Abstract
Hepatitis E virus (HEV, genus Orthohepevirus) is a common cause of hepatitis worldwide. Human-infecting HEV strains (Orthohepevirus A) include human-restricted and enzootic genotypes. Viruses in the Orthohepevirus A species also infect rabbits (HEV-3ra), camels, and swine. Using a selection-informed method, we dated the origin of the Orthohepevirus genus at least 21 million years ago, whereas the Orthohepevirus A species originated in Asia, most likely from a human-infecting ancestor that existed ~4500 to 6800 years ago. In this period, the appearance of large human settlements probably facilitated HEV emergence and spread. The earliest events in Orthohepevirus A evolutionary history involved the separation of the enzootic and human-restricted genotypes, as well as the split of the camel-infecting genotypes, which occurred during the time-frame of camel domestication. The place and timing of HEV-3ra divergence also correspond to the circumstances of rabbit domestication. This study clarifies the origin and historical events underlying HEV dispersal.
Introduction
Hepatitis E virus (HEV) is the most common cause of enterically-transmitted viral hepatitis worldwide1. HEV infection usually causes acute self-limiting hepatitis, but fulminant hepatic failure can occur in pregnant women, elderly patients or individuals suffering from underlying chronic liver disease1. In immunocompromised patients, HEV infection can develop into chronic hepatitis1. Overall, HEV imposes a significant health burden, with 20 million estimated annual infections, 3.3 million symptomatic cases, and more than 50,000 HEV-related deaths (http://www.who.int/mediacentre/factsheets/fs280/en/).
HEV is a single stranded, positive RNA virus belonging to the Hepeviridae family. Members of this family are classified into two genera, Orthohepevirus and Piscihepevirus2. The Piscihepevirus genus includes only one species with one member (cutthroat trout virus), whereas the Orthohepevirus genus is divided into four species (Orthohepevirus A to D)2. Human-infecting HEV strains belong to the Orthohepevirus A species: genotypes 1 and 2 (HEV-1 and HEV-2) infect only humans, whereas genotypes 3 and 4 (HEV-3 and HEV-4) have been described in humans and other domestic (mainly pigs) and wild animals1. Additional Orthohepevirus A genotypes were detected in rabbits (HEV-3ra), boars (HEV-5 and HEV-6), and camels (HEV-7 and HEV-8)1. Other species in the Orthohepevirus genus infect birds (Orthohepevirus B), rats and ferrets (Orthohepevirus C), as well as bats (Orthohepevirus D)1. Additional HEV sequences identified in mammals and birds are distantly related to other HEV species and remain unclassified1.
Human-infecting HEV genotypes display distinct epidemiological patterns: HEV-1 and HEV-2 cause waterborne outbreaks mainly in tropical and subtropical regions1, whereas the zoonotic transmission of HEV-3 and HEV-4 accounts for the majority of hepatitis E human cases in industrialized countries1. Phylogenetic analyses of HEV-3 and HEV-4 sequences from humans and swine revealed no clustering by host species3. This observation, and the ability of viruses derived from swine to infect non-human primates1, suggest that pig-infecting HEV-3 and HEV-4 can readily cross the species barrier and infect humans. Likewise, cynomolgus macaques can be infected with rabbit HEV, and a human HEV strain closely related to HEV-3ra was isolated, suggesting inter-species transmission of the virus from rabbits to humans4, 5. Recently, the identification of HEV-7 in a patient who regularly consumed camel meat and milk suggested that this genotype is also able to infect our species6. To summarize, whereas it seems that all Orthohepeviruses A are transmissible to humans, experimental infection with human-derived HEV-1 and HEV-2 strains indicated that these viruses have a limited host range, which is virtually limited to primates1. HEV genotypes are therefore usually referred to as enzootic (HEV-3 and 4) or human-restricted/anthropotropic (HEV-1 and 2).
The evolutionary events that led to the origin and radiation of the major Orthohepevirus species, as well of the Orthohepevirus A genotypes remain poorly understood. An analysis conducted before the identification of the boar and camel-derived genotypes indicated that the ancestor of HEV genotypes 1–4 split into human-restricted and enzootic lineages in relatively recent times, about 536 to 1344 ya7. The same study suggested that the ancestor of all Orthohepevirus A was enzootic7.
Herein we used a large set of HEV sequences to investigate the geographic origin and ancestral host range of Orthohepevirus A, as well as to estimate the timing of speciation and genotype radiation.
Materials and methods
Geographic distribution of HEV genotypes
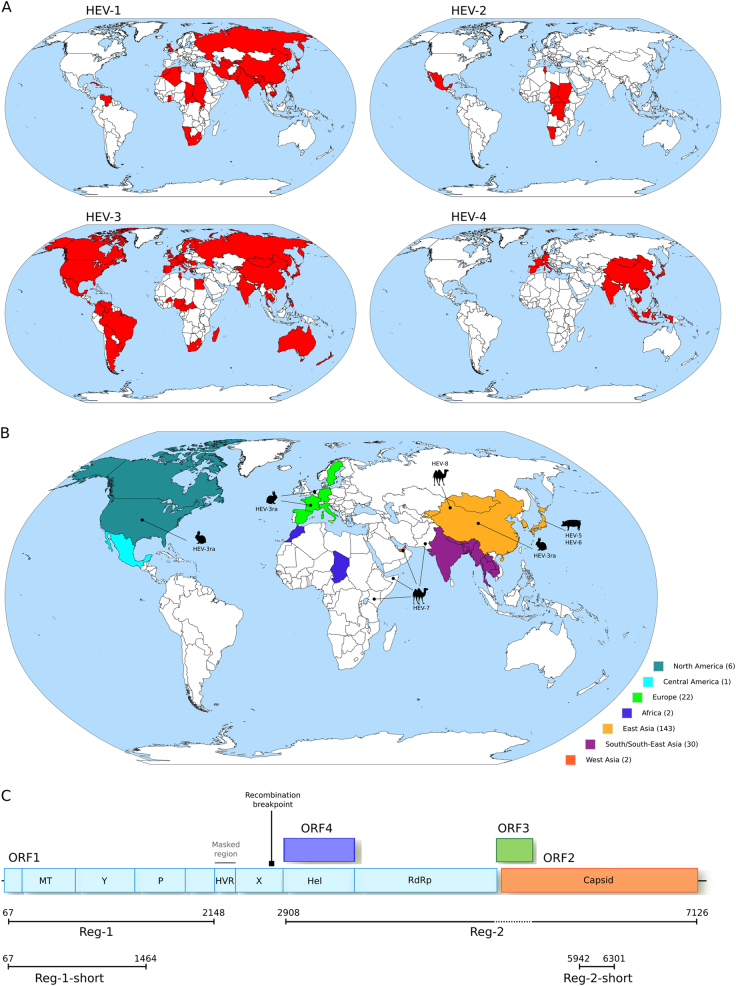
To obtain an overview of the geographic distribution of Orthohepevirus A genotypes, we updated epidemiological surveys8–14 (Supplementary Table S1) with recent literature reports of molecular-typed HEV infections, as well as with information on HEV strains identified in animals (Figs. 1a, b). Genotypes were assigned to countries irrespective of their prevalence. Thus, even if a single case was reported in a given country, the genotype was recorded as present. Cases that could be clearly ascribed to migration/travels were excluded.
