Why was the cohort set up?
During the past century, there have been dramatic improvements in life expectancy in Taiwan, with the average life span increasing from 30.0 and 32.1 years for men and women in 1908 to 77 and 83.5 years in 2016, respectively.1 As a consequence of this demographic transition, the population in Taiwan has rapidly been ageing. Currently, persons aged 65 and older comprise about 12.5% of the Taiwanese population; this proportion is projected to reach 14% in 2018 and 20% in 2025.2 With the ageing of a population comes an increase in prevalence of chronic diseases and geriatric syndromes, and an expansion in healthcare costs that impose a huge burden on the whole society.3 For example, cancer, coronary heart disease, stroke, diabetes, hypertension and chronic kidney disease have been listed among the 10 leading causes of death among the elderly in Taiwan for the past decade.4 The high prevalence of chronic kidney disease in the elderly (estimated to be over 37%5) is another example indicating that an ageing population is one of the most important factors behind the high incidence and prevalence of end-stage renal disease (ESRD) in Taiwan.6
Thus, it is essential to understand more about risk factors attributable to the ethnicity-specific ageing process so that effective prevention programmes can be developed for the elderly. To address different age-related health issues, several longitudinal Chinese ageing studies have previously been conducted in Taiwan or other Asian countries, such as the Chinese Longitudinal Health Longevity Survey (CLHLS),7 the China Health & Retirement Longitudinal Study (CHARLS),8 the Beijing Longitudinal Study of Aging (BLSA),9 the Taiwan Longitudinal Study of Aging (TLSA)10 and the Singapore Chinese Longitudinal Aging Study.11 However, most of these established senescent cohorts, which were mainly followed up by collecting self-reported information, had not acquired comprehensive biomedical profiles for their participants. To further understand determinants of healthy longevity and geriatric issues, additional functional status measurements and biochemical data collections have been appended to some sub-studies7,8,12 but their sample sizes were far smaller compared with their original cohorts. For example, lack of statistical power (n = 639) is one of major limitations of the Social Environment and Biomarkers of Aging Study (SEBAS),12 a sub-study of the TLSA, in spite of its enrichment with biochemical, genetic and functional measurements. To overcome the common barriers in geriatric cohort research, the Healthy Aging Longitudinal Study in Taiwan (HALST) was therefore established and funded by the National Health Research Institutes in Taiwan to address issues related to healthy ageing (ClinicalTrials.gov: NCT02677831).
The main study objectives of the HALST are to investigate: (i) factors that may influence trajectories of physical functioning; (ii) impacts of healthy lifestyles on incidence of chronic diseases, quality of life and mortality; (iii) individual, social and environmental determinants of cardiovascular diseases; (iv) association of neuropsychiatric risk factors and well-being; and (v) interaction between genetic traits and environmental risk factors in frailty versus successful ageing processes in older adults. The project also involves a variety of ancillary sub-studies focusing on important health-related issues that are unique to local people (such as betel quid chewing, dietary pattern, hepatitis due to viral infection and chronic renal disease). These issues are frequently overlooked but are crucial for the development of health-promotion programmes in older populations.
Who is in the cohort?
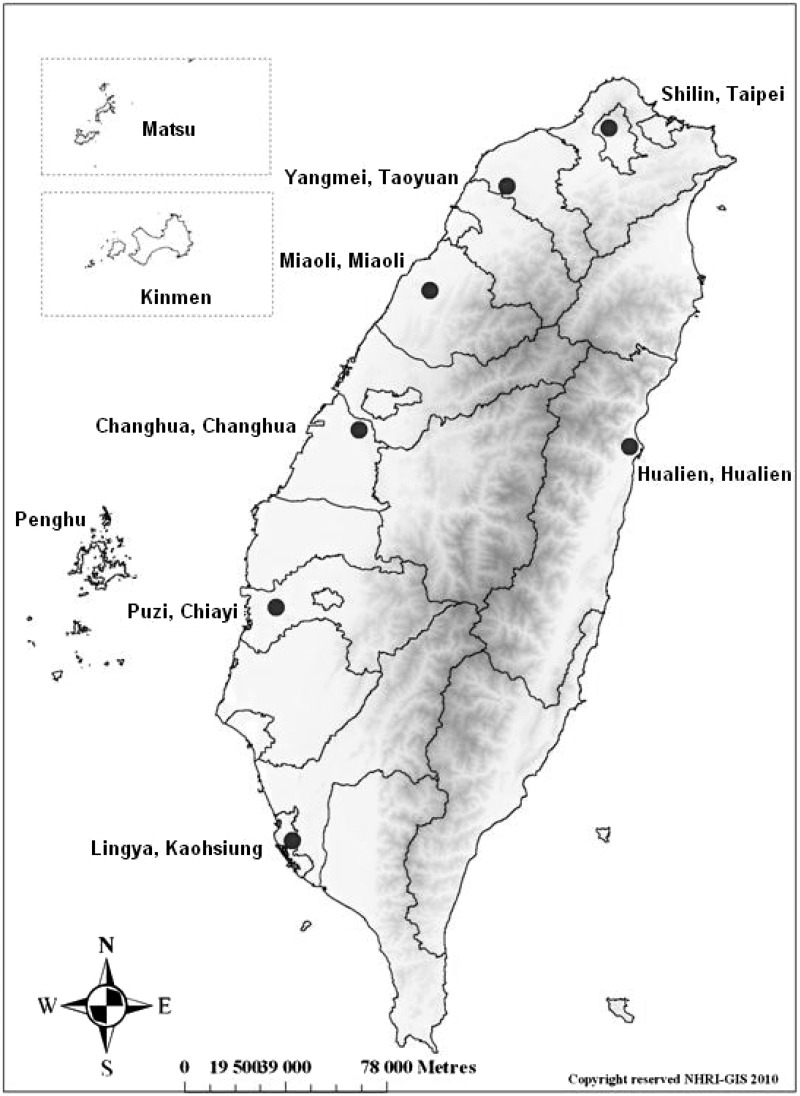
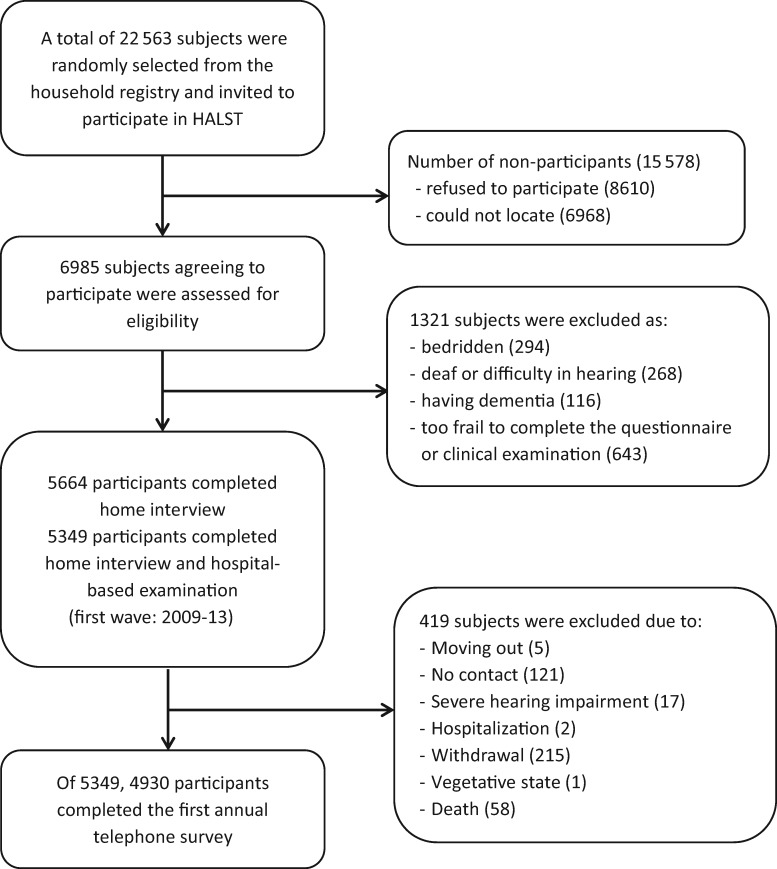
