This editorial refers to ‘Predictive value of telomere length on outcome following acute myocardial infarction: evidence for contrasting effects of vascular vs. blood oxidative stress’†, by M. Margaritis et al., on page 3094.
Telomeres are nucleotide sequences at the end of each eukaryotic chromosome, which confer a protective role during cell division. These segments are shortened with each cell division, unless replenished by the enzyme telomerase. When the length of telomeres shortens beyond a point, the cell becomes senescent and is no longer able to proliferate, ultimately undergoing apoptosis. Telomere length (TL) is determined by direct inheritance of telomeres through parental gametes, and genetic and non-genetic factors that affect the balance between telomere shortening and telomerase activity.1 As such, TL varies significantly in different populations, tissues, and cells. Immune cells, endothelial cells, and vascular smooth muscle cells (VSMCs) are all involved in the pathogenesis of atherosclerosis, and a short TL in these cell types has been associated with atherosclerosis.2 While short leucocyte TL (LTL) is correlated with increased mortality and risk of coronary atherosclerotic disease (CAD),3 it is unclear whether this increased risk is predominantly driven by genetic factors or non-genetic factors correlated with CAD which may also affect TL, such as increased oxidative stress and inflammation.4,5
In this issue of the journal, Margaritis etal. present exciting and thought-provoking results on the use of TL as a prognostic marker following acute myocardial infarction (AMI) as well as the interactions between oxidative stress and TL in whole blood vs. vascular tissue.6 In a first group of patients, TL was measured by quantitative PCR in whole blood samples (BTL) in patients post-AMI who were then followed for 1 year. Using a dichotomous cut-off based on receiver operating characteristic (ROC) analysis for predicting mortality, short BTL was a strong independent predictor of all-cause and cardiovascular mortality 1-year after AMI. The authors then used a second group of patients with ischaemic heart disease undergoing coronary artery bypass grafting to assess the relationship between oxidative stress and short TL. In this group, the authors used isolated peripheral blood mononuclear cells (PBMCs) to measure NADPH-stimulated superoxide (O2·–) production ex vivo, and show that PBMCs from patients with short BTL produced more O2·– upon stimulation. Single nucleotide polymorphisms (SNPs) of the CYBA gene encoding the p22phox subunit of NADPH-oxidases (NOXs) was used to group the patients based on NOX activity. As expected, PBMCs with higher NOX activity produced more O2·– upon stimulation, but also had a shorter BTL. Saphenous venous graft (SV) and internal mammary artery (IMA) samples were also collected, and vascular tissue TL (VTL) and vascular O2·– production were measured. There was no significant correlation between BTL and VTL in this group. Furthermore, VTL (but not BTL) was inversely correlated with vascular O2·– production. Flow-mediated dilation (FMD) of the brachial artery was used as a measure of endothelial function, and again VTL from IMA samples correlated with FMD, but BTL showed no significant correlation. VSMCs were cultured from SV samples collected from patients with the lowest NOX activity SNPs (0 risk alleles) and the highest NOX activity SNPs (4 risk alleles), and angiotensin II (Ang II) was used to stimulate O2·– production. While VSMCs produced significantly higher levels of O2·– in response to Ang II stimulation as previously shown7 in cells with the highest NOX activity SNPs; this was not seen in cells with the lowest NOX activity SNPs. Moreover, TL showed significant shortening in the highest NOX activity cells along with evidence of increased DNA damage upon chronic exposure to Ang II, but not in the lowest NOX activity cells.
