Abstract
Human adenoviruses (HAdVs) are uniquely important “model organisms” as they have been used to elucidate fundamental biological processes, are recognized as complex pathogens, and are used as remedies for human health. As pathogens, HAdVs may effect asymptomatic or mild and severe symptomatic disease upon their infection of respiratory, ocular, gastrointestinal, and genitourinary systems. High-resolution genomic data have enhanced the understanding of HAdV epidemiology, with recombination recognized as an important and major pathway in the molecular evolution and genesis of emergent HAdV pathogens. To support this view and to actualize an algorithm for identifying, characterizing, and typing novel HAdVs, we determined the DNA sequence of 95 isolates from archives containing historically important pathogens and collections housing currently circulating strains to be sequenced. Of the 85 samples that were completely sequenced, 18 novel recombinants within species HAdV-B and D were identified. Two HAdV-D genomes were found to contain novel penton base and fiber genes with significant divergence from known molecular types. In this data set, we found additional isolates of HAdV-D53 and HAdV-D58, two novel genotypes recognized recently using genomics. This supports the thesis that novel HAdV genotypes are not limited to “one-time” appearances of the prototype but are of importance in HAdV epidemiology. These data underscore the significance of lateral genomic transfer in HAdV evolution and reinforce the potential public health impact of novel genotypes of HAdVs emerging in the population.
Introduction
Human adenoviruses (HAdVs) occupy an important unique niche in biology and medicine as they were not only among the first respiratory viral pathogens to be isolated, identified, and characterized, but were also model organisms for fundamental discoveries and insights into molecular and cellular biology, immunology, and systems biology. These include messenger RNA splicing1, eukaryotic DNA replication2, and antigen presentation to T-cells3. Remarkably, this double-stranded (ds) DNA virus, with a capsid comprising nearly one-million amino acids and 150-megadalton molecular weight, has been crystallized and its structure resolved at 3.5 angstroms, allowing for insights into virus assembly and cell entry mechanisms4. This provides opportunities for improving and refining adenovirus-mediated gene transfer as vectors for vaccination and gene therapy4,5; and enhancing the role of HAdVs in oncolytics6.
As human pathogens, a wide spectrum of diseases is associated with HAdVs, involving the respiratory, ocular, gastrointestinal, and genitourinary systems, as well as a metabolic disorder (obesity)7. These have been documented extensively in the literature and many pathogenic strains have been collected into archives. Based on their biology, pathogenic attributes, and DNA sequence similarities, HAdVs are divided phylogenetically into seven species, A through G7. Adenovirus genotypes classified as HAdV-B, -C, and -E are associated principally with respiratory disease; HAdV-A, -D, -F, and -G with gastrointestinal disease; and HAdV-D and E with ocular diseases, including epidemic keratoconjunctivitis, a severe ocular surface infection7. HAdV-A genotypes have historical significance as oncogenic in certain rodent model organisms8,9. In immunocompetent individuals, HAdV infections are usually self-limiting and death is relatively uncommon7, but epidemics involving certain historically important strains and genotypes, e.g., “Ad-7h”, recently relabeled as type 66 (GenBank accession no. JN860676), “prove the exception”10–12. In immunocompromised individuals, HAdV infections are of significant concern, causing fatalities7. Specifically, HAdV-A, -B, and -C are all associated with infections of allogenic transplant recipients7, while many novel types within HAdV-D were first identified in patients with AIDS13 and other immunocompromised patients as potential opportunistic pathogens14. Thus, HAdV is a major public health concern in both immunocompetent and immunocompromised individuals.
As a model organism, adenoviruses are proving useful for demonstrating the power and application of high-resolution data from genomics and bioinformatics in pathogen detection15, analysis, and characterization. HAdVs are examples in which high-resolution sequence data are applied to resolve viral taxonomy16. Whole-genome sequencing and phylogenomics have now fully supplanted serology as the system by which HAdVs are typed16,17. To date, 84 unique HAdV genotypes (http://hadvwg.gmu.edu) are recognized by the adenovirus research community and by NCBI, with all genome-associated data deposited in GenBank. The availability of high-resolution genomic data has provided insights into the molecular evolution of this human viral pathogen. The genome of this presumably “stable” dsDNA virus, with respect to point mutations and genetic drift, is remarkably “unstable” as co-infections allow for homologous recombination to be a major pathway in the molecular evolution of new types and emergent pathogens14,18–24. This mechanism allows for “non-pathogenic” types to be “converted” into highly contagious pathogens21,23 and for a “renal pathogenic” type to be “converted” into a highly contagious respiratory pathogen22. Recent genomic analyses of thirty-two newly emergent adenoviral pathogens have demonstrated this mechanism in six HAdV species, with most reporting on the two HAdV species with the largest number of members, HAdV-B (11/84; 13.1%) and -D (54/84; 64.3%). Novel simian adenoviruses (SAdVs) also have been shown to arise via recombination25. These studies supplement the high-resolution re-analysis of the original 52 serotypes and confirm they are unique genotypes19, even among “major serological” cross-reacting types such as HAdV-D15 and -D29, which were controversial as they shared the identical serotyping epsilon antigen26.
In order to determine the degree to which previously untyped novel genotypes of HAdVs may be isolated from patients, two clinical centers evaluating ocular diseases were recruited (Sankara Nethralaya and University of Pittsburgh). Two collections of respiratory HAdV pathogens were included, providing a comparison of another disease system (Guangzhou and NHRC). To complement these, three archives containing historically important HAdV pathogens were included (Loveless Respiratory Research Institute, University of Florida, and Germany). The archives include serotyped but not genome-sequenced deposits, for example, “Ad-7h” which caused an unusual and atypical high rate of morbidity and mortality in the 1980 and 1990s10–12. Collectively, these efforts resulted in the first large-scale adenovirus study in which whole-genome data and DNA sequence analyzes were used to discriminate, type, and characterize HAdV pathogens. Two recently recognized HAdV genotypes, HAdV-D53 and -D58, were sampled serendipitously. These analyses confirm genomic recombination as a major molecular evolution mechanism in the genesis of novel HAdV pathogens and reaffirm HAdVs as on-going threats to public health, as well as provide an algorithm for rapid genomic sequencing and in silico identification and characterization of emergent viral pathogens.
Materials and methods
Sample selection and processing
Adenoviral samples were obtained from one researcher and six contemporary collections and archives. Selection criteria included sampling then-currently circulating pathogens of interest to the researchers and sampling “intriguing” historical isolates that were archived. The Germany archive included adenoviruses collected by two researchers (Drs. T. Adrian and R. Wigand) who have retired and have gifted the isolates to Dr. Albert Heim. From the Emerging Pathogens Institute (University of Florida; Gainesville, FL), a collection that was gathered from other researchers was made available. All adenoviruses were identified by serotyping methods by the original researchers and supplied as samples from eye, nasopharynx, pharynx, endotracheum, and feces, with the exception of the Germany archival samples and China samples, which were supplied as purified DNA, and the Florida archival samples, which were supplied as purified viruses. Samples were processed by several study centers: U.S. Naval Health Research Center collection (San Diego, CA) (processed at the Lovelace Respiratory Research Institute; Albuquerque, NM); University of Pittsburgh collection (Pittsburgh, PA) and Sankara Nethralaya collection (Chennai, India) (processed at the Massachusetts Eye and Ear Infirmary; Boston, MA); Biosafety Level-3 Laboratory, School of Public Health, Southern Medical University (Guangzhou, China); archives of Drs. T. Adrian and R. Wigand (Homburg, Germany) (processed at the Institut für Virologie, Medizinische Hochschule; Hannover, Germany); and one isolate from Dr. Leonardo Ferreyra (Cordoba, Argentina) (processed at the California Public Health Department; Richmond, CA). Additional historically important isolates were purchased from a NIH-funded archive at the Emerging Pathogens Institute of the University of Florida, Gainesville (Dr. Gary L. Heil). N.B., This collection is no longer available.
Samples were processed similarly at each collaborating laboratories14,21,27,28; for example, at the MEEI each virus was grown in either A549 cells, a human alveolar carcinoma cell line or HEp-2, a human laryngeal carcinoma cell line, and purified by CsCl gradient ultra-centrifugation. DNA was extracted either using a phenol–chloroform method or with a QIAmp MinElute Virus Spin Kit (Qiagen GmbH; Hilden, Germany). Most eye specimens were unpassaged and directly used for DNA extraction. The MEEI Human Studies Committee exempted this study from requiring informed consent as the samples were de-identified and would otherwise have been discarded.
Genome sequencing
Purified genomic DNA was simultaneously PCR-amplified and bar-coded in two separate reactions using sequence independent single-primer amplification (SISPA) (Djikeng et al.29). All genomes were sequenced using a two-platform strategy: Ilumina HiSeq (San Diego, CA) and Roche 454 GS-FLX (Branford, CT). The SISPA products were normalized and pooled into a single sample that was purified using a PCR purification kit (Qiagen). This sample was subsequently gel purified to select for SISPA products that were 300–500 bp in size for Illumina HiSeq-based sequencing and 500–800 bp in size for 454 GS-FLX-based sequencing. In preparation for sequencing, two aliquots each were used to construct the 454 and Illumina libraries, and sequenced on the respective platforms. Following DNA sequencing, sequence reads from each platform were deconvoluted by barcode identity, and the sequences trimmed for quality and for removal of the SISPA hexamer primer sequences. All reads were then assembled de novo using “clc_novo_assembly”, a command-line assembly algorithm (https://www.qiagenbioinformatics.com/products/clc-genomics-workbench/) and the resulting contigs were BLAST-searched against a database of complete HAdV sequences available at GenBank to find the closest reference sequence. Both 454 GS-FLX and Illumina sequence reads were then mapped to the selected reference genome using the “clc_ref_assembly_long” command-line assembly algorithm. At loci where both 454 GS-FLX and Illumina sequence data agreed on a variation (as compared to the reference sequence), the reference sequence was updated to reflect the variation. A final mapping of all next-generation sequences to the updated reference sequences was performed with “clc_ref_assemble_long” command-line assembly algorithm. As most genomes showed significant variation compared to selected reference sequences, manual reference extension and editing was performed based on sequencing reads, followed by another round of mapping assembly as mentioned above. Furthermore, to improve resulting genome consensus and fill in sequencing gaps, custom primers were designed using the automated primer design software30, and targeted PCR-based DNA sequencing reactions were conducted. The PCR products were sequenced using Sanger dideoxy chemistry for short-range amplicons (up to 1 kb) or using IonTorrent sequencing platform (Thermo Fisher Scientific) for long-range amplicons (2–4 kb). These finishing reads were then merged with initial data using clc command-line assembly algorithm as mentioned above. The sequences were verified for functional completeness and adequate sequence coverage using in-house QA software tools (JCVI; Rockville, MD). Resultant finished genome sequences from this pipeline had average genome coverages of 214.2x.
Preliminary high-throughput genome annotation and identification of penton base, hexon, and fiber genes
The sequences were annotated with a viral annotation software, Viral Genome ORF Reader (VIGOR)31, prior to GenBank submission. To identify and to query for novel penton base, hexon, and fiber genes, BLAST was used as well as a genotyping software tool for the hexon hypervariable regions, loops 1 and 2. This genotyping tool was developed by Kalpana Dommaraju (Ph.D. dissertation, in preparation) following the criteria published by Madisch et al.32. It also included typing of the penton base using the hypervariable region 1 and the RGD loop sequences and typing of the fiber by its knob sequence. Following visual inspection and editing, genome sequence and annotation data were deposited into the GenBank as part of Bioproject id PRJNA70469.
Following GenBank retrieval, additional higher resolution annotation of individual genomes of interest was performed using GATU (https://www.viprbrc.org) with manual methods to ensure accuracy. GenBank records will be updated to reflect these refined annotations.
Computational genome analyses
Publicly accessible software tools were used to perform DNA sequence analyses, as described earlier21. Exceptions are noted below in the discussion of specific analyses. The NIH version of BLAST was used to query for sequences similarities against the GenBank database (http://blast.ncbi.nlm.nih.gov/Blast.cgi).