The HALST is designed as a longitudinal study recruiting community-dwellers aged 55 and above in seven selected areas in Taiwan: two in the north (Shilin District and Yangmei Township), two in the central region (Miaoli City and Changhua City), two in the south (Puzi Township and Lingya District) and one in the east (Hualien). These seven locations (Figure 1) cover both urban and rural areas, as well as different ethnic groups speaking different dialects, representing the diverse socio-demographic characteristics of the Taiwanese population. In each catchment area, a regional hospital was selected to be the medical facility for clinical examinations, and all eligible residents living within about a 2-km radius of this local hospital were ascertained from the household registry archives. By using a systemic sampling method, beginning with around 3000–3500 residents aged 55 and above in each catchment area, we created a recruitment roster within the target population. To ensure that our study sample covered a sufficient number of the elderly with different socio-demographic backgrounds, the older adults (≥ 65 years of age) were over-sampled (70% for those ≥ 65 years and 30% for those in 55–64 years); on the other hand, the sampling distributions of gender and educational level (none, primary school, high school and above) are based on the demographic distribution within a li (village), a basic house registration unit defined by the Taiwan government. Individuals with any of the following conditions at the recruiting interview were excluded: highly contagious infectious diseases (including scabies and open tuberculosis), severe illnesses (including cancer under treatment), physician-diagnosed dementia, bedridden and/or too frail to stand and walk, severe mental disorder or cognitive impairment with a Mini-Mental Status Score < 16,13 mental retardation or severe hearing loss. Individuals who were then hospitalized or institutionalized were also excluded. Figure 2 presents a flow chart with details of the subject selection process and the number of participants who completed the first annual follow-up telephone interview. Compared with the recruited subjects, the non-participants were more likely to be women, older and illiterate. In the first-wave survey (2009–13), we enrolled 5664 community-dwellers aged 55 or above.
Figure 1.
Map of participating sites in the HALST.
Figure 2.
Selection flow chart for the HALST participants.
How often have they been followed up?
The longitudinal assessments conducted in the HALST consist of home interviews and hospital-based clinical examinations every 5 years. The first-wave survey (recruitment and baseline survey) was carried out in 2009–13. The field study team took about 6–8 months to finish the work in one catchment area, starting in Miaoli City and then moving to the next (Shilin District was the last) for the processes of recruitment, interview and examination. The second-wave follow-up (2014–19) of home interviews and examinations is currently under way. After enrolment, those who have completed both home interviews and hospital-based examinations (n = 5349) are to be followed up by telephone contact every year for updates on vital status and health-related conditions.
What has been measured?
In the sampling area of each site, community residents who met the inclusion criteria were invited to participate in the HALST study. A home interview was arranged for those who completed the consent form. Within 2 weeks after the home visit, study participants received a physical examination and provided morning spot urine and up to 30 ml of fasting blood specimens in one of the local hospitals. The home interview took about 90 to 120 min to complete; and the clinical examination required about 120 min. All interviewing and examination processes are based on the standardized manual of operation; the field sites are periodically inspected by the responsible investigators every season; and a routine call-back interview for quality and reliability control is performed for around 8% of the enrolled subjects by random selection.