Fig. 1. Worldwide distribution of HEV genotypes.
a Geographic distribution of the four major HEV genotypes (HEV-1 to HEV-4). b Geographic origin of complete Orthohepevirus A genomes analyzed in this study. The number of sequences deriving from each continent or region is reported in parentheses. The location where animal-infecting Orthohepevirus A were collected is also shown. c Schematic representation of the Orthohepevirus A genome. Positions refer to the Burma (GenBank accession: M73218) reference strain for genotype 1. Note that ORF4 has been described only in HEV-1 and not in other HEV genotypes25. The regions we used in different analyses, as detailed in the text, are shown. The region we filtered from the Orthohepevirus A genome alignment is indicated. The location of the recombination breakpoint is also reported. MT methyltransferase, P papain-like cysteine protease, Hel helicase, RdRp RNA-dependent RNA polymerase, Y Y domain, HVR hypervariable region, also known as polyproline region (PPR); X, macro domain
Sequences, alignments, and recombination
All complete or almost complete Orthohepevirus genomes were retrieved from the ViPR database (https://www.viprbrc.org/)15. As of 1 March 2017, 289 Orthohepevirus A sequences were available. Information on collection date and host, as well as geographic origin, were retrieved either via ViPR or through manual inspection of the literature. We discarded sequences with incomplete information. HEV strains deriving from cell culture adaptation, recombinants, and sequences isolated from experimentally inoculated animals were also pruned. The final dataset comprised 206 Orthohepevirus A sequences and included most strains proposed as references for HEV genotypes (with the exclusion of reference strains for genotypes 2b and 3d, as only partial sequences are available) (Supplementary Table S2). As for Orthohepevirus B to D, a total of 31 complete genomes were available, all of them with complete information about sampling date and location, as well as host (Supplementary Table S2). The sequences of three unclassified Orthohepeviruses deriving from a Swedish moose, a little egret, and a common kestrel16–18 were also downloaded from ViPR, as well as the genome sequence of cutthroat trout virus19 (Supplementary Table S2).
To root phylogenies, the outgroup method was used. In particular, the viral sequence falling outside the ingroup and showing the greatest phylogenetic proximity was selected20. Thus, the cutthroat trout virus19, which is the only member of the Piscihepevirus genus, was selected as the outgroup to root the Orthohepevirus phylogeny. As for analyses restricted to Orthohepeviruses A, the moose-derived virus was used for rooting. In fact, this virus falls outside the Orthohepevirus A species, but is more closely related to Orthohepeviruses A than other Orthohepevirus species (B to D)16.
A whole genome alignment of Orthohepevirus A sequences was generated using MAFFT21. The region corresponding to the hypervariable region (HVR) was filtered due to very poor alignment quality and recombination was searched for using the genetic algorithm GARD22 implemented in the HYPHY package23. GARD uses phylogenetic incongruence among segments in an alignment to detect the best-fit number and location of recombination breakpoints and evaluates the statistical significance of putative breakpoints through Kishino-Hasegawa tests. Because we detected one statistically significant (P < 0.01) breakpoint, two subregions, Region-1 (Reg-1) and Region-2 (Reg-2) were used in all analyses. In particular, Reg-1 includes the coding portion showing good alignment quality located 5′ of the recombination breakpoint (Fig. 1c). Reg-2 covers the helicase and RdRp domains in ORF1 plus the ORF2 region that shows no overlap with ORF3 (Fig. 1c). To increase the alignment length and, consequently, the confidence in phylogenetic reconstruction24, the helicase domain was included even though an alternative reading frame ORF (ORF4) was described (Fig. 1c); however ORF4 was only reported for HEV-125. The ORF1 and ORF2 segments were concatenated.
For phylogeographic analysis, we also used a sub-region of Reg-2 (denoted Reg-2-short). Reg-2-short was selected to maximize the number of available African and South/Central American sequences. In particular, we downloaded from ViPR all HEV genome fragments longer than 300 nucleotides and deriving from sequences isolated in Africa and South/Central America. We aligned these fragments to complete HEV genomes and selected the region of highest coverage with a minimum alignment length of 300 bp. This region corresponded to Reg-2-short, with an alignment length of 360 nucleotides.
Alignments of all regions were generated using RevTrans 2.026, which uses the protein alignment as a scaffold to build the nucleotide alignment.
Phylogeographic analysis and reconstruction of ancestral host range
For the phylogeographic analysis of complete HEV genomes, sequences were assigned to continents. As the majority of complete genomes came from Asia, this continent was divided into sub-region, namely East Asia, West Asia, and South/South-East Asia, based on the United Nation geographical sub-regions (http://unstats.un.org/unsd/methods/m49/m49regin.htm). Areas and the number of sequences per area are shown in Fig. 1b.
As for the phylogeographic analysis of Reg-2-short, 48 African sequences were available. We thus selected 48 Reg-2-short sequences from those sampled in South/Central America (out of a total of 61) and the same number from Asian, European, and North American sequences. All these sequences were selected to be distinct from those used in the full genome analyses and to be representative of collection date and country. Because only 31 South/South-East Asian sequences were available (and fewer for West Asia), East, West, and South/South-East Asia were collapsed in a single area (Asia) (Supplementary Table S3).
Inference of evolutionary rates, geographical origin, and ancestral host range were obtained using the Bayesian Evolutionary Analysis by Sampling Trees (BEAST, version 2.4.4) software27. Analyses were performed using the Bayesian Markov Chain Monte Carlo (MCMC) method with a General Time Reversible (GTR) substitution model and a gamma distribution (G) rate with 4 categories among sites. The GTR + G model was selected using the “ModelTest” utility28 implemented in the HYPHY package. A strict molecular clock was used.
The geographical locations and ancestral hosts at internal nodes were estimated using the discrete model implemented in BEAST29. Two different runs, one hundred million iterations each, were performed and sampled every 10 000 steps with a 10% burn-in. Runs were then combined after checking for convergence. Maximum clade credibility trees were summarized using TreeAnnotator27. All analyzed nodes had a posterior probability higher than 0.99. Trees were visualized with FigTree (http://tree.bio.ed.ac.uk/).
