreated confluent monolayers of allogeneic human VEC isolated from umbilical cord veins (positive controls). The test groups consisted of the same confluent donor endothelial cells used for the positive controls but they were treated with the ImmunoCloak membrane prior to introducing the allogeneic MNC.
Antigen Presentation
Following 72 hours in culture, antigen presentation was evaluated by measuring the cytokines in the supernatants that were produced by the cells in each of the groups listed above. Cytokine synthesis was measured using the Luminex xMap platform to determine the impact of the placement of the ImmunoCloak membrane and included: IL-1β, TNF-α, IL-8, γ-IFN, IP-10, MIP-1α and MIP-1β. All testing was performed in triplicate in multiple experiments with different combinations of human peripheral blood mononuclear cells (PBMCs) from 5 donors and human endothelial cells isolated from 5 umbilical cords (n=5).
Early T Cell Allogeneic Response
T cell activation was measured following 24 hours of culture in each of the control and test groups. Quantification of CD8+, CD69+ lymphocytes was performed by flow cytometry. The MNCs were used at a working concentration of 2×105 cells that were incubated with 20μL of anti-CD3(PECY5), anti-CD8(APC), anti-CD69(PE) antibodies (Abcam) for 1 hour at 4°C. The cells were then washed 3 times with Dulbecco’s Phosphate Buffered Saline (Sigma-Aldrich) and fixed for 10 minutes in 4% Formaldehyde. The fixed cells were washed again in dPBS and resuspended in 1 ml of running buffer (5% fetal bovine serum in dPBS) and analyzed using a FACScan (Becton Dickinson, Franklin Lakes, NJ) and Cellquest software (Cellquest Inc. Largo, FL, USA). The baseline channel fluorescence was determined using the negative controls consisting of MNCs alone. The median channel fluorescence (MCF) shift used for determining positivity was calculated as the standard deviation based upon the average of the median channel fluorescence of the negative controls. All testing was performed in triplicate in multiple experiments with different combinations of human PBMCs from 5 donors and human endothelial cells isolated from 5 umbilical cords (n=5). The fixed cells from each experiment were pooled for the flow cytometric analysis.
In addition, cytokine synthesis was evaluated to further determine early T cell allogeneic response. The concentration of the cytokines: IL-2, IL-6, MIG, and MCP-1 released into the supernatants in the cultures were quantified also using the Luminex xMap platform (n=10).
MNCs Proliferative Response
Using a standard ELISA BrdU proliferation assay the testing involved MNCs stimulated by confluent allogeneic VEC without the ImmunoCloak membrane (positive control) and the same VEC that were treated with the ImmunoCloak membrane prior to introducing the responding MNCs (test). The 5-BrdU assay is routinely used to measure DNA synthesis and to label dividing cells. All testing was performed in triplicate in multiple experiments with human PBMCs from 5 donors and human endothelial cells isolated from 5 umbilical cords (n=5).
Effect of ImmunoCloak Membrane On Chemotactic Signaling of Transendothelial Diapedesis
The human umbilical vein endothelial cells were seeded into Transwell® plates and then maintained in cell culture until confluence. The endothelial cells were grown on the top of a porous filter that separated 2 distinct fluid compartments simulating a static model of chemotactic leukocyte transendothelial migration. Following confluence for 48 hours, MNC (1×104) were added to the upper chambers and the chemoattractant SDF-1 was added to the lower chambers to result in a chemokine gradient. SDF-1 was selected because it is recognized as a potent stimulator of lymphocyte migration through vascular endothelium.
The plates were incubated for 3 hours at 37°C and any cells that had migrated through the endothelial layer into the lower chambers where collected and enumerated using a Nexcelom Cellometer. Negative controls consisted of MNC seeded into the upper chambers on top of the endothelial cell layer alone; while the positive controls consisted of the MNC in the upper chamber on top of the endothelial cell layers with 10 μg of SDF-1 placed in the lower chambers. The test groups consisted of the same combination of MNC/VEC monolayers but the endothelial cells were first treated with the ImmunoCloak membrane that was allowed to polymerize before introducing the MNC into the upper chambers and the SDF-1 in the lower chambers. The testing was done in triplicate in 3 experiments and the migration results were represented as the mean of the 3 experiments.