As seen in Table 1, information obtained through the measurements and analyses employed in the HALST can be organized into four parts: home visit, clinical examination, laboratory analysis and follow-up telephone survey. ‘Home visit’ and ‘clinical examination’, including blood and urine samples collection, constitute the formal investigation conducted every 5 years; ‘follow-up telephone survey’ is the annual survey of vital status and new health events occurring between formal home visit and clinical examination. The main measures are aimed at collecting information on physical function and geriatric conditions (e.g. lifestyle profiles, cardiovascular diseases, cognitive and mental health and longevity-related genetic factors) necessary for our research interests. In addition, the design of measures and instruments is mainly based on three practical considerations: (i) our results could be compared with those from well-recognized ageing-related studies, such as the Chicago Healthy Aging Study and Baltimore Longitudinal Study of Aging; (ii) the instruments have been used in similar population-based studies in ethnic Chinese communities; and (iii) the Chinese-language version questionnaires were chosen from those validated and widely recognized for use in community-based studies such as assessments of leisure time physical activity,14 the Chinese version of Lawton and Brody’s measure15 for instrumental activities of daily living (IADLs),16 the Mini-Mental State Exam (MMSE),17 the Center for Epidemiologic Studies Depression Scale (CES-D)18 and the Short-Form 12 Health Survey (SF-12).19,20 The food intakes were measured from a food frequency questionnaire (FFQ) containing more than 80 items of Chinese food. The validation of this FFQ has been reported elsewhere.21 Specifically, we show participants wooden blocks representing the volume of the food to approximately measure for each food item; then the frequency and the volume are converted accordingly. The final results are the amount consumed per day.
Table 1.
Measurements in the Healthy Aging Longitudinal Study in Taiwan (HALST)
| Type | Measures | Instruments |
|---|---|---|
| Interviewer-administered home visit | ||
| Questionnaires | Physical functioning | Barthel Index, Lawton-Brody IADL Scale |
| Frailty | CHS frailty phenotype, the Edmonton frail scale, CSHA-CFA, SOF | |
| Cognitive function | MMSE | |
| Mental health | 20-item CES-D | |
| Health-related quality of life | SF-12 | |
| Diet assessment | FFQ | |
| Others: evaluation of general conditions, social demography, health conditions, geriatric conditions (fall, pain), chronic disease risk factor, sleep, use of healthcare, lifestyle (smoking, alcohol, betel, physical activity, nutritional supplements) and family history of chronic diseases | ||
| Physical assessment | Performance-based measures | Peak flow test, grip strength, SPPB |
| Clinical examination | ||
| Examinations | Anthropometry | Height and weight, and hip circumference |
| Brief physical examination | ||
| Lower extremity function | Single-leg stand test, timed up-and-go test, 6-min walk | |
| Cardiovascular function | Blood pressure, heart rate, electrocardiogram, ankle-brachial index measurement, heart rate variability | |
| Cognitive function | Digit symbol substitution test, clock drawing test | |
| Vision | Visual acuity | |
| Mental health | PRIME-MD | |
| Questionnaires | Clinical assessment of cardiovascular symptoms | Rose questionnaire, TIA questionnaire |
| Other: vision, hearing, incontinence | ||
| Laboratory analysis | ||
| Blood tests | Routine biochemistry | Cholesterol, triglyceride, HDLC, LDLC, globulin, albumin, total protein, AST, ALT, GGT, insulin, glucose, creatinine, BUN, uric acid |
| Haematology | HbA1c, complete blood count (RBC, WBC, platelet), haemoglobin, HCT, MCV, MCH, MCHC, differential count of WBC | |
| Inflammation-related | High-sensitivity CRP (hsCRP), intact PTH, ionized calcium, vitamin B12, folic acid | |
| Hepatitis virus titre | HBsAg, Anti-HCVAb | |
| Coagulation factor | D-dimer, fibrinogen | |
| DNA | Genes associated with ageing (SNPs) | |
| Other | IL-6, TNF-R1, IGF-1, sIL-6r, vitamin D | |
| Urine test | Routine urinalysis | Colour, clarity, specific gravity, pH, glucose, protein, occult blood, urobilinogen, bilirubin, nitrite, ketone body, RBC, WBC, epithelial cells, casts, crystals, bacteria, parasites, urinary albumin, urine creatinine, leukocyte esterase |
| Follow-up telephone questionnaire | ||
| Questionnaires | Self-rated health status, physical functioning, pain, weight changes, smoking, physical activity, vision, falls and fractures, depressive symptoms, new events and health conditions, specific examinations and surgeries | BSRS-5 |
IADL, Instrumental Activity of Daily Living; CHS, Cardiovascular Health Study; CSHA-CFA, Chinese-Canadian Study of Health and Ageing Clinical Frailty scale physical version; SOF, Study of Osteoporotic Fractures; MMSE, Mini-Mental State Examination; CES-D, Center for the Epidemiologic Studies Depression Scale; SF-12, Short Form 12; FFQ, Food Frequency Questionnaire; SPPB, Short Physical Performance Battery; PRIME-MD, Primary Care Evaluation of Mental Disorders; TIA, transient ischaemic attack; HDLC, high-density lipoprotein cholesterol; LDLC, low-density lipoprotein cholesterol; AST, aspartate aminotransferase; ALT, alanine aminotransferase; GGT, gamma-glutamyl transpeptidase; BUN, blood urea nitrogen; HbA1c, glycated haemoglobin; RBC, red blood cells; WBC, white blood cells; HCT, haematocrit; MCV, mean cell volume; MCH, mean corpuscular haemoglobin; MCHC, mean corpuscular haemoglobin concentration; CRP, C-reactive protein; PTH, parathyroid hormone; HBsAg, hepatitis B surface antigen; Anti-HCVAb, anti-hepatitis C virus antibody; IL-6, interleukin-6; TNF-R1, tumour necrosis factor-R1; IGF-1, insulin-like growth factor-1; SNPs, single nucleotide polymorphisms; sIL-6r, soluble interleukin-6 receptor; BSRS-5, Brief Symptom Rating Scale-5.