Both ancestral characters (geographic origin and host range) were also inferred using the BBM (Bayesian Binary MCMC) method implemented in RASP (Reconstruct Ancestral State in Phylogenies)30, 31. For BBM analyses, 10 000 BEAST-generated trees and consensus tree were used as topology input. Two BBM chains were run for 100 000 generation with estimated state frequencies (F81), a gamma distributed among-site rate variation, sampling every 100 generations, and null character state for the outgroup (Swedish moose-derived Orthohepevirus).
BBM was preferred over other methods in the RASP suite for several reasons: (1) DEC and S-DEC (Statistical Dispersal–Extinction–Cladogenesis) could not handle the large number of nodes; (2) S-DIVA (Statistical-Dispersal Vicariance Analysis) favors models of vicariance and ancestral areas can be wrongly identified when evolutionary patterns are more complex30, 32; 3) BBM allows null character status information for a portion of input sequences. This property was exploited to run 100 analyses to check for consistency against the skewed geographic origin and host species of available sequences. In particular, for ancestral host range, most viruses were isolated from humans (n = 108) and swine (n = 60); we thus generated 100 distributions by randomly omitting character state for 22 human-derived sequences sampled from genotypes 3 and 4. For each distribution, we recorded the two highest probabilities, which always corresponded to human and swine.
For phylogeographic analysis of complete HEV genomes, 100 distributions were generated for BBM analysis so as to include a similar number of sequences from East-Asia, South/South-East Asia, and Europe. For each of these distribution, location probabilities were recorded for the MRCA and selected internal nodes. Probabilities were averaged across the 100 runs and the result displayed as pie charts.
Time estimates
The action of purifying selection can bias tMRCA evaluation, and selection-informed models can improve branch length estimation33, 34. We thus applied a branch-site model (aBS-REL, adaptive branch-site random effects likelihood)35 to estimate branch lengths while taking into account the effect of different selective pressures among sites and lineages in the phylogeny.
To determine whether there was sufficient temporal structure in the HEV phylogenies to estimate divergence times, we used TEMPEST to perform a regression of root-to-tip genetic distances against year of sampling36. Evidence for temporal structure was obtained for both Reg-1 (R2 = 0.26) and Reg-2 (R2 = 0.24).
Estimates of divergence times were performed with the LSD (least-squares dating) software v0.237. The aBS-REL tree was used as the input tree and in the case of extremely long terminal branches, most likely resulting from low precision in point estimates of dN/dS, branch lengths calculated using the GTR + G model were used instead of aBS-REL lengths.
A latin hypercube sampling scheme was used to sample from the aBS-REL parameter distributions so as to estimate confidence intervals, as previously suggested33, 34. Briefly, 500 samples were drawn from aBS-REL analyses to estimate branch length variance, 500 trees were generated, and then used as input trees for LSD. The upper and the lower 95% bounds were used as confidence intervals.
As a comparison, timescale trees were also estimated using the MCMC algorithm implemented in the BEAST package in combination with the GTR + G substitution model; we ran 2 chains for 100 million states with a step of 10 000. After discarding a 10 % burn-in and merging the two runs, the output was analyzed using TreeAnnotator.
tMRCA estimates of the origin of the Orthohepevirus genus were obtained using a phylogeny that included all available Orthohepevirus complete sequences that are not classified as Orthohepevirus A plus a sub-sample of Orthohepevirus A (n = 82, Supplementary Table S2), which were selected to be representative of different collection dates, geographic origins and hosts. Branch lengths were estimated using phyML with a maximum-likelihood approach, a General Time Reversible (GTR) model plus gamma-distributed rates, and four substitution rate categories38. Substitution rates and the dates of ancestral nodes were estimated using LSD. Branch lengths were also obtained using aBS-REL to account for the effect of variation in selective pressure along branches and to re-estimate ancestral node dating. The cutthroat trout virus sequence was used to root the phylogeny.
Results
Worldwide distribution of HEV genotypes and phylogeographic reconstruction
To obtain an overview of the geographic distribution of Orthohepevirus A genotypes, we combined data from human infections with information on HEV strains identified in animals (Supplementary Table S1). Results were consistent with known patterns1: HEV-1 and HEV-2 mainly occur in tropical and subtropical regions of Asia, Africa, and Latin America; HEV-4 is mostly observed in Asia and Europe; and HEV-3 is distributed worldwide (Fig. 1a). Inclusion of geographic information for HEV-3ra, HEV-5/6, and HEV-7/8 indicated that East Asia hosts the largest diversity of HEV genotypes (Fig. 1b). Although this distribution may reflect recent events or more intense sampling in Asia, it is consistent with an East Asian origin of Orthohepevirus A. We thus decided to formally test this hypothesis using phylogeographic analyses.
We compiled a list of 206 Orthohepevirus A complete or almost complete genomes with information concerning date and place of collection, as well as host species (Supplementary Table S2). A genome alignment was generated and the HVR in ORF1 was filtered due to very poor alignment reliability. The resulting alignment was screened for the presence of recombination events using GARD22 and one breakpoint was detected immediately upstream the helicase domain of ORF1 (Fig. 1c). The location of this breakpoint corresponds to those previously identified using other methods and HEV sequence sets39, 40, suggesting that the two regions defined by the breakpoint have distinct evolutionary histories. Subsequent analyses were thus carried out on two regions: one that covers the 5′ region of ORF1 (Reg-1) and the other that comprises the helicase and RdRp domains of ORF1 plus ORF2 with the exclusion of the region of overlap with ORF3 (Reg-2) (Fig. 1c).