The first important finding of this study is that patients with a short BTL had higher 1-year mortality rates following AMI, which has not been previously reported. This finding is concordant with previous reports that a short LTL is associated with increased mortality and risk of CAD.3,8,9 While further studies are needed to confirm the results in validation cohorts, the findings suggest that BTL may be useful as a prognostic factor in patients following AMI. Multiple methods for measuring TL have been developed, including but not limited to quantitative PCR assay of telomere repeats [in which TL is expressed as a ratio of telomeric repeat amplification to a known single-copy gene (T/S) as used in the current study10], and terminal restriction fragment (TRF) analysis by Southern blot (used in this study to estimate the absolute TL corresponding to T/S ratios by linear regression). It is reported that the TL measurement method used11 as well as different DNA extraction methods12 and pre-analysis handling of samples13 may influence TL measurements. These differences introduce challenges and need to be addressed when comparing TLs across various studies. Interestingly, the Kaplan–Meier survival curves show a pattern of early separation, implying that a short BTL associated with increased mortality post-AMI is more pronounced during the early post-AMI period and diminishes with time, which warrants further investigation. The BTL did not show a negative correlation with age, possibly due to the effect of CAD overshadowing that of ageing, given the narrow age ranges in this study, similar to what was noted in a previous study of TL in abdominal aortic aneurysm (AAA).14 In the same study, LTL showed a positive correlation with VTL from aortic samples; however, in the current study, BTL did not correlate with VTL from SV or IMA tissue samples. This may be in part due to varying degrees of immune cell infiltration in the aorta compared with the IMA or SV, vs. focal variations of endothelial and VSMC TL. It has been reported that VTL was shorter in the aorta compared with vascular segments, which did not develop atherosclerosis such as IMA and SV, and was shorter in atherosclerotic aortic tissue compared with non-atherosclerotic aortic tissue.15 The pathogenesis of atherosclerosis involves VSMC proliferation, so it is possible that VSMCs in atherosclerotic segments of the vasculature have shorter TLs due to replicative attrition compared with VSMCs in non-atherosclerotic segments. The same concept can be applied to endothelial cells as well, although to a lesser degree. The authors also show that VSMC response to Ang II stimulation in regards to O2·– production and TL shortening was significantly different when comparing cells with SNPs conferring the lowest vs. the highest NOX activity. This suggests that the relationship between oxidative stress and TL shortening is affected by a genetic predisposition to produce higher O2·– in response to certain stimuli which may be under the influence of various epigenetic and focal factors in a cell-specific manner.
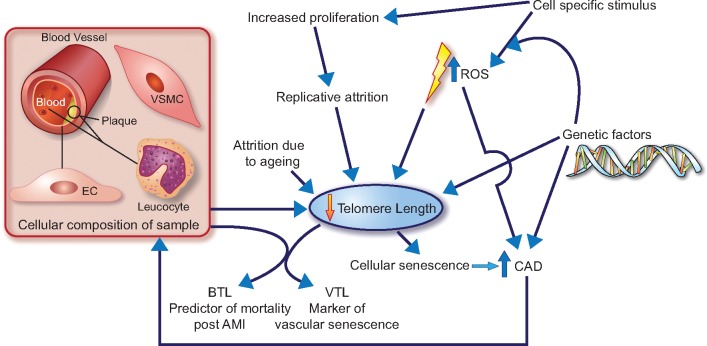
As Margaritis etal. propose, BTL may be viewed as a biomarker of oxidative stress in PBMCs but not vascular cells, while VTL may be a marker of oxidative stress in vascular cells. It is likely that there is significant interaction between genetic and non-genetic factors determining TL in various cells. Short TL itself leads to cell senescence, which in turn may contribute to mechanisms involved in atherosclerosis (Figure 1). The study by Margaritis etal. adds new insight to the relationship between TL, oxidative stress, and mortality following AMI, yet further studies are needed to better understand this complex relationship.
Figure 1.
A simplified proposed schematic of the complex interactions between oxidative stress, telomere length, and coronary atherosclerotic disease (CAD). AMI, acute myocardial infarction; BTL, blood telomere length; EC, endothelial cell; ROS, reactive oxygen species; VSMC, vascular smooth muscle cell; VTL, vascular telomere length.
Funding
This work was supported by the National Heart, Lung and Blood Institute at the National Institutes of Health [K08HL124292 to A.R.].
Conflict of interest: none declared.