Results
Electron Microscopy of Immunocloaked Vascular Endothelium
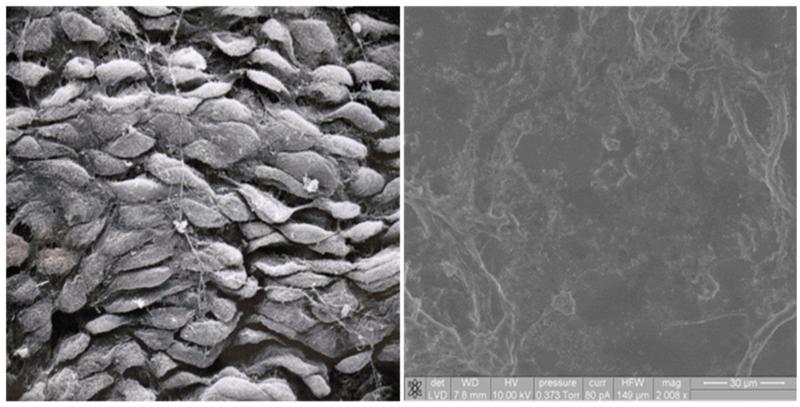
Confluent monolayers of VEC were assessed using scanning electron microscopy. A micrograph of confluent VEC is pictured in Figure 1a. A simple end-to-end cobblestone pattern can be typically seen. Applying the ImmunoCloak membrane to the confluent monolayer of endothelial cells results in a uniform coated surface (Figure 1b). While the normal functions of the endothelial cells continue unabated, the luminal surface becomes immunocloaked to the MNC that come in contact with the endothelial cell monolayer. In addition to providing a tissue engineered non-immunogenic luminal surface, the immunocloaked VEC surface remains non-thrombogenic.
Figure 1.
Scanning Electron Microscopy (SEM) of VEC in a confluent monolayer (left). SEM of ImmunoCloak treated VEC (right)
Antigen Presentation
Application of the ImmunoCloak membrane onto the surface of confluent cultures of VEC resulted in statistically significant inhibition of the cytokines/chemokines: IL-1β, γ-IFN, TNF-α, IL-8, IP-10 and MIP-1α & MIP-1β (p<0.05) (Table 1). This inhibition in the synthesis of cytokines that are normally produced by antigen presenting cells following stimulation with allogeneic endothelial cells suggests that the presence of the ImmunoCloak membrane prevents antigen presentation. The cytokines listed in Table 1 are recognized as pro-inflammatory. Therefore, the placement of the ImmunoCloak membrane prevented the synthesis of pro-inflammatory cytokines and provides evidence of the protective effect of the membrane in preventing the initial phase involved in allo-recognition.
Table 1.
Antigen Presentation
| IL-lβ | MIP-lα | MIP-lβ | IP-10 | TNF-α | IFN-γ | IL-8 | |
|---|---|---|---|---|---|---|---|
| MNC Alone | 3.6 ± 1.1 | 94.3 ± 2.7 | 66.6 ± 1.5 | 1282.5 ± 1666 | 8.8 ± 1.1 | 2.5 ± 1.3 | 1467 ± 61 |
| MNC + VEC | 4468 ± 48 | 3533 ± 212 | 2262 ± 181 | 13099 ± 326 | 1678 ± 16.0 | 1195 ± 57 | 14684 ± 140 |
| MNC + IC/VEC | 6.5 ± 1.4 | 4.6 ± 0.9 | 6.8 ± 1.1 | 57 ± 2.4 | 5.2 ± 1.2 | 1.5 ± 0.5 | 1917 ± 15 |
MNC= Allogeneic Mononuclear Cells
VEC = Confluent Vascular Endothelial Cells
IC/VEC = Immunocloaked VEC
Units in Net MFI (Mean Fluorescent Intensity)
Early T Cell Allogeneic Response
Similar to the observed inhibition of antigen presentation, the up-regulation of markers of T cell activation, IL-6, IL-2, MCP-1 and MIG were prevented following 24 hours of exposure to allogeneic VEC that had been treated with the ImmunoCloak membrane. Therefore, the presence of the ImmunoCloak membrane on the surface of the VEC leads to a blockade of T cell mediated responses (Table 2). These data demonstrate that the prevention of the primary antigen exposure provided by treatment with the ImmunoCloak membrane in turn prevents both antigen presentation and T cell activation.
Table 2.