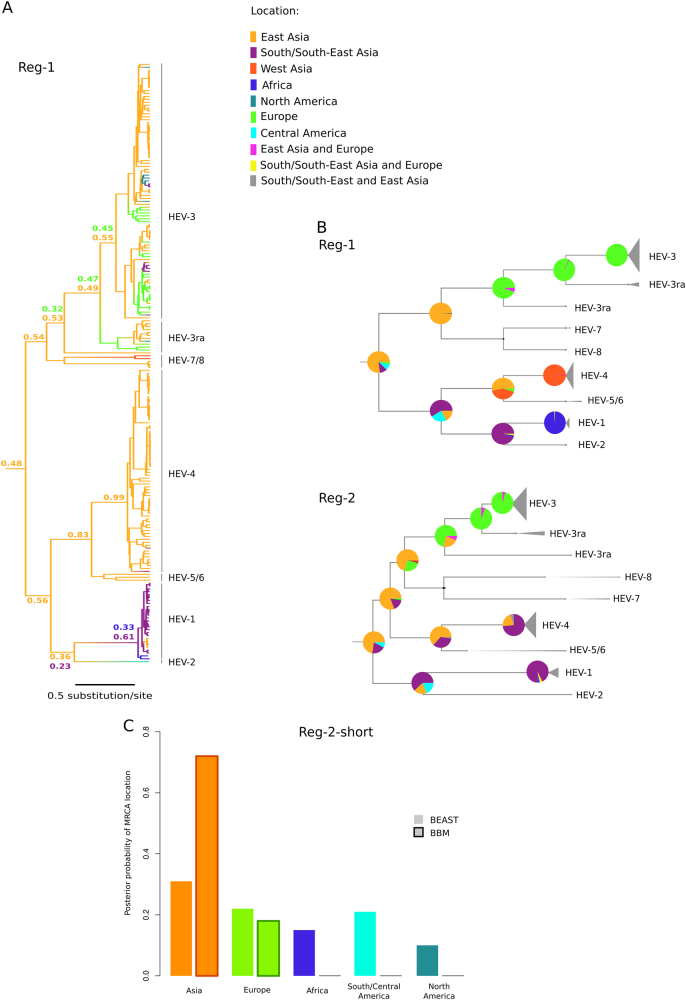
We used two independent phylogeography methods to determine the likely location of Orthohepevirus A origin. Sequences were assigned to geographic areas (Fig. 1b), which represent the character states for ancestral state reconstruction. The discrete phylogeography analysis in BEAST27, 29 assigned the highest probability to East Asia as the origin of Orthohepevirus A, with posterior probabilities of 0.48 for Reg-1 and 0.53 for Reg-2. BEAST also inferred a likely East Asian origin for several internal nodes of the phylogeny (Fig. 2a, Supplementary Figure S1). The ancestors of extant HEV-3 strains, as well as the MRCA (most recent common ancestor) of HEV-3ra and HEV-3, were assigned roughly equal probabilities of European or East Asian origin (Fig. 2a, Supplementary Figure S1). Very similar results were obtained with the BBM method31, which assigned high probabilities of an East Asian origin to the ancestral node of the Orthohepevirus A phylogeny (0.93 for Reg-1 and 0.90 for Reg-2) (full tree not shown).
Fig. 2. Phylogeographic analysis of Orthohepevirus A.
a Maximum clade credibility tree for Reg-1. Branches are colored according to inferred ancestral location; posterior support for inferred locations at relevant nodes is shown. b The topologies of the Reg-1 and Reg-2 trees are shown. Pie charts represent the ancestral location probability of the respective area. Probabilities were averaged over 100 BBM runs with a similar number of sequences from East Asia, South/South-East Asia, and Europe. In BBM analyses, combined regions appear because we allowed two areas per node. c Posterior probability distributions for the location of the Orthohepevirus A MRCA obtained using BEAST and BBM. Results refer to the Reg-2-short region
Most Orthohepevirus A sequences were collected in East Asia (mainly in China and Japan) (Fig. 1b). We thus checked whether this skewed distribution affected phylogeographic inference. To this aim, we run 100 BBM analyses by omitting the character state for 113 randomly selected East Asian strains. This number was selected to obtain a data set that included a similar number of sequences from East Asia (n = 30), South/South-East Asia (n = 30), and Europe (n = 22). We calculated the mean probabilities at selected nodes by averaging over the 100 BBM runs (Fig. 2b). For both ORFs, East Asia was identified as the most likely origin of Orthohepevirus A, with high average probabilities. These analyses also indicated that HEV-3 originated in Europe, in agreement with previous studies41, 42, and supported the notion that HEV-3ra is also of European origin. The MRCA of HEV-1 and HEV-2 were inferred to have originated in South/South-East Asia (Fig. 2b). This finding should however be interpreted with caution due to the availability of a single HEV-2 sequence.
The dataset of complete Orthohepevirus A genomes includes only few samples from Africa and South/Central America. To further confirm the Asian origin of Orthohepevirus A, we performed an additional analysis on a sub-region of Reg-2 (Reg-2-short, 360 nucleotide long) for which African and South/Central American sequences are available. The dataset for Reg-2-short included the same number of sequences (n = 48) for Asia, Africa, Europe, South/Central America, and North America (total sequences: 240) (see Methods and Supplementary Table S3). A drawback of this approach is that phylogenetic relationships can be reconstructed with limited confidence when small regions are analyzed24. Thus, we limited inference to the MRCA of the whole phylogeny, with no attempt to reconstruct the ancestral location of internal nodes. Both BEAST and BBM analyses inferred an Asian origin for the Orthohepevirus A MRCA (Fig. 2c). The second most likely location was Europe, with definitely lower probability, especially in BBM analysis (Fig. 2c). Overall, these data strongly support the hypothesis that Orthohepevirus A originated in Asia.
Ancestral host of Orthohepevirus A
As mentioned above, the major HEV genotypes differ in their host range, although most of them seem to be able to infect humans. Thus, an interesting question is whether the ancestor of extant Orthohepeviruses A infected humans or other mammals. We investigated this issue by applying an approach similar to the one described above. In this case, host information was entered as character state in BEAST and BBM analyses. BEAST indicated that the most likely ancestral host of Orthohepevirus A was human, although posterior probabilities were only slightly higher than those for swine (Fig. 3a). Similar results were obtained with BBM, with definitely higher probabilities of 0.94 and 0.86 for a human host for both regions (full tree not shown).
Fig. 3. Ancestral host range of Orthohepevirus A.
a Maximum clade credibility trees for Reg-1 and Reg-2. Branches are colored according to inferred ancestral host; posterior support for the host range of the MRCA is reported. b Posterior probability distributions of ancestral host state for the MRCA of the Orthohepevirus A phylogeny. Probabilities derive from 100 BBM runs with omission of character state information for 22 randomly sampled human sequences belonging to genotypes 3 and 4
However, as is the case for geographic location, the source of HEV sequences is biased, with an over representation of human-derived viruses. We thus generated 100 character state distributions by omitting the information for 22 randomly selected human-derived strains belonging to genotypes 3 and 4. We did not omit character states for genotype 1 and 2, as these viruses exclusively infect humans (thus the ancestor of HEV-1 and HEV-2 is always inferred with high confidence to be human-derived). The resulting distributions, used as an input for BBM, have the same number of HEV-3 and HEV-4 sequences derived from humans and swine. For both regions, BBM always estimated the probability of a human ancestral host to be definitely higher than that of an ancestral swine host (Fig. 3b). For both regions, the distribution of probabilities was definitely skewed towards a human ancestral host (Fig. 3b).
Dating the origin of Orthohepevirus A
Evolutionary rates scale negatively with the time-frame of the measurement for different viral taxa43, 44. This phenomenon often results in underestimation of the age of viral lineages43, 44 and is strongly associated with purifying selection and substitution saturation43. We thus applied a selection-informed method to estimate the tMRCA (time to the most recent common ancestor) of Orthohepeviruses A. Specifically, we calculated branch lengths in Orthohepevirus A phylogenies using the aBS-REL method, which allows for site- and branch-specific variation in selective pressure and is more robust than other evolutionary models to substitution saturation34, 45. These lengths were converted into tMRCA estimates using LSD (least-squares dating)37. Confidence intervals were calculated by estimating the variance in branch lengths produced by the aBS-REL model using a Latin hypercube sampling importance resampling scheme.