References
- 1. Blackburn EH, Epel ES, Lin J.. Human telomere biology: a contributory and interactive factor in aging, disease risks, and protection. Science 2015;350:1193–1188. [DOI] [PubMed] [Google Scholar]
- 2. Yeh JK, Wang CY.. Telomeres and telomerase in cardiovascular diseases. Genes (Basel) 2016;7:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Haycock PC, Heydon EE, Kaptoge S, Butterworth AS, Thompson A, Willeit P.. Leucocyte telomere length and risk of cardiovascular disease: systematic review and meta-analysis. BMJ 2014;349:g4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kurz DJ, Decary S, Hong Y, Trivier E, Akhmedov A, Erusalimsky JD.. Chronic oxidative stress compromises telomere integrity and accelerates the onset of senescence in human endothelial cells. J Cell Sci 2004;117:2417–2426. [DOI] [PubMed] [Google Scholar]
- 5. Samani NJ, van der Harst P.. Biological ageing and cardiovascular disease. Heart 2008;94:537–549. [DOI] [PubMed] [Google Scholar]
- 6. Margaritis M, Sanna F, Lazaros G, Akoumianakis I, Patel S, Antonopoulos AS, Duke C, Herdman L, Psarros C, Oikonomou EK, Shirodaria C, Petrou M, Sayeed R, Krasopoulos G, Lee R, Tousoulis D, Channon KM, Antoniades C.. Predictive value of telomere length on outcome following acute myocardial infarction: evidence for contrasting effects of vascular vs. blood oxidative stress. Eur Heart J 2017;38:3094–3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Herbert KE, Mistry Y, Hastings R, Poolman T, Niklason L, Williams B.. Angiotensin II-mediated oxidative DNA damage accelerates cellular senescence in cultured human vascular smooth muscle cells via telomere-dependent and independent pathways. Circ Res 2008;102:201–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Carty CL, Kooperberg C, Liu J, Herndon M, Assimes T, Hou L, Kroenke CH, LaCroix AZ, Kimura M, Aviv A, Reiner AP.. Leukocyte telomere length and risks of incident coronary heart disease and mortality in a racially diverse population of postmenopausal women. Arterioscler Thromb Vasc Biol 2015;35:2225–2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brouilette S, Singh RK, Thompson JR, Goodall AH, Samani NJ.. White cell telomere length and risk of premature myocardial infarction. Arterioscler Thromb Vasc Biol 2003;23:842–846. [DOI] [PubMed] [Google Scholar]
- 10. Cawthon RM. Telomere length measurement by a novel monochrome multiplex quantitative PCR method. Nucleic Acids Res 2009;37:e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Martin-Ruiz CM, Baird D, Roger L, Boukamp P, Krunic D, Cawthon R, Dokter MM, van der Harst P, Bekaert S, de Meyer T, Roos G, Svenson U, Codd V, Samani NJ, McGlynn L, Shiels PG, Pooley KA, Dunning AM, Cooper R, Wong A, Kingston A, von Zglinicki T.. Reproducibility of telomere length assessment: an international collaborative study. Int J Epidemiol 2015;44:1673–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cunningham JM, Johnson RA, Litzelman K, Skinner HG, Seo S, Engelman CD, Vanderboom RJ, Kimmel GW, Gangnon RE, Riegert-Johnson DL, Baron JA, Potter JD, Haile R, Buchanan DD, Jenkins MA, Rider DN, Thibodeau SN, Petersen GM, Boardman LA.. Telomere length varies by DNA extraction method: implications for epidemiologic research. Cancer Epidemiol Biomarkers Prev 2013;22:2047–2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tolios A, Teupser D, Holdt LM.. Preanalytical conditions and DNA isolation methods affect telomere length quantification in whole blood. PLoS One 2015;10:e0143889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wilson WR, Herbert KE, Mistry Y, Stevens SE, Patel HR, Hastings RA, Thompson MM, Williams B.. Blood leucocyte telomere DNA content predicts vascular telomere DNA content in humans with and without vascular disease. Eur Heart J 2008;29:2689–2694. [DOI] [PubMed] [Google Scholar]
- 15. Nzietchueng R, Elfarra M, Nloga J, Labat C, Carteaux JP, Maureira P, Lacolley P, Villemot JP, Benetos A.. Telomere length in vascular tissues from patients with atherosclerotic disease. J Nutr Health Aging 2011;15:153–156. [DOI] [PubMed] [Google Scholar]