Novel H5N6 viruses were first documented in Laos in 2013 and subsequently reported in Vietnam and China1, but these viruses did not initially attract a great deal of attention. Instead, there was a greater focus on known H5N6 subtype AIVs until a fatal human infection with a novel H5N6 virus was confirmed in Sichuan Province, China2. As of May 2017, 17 human infections with these novel H5N6 highly pathogenic avian influenza viruses (HPAIVs), including 12 deaths, have been identified3.
H5N6 was identified as one of the dominant AIV subtypes among poultry in southern China4. However, as of June 2017, few cases involving wild birds infected with H5N6 AIVs have been reported in China, and these cases have been reported only in central (Hubei) and eastern China (Jilin, Guangdong)2,4,5. We first reported novel H5N6 HPAIVs infections in eight species of migratory birds in western China.
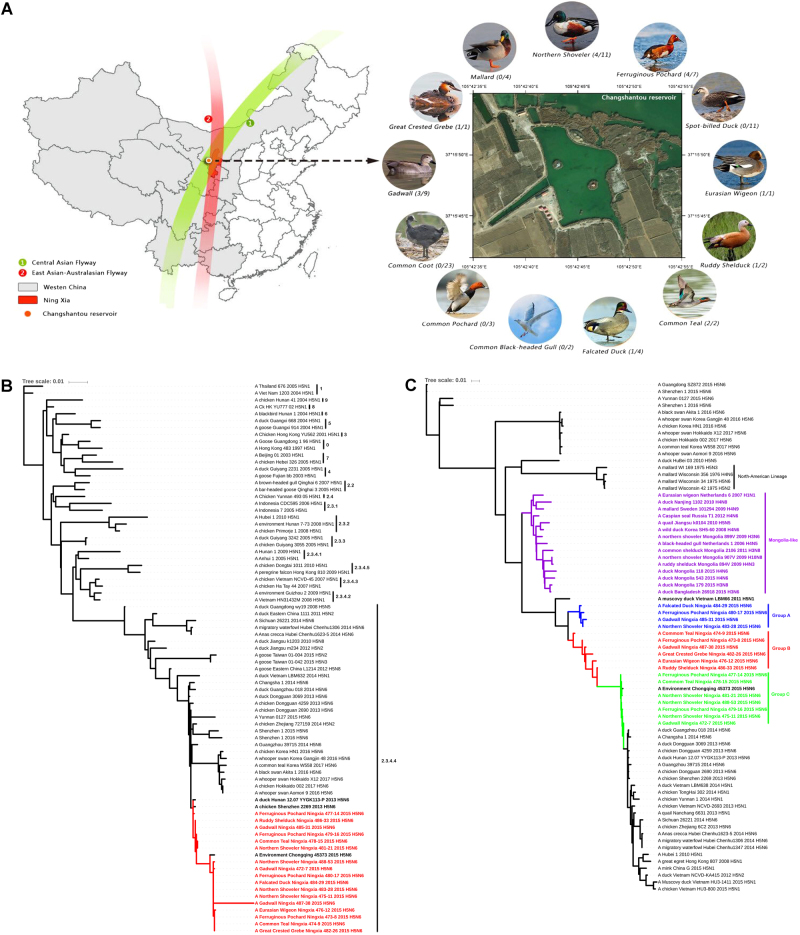
In November 2015, oropharyngeal and cloacal swabs from 80 wild waterfowl were collected during active surveillance at Changshantou reservoir, Ningxia, western China (Fig. 1a). Viruses were isolated in 10-day-old specific pathogen-free chicken embryos, and 17 samples were positive for H5N6 avian influenza virus according to RT-PCR detection; thus, the separation rate was 21.25% (17/80). All sequences determined in this study were confirmed by Sanger sequencing and have been submitted to GenBank under accession numbers MF399537–MF399672. We performed further experiments, including a receptor-binding specificity assay, a mouse pathogenicity test, and a guinea pig transmission test, to evaluate the pathogenicity and transmissibility of the isolated viruses (Supplementary Materials).
Fig. 1. The sampling location and phylogenetic analysis of HA and PB1 genes.
a The sampling location in Ningxia, western China. The ratio of the number of isolated viruses to the number of samples is indicated in parentheses. b Phylogenetic tree of HA showing relationships of emergent influenza A(H5N6) viruses with clade 2.3.4.4 H5 avian influenza viruses and c phylogenetic tree of PB1 showing that 17 PB1 genes cluster into three groups named Group A (red), Group B (green), and Group C (blue), evolving from Mongolia-like LPAIVs
The HA proteins of all 17 H5N6 AIVs contain the HPAIV amino acid sequence RERRRKR/GLF at the site of cleavage into HA1 and HA26. In these viruses’ HA proteins, although the amino acid substitutions Q222L and G224S (H5 numbering) were not found, the substitutions D94N, S123P, and S133A (H5 numbering), which are associated with increased binding to α-2, 6-linked sialic acid were identified7. Moreover, an 11-aa deletion at residues 59–69 of the neuraminidase (NA) protein was identified in all isolated viruses; this deletion could enhance virulence in mammals8. Mutations associated with increased virulence in mice were observed in matrix 1 protein (N30D)9, polymerase basic 2 protein (L89V), and nonstructural protein 1 (P42S, D87E, L98F, I101M, and the 80–84 deletion) in all isolated viruses (Supplementary Table S1)10,11.