As above, we analyzed two regions, Reg-1 and Reg-2. Saturation of synonymous substitution rates (dS) was not prominent in the two regions, as we found 3.6 and 4.3% of branches (most of them terminal branches) showing dS saturation for Reg-1 and Reg-2, respectively. We estimated the tMRCA of Orthohepevirus A genotypes to be 6795 years ago (ya) for Reg-1 (95% IC:13871–4011) and 4596 ya for Reg-2 (95% IC:13806–2404). In line with previous results45, much shallower tMRCAs were obtained using the GTR tree lengths for LSD (Table 1) or BEAST (for Reg-1, tMRCA = 1484 ya, CI: 2049–1132; for Reg-2, tMRCA = 975 ya, CI: 1383–242) analyses.
Table 1.
Branch lengths and tMRCA estimates for the Orthohepevirus genus
| Length (substitution/site) | Expansion (CI)a | tMRCA (ya) | |||
|---|---|---|---|---|---|
| Orthohepevirus | |||||
| GTR | aBS-REL (CI) | GTR | aBS-REL b (CI) | ||
| Reg-1-short | 45.99 | 91 009 | 1978.89 | 24 812 | 49 100 219 |
| (40 200–103 923) | (874.10–2 259.69) | (21 688 169–56 067 428) | |||
| Reg-2 | 36.54 | 168 856 | 4621.13 | 7300 | 33 734 249 |
| (122 782–380 992) | (3 360.21–10 426.72) | (24 529 533–74 115 056) | |||
| Orthohepevirus A | |||||
| GTR | aBS-REL | GTR | aBS-REL b (LSD calculated) c | ||
| Reg-1 | 14.22 | 60.13 | 4.23 | 1884 | 7969 (6795) |
| Reg-2 | 12.19 | 86.72 | 7.11 | 878 | 6242 (4596) |
a Total branch length expansion (aBS-REL/GTR + G)
b tMRCA inferred by multipling the time obtained with the GTR + G model (and LSD) by branch length expansion
c tMRCA estimated using aBS-REL branch lengths as an input for LSD
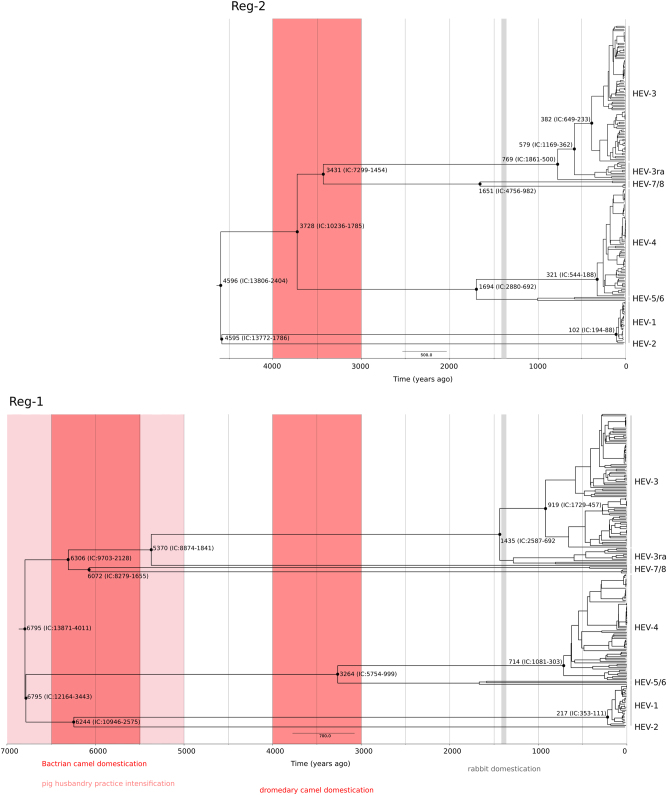
Data for both regions indicated that the earliest splits included the separation of enzootic and human-restricted genotypes (6795 to 4595 ya, depending on the region considered, plus confidence intervals), as well as the radiation of the camel-infecting genotypes 7 and 8 (6306–3431 ya) (Fig. 4). The most common genotypes were inferred to have appeared definitely earlier than previously estimated7, 41, 42, 46: the tMRCA of genotype 3 strains was estimated at 919–382 ya and genotype 4 originated 714–321 ya (Fig. 4). The analysis of HEV-3ra for Reg-1 is complicated by the presence of a highly divergent rabbit-derived sequence (accession: KJ013415) that, in analogy to previous analyses47, was basal to HEV-3 and HEV-3ra; excluding this sequence, the tMRCA of HEV-3 and HEV-3ra dates between 580 and 1436 CE (plus confidence intervals) (Fig. 4). The shallower tMRCA was observed for genotype 1, dating around 217–102 ya, whereas the split of the two human restricted genotypes occurred at least 4500 ya (Fig. 4).
Fig. 4. Orthohepevirus A timescaled phylogenetic trees.
Timescaled phylogenetic tree estimated using the Reg-1 and Reg-2 regions are shown. Branch lengths represent the evolutionary time measured by the grids corresponding to the timescale shown at the tree base (in years). The tMRCA of selected nodes is reported with 95% confidence intervals. The time-frames of historical events mentioned in the text are reported
Dating the origin of the Orthohepevirus genus
Finally, we estimated the tMRCA of the Orthohepevirus genus using the same approach described above. To this aim, 31 Orthohepevirus B to D sequences, plus unclassified Orthohepevirus, were retrieved and included in the phylogeny with a subset of 82 Orthohepevirus A genomes (Supplementary Table S2). Due to poor alignment quality, a sub-region of Reg-1 (Reg-1-short, Fig. 1C) was used for dating.
Direct inference of the tMRCA with LSD was not possible due to extreme heterogeneity of branch lengths estimated by aBS-REL. We thus calculated branch lengths using a GTR model, and we used these lengths to estimate the tMRCA of extant Orthohepeviruses (Table 1). We next calculated the total branch length expansion of the aBS-REL model compared to the GTR model. This expansion was used to approximate the Orthohepevirus tMRCA. We observed a 1978 fold expansion of Reg-1-short and a 4621 fold expansion for Reg-2. The tMRCAs calculated using GTR branch lengths (Reg-1-short tMRCA: 24812 ya, Reg-2 tMRCA: 7300 ya) were thus adjusted by these expansions to obtain tMRCAs of ~49 million ya (IC: 21–56 million ya) for Reg-1-short and ~33 million ya (IC: 24–74 million ya) for Reg-2. As a consistency check, we used the same approach for Orthohepevirus A Reg-1 and Reg-2 and obtained comparable results to those obtained using LSD with aBS-REL branch lengths (Table 1).