T Cell Allogeneic Response
| IL-2 | MIG | IL-6 | MCP-1 | CD69% | |
|---|---|---|---|---|---|
| MNC Alone | 6.0 ± 0.8 | 0.9 ± 0.6 | 209.4 ± 7.6 | 51.2 ± 2.6 | 3.0 |
| MNC + VEC | 2154 ± 49 | 2,014 ± 51 | 1105± 62 | 13638 ± 81 | 12.4 |
| MNC + IC/VEC | 8.3 ± 2.0 | 1.7 ± 0.9 | 121 ± 14 | 278 ± 68 | 3.6 |
MNC= Allogeneic Mononuclear Cells
VEC = Confluent Vascular Endothelial Cells
IC/VEC = Immunocloaked VEC
CD69 Expression
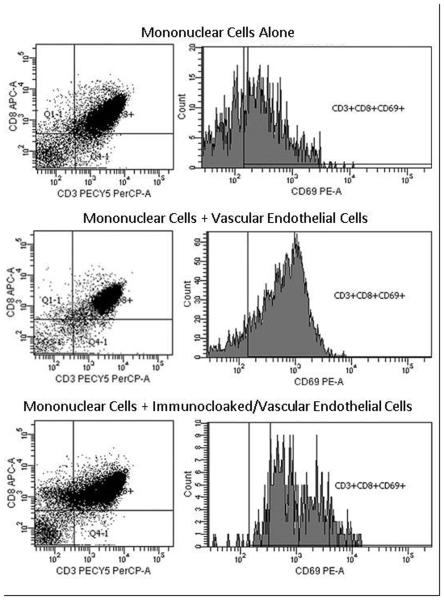
The results of the flow cytometric analysis demonstrated an up-regulation in the expression of the early T cell activation marker, CD69 when the MNCs were in the presence of allogeneic VEC. When the ImmunoCloak membrane was first bound to the allogeneic VEC CD69 was not significantly up-regulated and the number of cells positive for CD69 were equivalent to that of the negative controls (Figure 2).
Figure 2.
Flow cytometric analysis of expression of CD3 (PerCP), CD8 (APC) and CD69 (PE) on peripheral blood MNC. Negative control was represented as MNC, allogeneic VEC were used as stimulator cells in positive control and compared to immunocloaked VEC with MNC. Tests were performed in a 96-well plate in triplicate, pooled and analyzed (N=5). Scatterplots show an inhibition of CD69 activated cytotoxic T-cells in immunocloaked wells compared to positive control.
MNCs Proliferative Response
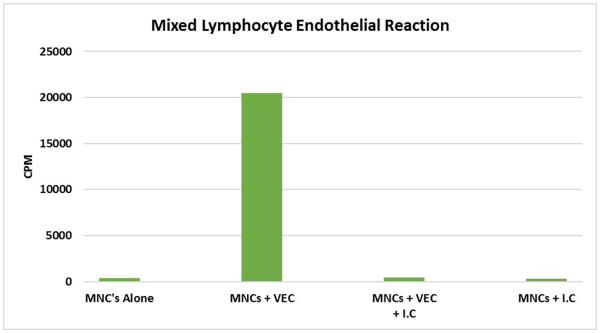
When human MNCs are stimulated with confluent monolayers of human allogeneic vascular endothelial cells isolated from umbilical cord veins the VEC strongly stimulated allogeneic MNCs (Figure 3). As expected the negative controls consisting of the MNCs alone did not proliferate. Immunocloaking the same donor VEC prior to introducing the allogeneic MNCs inhibited these in vitro allo-responses by 99% (Fig 3). Further evidence supporting the non-immunogenicity of the ImmunoCloak membrane is provided by the absence of a proliferative response when the responding MNCs were stimulated with the ImmunoCloak membrane alone. These results demonstrate that the ImmunoCloak membrane is non-immunogenic and its presence along the surface of the endothelial cell monolayer prevented a T cell proliferative response.
Figure 3.
A mixed lymphocyte-endothelial reaction was performed in a standard 96-well plate. MNC were plated alone, with allogeneic vascular endothelial cells (VEC) or on Immunocloaked (IC) VEC. Cells were allowed to proliferate for 4 days prior to pulsing with [3H] thymidine. The cells were then allowed to incubate for 18 hours and read on a scintillation counter (cpm).