Phylogenetic analysis
Sequences were aligned using the ClustalW tool in the Molecular Evolutionary Genetic Analysis version 6 software package (MEGA6; www.megasoftware.net/). Phylogenetic trees were constructed using the maximum-parsimony method with a bootstrap test of 1000 replicates and the Tree-Bisection-Reconnection (TBR) model. The whole-genome tree was drawn using the “Interactive Tree of Life” software (iTOL v3; itol.embl.de).
Nucleotide diversity
Nucleotide diversity plots were constructed using the DNA Sequence Polymorphism software (DnaSP v5.10.01; www.ub.edu/dnasp/). Sites with alignment gaps were excluded. The analysis was performed with a sliding window length of 100 bps and step size of 25 bps. Pairwise sequence identities from multiple sequence alignments were calculated using the Sequence Identities and Similarities software (SIAS; http://imed.med.ucm.es/Tools/sias_help.html).
Recombination analysis
Simplot, a web-accessible software tool, was used to query the genomes and genes for nucleotide sequence recombination. This software includes Bootscan, a tool that was used to complete the nucleotide sequence recombination assessment of the ClustalW-aligned genes33. For genes, default parameter settings were used for the window size (200 nucleotides [nt]), step size (20 nt), replicates used (n = 100), gap stripping (on), distance model (Kimura), and tree model (neighbor-joining). Similarly, whole genomes were analyzed, starting with an initial alignment using ClustalW and following with recombination analysis using Simplot and Bootscan. For this much larger DNA sequence, only the window size and step size were altered (1000 and 200, respectively), with the remainder of the default parameters unchanged.
Homology modeling
A homology penton base protein model was built in the Swiss-Model ExPASy software (swissmodel.expasy.org) using the crystal structure of HAdV-C2 as the template (Protein Data Bank [PDB] code 1X9TA). UCSF Chimera v1.9 (www.cgl.ucsf.edu/chimera/) was used for visualization and root-mean-square deviation (RMSD) analysis.
Statistical analysis
Nucleotide identity differences between HAdV (A–D) species were analyzed using Kruskal–Wallis test and the data represented as a boxplot. A P-value of < 0.05 was considered statistically significant. This analysis was performed using GraphPad Prism v6.0 (GraphPad Software; San Diego, CA).
Results
Large-scale genomic and bioinformatic analysis of 95 adenoviruses
Ninety-five HAdV genomes isolated from currently circulating and historically intriguing pathogens were sequenced, albeit ten genomes were only partially completed due to template quality and quantity. However, the partial genomic sequences allow identification of the “marker” genes for determining HAdV molecular types, in accordance with GenBank- and ICTV-accepted practices of metagenomics17,34, and provided valuable information. As shown in Table 1, virus samples, stocks, or purified genomic DNA were obtained from one researcher and six collections and archives, with selection criteria determined by the collaborator and included sampling then-currently circulating pathogens of interest and “intriguing” historical isolates from archives. All of the adenoviruses were identified by serotyping methods by the original researchers and supplied as samples from eye, nasopharynx, pharynx, endotracheum, and feces, or noted as “unknown”.
Table 1.
Inventory of HAdV isolates
| Sequence identity | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Taxon | GenBank name | Serotype | Accession no. | Collection date | Location | Sample origin | Collection | Genome | Penton base | Hexon | Fiber |
| Species A | USA/UFL_Ad31/2005/31[P31H31F31] | Ad31 | KF268119 | 2005 | CO, USA | Fecal isolate | U Florida | HAdV-A31 | HAdV-A31 | HAdV-A31 | HAdV-A31 |
| Species B | DEU/Ad34/1985/34[P35H34F7] | Ad34 | KF268328 | 1985 | DEU | Unknown | Germany | Novel | HAdV-B35 | HAdV-B34 | HAdV-B7 |
| DEU/Ad3/1988/3[P16H3F16] | Ad3 | KF268315 | 1988 | DEU | Unknown | Germany | Novel | HAdV-B16 | HAdV-B3 | HAdV-B16 | |
| USA/Ad21 + 16H16/1965/76[P21H21F16] | Ad21 + 16H16 | KF633445 | 1965 | TX, USA | Lung sample | Germany | Novela | HAdV-B21 | HAdV-B21 | SAdV-B35 | |
| USA/UFL_Ad3/2004/3[P3/H3/F3] | Ad3 | KF268195 | 2004 | MO, USA | Nasal wash | U Florida | HAdV-B3 | HAdV-B3 | HAdV-B3 | HAdV-B3 | |
| USA/UFL_Ad3a50/2007/3[P3H3F3] | Ad3a50 | KF268133 | 2007 | CT, USA | Nasal wash | U Florida | HAdV-B3 | HAdV-B3 | HAdV-B3 | HAdV-B3 | |
| USA/CL_45/1988/3[P3H7F3] | Ad3 | KF268132 | 1988 | PA, USA | Ocular isolate | U Pittsburgh | HAdV-B3 | HAdV-B3 | HAdV-B3 | HAdV-B3 | |
| USA/UFL_Ad3a17/2007/3[P3H3F3] | Ad3a17 | KF268131 | 2007 | CT, USA | Nasal wash | U Florida | HAdV-B3 | HAdV-B3 | HAdV-B3 | HAdV-B3 | |
| USA/CL_46/1988/3[P3H3F3] | Ad3 | KF268128 | 1988 | PA, USA | Ocular isolate | U Pittsburgh | HAdV-B3 | HAdV-B3 | HAdV-B3 | HAdV-B3 | |
| USA/UFL_Ad3a51/2007/3[P3H3F3] | Ad3a51 | KF268123 | 2007 | CT, USA | Nasal wash | U Florida | HAdV-B3 | HAdV-B3 | HAdV-B3 | HAdV-B3 | |
| USA/UFL_Ad3a2/2007/3[P3H3F3] | Ad3a2 | KF268120 | 2007 | CT, USA | Nasal wash | U Florida | HAdV-B3 | HAdV-B3 | HAdV-B3 | HAdV-B3 | |
| IND/Ad3/2011/3[P7H3F3] | Ad3 | KF268212 | 2011 | Tamil Nadu, INDIA | Ocular isolate | India | HAdV-B3 | HAdV-B7 | HAdV-B3 | HAdV-B3 | |
| IND/Ad3/2011/3[P7H3F3] | Ad3 | KF268210 | 2011 | Tamil Nadu, INDIA | Ocular isolate | India | HAdV-B3 | HAdV-B7 | HAdV-B3 | HAdV-B3 | |
| USA/Ad3/X/3[P3H3F3] | Ad3 | KF268202 | 2003 | PA, USA | Ocular isolate | U Pittsburgh | HAdV-B3 | HAdV-B3 | HAdV-B3 | HAdV-B3 | |
| USA/ak34_Ad3a2/2008/3[P3H3F3] | Ad3a2 | JX423382 | 2008 | Philadelphia, USA | Unknown | LRRI/NHRC | HAdV-B3 | HAdV-B3 | HAdV-B3 | HAdV-B3 | |
| USA/ak33_Ad3a/2003/3[P3H3F3] | Ad3a variant | JX423381 | 2008 | Philadelphia, USA | Unknown | LRRI/NHRC | HAdV-B3 | HAdV-B3 | HAdV-B3 | HAdV-B3 | |
| USA/ak32_Ad3a/2004/3[P3H3F3] | Ad3a variant | JX423380 | 2004 | San Diego, USA | Unknown | LRRI/NHRC | HAdV-B3 | HAdV-B3 | HAdV-B3 | HAdV-B3 | |
| USA/Ad3/1988/NEW[P3H3F7] | Ad3 | KF429752 | 1988 | PA, USA | Ocular isolate | U Pittsburgh | HAdV-B3 | HAdV-B3 | HAdV-B3 | HAdV-B3 | |
| CHN/Ad4/2007/NEW[P3H3F7] | Ad4 | KF268311 | 2007 | Guangzhou, CHINA | Throat swab | So Med Univ | HAdV-B3 | HAdV-B3 | HAdV-B3 | HAdV-B3 | |
| USA/UFL_Ad7d2-3/unknown/7[P7H7F7] | Ad7d2–4 | KF268135 | 2002 | IA, USA | Nasal wash | U Florida | HAdV-B7 | HAdV-B7 | HAdV-B7 | HAdV-B7 | |
| USA/Ad4/1988/7[P7H7F7] | Ad4 | KF268134 | 1988 | PA, USA | Ocular isolate | U Pittsburgh | HAdV-B7 | HAdV-B7 | HAdV-B7 | HAdV-B7 | |
| USA/Ad7a/1988/7[P7H7F7] | Ad7a | KF268125 | 1988 | PA, USA | Ocular isolate | U Pittsburgh | HAdV-B7 | HAdV-B7 | HAdV-B7 | HAdV-B7 | |
| USA/UFL_Ad7d2-2/unknown/7[P7H7F7] | Ad7d2-1 | KF268117 | 2001 | IA, USA | Nasal wash | U Florida | HAdV-B7 | HAdV-B7 | HAdV-B7 | HAdV-B7 | |
| USA/Ad4/1988/7[P7H7F7] | Ad4 | KF429748 | 1988 | PA, USA | Ocular isolate | U Pittsburgh | HAdV-B7 | HAdV-B7 | HAdV-B7 | HAdV-B7 | |
| CHN/Ad7/2011/7[P7H7F7] | Ad7 | KF268316 | 2011 | Guangzhou, CHINA | Throat swab | So Med Univ | HAdV-B7 | HAdV-B7 | HAdV-B7 | HAdV-B7 | |
| CHN/Ad7/2011/7[P7H7F7] | Ad7 | KF268314 | 2011 | Dongguan, CHINA | Throat swab | So Med Univ | HAdV-B7 | HAdV-B7 | HAdV-B7 | HAdV-B7 | |
| USA/ak39_Ad7d2/1997/7[P7H7F7] | Ad7d2 | JX423387 | 1997 | IL, USA | Pharyngeal swab | LRRI/NHRC | HAdV-B7 | HAdV-B7 | HAdV-B7 | HAdV-B7 | |
| USA/ak40_Ad7b/1997/7[P7H7F7] | Ad7b | JX423388 | 1997 | IL, USA | Unknown | LRRI/NHRC | HAdV-B7 | HAdV-B7 | HAdV-B7 | HAdV-B7 | |
| USA/ak35_Ad7d2/2006/7[P7H7F7] | Ad7d2 | JX423383 | 2006 | MO, USA | Pharyngeal swab | LRRI/NHRC | HAdV-B7 | HAdV-B7 | HAdV-B7 | HAdV-B7 | |
| USA/UFL_Ad11/2005/11[P11H11F11] | Ad11 | KF268121 | 2005 | WI, USA | Urine isolate | U Florida | HAdV-B11 | HAdV-B11 | HAdV-B11 | HAdV-B11 | |
| USA/UFL_Ad34/2005/34[P34/H34/F34] | Ad34 | KF268196 | 2005 | TX, USA | Nasal wash | U Florida | HAdV-B34 | HAdV-B34 | HAdV-B34 | HAdV-B34 | |
| USA/UFL_Ad35/2004/35[P35H35F35] | Ad35 | KF268124 | 2004 | IA, USA | Urine isolate | U Florida | HAdV-B35 | HAdV-B35 | HAdV-B35 | HAdV-B35 | |
| EGY/ak37_Ad11a/2001/55[P14H11F14] | Ad11a | JX423385 | 2001 | EGY | Pharyngeal swab | LRRI/NHRC | HAdV-B55 | HAdV-B14 | HAdV-B11 | HAdV-B14 | |
| ARG/ak36_Ad11a/2005/55[P14H11F14] | Ad11a | JX423384 | 2001 | ARG | Unknown | LRRI/NHRC | HAdV-B55 | HAdV-B14 | HAdV-B11 | HAdV-B14 | |
| USA/UFL_Ad7h/2005/66[P7H7F3] | Ad7h | KF268126 | 2005 | CT, USA | Nasal wash | U Florida | HAdV-B66 | HAdV-B7 | HAdV-B7 | HAdV-B3 | |
| ARG/ak38_Ad7h/2003/7[P7H7F7] | Ad7h | JX423386 | 2003 | ARG | Unknown | LRRI | HAdV-B66 | HAdV-B7 | HAdV-B7 | HAdV-B3 | |
| Species C | ARG/Ad1/2000/1[P1H1F1] | Ad1 | JX173078 | 2000 | ARG | Unknown | LRRI | HAdV-C1 | HAdV-C1 | HAdV-C1 | HAdV-C1 |
| USA/Ad1/2004/1[P1H1F1] | Ad1 | JX173086 | 2004 | USA | Unknown | LRRI/NHRC | HAdV-C1 | HAdV-C1 | HAdV-C1 | HAdV-C1 | |
| USA/Ad1/2003/1[P1H1F1] | Ad1 | JX173085 | 2003 | USA | Unknown | LRRI/NHRC | HAdV-C1 | HAdV-C1 | HAdV-C1 | HAdV-C1 | |