For the laboratory analysis, routine fasting blood and morning urine tests are analysed at a certified clinical laboratory. In addition to the routine standardization and calibration tests performed by the laboratory, duplicate samples for about 5% of the specimens blinded to the laboratory are submitted together with other control samples to test reliability. We created three different levels (high, medium and low) of serum pools from control samples to assess accuracy of the assays operated by the central laboratory. All the remaining blood is centrifuged, aliquoted and stored in a -80°C freezer at the National Health Research Institutes, where other blood tests—including inflammatory markers, blood clotting markers, hepatitis B and hepatitis C markers and genetic assays—are undertaken in the principal investigators’ laboratories. The results of annual validity and reliability tests regarding between-run and within-run quality control of some major laboratory items are acceptable.
In addition to the measures set in the routine investigations, a number of measures in relation to our main research interests are also conducted through ancillary sub-studies by collaborative researchers. For example, to better understand the relationship between bone/muscle mass and older adults’ health, examinations of bone mineral density and whole body scan using dual-energy X-ray absorptiometry were carried out at the Puzi, Changhua and Hualien sites.
In addition to the home interviews and hospital-based examinations which are conducted every 5 years, we also perform annual telephone contact to update participants’ health conditions such as changes in body weight, lifestyle behaviours, newly diagnosed diseases and conditions and new events of fall and fall-induced fractures and hospitalizations. To ascertain mortality and cause of death, we link the identification number of the HALST participants to the National Death Registry Database on a yearly basis. Similarly, medical records are requested for the ascertainment of any hospitalized events. In addition, we also assess health outcomes, healthcare utilization and medical costs from the National Health Insurance database (NHID) for those who have signed an informed consent (n = 5152, 91%) for the data linkage.
What has been found? Key findings and publications
Table 2 shows some selected baseline socio-demographic characteristics by different age groups. Women outnumbered men for those younger than 75. The prevalence of widowhood increased with older ages. The percentages of self-identified ‘mainlanders’ (immigrants from China) and no education were the highest (20.0% and 24.6%, respectively) in the oldest group. As regards lifestyle characteristics, about 13% of the study participants were current smokers and 3% were betel-quid chewers. The prevalence of these risky behaviours declined as the participants became older. The percentage in each age group that engaged in leisure-time physical activity was about the same (around 71%). With regard to the self-reported major cardiovascular diseases (such as heart disease and stroke) and some age-related conditions (such as cataract, arthritis and prostatic disorders in males), the prevalence generally increased with age.
Table 2.
Numbers of study subjects in the HALST cohort presenting with selected socio-demographic characteristics (and percentages in parentheses) and reported health conditions
| Total (n = 5664) | 55–64 years (n = 1686) | 65–74 years (n = 2497) | ≥ 75 years (n = 1481) | P | |
|---|---|---|---|---|---|
| Sex | 0.001 | ||||
| Male | 2676 (47.2) | 800 (47.4) | 1121 (44.9) | 755 (51.0) | |
| Female | 2988 (52.8) | 886 (52.6) | 1376 (55.1) | 726 (49.0) | |
| Marital status | < 0.001 | ||||
| Married | 4140 (73.1) | 1428 (84.7) | 1823 (73.0) | 889 (60.0) | |
| Widowed | 1235 (21.8) | 121 (7.2) | 570 (22.8) | 544 (36.7) | |
| Othera | 289 (5.1) | 137 (8.1) | 104 (4.2) | 48 (3.2) | |
| Ethnicityb | < 0.001 | ||||
| Fukien | 3326 (58.8) | 1017 (60.4) | 1573 (63.0) | 736 (49.8) | |
| Hakka | 1638 (28.9) | 475 (28.2) | 746 (29.9) | 417 (28.2) | |
| Mainlander | 574 (10.1) | 157 (9.3) | 121 (4.8) | 296 (20.0) | |
| Aborigine | 122 (2.2) | 36 (2.1) | 56 (2.2) | 30 (2.0) | |
| Level of education | < 0.001 | ||||
| No education | 799 (14.1) | 49 (2.9) | 386 (15.5) | 364 (24.6) | |
| Primary school | 2322 (41.0) | 570 (33.8) | 1135 (45.5) | 617 (41.7) | |
| High school | 1628 (28.8) | 643 (38.2) | 648 (26.0) | 337 (22.8) | |
| University | 911 (16.1) | 422 (25.1) | 328 (13.1) | 161 (10.9) | |
| Smoking status | < 0.001 | ||||
| Current smoker | 723 (12.8) | 290 (17.2) | 293 (11.7) | 140 (9.5) | |
| Past smoker | 894 (15.8) | 208 (12.3) | 350 (14.0) | 336 (22.7) | |
| Non-smoker | 4047 (71.5) | 1188 (70.5) | 1854 (74.2) | 1005 (67.9) | |
| Betel quid | < 0.001 | ||||
| Current chewer | 180 (3.2) | 86 (5.1) | 76 (3.0) | 18 (1.2) | |
| Past chewer | 512 (9.0) | 194 (11.5) | 218 (8.7) | 100 (6.8) | |
| Non-chewer | 4972 (87.8) | 1406 (83.4) | 2203 (88.2) | 1363 (92.0) | |
| Engage in physical activityc | 4039 (71.3) | 1196 (70.9) | 1799 (72.0) | 1044 (70.5) | 0.533 |
| Falls in previous year | 1103 (19.5) | 247 (14.7) | 488 (19.5) | 368 (24.8) | < 0.001 |
| Chronic diseased | |||||
| Heart disease | 1215 (21.5) | 248 (14.7) | 532 (21.3) | 435 (29.4) | < 0.001 |
| Stroke | 303 (5.3) | 44 (2.6) | 147 (5.9) | 112 (7.6) | < 0.001 |
| Cataract | 2214 (39.1) | 236 (14.0) | 1045 (41.9) | 933 (63.0) | < 0.001 |
| Arthritis | 986 (17.4) | 207 (12.3) | 441 (17.7) | 338 (22.8) | < 0.001 |
| Osteoporosis | 1118 (19.7) | 300 (17.8) | 538 (21.5) | 280 (18.9) | 0.007 |
| Prostatic disorderse | 875 (32.7) | 143 (17.9) | 384 (34.3) | 348 (46.1) | < 0.001 |
aIncludes divorced, separated, and single.