Chemotactic Transmigration
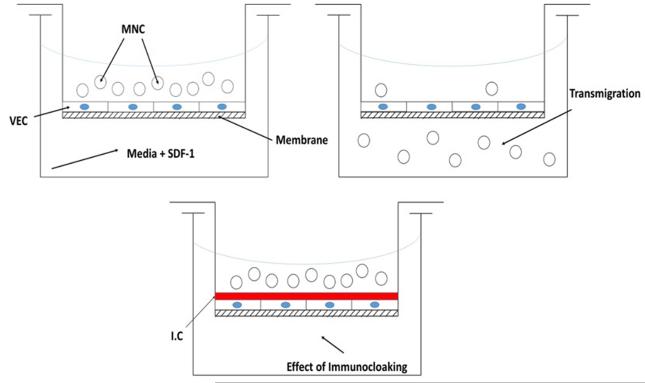
Theoretically, preventing endothelial cell activation would likewise prevent the externalization of the pre-formed Weibel-Palade bodies that contain P-selectin adhesion molecules. Without a pro-inflammatory signal, the multi-step leukocyte extravasation process would be averted while the ImmunoCloak membrane masks the allograft. The testing system is diagramed in Figure 4.
Figure 4.
Transmigration Study Diagram
MNCs= Allogeneic Mononuclear Cells
VEC = Confluent Vascular Endothelial Cells
SDF-1 = 10 ug Stromal Cell-Derived Factor 1,
IC = Immunocloaking Membrane
Membrane = 6.5mm Transwell with a 3.0μM polyester membrane
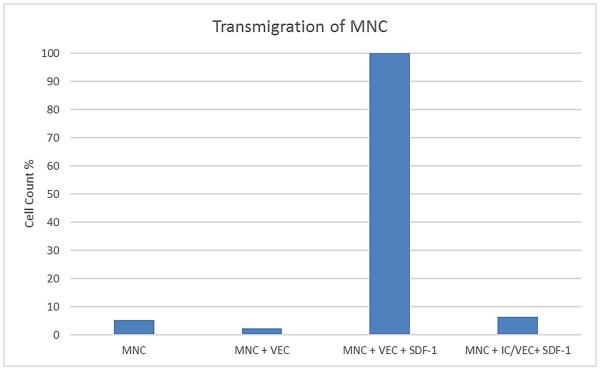
In the negative controls without SDF-1 very few MNC were found to have migrated through the endothelial cell layer into the lower chambers (Figure 5). In the case of the positive controls 65% of the cells were found to have migrated into the lower chambers containing the SDF-1 cytokine within 3 hours. However, when the endothelial cell monolayer was first immunocloaked, the leukocyte migration into the lower chambers containing the SDF-1 was inhibited by a mean of 93% Figure 5.
Figure 5.
MNC were plated into 6.5 mm Transwell with a 3.0 μM power polyester membrane alone, with allogenic VEC or with immunocloaked (IC) VEC. The MNC were stimulated using 10 ug/mL SDF-1 and allowed to incubate for 3 hours prior to enumeration using a hemacytometer.
The results of these immunologic screenings support our working hypothesis that immunocloaking can be successfully achieved in an organ-specific manner to prevent the cascade of events resulting in allo-recognition that normally occurs upon reperfusion.
Conclusions
We have previously shown that the application of the ImmunoCloak, significantly prolongs graft survival (approximately 5-fold) in the absence of systemic immunosuppression in a canine renal allograft model.10 While it was documented that the ImmunoCloak membrane provides a physical barrier between the graft vasculature and the recipient’s immune cells in the circulating blood during the early posttransplant period, specific mechanisms for how the ImmunoCloak membrane prevented the early allo-activation was previously not known.