| USA/Ad1/2003/1[P1H1F1] | Ad1 | JX173083 | 2003 | USA | Unknown | LRRI/NHRC | HAdV-C1 | HAdV-C1 | HAdV-C1 | HAdV-C1 | |
| USA/Ad1/2003/1[P1H1F1] | Ad1 | JX173082 | 2003 | USA | Unknown | LRRI/NHRC | HAdV-C1 | HAdV-C1 | HAdV-C1 | HAdV-C1 | |
| EGY/Ad1/2001/1[P1H1F1] | Ad1 | JX173080 | 2001 | EGY | Unknown | LRRI/NHRC | HAdV-C1 | HAdV-C1 | HAdV-C1 | HAdV-C1 | |
| USA/UFL_Ad1/2005/1[P1H1F1] | Ad1 | KF268331 | 2005 | CO, USA | Nasal wash | U Florida | HAdV-C1 | HAdV-C1 | HAdV-C1 | HAdV-C1 | |
| USA/Ad1/1988/1[P1H1F1] | Ad1 | KF429744 | 1988 | PA, USA | Ocular isolate | U Pittsburgh | HAdV-C1 | HAdV-C1 | HAdV-C1 | HAdV-C1 | |
| USA/UFL_Ad2/2004/2[P2H2F2] | Ad2 | KF268130 | 2004 | NY, USA | Nasal wash | U Florida | HAdV-C2 | HAdV-C2 | HAdV-C2 | HAdV-C2 | |
| USA/Ad2/2003/2[P2H2F2] | Ad2 | JX173084 | 2003 | USA | Unknown | LRRI/NHRC | HAdV-C2 | HAdV-C2 | HAdV-C2 | HAdV-C2 | |
| EGY/Ad2/2001/2[P2H2F2] | Ad2 | JX173081 | 2001 | EGY | Unknown | LRRI/NHRC | HAdV-C2 | HAdV-C2 | HAdV-C2 | HAdV-C2 | |
| ARG/Ad2/2002/2[P2H2F2] | Ad2 | JX173079 | 2002 | ARG | Unknown | LRRI | HAdV-C2 | HAdV-C2 | HAdV-C2 | HAdV-C2 | |
| ARG/Ad2/2005/2[P2H2F2] | Ad2 | JX173077 | 2005 | ARG | Unknown | LRRI | HAdV-C2 | HAdV-C2 | HAdV-C2 | HAdV-C2 | |
| USA/Ad2/1992/2[P2H2F2] | Ad2 | KF268310 | 1992 | PA, USA | Ocular isolate | U Pittsburgh | HAdV-C2 | HAdV-C2 | HAdV-C2 | HAdV-C2 | |
| USA/UFL_Ad5/2008/5[P2/H5/F5] | Ad5 | KF268199 | 2008 | CT, USA | Nasopharyngeal asp. | U Florida | HAdV-C5 | HAdV-C2 | HAdV-C5 | HAdV-C5 | |
| USA/Ad2/1988/5[P5H5F5] | Ad2 | KF268127 | 1988 | PA, USA | Ocular isolate | U Pittsburgh | HAdV-C5 | HAdV-C5 | HAdV-C5 | HAdV-C5 | |
| USA/Ad8/1990/5[P5H5F5] | Ad8 | KF429754 | 1990 | PA, USA | Ocular isolate | U Pittsburgh | HAdV-C5 | HAdV-C5 | HAdV-C5 | HAdV-C5 | |
| USA/ak31_Ad6/2007/6[P6H6F6] | Ad6 | JX423389 | 2007 | Philadelphia, USA | Unknown | LRRI/NHRC | HAdV-C6 | HAdV-C6 | HAdV-C6 | HAdV-C6 | |
| USA/UFL_Ad6/2005/6[P6H6F6] | Ad6 | KF268129 | 2005 | CO, USA | Fecal isolate | U Florida | HAdV-C6 | HAdV-C6 | HAdV-C6 | HAdV-C6 | |
| Species D | DEU/Ad9/1984/9[P67H9F15] | Ad9 | KF268206 | 1984 | DEU | Unknown | Germany | Novel | HAdV-D67 | HAdV-D9 | HAdV-D15 |
| DEU/Ad32/1988/32[P23H32F62] | Ad32 | KF268327 | 1988 | DEU | Unknown | Germany | Novel | HAdV-D23 | HAdV-D32 | HAdV-D62 | |
| DEU/Ad28/1987/28[P67H28F60] | Ad28 | KF268320 | 1987 | DEU | Unknown | Germany | Novel | HAdV-D67 | HAdV-D28 | HAdV-D60 | |
| DEU/Ad46/1986/46[P49H46F65] | Ad46 | KF268332 | 1986 | DEU | Unknown | Germany | Novel | HAdV-D49 | HAdV-D46 | HAdV-D9 | |
| DEU/Ad46/1987/46[P9H46F39] | Ad46 | KF268211 | 1987 | DEU | Unknown | Germany | Novel | HAdV-D9 | HAdV-D46 | HAdV-D39 | |
| DEU/Ad17/1986/17[P17H17F17] | Ad17 | KF268330 | 1986 | DEU | Unknown | Germany | Novel | HAdV-D48 | HAdV-D17 | HAdV-D30 | |
| DEU/Ad37/1988/37[P67H37F45] | Ad37 | KF268324 | 1988 | DEU | Unknown | Germany | Novel | HAdV-D67 | HAdV-D37 | HAdV-D45 | |
| DEU/Ad38/1984/38[P22H38F17] | Ad38 | KF268312 | 1984 | DEU | Unknown | Germany | Novel | HAdV-D42 | HAdV-D38 | HAdV-D30 | |
| DEU/Ad33/1986/33[P62H33F17] | Ad33 | KF268322 | 1986 | DEU | Unknown | Germany | Novel | HAdV-D48 | HAdV-D33 | HAdV-D30 | |
| DEU/Ad37/X/NEW[P37H37F17] | Ad37 | KF268208 | Unknown | DEU | Unknown | Germany | Novel | HAdV-D37 | HAdV-D37 | HAdV-D17 | |
| DEU/Ad32/1986/NEW[P38H32F27] | Ad32 | KF268325 | 1986 | DEU | Unknown | Germany | Novel | HAdV-D38 | HAdV-D32 | HAdV-D27 | |
| DEU/Ad37/1988/37[P28H37F38] | Ad37 | KF268334 | 1988 | DEU | Unknown | Germany | Novel | HAdV-D28 | HAdV-D37 | HAdV-D38 | |
| DEU/Ad15/1982/NEW[P33H56F56] | Ad15 | KF268201 | 1982 | DEU | Unknown | Germany | Novel | HAdV-D33 | HAdV-D15 | HAdV-D9 | |
| DEU/Ad71/1987/71[P9H20F67] | Ad9 | KF268207 | 1987 | DEU | Unknown | Germany | Novelb | HAdV-D9 | HAdV-D20 | Novelb | |
| DEU/Ad30/1985/30[P38H63F44] | Ad30 | KF268335 | 1985 | DEU | Unknown | Germany | Novelc | Novelc | HAdV-D30 | Novelc | |
| USA/Ad8/1991/8[P8/H8/F53] | Ad8 | KF268198 | 1991 | PA, USA | Ocular isolate | U Pittsburgh | HAdV-D8 | HAdV-D8 | HAdV-D8 | HAdV-D8 | |
| USA/Ad8/1991/8[P8/H8F/F53] | Ad8 | KF268118 | 1991 | PA, USA | Ocular isolate | U Pittsburgh | HAdV-D8 | HAdV-D8 | HAdV-D8 | HAdV-D8 | |
| USA/Ad8/X/8[P8H8F8] | Ad8 | KF268321 | Unknown | PA, USA | Ocular isolate | U Pittsburgh | HAdV-D8 | HAdV-D8 | HAdV-D8 | HAdV-D8 | |
| USA/Ad8/1992/8[P8H8F8] | Ad8 | KF429751 | 1992 | PA, USA | Ocular isolate | U Pittsburgh | HAdV-D8 | HAdV-D8 | HAdV-D8 | HAdV-D8 | |
| USA/Ad8/X/8[P8H8F8] | Ad8 | KF268205 | Unknown | PA, USA | Unknown | U Pittsburgh | HAdV-D8 | HAdV-D8 | HAdV-D8 | HAdV-D8 | |
| DEU/Ad12/1988/15[P15H15F15] | Ad12 | KF268204 | 1988 | DEU | Unknown | Germany | HAdV-D15 | HAdV-D15 | HAdV-D15 | HAdV-D15 | |
| USA/Ad10-Ad19/1989/37[P37/H37/F37] | Ad10/Ad19 | KF268122 | 1989 | PA, USA | Ocular isolate | U Pittsburgh | HAdV-D37 | HAdV-D37 | HAdV-D37 | HAdV-D37 | |
| USA/Ad37/X/37[P37H37F37] | Ad37 | KF268203 | Unknown | PA, USA | Ocular isolate | U Pittsburgh | HAdV-D37 | HAdV-D37 | HAdV-D37 | HAdV-D37 | |
| USA/UFL_Ad22/2005/53[P53/H53/F53] | Ad22 | KF268197 | 2005 | CT, USA | Conjunctival swab | U Florida | HAdV-D53 | HAdV-D37 | HAdV-D22 | HAdV-D8 | |
| USA/Ad8/1992/56[P56/H56/F56] | Ad8 | KF268333 | 1992 | PA, USA | Ocular isolate | U Pittsburgh | HAdV-D56 | HAdV-D9 | HAdV-D15 | HAdV-D9 | |
| DEU/Ad15/1982/56[P56H56F56] | Ad15 | KF268329 | 1982 | DEU | Unknown | Germany | HAdV-D56 | HAdV-D9 | HAdV-D15 | HAdV-D9 | |
| USA/Ad4/1992/NEW[P26H56F56] | Ad4 | KF268313 | 1992 | PA, USA | Ocular isolate | U Pittsburgh | HAdV-D56 | HAdV-D9 | HAdV-D15 | HAdV-D9 | |
| USA/Ad56/X/56[P9H15F9] | Ad9 | KF268209 | Unknown | PA, USA | Ocular isolate | U Pittsburgh | HAdV-D56 | HAdV-D9 | HAdV-D15 | HAdV-D9 | |
| ARG/Ad58/2010/58[P58H58F58] | unknown | KF268319 | 2010 | Cordoba, ARG | Fecal isolate | Ferreyra/CaPH | HAdV-D58 | HAdV-D49 | HAdV-D58 | HAdV-D29 | |
| USA/Ad64/X/64[P64H64F64] | Ad22 | KF268213 | Unknown | PA, USA | Ocular isolate | U Pittsburgh | HAdV-D64 | HAdV-D22 | HAdV-D19 | HAdV-D37 | |
| USA/Ad/1989/8[P8H8F8] | Ad8 | KF429753 | 1989 | PA, USA | Ocular isolate | U Pittsburgh | N/A- partial | HAdV-D8 | HAdV-D8 | HAdV-D8 | |
| USA/Ad8/1992/8[P8H8F8] | Ad8 | KF429749 | 1992 | PA, USA | Ocular isolate | U Pittsburgh | N/A- partial | HAdV-D8 | Unsequenced | HAdV-D8 | |
| USA/Ad8/1991/8[P8H8F8] | Ad8 | KF429746 | 1991 | PA, USA | Ocular isolate | U Pittsburgh | N/A- partial | HAdV-D8 | HAdV-D8 | HAdV-D8 | |
| USA/Ad8/1991/8[P8H8F8] | Ad8 | KF429743 | 1991 | PA, USA | Ocular isolate | U Pittsburgh | N/A- partial | HAdV-D8 | HAdV-D8 | HAdV-D8 | |
| USA/Ad8/1992/NEW[P26H56F56] | Ad8 | KF429747 | 1992 | PA, USA | Ocular isolate | U Pittsburgh | N/A- partial | HAdV-D9 | HAdV-D15 | HAdV-D9 | |
| USA/Ad8/1992/NEW[P26H56F56] | Ad8 | KF429750 | 1992 | PA, USA | Ocular isolate | U Pittsburgh | N/A- partial | HAdV-D9 | HAdV-D15 | Unsequenced | |
| USA/UFL_Ad19/2005/64[P64H64F64] | Ad19 | KF268323 | 2005 | TN, USA | Bronchoscopy sample | U Florida | N/A- partial | HAdV-D22 | HAdV-D19 | HAdV-D37 | |
| USA/UFL_Ad45/2005/[P-NEW/H45/F-NEW] | Ad45 | KF268200 | 2005 | IN, USA | Fecal isolate | U Florida | N/A- partial | HAdV-D48 | HAdV-D45 | Novel | |
| DEU/Ad17/1988/17[P29H17F30] | Ad17 | KF268326 | 1988 | DEU | Unknown | Germany | N/A- partial | HAdV-D29 | HAdV-D17 | HAdV-D29 | |
| USA/Ad8/1992/NEW[P9H22F44] | Ad8 | KF429745 | 1992 | PA, USA | Ocular isolate | U Pittsburgh | N/A- partial | HAdV-D9 | HAdV-D22 | Unsequenced | |
ARG Argentina, DEU Germany, EGY Egypt. GenBank accession number, serotype, sample origin, collection information, and preliminary sequence characterization of 85 currently circulating and historically intriguing human adenoviruses (HAdVs) are presented. Complete and partial genome sequences were analyzed with respect to sequence percent identities for genotyping using the three major capsid genes: penton base, hexon, and fiber. Sequence identities to prototypes are noted, with the HAdV name noted as species and type. Novel genome and gene sequences are noted as “novel”, with three genomes re-named as accepted genotypes (1, HAdV-B76; 2, HAdV-D71; and 3, HAdV-D72). The current GenBank name is included for reference; these will be updated with regards to the sequence analyses. Collections sampled include the School of Public Health, Southern Medical University, Guangzhou, China (So Med Univ); Sankara Nethralaya, Chennai, India (India); Institut für Virologie, Medizinische Hochschule, Hannover, Germany (Germany); (U Florida); Charles T. Campbell Ophthalmic Microbiology Laboratory, Ear and Eye Institute, School of Medicine, University of Pittsburgh, Pittsburgh, PA (U Pittsburgh); and two collections processed at the Lovelace Respiratory Research Institute, Albuquerque, NM, including samples from Dr. Adriana Kajon and the U.S. Naval Health Research Center, San Diego, CA (LRRI and LRRI/NHRC, respectively). One sample was provided by Dr. Leonardo Ferreyra (Cordoba, Argentina) and processed at the California Public Health Dept., Richmond, CA (Ferreyra/CaPH).