Phylogenetic analysis was performed using RAxML with 1000 bootstrap replicates, which revealed that all 17 isolated virus genomes belong to the Eurasian lineage (Fig. 1b–c and Supplementary Fig. S1A–F), and these viruses’ hemagglutinin (HA) genes clustered into clade 2.3.4.4 (Fig. 1b). Seven genes of the 17 isolated viruses, except the polymerase basic 1 (PB1) gene, originate from A/duck/Hunan/12.07 YYGK113-P/2013(H5N6) (HN113-P) and share the highest nucleotide identity with A/Environment/Chongqing/45373/2015(H5N6) (CQ45373), with identities ranging from 98.9 to 99.6% (for the HA gene, with the exception of the HA gene of NX488-38) to 99.8–100% (for the NS gene), indicating the same genotype. In particular, the HA gene of A/Gadwall/Ningxia/487-38/2015(H5N6) (NX487-38) shares less than 97.6% nucleotide identity with all known strains. All isolated viruses also share high homology with A/Changsha/1/2014(H5N6) (CS1), with 98.2–99.2% nucleotide identity for the HA gene and 98.4–99.7% nucleotide identity for other genes, with the exception of the PB1 gene and HA gene of NX487-38.
The nucleotide homologies for the PB1 genes of the 17 isolated viruses were 92.8–99.9%; therefore, these PB1 genes exhibit diversity. A phylogenetic tree of PB1 genes showed that all 17 isolated viruses clustered into three groups (Groups A, B, and C) (Fig. 1c). Group A shares less than 93% nucleotide homology with Group C, and Group B is a transition group between Group A and Group C. Group B and Group C share the highest nucleotide homologies with CQ45373. A phylogenetic tree showed that the PB1 gene may originate from unidentified Mongolia-like low pathogenic avian influenza viruses (LPAIVs), evolving from A/duck/Mongolia/179/2015 (H3N8) and A/duck/Bangladesh/26918/2015 (H3N6), but the detailed evolutionary history of PB1 genes remains unclear.
The isolates are closely related to human virus CS1, and sequence analysis showed that they possess the same key amino acids associated with mammalian infectivity and pathogenicity. Therefore, we selected NX488-53 (because all isolated viruses have the same key amino mutations, as shown in Supplementary Table S1) as a representative strain and performed further experiments. The receptor-binding specificity was determined by hemagglutinin assays, revealing that the NX488-53 virus preferentially binds α-2, 3-linked avian sialic acid receptors (Supplementary Fig. S2). We further evaluated the pathogenicity of NX488-53 in a mouse model (Supplementary Materials). The body weights of the virus-inoculated BALB/c mice first decreased and then increased gradually. At 14 dpi, the body weights of the infected mice were comparable to those of the controls (Supplementary Fig. S3A). The pathogenicity of the NX488-53 virus in the inoculated BALB/c mice was low and thus resulted in non-lethal infections (Supplementary Fig. S3B). The virus replicated well in the lungs of infected mice at 1, 3, 5, and 7 dpi, with titers ranging from 101.8 to 103.2 EID50 (Supplementary Fig. S3C). The virus was also detected in the kidney, heart, and liver at 5 dpi, but no virus was detected in the brain or spleen throughout the course of infection (Supplementary Fig. S3C). Histological analysis showed that the NX488-53 virus was capable of systemic infection and induced slight pathological changes (Supplementary Fig. S4). These results indicate that mice could be infected by this virus without prior adaption, but the virus showed low pathogenicity in BALB/c mice. A guinea pig transmission test showed that the virus was detected in only nasal washes of the group inoculated intranasally (Supplementary Fig. S5), indicating that it had not yet acquired transmissibility among guinea pigs via direct contact or aerosol.
We report the first case of novel H5N6 HPAIV infection in migratory birds in western China. Phylogenetic and molecular analyses indicated that all novel H5N6 HPAIVs have been generated by the reassortants of Mongolia-like LPAIVs and HN113-P, which donated their PB1 gene and another seven genes, respectively. These viruses’ HA genes cluster into clade 2.3.4.4. The PB1 genes from the isolated viruses exhibit diversity.
Although NX488-53 showed low pathogenicity in BALB/c mice, mice could be infected by this virus without prior adaption. These results indicate that the isolated viruses may be able to infect other mammals, including humans. In addition, an examination of the transmissibility of the NX488-53 virus in guinea pigs showed that it has not acquired the ability to be transmitted among guinea pigs via direct contact or aerosol, indicating that these viruses may not yet have the capacity for mammal-to-mammal transmission, which is one possible reason for the sporadic H5N6 infections in humans, as opposed to large-scale outbreaks.