The luminal surface along the vasculature within a renal allograft consists of a single monolayer of VEC. In vivo, VEC express both MHC class I & II antigens. As a result, endothelial cells are also capable of functioning as antigen presenting cells. Using a proliferative assay, endothelial cells were first recognized to stimulate T cell proliferation more than 30 years ago.11 Endothelial cells isolated from both large and small blood vessels express a variety of co-stimulating molecules resulting in increased receptor expression on activated and memory T cells. These inducible up-regulated receptors include LFA-3, CD58, OX40L, CD137, ICOS-L and GITR-L.12-15 In general, arterial blood vessels tend to be less responsive to the inflammatory cytokines than are endothelial cells located in the venous compartment.16 Some of this variability in responsiveness can be attributed to the difference in shear forces, PaO2, vascular flow rates as well as local tissue signaling. Although it has been reported that the interaction between naïve T cells and VEC can lead to tolerance, endothelial cells have also been shown to prime naïve as well as antigen-specific alloreactive T cells.17 Throughout the posttransplant period there is a continued stimulation of effector pathways mediated by the vascular endothelium that likely contributes to the development of chronic graft rejection.18 The up-regulation of integrins such as LFA-1, MAC-1 and VLA-4 facilitates the firm adherence of activated T cells to the surface of the graft vascular endothelium.19 Consequently, the endothelium is a major site of inflammation, allorecognition, T cell alloreactivity, leukocyte recruitment, diapedesis and the target of both cellular and humoral mediated immune responses. Therefore, the design and development of an organ-specific therapy will have to address the multifaceted intricacy of the interactions between donor vascular endothelium and the recipient’s T cells. The results of the present immunologic screenings suggest that placement of the ImmunoCloak membrane along the surface of VEC prevents antigen presentation and T cell activation for a period of time.
The targets of rejecting allografts were described 32 years ago by focusing on whether there was principal damage to parenchymal cells or the vascular cells.20 The morphological evidence supports that whether the nature of the rejection process was early, intermediate or late, the vasculature was always the primary target.21,22 The morphological patterns of injury observed included widespread destruction of the vascular endothelium.
In addition to VEC being the primary target of the rejection process, they are also active participants. The endothelium can initiate rejection by functioning as antigen presenting cells to circulating T cells. Similarly, the vascular endothelium plays an active role in the development of inflammation and thrombosis. The up-regulation of adhesion molecules on the surface of the endothelial cells leads to increased leukocyte adhesion resulting in enhanced diapedesis into the allograft.23 This extravasation of the activated leukocytes occurs through the endothelial monolayer both within individual cells and between the cellular junctions.24 In another study using high-resolution confocal imaging of the distribution of adhesion receptors known to play a role in diapedesis, a transcellular route was identified as well as the established paracellular route of transmigration. Both routes of extravasation involved endothelial “cuplike” transmigratory structures that were enriched with ICAM-1 and VCAM-1 projections following stimulation by cytokines.25 The kinetics involved with immune cells first rolling followed by firm adhesion to the vascular endothelium along with subsequent diapedesis can be measured in seconds and minutes.26,27 Therefore, the development of an organ-specific therapy to prevent allograft rejection must not only protect the vascular endothelium but also prevent direct T cell interactions during the immediate posttransplant period. The results of the transwell studies described herein demonstrate a significant reduction in leukocyte migration through the endothelial cell monolayer even in the face of a cytokine gradient if the endothelial cells are first treated with the ImmunoCloak membrane.
While an organ-specific therapy could represent an advancement over systemic immunosuppressive drug regimens, such a therapy could not interfere with the critical events that are involved in normal vascular biology. Microvessel endothelial cells do not interact with circulating T cells under normal conditions.28 However, T cells once activated can modulate VEC functions mediated by both direct contact and soluble signals. Therefore, the interactions between T cells and the vascular endothelium are bidirectional. If an organ-specific therapy that targets the allograft vasculature is to be achieved, it will be mandatory to prevent the allorecognition that is inherent in the bidirectional interaction between the immune and vascular systems.
The results of our studies demonstrate that applying the ImmunoCloak membrane to the most immunogenic component of a renal allograft prevents the allo-recognition that normally occurs immediately upon reperfusion. Our preclinical studies using the ImmunoCloak membrane have demonstrated a protective effect in the early posttransplant period by interrupting the recipient/donor interface.10 Using a canine kidney allograft model, a statistically significant prolongation of graft survival in comparison to controls was observed in the absence of systemic immunosuppression. This work also demonstrated that ubiquitous application of the membrane during the pretransplant period of ex vivo perfusion could be achieved. The application of the ImmunoCloak membrane to the vasculature provides a window of opportunity where immune activation is prevented in the absence of systemic immunosuppression. This interpretation is supported by the results of the flow cytometry, Luminex screening studies and chemotactic driven diapedesis studies demonstrating the absence of antigen presentation, T cell activation and proliferation or chemokine stimulated immune cell transmigration.