aHAdV-B76
bHAdV-D71
cHAdV-D72
Whole-genome analysis of sequenced HAdVs species (A–D)
Initial post-sequencing, JCVI pipeline-based, automated annotation, supplemented with two HAdV genotyping tools (in beta testing), provided a “first-pass” characterization of these genomes. These were submitted to GenBank, ahead of publication, and assigned accession numbers, as shown in Table 1.
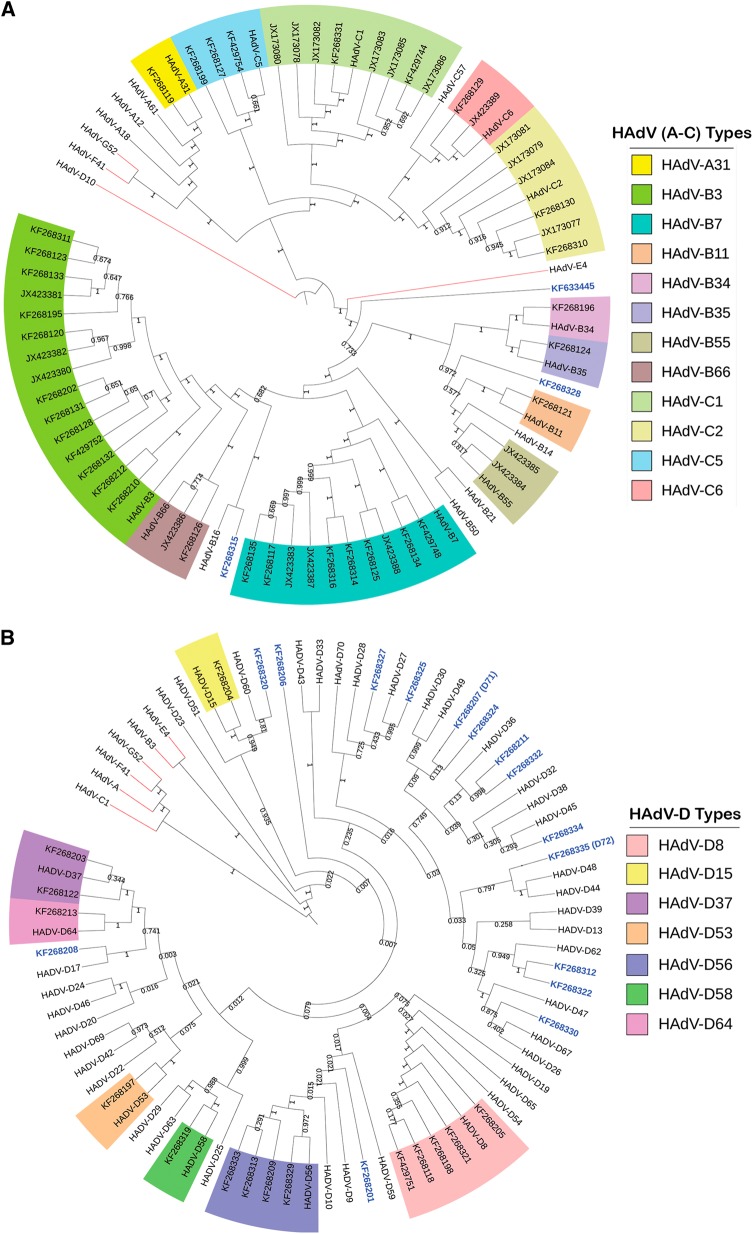
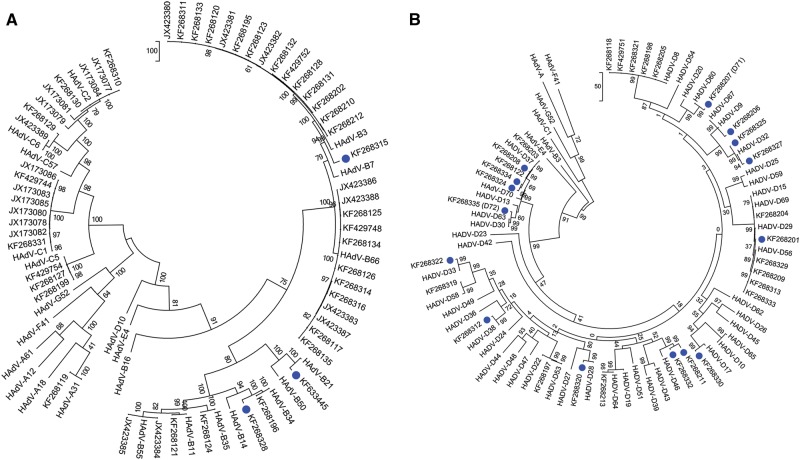
To provide a more thorough and individualized analysis of each whole-genome sequence, additional computational analyses were performed. The newly obtained sequences were typed to four HAdV species, including species A (n = 1), species B (n = 35), species C (n = 19), and species D (n = 40). Upon BLAST and whole-genome phylogenetic analysis, 67 sequences were characterized as previously known genotypes, as shown in the whole-genome phylogenetic trees (Fig. 1a, b). In these trees, the newly obtained genomes are noted by their GenBank accession numbers; there are two trees presented in order to highlight the larger HAdV-D sample size. Representative genomes from species E, F, and G are included for reference. The remaining 18 sequences showed 1.65 to 7.48% nucleotide differences and diverged into separate subclades from existing HAdV types. These sequences were considered as novel genotypes, and were identified as either HAdV-B or D. One novel genome, KF633445, clades with species B and appears to have a zoonotic origin (data not shown).
Fig. 1. Human adenovirus whole-genome phylogenetic trees for (a) HAdV-A through C and (b) HAdV-D complete genomic sequences.
Two trees are presented in order to display the larger number of species D clearly. Representatives of species E, F, and G are presented for reference. Trees were constructed using the maximum-parsimony analysis, following alignment using the ClustalW tool in the Molecular Evolutionary Genetic Analysis version 6 software package (MEGA6; www.megasoftware.net/), with a bootstrap test of 1000 replicates and default parameters and Tree-Bisection-Reconnection (TBR) method. Each clade is highlighted according to specific HAdV species using a color code as shown. Novel sequences are shown in blue and displayed as GenBank accession numbers (acc. nos.). Bootstrap values are displayed on the branches and nodes for different HAdV species used for references are shown in red
By maximum-parsimony phylogenetic analysis, one sequence in HAdV-A grouped with type 31, an important pathogen in allotransplant recipients7. Within the HAdV-B genotypes, the majority of isolates segregated with types B3 (n = 15), B7 (n = 11), and B55 (n = 2). This reflected the collections and respiratory pathogen interests of two of the collaborators, and comprised the majority of samples in the University of Florida archive of historically intriguing pathogens. The genotype distribution in species HAdV-C were C1 (n = 7), C2 (n = 7), C5 (n = 3), and C6 (n = 2), again reflecting the above respiratory disease interests. For two other collaborators, ocular disease-associated pathogens are represented by the majority of submitted samples; these genotypes are in species HAdV-D (43%) and segregate with isolates associated with epidemic keratoconjunctivitis, including HAdV-D8 (n = 5), -D37 (n = 2), -D53 (n = 1), -D56 (n = 4), and -D64 (n = 1), reflecting the clinical settings in which the samples were obtained. This multi-center, large-scale genomics study identifies a wide distribution of HAdV-A. B, C, and D genotypes. The genomes identified are consistent with those noted as respiratory and ocular pathogens in the literature, and were available given the interests of individual centers in these diseases, for example, HAdV-B7 with respiratory diseases and HAdV-D8 with ocular diseases.