bThe ethnicity of participants was classified based on the origin of the participants’ fathers.
cEngage in physical activity: having any leisure-time physical activity in the past year.
dPhysician-diagnosed chronic disease.
eThe percentage was calculated for males.
Table 3 reveals baseline biomarker profiles of the HALST participants. Systolic blood pressure increased but diastolic blood pressure decreased along with age. In addition, the levels of haemoglobin, albumin, glomerular filtration rate, cholesterol and triglyceride, and gait speed also all decreased with age, whereas the prevalence of under-weight [body mass index (BMI) < 20 kg/m2] increased with age. For those older than 75 years, > 11% and > 6% had BMI < 20 kg/m2 and serum albumin < 4 g/dl, respectively, indicating a risk of malnutrition that medical personnel should be on the alert for. Some common chronic diseases—such as hypertension, diabetes, chronic kidney disease and anaemia—also increased with age. In general, the intake of food and nutrients decreases in older participants, except for beans and dairy intake in males (data not shown). Regarding the physical function performance, the mean gait speed of those older than 75 (0.7 m/s) was slower than the cutoff point suggested in the European consensus, indicating the need for refining the definition of sarcopenia for the Asian population.22 Similar situations can also be found with gender-specific handgrip strength (about 5 kg lower than that of Caucasian counterparts)22 and distance in 6-min walking test (> 15% of those older than 75 years could walk no farther than 250 m).
Table 3.
Baseline biomarker profiles for the study subjects in the HALST cohort
| Total (n = 5664) | 55–64 years (n = 1686) | 65–74 years (n = 2497) | ≥ 75 years (n = 1481) | P | |
|---|---|---|---|---|---|
| BMI (kg/m2) | 24.6 (3.5) | 24.7 (3.5) | 24.7 (3.5) | 24.2 (3.5) | < 0.001 |
| SBP(mmHg) | 128.6 (18.8) | 122.6 (17.3) | 129.4 (18.0) | 134.4 (19.7) | < 0.001 |
| DBP(mmHg) | 70.6 (10.8) | 72.2 (11.1) | 70.7 (10.6) | 68.4 (10.6) | < 0.001 |
| Fasting glucose (mg/dl) | 111.9 (31.4) | 110.0 (29.8) | 113.2 (33.8) | 111.6 (28.7) | 0.007 |
| HbA1c (%) | 6.2 (1.1) | 6.1 (1.0) | 6.3 (1.1) | 6.3 (1.1) | < 0.001 |
| Haemoglobin (g/dl) | 13.6 (1.5) | 14.0 (1.5) | 13.7 (1.4) | 13.2 (1.5) | < 0.001 |
| Albumin (g/dl) | 4.4 (0.2) | 4.4 (0.2) | 4.4 (0.2) | 4.3 (0.2) | < 0.001 |
| GFR (ml/min/1.73 m2) | 83.2 (22.0) | 91.2 (20.4) | 83.6 (21.1) | 72.8 (21.2) | < 0.001 |
| ALT (U/l) | 27.0 (19.0) | 29.3 (21.6) | 27.3 (19.2) | 23.7 (14.2) | < 0.001 |
| AST (U/l) | 28.9 (14.6) | 28.8 (13.8) | 29.2 (16.8) | 28.4 (11.1) | 0.228 |
| Uric acid (mg/dl) | 6.0 (1.6) | 5.8 (1.5) | 5.9 (1.5) | 6.3 (1.6) | < 0.001 |
| LDL-C (mg/dl) | 118.1 (33.1) | 121.4 (33.9) | 118.6 (32.7) | 113.0 (32.3) | < 0.001 |
| HDL-C (mg/dl) | 52.5 (13.7) | 52.8 (13.9) | 52.6 (13.4) | 52.0 (13.8) | 0.288 |
| TG (mg/dl) | 124.1 (87.6) | 131.5 (102.6) | 123.2 (86.6) | 116.7 (66.4) | < 0.001 |
| Gait speed (m/s)a | 0.9 (0.3) | 1.0 (0.3) | 0.9 (0.3) | 0.7 (0.3) | < 0.001 |
| Handgrip strength (kg)b | 28.4 (10.2) | 32.5 (10.3) | 28.3 (9.6) | 23.8 (9.0) | < 0.001 |
| SPPBc | 10.0 (2.0) | 11.0 (1.0) | 11.0 (2.0) | 9.0 (3.0) | < 0.001 |
| 6-min walk (m)d | 382.2 (82.0) | 421.5 (69.6) | 381.2 (74.9) | 328.0 (80.8) | < 0.001 |
| BMI (kg/m2) | < 0.001 | ||||
| < 20 | 432 (8.1) | 107 (6.6) | 174 (7.3) | 151 (11.3) | |
| 20–24.9 | 2672 (50.0) | 824 (50.6) | 1175 (49.5) | 673 (50.3) | |
| 25–29.9 | 1876 (35.1) | 574 (35.3) | 870 (36.6) | 432 (32.3) | |
| ≥ 30 | 360 (6.7) | 123 (7.6) | 156 (6.6) | 81 (6.1) | |
| Hypertensione | 3017 (53.3) | 657 (39.0) | 1370 (54.9) | 990 (66.9) | < 0.001 |
| Diabetesf | 1365 (25.5) | 343 (21.1) | 652 (27.4) | 370 (27.5) | < 0.001 |
| Haemoglobin < 12 g/dl | 609 (11.4) | 118 (7.3) | 242 (10.2) | 249 (18.5) | < 0.001 |
| Albumin < 4 g/dl | 186 (3.5) | 31 (1.9) | 67 (2.8) | 88 (6.6) | < 0.001 |
| GFR < 60 ml/min/1.73 m2 | 696 (13.0) | 91 (5.6) | 255 (10.7) | 350 (26.0) | < 0.001 |
| ACR ≥ 30 mg/g | 1278 (24.0) | 252 (15.6) | 535 (22.6) | 491 (36.7) | < 0.001 |
| LDL-C ≥ 200 mg/dl | 74 (1.4) | 30 (1.8) | 34 (1.4) | 10 (0.7) | 0.037 |