Our current efforts are focused on modifying the chemistry of the ImmunoCloak membrane to enhance the intravascular retention times by understanding how the membrane deteriorates along the vasculature. While our previous studies demonstrated the retention of normal renal function for as much as six months posttransplantation of immunocloaked renal autografts, further studies on vascular biology are warranted.10 Another concept worthy of investigation would be to use the window of opportunity where allorecognition does not occur to induce donor-specific tolerance. Alternatively, developing strategies to optimize costimulatory blockade in combination with low dose systemic immunosuppression during the period the ImmunoCloak membrane is beginning to degrade could positively impact long-term outcomes. Certainly eliminating the need for calcineurin inhibitors during the early posttransplant period could help to make transplanting ischemically damaged allografts more feasible.
Acknowledgments
This work was funded by the National Institute of Health R44 Grant Grant # R44AI081372-05
Abbreviations
- DCD
Donation after Cardiac Death
- VEC
Vascular Endothelial Cells
- SDF-1
Stromal Derived Factor-1
- MNC
Mononuclear Cells
Footnotes
Authorship
- Participated in research design
- Participated in the writing of the paper
- Participated in the performance of the research
- Contributed new reagents or analytic tools
- Participated in data analysis
- Participated in research design
- Participated in the performance of the research
- Participated in data analysis
- Participated in research design
- Participated in the writing of the paper
- Participated in data analysis
The authors declare no conflicts of interest.
References
- 1.Taflin C, Charron D, Glotz D, Mooney N. Immunological function of the endothelial cell within the setting of organ transplantation. Immunol Lett. 2011;139:1–6. doi: 10.1016/j.imlet.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 2.Hirschberg H, Evensen SA, Henriksen T, Thorsby E. Stimulation of Human Lymphocytes by Allogeneic Endothelial Cells in Vitro. Tissue Antigens. 1974;4:257–261. doi: 10.1111/j.1399-0039.1974.tb00249.x. [DOI] [PubMed] [Google Scholar]
- 3.Cerilli J, Brasile L. Abouna G, White A, editors. The role of vascular endothelial cell antigens in renal transplantation. In current status of clinical organ transplantation. Martinus Nijhoff (pub) 1984:15. [Google Scholar]
- 4.Preston EH, Light JA, Kampen RL, Kirk AD. Human renal allograft rejection despite the absence of allogeneic passenger leukocytes. Am. J. Transplant. 2004;4:283–285. doi: 10.1046/j.1600-6143.2003.00320.x. [DOI] [PubMed] [Google Scholar]
- 5.Divate SA. Acute renal allograft rejection: progress in understanding cellular and molecular mechanisms. J Postgrad Med. 2000;46:293. [PubMed] [Google Scholar]
- 6.Lechler R, Batchelor J. Immunogenicity of retransplanted rat kidney allografts. Effect of inducing chimerism in the first recipient and quantitative studies on immunosuppression of the second recipient. J Exp Med. 1982;156:1835–1841. doi: 10.1084/jem.156.6.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Watschinger B. Indirect recognition of allo MHC peptides - potential role in human transplantation. Nephr Dial Transplant. 1999;14:8–11. doi: 10.1093/ndt/14.1.8. [DOI] [PubMed] [Google Scholar]
- 8.Rothstein DM, Sayegh MH. T cell costimulatory pathways in allograft rejection and tolerance. Immunol Rev. 2003;196:85–108. doi: 10.1046/j.1600-065x.2003.00088.x. [DOI] [PubMed] [Google Scholar]
- 9.Kreisel D, Krupnick AS, Gelman AE, Engels FH, Popma SH, Krasinskas AM, Balsara KR, Szeto WY, Turka LA, Rosengard BR. Non-hematopoietic allograft cells directly activate CD8+ T cells and trigger acute rejection: an alternative mechanism of allorecognition. Nat Med. 2002;8:233–239. doi: 10.1038/nm0302-233. [DOI] [PubMed] [Google Scholar]