Sequence diversity of HAdVs genotypes in species A through D
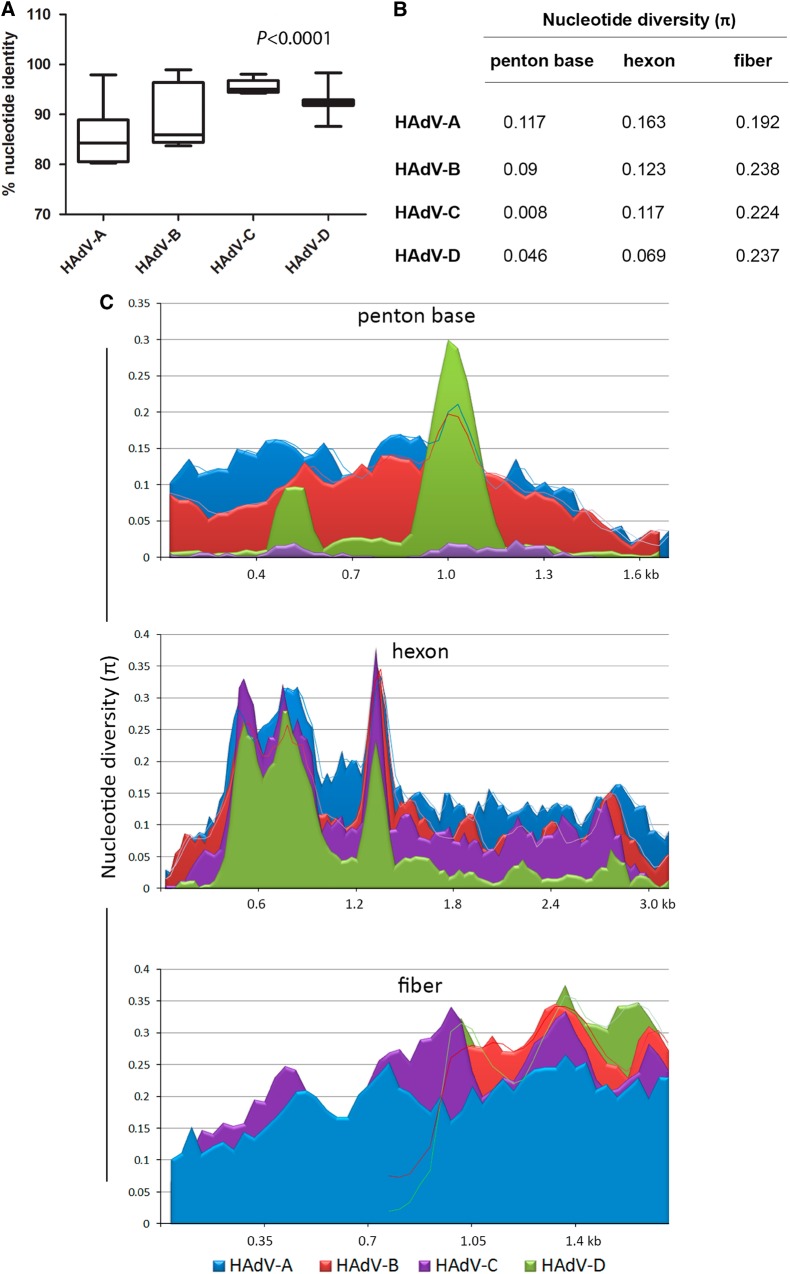
HAdV genome sequences are largely conserved from isolate to isolate, of the same type, e.g., HAdV-B7, over time35–37, as expected for dsDNA genomes. An analysis that included all of the prototype genome sequences for HAdV-A, -B, -C, and -D genotypes shows the average nucleotide identities within species were 85.5, 89.7, 95.5, and 92.3%, respectively (Fig. 2a). It was reconfirmed with this large data set that the genomic regions that primarily influence sequence and genome diversity include the three major capsid genes: penton base, hexon, and fiber, along with the E3 transcription unit (at distinct loci). These capsid genes are the same regions employed for HAdV genotyping and identification of recombinants16,17.
Fig. 2. Nucleotide sequence identity and diversity data for HAdV species (A–D) prototype sequences.
a “Box-and-whiskers” plots show percent nucleotide identity of prototype HAdV complete genome sequences. The “box” represents the median and interquartile range (IQR), and the “whiskers” show both minimum and maximum values. The spacing between the boxes indicates the degree of spread; as depicted, the HAdV-C and D species are relatively homogenous. The nucleotide identities are significantly different for HAdV species analyzed (Kruskal–Wallis, P < 0.0001). b Average nucleotide diversity of the major capsid genes between each type within HAdV-A through D. c Nucleotide diversity (π) plots showing the average number of nucleotide differences per site along each gene for HAdV-A through D, calculated for penton base, hexon, and fiber prototype sequences. The plot was constructed using DnaSP v5 ((http://www.ub.edu/dnasp/), with a 100 nucleotide window and 25 nucleotide step size. Nucleotide alignments with gaps were excluded and graphs were constructed using Microsoft Excel software. The lines in the graph represent “trend lines”. Viruses within HADV-B and -D had shorter fiber genes than HAdV-A or -C and the trend lines are denoted accordingly
Nucleotide sequence diversity analyses and graphs comparing the major capsid genes for each type in HAdV-A through -D reaffirm the relationships reported in the literature (Fig. 2b, c). The nucleotide diversity plot (Fig. 2c) depicts the HAdV-C penton base genes to be relatively conserved, reflecting previous observations for these species C genotypes38. For HAdV-D, there are two distinct hypervariable regions (HVR-1 and 2) within the penton base gene; these correspond to the two hypervariable loop domains (HVL1 and HVL2) on the protein. In contrast, the penton base gene of HAdV-A and -B are entirely hypervariable. This is reflected also in average intra-species sequence divergence rates for the HAdV-A, -B, -C, and -D penton base gene of 11.7, 9.0, 0.8, and 4.6%, respectively (Fig. 2b).
The hexon protein contains two hypervariable loop domains (HVL1 and HVL2) that form the “epsilon” epitope, determinants that are recognized by neutralizing antibodies and are the basis for serum or virus neutralization (SN or VN) and serotyping7,37,39. For this data set, the average nucleotide divergence rates for the hexon gene was 16.3, 12.3, 11.7, and 6.9% for HAdV-A, -B, -C, and -D, respectively.
The trimeric fiber protein contains a N-terminal tail, a central shaft, and a C-terminal knob; the latter mediates the primary interaction with host cells, i.e., cell tropism. The fiber knob contains the “gamma” epitope, which was useful for serotyping through hemagglutination inhibition (HI)7. In comparison to the other major capsid genes, the fiber was found to be entirely hypervariable in all four HAdV species (Fig. 2c). Notably, the fiber open-reading frames for HAdV-B and D are just over half the length of those for HAdV-A and C, consistent with the literature, but all four species show a similar degree of diversity (Fig. 2b, c).
Characterization of hypervariable capsid genes
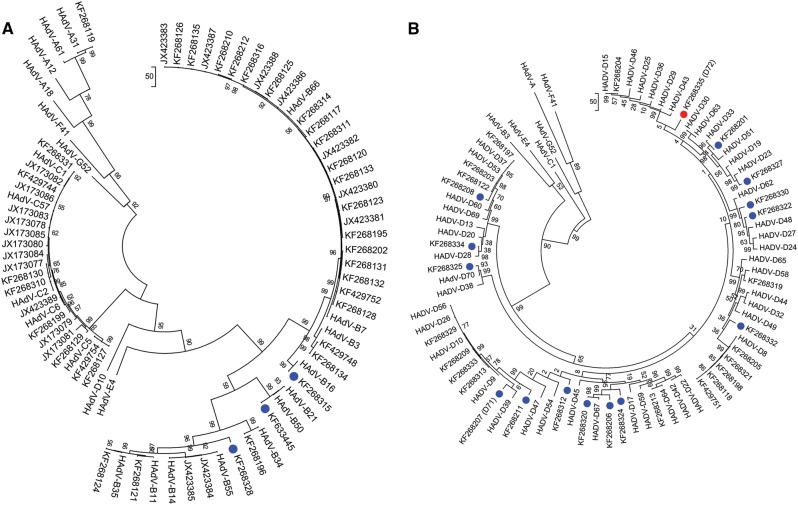
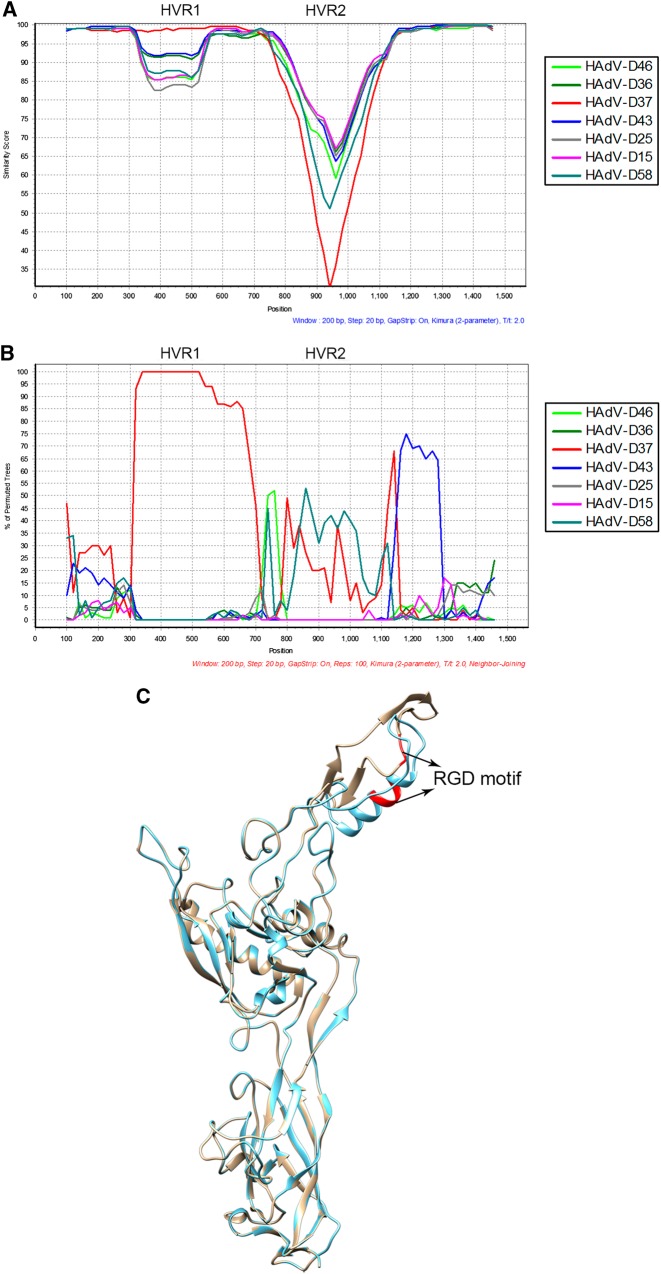
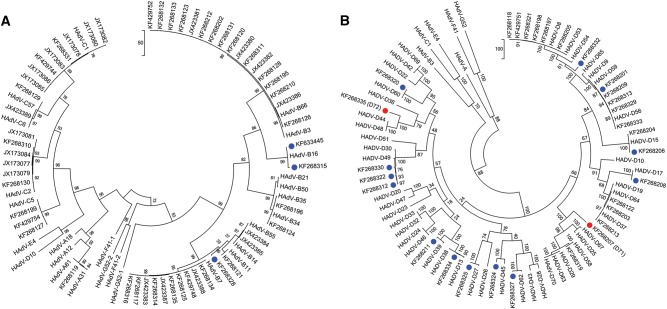
To classify the molecular types for each adenoviral genome and to characterize in detail the 18 novel genotype sequences identified by whole-genome analysis (Fig. 1), maximum-parsimony phylogenetic analyses were performed for the capsid genes. The penton base phylogenetic tree for HAdV-A through -C and, separately, for HAdV-D are displayed in Fig. 3a, b, respectively. All three novel sequences in HAdV-B clustered to one of the previously known types B16, B35, and B21, with 99% bootstrap support (Fig. 3a). Among the 15 novel sequences in HAdV-D, 14 are clustered with the previously known types, with the majority showing 80–99% bootstrap support (blue dots, Fig. 3b). HAdV-D72 formed a separate subclade and is recognized as a novel genotype (Fig. 3b). Using Simplot and Bootscan software for sequence recombination analyses, the penton base sequence of HAdV-D72 was found to be recombinant, with its HVR-1 deriving from HAdV-D37 and its HVR-2 comprising a novel sequence (18% nucleotide divergence from nearest HAdV-D15), as shown in Fig. 4a, b, respectively. Penton base HVR-2 contains the canonical Arg-Gly-Asp (RGD) motif that interacts with host cell integrins to mediate virus endocytosis40,41. To examine this closer, a penton base homology protein model for this novel sequence was generated, superimposed with HAdV-D37 for visualizing the structural variations (Fig. 4c). The RMSD for the two superimposed structures was 0.419 with structural distance 8.54 and Q-value 0.869. In the structural model, crucial β-sheets for the novel RGD loop are absent and an additional α-helix is projected. Intriguingly, the RGD motif falls within the crucial α-helix. Novelties in the penton base and fiber (noted below) genes led to the approval by the Human Adenovirus Working Group and NCBI of a new type number (HAdV-D72) (Accession no. KF268335).