| TG ≥ 200 mg/dl | 594 (11.1) | 223 (13.7) | 252 (10.6) | 119 (8.9) | < 0.001 |
| Uric acid ≥ 8 mg/dl | 591 (11.1) | 138 (8.5) | 246 (10.4) | 207 (15.4) | < 0.001 |
| Hepatitis B carrier | 498 (9.4) | 210 (12.9) | 202 (8.5) | 86 (6.5) | < 0.001 |
| Hepatitis C carrier | 273 (5.1) | 73 (4.5) | 119 (5.0) | 81 (6.1) | 0.141 |
| Slow gait speed | |||||
| < 0.8 m/s | 1994 (35.7) | 281 (16.8) | 848 (34.3) | 865 (60.3) | < 0.001 |
| < 0.7 m/s | 1298 (23.3) | 131 (7.8) | 523 (21.1) | 644 (44.9) | < 0.001 |
| Low handgrip strength (kg) | |||||
| male < 30, female < 20 | 1520 (27.1) | 155 (9.3) | 594 (23.9) | 771 (52.8) | < 0.001 |
| male < 25, female < 15 | 616 (11.0) | 49 (2.9) | 184 (7.4) | 383 (26.2) | < 0.001 |
| SPPB ≤ 9 | 1199 (21.8) | 105 (6.3) | 452 (18.5) | 642 (45.9) | < 0.001 |
| 6-min walk < 250 m | 293 (5.9) | 18 (1.1) | 103 (4.6) | 172 (15.4) | < 0.001 |
Data are n (%) unless indicated otherwise.
BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; GFR, glomerular filtration rate; ALT, alanine aminotransferase; AST, aspartate aminotransferase; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; TG, triglyceride; ACR, urine albumin-to-creatinine ratio.
a5580 study subjects had received measurement of gait speed.
b5612 study subjects had received measurement of handgrip strength.
c5490 study subjects had received measurement of SPPB.
d4954 study subjects had received measurement of 6-min walk.
eHypertension: SBP ≥ 140 mmHg, or DBP ≥ 90 mmHg, or taking anti-hypertensive drugs.
fDiabetes: fasting glucose ≥ 126 mg/dl, or HbA1c ≥ 6.5%, or taking anti-diabetic drugs.
Among those who had completed the first annual telephone interview (n = 4930), 123 (2.49%) had a new diagnosis of hypertension, 99 (2.01%) developed diabetes, 34 (0.69%) had stroke and 38 (0.77%) had cancer diagnosed in the previous year of the first telephone survey. We also found, in the previous year, 752 (15.26%) had falls, 589 (11.95%) had been admitted to hospitals, 267 (5.53%) had body weight loss of more than 3 kg and 52 (1.09%) had various degrees of depression syndrome [BSRS-5 score 10–14: 45 (0.94%), score ≥ 15: 7 (0.15%)].
In addition to the unique characteristics described above, several interesting results have been found in the HALST study. For example, we found a strong relationship between dietary fibre intake and physical performance in the elderly, providing potential practical preventive strategies for frail older adults.23 Those who had higher education, higher BMI and lower fish and milk intake were found to be more likely to have vitamin D insufficiency.24 The results of gait speed and handgrip strength performed by the HALST participants were adopted to refine cutoffs and prevalence of sarcopenia in Taiwan.25 We also illustrated a synergistic impact of sarcopenia and obesity on elders’ physical performance.26
The HALST study is a member of the TaiChi consortium, joining international efforts to identify genetic determinants of atherosclerosis and metabolic-related traits in multi-ethnic populations. For the past 3 years, collaboration within the TaiChi consortium has been fruitful. For example, four new genetic loci have been found related to obesity;27 some novel genetic variants associated with HbA1c, plasma triglycerides and risk of coronary artery disease were identified;28,29 a novel independent type 2 diabetes locus was found in the Chinese population;30 and some other important findings were also published in renowned journals.31–37 By linking with the NHID, we have recently conducted a prospective study and found that the older adults performing a healthy lifestyle (higher diet score, physical activity and psychosocial score) would be less likely to develop diabetes (manuscript under revision). More findings based on the follow-up data will be realized when we finish the second wave which started in 2014. The HALST, because of its prospective nature and extended data linkage, is a good epidemiological research platform to better understand multidimensional health risks in the elderly.
What are the main strengths and weaknesses?