Discussion
HEV has a worldwide diffusion and causes a substantial health burden48. Investigating the origin and historical dissemination of this virus is important for understanding its present distribution and epidemic/pandemic potential.
Time inferences obtained using a selection-informed method were markedly different from those reported in other studies. Previous analyses focusing on the enzootic HEV strains dated the origin of genotype 4 in the Twentieth Century (1909, lower-bound estimate 1871)46, whereas different estimates were provided for genotype 3, with tMRCAs ranging from 320 to 199 ya41, 42. A study that analyzed HEV strains available in 2010 reached similar conclusions, with tMRCAs of 265–342 and 130–266 ya for genotypes 3 and 4, respectively7. In that analysis, the whole HEV phylogeny had a tMRCA of 536 ya, with a lower bound estimate of 1344 ya. The authors however noted that, because of sparse sampling and unaccounted variation in substitution rates, their dating strategy could have underestimated the true ages of these viral lineages7. Whereas the problem of incomplete sampling also applies to analyses herein, as several extant HEV lineages may remain undescribed and many others may have gone extinct, we applied a method that at least partially corrects for temporal variation in substitution rates. This approach was previously used to revise the evolutionary time frames of other RNA viruses, which resulted much deeper than those obtained with classical models34, 45. Although this method accounts for the effect of purifying selection, and we detected limited evidence of substitution saturation, we most likely failed to fully correct for time-dependent substitution rate variation49. As a consequence, the time frames we report may still underestimate the true timing of Orthohepevirus evolution.
Therefore, the methodology we applied for time inferences most likely accounts for the discrepancy between our findings and the previous ones7, 41, 42, 46. Indeed, when we performed molecular dating with models that do not account for selection (BEAST or LSD with GTR branch lengths), the tMRCA for the Orthohepevirus A phylogeny was not so different from that obtained by Purdy and coworkers7. Clearly, an additional source of diversity from that previous study derives from the dataset, as Purdy et al. performed their analyses in 2010, when several HEV genotypes (i.e., HEV-5 to HEV-8) had still to be discovered. As for phylogeography, our results are consistent with previous reports indicating a European origin for HEV-341, 42. Conversely, data for HEV-4 are more difficult to interpret. Recently, this genotype was shown to have originated in Japan, although the skewed sampling of HEV-4 sequences was not accounted for46. We also obtained evidence of an East-Asian origin for this genotype using both BEAST and BBM. However, resampling with omission of character states for subsets of East-Asian strains yielded different results depending on the analyzed region and assigned HEV-4 to either West or South/South-East Asia. Overall, these data suggest that additional unbiased sampling of HEV-4 sequences will be required to correctly infer to geographic origin of this genotype (see also below).
Data herein indicate that Orthohepevirus A most likely originated in East Asia, from a human-infecting ancestor that existed ~6800 to ~4500 ya. This time frame corresponds to a period when intensive, sedentary agriculture (mainly rice and millet) was fully developed and spreading across China and neighboring regions50–52. The same period witnessed a rapid population growth in several East and South-East Asian regions and the appearance of relatively large human settlements53. These conditions, most probably characterized by the close proximity of habitation areas and waste deposits, as well as by the frequent contamination of drinking water54, may have facilitated the emergence of HEV strains with epidemiological features similar to extant human-restricted genotypes. Domesticated pigs were already present in China by 6000 BCE55, although husbandry practices probably intensified around 5000 to 3000 BCE52, 56. The close contact between humans and pigs was possibly responsible for the origin of the enzootic HEV strains, which represent one of the earliest splits in Orthohepevirus A phylogenies.
Our time estimates indicate that another early event in the Orthohepevirus A evolutionary history accompanied the emergence of camel-infecting genotypes. This observation is in line with a recent report that, based on HEV-7 genetic diversity and broad geographic distribution, suggested a long evolution of Orthohepevirus A in dromedary camels57. We dated the split of HEV-7 and HEV-8 from other genotypes in a wide time frame ranging from 4291 BCE to 1416 BCE (plus confidence intervals). This period encompasses the time of domestication of Bactrian camels (earliest evidences dating around 4500–3500 BCE) and dromedary camels (1000–2000 BCE)58, 59. The place and timing of HEV-3/HEV-3ra divergence also correspond remarkably well with the circumstances of rabbit domestication, which started in France around 600 CE60. However, the correspondence between domestication processes and divergence dates does not necessarily imply that the animal viruses originated from human transmission events. Husbandry practices commonly create animal colonies isolated from the other wildlife and living in crowded facilities. These conditions may have favored the spread of pre-existing animal viruses and their divergence into specific genotypes. With respect to phylogeographic analyses, we mention that an important limitation of our study is the underlying bias in sequence origin. We addressed this problem by both resampling with omission of character states for subsets of Asian strains and by analysis of an independent set of sequences which were selected to be equally representative of the continents. However, these approaches cannot compensate for areas where very few sequences were reported such as Central Asia and the Middle East. Sampling efforts in these regions will be pivotal to refine the geographic origin of HEV.
Finally, we note that the identification of humans as the likely ancestral hosts for Orthohepevirus A is consistent with the observation that most, if not all, HEV genotypes can infect our species. This is not merely a result of ecological factors, as inter-species transmission experiments confirmed the strict host specificity of HEV-1 and HEV-21. Clearly, our inference concerning an ancestral human host for extant Orthohepevirus A strains does not exclude the possibility that humans acquired HEV through cross-species transmission from other animals. However, known Orthohepeviruses that infect mammals and birds are distantly related to Orthohepevirus A, suggesting that none of them represents the source of human-infecting HEV. To gain insight into the time of origin of the Orthohepevirus genus we exploited a strategy that was previously applied to Coronaviruses and is based on extrapolating over branch lengths obtained under different models45. In the case of Coronaviruses the authors obtained time estimates that were broadly consistent with the divergence time of birds and mammals, suggesting co-speciation of mammal-infecting and bird-infecting Coronaviruses with their hosts45. Conversely, the estimates we obtained for the tMRCA of the Orthohepevirus genus are much shallower than the divergence times of the host species that these viruses infect. Although these time frames may represent severe underestimates, the lack of virus-host co-speciation is also supported by the poor congruence of the viral and host phylogenies. Thus, ancient events of co-evolution and host-shifts probably contributed to originate extant Orthohepevirus species.
Several Orthohepeviruses have been described only recently1 and the current sampling of this viral genus is most likely limited in terms of host species. For instance, anti-HEV antibodies were detected in different animal species such as sheep, horses, cats, and dogs. Although no viral RNA has been isolated from these animals, it is likely that additional mammalian and non-mammalian HEV species or strains exist1. Analyses of more diverse potential hosts may thus shed further light into the ultimate origin of Orthohepevirus A.