Fig. 3. Maximum-parsimony phylogenetic analysis of the penton base.
Penton base gene sequences of a HAdV species A–C genotypes and b HAdV species D genotypes are presented; two trees allow for discrimination of the larger numbers of species D genotypes. Representatives of species E, F, and G are included for reference. Sequences obtained from this study are displayed as GenBank accession numbers. Novel HAdVs in this data set, identified by whole-genome analysis, are marked with blue dots. A novel penton base gene (KF268355; HAdV-D72) that diverged significantly from previously described HAdV-D sequences is identified by a red dot. Trees were constructed using the maximum-parsimony analysis, following alignment using the ClustalW tool in the Molecular Evolutionary Genetic Analysis v6 software package (MEGA6;www.megasoftware.net/), with a bootstrap test of 1000 replicates and default parameters, and Tree-Bisection-Reconnection (TBR) method in MEGA 6.0
Fig. 4. Recombination and structural modeling analysis of putative novel penton base gene in HAdV-D72.
a SimPlot analysis demonstrates the genetic distances to HAdV reference sequences across the penton base gene, in which the x-axis denotes gene nucleotide position and the percentages of permutated trees that supported grouping are marked along the y-axis (http://sray.med.som.jhmi.edu/SCRoftware/simplot/). b Bootscan analysis demonstrates phylogenetic relationships to the reference strains. Each HAdV reference genotype is color coded; only closely related HAdV types are presented for clarity. Penton base gene recombination between hypervariable region (HVR)-1 and the RGD motif-containing HVR-2 is shown. The HVR-1 fragment showed high similarity with HAdV-D37, while HVR-2 was dissimilar to existing reference sequences, indicating both recombination and a novel gene segment. Prior to recombination analysis, sequences were aligned using the ClustalW tool in the Molecular Evolutionary Genetic Analysis v6 software package (MEGA6; www.megasoftware.net/). Default parameter settings for the Simplot software were used for analyzing the hexon sequences: window size (200 nucleotides [nt]), step size (20 nt), replicates used (n1/4100), gap stripping (on), distance model (Kimura) and tree model (neighbor-joining). c Homology modeling of the penton base in which the HAdV-D72 amino-acid sequences (blue) is superimposed over that of HAdV-D37 (cyan). Superimposition of structural models showed significant structural variation in RGD motif location (indicated in red). Homology model was built in Swiss ExPASy (http://swissmodel.expasy.org) using the crystal structure of HAdV-C2 as the template ([PDB] code 1X9TA)
The hexon gene sequences were analyzed by maximum-parsimony phylogenetic analysis, with trees for HAdV-A, -B, and -C and for HAdV-D, separately, presented in Fig. 5a, b, respectively. Three novel HAdV-B genotypes contain hexon sequences that clustered with previously typed HAdV-B3, -B34, and -B21. Similarly, all HAdV-D hexon genes clustered with previously known types. Therefore, no unique hexon molecular types were identified in the whole and partial genome sequences of this data set.
Fig. 5. Maximum-parsimony phylogenetic analysis for hexon.
Gene sequences of hexons from a HAdV-A, -B, and -C and b HAdV-D are presented. Trees were constructed, following sequence alignment with ClustalW, using the maximum-parsimony option of the Molecular Evolutionary Genetic Analysis v6 software package (MEGA6; www.megasoftware.net/) and implementing a bootstrap test of 1000 replicates and default parameters. Sequences derived from this large-scale sequencing study are identified by their GenBank accession codes. Putative novel genotypes are denoted by blue dots. No novel hexon sequence was found
Phylogenetic trees for the fiber genes of viruses sequenced in species HAdV-A, -B, and -C and for HAdV-D, separately, are shown in Fig. 6a, b, respectively. As displayed, the three novel HAdV-B genotypes contain fiber sequences that clustered with the HAdV-B7 (one) and -B16 fiber genes (two). Among the 15 novel sequences in HAdV-D, 13 contained fiber genes with homology to published counterpart sequences. The remaining two sequences each formed distinct clades with significant bootstrap support (red dots, Fig. 6b). Calculated nucleotide sequence differences to the nearest fiber sequences (HAdV-D67 and -D44) were 16% and 9%, respectively. These two emergent adenoviruses, with putatively novel fiber genes, were recognized with new type numbers by NCBI as HAdV-D71 and -D72 (KF268207 and KF268335, respectively). It is noteworthy that while HAdV-D72 contains both a novel penton base protein and novel fiber protein, it has a hexon gene sequence that is highly similar to HAdV-D30.
Fig. 6. Maximum-parsimony phylogenetic analysis for the fiber genes.
Sequences of a HAdV-A, -B, and -C and b HAdV-D are presented as phylogenetic trees. Sequences derived from this large-scale sequencing study are identified by their GenBank accession codes. Novel HAdV genotype sequences identified by whole-genome analysis are denoted with blue dots. Novel fiber genes (KF268207, HAdV-D71, and KF268355, D72) with sequences that diverged significantly with known HAdV-D type sequences are identified by red dots. Trees were constructed, following sequence alignment with ClustalW, using the maximum-parsimony option of the Molecular Evolutionary Genetic Analysis v6 software package (MEGA6; www.megasoftware.net/) and implementing a bootstrap test of 1000 replicates and default parameters
HAdV molecular types and novel genotypes
Eighty-five newly obtained whole-genome sequences isolated from respiratory, ocular, and gastrointestinal pathogens partition into four HAdV species: A (n = 1), B (n = 35), C (n = 19), and D (n = 40). The numbers are arbitrarily skewed due to the number of samples provided by collaborators and the success of the sequencing run due to the quality and quantity of the DNA. These genomes and their genotype-defining markers, i.e., the three major capsid genes, are presented in Fig. 7. It is clear that recombination is a major evolution pathway by which novel and emergent HAdV pathogens arise. Even with a skewed and “small” sample size of 85, recombination is observed in significant numbers. Of the 30 HAdV-D whole-genomes sequenced, 22 (73.3%) were clearly recombinants; for HAdV-B and -C, 07/35 (20%) and 01/19 (5.3%), respectively, were clear recombinants. In summary, 18 novel genotypes were found.
Fig. 7. Summary of genotypes and molecular types.
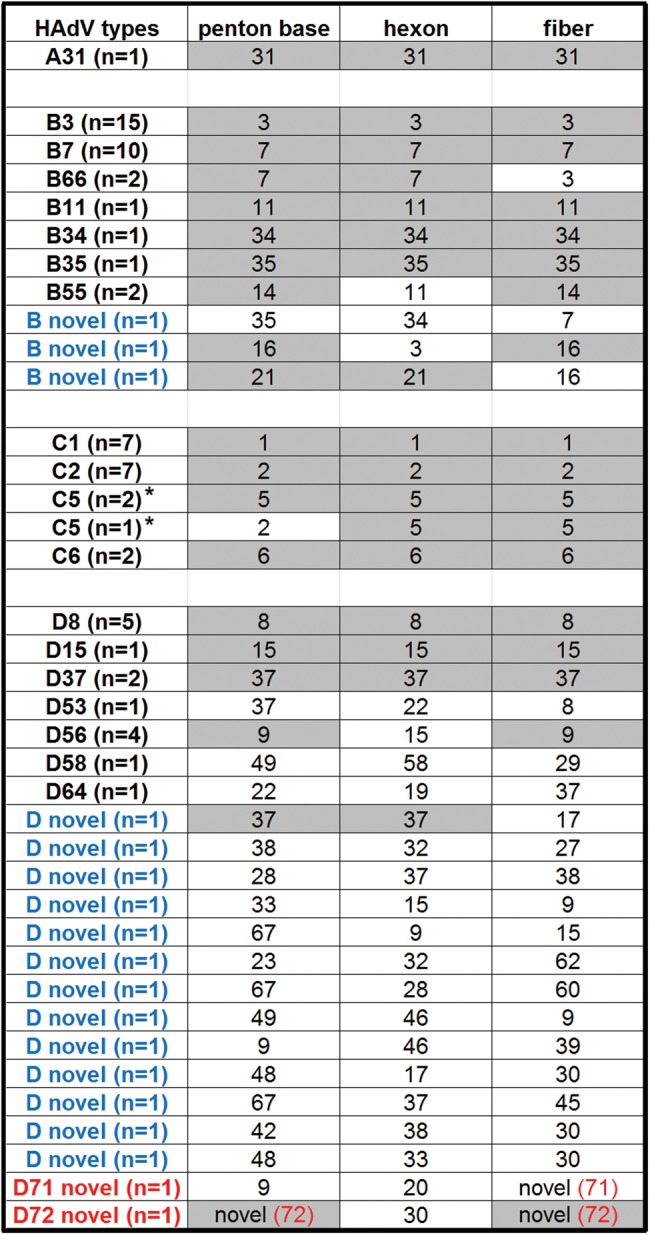
For each isolate sequenced and providing whole-genome sequence, their capsid genes are noted, with the number of related isolates in parenthesis as “n = ”. Capsid genes that were shared within each HAdV type are highlighted. HAdV genotypes that are novel recombinants are indicated in blue and HAdV types containing novel genes indicated in red; novel genotype number is indicated in red and in parenthesis. *The penton bases for HAdV-C species are highly conserved and, therefore, these penton base genes did not contribute to HAdV typing in this species by whole-genome analysis
Discussion
The Human Adenovirus (HAdV) Genome Sequencing Project was a collaborative project between the Adenovirus Genome Sequencing Consortium and the J. Venter Institute (JCVI) Genomic Sequencing Center for Infectious Diseases (GSC) (Rockville, MD). Using HAdV as a model organism, several sequencing platforms, sequencing strategies, and software tools were tested, modified, and/or developed. After test runs, the optimal small-genome sequencing strategy was a two-platform protocol: Ilumina HiSeq (San Diego, CA) and Roche 454 GS-FLX (Branford, CT). The advantages of each provided for the shortfalls inherent with the other. The protocol is designed to identify and type rapidly an unknown HAdV pathogen.
Whole-genome sequencing and preliminary bioinformatics analysis of 95 samples were performed to characterize the predominance of genotypes under certain conditions and to study the molecular evolution of HAdV pathogens, e.g., the numbers of recombinant viruses. Of the 85 whole-genomes sequenced, 18 are of previously uncharacterized HAdVs, all of which evolved by homologous recombination, including three novel HAdV-B types and 15 novel HAdV-D types. Partially sequenced viral genomes are hallmarks of metagenomics survey projects and have been accepted by both NCBI and ICTV recently34. In this survey, there are ten partial genome sequences, all of which were from samples collected in eye clinics. The large-scale sampling and processing, including variable viral titers, likely contributed to the genomic DNA quality and quantity. Even with partial sequences, three ocular isolates were characterized as highly similar to HAdV-D8, a known EKC pathogen, with penton base, hexon, and fiber sequences (P8H8F8) (KF429753, KF429746, and KF429743). For KF429749, the penton and fiber are also highly similar to HADV-D8, however, the hexon sequence is incomplete. KF429747 and KF429750 are both recombinants at the three capsid genes used for HAdV typing, having sequence similarities corresponding to a penton base of HAdV-D9 and a hexon of HAdV-D15. The latter has an incomplete fiber gene sequence and the former has a fiber sequence with identity to HAdV-D9. KF268323 is similar to HAdV-D64 with a preliminary recombinant genotyping of P22H19F37. Similarly, KF268326 is similar to HAdV-D17, with a preliminary genotyping as P29D17F29. KF268200 has a preliminary genotype of P48H45 with a novel fiber. The final partial genome KF429745 was noted with a preliminary genotype of P9H22 and an unsequenced fiber gene.