Wild aquatic birds have been suspected to play a key role in the dissemination of H5 HPAIVs to various regions during their migration, such as clade 2.2 H5N1 HPAIV in 2005, clade 2.3.2.1 H5N1 HPAIV in 2009, and clade 2.3.4.4 H5N8 HPAIV in 201412,13. Ningxia, which is located in western China, is where the East Asian/Australasian and Central Asian flyways overlap and therefore represents an important ecological niche for migratory birds (Fig. 1a). In this study, PB1 genes were found to involve from unidentified Mongolia-like LPAIVs; thus, we speculate that the isolated viruses may have been generated by the reassortment of circulating H5N6 viruses in China with Mongolia-like LPAIVs, suggesting possible close contact during migration of birds carrying LPAIVs from Mongolia to wintering sites. Therefore, in addition to intensification of the surveillance of AIVs in migratory birds in western China, international surveillance and information sharing should be strengthened.
Electronic supplementary material
Acknowledgements
This research was supported by the National Natural Science Foundation of China (Grant No. 31601873), Fundamental Research Funds for the Central Universities (2572017CA19 and DL13EA01-03) and the 13th Five-Year National Key Research Project: the Migration of Wild Animal Epidemic Disease Transmission Risk Research Project (Grant No. 2016YFC1201600). This work was also funded by the Surveillance of Wildlife Diseases from the State Forestry Administration of China. We are also grateful to other related laboratories for their technical support. We are thankful to Yanbing Li (Harbin Veterinary Research Institute, Chinese Academy of Agriculture Sciences, Harbin, China) for her guidance in data analysis. We also thank Yuping Hua (College of Wildlife Resources, Northeast Forestry University, Harbin, China) for his assistance with experimental design.
Competing interests
The authors declare that they have no competing interests.
Contributor Information
Hongliang Chai, Email: 17758625@163.com.
Linna Liu, Email: liulinna7@126.com.
Jun Qian, Email: qianj1970@126.com.
Jianzhang Ma, Email: jianzhangma@163.com.
Electronic supplementary material
Supplementary Information accompanies this paper at 10.1038/s41426-017-0001-1.
References
- 1.Bi Y, et al. Genesis, evolution and prevalence of H5N6 avian influenza viruses in China. Cell Host Microbe. 2016;20:810–821. doi: 10.1016/j.chom.2016.10.022. [DOI] [PubMed] [Google Scholar]
- 2.Bi Y, et al. Novel avian influenza A (H5N6) viruses isolated in migratory waterfowl before the first human case reported in China, 2014. Sci. Rep. 2016;6:29888. doi: 10.1038/srep29888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jiang H, et al. Preliminary epidemiologic assessment of human infections with highly pathogenic avian influenza A(H5N6) virus, China. Clin. Infect. Dis. 2017;65:383–388. doi: 10.1093/cid/cix334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu Z, et al. Fatal H5N6 avian influenza virus infection in a domestic cat and wild birds in China. Sci. Rep. 2015;5:10704. doi: 10.1038/srep10704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kang Y, et al. Highly pathogenic H5N6 influenza A viruses recovered from wild birds in Guangdong, southern China, 2014-2015. Sci. Rep. 2017;7:44410. doi: 10.1038/srep44410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Y, et al. A single amino acid at the hemagglutinin cleavage site contributes to the pathogenicity and neurovirulence of H5N1 influenza virus in mice. J. Virol. 2012;86:6924–6931. doi: 10.1128/JVI.07142-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang W, et al. Glycosylation at 158N of the hemagglutinin protein and receptor binding specificity synergistically affect the antigenicity and immunogenicity of a live attenuated H5N1 A/Vietnam/1203/2004 vaccine virus in ferrets. J. Virol. 2010;84:6570–6577. doi: 10.1128/JVI.00221-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsuoka Y, et al. Neuraminidase stalk length and additional glycosylation of the hemagglutinin influence the virulence of influenza H5N1 viruses for mice. J. Virol. 2009;83:4704–4708. doi: 10.1128/JVI.01987-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fan S, et al. Two amino acid residues in the matrix protein M1 contribute to the virulence difference of H5N1 avian influenza viruses in mice. Virology. 2009;384:28–32. doi: 10.1016/j.virol.2008.11.044. [DOI] [PubMed] [Google Scholar]
- 10.Gao R, et al. Human infection with a novel avian-origin influenza A (H7N9) virus. N. Engl. J. Med. 2013;368:1888–1897. doi: 10.1056/NEJMoa1304459. [DOI] [PubMed] [Google Scholar]
- 11.Monne I, et al. Highly pathogenic avian influenza A(H5N1) virus in poultry, Nigeria, 2015. Emerg. Infect. Dis. 2015;21:1275–1277. doi: 10.3201/eid2107.150421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lycett SJ, et al. Role for migratory wild birds in the global spread of avian influenza H5N8. Science. 2016;354:213–217. doi: 10.1126/science.aaf8852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olsen B, et al. Global patterns of influenza A virus in wild birds. Science. 2006;312:384–388. doi: 10.1126/science.1122438. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.