Strengths
The HALST study has several strengths. First, composed of comprehensive geriatric assessment and extended biochemical and genetic measurements, the HALST is more feasible, compared with other Chinese longitudinal ageing studies, to investigate factors related to healthy ageing. We have established some international collaboration to conduct genetic and biomarker studies for ageing-related genetic traits. Results from the HALST study will provide information unique to Asian societies and allow a direct comparison with those from Western countries which differ in lifestyle and in genetic and environmental characteristics. Second, the study design includes data linkage with National Health Insurance databases, the mortality registry, the cancer registry, and medical records, which allows a tracking of participant incidence of health-related events and use of healthcare. Third, the HALST has a close link with the Chicago Healthy Aging Study.38 Most methods of procedures (MOP) between these two studies are similar. This provides opportunities to make ethnicity and cross-country comparisons in various geriatric research issues. For example, researchers on both sides have recently been working on developing and cross-validating a sensitive but important questionnaire about filial piety which is unique to Chinese culture. Finally, all measurements in the HALST have been conducted by a well-trained team containing 15 fieldworkers who are trained to strictly follow the study protocols. Data collection, management, validation and processing are also being carried out to an exceptionally high standard.
Weaknesses
First, the HALST cohort is not a completely random sample from the elderly population in Taiwan; instead, the study is targeted at recruiting enough people with different socio-demographic backgrounds. This incomplete representation of Taiwan’s general population limits the data applicability for estimation of disease prevalence. However, our study focuses on searching for the risk factors of ageing-related diseases and conditions, so the sampling effects would be minimal. Second, as with other longitudinal studies for ageing, response rate, sample attrition and missing data are always great concerns in the interpretation of the results. Third, although our study has a relatively large sample size, several years of longitudinal observations are necessary before it can obtain statistical power for new outcomes.
Can I get hold of the data? Where can I find out more?
The HALST study group encourages domestic and international research collaboration. To learn more about the HALST study, access the data and explore potential collaboration, please contact the principal investigator, Dr. Chao A. Hsiung [hsiung@nhri.org.tw].
HALST in a nutshell
HALST is a prospective cohort study aiming at investigating multidimensional determinants of healthy aging—including lifestyle behaviours and genetic, metabolic and inflammatory factors—in an older Asian population.
A total of 5664 community-dwellers aged 55 and over, recruited from seven selected cities/counties to represent the socio-demographic diversity of the Taiwanese population, participated in home interviews and hospital-based clinical examinations in the first-wave survey (2009–13).
Participants have annual telephone contact to update health-related conditions and hospitalized events. The HALST dataset has been linked to Taiwan’s National Health Insurance database, the national mortality registry, the national cancer registry and medical records.
The HALST dataset comprises a broad scope of measurements, including socio-demographic information, lifestyle pattern, dietary habit, metabolic profile, inflammatory biomarkers, cognitive function, depression assessment, physical function, medication and genetic components.
The first-wave HALST data (2009–13) have been compiled and are available for analysis; the second-wave survey (2014–19) is ongoing. Further enquiries about research collaboration should be addressed to the principal investigator, Dr. Chao A. Hsiung [hsiung@nhri.org.tw].
Funding
This study was supported by the National Health Research Institutes in Taiwan [project no. BS-097-SP-04, PH-098-SP-02, PH-099-SP-01, PH-100-SP-01, PH-101-SP-01, PH-102-SP-01, PH-103-SP-01, PH-104-SP-01].
Acknowledgements
We would like to thank the members of the advisory committee for the HALST study (Drs Luigi Ferrucci, Jack M. Guralnik, Dilip V. Jeste and Kung-Yee Liang) who have continued to monitor the study’s progress and provide valuable suggestions. We are also grateful to the HALST participants for their willingness to join this study and to the HALST staff for their high-quality work.
Conflict of interest: None declared.
References
- 1. Central Intelligence Agency. (2016). Life expectancy at birth in Taiwan (2016 est.) In The world factbook. Retrieved from https://www.cia.gov/library/publications/the-world-factbook/fields/2102.html#tw.
- 2. National Development Council, Department of Human Resources Development. Population Projections for Republic of China (Taiwan), 2014–2060. http://www.ndc.gov.tw/Content_List.aspx?n = 5B78EEBCE18CBE9F (14 October 2015, date last accessed).
- 3. Lan TY. Population aging in Taiwan: future health implications. Taiwan J Public Health 2003;22:237–44. [Google Scholar]
- 4. Ministry of Health and Welfare. 2013 Statistics of Cause of Death. http://www.mohw.gov.tw/EN/Ministry/Statistic_P.aspx?f_list_no = 474&fod_list_no = 5045&doc_no = 45981 (14 October 2015, date last accessed).
- 5. Wen CP, Cheng TY, Tsai MK. et al. All-cause mortality attributable to chronic kidney disease: a prospective cohort study based on 462 293 adults in Taiwan. Lancet 2008;371:2173–82. [DOI] [PubMed] [Google Scholar]
- 6. United States Renal Data System. 2014 USRDS Annual Data Report. http://www.usrds.org/adr.aspx (14 October 2015, date last accessed).
- 7. Zeng Y. Introduction to the Chinese Longitudinal Healthy Longevity Survey (CLHLS). In: Healthy Longevity in China: Demographic, Socioeconomic, and Psychological Dimensions. Dordrecht, The Netherlands: Springer Publishing, 2008. [Google Scholar]
- 8. Zhao Y, Hu Y, Smith JP, Strauss J, Yang G. Cohort Profile: The China Health and Retirement Longitudinal Study (CHARLS). Int J Epidemiol 2014;43:61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jiang J, Tang Z, Meng XJ, Futatsuka M. Demographic determinants for change in activities of daily living: a cohort study of the elderly people in Beijing. J Epidemiol 2002;12:280–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bureau of Health Promotion at the Department of Health in Taiwan. Taiwan Longitudinal Study on Aging (TLSA). http://www.hpa.gov.tw/English/ClassShow.aspx?No = 200803270009 (3 October 2016, date last accessed).