Electronic supplementary material
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version of this article (10.1038/s41426-017-0009-6) contains supplementary material.
References
- 1.Doceul V, Bagdassarian E, Demange A, Pavio N, Zoonotic Hepatitis E. Virus: Classification, Animal Reservoirs and Transmission Routes. Viruses. 2016;8:E270. doi: 10.3390/v8100270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith DB, Simmonds P. Consensus proposals for classification of the family Hepeviridae. J. Gen. Virol. 2014;95:2223–2232. doi: 10.1099/vir.0.068429-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bouquet J, Tesse S, Lunazzi A, et al. Close similarity between sequences of hepatitis E virus recovered from humans and swine, France, 2008-2009. Emerg. Infect. Dis. 2011;17:2018–2025. doi: 10.3201/eid1711.110616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu P, Bu QN, Wang L, et al. Transmission of hepatitis E virus from rabbits to cynomolgus macaques. Emerg. Infect. Dis. 2013;19:559–565. doi: 10.3201/eid1904.120827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Izopet J, Dubois M, Bertagnoli S, et al. Hepatitis E virus strains in rabbits and evidence of a closely related strain in humans, France. Emerg. Infect. Dis. 2012;18:1274–1281. doi: 10.3201/eid1808.120057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee GH, Tan BH, Teo EC, et al. Chronic infection with camelid hepatitis E virus in a liver transplant recipient who regularly consumes camel meat and milk. Gastroenterology. 2016;150:355–357. doi: 10.1053/j.gastro.2015.10.048. [DOI] [PubMed] [Google Scholar]
- 7.Purdy MA, Khudyakov YE. Evolutionary history and population dynamics of hepatitis E virus. PLoS ONE. 2010;5:e14376. doi: 10.1371/journal.pone.0014376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aggarwal R. Hepatitis e: epidemiology and natural history. J. Clin. Exp. Hepatol. 2013;3:125–133. doi: 10.1016/j.jceh.2013.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okamoto H. Genetic variability and evolution of hepatitis E virus. Virus Res. 2007;127:216–228. doi: 10.1016/j.virusres.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 10.Geng Y, Wang Y. Epidemiology of Hepatitis E. Adv. Exp. Med Biol. 2016;948:39–59. doi: 10.1007/978-94-024-0942-0_3. [DOI] [PubMed] [Google Scholar]
- 11.Pauli G, Aepfelbacher M, Bauerfeind U, et al. Hepatitis E Virus. Transfus. Med Hemother. 2015;42:247–265. doi: 10.1159/000431191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Echevarria JM, Gonzalez JE, Lewis-Ximenez LL, et al. Hepatitis E virus infection in Latin America: a review. J. Med Virol. 2013;85:1037–1045. doi: 10.1002/jmv.23526. [DOI] [PubMed] [Google Scholar]
- 13.Kim JH, et al. Erratum to: A systematic review of the epidemiology of hepatitis E virus in Africa. BMC Infect. Dis. 2017;17:187. doi: 10.1186/s12879-017-2274-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim JH, et al. A systematic review of the epidemiology of hepatitis E virus in Africa. BMC Infect. Dis. 2014;14:308–2334-14-308. doi: 10.1186/1471-2334-14-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pickett BE, Sadat EL, Zhang Y, et al. ViPR: an open bioinformatics database and analysis resource for virology research. Nucleic Acids Res. 2012;40:D593–D598. doi: 10.1093/nar/gkr859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin J, Norder H, Uhlhorn H, Belak S, Widen F. Novel hepatitis E like virus found in Swedish moose. J. Gen. Virol. 2014;95:557–570. doi: 10.1099/vir.0.059238-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reuter G, et al. Divergent hepatitis E virus in birds of prey, common kestrel (Falco tinnunculus) and red-footed falcon (F. vespertinus), Hungary. Infect. Genet Evol. 2016;43:343–346. doi: 10.1016/j.meegid.2016.06.013. [DOI] [PubMed] [Google Scholar]
- 18.Reuter G, et al. A novel avian-like hepatitis E virus in wild aquatic bird, little egret (Egretta garzetta), in Hungary. Infect. Genet Evol. 2016;46:74–77. doi: 10.1016/j.meegid.2016.10.026. [DOI] [PubMed] [Google Scholar]
- 19.Batts W, Yun S, Hedrick R, Winton J. A novel member of the family Hepeviridae from cutthroat trout (Oncorhynchus clarkii) Virus Res. 2011;158:116–123. doi: 10.1016/j.virusres.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 20.Huelsenbeck JP, Bollback JP, Levine AM. Inferring the root of a phylogenetic tree. Syst. Biol. 2002;51:32–43. doi: 10.1080/106351502753475862. [DOI] [PubMed] [Google Scholar]
- 21.Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 2013;30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kosakovsky Pond SL, Posada D, Gravenor MB, Woelk CH, Frost SD. Automated phylogenetic detection of recombination using a genetic algorithm. Mol. Biol. Evol. 2006;23:1891–1901. doi: 10.1093/molbev/msl051. [DOI] [PubMed] [Google Scholar]
- 23.Delport W, et al. CodonTest: modeling amino acid substitution preferences in coding sequences. PLoS Comput. Biol. 2010;6:e1000885. doi: 10.1371/journal.pcbi.1000885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Purdy MA, Sue A. The effect of phylogenetic signal reduction on genotyping of hepatitis E viruses of the species Orthohepevirus A. Arch. Virol. 2017;162:645–656. doi: 10.1007/s00705-016-3135-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nair VP, Anang S, Subramani C, et al. Endoplasmic reticulum stress induced synthesis of a novel viral factor mediates efficient replication of genotype-1 hepatitis E virus. PLoS Pathog. 2016;12:e1005521. doi: 10.1371/journal.ppat.1005521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wernersson R, Pedersen AG. RevTrans: Multiple alignment of coding DNA from aligned amino acid sequences. Nucleic Acids Res. 2003;31:3537–3539. doi: 10.1093/nar/gkg609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bouckaert R, Heled J, Kuhnert D, et al. BEAST 2: a software platform for Bayesian evolutionary analysis. PLoS Comput. Biol. 2014;10:e1003537. doi: 10.1371/journal.pcbi.1003537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Posada D, Crandall KA. MODELTEST: testing the model of DNA substitution. Bioinformatics. 1998;14:817–818. doi: 10.1093/bioinformatics/14.9.817. [DOI] [PubMed] [Google Scholar]