- 10.Brasile L, Glowacki P, Castracane J, Stubenitsky BM. Pretransplant kidney-specific treatment to eliminate the need for systemic immunosuppression. Transplantation. 2010;90(12):1294–8. doi: 10.1097/TP.0b013e3181ffba97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirschberg H, Evensen SA, Henriksen T, Thorsby E. Stimulation of human lymphocytes by cultured allogeneic skin and endothelial cells in vitro. Transplantation. 1975;19:191–194. doi: 10.1097/00007890-197502000-00016. [DOI] [PubMed] [Google Scholar]
- 12.Omari KI, Dorovini-Zis K. Expression and function of the costimulatory molecules B7-1 (CD80) and B7-2 (CD86) in an in vitro model of the human blood–brain barrier. J. Neuro immunol. 2001;113:129–141. doi: 10.1016/s0165-5728(00)00435-5. [DOI] [PubMed] [Google Scholar]
- 13.Epperson DE, Pober JS. Antigen-presenting function of human endothelial cells. Direct activation of resting CD8 T Cells. J. Immunol. 1994;153:5402–5412. [PubMed] [Google Scholar]
- 14.Khayyamian S, Hutloff A, Büchner K. ICOS-ligand, expressed on human endothelial cells, costimulates Th1 and Th2 cytokine secretion by memory CD4+ T cells. Proc. Natl. Acad. Sci. U.S.A. 2002;99:6198–6203. doi: 10.1073/pnas.092576699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nocentini G, Riccardi C. GITR: a multifaceted regulator of immunity belonging to the tumor necrosis factor receptor superfamily. Eur. J. Immunol. 2005;35:1016–1022. doi: 10.1002/eji.200425818. [DOI] [PubMed] [Google Scholar]
- 16.Valujskikh A, Heeger PS. Emerging roles of endothelial cells in transplant rejection. Current Opinion in Immunology. 2003;15:493–498. doi: 10.1016/s0952-7915(03)00110-9. [DOI] [PubMed] [Google Scholar]
- 17.Ma W, Pober JS. Human endothelial cells effectively costimulate cytokine production by, but not differentiation of, naïve CD4+ T cells. J. Immunol. 1998;161:2158–2167. [PubMed] [Google Scholar]
- 18.Rose ML. Endothelial cells as antigen-presenting cells: role in human transplant rejection. Cell Mol Life Sci. 1998;54(9):965–78. doi: 10.1007/s000180050226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272:60–66. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- 20.McCluskey RT. Comments on targets in rejecting allografts. Transplant Proc. 1980;12:22–25. [PubMed] [Google Scholar]
- 21.Busch GJ, Reynolds ES, Galvanek EG, Braun WE, Dammin GJ. Human renal allografts. The role of vascular injury in early graft failure. Medicine. 1971;50:29–83. [PubMed] [Google Scholar]
- 22.Busch GJ, Garovoy MR, Tilney NL. Variant forms of arteritis in human renal allografts. Transplantation. 1979;11:100–103. [PubMed] [Google Scholar]
- 23.Bevilaqua MP. Endothelial leukocyte adhesion molecules. Annu Rev Immunol. 1993;11:767–804. doi: 10.1146/annurev.iy.11.040193.004003. [DOI] [PubMed] [Google Scholar]
- 24.Shivizu Y, Shaw S, Graver N. Activation-indepentent binding of human memory T cells to adhesion molecule ELAM-1. Nature. 1996;349:786–799. doi: 10.1038/349799a0. [DOI] [PubMed] [Google Scholar]
- 25.Carman CV, Springer TA. A transmigratory cup in leukocyte diapedesis both through individual VEC and between them. J Cell Biol. 2004;167:377–388. doi: 10.1083/jcb.200404129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carman CV, Jun CD, Salas A, Springer TA. Endothelial cells proactively form microvilli-like membrane projection upon ICAM-1 engagement of leukocyte LFA-11. J Immunol. 2003;171:6135–6144. doi: 10.4049/jimmunol.171.11.6135. [DOI] [PubMed] [Google Scholar]
- 27.Luu NT, Rainger GE, Nash GB. Kinetics of the different steps during neutrophil migration through cultured endothelial monolayers treated with tumor necrosis factor-alpha. J Vasc Res. 1999;36:477–485. doi: 10.1159/000025690. [DOI] [PubMed] [Google Scholar]
- 28.Hancock WW, Kraft N, Atkins RC. The immunohistochemical demonstration of major histocompatibility antigens in the human kidney using monoclonal antibodies. Pathology. 1982;31:75–78. doi: 10.3109/00313028209092120. [DOI] [PubMed] [Google Scholar]