In the continuation of understanding sequence divergence and proteotypes19, two novel fiber genes and one novel penton base gene were defined in two viruses within species HAdV-D. These analyses confirm genome recombination and lateral genomic transfer as a major molecular evolution mechanism in the genesis of novel HAdV pathogens and provide an algorithm for rapid genomic sequencing and in silico identification and characterization of emergent viral pathogens. Genomics and bioinformatics allow a thorough and high-resolution analysis of currently circulating pathogens, in some cases, providing insights into why a particular isolate is either more or less pathogenic, infectious, or contagious42. This approach is invaluable to understanding pathogens that were similarly historically intriguing but no longer circulating. As an example, a respiratory adenoviral pathogen causing a fatality and isolated in the U.S. was studied and archived in a Germany repository. It was identified as an adenovirus that serotyped at the epsilon epitope as both HAdV-B21 and -B16, both acute respiratory disease (ARD) pathogens, and at the gamma epitope as HAdV-B16. It was named “Ad21 + 16H16”, which was not standard. Following its genome determination in this study, subsequent analysis beyond serotyping the hexon and fiber reveals it was an emergent and is a predicted “coming” human pathogen. Uniquely, it contains a multi-recombinant genome that incorporates elements of two SAdVs along with two HAdVs, suggesting multi-directional and reciprocal zoonosis and anthroponosis (prototype (HQ883276 preparation).
Another goal was to assist NCBI/GenBank in standardizing the HAdV genome records by providing an example to the research community, in providing a large set of similarly annotated and formatted HAdV genome data, including an informative GenBank “universal” name that included “adenovirus species/host/location/lab name/year/type number [serological/genome markers]”; and example of this is “Adenovirus D human/DEU/IAI-1/2005/53[P37H22F8].”
This is the first large-scale adenovirus study in which whole-genome data and DNA sequence analyses were used to discriminate and to type and characterize HAdV pathogens. In the past, only serology-based typing of the epsilon (SN) and/or gamma (HI) epitopes were reported. The gamma epitope is notoriously difficult to determine let alone repeat, even by “experts”26,43. Given the numbers of recombinants, the past surveys are flawed for a deeper understanding of this pathogen. For a comprehensive understanding of what makes a pathogen a pathogen, and to what degree, a solid definition of what exactly constitutes a pathogen is critical. Three examples illustrate this clearly. A novel recombinant strain of HAdV-D22, (“22H8”) with a penton sequence identical to type 37 was reported as a highly contagious epidemic keratoconjunctivitis (EKC) pathogen in Germany44. Since its first isolation in 1960 and to date, HAdV-D22 had never been associated with EKC45. Genome analysis showed the emergent pathogen was a recombinant that incorporated only the epsilon epitope of HAdV-D22 into the genome chassis that contained a penton based derived from HAdV-D37 and a fiber transferred from HAdV-D8, both of which are EKC pathogens21; it was named HAdV-D53 in recognition of a novel adenoviral pathogen. Furthermore, in a subsequent re-analysis of a collection of EKC pathogens at the Japan National Institute of Infectious Diseases, based on data from Walsh et al.21, HAdV-D53 was found to have been a major circulating ocular pathogen since 1996 and was the third most common EKC pathogen, having been mis-identified “as types 8, 22, or 37” (ref.46). The correct identification focuses efforts to prevent or remedy HAdV-D37-caused EKC infections to be directed at HAdV-37 chassis rather than the non-pathogenic HAdV-D22. A similar example was reported for the emergent EKC pathogen and recombinant HAdV-D64, comprising the epsilon epitope of non-pathogenic HAdV-D19 and the EKC pathogen HAdV-D3723. A third example is that of the “Trojan Horse” pathogen HAdV-B5522. This is a highly contagious ARD pathogen that is a recombinant that presents the epsilon epitope of a renal pathogen, HAdV-B11 in a genome and proteome of an ARD pathogen, HAdV-B14. No other HAdV-B11 strain or genome types are respiratory pathogens. HAdV-B11 has been associated with renal disease in immunocompromised renal transplant patients7 and likely has limited circulation in the general population, hence an immunologically naive population as HAdV-B14 circulates currently47–49.
This study provides support that the novel recombinant genotypes are relevant pathogens despite possible sporadic reports subsequent to the initial identification and recognition of the prototype. As noted earlier for HAdV-D53, recognition of a novel pathogen may be followed by re-examination of archives and subsequent identification of mistyped pathogens46. Interestingly, HAdV-D53 was found in an archive of historically intriguing pathogens, collected in Connecticut (USA; 2005). It was typed serologically and mis-identified as HAdV-D22, a non-EKC pathogen of low virulence. The genome data (KF268197) presented in this study shows identity to the prototype HAdV-D53, and reinforces the importance of high-resolution data in identifying and typing pathogens for a better understanding of their molecular evolution, distribution, and epidemiology. This recognition of a third sampling and third country of isolation indicates that HAdV-D53 and other novel HAdV genotypes may be underreported and underappreciated as important human pathogens, particularly if only the hexon and/or fiber serotyping epitopes are used for identity and typing.
Additionally, a specimen associated with gastrointestinal disease was included. It was isolated from a stool sample of an AIDS patient (aged 46) in 1996 and was initially typed by hexon sequencing as HAdV-F41. The virus isolated and amplified in cell culture was thought to be interesting as it was not serum neutralized by antiserum to HAdV-F41. However, upon genome sequencing, this isolate shows sequence identity to prototype HAdV-D58 (HQ883276), isolated in 1996 from the stool of a 31-year-old AIDS patient who presented with severe chronic diarrhea. Interestingly, HAdV-D58 (KF268319) contained a serologically unique hexon and a recombinant fiber that has a partial proximal shaft sequence derived from HAdV-D25 and a distal shaft plus knob contributed by HAdV-D29. A comparison of the sequences of KF268319 to the prototype reveals a nearly identical virus: Genome (99.4%); penton base (99.3%); hexon (99.8%); and fiber (100%). In retrospect, the patient, with chronic diarrhea, had a co-infection of two HAdVs; apparently, HAdV-F41 did not replicate well in cell culture prior to DNA isolation for sequencing. It should be noted that one objection to using genome data for recognizing novel HAdV types, as opposed to only hexon and fiber epitopes, was that many “newly-recognized” HAdV species D serotypes from AIDS patients in the 1990s were never reported again. In the context of HAdV evolution and pathogen genesis, this objection is irrelevant, as demonstrated for this isolate of HAdV-D58 and for the previously noted HAdV-D53.
Viruses represent a highly tractable model system for studying evolutionary biology. The HAdV genome is largely conserved, but interrupted in stereotypical fashion by hypervariability at four major regions of the genome, specifically the three major capsid genes and the E3 transcription unit. By comparison of genomic differences between HAdV major capsid regions, marked differences across species were noted. For example, the penton base genes of HADV-C types are relatively conserved. In contrast, HAdV-A and -B penton base genes are largely hypervariable across their entire open-reading frames. HAdV-D penton base genes show overall conservation interrupted by two distinct hypervariable regions, accounting for two distinct hypervariable loops on the external capsid surface, HVL1 and HVL2. We previously showed that these two hypervariable regions of the penton base gene often undergo homologous recombination, and at times, independent of one another50. HAdV-D72 was typed as a novel genotype on the basis of differential recombination within the penton base gene, specifically involving RGD loop (HVL2). Thus, it appears that viruses within HAdV-D may more frequently utilize penton base gene recombination for their evolution than other HAdV species. Also notable was the fiber gene. Viruses within HADV-B and -D had shorter fiber genes than HAdV-A or -C, but for all the four species, the fiber sequences showed a similar degree of diversity. A short trimeric fiber protein is thought to be more rigid, perhaps with more strict receptor interactions and cellular specificity. However, a short fiber may permit docking of penton base proteins to host cell integrins independent of fiber knob binding to a primary adenovirus receptor, and allow for fiber-independent cell entry51. These data suggest that fiber length matters, but in a complex fashion. Furthermore, we and others have shown a correlation between fiber knob amino acids and corneal tropism42,52. Altogether, these data are consistent with an important role for variations in fiber length and nucleotide content in infectivity and cellular tropism.
The range of HAdV types in circulation reflects an indeterminate combination of asymptomatic carrier states and the global burden of disease caused by adenoviruses, and we lack sufficient information about the human source of each sample and their condition to draw conclusions. However, HAdV-A31, the only HAdV-A identified in this study, is a pathogen of allogeneic hematopoietic stem cell transplant recipients. In contrast, a majority of HAdV-B types identified were -B7 and -B3, both associated with severe respiratory infections, along with the HAdV-B55 discussed above. HAdV-Cs are important pathogens in immunocompromised persons and are primarily associated with respiratory infection. Infections from viruses within HAdV-C are all commonly seen in young children. In our analysis, all the HAdV-Cs we identified had been previously characterized.
Identification of two HAdV-D types with novel fiber genes suggests as yet unidentified parent viruses for which the full genomes have not yet been characterized. Though the RGD loop was not solved in the original crystal structure (1X9T), our modeling analysis also suggested significant structural variation in the penton base protein RGD loop of the aforementioned HAdV-D72. The RGD motif is thought to be crucial to adenovirus internalization, and the RGD motif predicted within a novel α-helix structure in HAdV-D72 likely has an impact on cellular interactions. Though the conformation of the protein in this disordered loop is not informative, identification of α-helix over β-sheets in the comparative modeling analysis could be significant. This further warrants the need for deeper high-resolution structural analysis. Notably, the fiber protein of HAdV-D72 was also novel.
Altogether, HAdV types characterized in this survey were all from the four HAdV species with the most members, and were either replicates of viruses already whole-genome sequenced and characterized or newly identified recombinants, and two of the latter contained novel penton base and/or fiber genes. All HAdV-Ds with archived whole-genome sequences show evidence for homologous recombination in at least two loci in their genomes. In our study, recombination among HAdV-Ds was much more common than finding novel capsid genes, supporting the primacy of homologous recombination in the molecular evolution of HAdV-D. Our data further support evolution of HAdV-B by homologous recombination, but less assuredly of HAdV-C. However, the numbers of total viruses within HAdV-C was too few to allow for a conclusion. Finally, the clinical significance of those novel HAdV types identified in this analysis remains unknown. Regardless, our results taken together with recent descriptions of putatively new HAdV types causing serious diseases, highlights HAdV evolution as a persistent threat to public health.
Acknowledgements
This project has been funded in whole or part with federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services under Contract Number HHNS272200900007C and Grant Number U19AI110819. J.C. and J.R. are supported by NIH grants EY013124, EY021558, and EY014104, a Senior Scientific Investigator Award grant from Research to Prevent Blindness, Inc., New York, NY, The Falk Foundation, and the Massachusetts Lions Eye Research Fund.
Competing interests
The authors declare that they have no competing interests.
Footnotes
The original version of this article was revised: The author R. Halpin has been added in the author group.
Ashrafali M. Ismail and Tiange Cui contributed equally to this work.
Change history
12/8/2018
The original publication of this article [1] was missing the below author that made contributions to the research and the published article:
Contributor Information
James Chodosh, Email: james_chodosh@meei.harvard.edu.
Donald Seto, Email: dseto@gmu.edu.