- 29.Lemey P, Rambaut A, Drummond AJ, Suchard MA. Bayesian phylogeography finds its roots. PLoS Comput. Biol. 2009;5:e1000520. doi: 10.1371/journal.pcbi.1000520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu Y, Harris AJ, Blair C, He X. RASP (Reconstruct Ancestral State in Phylogenies): a tool for historical biogeography. Mol. Phylogenet Evol. 2015;87:46–49. doi: 10.1016/j.ympev.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 31.Ronquist F, Huelsenbeck JP. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- 32.Kodandaramaiah Ullasa. Use of dispersal–vicariance analysis in biogeography – a critique. J. Biogeogr. 2010;37:3–11. doi: 10.1111/j.1365-2699.2009.02221.x. [DOI] [Google Scholar]
- 33.Wertheim JO, Smith MD, Smith DM, Scheffler K, Kosakovsky Pond SL. Evolutionary origins of human herpes simplex viruses 1 and 2. Mol. Biol. Evol. 2014;31:2356–2364. doi: 10.1093/molbev/msu185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wertheim JO, Kosakovsky Pond SL. Purifying selection can obscure the ancient age of viral lineages. Mol. Biol. Evol. 2011;28:3355–3365. doi: 10.1093/molbev/msr170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith MD, et al. Less is more: an adaptive branch-site random effects model for efficient detection of episodic diversifying selection. Mol. Biol. Evol. 2015;32:1342–1353. doi: 10.1093/molbev/msv022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rambaut A, Lam TT, Max Carvalho L, Pybus OG. Exploring the temporal structure of heterochronous sequences using TempEst (formerly Path-O-Gen) Virus Evol. 2016;2:vew007. doi: 10.1093/ve/vew007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.To TH, Jung M, Lycett S, Gascuel O. Fast Dating Using Least-Squares Criteria and Algorithms. Syst. Biol. 2016;65:82–97. doi: 10.1093/sysbio/syv068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guindon S, Delsuc F, Dufayard JF, Gascuel O. Estimating maximum likelihood phylogenies with PhyML. Methods Mol. Biol. 2009;537:113–137. doi: 10.1007/978-1-59745-251-9_6. [DOI] [PubMed] [Google Scholar]
- 39.Chen X, Zhang Q, He C, et al. Recombination and natural selection in hepatitis E virus genotypes. J. Med Virol. 2012;84:1396–1407. doi: 10.1002/jmv.23237. [DOI] [PubMed] [Google Scholar]
- 40.Brayne AB, Dearlove BL, Lester JS, Kosakovsky Pond SL, Frost SD. Genotype-specific evolution of hepatitis E virus. J. Virol. 2017;91:e02241–16. doi: 10.1128/JVI.02241-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mirazo S, et al. New insights into the hepatitis E virus genotype 3 phylodynamics and evolutionary history. Infect. Genet Evol. 2016;43:267–273. doi: 10.1016/j.meegid.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 42.Zehender G, Ebranati E, Lai A, et al. Phylogeography and phylodynamics of European genotype 3 hepatitis E virus. Infect. Genet Evol. 2014;25:138–143. doi: 10.1016/j.meegid.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 43.Duchene S, Holmes EC, Ho SY. Analyses of evolutionary dynamics in viruses are hindered by a time-dependent bias in rate estimates. Proc. Biol. Sci. 2014;281:20140732. doi: 10.1098/rspb.2014.0732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aiewsakun P, Katzourakis A. Time-Dependent Rate Phenomenon in Viruses. J. Virol. 2016;90:7184–7195. doi: 10.1128/JVI.00593-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wertheim JO, Chu DK, Peiris JS, Kosakovsky Pond SL, Poon LL. A case for the ancient origin of coronaviruses. J. Virol. 2013;87:7039–7045. doi: 10.1128/JVI.03273-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nakano T, Takahashi K, Takahashi M, et al. Investigating the origin and global dispersal history of hepatitis E virus genotype 4 using phylogeographical analysis. Liver Int. 2016;36:31–41. doi: 10.1111/liv.12880. [DOI] [PubMed] [Google Scholar]
- 47.Smith DB, Ijaz S, Tedder RS, et al. Variability and pathogenicity of hepatitis E virus genotype 3 variants. J. Gen. Virol. 2015;96:3255–3264. doi: 10.1099/jgv.0.000264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kamar N, Dalton HR, Abravanel F, Izopet J. Hepatitis E virus infection. Clin. Microbiol Rev. 2014;27:116–138. doi: 10.1128/CMR.00057-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ho SY, Duchene S, Molak M, Shapiro B. Time-dependent estimates of molecular evolutionary rates: evidence and causes. Mol. Ecol. 2015;24:6007–6012. doi: 10.1111/mec.13450. [DOI] [PubMed] [Google Scholar]
- 50.Fuller DQ, Qin L, Zheng Y, et al. The domestication process and domestication rate in rice: spikelet bases from the Lower Yangtze. Science. 2009;323:1607–1610. doi: 10.1126/science.1166605. [DOI] [PubMed] [Google Scholar]
- 51.Gross BL, Zhao Z. Archaeological and genetic insights into the origins of domesticated rice. Proc. Natl. Acad. Sci. U. S. A. 2014;111:6190–6197. doi: 10.1073/pnas.1308942110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barton L, et al. Agricultural origins and the isotopic identity of domestication in northern China. Proc. Natl. Acad. Sci. U. S. A. 2009;106:5523–5528. doi: 10.1073/pnas.0809960106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bocquet-Appel J., Bar-Yosef O. The Neolithic demographic transition and its consequences. Springer Science & Business Media, Dordrecht, The Netherlands, 2008.
- 54.Armelagos GJ, Barnes KC, Lin J. Disease in human evolution: the re-emergence of infectious disease in the third epidemiological transition. AnthroNotes. 1996;18:1–7. doi: 10.5479/10088/22354. [DOI] [Google Scholar]
- 55.Cucchi T, Hulme-Beaman A, Yuan J, Dobney K. Early Neolithic pig domestication at Jiahu, Henan Province, China: clues from molar shape analyses using geometric morphometric approaches. J. Archaeol. Sci. 2011;38:11–22. doi: 10.1016/j.jas.2010.07.024. [DOI] [Google Scholar]
- 56.Cucchi T, Dai L, Balasse M, et al. Social complexification and pig (Sus scrofa) husbandry in Ancient China: A combined geometric morphometric and isotopic approach. PLoS ONE. 2016;11:e0158523. doi: 10.1371/journal.pone.0158523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rasche A, Saqib M, Liljander AM, et al. Hepatitis E virus infection in dromedaries, North and East Africa, United Arab Emirates, and Pakistan, 1983-2015. Emerg. Infect. Dis. 2016;22:1249–1252. doi: 10.3201/eid2207.160168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kuzmina E. E. The prehistory of the Silk Road. University of Pennsylvania Press, Philadelphia, 2008.
- 59.Uerpmann H, Uerpmann M. The appearance of the domestic camel in south-eastArabia. J. Oman Stud. 2002;12:235–260. [Google Scholar]
- 60.Clutton-Brock J. A natural history of domesticated mammals. Cambridge University Press, Cambridge, UK, 1999.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.