- 11. Niti M, Yap KB, Kua EH, Tan CH, Ng TP. Physical, social and productive leisure activities, cognitive decline and interaction with APOE-epsilon4 genotype in Chinese older adults. Int Psychogeriatr. 2008;20:1–15. [DOI] [PubMed] [Google Scholar]
- 12. Cornman JC, Glei DA, Goldman N. et al. Cohort Profile: The social environment and biomarkers of aging study (SEBAS) in Taiwan. Int J Epidemiol 2016;45:54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Folstein MF, Folstein SE, McHugh PR. ‘Mini-mental state’. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–98. [DOI] [PubMed] [Google Scholar]
- 14. Pan WH, Hung YT, Shaw NS. et al. Elderly Nutrition and Health Survey in Taiwan (1999–2000): research design, methodology and content. Asia Pac J Clin Nutr 2005;14:203–10. [PubMed] [Google Scholar]
- 15. Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist 1969;9:179–86. [PubMed] [Google Scholar]
- 16. Chen YJ, Dai YT, Yang CT. et al. A Review and Proposal on Patient Classification in Long-term Care System. Taipei: Department of Health, 1995. [Google Scholar]
- 17. Ofstedal MB, Zimmer ZS, Lin HS. A comparison of correlates of cognitive functioning in older persons in Taiwan and the United States. J Gerontol B Psychol Sci Soc Sci 1999;54:S291–301. [DOI] [PubMed] [Google Scholar]
- 18. Chien CP, Cheng TA. Depression in Taiwan: epidemiological survey utilizing CES-D. Seishin Shinkeigaku Zasshi 1985;87:335–38. [PubMed] [Google Scholar]
- 19. Lu JFR, Tseng HM, Tsai YJ. Assessment of health related quality of life in Taiwan. (I): development and psychometric testing of SF-36 Taiwan Version. Taiwan J Public Health 2002;22:501–11. [Google Scholar]
- 20. Tseng HM, Lu JFR, Tsai YJ. Assessment of health-related quality of life (II): norming and validation of SF-36 Taiwan version. Taiwan J Public Health 2002;22:512–18. [Google Scholar]
- 21. Lee MM, Chang IY, Horng CF, Chang JS, Cheng SH, Huang A. Breast cancer and dietary factors in Taiwanese women. Cancer Causes Control 2005;16:929–37. [DOI] [PubMed] [Google Scholar]
- 22. Cruz-Jentoft AJ, Baeyens JP, Bauer JM. et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010;39:412–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wu IC, Chang HY, Hsu CC. et al. Association between dietary fiber intake and physical performance in older adults: a nationwide study in Taiwan. PLoS One 2013;8:e80209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chuang SC, Chen HL, Tseng WT. et al. Circulating 25-hydroxyvitamin D and physical performance in older adults: a nationwide study in Taiwan. Am J Clin Nutr 2016;104:1334–44. [DOI] [PubMed] [Google Scholar]
- 25. Wu IC, Lin CC, Hsiung CA. et al. Sarcopenia and Translational Aging Research in Taiwan Team. Epidemiology of sarcopenia among community-dwelling older adults in Taiwan: a pooled analysis for a broader adoption of sarcopenia assessments. Geriatr Gerontol Int 2014;14(Suppl 1):52–60. [DOI] [PubMed] [Google Scholar]
- 26. Chang CI, Huang KC, Chan DC. et al. The impacts of sarcopenia and obesity on physical performance in the elderly. Obes Res Clin Pract 2015;9:256–65. [DOI] [PubMed] [Google Scholar]
- 27. Wen W, Zheng W, Okada Y. et al. Meta-analysis of genome-wide association studies in East Asian-ancestry populations identifies four new loci for body mass index. Hum Mol Genet 2014;23:5492–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chen P, Takeuchi F, Lee JY. et al. Multiple non-glycemic genomic loci are newly associated with blood level of glycated hemoglobin in East Asians. Diabetes 2014;63:2551–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Do R, Willer CJ, Schmidt EM. et al. Common variants associated with plasma triglycerides and risk for coronary artery disease. Nat Genet 2014;45:1345–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kuo JZ, Sheu WH, Assimes TL. et al. Trans-ethnic fine mapping identifies a novel independent locus at the 3’ end of CDKAL1 and novel variants of several susceptibility loci for type 2 diabetes in a Han Chinese population. Diabetologia 2013;56:2619–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Global Lipids Genetics Consortium; Willer CJ, Schmidt EM, Sengupta S. et al. Discovery and refinement of loci associated with lipid levels. Nat Genet 2013;45:1274–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wu Y, Waite LL, Jackson AU. et al. Trans-ethnic fine-mapping of lipid loci identifies population-specific signals and allelic heterogeneity that increases the trait variance explained. PLoS Genet 2013;9:e1003379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Miller CL, Anderson DR, Kundu RK. et al. Disease-related growth factor and embryonic signaling pathways modulate an enhancer of TCF21 expression at the 6q23.2 coronary heart disease locus. PLoS Genet 2013;9:e1003652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wu Y, Gao H, Li H. et al. A meta-analysis of genome-wide association studies for adiponectin levels in East Asians identifies a novel locus near WDR11-FGFR2. Hum Mol Genet 2014;23:1108–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Assimes TL, Lee IT, Juang. et al. Genetics of coronary artery disease in Taiwan: A cardiometabochip study by the TAICHI consortium. PloS One 2016;11:e0138014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Knowles JW, Xie W, Zhang Z. et al. Identification and validation of N-acetyltransferase 2 as an insulin sensitivity gene. J Clin Invest 2015;125:1739–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Voight BF, Kang HM, Ding J. et al. Correction: The metabochip, a custom genotyping array for genetic studies of metabolic, cardiovascular, and anthropometric traits. PLoS Genet 2012;8:e1002793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pirzada A, Reid K, Kim D. et al. Chicago healthy aging study: objectives and design. Am J Epidemiol 2013;178:635–44. [DOI] [PMC free article] [PubMed] [Google Scholar]