References
- 1.Sharp PA. Split genes and RNA splicing. Cell. 1994;77:805–815. doi: 10.1016/0092-8674(94)90130-9. [DOI] [PubMed] [Google Scholar]
- 2.Stillman BW. The replication of adenovirus DNA with purified proteins. Cell. 1983;35:7–9. doi: 10.1016/0092-8674(83)90201-5. [DOI] [PubMed] [Google Scholar]
- 3.Burgert HG, Kvist S. An adenovirus type 2 glycoprotein blocks cell surface expression of human histocompatibility class I antigens. Cell. 1985;41:987–997. doi: 10.1016/S0092-8674(85)80079-9. [DOI] [PubMed] [Google Scholar]
- 4.Reddy VS, Natchiar SK, Stewart PL, Nemerow GR. Crystal structure of human adenovirus at 3.5 A resolution. Science. 2010;329:1071–1075. doi: 10.1126/science.1187292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Graham FL, Prevec L. Adenovirus-based expression vectors and recombinant vaccines. Biotechnology. 1992;20:363–390. doi: 10.1016/b978-0-7506-9265-6.50022-1. [DOI] [PubMed] [Google Scholar]
- 6.Larson C, Oronsky B, Scicinski J, et al. Going viral: a review of replication-selective oncolytic adenoviruses. Oncotarget. 2015;6:19976–19989. doi: 10.18632/oncotarget.5116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lion T. Adenovirus infections in immunocompetent and immunocompromised patients. Clin. Microbiol. Rev. 2014;27:441–462. doi: 10.1128/CMR.00116-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuhlmann I, Achten S, Rudolph R, Doerfler W. Tumor induction by human adenovirus type 12 in hamsters: loss of the viral genome from adenovirus type 12-induced tumor cells is compatible with tumor formation. EMBO J. 1982;1:79–86. doi: 10.1002/j.1460-2075.1982.tb01128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yabe Y, Samper L, Bryan E, Taylor G, Trentin JJ. Oncogenic effect of human adenovirus type 12, in mice. Science. 1964;143:46–47. doi: 10.1126/science.143.3601.46. [DOI] [PubMed] [Google Scholar]
- 10.Kajon AE, Larranaga C, Suarez M, Wadell G, Avendano LF. Genome type analysis of Chilean adenovirus strains isolated in a children’s hospital between 1988 and 1990. J. Med. Virol. 1994;42:16–21. doi: 10.1002/jmv.1890420104. [DOI] [PubMed] [Google Scholar]
- 11.Kajon AE, et al. A new genome type of adenovirus 3 associated with severe lower acute respiratory infection in children. J. Med. Virol. 1990;30:73–76. doi: 10.1002/jmv.1890300116. [DOI] [PubMed] [Google Scholar]
- 12.Niel C, Moraes MT, Mistchenko AS, Leite JP, Gomes SA. Restriction site mapping of four genome types of adenovirus types 3 and 7 isolated in South America. J. Med. Virol. 1991;33:123–127. doi: 10.1002/jmv.1890330211. [DOI] [PubMed] [Google Scholar]
- 13.De Jong JC, Wermenbol AG, Verweij-Uijterwaal MW, et al. Adenoviruses from human immunodeficiency virus-infected individuals, including two strains that represent new candidate serotypes Ad50 and Ad51 of species B1 and D, respectively. J. Clin. Microbiol. 1999;37:3940–3945. doi: 10.1128/jcm.37.12.3940-3945.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hage E, Gerd Liebert U, Bergs S, Ganzenmueller T, Heim A. Human mastadenovirus type 70: a novel, multiple recombinant species D mastadenovirus isolated from diarrhoeal faeces of a haematopoietic stem cell transplantation recipient. J. Gen. Virol. 2015;96:2734–2742. doi: 10.1099/vir.0.000196. [DOI] [PubMed] [Google Scholar]
- 15.Lin B, Wang Z, Vora GJ, et al. Broad-spectrum respiratory tract pathogen identification using resequencing DNA microarrays. Genome Res. 2006;16:527–535. doi: 10.1101/gr.4337206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seto D, Chodosh J, Brister JR, Jones MS. Members of the Adenovirus Research C. Using the whole-genome sequence to characterize and name human adenoviruses. J. Virol. 2011;85:5701–5702. doi: 10.1128/JVI.00354-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seto D, Jones MS, Dyer DW, Chodosh J. Characterizing, typing, and naming human adenovirus type 55 in the era of whole genome data. J. Clin. Virol. 2013;58:741–742. doi: 10.1016/j.jcv.2013.09.025. [DOI] [PubMed] [Google Scholar]
- 18.Robinson CM, Singh G, Henquell C, et al. Computational analysis and identification of an emergent human adenovirus pathogen implicated in a respiratory fatality. Virology. 2011;409:141–147. doi: 10.1016/j.virol.2010.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robinson CM, Singh G, Lee JY, et al. Molecular evolution of human adenoviruses. Sci. Rep. 2013;3:1812. doi: 10.1038/srep01812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robinson CM, Zhou X, Rajaiya J, et al. Predicting the next eye pathogen: analysis of a novel adenovirus. MBio. 2013;4:e00595–00512. doi: 10.1128/mBio.00595-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walsh MP, Chintakuntlawar A, Robinson CM, et al. Evidence of molecular evolution driven by recombination events influencing tropism in a novel human adenovirus that causes epidemic keratoconjunctivitis. PLoS ONE. 2009;4:e5635. doi: 10.1371/journal.pone.0005635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walsh MP, et al. Computational analysis identifies human adenovirus type 55 as a re-emergent acute respiratory disease pathogen. J. Clin. Microbiol. 2010;48:991–993. doi: 10.1128/JCM.01694-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou X, Robinson CM, Rajaiya J, et al. Analysis of human adenovirus type 19 associated with epidemic keratoconjunctivitis and its reclassification as adenovirus type 64. Invest. Ophthalmol. Vis. Sci. 2012;53:2804–2811. doi: 10.1167/iovs.12-9656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoshitomi H, Sera N, Gonzalez G, Hanaoka N, Fujimoto T. First isolation of a new type of human adenovirus (genotype 79), species Human mastadenovirus B (B2) from sewage water in Japan. J. Med. Virol. 2017;89:1192–1200. doi: 10.1002/jmv.24749. [DOI] [PubMed] [Google Scholar]
- 25.Dehghan S, et al. Simian adenovirus type 35 has a recombinant genome comprising human and simian adenovirus sequences, which predicts its potential emergence as a human respiratory pathogen. Virology. 2013;447:265–273. doi: 10.1016/j.virol.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wigand R. Pitfalls in the identification of adenoviruses. J. Virol. Methods. 1987;16:161–169. doi: 10.1016/0166-0934(87)90001-2. [DOI] [PubMed] [Google Scholar]
- 27.Kajon AE, Lamson D, Shudt M, et al. Identification of a novel intertypic recombinant species D human adenovirus in a pediatric stem cell transplant recipient. J. Clin. Virol. 2014;61:496–502. doi: 10.1016/j.jcv.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao S, Wan C, Ke C, et al. Re-emergent human adenovirus genome type 7d caused an acute respiratory disease outbreak in Southern China after a twenty-one year absence. Sci. Rep. 2014;4:7365. doi: 10.1038/srep07365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Djikeng A, Halpin R, Kuzmickas R, Depasse J, Feldblyum J, Sengamalay N, Afonso C, Zhang X, Anderson NG, Ghedin E, Spiro DJ. Viral genome sequencing by random priming methods. Virol. J. 2008;9:5. doi: 10.1186/1471-2164-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li K, Shrivastava S, Brownley A, et al. Automated degenerate PCR primer design for high-throughput sequencing improves efficiency of viral sequencing. Virol. J. 2012;9:261. doi: 10.1186/1743-422X-9-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang S, Sundaram JP, Stockwell TB. VIGOR extended to annotate genomes for additional 12 different viruses. Nucl. Acids Res. 2012;40:W186–192. doi: 10.1093/nar/gks528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Madisch I, Harste G, Pommer H, Heim A. Phylogenetic analysis of the main neutralization and hemagglutination determinants of all human adenovirus prototypes as a basis for molecular classification and taxonomy. J. Virol. 2005;79:15265–15276. doi: 10.1128/JVI.79.24.15265-15276.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lole KS, Bollinger RC, Paranjape RS, et al. Full-length human immunodeficiency virus type 1 genomes from subtype C-infected seroconverters in India, with evidence of intersubtype recombination. J. Virol. 1999;73:152–160. doi: 10.1128/jvi.73.1.152-160.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simmonds P, Adams MJ, Benko M, et al. Consensus statement: Virus taxonomy in the age of metagenomics. Nat. Rev. Microbiol. 2017;15:161–168. doi: 10.1038/nrmicro.2016.177. [DOI] [PubMed] [Google Scholar]
- 35.Seto J, Walsh MP, Metzgar D, Seto D. Computational analysis of adenovirus serotype 5 (HAdV-C5) from an HAdV coinfection shows genome stability after 45 years of circulation. Virology. 2010;404:180–186. doi: 10.1016/j.virol.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 36.Mahadevan P, Seto J, Tibbetts C, Seto D. Natural variants of human adenovirus type 3 provide evidence for relative genome stability across time and geographic space. Virology. 2010;397:113–118. doi: 10.1016/j.virol.2009.10.052. [DOI] [PubMed] [Google Scholar]
- 37.Crawford-Miksza L, Schnurr DP. Analysis of 15 adenovirus hexon proteins reveals the location and structure of seven hypervariable regions containing serotype-specific residues. J. Virol. 1996;70:1836–1844. doi: 10.1128/jvi.70.3.1836-1844.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walsh MP, Seto J, Liu EB, et al. Computational analysis of two species C human adenoviruses provides evidence of a novel virus. J. Clin. Microbiol. 2011;49:3482–3490. doi: 10.1128/JCM.00156-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yuan X, Qu Z, Wu X, et al. Molecular modeling and epitopes mapping of human adenovirus type 3 hexon protein. Vaccine. 2009;27:5103–5110. doi: 10.1016/j.vaccine.2009.06.041. [DOI] [PubMed] [Google Scholar]
- 40.Lord R, Parsons M, Kirby I, et al. Analysis of the interaction between RGD-expressing adenovirus type 5 fiber knob domains and alphavbeta3 integrin reveals distinct binding profiles and intracellular trafficking. J. Gen. Virol. 2006;87:2497–2505. doi: 10.1099/vir.0.81620-0. [DOI] [PubMed] [Google Scholar]
- 41.Li E, Stupack D, Klemke R, Cheresh DA, Nemerow GR. Adenovirus endocytosis via alpha(v) integrins requires phosphoinositide-3-OH kinase. J. Virol. 1998;72:2055–2061. doi: 10.1128/jvi.72.3.2055-2061.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ismail AM, et al. Selection pressure in the human adenovirus fiber knob drives cell specificity in epidemic keratoconjunctivitis. J. Virol. 2016;90:9598–9607. doi: 10.1128/JVI.01010-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wigand R, Sehn N, Hierholzer JC, de Jong JC, Adrian T. Immunological and biochemical characterization of human adenoviruses from subgenus B. I. Antigenic relationships. Arch. Virol. 1985;84:63–78. doi: 10.1007/BF01310554. [DOI] [PubMed] [Google Scholar]
- 44.Engelmann I, Madisch I, Pommer H, Heim A. An outbreak of epidemic keratoconjunctivitis caused by a new intermediate adenovirus 22/H8 identified by molecular typing. Clin. Infect. Dis. 2006;43:e64–66. doi: 10.1086/507533. [DOI] [PubMed] [Google Scholar]
- 45.Bell SD, Jr., Rota TR, McComb DE. Adenoviruses isolated from Saudi Arabia. III. Six new serotypes. Am. J. Trop. Med. Hyg. 1960;9:523–526. doi: 10.4269/ajtmh.1960.9.523. [DOI] [Google Scholar]
- 46.Kaneko H, Aoki K, Ohno S, et al. Complete genome analysis of a novel intertypic recombinant human adenovirus causing epidemic keratoconjunctivitis in Japan. J. Clin. Microbiol. 2011;49:484–490. doi: 10.1128/JCM.01044-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carr MJ, Kajon AE, Lu X, et al. Deaths associated with human adenovirus-14p1 infections, Europe, 2009-2010. Emerg. Infect. Dis. 2011;17:1402–1408. doi: 10.3201/1708.101760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kajon AE, Lu X, Erdman DD, et al. Molecular epidemiology and brief history of emerging adenovirus 14-associated respiratory disease in the United States. J. Infect. Dis. 2010;202:93–103. doi: 10.1086/653083. [DOI] [PubMed] [Google Scholar]
- 49.Zhang Q, et al. Genome sequence of the first human adenovirus type 14 isolated in China. J. Virol. 2012;86:7019–7020. doi: 10.1128/JVI.00814-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Robinson CM, Rajaiya J, Walsh MP, et al. Computational analysis of human adenovirus type 22 provides evidence for recombination among species D human adenoviruses in the penton base gene. J. Virol. 2009;83:8980–8985. doi: 10.1128/JVI.00786-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu E, et al. Flexibility of the adenovirus fiber is required for efficient receptor interaction. J. Virol. 2003;77:7225–7235. doi: 10.1128/JVI.77.13.7225-7235.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huang S, Reddy V, Dasgupta N, Nemerow GR. A single amino acid in the adenovirus type 37 fiber confers binding to human conjunctival cells. J. Virol. 1999;73:2798–2802. doi: 10.1128/jvi.73.4.2798-2802.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]